Abstract
Elevated levels of plasma homocysteine (Hcy) called hyperhomocysteinemia (HHcy) have been implicated in inflammation and remodeling in intestinal vasculature, and HHcy is also known to aggravate the pathogenesis of inflammatory bowel disease (IBD). Interestingly, colon is the pivotal site that regulates Hcy levels in the plasma. We hypothesize that HHcy decreases intestinal motility through matrix metalloproteinase-9 (MMP-9)-induced intestinal remodeling leading to constipation. To verify this hypothesis, we used C57BL/6J or wild-type (WT), cystathionine β-synthase (CBS+/−), MMP-9−/−, and MMP-9−/− + Hcy mice. Intestinal motility was assessed by barium meal studies and daily feces output. Plasma Hcy levels were measured by HPLC. Expression of ICAM-1, inducible nitric oxide synthase, MMP-9, and tissue inhibitors of MMPs was studied by Western blot and immunohistochemistry. Reactive oxygen species (ROS) including super oxide were measured by the Invitrogen molecular probe method. Tissue nitric oxide levels were assessed by a commercially available kit. Plasma Hcy levels in the treated MMP-9 group mice were comparable to CBS+/− mice. Barium meal studies suggest that intestinal motility is significantly decreased in CBS+/− mice compared with other groups. Fecal output-to-body weight ratio was significantly reduced in CBS+/− mice compared with other groups. There was significant upregulation of MMP-9, iNOS, and ICAM-1 expression in the colon from CBS+/− mice compared with WT mice. Levels of ROS, superoxide, and inducible nitric oxide were elevated in the CBS+/− mice compared with other groups. Results suggest that HHcy decreases intestinal motility due to MMP-9-induced intestinal remodeling leading to constipation.
Keywords: matrix metalloproteinase-9, intestinal remodeling
hyperhomocysteinemia (HHcy) is well-established risk factor for various disease conditions including ischemic heart disease, chronic kidney disease, cerebrovascular disease, and various vascular pathologies. It has been recognized that HHcy is a risk factor for both arterial and venous thrombosis including mesenteric ischemia and thrombosis (2, 8, 15, 35). Recent studies (10, 33) have explored the relation between HHcy and inflammatory bowel disease (IBD) condition. According to Centers for Disease Control and Prevention, IBD is one of the most common gastrointestinal diseases in the United States, with healthcare cost averaging more than $1.7 billion. Globally, the prevalence of IBD has reached up to 396/100,000 persons (23) affecting the quality of life.
Interestingly, clinical studies (38) have reported that HHcy is more common in IBD patients compared with healthy controls and is associated with lower vitamin B12 levels. The gastrointestinal tract (GIT) is a significant site of sulfur amino acid metabolism. The gastrointestinal tract accounts for ∼25% of whole body transmethylation and transsulfuration pathways and is a prominent site of net Hcy release (3, 36). Previous studies (7) have shown that exposure of Hcy to isolated human intestinal microvascular endothelial cells triggers surface expression of cell adhesion molecules (ICAM-1) and contributes to the accumulation of inflammatory infiltrate and also aggravates IBD condition. HHcy due to methylene tetrahydrofolate reductase (MTHFR) gene polymorphism was reported as one of the risk factors of mesenteric venous thrombosis leading to infarction of the bowel (18). In addition, increased prevalence of MTHFR gene polymorphism has been reported in colorectal cancer and IBD patients (4, 34). As the enzyme MTHFR plays a critical role in the remethylation pathway of Hcy metabolism, polymorphism/mutation of MTHFR results in elevated levels of Hcy in the plasma, particularly in folate deficiency (43). Furthermore, IBD patients were found to have lower levels of folate and higher levels of Hcy in the circulation (20).
HHcy has been associated with inflammation and remodeling. The inflammatory response represents coordinative proteolytic activity in a facet of remodeling and disintegration. Studies (31) have reported that during experimental colitis inflammation induces endothelial-dependent arteriolar dysfunction and results in decreased blood flow. Similarly, we (14, 32) reported that during HHcy there is rarefaction and narrowing of vessels in the gut microvasculature. Furthermore, studies (46) have shown Hcy causes upregulation of inducible nitric oxide synthase (iNOS), which further contributes towards the inflammatory response. iNOS was further shown to initiate complex inflammatory changes during hemorrhagic shock leading to morphological and functional intestinal injury (16). Along the same line, another inflammatory molecule, ICAM-1, was shown to impair intestinal smooth muscle contractility during hemorrhagic shock (21). Interestingly, anti-inflammatory medications such as resveratrol and aspirin have been found to have beneficial role in lowering Hcy levels (40).
Matrix metalloproteinases (MMPs) were found to play an important role in the pathophysiology of several intestinal inflammatory disorders (28). MMP-9 has been associated with mucosal damage during intestinal inflammation, whereas MMP-2 was found to be protective (12). Previous reports (9) suggest that inhibiting MMPs has a therapeutic potential in targeting intestinal inflammation such as during colitis. Besides the regular symptoms of abdominal pain and diarrhea, constipation can also be a symptom of IBD. The constipation that is present in these cases is due to bowel obstruction and slowed transit. Drug-induced mal absorption of vitamins during active IBD results in deficiency of folate as well as vitamin B12. Furthermore, vitamin B12 deficiency has also been implicated as a digestive disorder resulting in constipation due to reduced gastrointestinal motility (43).
In current study, we tried to evaluate the role of MMP-9 in inducing intestinal remodeling during HHcy and thus leading to decreased intestinal motility or constipation. To our knowledge, this is the first study to explore the effect HHcy on colon transit time and its role in intestinal remodeling.
MATERIALS AND METHODS
Antibodies and reagents.
Rabbit polyclonal antibodies to MMP-9, tissue inhibitors of MMP-1 (TIMP-1), and iNOS were purchased from Abcam (Cambridge, MA), and rabbit polyclonal antibodies to TIMP-4 were from Sigma-Aldrich (St. Louis, MO). Goat polyclonal and rabbit polyclonal antibodies to ICAM-1 and SOD-1 antibody were procured from Santa Cruz Biotechnology (Santa Cruz, CA). PVDF membrane was from Bio-Rad (Hercules, CA).
Animals.
Male C57BL/6J wild-type mice (8–10 wk old), cystathionine β-synthase [CBS+/−] heterozygous mice (HHcy), and MMP-9−/− mice were obtained from Jackson Laboratory (Bar Harbor, ME) and kept in the animal care facility of University of Louisville where ambient environmental conditions (12:12-h light-dark cycle, 22–24°C) were maintained. CBS heterozygous knockout mice on standard chow are well-known models of HHcy (26). The background strain for both CBS+/− and MMP-9−/− is C57BL6/J, and it serves as control for both groups (Jackson mice data reference). Recent literature also confirmed the C57BL/6 background for CBS+/− mice. This is reported in the article under the animal detail section in Ref. 26.
