Abstract
Hyaluronic acid (HA), a component of the extracellular matrix, affects gastrointestinal epithelial proliferation in injury models, but its role in normal growth is unknown. We sought to determine the effects of exogenous HA on intestinal and colonic growth by intraperitoneal injection of HA twice a week into C57BL/6 mice from 3 to 8 wk of age. Similarly, to determine the effects of endogenous HA on intestinal and colonic growth, we administered PEP-1, a peptide that blocks the binding of HA to its receptors, on the same schedule. In mice treated with exogenous HA, villus height and crypt depth in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon were increased. In mice treated with PEP-1, intestinal and colonic length were markedly decreased and crypt depth and villus height in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon were decreased. Administration of HA was associated with increased levels of EGF (intestine) and IGF-I (colon), whereas administration of PEP-1 was associated with decreased levels of IGF-I (intestine) and epiregulin (colon). Exogenous HA increases intestinal and colonic epithelial proliferation, resulting in hyperplasia. Blocking the binding of endogenous HA to its receptors results in decreased intestinal and colonic length and a mucosal picture of hypoplasia, suggesting that endogenous HA contributes to the regulation of normal intestinal and colonic growth.
Keywords: hyaluronic acid synthase, PEP-1
hyaluronic acid (HA), a glycosaminoglycan polymer with repeating units of disaccharides composed of d-glucuronic acid and N-acetyl-d-glucosamine, is an important constituent of the extracellular matrix. HA, which is secreted by many cell types, is assembled at the plasma membrane by HA synthases (HASs) and extruded into the extracellular space (9, 18, 36). The HA chain can extend up to 2 × 105 disaccharides and up to 25 μm long. Three HAS isoforms (HAS1, HAS2, and HAS3) synthesize HA of different chain lengths and are subject to differential regulation (14, 15, 29).
The biological activity of HA is a function of its molecular mass (35). High-molecular-mass HA, such as that found in synovial fluids, supports tissue hydration and volume expansion. In response to injury and inflammation, HA undergoes increased synthesis through induction of HASs and degradation to smaller-molecular-mass fragments by hyaluronidases (HYALs) (23, 24). Shorter-chain-length HA (2.5 × 102 kDa) induces the expression of inflammatory genes in macrophages. Overall, lower-molecular-mass HA fragments are angiogenic, inflammatory, and immunostimulatory. HA expression is increased in many injury states, including Crohn's disease in humans and dextran sodium sulfate (DSS)-induced colitis in mice (22, 38). Increased levels of HA and HAS1 are seen in a genetic model of ileitis in which TNFα is overexpressed (4).
The angiogenic, inflammatory, and immunostimulatory effects of HA are mediated by binding to its receptors. The HA receptor CD44 is expressed on the plasma membrane of most cells, including fibroblasts, smooth muscle cells, epithelial cells, and immune cells (32). In addition to binding to CD44, HA also binds to Toll-like receptors (TLRs) 2 and 4 (TLR2 and TLR4), which are components of the innate immune system (17, 37, 38). TLR2 and TLR4 are widely distributed in the gastrointestinal tract and are important in mediating the host response to commensal and pathogenic bacteria (34). Although TLRs are usually thought of as responding to microbial products, TLR2 and TLR4 also ligate host molecules, including HA.
TLR4 signaling plays a role in the host response to DSS-induced colitis (1, 11). DSS-induced colitis is more severe in mice deficient in TLR4 or MyD88, a TLR adaptor molecule. In DSS-induced colitis, signaling through TLR4 results in increased cyclooxygenase-2 expression and prostaglandin synthesis; these downstream signaling events promote increased epithelial cell survival and proliferation. The expression of HA is induced in DSS-induced colitis through increased expression of HAS2 and HAS3, enzymes involved in HA synthesis (38). Moreover, exogenous HA is protective in wild-type mice in DSS-induced colitis; in mice, intraperitoneal administration of HA prior to DSS results in reduced weight loss and improved histology compared with administration of DSS alone (38). HA expression is also induced in the intestine by irradiation, and intraperitoneal administration of HA prior to radiation blocks radiation-induced apoptosis (31).
Having found that HA promotes epithelial protection and repair in the intestine and colon, we sought to determine if HA was involved in the regulation of epithelial proliferation and mucosal growth during normal development. To address the effects of exogenous HA on intestinal and colonic growth, we treated weanling mice with intraperitoneal HA twice a week for 5 wk. To address the effects of endogenous HA on intestinal and colonic growth, we treated weanling mice with intraperitoneal PEP-1, a peptide that blocks HA binding to its receptors (25, 38).
MATERIALS AND METHODS
Chemicals and reagents.
HA (750 kDa) was obtained from Sigma-Aldrich (St. Louis, MO). We previously demonstrated that this HA preparation was not contaminated with LPS (38). The HA-binding peptide PEP-1 (H2N-GAHWQFNALTVR-OH) (21) and scrambled control peptide (H2N-WRHGEALTAVNQ-OH) were obtained from New England Peptide (Gardner, MA).
Animals.
Wild-type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in-house. Mice were maintained on a 12:12-h light-dark schedule in a temperature-controlled specific pathogen-free facility and fed standard laboratory mouse chow. Animal procedures were carried out in accordance with the Washington University School of Medicine Animal Studies Committee, which approved the protocols. Intraperitoneal injections of HA (30 mg/kg) or PEP-1 (40 mg/kg) were administered to male wild-type mice starting at weaning (3 wk of age) and continuing twice a week for 5 wk. Control mice received 0.9% saline. At 8 wk of age, mice from all treatment groups were given an intraperitoneal injection of a mixture of 5-bromo-2′-deoxyuridine (BrdU, 120 mg/kg) and 5-fluoro-2′-deoxyuridine (12 mg/kg) 90 min before they were euthanized to label S phase cells. Small intestines and colons were removed and measured for length, flushed with PBS, and cut open longitudinally. Strips of tissue were cut from the lengths of the small intestines and colons and placed in RiboZol RNA extraction reagent (catalog no. N580-200ML, Amresco, Solon, OH). The remaining intestinal and colonic tissues were fixed in fresh 10% formalin and embedded in 2% agar and then in paraffin for use in immunohistochemical studies.
Immunohistochemical analysis.
Formalin-fixed paraffin-embedded proximal jejunum (PJ) and distal colon (DC) tissue sections were used in all immunohistochemical analyses. Hematoxylin-eosin-stained slides were used to obtain measurements of intestinal villus heights, intestinal and colonic crypt depths, and muscularis thickness. Villus heights and crypt depths were measured from images taken at ×100 magnification and saved as Axiovision zvi files using the Scalings program (Carl Zeiss Imaging Systems, Maple Grove, MN). Thirty villi and 30 crypts were measured in each of 4 mice per treatment group. Muscularis thickness measurements were obtained from images taken at ×200 magnification and saved as described above. Fifty measurements were made in the PJ and DC of each of four mice per treatment group. For assessment of epithelial proliferation, tissue sections were stained using a goat antibody against BrdU (1:1,000 dilution) followed by biotin-labeled donkey anti-goat IgG (1:500 dilution; Jackson ImmunoResearch, West Grove, PA) and signal detection with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich). The numbers of BrdU-labeled epithelial cells in PJ and DC crypts were scored, with 30 crypts counted in each of 4 mice per treatment group. The number of intestinal villi and crypts was counted from ×100 magnification photographs of hematoxylin-eosin-stained longitudinal sections of PJ. At this magnification, the length of the intestine encompassed in each image is 900 μm, and the height is 725 μm, and this defines a full field of view. The number of villi and crypts per full field view was counted. The ratio of villi to crypts was then calculated.
