Abstract
Animal studies suggest that the small (SK) and intermediate (IK) conductance Ca2+-activated K+ channels may contribute to detrusor smooth muscle (DSM) excitability and contractility. However, the ability of SK and IK channels to control DSM spontaneous phasic and nerve-evoked contractions in human DSM remains unclear. We first investigated SK and IK channels molecular expression in native human DSM and further assessed their functional role using isometric DSM tension recordings and SK/IK channel-selective inhibitors. Quantitative PCR experiments revealed that SK3 channel mRNA expression in isolated DSM single cells was ∼12- to 44-fold higher than SK1, SK2, and IK channels. RT-PCR studies at the single-cell level detected mRNA messages for SK3 channels but not SK1, SK2, and IK channels. Western blot and immunohistochemistry analysis further confirmed protein expression for the SK3 channel and lack of detectable protein expression for IK channel in whole DSM tissue. Apamin (1 μM), a selective SK channel inhibitor, significantly increased the spontaneous phasic contraction amplitude, muscle force integral, phasic contraction duration, and muscle tone of human DSM isolated strips. Apamin (1 μM) also increased the amplitude of human DSM electrical field stimulation (EFS)-induced contractions. However, TRAM-34 (1 μM), a selective IK channel inhibitor, had no effect on the spontaneous phasic and EFS-induced DSM contractions suggesting a lack of IK channel functional role in human DSM. In summary, our molecular and functional studies revealed that the SK, particularly the SK3 subtype, but not IK channels are expressed and regulate the spontaneous and nerve-evoked contractions in human DSM.
Keywords: apamin, TRAM-34, single-cell RT-PCR, qPCR, Western blot, immunohistochemistry, electrical field stimulation
overactive bladder (OAB), occurring in ∼17% of the American population, is a pathophysiological condition that impairs quality of life (36). The current pharmacological treatment of OAB is primarily based on antimuscarinic drugs, which are not universally effective and have many unwanted side effects (2, 31). To develop novel effective pharmacological treatments for OAB, it is crucial to improve our understanding of the basic physiology, pathophysiology, and pharmacology of the bladder function. Previous studies already demonstrated that various K+ channels including the large-conductance Ca2+-activated K+ (BK) channel (3, 5, 10, 14, 21, 23, 24, 32, 35), the voltage-gated K+ (KV) channels (6, 12, 14, 20), and the ATP-sensitive K+ (KATP) channels (10, 33) are essential contributors to urinary bladder physiology. However, other important channels such as the small (SK) and intermediate (IK) conductance Ca2+-activated K+ channels remain largely unexplored in human detrusor smooth muscle (DSM), although some studies outline a potential role for these channels in DSM phasic contractions (8, 10, 13, 17, 18, 26, 27, 38). The SK channel family consists of three isoforms: SK1, SK2, and SK3 (30). IK channel, initially classified as SK4, is the only member of its family (30). SK1–3 channels are selectively blocked by apamin, a peptide isolated from Apis mellifera (30, 37), whereas the IK channels are selectively blocked by TRAM-34 (38). In guinea pig DSM isolated strips, apamin significantly increased the amplitude and duration but decreased the frequency of the spontaneous phasic contraction (16). In pig DSM, which has similar characteristics to that of human DSM (9), apamin increased both amplitude and frequency of the DSM spontaneous contractions (4). In mice, genetic ablation of the SK3 channels caused an increase in the spontaneous phasic contractions in vitro and nonvoiding contractions in vivo (18).
Notably, it has been demonstrated that activation of SK and IK channels has the opposite effect in the DSM of various species. NS4591, a nonselective SK and IK channel opener, inhibited electrical field stimulation (EFS)-induced and carbachol-induced phasic contractions in rat, pig, and human DSM (26). In rats, an increase in bladder capacity and micturition volume as well as a reduction in bladder overactivity were observed after application of NS309, an activator of SK/IK channels (28). Similarly, in rats and cats, NS4591 (30 mg/kg) inhibited bladder overactivity induced by capsaicin and acetic acid in vivo (19). Furthermore, SKA-31, the most potent SK/IK channel activator known to date (34), has been shown to decrease excitability and contractility in guinea pig DSM via selective SK channel activation (29). Collectively, these data suggest an important role played by SK and IK channels in DSM contractility in nonhuman tissues. Indeed, the majority of the current knowledge about the functional role of SK and IK channels comes principally from animals and is likely not directly translatable to humans due to species differences in DSM ion channel expression, action potential shape, and pattern of contractility. Furthermore, the limited number of functional studies performed on human DSM specimens has not addressed the role of SK and IK channels under normal physiological conditions of spontaneous activity but rather after addition of depolarizing agents such as KCl or carbachol (10, 26, 27). An investigation of human DSM strips exhibiting spontaneous phasic contractions would provide a more physiologically relevant condition for the determination of the role of SK and IK channels.
The aim of this study was twofold: 1) to investigate the molecular expression of SK and IK channels in human DSM using RT-PCR, quantitative PCR (qPCR), Western blot, and immunohistochemistry and 2) to evaluate the functional role of SK and IK channels in spontaneous and nerve-evoked contractions using isometric DSM tension recordings and the selective SK and IK channel inhibitors, apamin and TRAM-34, respectively.
MATERIALS AND METHODS
Human DSM tissue collection.
All human studies were reviewed and approved by the institutional review board of the Medical University of South Carolina (MUSC) (protocol, HR 16918). In total, DSM tissue samples from 36 (25 males and 11 females) patients (28–85 yr old; average age 61.9 ± 2.2) including 29 Caucasians and 7 African-Americans without a history of OAB were obtained during radical cystectomy for bladder cancer or other open bladder surgeries for malignant or nonmalignant conditions of the lower urinary tract. Two types of DSM samples were collected from each patient. The first sample was stored in ice-cold Ca2+-free HEPES-buffered dissection solution (§Solutions and Drugs) and was used to conduct functional studies on DSM contractility. The second sample was kept in RNA-later (QIAGEN Sciences, Hilden, Germany) and was used for qPCR and RT-PCR experiments. Both samples were transported to the laboratory immediately after surgery.
Detection and quantification of mRNA message by RT-PCR and qPCR.