The animals were fed standard chow and water ad libitum. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville, School of Medicine in accord with Animal Care and Use Program Guidelines of the National Institutes of Health.
Groups.
Wild-type (C57BL/6J), CBS+/−, MMP-9−/−, and MMP-9−/− mice with high Hcy diet. Hcy was supplemented through drinking water and high methionine diet. A minimum number of five animals were used for each of the studies performed.
Body weight/stool weight.
Animals were fed similar amount of feed, and daily fecal output was measured and normalized with body weight ratio.
Tissue extraction.
Abdominal cavity was exposed by a midline laparotomy, and the entire colon was removed from the caecum to the anus. The length of the colon was measured, the colon was flushed with cold PBS, and tissue obtained from each colon was processed for further investigations.
Immunoblot studies of MMP-9, TIMP-4, iNOS, and ICAM-1.
Western blot analyses were performed on colonic tissue homogenates using 10% SDS-PAGE as described previously (44). The BCA method was used to estimate total protein, and 25 μg of protein were loaded in each well of electrophoresis gels. After electrophoresis, proteins were transferred to PVDF, blocked with 5% fat-free milk, and blotted with respective antibodies. As a loading control, GAPDH was used. The bands were normalized with GAPDH controls.
Gelatin-zymography.
Colon was harvested and cleaned minutely of any fecal attachment and from blood by being flushed with PBS and observed under microscope. For each zymogram, four colons were homogenized in buffer containing 50 mM Tris·HCl pH 7.6, 5 mM CaCl2, 150 mM NaCl, 0.05% Brij35 (Sigma, St. Louis, MO), and 0.02% NaN3. The supernatant was collected, and estimation of protein was done using BCA assay kit (Pierce, Rockford, IL). The supernatant containing eluted protein was then collected after centrifugation (10 min at 10,000 g). Each sample was mixed with an equal volume of 2× zymography loading buffer (Bio-Rad), incubated for 10 min at room temperature, and then separated on a 10% gelatin zymogram gel (Bio-Rad). Gels were soaked in 1× zymogram renaturation buffer for 15 min at room temperature; this was repeated three times. The gel was incubated in 1× zymogram developing buffer for 24 h at 37°C, stained with Coomassie blue (1%) in a 30% methanol and 10% acetic acid 1 h, destained in water, and developed using Gel-Doc.
Barium meal method.
To study the gut motility in mice, we used barium meal preparation. Barium meal is very popular method of studying the gut abnormalities in pediatric and adult populations. For the first time we used similar technique in mice to study the motility differences between the study groups. Briefly, 1 g of barium sulfate (BaSo4) contrast is mixed in 5 ml of water and thoroughly mixed to make an aqueous mixture. Mice weighing 30 g were used and were deprived of food for overnight and water for 2 h. Under pentobarbital anesthesia, animals were instilled with 0.3 ml of BaSo4 aqueous mixture by oral gavage. Mice were then killed after watching for a fixed time period of 7 h. The whole intestine from duodenum to rectum was harvested and radiographed using Kodak In-Vivo Imaging system FX Pro (Care stream Health). Radio opaque barium sulfate is visualized through X-ray images. The distance barium travelled measures the intestinal motility.
Cryosectioning.
The colon tissue was excised and cryopreserved in Peel-A-Way disposable plastic tissue-embedding molds (Polysciences, Warrington, PA) containing tissue-freezing media (Triangle Biomedical Sciences, Durham, NC). These molds were kept frozen (−70°C) until serial 8-μm tissue sections were made in Cryocut 1800 (Reichert-Jung). Cryosections were placed on Superfrost/plus microscope slides and air dried.
Histological studies.
Hematoxylin and eosin staining showing general colon histology in all groups was done using commercially available kit. Similarly elastin and collagen staining was done on frozen colon sections using commercially available kits from Fisher Scientific/Richard Allan Scientific following their staining protocol.
Immunofluorescence.
Immunofluorescence staining was performed on 5-μm thick frozen sections of the colon tissue with MMP-9 and TIMP-1 primary antibodies and secondarily stained with Alexa Fluor 488 (Invitrogen) to detect protein expression.
RNA isolation and real-time PCR.
RNA was isolated from colon samples using commercially available kit from Promega (catalog no. A3800). The purity of RNA was assessed by nano drop, and only pure RNA was used for real-time PCR by the Syber green method. PCR for cDNA and real time was set up as mentioned elsewhere (30).
Plasma Hcy measurement.
Plasma Hcy levels in all the animal groups were measured using high-performance liquid chromatography as previously described (41).
Nitric oxide assay.
Nitric oxide (NO) assay was performed as mentioned elsewhere (26). Briefly, tissue homogenates were prepared from the animal groups and filtered through 30-kDa molecular mass cutoff microfuge ultrafiltration device (Amicon, Millipore, MA) and filtrate was used for analysis. Total nitrate and nitrite was measured using nitrate and nitrite calorimetric assay kit (780001; Cayman chemical, Ann Arbor, MI) according to manufacturer's instructions. After the assay was performed, absorbance at 540 nm was measured in the samples and standards using spectrophotometer and results were expressed as NO in μM.
Superoxide and reactive oxygen species measurements.
Superoxide and reactive oxygen species (ROS) were measure in frozen tissue sections using molecular probes from Invitrogen as described earlier (1, 24). Fluorescence intensity was measured using confocal microscope, and images were analyzed using Image Proplus software.
Statistics.
All data are expressed as means ± SE. One-way ANOVA was performed to test for treatment effects, and differences between groups were determined using Tukey's post hoc test. A P value <0.05 was considered to be significant.
RESULTS
Fecal output is decreased in HHcy mice.