For determination of the cellular composition of the epithelium in PJ and DC, the total numbers of villus (PJ) and crypt (PJ and DC) epithelial cells were counted using hematoxylin-eosin-stained slides, while the numbers of different secretory epithelial cell types were scored from tissue sections stained for specific cell markers. Goblet cells were stained using the periodic acid-Schiff procedure. Enteroendocrine cells were identified using rabbit anti-chromogranin-A (1:500 dilution; Abcam, Cambridge, MA) followed by biotin-labeled donkey anti-rabbit IgG (1:250 dilution; Jackson ImmunoResearch). Paneth cells were identified using rabbit anti-lysozyme (1:1,000 dilution; Abcam) followed by biotin-labeled donkey anti-rabbit IgG (1:500 dilution; Jackson ImmunoResearch). Signal detection for enteroendocrine and Paneth cells was carried out with DAB. Cells in PJ villi and crypts and DC crypts were counted in 10 fields of view at ×100 magnification in each of 4 mice per treatment group. The number of enterocytes in PJ and colonocytes in DC was determined by subtraction of the total secretory cell counts from the total epithelial cell counts. Data for each cell type are presented as a percentage of the total epithelial cell count per villus (PJ) or crypt (PJ and DC). Apoptotic cells were counted on hematoxylin-eosin-stained longitudinal sections of PJ. One hundred well-oriented crypts were analyzed in each of the four mice per treatment group.
Immunofluorescence detection for HA was carried out using biotinylated HA-binding protein (1:800 dilution; Associates of Cape Cod-Northstar Bio Products, East Falmouth, MA) followed by Alexa Fluor 594-conjugated streptavidin (1:1,000 dilution; Invitrogen, Carlsbad, CA).
Total collagen was stained with Sirius red (Sigma, St. Louis, MO), and fast green (Sigma) was used to stain noncollagenous proteins.
Quantitative RT-PCR.
Total cellular RNA was extracted from distal jejunum and DC by RiboZol RNA extraction reagent according to the manufacturer's instruction, and its quality was evaluated using an Agilent Technologies 2100 Bioanalyzer. Random primed cDNA synthesis was used as a template for quantitative RT-PCR (qRT-PCR), which was performed in triplicate for each biological sample using PerfeCta SYBR Green FastMix for iQ (catalog no. 95071-012, Quata Bioscience, Gaithersburg, MD). The cycle number at threshold for each amplicon was determined as the PCR cycle at which the fluorescence intensity crosses a user-established threshold. The primers are shown in Table 1.
Table 1.
Mouse gene probes used in quantitative RT-PCR with forward and reverse primer sequences
| Gene Probe | Primer Sequences |
|---|---|
| mIGF1 | F: 5′ CCC AAG ACT CAG AAG TCC CCG T 3′ |
| R: 5′ GGA TCC TGC GGT GAT GTG GCA 3′ | |
| mIGFBP5 | F: 5′ GGT GCC CCG TGC TGT GTA CC 3′ |
| R: 5′ CTG CTG TCG AAG GCG TGG CA 3′ | |
| mFGF2 | F: 5′ AGG GAG TGT GTG CCA ACC GG 3′ |
| R: 5′ ATG GCC TTC TGT CCA GGT CCC G 3′ | |
| mFGF4 | F: 5′ TGT GCA CGC AGA CAC GAG GG 3′ |
| R: 5′ TCG TCG GTA AAG AAA GGC ACA CCG 3′ | |
| mFGF8 | F: 5′ TGC AGG TCC TGG CCA ACA AGC 3′ |
| R: 5′ GTC CTT GCC TTT GCC GTT GCT CTT G 3′ | |
| mEGF | F: 5′ CCC TGG ACT GGA TTG GCC GGA 3′ |
| R: 5′ CAG TCT CCT GGC CCT TGG CA 3′ | |
| mEpiregulin | F: 5′ TTG TGC TGA TAA CTG CCT GTA GAA 3′ |
| R: 5′ CAC CGA GAA AGA AGG ATG GAG AC 3′ | |
| mHAS1 | F: 5′ GCA TCA GTG GTC CTC TGG GTC TAT 3′ |
| R: 5′ GGA GGG CGT CTC CGA GTA GCA 3′ | |
| mHYAL3 | F: 5′ GGA GTA CTG TGT CTG CGG AGC C 3′ |
| R: 5′ GCA GCA AAG CTT GGC CAT GCA TC 3′ | |
| mHYAL3 | F: 5′ CAA CGA GCG GTT CCG ATG 3′ |
| R: 5′ GCC ACA GGA TTC CAT ACC CA 3′ |
IGFBP, insulin-like growth factor-binding protein; HAS, hyaluronic acid synthase; HYAL, hyaluronidase; F, forward; R, reverse.
Epithelial cell proliferation in vitro.
IEC-6 cells, a nontransformed epithelial cell line derived from neonatal rat intestine, were obtained from the American Type Culture Collection (Rockville, MD). They were propagated in 75- or 150-cm2 flasks at 37°C in 5% CO2 in 95% humidity in growth medium (DMEM) supplemented with 10% heat-inactivated FCS, antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin), and ITS [insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 ng/ml); Roche, Indianapolis, IN]. All experiments were performed on IEC-6 cells between passages 14 and 20. Human recombinant IGF-I was obtained from Sigma Chemical. Cell proliferation was assessed using a colorimetric 3-(4,5-dimethlythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche), as described previously (2). Briefly, 5 × 103 IEC-6 cells were seeded into 96-well plates (Costar 3595, Corning, NY) in the presence of the above-described medium for 48 h. Cells were switched to 0.05% FCS-DMEM supplemented with 0.1% BSA and 5 μg/ml transferrin, along with HA and/or IGF-I, and incubated for 24 h. MTT labeling reagent was added, and incubation was continued for 4 h at 37°C.
Statistical analysis.
One-way analysis of variance was used to assess differences in measured variables between the treatment and control groups. Values are means ± SE. Statistical difference was accepted at P < 0.05.
RESULTS
Exogenous HA and PEP-1 affect intestinal and colonic growth.
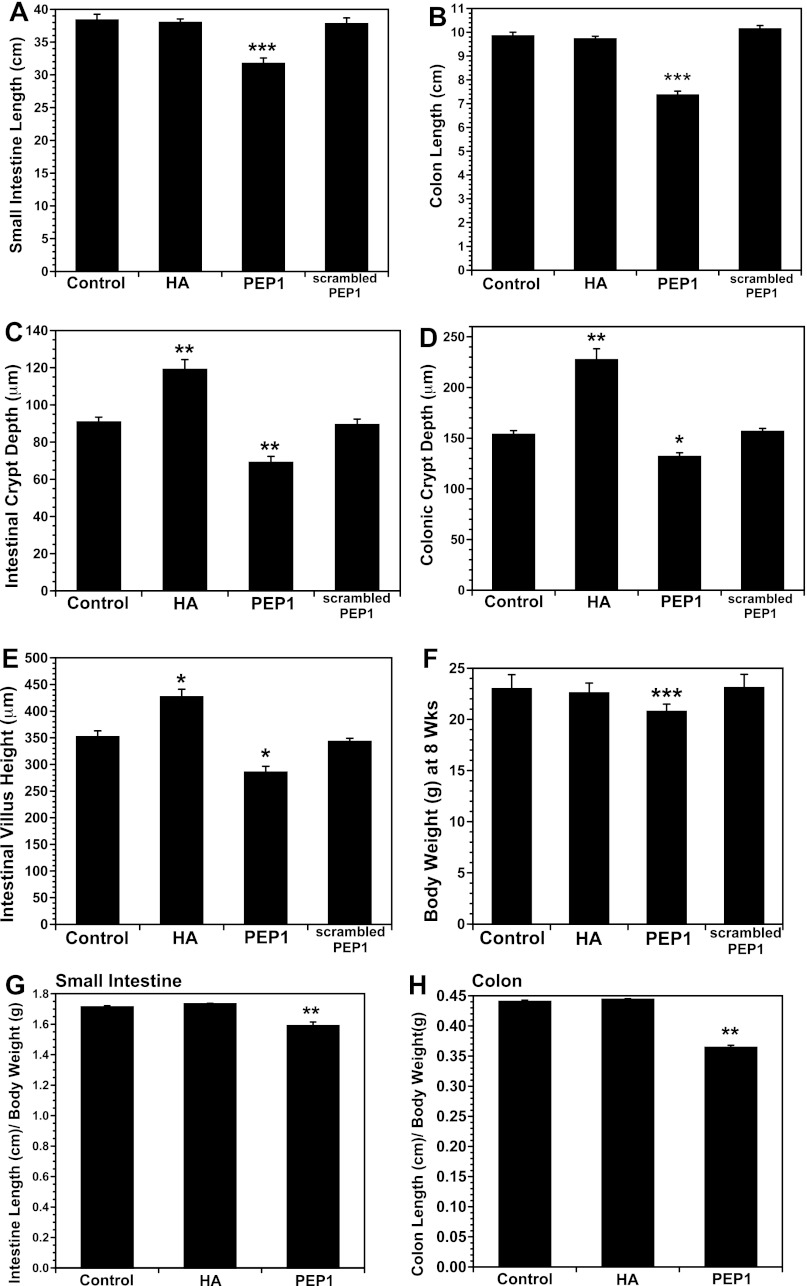
Mice were given intraperitoneal HA or PEP-1 twice a week for 5 wk beginning at 3 wk of age. Exogenous HA had no effect on the length of the small intestine or colon, but PEP-1 resulted in ∼20% decrease in small intestinal length and a 25% decrease in colonic length (Fig. 1, A and B). Scrambled PEP-1 had no effect on intestinal or colonic length, indicating the specificity of PEP-1 binding for HA. Exogenous HA resulted in increased crypt depth and villus height in the small intestine and increased crypt depth in the colon (Fig. 1, C–E). In contrast, PEP-1 resulted in decreased villus height and crypt depth in the intestine and decreased crypt depth in the colon. Scrambled PEP-1 had no effect on villus height or crypt depth. Although exogenous HA and PEP-1 had profound effects on the intestine and colon, they had little effect on the growth of the mouse in total. The weights of HA-treated mice were identical to those of vehicle-treated mice, whereas the weights of PEP-1-treated mice were 10% lower (Fig. 1F). In mice treated with PEP-1, the decreases in intestinal and colonic length were proportionally greater than the decrease in body weight (Fig. 1, G and H).