Expression of the genes coding for SK and IK channels in human DSM was analyzed using both qPCR and RT-PCR. Human whole DSM tissue and freshly isolated DSM single cells total RNA were extracted using the RNeasy Mini Kit (QIAGEN) and then reverse-transcribed into cDNA as previously described (1, 6, 7, 21, 22). The native human whole DSM tissue was dissected under a Stemi 200-C dissection microscope (Carl Zeiss). The serosa and mucosa (including the urothelium) as well as the major visible blood vessels were removed using micro scissors before RNA extraction. DSM single cells for qPCR and RT-PCR experiments were collected as previously described (1, 6, 7, 21, 22, 24). Briefly, one to two strips (3–5 mm long, 2–4 mm wide) of urothelium and mucosa-free DSM were cut and placed in dissection solution (2 ml) supplemented with BSA (1 mg/ml), papain (1 mg/ml), and DL-dithiothreitol (1 mg/ml), and then incubated for 25–30 min at 37°C. Next, the DSM strips were transferred into dissection solution (2 ml) containing BSA (1 mg/ml), collagenase type II (1 mg/ml), and CaCl2 (100 μM), and then incubated for 7–10 min at 37°C. DSM strips were then washed with fresh BSA containing dissection solution. The isolated single DSM cells were washed four to five times using a perfusion system that removes the majority of the non-DSM cells that do not adhere to the glass-bottom of the chamber. Then, we performed individual DSM cell collection based on DSM cell morphology using an Axiovert 40CFL microscope (Carl Zeiss) with Nomarski interference contrast. Next, ∼100–150 DSM cells were immediately collected individually by suction into a glass micropipette using an MP-285/ROE micromanipulator (Sutter Instruments, San Rafael, CA). Only spindle-shaped, bright, and shiny DSM cells (when viewed under the microscope) were collected for further RNA extraction. The concentration of total RNA extracted from whole DSM tissue and isolated DSM single cells was determined by BioPhotometer 6131 (Eppendorf, Hamburg, Germany) and most samples had a concentration greater than 100 ng/μl. The extracted total RNA was reverse-transcribed into cDNA using oligo d(T) primers (Promega, Madison, WI), M-MLV Reverse Transcriptase (Promega), dNTP mix (Fermentas Life Sciences), and RNase inhibitor (Applied Biosystems). Specific primers for SK1–3/IK channels and GAPDH (Table 1) were designed based on the cDNA complete sequences of human genes in Genbank and synthesized by Integrated DNA Technologies (IDT, Coralville, IA). For qPCR experiments, a two-step amplification followed by melting curve protocol was performed using the IQ5 Thermo Cycler system (Bio-Rad, Hercules, CA). qPCR experiments were carried out on human whole DSM tissue and isolated DSM single cells cDNA (10 ng/μl) using IQ SYBR Green Supermix (Bio-Rad) and specific primers for SK and IK channels. GAPDH was chosen as an internal control gene to analyze SK and IK channels mRNA relative expression and each sample was run in duplicates. The parameters of the qPCR experiments were as follows: cycle 1, 95°C for 3 min; cycle 2, 95°C for 10 s and then 55°C for 30 s (repeated 40 times); cycle 3, 95°C for 1 min; cycle 4, 55°C for 1 min; cycle 5, 55°C for 10 s (repeated 81 times to generate a melting curve). For RT-PCR, the cDNA products from whole DSM tissue and isolated DSM single cells were PCR amplified in the presence of GoTaq Green Master Mix (Promega) and specific primers for SK1, SK2, SK3, and IK channels using the mastercycler gradient from Eppendorf. cDNA was heated for 3 min at 94°C and then amplified by 35 cycles (94°C for 30 s, 60.1°C for 30 s, 72°C for 15 s) followed by 5 min of extension at 72°C. The sizes of the PCR products obtained were 389, 359, 470, and 378 bp for SK1, SK2, SK3, and IK channels, respectively. RT-PCR products were visualized after electrophoresis was performed using 2% agarose ethydium bromide-stained gels. Product sizes were confirmed using a 100-bp extended range DNA ladder (Lonza, Rockland, ME). Total RNA extracted from human brain was purchased from Clontech (Mountain View, CA) and used as a positive control. RT-PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma, St. Louis, MO) and sequenced at the University of South Carolina Environmental Genomics Core Facility to confirm their identity.
Table 1.
RT-PCR and qPCR primers for SK and IK channel identification
| Channel | Sense | Anti-sense | Product, bp | Accession Number |
|---|---|---|---|---|
| SK1 | TCAAATGCCTCATCAGCCTCTCCA | GCGCGTGTTGAAGGTGATCTTGTT | 389 | NM002248 |
| SK2 | TGGTGGACAATGGAGCAGATGACT | AACCAAGAGTACAGTTCCTGGGCA | 359 | NM170775 |
| SK3 | CTGCCTGTGGGAAATTGAATGGCA | AGGAGCACCATTCTTGGGACATGA | 470 | NM170782 |
| IK | CTCATCGTGGCCTTTCATGCCAAA | CATGTAAAGCTTGGCCACGAACCA | 378 | NM002250 |
| GAPDH | GGATTTGGTCGTATTGGG | GGAAGATGGTGATGGGATT | 205 | NM002046 |
qPCR, quantitative PCR; SK and IK, small and intermediate conductance Ca2+-activated K+ channel; bp, base pairs.
Detection and localization of SK and IK channel proteins by Western blot and immunohistochemistry.
Western blot experiments were conducted on freshly isolated mucosa-free human DSM tissue as previously described (20, 21). Briefly, ∼30 μg of total protein extracted from human whole DSM tissue were loaded into 10% Tris-HEPES-SDS precast polyacrylamide mini gels (Thermo Scientific, Rockford, IL) and run at 130 V for 50 min using a PowerPac Universal power supply from Bio-Rad. Proteins were then transferred from the gel onto an immobilon-P PVDF membrane by semi-dry transfer technique using a Hoefer semi-dry blotter TE70XP (Fisher Scientific). Next, the membrane was blocked by 5% nonfat dry milk in 0.5% TBST (0.5% Tween 20 in TBS) buffer for 1 h. Following the block, the membrane was incubated with the affinity-purified rabbit polyclonal antibodies anti-KCa2.3 (SK3; 1:200) or anti-KCa3.1 (IK; 1:300; Alomone Labs, Jerusalem, Israel) overnight at 4°C. Next, the membrane was washed with 0.5% TBST four times and reincubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (diluted to 1:1,500) in the blocking buffer for 1 h at room temperature. Bound antibodies were detected by the enhanced chemiluminescence substrate kit (Amersham, Piscataway, NJ) according to the manufacturer's instructions. The same experimental procedures were repeated in the presence of competing peptides for rabbit polyclonal antibodies anti-KCa2.3 (1:200) or anti-KCa3.1 (IK; 1:300; Alomone Labs). Human heart protein medley was purchased from Clontech Laboratories and used as a positive control.
Following Western blot experiments, immunohistochemistry experiments were performed on freshly isolated mucosa-free native human DSM tissue to further identify the localization of SK3 and IK channels, respectively. Human DSM tissue was sliced into 2-mm-long, 2-mm-wide, and 6-μm-thick sections using a vibratome model G tissue slicer (Oxford Laboratories, Foster City, CA). Sections were then transferred in individual dishes, stained, and imaged using confocal microscopy (LSM 510 META, Carl Zeiss). Tissue sections were incubated with primary antibody anti-KCa2.3 (1:100) or anti-KCa3.1 (1:100) in 1% BSA/PBS at 37°C for 1 h. Tissue sections were then rinsed multiple times with 1% BSA/PBS, incubated for 1 h in 5% normal donkey serum in 1% BSA/PBS, and then reincubated with secondary antibody (1:100; Cy3-conjugated anti-rabbit IgG; Jackson ImmunoResearch, West Grove, PA) in the dark for another hour. Human DSM tissue sections were subsequently washed with 1% BSA/PBS then PBS and then incubated with phalloidin 488 (1:50) in PBS for 2 h in the dark. Then, DSM tissue sections were rinsed three more times with PBS, incubated with 4′,6-diamidino-2-phenylindole (1:5,000) in PBS for 15 min, and then mounted with DABCO (Sigma). Control experiments were performed by incubating tissue sections without addition of secondary antibody or with primary antibody in the presence of a competing peptide.
Isometric DSM tension recordings.