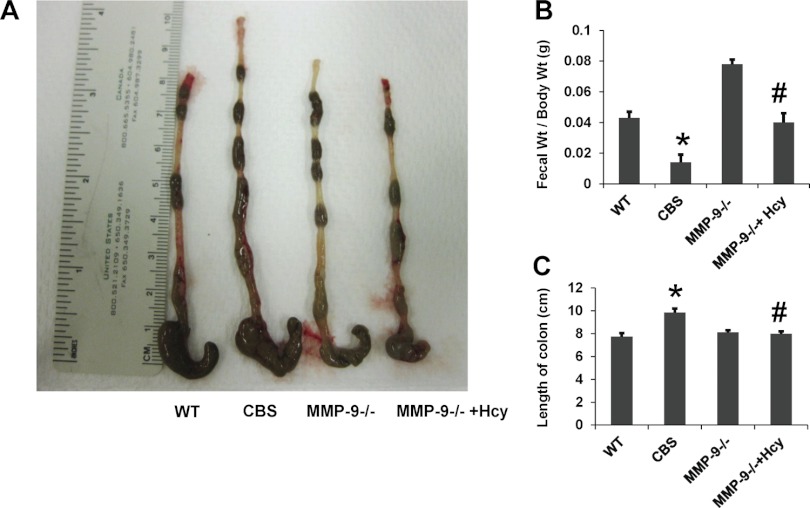
Differences in morphological parameters such as the body weight, stool, and colon length measurement are examined in all the four groups. We found that colon length in the CBS mice was significantly greater than the rest of the groups with a large amount of residual stool (Fig. 1, A and C). Although the animals used in this study were ∼27–30 g in weight, we used the stool weight and body weight ratio to normalize the stool weight data. The body weight-to-stool weight ratio was significantly less in CBS mice compared with remaining groups and normalized in Hcy-treated MMP-9 mice (Fig. 1B).
Fig. 1.
A: gross images of colon from all the 4 groups of mice showing residual stool content. B: bar graph showing the data from stool/fecal weight (FWt) over body weight (BWt) in grams. C: bar graph depicting the data from length of the colon in all groups of mice. *P < 0.05, compared with wild-type (WT) group and #P < 0.05, compared with cystathionine β-synthase (CBS+/−) group. MMP-9, matrix metalloproteinase-9; Hcy, homocysteine. Data represent means ± SE from n = 5 per group.
Intestinal motility is decreased in HHcy due to MMP-9-induced intestinal remodeling.
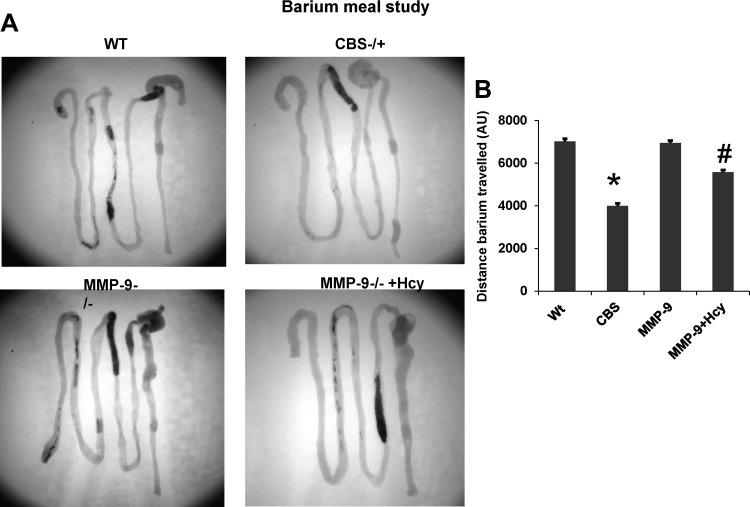
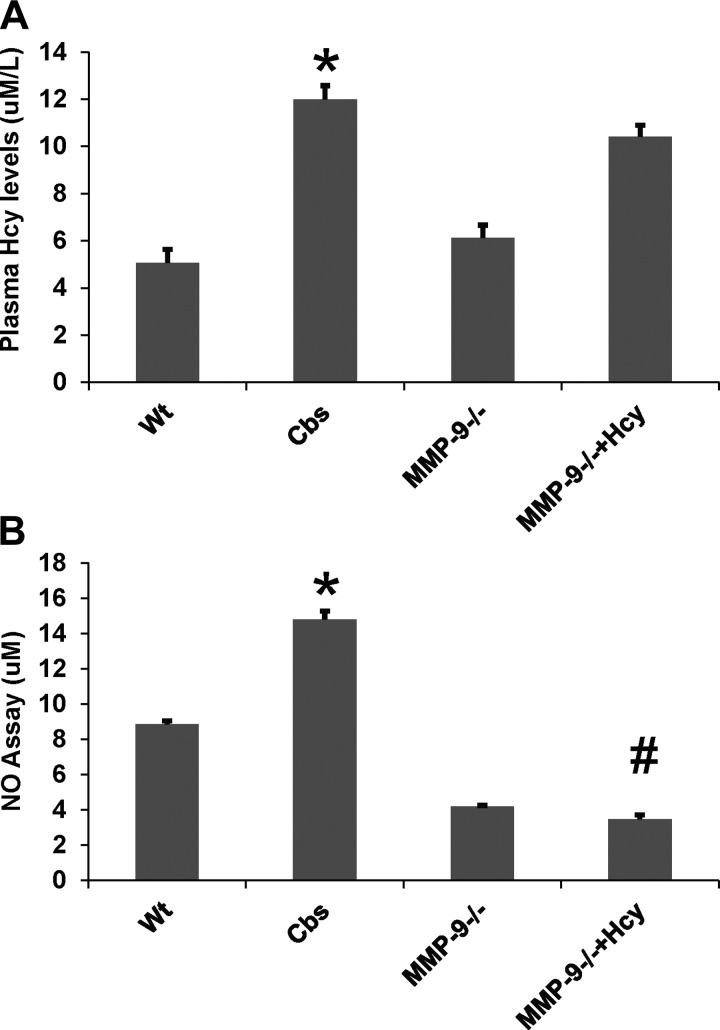
Intestinal motility was assessed by the barium meal study with X-ray machine, and the farther the barium travelled in given time, the faster is the intestinal motility. The data suggest that barium travelled at a slower rate in HHcy CBS+/− mice, suggesting decreased intestinal motility compared with other groups. Interestingly, we found that intestinal motility is increased in Hcy-treated MMP-9−/− mice, suggesting the role of MMP-9 in decreased intestinal motility during HHcy (Fig. 2). We also report that plasma Hcy levels assessed by HPLC method showed elevated levels of Hcy in MMP-9−/−-treated mice that are comparable to HHcy CBS+/− mice (see Fig. 13A).
Fig. 2.
Barium meal study showing the differences in intestinal transit among the groups of mice. The farther the barium travelled, the faster is the motility. The distance barium travelled is measured with Image Pro software, and the data are depicted in arbitrary units (AU). Barium travelled at a much slower rate in CBS+/− mice representing decreased motility or constipation. Quantified data from the image are depicted in bar diagram. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Muscularis mucosa and elastin is decreased in HHcy mice.