Fig. 1.
A and B: intraperitoneal administration of exogenous hyaluronic acid (HA) starting at weaning and continuing twice a week for 5 wk had no effect on length of small intestine or colon, but blockade of endogenous HA by intraperitoneal administration of PEP-1 using the same regimen resulted in a significant decrease in length of small intestine and colon compared with control mice. Scrambled PEP-1 had no effect on length of small intestine or colon. Values are means ± SE for 12 control, 18 HA-treated, 23 PEP-1-treated, and 6 scrambled PEP-1-treated mice. ***P < 0.0001 vs. control. C–E: intraperitoneal injection of HA twice weekly from 3 to 8 wk of age resulted in an increase in intestinal villus height and intestinal and colonic crypt depth compared with control mice. Administration of PEP-1 using the same regimen resulted in a decrease in intestinal villus height and intestinal and colonic crypt depth compared with control mice. Scrambled PEP-1 had no effect on villus height or crypt depth. Values are means ± SE for 4 mice per group. *P < 0.005, **P < 0.002 vs. control. F: HA treatment from weaning to 8 wk of age had no effect on total body weight, but average weight was 10% lower in mice treated with PEP-1 twice a week for 5 wk than in control or HA-treated mice. G and H: mice given PEP-1 showed reductions in intestine and colon length out of proportion to decrease in body weight. Values are means ± SE for 10 mice per group. ***P < 0.0002 vs. control.
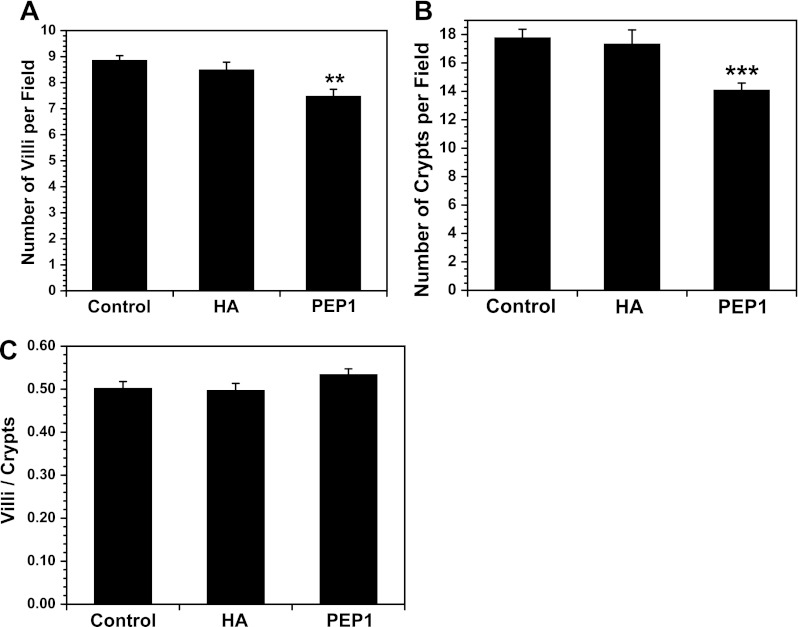
We next sought to determine if treatment with PEP-1 or HA affected the number of crypts and villi in the small intestine. PEP-1 reduced the number of crypts and the number of villi in the small intestine without altering the villus-to-crypt ratio (Fig. 2). HA had no effect on the number of crypts or villi.
Fig. 2.
Intraperitoneal administration of PEP-1 starting at weaning and continuing twice a week for 5 wk reduced the number of intestinal villi (A) and intestinal crypts (B) without altering the villus-to-crypt ratio (C). Values are means ± SE for 8 mice per group. **P < 0.003, ***P < 0.0004 vs. control.
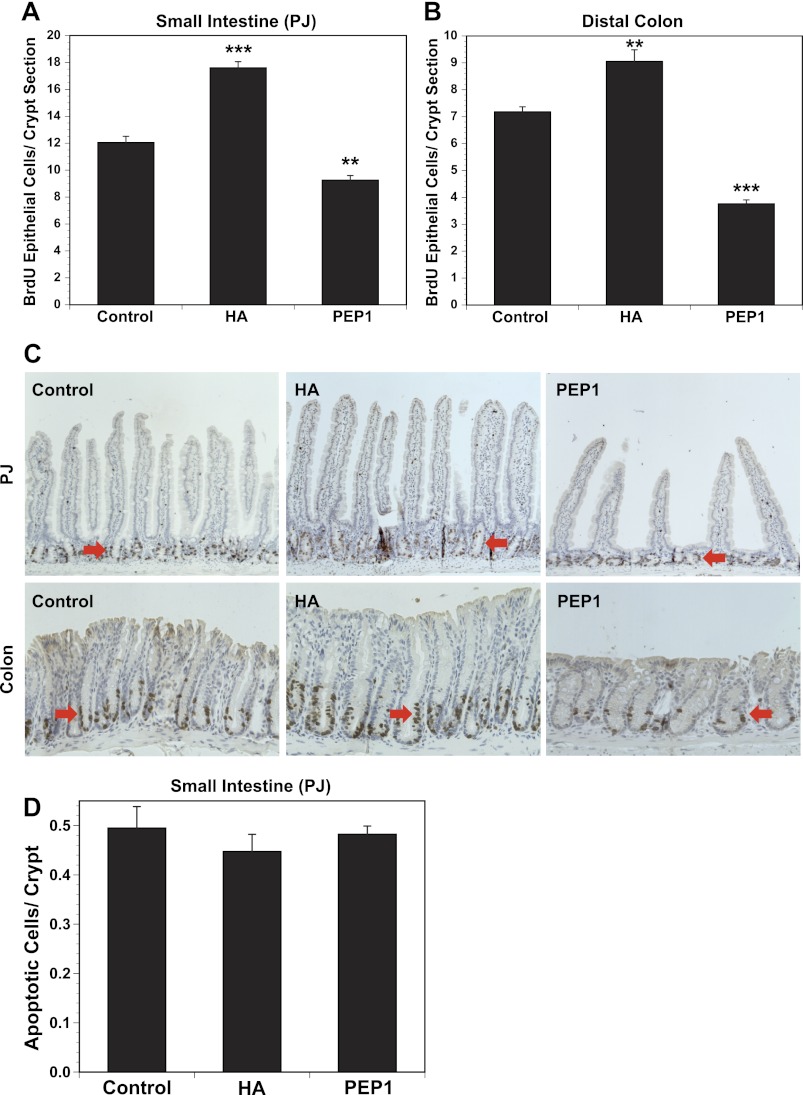
Exogenous HA increases and PEP-1 decreases epithelial proliferation in the intestine and colon.