Human DSM strips (5–7 mm long and 1–2 mm wide) were isolated for functional studies on DSM contractility as previously described (20, 21). Briefly, human DSM isolated strips were suspended between clips and connected to a force displacement transducer and a stationary hook. The strips were then submerged in thermostatically controlled tissue baths with Ca2+-containing physiological saline solution (PSS) at 37°C. Next, the DSM strips were stretched to ∼1.0-g force and were allowed to equilibrate for 1 h. During this equilibration period, strips were washed out with fresh PSS every 15 min. Next, strips were separated into two groups based on the amplitude of the spontaneous contractions after the initial stretch to 1 g. The first group was characterized by DSM strips exhibiting stable spontaneous contraction amplitude >0.1 g after the initial equilibration period. On these strips, tetrodotoxin (TTX; 1 μM) was added into the baths to block the potential contribution of neurotransmitter release. The second group was characterized by DSM strips phasic contraction amplitude <0.1 g. These strips were exposed to EFS to generate nerve-evoked contractions in the absence of TTX. EFS pulses were generated using a PHM-152I stimulator (MED Associates, Georgia, VT) and had the following parameters: pulse amplitude was 20 V, pulse width was 0.75 ms, stimulus duration was 3 s, and polarity was reversed for alternating pulses.
Solutions and drugs.
The Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, 2 MgCl2, and pH 7.3 adjusted with NaOH. The Ca2+-containing PSS was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose, and aerated with 95% O2-5% CO2 to obtain pH 7.4. Apamin, TRAM-34, and TTX were purchased from Sigma.
Data analysis and statistics.
mRNA relative expression was analyzed using the comparative threshold (Ct) method (ΔΔCt, delta-delta Ct) after determining the Ct values for reference (GAPDH) and target genes (SK1, SK2, SK3, or IK) for each sample (25). Fold changes in target mRNA expression level were calculated after normalization to GAPDH and the SK3 channel expression level was arbitrarily chosen as the calibrator. MiniAnalysis (Synaptosoft, Decatur, GA) software version 6.0.7 was used to analyze data from DSM phasic contractions and EFS-induced contractions. GraphPad Prism 4.03 software (GraphPad Software, San Diego, CA) was used for further statistical analysis. To compare the phasic contraction parameters, data were normalized to the control spontaneous contraction parameters (taken to be 100%) and expressed as percentages. For the EFS-induced contractions, the maximal contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%. CorelDraw Graphic Suite X3 (Corel, Ottawa, Canada) software was used for data illustration. qPCR and spontaneous phasic contraction data were compared using paired Student's t-test. Two-way ANOVA followed by the Bonferroni posttest was used to compare frequency-response curves. Results are summarized as means ± SE where n represents the number of DSM isolated strips or PCR samples; and N, the number of patients; P value <0.05 was considered statistically significant.
RESULTS
Detection and relative expression level of mRNA messages for SK1, SK2, SK3, and IK channels in human DSM.
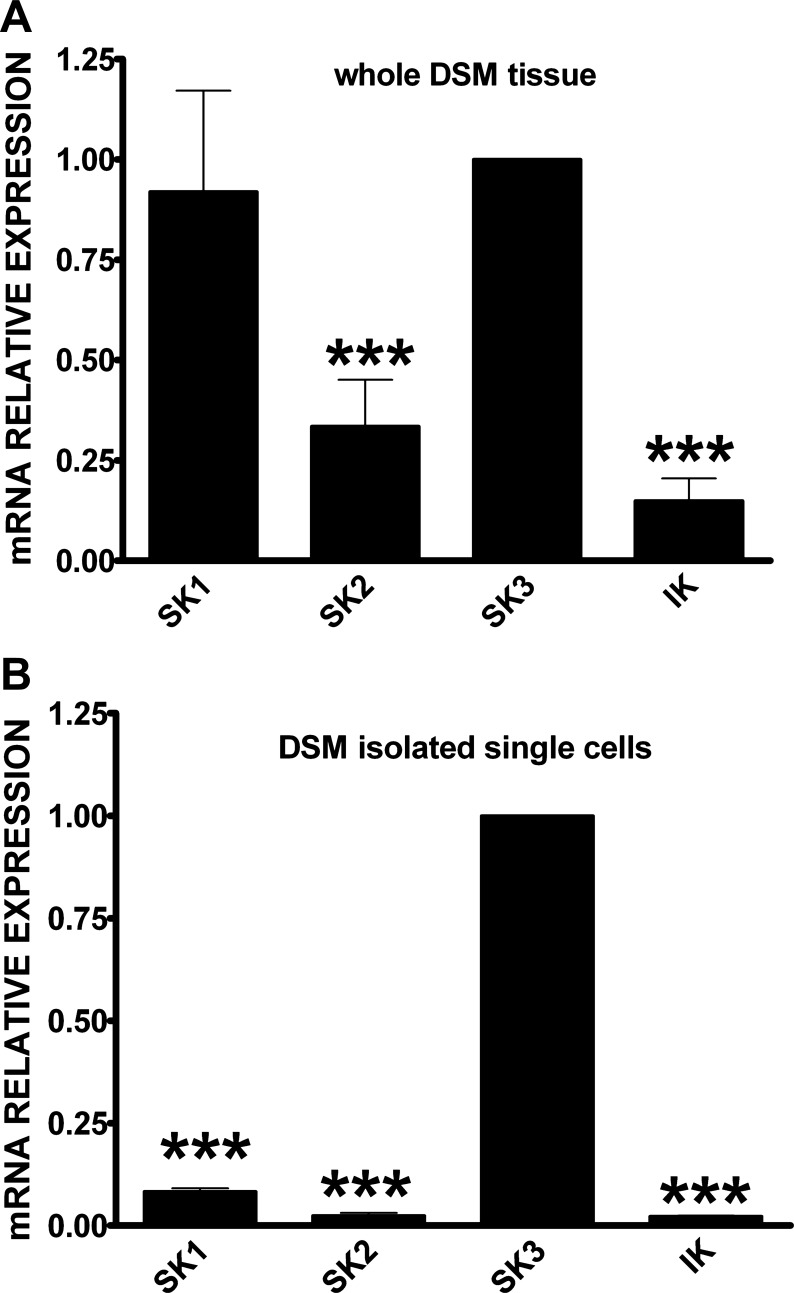
First, qPCR experiments were performed to evaluate the mRNA relative expression for all SK1–3 and IK channels. In human whole DSM tissue, we found that SK1 and SK3 channel mRNA expressions were higher compared with SK2 and IK channels (Fig. 1A). SK3 channel mRNA expression was three- and sevenfold higher than SK2 and IK channel mRNA expression, respectively (n = 14, N = 7, P < 0.005; Fig. 1A). However, SK1 and SK3 channel mRNA expression was comparatively similar in whole DSM tissue (n = 14, N = 7, P < 0.05; Fig. 1A). Next, we conducted qPCR experiments on human DSM freshly isolated single cells to confirm that the mRNA messages detected in the whole DSM tissue originated directly from DSM cells but not from other non-DSM cell types such as neurons, fibroblasts, vascular, and endothelial cells present within the DSM layers (6, 7, 20, 21). The single-cell qPCR experiments confirmed a significantly higher SK3 channel mRNA relative expression compared with other SK and IK channels. SK3 channel mRNA expression was 12-, 41-, and 44-fold higher than SK1, SK2, and IK channels, respectively (n = 16, N = 8, P < 0.005; Fig. 1B). These results demonstrate that in human DSM cells, the SK3 channel expression is predominant among SK and IK channels at the mRNA level.