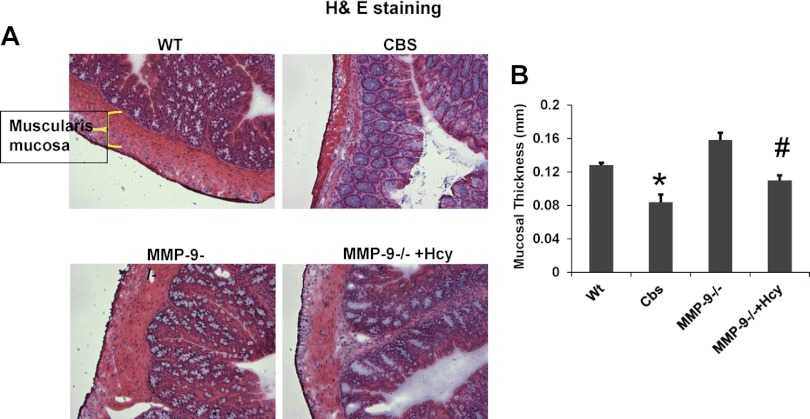
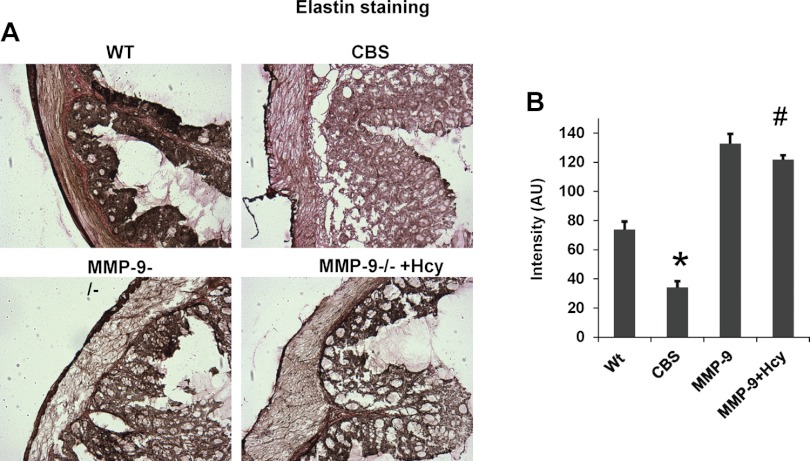
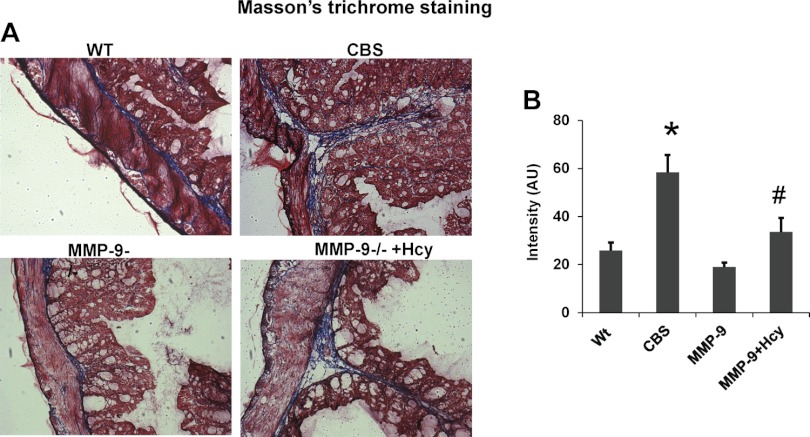
Histological examination of the colonic sections by hematoxylin and eosin, elastin staining, and Masson's trichrome staining suggests gross histological variation in CBS+/− mice compared with other group of mice. The thickness of muscularis mucosa and elastin content was significantly decreased in CBS+/− mice whereas collagen content assessed by Masson's trichrome staining was significantly increased compared with rest of the groups. Other histological inflammatory changes such as ulceration, crypt dilation, and goblet cell depletion are found in CBS colonic architecture. Interestingly, muscularis mucosa and elastin were restored to normal and collagen content is decreased in Hcy-treated MMP-9−/− mice, suggesting the role of MMP-9 in inducing these changes (Figs. 3, 4, and 5).
Fig. 3.
Hematoxylin and eosin (H&E) staining in frozen colon sections in WT, CBS+/−, matrix metalloproteinase-9 (MMP-9−/−) and Hcy-treated MMP-9−/− groups. The thickness of muscularis mucosa assessed by Image Proplus software is depicted in bar diagram. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 4.
A: elastin staining using commercially available kit was done in colon tissue sections from all the 4 groups. Elastin fibers are stained as brown-black color and collagen in red color. B: elastin content was assessed by Image Pro software, and intensity data (AU) are represented in bar graphs. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 5.
A: collagen content was assessed by Mas O2-n's trichrome staining kit. Collagen is stained as blue color. B: bar graphs represent the quantified blue color intensity by Image Pro software in AU. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Expressions of MMP-9, ICAM, and iNOS are increased in CBS+/− mice.
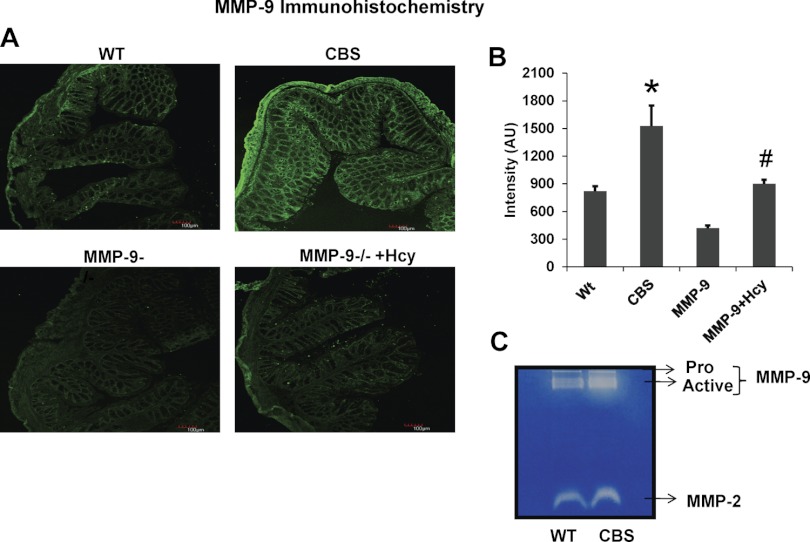
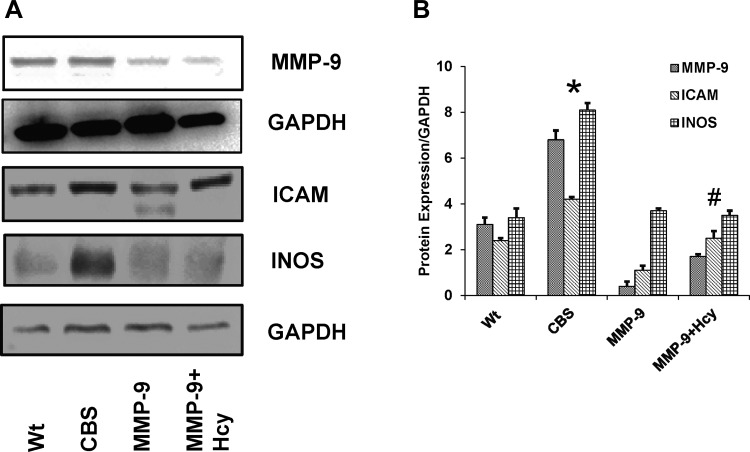
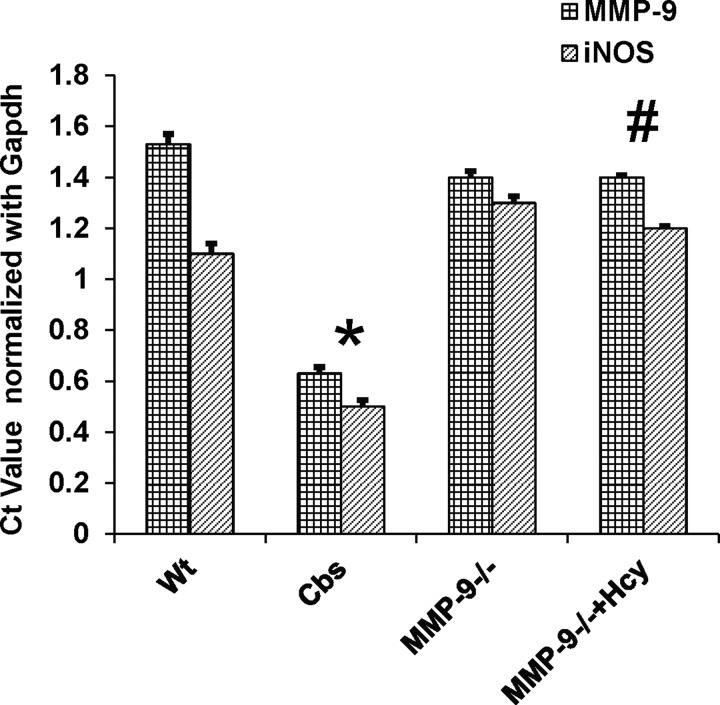
To provide further insight into the molecular mechanisms responsible for gross morphological changes in the colons from HHcy mice, protein expression of inflammatory cytokine, iNOS, and adhesion molecule ICAM-1 was measured. To understand the role of MMP-9 in colonic inflammation in CBS+/− mice immunoblot and immunohistochemistry assessing MMP-9, ICAM and INOS were performed (Figs. 6 and 7). The results showed significant upregulation of MMP-9 along with other inflammatory markers like ICAM and iNOS in the colons of CBS+/− mice compared with WT mice, suggesting the possible role of MMP-9 in induced inflammatory changes during HHcy. mRNA expression assessed by real time PCR showed increased expression of MMP-9 and iNOS in HHcy CBS+/− mice compared with other groups (Fig. 8).
Fig. 6.
A: immunohistochemistry data showing MMP-9 expression secondarily stained with Alexa fluor 488 (green intensity) in all the 4 groups. B: bar graphs representing the intensity (AU) data assessed by Image Pro software. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group. C: zymography data showing MMP-9 and -2 activity in WT and CBS+/− mice. MMP-9 activity is enormously increased in CBS+/− mice compared with WT.
Fig. 7.
A: Western blot data showing protein expression of MMP-9, ICAM, and inducible nitric oxide synthase (iNOS) and the corresponding GAPDH blots. B: bar graphs representing data from densitometry of the protein expression normalized with GAPDH assessed by Bio-Rad software. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 8.
Cycle threshold (Ct) value from real time PCR data was depicted in bar diagram. mRNA expression of MMP-9 and iNOS was significantly increased in CBS+/− mice compared with rest of the groups, which was normalized in MMP-9 knockout mice treated with Hcy. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
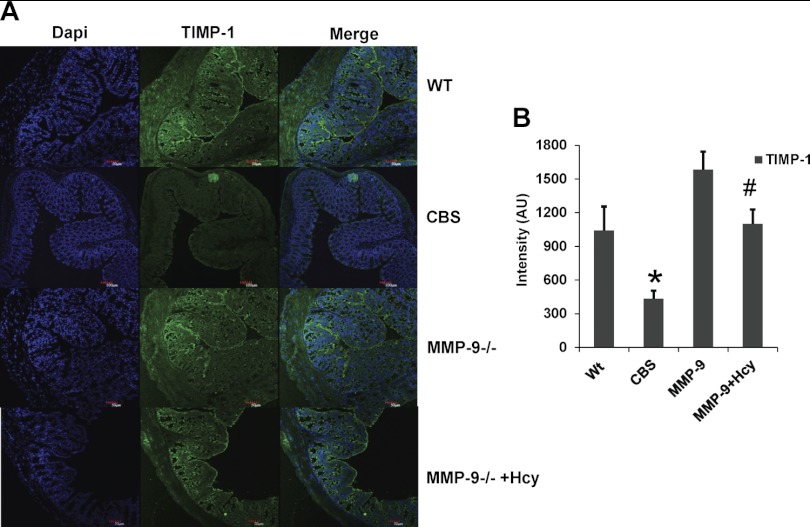
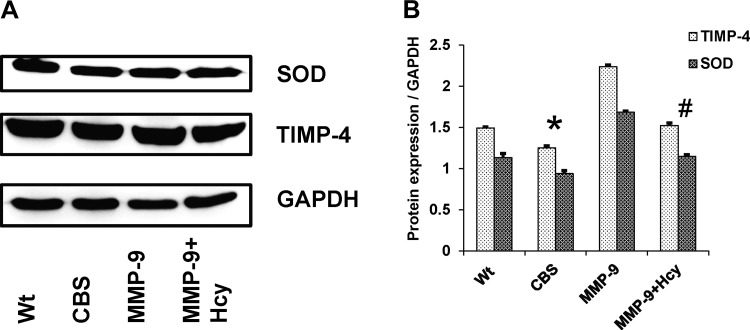
Expression of TIMP-1 and -4 and SOD were decreased in HHcy.
To elucidate possible causes of upregulation of MMP-9, TIMP-1, and TIMP-4 protein levels were determined along with SOD (Fig. Fig. 9). Levels of TIMP-1 and -4 and SOD are decreased in CBS+/− mice compared with other groups, suggesting the decreased antioxidant protection in HHcy (Fig. Fig. 10). Surprisingly, these effects were normalized in MMP-9 knockout mice that are supplemented with Hcy treatment.
Fig. 9.
A: immunohistochemistry data showing tissue inhibitor of MMP-1 (TIMP-1) expression secondarily stained with Alexa fluor 488 (Green intensity) in all the 4 groups. B: bar graphs representing the intensity (AU) data assessed by Image Pro software. *P < 0.05 compared with WT group and #P < 0.05 compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 10.