The number of BrdU-positive cells in the small intestine increased by 50% in the small intestine and by 28% in the colon in response to exogenous HA (Fig. 3, A and B). In response to PEP-1, epithelial proliferation decreased by 25% in the small intestine and 52% in the colon. Histology and immunohistochemistry for BrdU are shown in Fig. 3C. BrdU immunohistochemistry in the small intestine demonstrates an increase in crypt height and villus height after HA and a decrease in crypt height and villus height after PEP-1. The increased crypt height after HA is associated with an expanded zone of BrdU-positive cells. After PEP-1, BrdU-positive cells were observed throughout the shortened crypt. In the colon, administration of exogenous HA resulted in increased crypt height and expansion in the zone of BrdU-positive cells. In contrast, the colons of PEP-1-treated mice were shortened and the zone of BrdU-positive cells was diminished. Treatment with HA or PEP-1 had no effect on apoptosis in the small intestine (Fig. 3D).
Fig. 3.
A and B: intraperitoneal administration of exogenous HA starting at weaning and continuing twice a week for 5 wk promotes epithelial cell proliferation in small intestine and colon, while blockade of endogenous HA with PEP-1 using the same regimen reduces epithelial cell proliferation in small intestine [proximal jejunum (PJ)] and colon, compared with control mice. HA-treated mice have significantly increased numbers of 5-bromo-2′-deoxyuridine (BrdU)-positive crypt epithelial cells in small intestine and colon compared with control mice. PEP-1-treated mice have significantly fewer BrdU-positive crypt epithelial cells in small intestine and colon than control mice. Values are means ± SE for 4 control, 4 HA-treated, and 8 PEP-1-treated mice. **P < 0.001, ***P < 0.0001 vs. control. C: immunohistochemistry for BrdU shows proliferating epithelial cells (arrows) in crypts of small intestine (PJ) and colon. Average intestinal villus height and average intestinal and colonic crypt depths are greater in HA-treated than control mice, while average intestinal villus height and average intestinal and colonic crypt depths are less in PEP-1-treated than control mice. Original magnification: ×100 (PJ) and ×200 (colon). D: HA or PEP-1 treatment had no effect on apoptosis in small intestine.
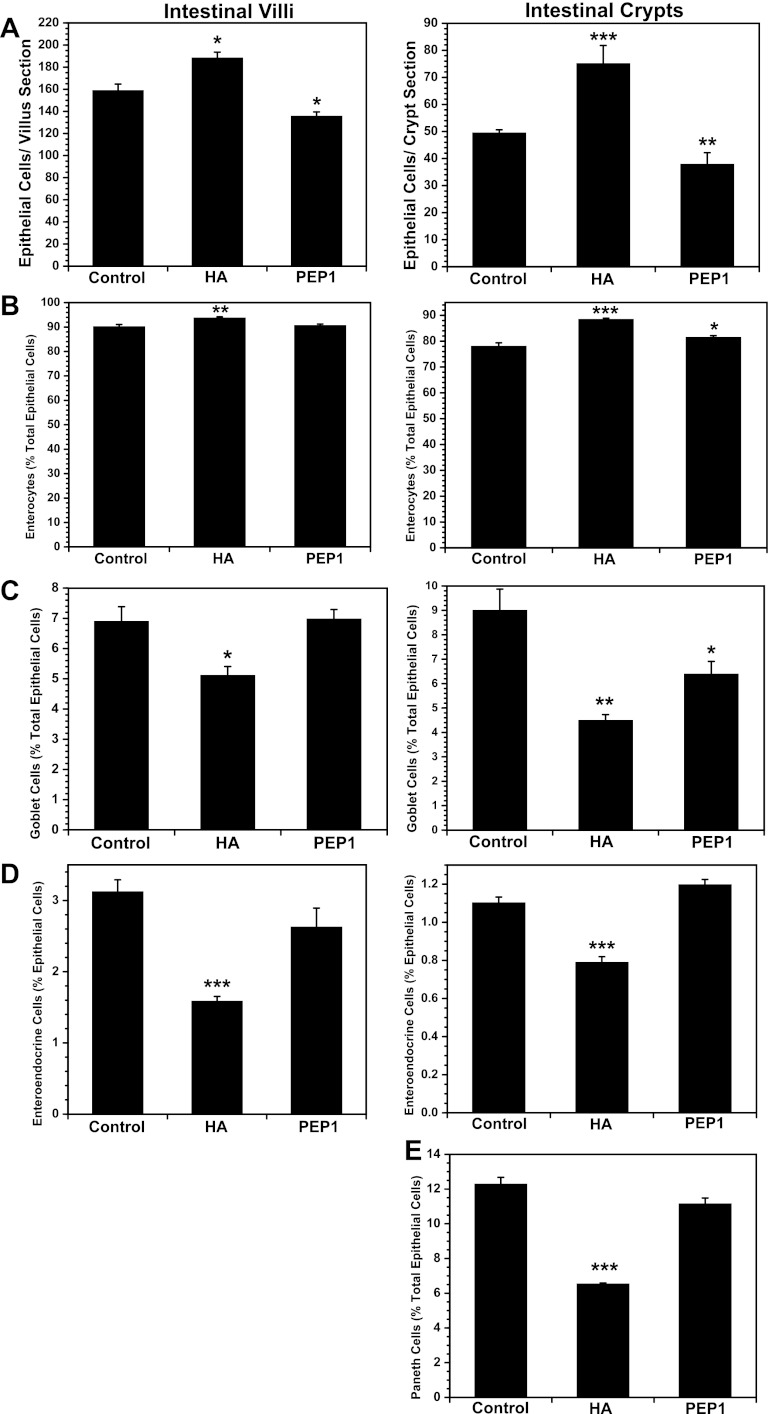
Exogenous HA increases the proliferation of enterocytes to a greater extent than secretory cells in the intestinal epithelium.
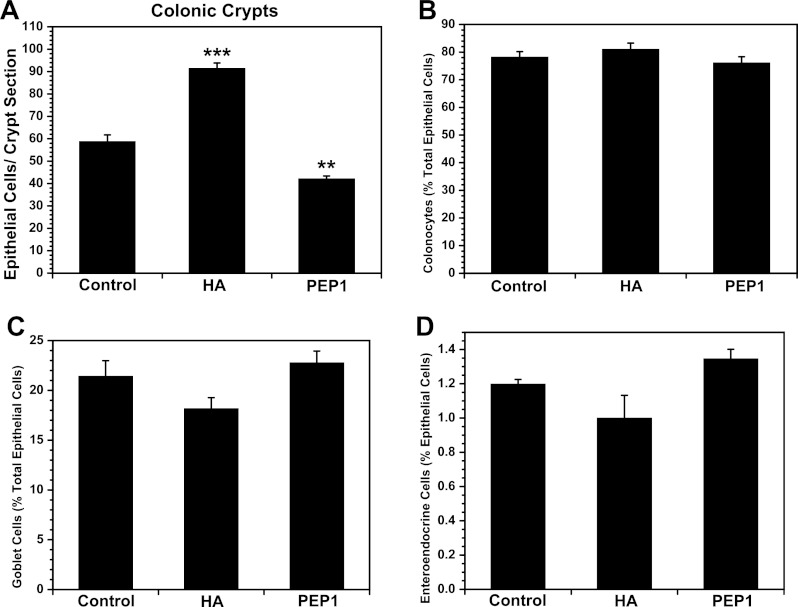
Exogenous HA increased the number of epithelial cells in the villi and crypts of the small intestine (Fig. 4A). In the villi and crypts, enterocytes as a percentage of epithelial cells were increased in response to exogenous HA (Fig. 4B), whereas goblet cells, enteroendocrine cells, and Paneth cells as a percentage of epithelial cells were diminished (Fig. 4, C–E). In contrast, PEP-1 diminished the number of epithelial cells per villus section and per crypt section in the small intestine. The effect of PEP-1 on the epithelial cell lineages was less that the effect of exogenous HA. PEP-1 administration resulted in a modest increase in enterocytes and decrease in goblet cells in the crypts without affecting lineages in the villi. In the colon, HA increased the number of cells per crypt and PEP-1 decreased the number of cells per crypt, but neither HA nor PEP-1 affected lineages (Fig. 5). Immunohistochemistry for goblet cells, enteroendocrine cells, and Paneth cells is shown in Fig. 6.