Fig. 1.
Quantitative PCR (qPCR) analyses for small (SK; SK1, SK2, SK3) and intermediate (IK) conductance Ca2+-activated K+ channel mRNA expression in human whole detrusor smooth muscle (DSM) tissue (n = 14, N = 7; A) and human DSM isolated single cells (n = 16, N = 8; B). Data were normalized to GAPDH and SK3 channel mRNA message was arbitrarily chosen as the calibrator. Comparative threshold (Ct) values are expressed as means ± SE. SK and IK channel mRNA levels that are statistically different from SK3 channel mRNA level are indicated by (***) for P < 0.005.
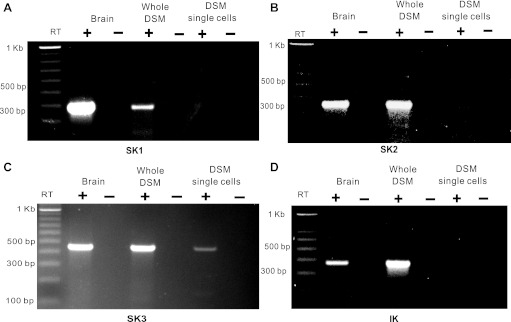
Next, we investigated SK1, SK2, SK3, and IK channel mRNA message expression using RT-PCR experiments on both human whole DSM tissue and freshly isolated DSM single cells. In human whole DSM tissue, our RT-PCR experiments demonstrated detectable expression of all SK and IK channel subtype mRNA messages (n = 6–14, N = 3–7; Fig. 2). At the DSM single-cell level, only the SK3 channel mRNA message was detected. There was no detection of SK1, SK2, or IK channels, which is consistent with our qPCR data in human isolated DSM single cells (n = 8–12, N = 4; Fig. 2). RT-PCR experiments were conducted on human DSM cDNAs equivalent to 100 ng of starting mRNA. Negative control experiments performed in the absence of the reverse transcriptase (−RT) demonstrated an absence of genomic DNA contamination. These data suggest that the SK3 channel is highly expressed at the mRNA level, whereas SK1, SK2, and IK channel mRNA is expressed at very low, perhaps nonphysiological, levels in human DSM cells.
Fig. 2.
RT-PCR detection of SK1 (A), SK2 (B), SK3 (C), and IK (D) channel mRNA messages in human whole DSM tissue (n = 6–14, N = 3–7) and isolated DSM single cells (n = 8–12, N = 4). No product was observed in the negative controls (−RT) in which reverse transcriptase was not added to the reaction.
Native human whole DSM tissue expresses SK3 but not IK channel proteins.
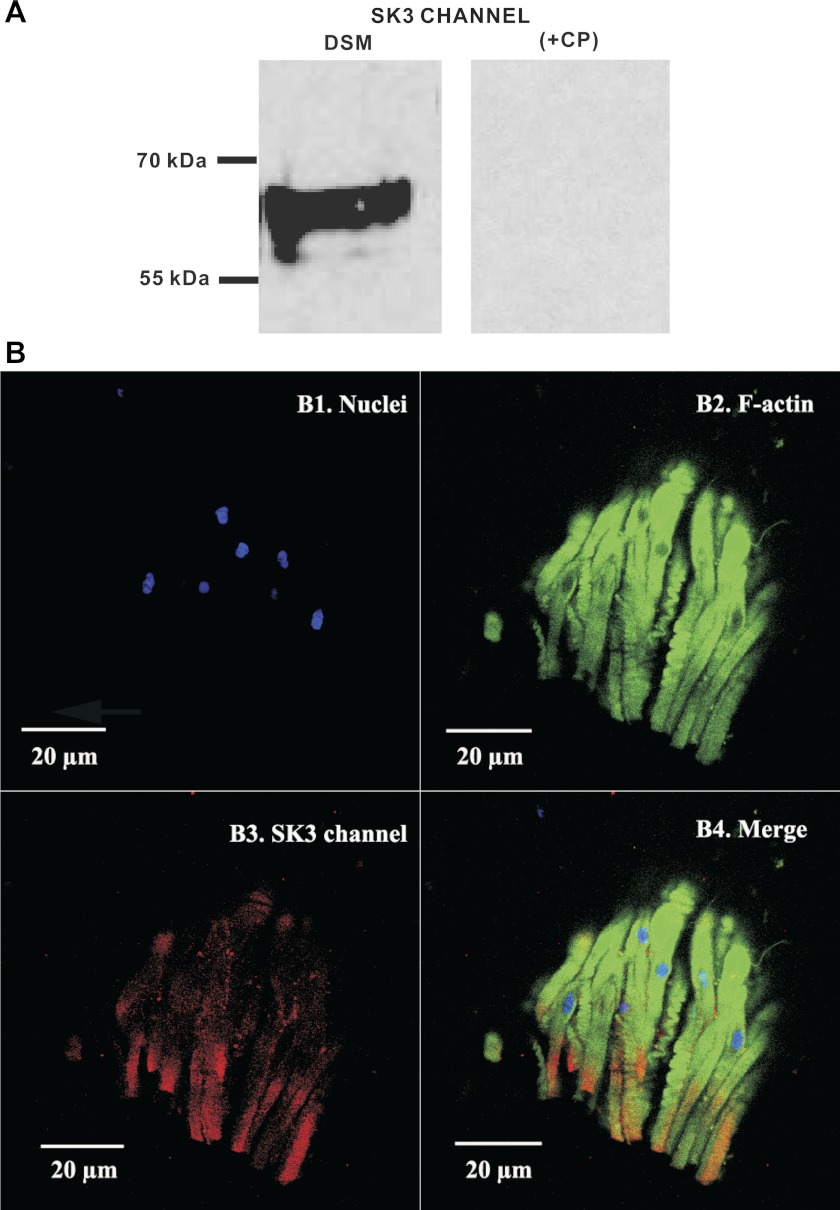
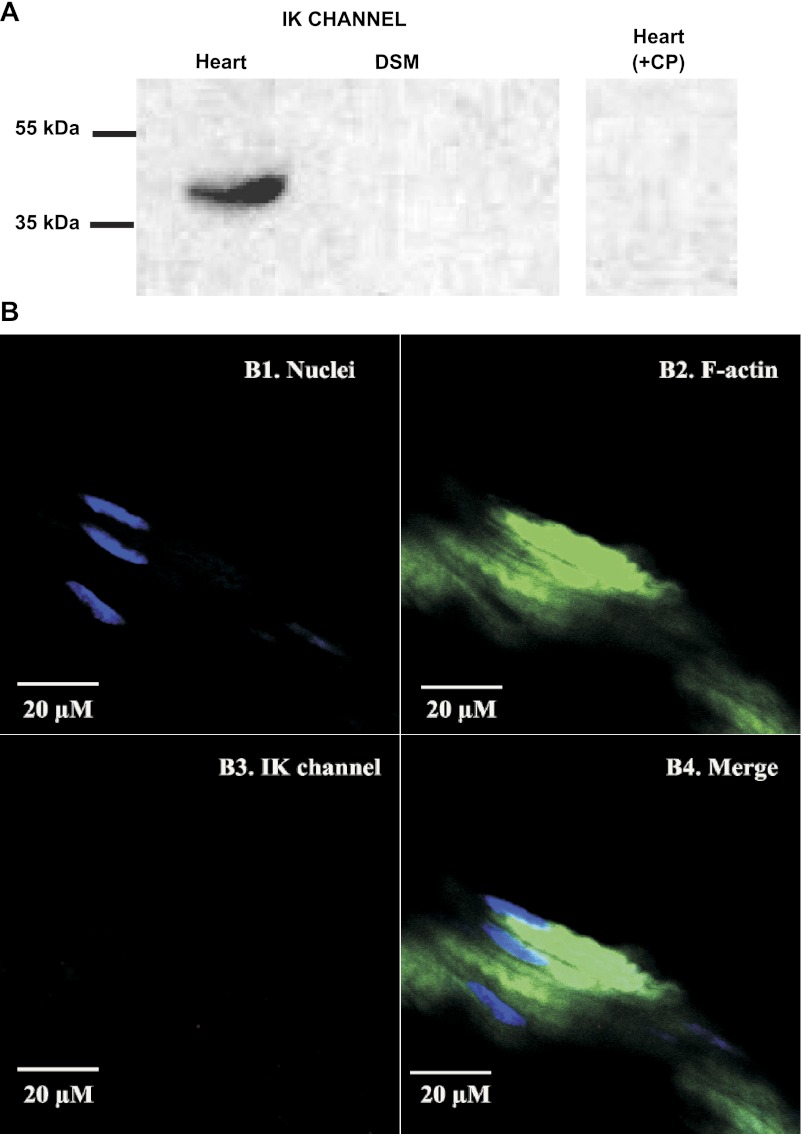
To further investigate the expression of SK/IK channel protein in native human DSM tissue, we performed Western blot experiments using specific antibodies against the SK3 or IK channels. The data showed that human whole DSM tissue expresses SK3 channel protein (Fig. 3A) but not IK channel protein (Fig. 4A). These data verified our RT-PCR results that showed an SK3 but not an IK channel mRNA message in human DSM single cells. Further immunohistochemical analysis also confirmed the expression of SK3 channel protein (Fig. 3B) and ruled out the expression of IK channel protein (Fig. 4B) in human whole DSM tissue. Western blot and immunohistochemistry control experiments were further conducted by preabsorption of the primary antibody with its antigenic competing peptide to verify the specificity of the antibodies for their intended epitope.
Fig. 3.
Western blot and immunohistochemical detection of SK3 channel protein expression in native human whole DSM tissues. A: protein expression for SK3 channels was detected by Western blot in native human whole DSM tissue. The immunoreactive band was eliminated by a competing peptide (+CP). Experiments were conducted in 3 separate Western blot reactions using protein isolated from 3 patients. B: immunohistochemical detection of SK3 channels in mucosa-free human whole DSM tissue using SK3 channel-specific antibody. Cells' nuclei are shown in blue (B1); F-actin is shown in green (B2). Red staining indicates detection of the SK3 channels (B3). The merged images of the nuclei, F-actin, and the expected SK3 channels are illustrated in B4. Images were captured with a Carl Zeiss LSM 510 META confocal microscope. Experiments were conducted on tissue samples isolated from 4 different patients.