A: Western blot data showing protein expression of SOD-1 and TIMP-4 and the corresponding GAPDH blots. B: bar graphs representing data from densitometry of the protein expression normalized with GAPDH assessed by Bio-Rad software. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
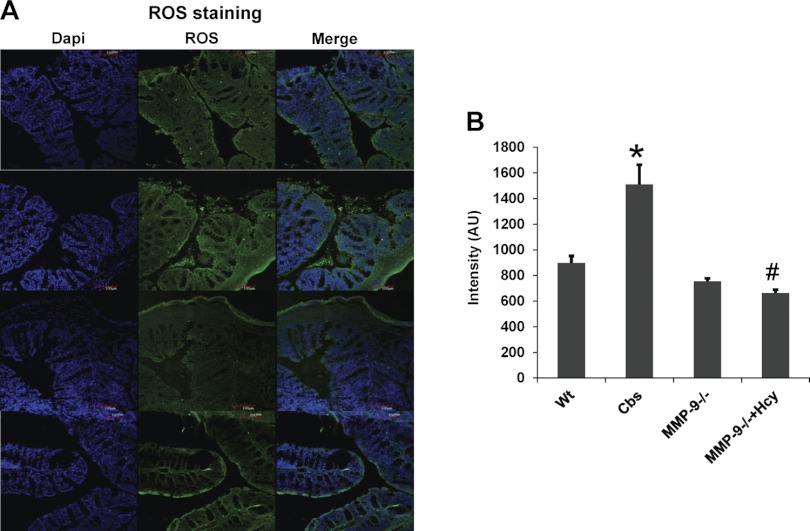
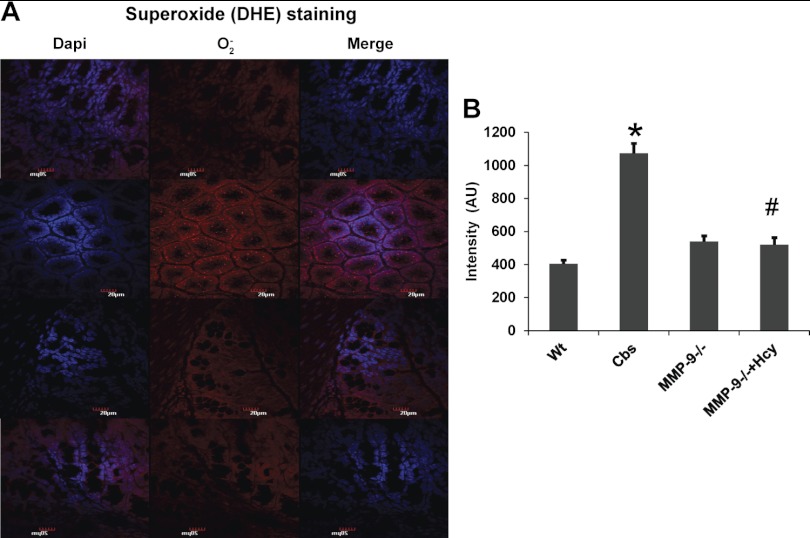
Induced NO and ROS including superoxide (O2·−) expressions were increased in HHcy.
NO assay performed by commercially available kit (Caymen) showed significant increase in expression of induced NO. Interestingly measurement of ROS including O2·− done by molecular probe staining (Invitrogen) showed increased expression in CBS+/− mice, suggesting their role during HHcy (Figs. 11, 12, and 13B).
Fig. 11.
Staining for reactive oxygen species (ROS) showing elevated levels in CBS+/− mice compared with other groups. ROS are stained with green fluorescence intensity and nuclei are stained with DAPI (blue). *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 12.
Superoxide staining with dihydroethidium (DHE) stain from Molecular Probes, Invitrogen showing increased superoxide expression in CBS+/− mice compared with rest of the groups. O2·− is stained with red fluorescence intensity and nuclei with DAPI (blue). *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
Fig. 13.
A: bar graph representing the quantified data from plasma Hcy levels. B: quantified data from nitric oxide (NO) assay are depicted in bar diagram. *P < 0.05, compared with WT group. #P < 0.05, compared with CBS+/− group. Data represent means ± SE from n = 5 per group.
DISCUSSION
The significant association of HHcy to the intestinal inflammation has been well established in previous studies (10, 33). Studies (28, 29) have also found the role of MMP-9 in inducing the inflammation in colitis and other intestinal pathology. Interestingly, MMP-9 inhibitors alleviated these intestinal inflammatory changes in preclinical studies (12). Decreased intestinal motility leading to constipation is also an important clinical symptom during intestinal inflammation or colitis. In our present study, we tried to evaluate the role of MMP-9 in inducing the decreased intestinal motility during HHcy and associated intestinal inflammatory changes by studying histological and molecular inflammatory determinants in WT, CBS+/−, MMP-9−/−, and Hcy-treated MMP-9−/− mice.
The present study showed that the CBS+/−, HHcy mice model has delayed intestinal transits due to elevated levels of inflammatory cytokines and increased expression and activity of MMP-9 in colonic mucosa. In addition, there was a marked reduction in expression of TIMP-1 and TIMP-4 along with SOD. It has been shown previously that colon lengthening slowed intestinal transit due to elongated longitudinal muscle (42). Findings from our study are consistent with this finding as the length of colon in CBS+/− mice is significantly elongated and these mice have lot of residual stool in their colon (Fig. 1). Fecal output is also is significantly low in CBS+/− mice compared with rest of the groups. In support, results from our barium meal study suggest that there is decreased motility in HHcy CBS+/− mice whereas the motility is much improved in MMP-9−/− mice treated with Hcy (Fig. 2).
Elevated levels of Hcy have also been implicated in increased cardiovascular risk and thromboembolic disorders in IBD patients (10). Although there is a higher prevalence of HHcy during intestinal inflammation, the mechanism by which Hcy causes colonic injury and hence reduced colonic motility has not been clearly elucidated. MMP/TIMP axis have been associated to have patho-physiological role in intestinal inflammation (28). MMP-9 expression and activity have been corelated with inflammation and ROS activation. The assessment of MMP-9 activity is a well-established marker for quantifying intestinal inflammation. Also, MMP-9 plays a pivotal role in the Hcy-induced formation of ROS that leads to tissue damage (45). In our study, we showed that Hcy induces MMP-9 activation in the colonic mucosa. Our results are consistent with previous reports (17, 32) showing that Hcy induces MMPs in heart and vascular tissue and hence participates in the remodeling process. Previous reports (12) demonstrated that increased expression of MMP-9 contributes to intestinal inflammation and colitis. In addition, MMP levels have been suggested to be marker for IBD condition (25). TIMP-1 plays a key role in regulating MMPs in the body. Others (37) have shown that Hcy induced upregulation of TIMP-1 in the liver tissue. Interestingly, our study showed decreased expression TIMP-1 and TIMP-4 in the colons of HHcy (CBS+/−) mice. Previous research (5, 39) showed that MMP-9 inhibitor has therapeutic potential in alleviating inflammation associated with IBD. Similarly our study showed that the inflammatory changes, loss of normal histological architecture, and function associated with HHcy CBS+/− mice were abrogated in Hcy-treated MMP-9 knockout mice. These findings suggest the plausible role of MMP-9 in inducing the inflammatory changes and thus decreased motility during HHcy.