Fig. 4.
Long-term treatment of mice with exogenous HA promotes epithelial proliferation, while long-term treatment with PEP-1 reduces epithelial proliferation, in small intestine. A: number of intestinal epithelial cells in villi and crypts are significantly increased in mice treated with intraperitoneal injections of exogenous HA starting at weaning and continuing twice a week for 5 wk compared with control mice. There are significantly fewer intestinal epithelial cells in villi and crypts of mice in which endogenous HA is blocked with intraperitoneal administration of PEP-1 using the same regimen than in control mice. B–E: in HA-treated mice, increased epithelial proliferation appears to favor formation of enterocytes over secretory cells, as suggested by consistently lower percentages of goblet cells, enteroendocrine cells, and Paneth cells making up the intestinal epithelium, while the percentage of enterocytes is increased. In PEP-1-treated mice, reduced epithelial proliferation appears to have a similar impact on formation of absorptive and secretory cells, as suggested by changes in percent composition of intestinal epithelium that are, for the most part, small and not statistically significant; one exception is a significant decrease in the percentage of goblet cells comprising the intestinal crypt epithelium (C). Values are means ± SE for 4 mice per group. *P < 0.02, **P < 0.001, ***P < 0.0001 vs. control.
Fig. 5.
Long-term treatment of mice with exogenous HA promotes epithelial proliferation, while long-term treatment with PEP-1 reduces epithelial proliferation, in the colon. A: number of colonic crypt epithelial cells is significantly increased in mice treated with intraperitoneal injections of exogenous HA starting at weaning and continuing twice a week for 5 wk compared with control mice. There are significantly fewer colonic crypt epithelial cells in mice in which endogenous HA is blocked with intraperitoneal administration of PEP-1 using the same regimen than in control mice. B–D: percent composition of the colonic epithelium shows a trend toward favoring formation of absorptive colonocytes over secretory cells in HA-treated compared with control mice, but the increased percentage of colonocytes and decreased percentages of goblet cells and enteroendocrine cells in the colonic epithelium are small and not statistically significant. In PEP-1-treated mice, reduced epithelial proliferation appears to have a slightly more negative impact on formation of absorptive colonocytes than secretory cells, as shown by a slightly decreased percentage of colonocytes and slightly increased percentages of goblet cells and enteroendocrine cells comprising the colonic crypt epithelium that are not statistically significant. Values are means ± SE for 4 mice per group. **P < 0.003, ***P < 0.0002 vs. control.
Fig. 6.
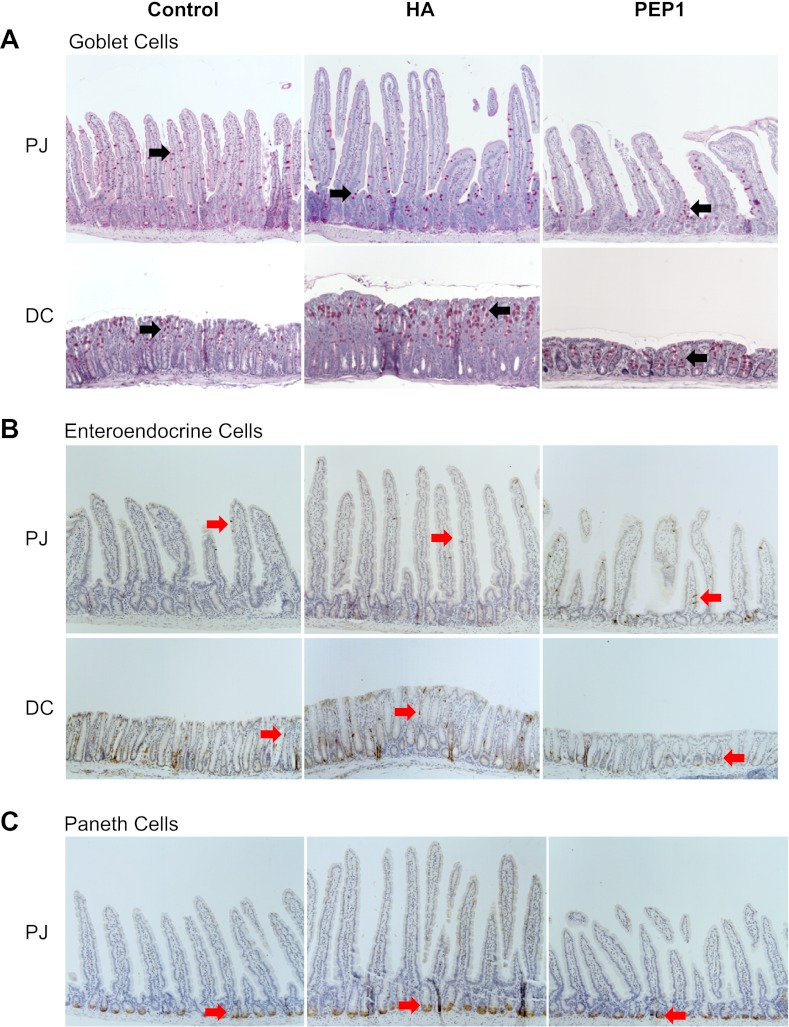
A: periodic acid-Schiff staining for goblet cells (arrows) in PJ and distal colon (DC) in wild-type control mice and mice treated with HA or PEP-1 twice a week for 5 wk starting at weaning at 3 wk of age. Percentage of goblet cells in intestinal epithelium was significantly lower in HA-treated than control or PEP-1-treated mice. Long-term HA treatment resulted in increased epithelial proliferation that favored formation of enterocytes at the expense of secretory cells. The same trend occurred, but to a lesser degree, in DC. Original magnification ×100. B: immunohistochemistry using anti-chromogranin-A for identification of enteroendocrine cells (arrows) in PJ and DC of wild-type control mice and mice treated with HA or PEP-1. Percentage of enteroendocrine cells in intestinal epithelium was significantly lower in HA-treated than control or PEP-1-treated mice. C: immunohistochemistry using anti-lysozyme for identification of Paneth cells (arrows) in small intestinal crypts in wild-type control mice and mice treated with HA or PEP-1. Percentage of Paneth cells in intestinal epithelium was significantly lower in HA-treated than control or PEP-1-treated mice. Long-term HA treatment resulted in increased epithelial proliferation that favored formation of enterocytes at the expense of secretory cells.
HA has no direct effect on intestinal epithelial cell proliferation.
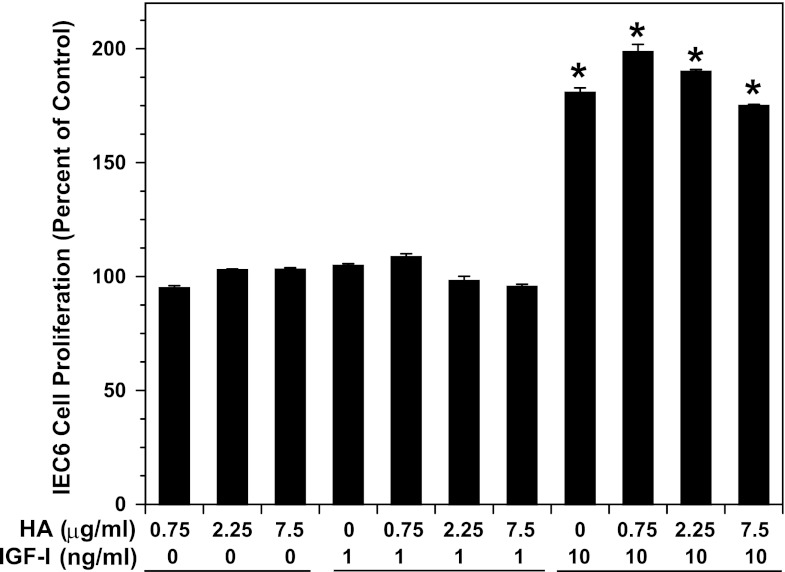
To determine if HA has a direct effect on epithelial cell proliferation, we incubated HA with a nontransformed rat duodenal epithelial cell line, IEC-6, in the presence and absence of IGF-I, which is known to promote IEC-6 proliferation (33) (Fig. 7). Exogenous HA had no effect on the proliferation of IEC-6 cells. IGF-I at 10 ng/ml induced a threefold increase in IEC-6 proliferation, whereas IGF-I at 1 ng/ml had no effect. The combination of IGF-I and HA had the same effect on IEC-6 proliferation as IGF-I alone.