Fig. 4.
Western blot and immunohistochemical detection of IK channel showing the lack of IK channel protein expression in native human whole DSM tissues. A: protein expression for IK channels was detected by Western blot in human heart protein medley (positive control) but not in native human whole DSM tissue. The immunoreactive band in human heart protein medley was eliminated by +CP. Experiments were conducted in 3 separate Western blot reactions using protein isolated from 4 patients. B: IK channel protein was not detected in mucosa-free human whole DSM tissue following immunohistochemical reaction using IK channel-specific antibody. Cells' nuclei are shown in blue (B1); F-actin is shown in green (B2). Lack of red staining in B3 indicates no expression of IK channel. The merged images of the nuclei and F-actin are illustrated in B4. Images were captured with a Carl Zeiss LSM 510 META confocal microscope. Experiments were conducted on tissue samples isolated from 4 different patients.
Role of SK and IK channels in spontaneous (myogenic) phasic and tonic DSM contractions.
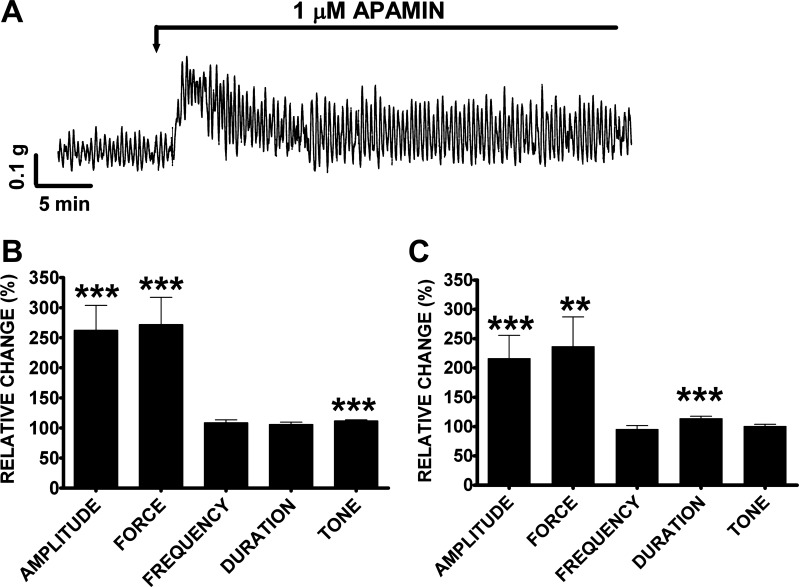
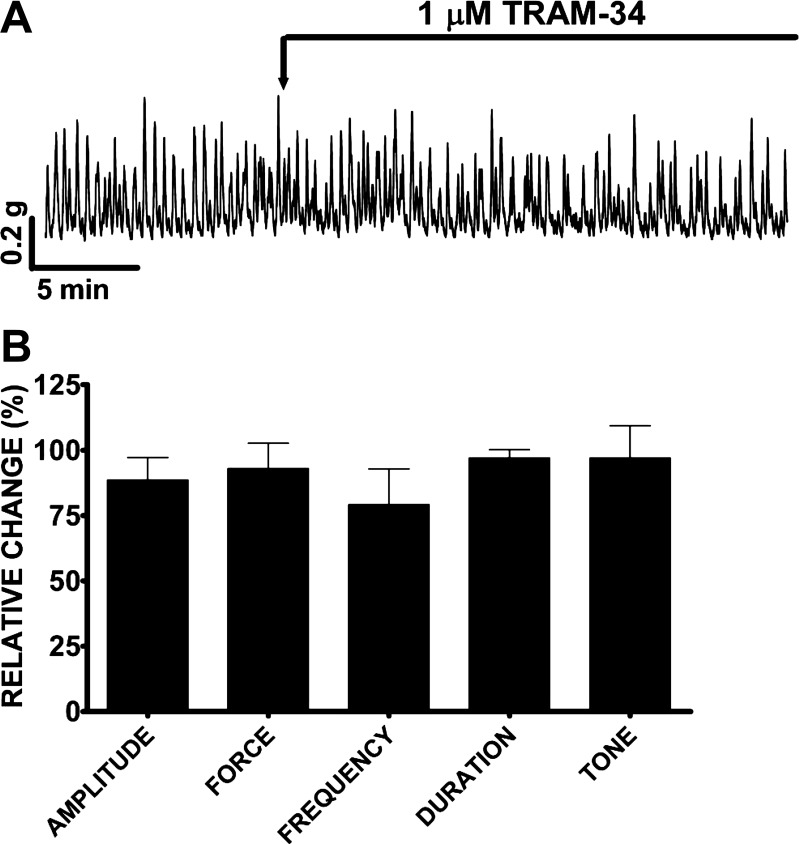
Here, we sought to evaluate the roles of SK and IK channels in human DSM spontaneous contractions by using the SK and IK channel-specific inhibitors apamin and TRAM-34, respectively. Apamin (1 μM) increased the DSM spontaneous phasic and tonic contractions and had a biphasic effect on DSM spontaneous contraction (Fig. 5A). Within the first 10 min, apamin (1 μM) increased the DSM spontaneous phasic contraction amplitude by 162.1 ± 41.7%, muscle force integral by 171.8 ± 45.5%, and muscle tone by 11.7 ± 1.8% (n = 28, N = 13, P < 0.005), without any significant effect on the spontaneous phasic contraction frequency and duration (Fig. 5B). We also evaluated the changes in the spontaneous phasic contractions, 30–40 min after apamin addition. During this second time period, the spontaneous phasic contraction amplitude was increased by 116.4 ± 39.2%, muscle force integral by 136.5 ± 50.7%, and phasic contraction duration by 13.8 ± 3.9% (n = 28, N = 13, P < 0.005); no significant effect was observed on the phasic contraction frequency and muscle tone (Fig. 5C). On the other hand, TRAM-34 (1 μM) did not have any significant effect on the spontaneous phasic and tonic contractions in the first 10 or 30–40 min after addition (n = 4, N = 4, P > 0.05; Fig. 6). Collectively, these results suggest that SK channels but not IK channels regulate human DSM myogenic activity.
Fig. 5.
Apamin increases the spontaneous phasic contraction amplitude, muscle force integral, phasic contraction duration, and muscle tone in human DSM isolated strips. A: original DSM tension recordings illustrating apamin (1 μM) effect on human DSM isolated strips. B: summary data showing significant increase in human DSM spontaneous phasic contractions amplitude, muscle force integral, and muscle tone within 10 min following apamin (1 μM) addition. C: summary data showing a significant increase in human DSM spontaneous phasic contractions amplitude, muscle force integral, and phasic contraction duration 30–40 min following apamin (1 μM) addition. The 10-min control period before apamin addition was taken to be 100% and data were normalized. Values are means ± SE (n = 28, N = 13; **P < 0.01, ***P < 0.005). Tetrodotoxin (TTX; 1 μM) was present throughout the experiments.