It has been postulated that oxy radicals are key mediator in colitis-associated intestinal injury (22). SOD-1 plays a critical role in regulating redox balance and capable of removing excess of oxy radicals such as H2O2. A previous report (19) demonstrated folic acid supplementation ameliorated Hcy-induced suppression of antioxidant enzyme. In the present study, we showed that colonic mucosa from HHcy mice is relatively deficient in antioxidant enzymes such as SOD-1 (Fig. 10). ROS including superoxide levels were increased during HHcy, suggesting their mechanistic role during MMP-9-induced inflammation (Figs. 11 and 12).
HHcy was shown to induce iNOS expression, which further exacerbates the overall inflammation associated with atherosclerosis (47). Furthermore, expression of iNOS and ICAM-1 was found to be elevated in inflamed colons in IBD (6). These reports are consistent with our findings of increased expression of iNOS and ICAM-1 in colon tissue from HHcy, which was normalized in Hcy-treated MMP-9−/− colons. Nitric oxide levels due to iNOS were significantly increased during HHcy, suggesting inflammatory mechanism (Fig. 13B).
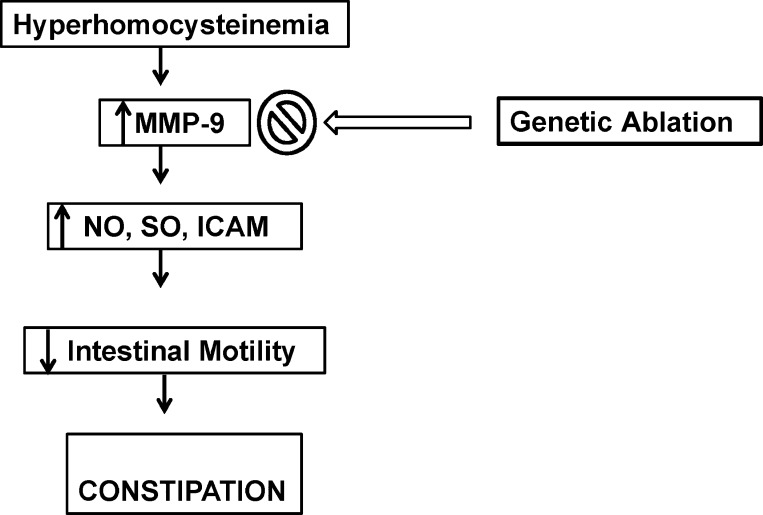
To summarize, the results from our study showed that MMP-9 plays an important role in inducing inflammatory changes and thus decreased intestinal motility (constipation) during HHcy. These changes were nullified in Hcy-treated MMP-9 knockout (MMP-9−/−) mice, suggesting the role of MMP-9. Overall hypothesis of our study is depicted in flow diagram (Fig. 14). To our knowledge, our study is the first to show the association of Hcy-induced constipation to MMP-9 and this further gives insight into the mechanisms involved in constipation during HHcy.
Fig. 14.
Hypothetical representation depicting mechanisms involved in MMP-9-induced intestinal remodeling during hyperhomocyteinemia leading to decreased motility and thus to constipation.
GRANTS
A part of this study was supported by National Institutes of Grants HL-71010 and NS-51568.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G. and C.M. conception and design of research; S.G., C.M., N.N., F.A., G.T., and N.M. performed experiments; S.G., N.N., F.A., and N.M. analyzed data; S.G. interpreted results of experiments; S.G. prepared figures; S.G., C.M., and N.N. drafted manuscript; S.G. and S.C.T. edited and revised manuscript; S.G. and S.C.T. approved final version of manuscript.
REFERENCES
- 1. Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of reactive oxygen species. Protocol Exchange 2008. doi:10.1038/nprot.2008.23 [Google Scholar]
- 2. Bar-Or D, Curtis CG, Sullivan A, Rael LT, Thomas GW, Craun M, Bar-Or R, Maclean KN, Kraus JP. Plasma albumin cysteinylation is regulated by cystathionine beta-synthase. Biochem Biophys Res Commun 325: 1449–1453, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bauchart-Thevret C, Stoll B, Burrin DG. Intestinal metabolism of sulfur amino acids. Nutr Res Rev 22: 175–187, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Cao HX, Gao CM, Takezaki T, Wu JZ, Ding JH, Liu YT, Li SP, Su P, Cao J, Hamajima N, Tajima K. Genetic polymorphisms of methylenetetrahydrofolate reductase and susceptibility to colorectal cancer. Asian Pac J Cancer Prev 9: 203–208, 2008 [PubMed] [Google Scholar]
- 5. Claramunt RM, Bouissane L, Cabildo MP, Cornago MP, Elguero J, Radziwon A, Medina C. Synthesis and biological evaluation of curcuminoid pyrazoles as new therapeutic agents in inflammatory bowel disease: effect on matrix metalloproteinases. Bioorg Med Chem 17: 1290–1296, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Terranova ML, Ciccolo A, Caputi AP. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J Pineal Res 30: 1–12, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Danese S, Sgambato A, Papa A, Scaldaferri F, Pola R, Sans M, Lovecchio M, Gasbarrini G, Cittadini A, Gasbarrini A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol 100: 886–895, 2005 [DOI] [PubMed] [Google Scholar]
- 8. den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost 80: 874–877, 1998 [PubMed] [Google Scholar]
- 9. Di Sebastiano P, di Mola FF, Artese L, Rossi C, Mascetta G, Pernthaler H, Innocenti P. Beneficial effects of Batimastat (BB-94), a matrix metalloproteinase inhibitor, in rat experimental colitis. Digestion 63: 234–239, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Erzin Y, Uzun H, Celik AF, Aydin S, Dirican A, Uzunismail H. Hyperhomocysteinemia in inflammatory bowel disease patients without past intestinal resections: correlations with cobalamin, pyridoxine, folate concentrations, acute phase reactants, disease activity, and prior thromboembolic complications. J Clin Gastroenterol 42: 481–486, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol 296: G175–G184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Givvimani S, Sen U, Tyagi N, Munjal C, Tyagi SC. X-ray imaging of differential vascular density in MMP-9-/-, PAR-1-/+, hyperhomocysteinemic (CBS-/+) and diabetic (Ins2-/+) mice. Arch Physiol Biochem 117: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gradman WS, Daniel J, Miller B, Haji-Aghaii M. Homocysteine-associated acute mesenteric artery occlusion treated with thrombectomy and bowel resection. Ann Vasc Surg 15: 247–250, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 286: G225–G233, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 107: 675–683, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hotoleanu C, Andercou O, Andercou A. Mesenteric venous thrombosis with bowel infarction and hyperhomocysteinemia due to homozygous methylenetetrahydrofolate reductase C677T genotype. Vasc Endovascular Surg 42: 477–481, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Hwang SY, Siow YL, Au-Yeung KK, House J, OK Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol 300: F189–F198, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y, Zhao J, Jiang T, Ge L, Zhou F, Chen Z, Lei Y, Huang S, Xia B. Genetic polymorphism of methylenetetrahydrofolate reductase