Fig. 7.
IEC-6 cell proliferation in response to incubation with HA and IGF-I. IEC-6 cells were grown in DMEM supplemented with 10% FCS, penicillin (50 μg/ml), streptomycin (50 μg/ml), and ITS [insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 ng/ml)]. For all experiments, cells were switched to DMEM supplemented with 0.05% FCS, 0.1% BSA, and 5 μg/ml transferrin, along with HA and/or IGF-I, and incubated for 24 h. Proliferation was assayed by 3-(4,5-dimethlythiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT). Values are means ± SE for 3 experiments. *P < 0.0001 vs. control.
Administration of exogenous HA induces growth factor expression, whereas PEP-1 reduces growth factor expression.
We extracted mRNA from the distal jejunum and DC of mice after treatment for 5 wk with vehicle or exogenous HA or PEP-1. We then performed qRT-PCR for genes related to growth: IGF-I, insulin-like growth factor-binding protein (IGFBP) 3 (IGFBP3), IGFBP5, macrophage colony-stimulating factor, FGF1, FGF2, FGF3, FGF4, FGF5, FGF6, FGF7, FGF8, FGF9, FGF10, FGF15, EGF, glucagon, hepatocyte growth factor, epiregulin, and amphiregulin. Table 2 shows results for genes that were affected by HA or PEP-1. Administration of exogenous HA was associated with increases in the expression of FGF4 (+1.5-fold) and EGF (+3.2-fold) in the distal jejunum. Administration of PEP-1 was associated with decreases in IGF-I (−1.8-fold) and IGFBP5 (−2.5-fold) in the distal jejunum. In the DC, administration of exogenous HA was associated with increases in IGF-I (+2.6-fold), IGFBP5 (+1.4-fold), FGF2 (+2.1-fold), and FGF8 (+2.4-fold). In the colon, administration of PEP-1 was associated with a decrease in the expression of the EGF receptor (EGFR) ligand epiregulin (−1.5-fold). None of the experimental conditions was associated with significant change in the other growth factors tested (macrophage colony-stimulating factor, FGF1, FGF3, FGF5, FGF6, FGF7, FGF9, FGF10, FGF15, glucagon, hepatocyte growth factor, and amphiregulin).
Table 2.
Quantitative RT-PCR for expression of genes affected by long-term treatment of mice with exogenous HA or PEP-1
| Distal Jejunum |
Distal Colon |
|||||||
|---|---|---|---|---|---|---|---|---|
| HA |
PEP-1 |
HA |
PEP-1 |
|||||
| Gene | Fold Δ | P value | Fold Δ | P value | Fold Δ | P value | Fold Δ | P value |
| IGF1 | NSD | −1.8 | 0.035 | +2.6 | 0.038 | NSD | ||
| IGFBP5 | NSD | −2.5 | 0.028 | +1.4 | 0.039 | NSD | ||
| FGF2 | NSD | NSD | +2.1 | 0.047 | NSD | |||
| FGF4 | +1.5 | 0.045 | NSD | NSD | NSD | |||
| FGF8 | NSD | NSD | +2.4 | 0.025 | NSD | |||
| EGF | +3.2 | 0.022 | NSD | NSD | NSD | |||
| Epiregulin | NSD | NSD | NSD | −1.5 | 0.040 | |||
| HAS1 | +6.3 | 0.004 | NSD | +6.0 | 0.002 | NSD | ||
| HYAL3 | +1.4 | 0.025 | NSD | +2.0 | 0.042 | NSD | ||
Values are means ± SE; n = 4 mice for all genes. Mice were given hyaluronic acid (HA) or PEP-1 intraperitoneally twice a week for 5 wk starting at weaning. mRNA was extracted from distal jejunums and distal colons and used in RT-PCR. In HA-treated mice, there was increased gene expression for growth factors in intestine (FGF4 and FGF8) and colon [IGF1 (IGF-I), IGFBP5, and FGF2]; there was also increased expression of genes for HA synthesis (HAS1) and HA degradation (HYAL3) in intestine and colon. In mice treated with PEP-1, there was decreased gene expression for growth factors in intestine (IGF-I and IGFBP5) and colon (epiregulin), but there was no change in HAS or HYAL genes. NSD, no significant difference.
Exogenous HA induces HAS1 and HYAL3 mRNA.
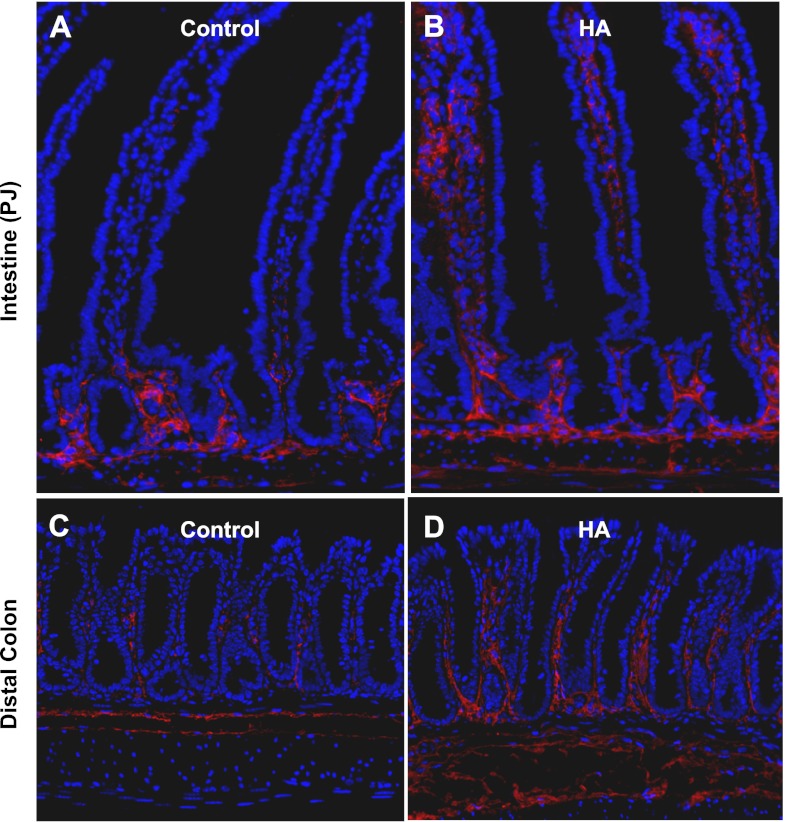
Immunofluorescence for HA in control animals demonstrates the presence of HA in the lamina propria surrounding the crypts in the small intestine (Fig. 8). In colons from control mice, HA is found in the lamina propria surrounding the lower crypts. After 5 wk of exogenous HA administration, there was more HA surrounding the crypts in the small intestine, with additional HA in the lamina propria of the villi. There was also extension of the distribution of HA in the colon, with HA found in the lamina propria more than half way up the crypts. Distribution of HA in the intestines and colons of PEP-1-treated mice was indistinguishable from that in control mice (data not shown). To assess the contribution of changes in HA synthesis or degradation to the changes in HA distribution, we performed qRT-PCR for HAS1, HAS2, HAS3, HYAL1, HYAL2, and HYAL3. The increase in HA distribution as seen on immunofluorescence in the intestines and colons of mice treated with exogenous HA was associated with a sixfold increase in HAS1 mRNA as measured by qRT-PCR (Table 1). There was no change in the expression of HAS2 or HAS3 in response to HA administration or in the expression of HAS1, HAS2, or HAS3 in response to PEP-1. The biological effects of HA are dependent on the molecular mass of the HA polymer; in general, lower-molecular-mass HA has more biological activity. Higher-molecular-mass forms of HA are degraded to lower-molecular-mass forms by HYALs. The administration of exogenous HA was associated with the increased production of HYAL3 in intestine and colon. HYAL3 expression was not affected by PEP-1. The expression of HYAL1 and HYAL2 was not affected in any experimental condition tested.
Fig. 8.