Fig. 6.
TRAM-34 does not affect the spontaneous phasic and tonic contractions in human DSM isolated strips. A: original DSM tension recordings illustrating the lack of TRAM-34 (1 μM) effect on human DSM isolated strips. B: summary data showing that TRAM-34 (1 μM) has no effect on any of the spontaneous phasic and tonic contraction parameters. TRAM-34 effect was evaluated within 10 min following TRAM-34 addition. The 10-min control period before TRAM-34 addition was taken to be 100% and data were normalized. Values are means ± SE (n = 4, N = 4; P > 0.05). TTX (1 μM) was present throughout the experiments.
Role of SK and IK channels in nerve-evoked contractions.
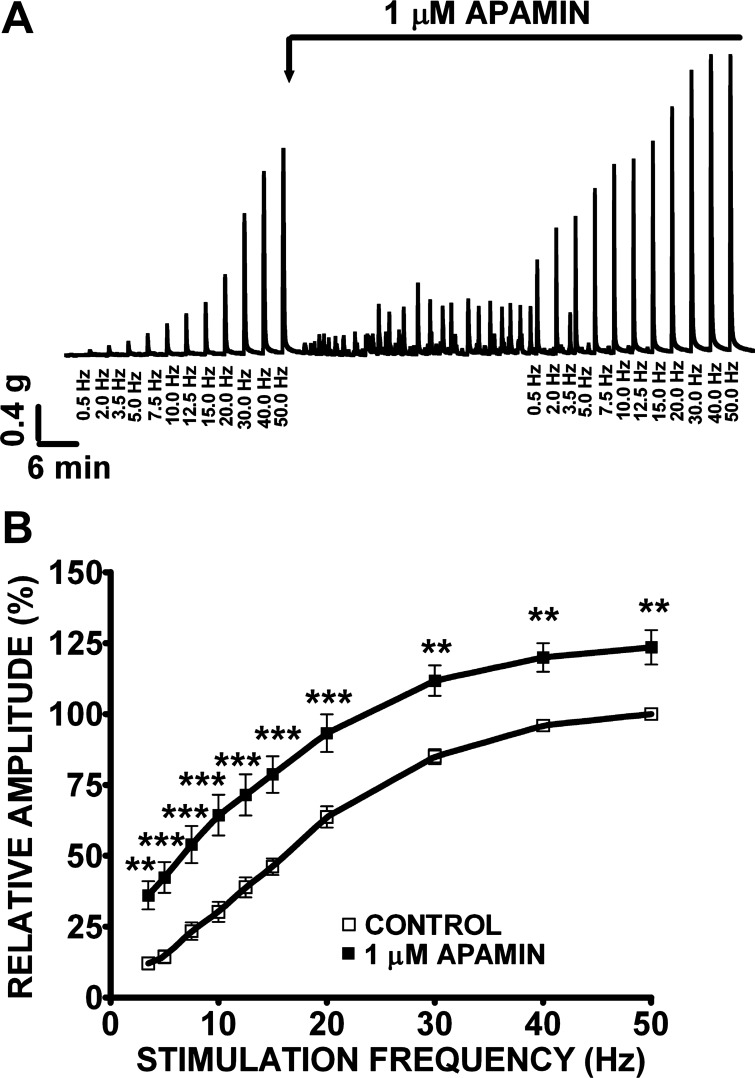
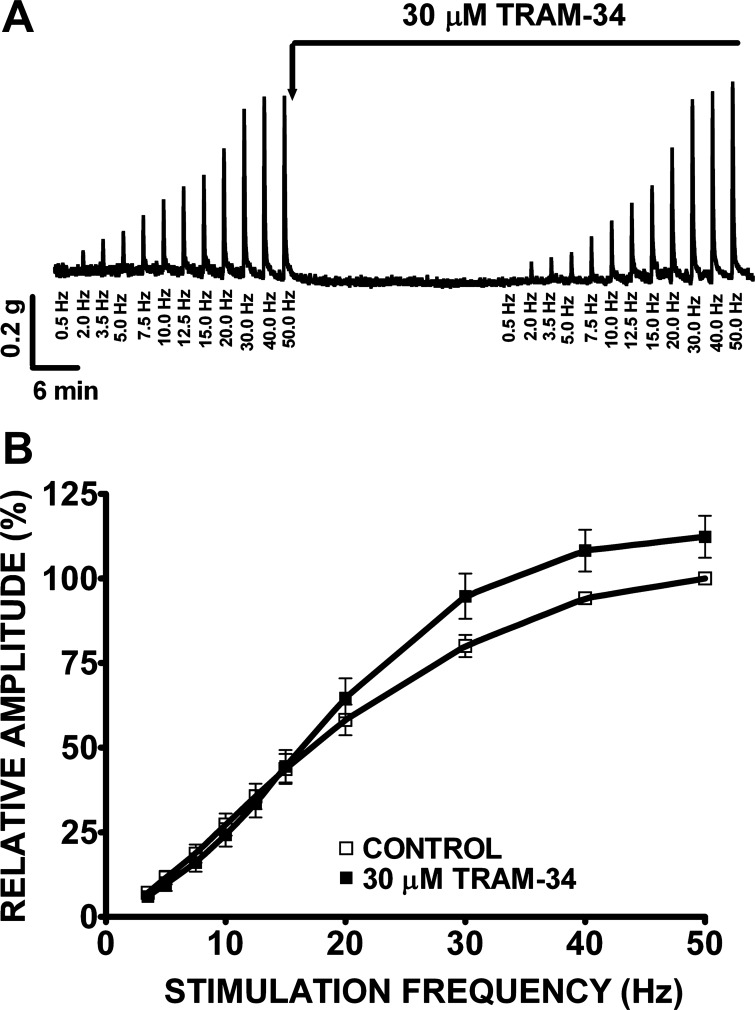
In this experimental series, we investigated the effects of apamin (1 μM) and TRAM-34 (1 or 30 μM) on nerve-evoked contractions of human DSM isolated strips (Figs. 7 and 8). Nerve-evoked contractions were generated by EFS and characterized in response to increasing EFS frequencies (0.5–50 Hz) in the presence or absence of apamin or TRAM-34, respectively. Increasing EFS frequencies (0.5, 2.0, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50 Hz) were first applied to each human DSM strip as a control. Then, apamin (1 μM) or TRAM-34 (1 or 30 μM) was added to the bath for 30 min. Next, the same EFS protocol (0.5–50 Hz) was applied to evaluate how these SK and IK channel inhibitors modulate EFS-induced contractions. The frequency-response curves showed that apamin (1 μM) significantly increased the amplitude of the EFS-induced contractions at stimulation frequencies ranging from 3.5 to 50 Hz (n = 23, N = 9, P < 0.01; Fig. 7B). At the maximal stimulation frequency of 50 Hz, apamin increased the EFS-induced contraction amplitude by 23.5 ± 6.1% (n = 23, N = 9, P < 0.01). However, TRAM-34 (1 μM) did not have any significant effect on EFS-induced contraction at all stimulation frequencies (0.5–50 Hz; n = 7, N = 2, P > 0.05). This lack of effect was also observed at TRAM-34 concentration as high as 30 μM (n = 21, N = 7, P > 0.05; Fig. 8B), suggesting that IK channels do not play a role in the nerve-evoked contractions.
Fig. 7.
Apamin increases the amplitude of the electrical field stimulation (EFS)-induced contractions in human DSM isolated strips. A: original recordings illustrating apamin (1 μM) effect on EFS-induced (0.5–50 Hz) contractions. As shown, apamin (1 μM) also induced myogenic contractions, consistent with data illustrated in Fig. 4. B: frequency-response curves showing a significant increase in the amplitude of the EFS-induced contractions following application of apamin (1 μM). The maximal EFS-contraction amplitude at a stimulation frequency of 50 Hz under control conditions was taken to be 100% and the contractions were normalized. Values are means ± SE (n = 23, N = 9; **P < 0.01, ***P < 0.005).
Fig. 8.
TRAM-34 has no effect on the amplitude of the EFS-induced contractions in human DSM isolated strips. A: original recordings illustrating the lack of TRAM-34 (30 μM) effect on EFS-induced (0.5–50 Hz) contractions. B: frequency-response curves showing no significant change in the amplitude of the EFS-induced contractions following application of TRAM-34 (30 μM). The maximal EFS-contraction amplitude at a stimulation frequency of 50 Hz under control conditions was taken to be 100% and the contractions were normalized. Values are means ± SE (n = 21, N = 7; P > 0.05).