G1793A, hyperhomocysteinemia, and folate deficiency correlate with ulcerative colitis in central China. J Gastroenterol Hepatol 25: 1157–1161, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Kalff JC, Hierholzer C, Tsukada K, Billiar TR, Bauer AJ. Hemorrhagic shock results in intestinal muscularis intercellular adhesion molecule (ICAM-1) expression, neutrophil infiltration, and smooth muscle dysfunction. Arch Orthop Trauma Surg 119: 89–93, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Korkina L, Suprun M, Petrova A, Mikhal′chik E, Luci A, De Luca C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and Aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors 18: 255–264, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol 12: 6102–6108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lauzier B, Sicard P, Bouchot O, Delemasure S, Menetrier F, Moreau D, Vergely C, Rochette L. After four hours of cold ischemia and cardioplegic protocol, the heart can still be rescued with postconditioning. Transplantation 84: 1474–1482, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Manfredi MA, Zurakowski D, Rufo PA, Walker TR, Fox VL, Moses MA. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 14: 1091–1096, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Mayo JN, Beard RS, Jr, Price TO, Chen CH, Erickson MA, Ercal N, Banks WA, Bearden SE. Nitrative stress in cerebral endothelium is mediated by mGluR5 in hyperhomocysteinemia. J Cereb Blood Flow Metab 32: 825–834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther 318: 933–938, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Medina C, Santana A, Paz MC, Diaz-Gonzalez F, Farre E, Salas A, Radomski MW, Quintero E. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukoc Biol 79: 954–962, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys 55: 153–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mori M, Stokes KY, Vowinkel T, Watanabe N, Elrod JW, Harris NR, Lefer DJ, Hibi T, Granger DN. Colonic blood flow responses in experimental colitis: time course and underlying mechanisms. Am J Physiol Gastrointest Liver Physiol 289: G1024–G1029, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Munjal C, Givvimani S, Qipshidze N, Tyagi N, Falcone JC, Tyagi SC. Mesenteric vascular remodeling in hyperhomocysteinemia. Mol Cell Biochem 348: 99–108, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Oussalah A, Gueant JL, Peyrin-Biroulet L. Meta-analysis: hyperhomocysteinaemia in inflammatory bowel diseases. Aliment Pharmacol Ther 34: 1173–1184, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Papa A, De Stefano V, Danese S, Chiusolo P, Persichilli S, Casorelli I, Zappacosta B, Giardina B, Gasbarrini A, Leone G, Gasbarrini G. Hyperhomocysteinemia and prevalence of polymorphisms of homocysteine metabolism-related enzymes in patients with inflammatory bowel disease. Am J Gastroenterol 96: 2677–2682, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Perry IJ, Refsum H, Morris RW, Ebrahim SB, Ueland PM, Shaper AG. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet 346: 1395–1398, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Riedijk MA, Stoll B, Chacko S, Schierbeek H, Sunehag AL, van Goudoever JB, Burrin DG. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci USA 104: 3408–3413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robert K, Nehme J, Bourdon E, Pivert G, Friguet B, Delcayre C, Delabar JM, Janel N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology 128: 1405–1415, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Romagnuolo J, Fedorak RN, Dias VC, Bamforth F, Teltscher M. Hyperhomocysteinemia and inflammatory bowel disease: prevalence and predictors in a cross-sectional study. Am J Gastroenterol 96: 2143–2149, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Santana A, Medina C, Paz-Cabrera MC, Diaz-Gonzalez F, Farre E, Salas A, Radomski MW, Quintero E. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol 12: 6464–6472, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schroecksnadel K, Winkler C, Wirleitner B, Schennach H, Weiss G, Fuchs D. Anti-inflammatory compound resveratrol suppresses homocysteine formation in stimulated human peripheral blood mononuclear cells in vitro. Clin Chem Lab Med 43: 1084–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol 293: C1779–C1787, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Southwell BR. Colon lengthening slows transit: is this the mechanism underlying redundant colon or slow transit constipation? J Physiol 588: 3343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sturtzel B, Dietrich A, Wagner KH, Gisinger C, Elmadfa I. The status of vitamins B6, B12, folate, and of homocysteine in geriatric home residents receiving laxatives or dietary fiber. J Nutr Health Aging 14: 219–223, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Tyagi N, Kandel M, Munjal C, Qipshidze N, Vacek JC, Pushpakumar SB, Metreveli N, Tyagi SC. Homocysteine mediated decrease in bone blood flow and remodeling: Role of folic acid. J Orthop Res 29: 1511–1516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tyagi N, Moshal KS, Sen U, Lominadze D, Ovechkin AV, Tyagi SC. Ciglitazone ameliorates homocysteine-mediated mitochondrial translocation and matrix metalloproteinase-9 activation in endothelial cells by inducing peroxisome proliferator activated receptor-gamma activity. Cell Mol Biol (Noisy-le-grand) 52: 21–27, 2006 [PubMed] [Google Scholar]
- 46. Welch GN, Upchurch GR, Jr, Farivar RS, Pigazzi A, Vu K, Brecher P, Keaney JF, Jr, Loscalzo J. Homocysteine-induced nitric oxide production in vascular smooth-muscle cells by NF-kappa B-dependent transcriptional activation of Nos2. Proc Assoc Am Physicians 110: 22–31, 1998 [PubMed] [Google Scholar]
- 47. Woo CW, Cheung F, Chan VW, Siow YL, OK Homocysteine stimulates inducible nitric oxide synthase expression in macrophages: antagonizing effect of ginkgolides and bilobalide. Mol Cell Biochem 243: 37–47, 2003 [DOI] [PubMed] [Google Scholar]