Immunofluorescence for HA-binding protein (red) shows increased regional expression of HA in small intestine and colon of wild-type (control) mice treated with exogenous HA. A and B: small intestine (PJ). Most HA in control mice is restricted to the crypt zone in the lamina propria and the muscularis layer (A), but in mice receiving HA intraperitoneally twice a week for 5 wk, HA expression extends into the lamina propria of the villi (B). C and D: DC. In control mice, HA expression occurs in a scattered pattern in the lamina propria between crypts (C), but HA has a more extensive coverage throughout the colonic lamina propria in mice treated with exogenous HA (D). Original magnification ×200.
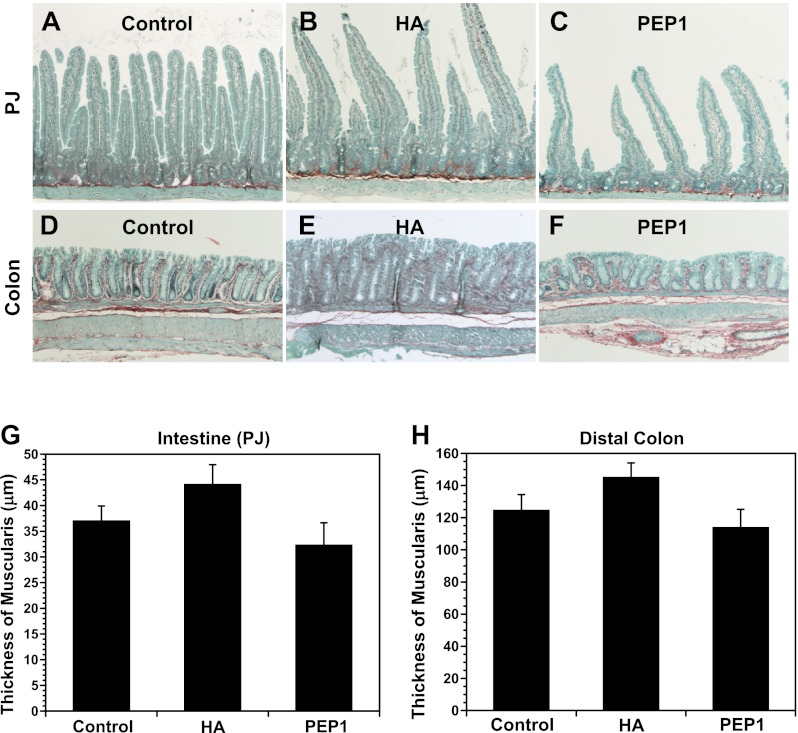
Growth effects of HA and PEP-1 are confined to the mucosa.
Figure 9 shows staining for total collagen and noncollagenous protein in the small intestine and colon of control, HA-treated, and PEP-1-treated mice. Staining for collagen in the lamina propria and muscularis of the small intestine and colon illustrates significant morphological changes in the mucosa due to the long-term treatments with HA or PEP-1 (Fig. 9, A–F). Average intestinal villus heights and intestinal and colonic crypt depths were greater in HA-treated than control mice, while average villus heights and crypt depths were reduced in PEP-1-treated mice compared with controls. There was no statistically significant effect of treatment with HA or PEP-1 on the thickness of the muscularis in the small intestine (Fig. 9G) or colon (Fig. 9H), although there was a trend toward increased thickness of the muscularis with HA treatment and a trend toward decreased thickness with PEP-1 treatment.
Fig. 9.
Sirius red staining for total collagen (red) and fast-green staining for noncollagenous protein (green) in small intestine (PJ, A–C) and DC (D–F). Collagen staining in the lamina propria shows changes in intestinal and colonic morphology due to HA or PEP-1 treatment confined to the mucosa. Average villus height (intestine) and crypt depth (intestine and colon) were greater in HA-treated mice than controls, while average villus height and crypt depth were smaller in PEP-1-treated mice than controls. Original magnification ×100. G and H: there was no significant effect on thickness of the muscularis with HA or PEP-1 treatment in intestine and colon. Values are means ± SE for 4 mice per group.
DISCUSSION
Intraperitoneal administration of exogenous HA twice a week for 5 wk beginning at weaning resulted in increased epithelial proliferation in the small intestine and colon. This increased proliferation was associated with increased crypt depth in the small intestine and colon and increased villus height in the small intestine. Exogenous HA had no effect on the length of the intestine or colon. In parallel studies, administration of PEP-1, a peptide that blocks HA binding to its receptors, resulted in a marked decrease in length of the small intestine and colon, decrease in epithelial proliferation in the small intestine and colon, decrease in crypt depth in the small intestine and colon, and decrease in villus height in the small intestine. Although HA and PEP-1 had profound effects on the gastrointestinal tract, they had little effect on the overall growth of the animal, with only marginal effects on body weight.
The histological picture seen with exogenous HA administration is consistent with the adaptive hyperplasia seen after ileocecal resection, which is also marked by increased crypt depth and villus height (8). Adaptive hyperplasia is associated with increased epithelial proliferation and may involve intestinal epithelial stem cell expansion and crypt fission (7, 12).
The mucosal picture seen with PEP-1 administration is consistent with the adaptive hypoplasia seen with malnutrition and total parenteral nutrition (TPN) (5, 26). Adaptive hypoplasia is characterized by decreased epithelial proliferation and decreased crypt depth and villus height. In addition to inducing hypoplasia, PEP-1 administration also resulted in a marked decrease in the length of the intestine and colon, suggesting that endogenous HA is involved in promoting the normal elongation of the intestine and colon from 3 to 8 wk after birth. The period from 2 to 4 wk after birth is associated with intestinal elongation by crypt fission (6); it is possible that the loss of endogenous HA binding to its receptors may block crypt fission at ∼3 wk after birth.
The mechanism by which exogenous HA induces hyperplasia is not completely clear. The increase in epithelial proliferation seen with exogenous HA could be due to a direct effect of HA on epithelial cells or the induction of growth factor production by other cell types. HA had no effect on the proliferation of IEC-6 cells in vitro. Whether this in vitro effect can be extrapolated to in vivo proliferation is not clear. HA administration is associated with increased levels of FGF4 and EGF mRNAs in the intestine and increased levels of IGF-I, IGFBP5, FGF2, and FGF8 mRNAs in the colon. IGF-I is the growth factor most closely associated with intestinal growth and promotion of epithelial proliferation (21). Circulating IGF-I, primarily produced by hepatocytes, and locally produced IGF-I, made by intestinal mesenchymal cells, promote intestinal epithelial proliferation. Exogenously administered IGF-I and transgene-derived IGF-I lead to increased epithelial proliferation and decreased apoptosis (13, 19, 27). IGFBP5 is highly active in orchestrating IGF-I action (10, 20). PEP-1 administration resulted in decreased IGF-I and IGFBP5 in the intestine. It is possible that the decreased intestinal length, crypt depths and villus heights, and epithelial proliferation associated with PEP-1 administration relate to the decrease in IGF-I and IGFBP5. Similarly, the increases in crypt depth and epithelial proliferation in the colon after exogenous HA may relate to the increase in IGF-I. EGF, which binds to EGFR, is also associated with increased intestinal and colonic epithelial proliferation and the epithelial response to injury (3, 30). Exogenous HA results in increased EGF in the intestine. PEP-1 administration results in decreased levels of epiregulin, another EGFR ligand, in the colon. Thus, EGFR signaling may also contribute to the increased proliferation seen with exogenous HA and the decreased proliferation seen with PEP-1. HA and PEP-1 were administered for 5 wk beginning at 3 wk of age, and growth factor mRNA levels were assessed only at 8 wk of age. It is possible that measurement of growth factor expression at earlier time points would have given different results. The cell types responding to HA and the cell types producing the growth factors are not defined. Myofibroblasts, macrophages, and mesenchymal stem cells could potentially respond to HA and produce growth factors. These studies also do not address whether the growth effects of HA are mediated through CD44 or TLR4 or a combination of the two.
HA increased and PEP-1 decreased the number of epithelial cells per crypt and per villus in the intestine and the number of epithelial cells per crypt in the colon. Examination of the epithelial cell lineages demonstrated that, in the intestine, exogenous HA resulted in a disproportionate increase in the number of enterocytes compared with the number of secretory cells (Paneth cells, goblet cells, and enteroendocrine cells) in the intestine. Enterocyte production is driven by the transcription factor hairy/enhancer of split homolog 1 (HES-1) (16). Whether HA administration affects the expression of HES-1 or other transcription factors has not been established.