DISCUSSION
In the present study, we used combined molecular (RT-PCR, qPCR, Western blot, and immunohistochemistry), functional (isometric DSM tension recordings), and pharmacological (SK and IK channel inhibitors) approaches to investigate the molecular expression of all SK and IK channels in freshly isolated human DSM cells and further evaluated their functional role in human DSM contractility. Single-cell RT-PCR experiments confirmed the expression of only the SK3 channel subtype at the mRNA level. qPCR experiments indicated that the SK3 channel is the most predominant subtype in native human DSM single cells at the mRNA level. Western blot and immunostaining further confirmed SK3 channel protein expression. Functional studies using apamin and TRAM-34 suggest that the SK channels but not the IK channels have an important role in human DSM contractility.
SK and IK channels mRNA expression in human DSM cells.
In human whole DSM tissue, RT-PCR showed that all SK and IK channels were expressed. Furthermore, the qPCR experiments not only confirmed mRNA expression for all SK and IK channel subtypes in whole DSM but also indicated that SK1 and SK3 channels are the most predominant. However, at the DSM single-cell level, only the SK3 channels were detected by RT-PCR, in all patients tested. Moreover, qPCR data from isolated DSM single cells indicate a much higher expression level for the SK3 channel. The difference in mRNA relative expression between whole DSM tissue and isolated DSM single cells could be explained by the fact that the detection of mRNA messages in isolated DSM single cells is a more accurate approach. The unique advantage of using freshly isolated DSM single cells in RT-PCR and qPCR is that this approach eliminates any possible contamination by other non-DSM cell types including neurons, fibroblasts, endothelial cells, and vascular cells present within the DSM layers (6, 7, 20, 21). It is possible that the detected SK1 channel mRNA in whole DSM originated from neuronal cells present in the DSM layers consistent with the findings from Chen et al. (8). The aforementioned study using qPCR experiments revealed that the SK3 channel is the most predominant SK channel subtype in human whole bladder tissue and that the SK1 channel is highly expressed in human neuronal tissues (brain) (8). This observation was confirmed by our RT-PCR, Western blot, and immunohistochemistry data showing mRNA message and protein expression for SK3 channel in native human DSM single cells and whole DSM tissue, respectively (Figs. 1–3). The presence of the SK3 channel protein in human whole DSM tissue confirms that SK3 channels play a critical physiological role in human bladder physiology, similar to rodent models (18). Studies have shown that selective suppression of SK3 channel expression increased the phasic contractions of mouse isolated DSM strips and increased nonvoiding contractions in vivo (18). Suppression of SK3 channel expression by genetic manipulations further elevated micturition pressure compared with control mice. Transgenic mice overexpressing the SK3 channels had greatly elevated bladder capacities and urine output compared with wild-type mice (18). In the present study, we detected high levels of SK3 channel mRNA message in human DSM single cells and further confirmed the SK3 channel protein expression in whole DSM tissue. Therefore, it is likely that SK3 channels play an important functional role in human DSM contractility.
SK channels regulate human DSM spontaneous phasic contractions.
Using channel-specific inhibitors apamin and TRAM-34, we determined the SK and IK channel functional roles in human DSM spontaneous phasic contractions. Our data show that apamin but not TRAM-34 significantly increased the phasic contraction amplitude, muscle force integral, phasic contraction duration, and muscle tone of the DSM spontaneous contraction, suggesting that SK channels but not IK channels are effective modulators of human DSM contractility. Consistent with our human data, studies in guinea pig DSM showed that apamin but not TRAM-34 increases DSM spontaneous contractions (29). Furthermore, apamin but not TRAM-34 blocked guinea pig DSM relaxation induced by SKA-31, a novel and potent SK channel opener, suggesting that IK channels have no functional role in guinea pig DSM physiology compared with SK channels (29). A previous study established a correlation between spontaneous contractile activity and electrical activity in guinea pig DSM (13). The study showed that the inhibition of SK channels with apamin increased spontaneous phasic contraction amplitude and duration, and reduced phasic contraction frequency while converting the electrical activity from individual action potentials into bursts without changing either the action potential shape or after hyperpolarization (13). Apamin also increased guinea pig and mouse DSM action potential frequency suggesting that SK channels are important regulators of DSM excitability (11, 14). These animal data are consistent with our human data since apamin increased spontaneous phasic contraction amplitude and duration without affecting the phasic contraction frequency of human DSM isolated strips. We propose that the initial burst in action potentials could explain the initial jump observed in spontaneous contraction observed immediately after apamin was added into the baths (Fig. 5A). Earlier studies further showed that apamin increased human DSM phasic contractions induced by 10 mM KCl, suggesting the involvement of SK channels in KCl-induced contractions (10). Studies by others also reported that apamin can partially reverse the relaxation caused by the selective SK channel opener NS4591 in rat, pig, and human DSM strips stimulated by 1 μM carbachol (26). The major advantage of our work compared with those conducted previously on human DSM is that herein we evaluated the SK and IK channels functional role on human DSM strips exhibiting spontaneous phasic contractions. The unique advantage of using these spontaneously contracting DSM strips is their direct physiological relevance compared with those in which contractions are artificially induced by depolarizing agents such as KCl or carbachol (10, 26).
SK channel role in human DSM nerve-evoked contractions.
During micturition, parasympathetic nerves release ATP and acetylcholine that activate purinergic P2X receptors and muscarinic receptors to cause DSM contraction. Using EFS, we activated the cholinergic and purinergic nerves present in the human DSM strips to induce contractions and then evaluated whether apamin or TRAM-34 could modulate EFS-induced contractions. Our data show that apamin but not TRAM-34 significantly increased the amplitude of the EFS-induced contraction at all stimulation frequencies between 3.5 and 50 Hz, suggesting that SK channels but not IK channels modulate human DSM nerve-evoked contraction. Our data on human DSM are consistent with previous studies on mouse and guinea pig DSM isolated strips (15, 29). In mouse and guinea pig, blocking SK channels with apamin resulted in an increase in the amplitude of nerve-evoked contractions (15, 29). However, in guinea pig DSM, inhibition of IK channels with TRAM-34 had no effect on nerve-evoked contractions suggesting the lack of a functional role for IK channels (29). Our findings suggest that SK channels participate in a negative feedback loop that limits the DSM excitability and contractility in response to nerve stimulation consistent with previous mouse and guinea pig DSM studies (15, 16, 18, 29).
In summary, the SK3 channels are the only SK channel subtype expressed in human DSM single cells at the mRNA level. SK3 channel protein expression was also confirmed in whole DSM tissue. DSM contractility studies using apamin and TRAM-34 showed that the SK channels but not the IK channels have a critical role in human DSM function. Taken together, we suggest that the SK channels, the SK3 subtype in particular, are important modulators of human DSM contractility. SK3 channels could therefore represent novel therapeutic targets for the pharmacological treatment of OAB.
GRANTS
This study was supported by National Institutes of Health Grants DK084284 and DK083687 to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.A., E.S.R., and G.V.P. conception and design of research; S.A.A. and G.V.P. performed experiments; S.A.A. and G.V.P. analyzed data; S.A.A. and G.V.P. interpreted results of experiments; S.A.A. and G.V.P. prepared figures; S.A.A. and G.V.P. drafted manuscript; S.A.A., E.S.R., and G.V.P. edited and revised manuscript; S.A.A., E.S.R., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Medical University of South Carolina (MUSC) Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, Ahmed M. El-Zawahry, and Jonathan Picard, as well as the MUSC Urology Residents: Drs. Avi C. Weiss, Gary W. Bong, Kelly Doyle, Matthew McIntyre, Matt Eskridge, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, Elizabeth Peacock, Lydia Labocetta, Matthew Young, Erin Burns, Taylor Vaughn, Samuel Nickles, and Vinh Q. Trang for help with human tissue collection; Drs. Joe Jones and Kim Creek for help with the qPCR experiments; and Drs. John Malysz, Kiril L. Hristov, Shankar Parajuli, Wenkuan Xin, Rupal P. Soder, Ms. Amy Smith, and Mr. Qiuping Cheng for the critical evaluation of the manuscript.
REFERENCES
- 1. Afeli SA, Hristov KL, Petkov GV. Do β3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am J Physiol Renal Physiol 302: F251–F263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson KE. Pharmacotherapy of the overactive bladder. Discov Med 8: 118–124, 2009 [PubMed] [Google Scholar]
- 3. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135: 639–648, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol 369: 602–615, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Crowe R, Burnstock G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra? J Urol 141: 414–422, 1989 [DOI] [PubMed] [Google Scholar]
- 10. Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol 99: 779–785, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayase M, Hashitani H, Kohri K, Suzuki H. Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181: 2355–2365, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hougaard C, Fraser MO, Chien C, Bookout A, Katofiasc M, Jensen BS, Rode F, Bitsch-Norhave J, Teuber L, Thor KB, Strobaek D, Burgard EC, Ronn LC. A positive modulator of KCa2 and KCa3 channels, 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591), inhibits bladder afferent firing in vitro and bladder overactivity in vivo. J Pharmacol Exp Ther 328: 28–39, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Hristov KL, Chen M, Afeli SA, Cheng Q, Rovner ES, Petkov GV. Expression and function of KV2-containing channels in human urinary bladder smooth muscle. Am J Physiol Cell Physiol 302: C1599–C1608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360–C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen JS, Rode F, Rahbek M, Andersson KE, Ronn LC, Bouchelouche K, Nordling J, Bouchelouche P. Effect of the SK/IK channel modulator 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591) on contractile force in rat, pig and human detrusor smooth muscle. BJU Int 108: 771–777, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108: 604–611, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Pandita RK, Ronn LC, Jensen BS, Andersson KE. Urodynamic effects of intravesical administration of the new small/intermediate conductance calcium activated potassium channel activator NS309 in freely moving, conscious rats. J Urol 176: 1220–1224, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petkov GV. Ion channels. In: Pharmacology: Principles and Practice, edited by Hacker M, Messer W, Bachmann K. New York: Elsevier, 2009, chapt. 16, p. 385–425 [Google Scholar]
- 31. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Kohler R, Wulff H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75: 281–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327–336, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ESR, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol 294: R1737–R1743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]