Exogenous HA induced the expression of HAS1 in the intestine and colon. Exogenous HA also resulted in a change in the amount and distribution of HA in the intestine. In control mice, extracellular HA is seen in the lamina propria surrounding the crypts. After 5 wk of exogenous HA, there is HA in the lamina propria of the villi. The distribution of HA expression after 5 wk of exogenous HA suggests that the production of increased endogenous HA through HAS1 occurs in lamina propria mesenchymal cells. The induction of HAS1 by exogenous HA suggests the existence of a feedforward loop in which exogenous HA induces the synthesis of endogenous HA (28, 38). Administration of exogenous HA for 5 wk also resulted in the induction of HYAL3, which should lead to production of smaller and more biologically active HA fragments.
This study demonstrates a role for the extracellular matrix, specifically endogenous HA, in regulating the normal growth of the intestine and colon from 3 to 8 wk after birth. Intestinal growth occurs in defined stages prenatally and postnatally. Whether the effects of HA on growth occurred uniformly over the 5-wk period or were confined to a portion of that period was not addressed. Whether the effects of HA on growth would also be seen if HA were given earlier or later was also not addressed. Blocking HA binding to its receptors creates a mucosal picture resembling the adaptive hypoplasia seen with TPN or starvation. In addition, the administration of exogenous HA creates a mucosal picture resembling the adaptive hyperplasia seen after intestinal resection. These observations raise the following question: Do changes in HA expression mediate adaptive hyperplasia in response to resection or adaptive hypoplasia in response to TPN or starvation?
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37 DK-33165 and R01-DK-55753 and Washington University Digestive Diseases Research Core Grant P30-DK-52574.
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.E.R. and W. F. S. designed the study; T.E.R. and X.E. acquired the data; T. E. R. and W. F. S. drafted the manuscript.
REFERENCES
- 1. Brown SL, Riehl TE, Walker M, Geske M, Doherty J, Stenson WF, Stappenbeck T. MyD88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmichael J, DeGraff WG, Gazdar AF, Minna J, Mitchell J. Evaluation of tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47: 936–942, 1987 [PubMed] [Google Scholar]
- 3. Cohen S, Carpenter G, King L. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem 255: 4834–4842, 1980 [PubMed] [Google Scholar]
- 4. Collins CB, Ho J, Wilson TE, Wermers J, Tlaxca J, Lawrence M, Solga M, Lannigan J, Rivera-Nieves J. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology 135: 1993–2002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-1 induced hyperplasia in rats. J Nutr 132: 2010–2014, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Dehmer JJ, Garrison AP, Speck KE, Dekaney CM, Van Landeghem L, Sun X, Henning SJ, Helmrath MA. Expansion of intestinal epithelial stem cells during murine development. PLos One 6: e27070, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dekaney CM, Fong JJ, Rigby RJ, Lund P, Henning S, Helmrath M. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol 293: G1013–G1022, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Dowling RH. Small bowel adaptation and its regulation. Scand J Gastrorenterol 74 Suppl 17: 53–74, 1983 [PubMed] [Google Scholar]
- 9. Flynn RS, Mahavadi S, Murthy KS, Kellum J, Kuemmerle J. Insulin-like growth factor-binding protein-5 stimulates growth of human intestinal muscle cells by activation of Gαi3. Am J Physiol Gastrointest Liver Physiol 297: G1232–G1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 242: 27–33, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan A, Thomas L, Xu R, Inoue H, Arditi M, Dannenberg A, Abreu M. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862–877, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrison AP, Dekaney CM, von Allmen DC, Lund P, Henning S, Helmrath M. Early but not late administration of glucagon-like peptide-2 following ileo-cecal resection augments putative intestinal stem cell expansion. Am J Physiol Gastrointest Liver Physiol 296: G643–G650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillingham MB, Dahly EM, Murali SG, Ney D. IGF-1 treatment facilitates transition from parenteral to enteral nutrition in rats with short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 284: R363–R371, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Itano N, Atsumi F, Sawai T, Miyaishi O, Taniguchi S, Kannagi R, Hamaguchi M, Kimata K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci USA 99: 3609–3614, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Itano N, Sawai T, Atsumi F, Sawai T, Miyaishi O, Taniguchi S, Kannagi R, Hamaguchi M, Kimata K. Selective expression and functional characteristics of three mammalian hyaluronan synthases in oncogenic malignant transformation. J Biol Chem 279: 18679–18687, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Jensen J, Pedersen EE, Galante P, Hald J, Heller R, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen O. Control of endodermal endocrine development by Hes-1. Nat Genet 24: 36–44, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Jiang D, Liang J, Fan J. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Karamanos NK, Axelsson S, Vanky P, Tzanakakis G, Hjerpe A. Determination of hyaluronan and galactosaminoglycan disaccharides by high-performance capillary electrophoresis at the attomole level. Applications to analyses of tissue and cell culture proteoglycans. J Chromatogr A 696: 295–305, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Knott AW, Juno RJ, Jarboe MD, Profitt S, Erwin C, Smith E, Fagin J, Warner B. Smooth muscle overexpression of IGF-1 induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol 287: G562–G570, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kuemmerle JF, Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-1 secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J Biol Chem 277: 20563–20571, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Lund PK. Insulin-like growth factors: gene structure and regulation. In: Handbook of Physiology. The Endocrine System. Hormonal Control of Growth. Bethesda, MD: Am. Physiol. Soc., 1999, sect. 7, p. 537–571 [Google Scholar]
- 22. Majors AK, Austin RC, de la Motte CA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem 78: 47223–47231, 2003 [DOI] [PubMed] [Google Scholar]
- 23. McKee CM, Penno MB, Cowman M, Burdick M, Strieter R, Bao C, Noble P. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98: 2403–2413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin B, Choi A, Noble P. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor κB-dependent mechanism. J Biol Chem 272: 8013–8018, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Mummert M, Mohamadzadeh M, Mummert D. Development of peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med 192: 769–779, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murali SG, Nelson DW, Draxler AK, Liu X, Ney D. Insulin-like growth factor-I (IGF-1) attenuates jejunal atrophy in association with increased expression of IGF-1 binding protein-5 in parenterally fed mice. J Nutr 135: 2553–2559, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Ohneda K, Ulshen MH, Fuller CR, D'Ercole A, Lund P. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112: 444–454, 1997 [DOI] [PubMed] [Google Scholar]
- 28. O'Neill L. A feed-forward loop involving hyaluronic acid and Toll-like receptor-4 as a treatment for colitis. Gastroenterology 137: 1889–1891, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Prehm P, Schumacher U. Inhibition of hyaluronan export from human fibroblasts by inhibitors of multidrug resistance transporters. Biochem Pharmacol 68: 1401–1410, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Riegler M, Sedivy R, Sogukogulu T, Cosentini E, Bischof G, Teleky B, Feil W, Schiessel R, Hamilton G, Wenzl E. Epidermal growth factor promotes rapid response to epithelial injury in rabbit duodenum in vitro. Gastroenterology 111: 28–36, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am J Physiol Gastrointest Liver Physiol 302: G309–G316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherman L, Sleeman J, Herrlich Pl. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6: 726–733, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Simmons J, Ling Y, Wilkins H, Fuller C, D'Ercole A, Fagin J, Lund P. Cell-specific effects of insulin receptor substrate-1 deficiency on normal and IGF-1-mediated colon growth. Am J Physiol Gastrointest Liver Physiol 293: G995–G1003, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stenson WF. Toll-like receptors and intestinal epithelial repair. Curr Opin Gastroenterol 24: 103–107, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Stern R, Asari A, Sugahara K. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85: 699–715, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Tammi R, Saamanen A, Maibach H, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol 97: 126–130, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Taylor K, Yamasaki K, Radek K. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on toll-like receptor 4, CD44, and MD-2. J Biol Chem 282: 18265–18275, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Zheng L, Riehl TE, Stenson WF. Regulation of colonic epithelial repair in mice by toll-like receptors and hyaluronic acid. Gastroenterology 137: 2041–2051, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]