Abstract
We demonstrated that nonselective PKC activation promotes mitochondrial function in renal proximal tubular cells (RPTC) following toxicant injury. However, the specific PKC isozyme mediating this effect is unknown. This study investigated the role of PKC-α in the recovery of mitochondrial functions in oxidant-injured RPTC. Wild-type PKC-α (wtPKC-α) and inactive PKC-α mutants were overexpressed in RPTC to selectively increase or block PKC-α activation. Oxidant (tert-butyl hydroperoxidel; TBHP) exposure activated PKC-α in RPTC but decreased PKC-α levels in mitochondria following treatment. Uncoupled and state 3 respirations and activities of complexes I and IV in TBHP-injured cells decreased to 55, 44, 49, and 65% of controls, respectively. F0F1-ATPase activity and ATP content in injured RPTC decreased to 59 and 60% of controls, respectively. Oxidant exposure increased reactive oxygen species (ROS) production by 210% and induced mitochondrial fragmentation and 52% RPTC lysis. Overexpressing wtPKC-α did not block TBHP-induced ROS production but improved respiration and complex I activity, restored complex IV and F0F1-ATPase activities, promoted recovery of ATP content, blocked mitochondrial fragmentation, and reduced RPTC lysis to 14%. In contrast, inhibiting PKC-α 1) induced mitochondrial hyperpolarization and fragmentation; 2) blocked increases in ROS production; 3) prevented recovery of respiratory complexes and F0F1-ATPase activities, respiration, and ATP content; and 4) exacerbated TBHP-induced RPTC lysis. We conclude that 1) activation of PKC-α prevents mitochondrial hyperpolarization and fragmentation, decreases cell death, and promotes recovery of mitochondrial respiration and ATP content following oxidant injury in RPTC; and 2) respiratory complexes I and IV and F0F1-ATPase are targets of active PKC-α.
Keywords: mitochondrial dysfunction, renal proximal tubular cells, reactive oxygen species, respiration, electron transport chain, complex I, complex IV, cytochrome oxidase, F0F1-ATPase, ATP
pkc-α is ubiquitously expressed in different cell types and is involved in a variety of physiological and pathological processes including proliferation, growth, differentiation, cell death, survival, malignant transformation, and cancer (4, 19). Activation of PKC-α has been implicated in the promotion of tumor cell survival and resistance to cell death induced by chemotherapeutic agents (4, 11, 30). Defective differentiation in skin cancer cells is associated with elevated PKC-α activity (4). Consequently, PKC-α has been a target of pharmacological approaches to inhibit proliferation of cancer cells as well as to treat a number of other conditions (3, 12, 13). Translocation of PKC-α to different subcellular compartments is critical to the functions of PKC-α in response to stimulation and is thought to be a key indicator of PKC-α function. A large body of evidence demonstrates that PKC isoforms play a direct role in regulating mitochondrial function. Translocation of PKC isoforms, including PKC-α, to mitochondria has been shown in several different cell types in response to a variety of different stimuli, including PMA, ischemia followed by reperfusion, heat shock, and toxicant exposure (10, 41, 42, 43). PKC-α appears to be localized to the inner mitochondrial membrane and cristae and is specifically targeted and anchored to mitochondria by the protein interacting with C kinase (PICK1) (41, 42). Mitochondrial localization of PKC-α is associated with its activation and resistance to drug-induced apoptosis in leukemia REH cells (35). The mechanism of resistance to apoptosis is through PKC-α-mediated phosphorylation of Bcl-2 on Ser70 and decreased dimerization of the proapoptotic Bax. These events prevent the loss of mitochondrial membrane potential and promote cell survival (35).
PKC-α-mediated signaling has prosurvival effects in some cell types, whereas in other tissues PKC-α activation is detrimental and contributes to cell death. In the U937 monocyte cell line, nontoxic concentrations of peroxynitrite induce survival signaling that involves mitochondrial translocation of PKC-α and redistribution of Bad from mitochondria to the cytoplasm (6). Loss of PKC-α from mitochondria and translocation of PKC-α from mitochondria to the nucleus in gastric cancer cells are associated with apoptosis, suggesting that PKC-α exerts protective effects only when present in mitochondria (44). PKC-α activation and translocation to mitochondria are also involved in cardioprotection from ischemic injury (2).
In contrast, PMA-induced mitochondrial localization of PKC-α in C6 glioma and COS-7 fibroblast-like cells and in C2C12 myoblasts activates mitochondrial PKC-α, alters cell morphology, collapses mitochondrial membrane potential, decreases complex I and pyruvate dehydrogenase activities, and increases mitochondrial reactive oxygen species (ROS) production (43). Activation of PKC-α by an anticancer agent, WJ9708012, in prostate cancer cells induces mitochondrial stress and loss of mitochondrial membrane potential (ΔΨm), leading ultimately to apoptosis (14). Ruvolo and colleagues (35) proposed that the mechanism responsible for the apparent dichotomy in the effects of PKC-α on cell fate is dependent on the localization of active PKC-α. Agents that induce mitochondrial localization of PKC-α and in vitro overexpression of exogenous PKC-α leading to its mitochondrial localization increase cell resistance to drug-induced cell death. On the other hand, agents that induce localization of PKC-α in other subcellular compartments but not in mitochondria result in mitochondrial toxicity and cell death (35). Thus this hypothesis (35) proposed protective actions of active PKC-α when localized to mitochondria, but not when active in other subcellular compartments.
It has been shown that several PKC isozymes, including PKC-α, are crucial for kidney development. Inhibition of these PKC isozymes disrupts nephron formation and growth and induces apoptosis in the developing kidney (36). PKC-α activation by nephrotoxicants such as cisplatin or S-(1,2-dichlorovinyl)-l-cysteine (DCVC) is associated with mitochondrial dysfunction, decreases in active Na+ transport, and apoptosis (16, 21). Interestingly, cisplatin-induced activation of PKC-α in renal proximal tubule cells (RPTC) is not associated with its translocation to mitochondria or phosphorylation of mitochondrial PKC-α despite profound effects on mitochondrial function (21). We have demonstrated that the repair of mitochondrial functions in RPTC following oxidant injury is mediated through PKC-dependent mechanisms and that isozyme-nonselective activation of PKC improves recovery of mitochondrial function after oxidant injury (20). However, specific PKC isozymes involved in the repair of oxidant-injured RPTC remain unknown. Therefore, we tested whether the recovery of mitochondrial function after oxidant injury is mediated by PKC-α. This study was designed to determine whether selective overexpression of PKC-α in RPTC plays a role in mitochondrial dysfunction induced by an oxidant and in the recovery of mitochondrial function in oxidant-injured RPTC. Specific goals of this study were to 1) examine whether selective overexpression of PKC-α in RPTC alters mitochondrial function and cell survival following oxidant-induced injury, 2) determine mitochondrial functions targeted by PKC-α in oxidant-injured RPTC, and 3) test whether overexpression of PKC-α improves recovery of mitochondrial function following oxidant injury in RPTC.
MATERIALS AND METHODS
Animals and materials.
Female New Zealand White rabbits (2.0–2.5 kg) were purchased from Myrtle's Rabbitry (Thompson Station, TN). All animal procedures involved in this study were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. The cell culture media (a 50:50 mixture of DMEM and Ham's F-12 nutrient mix without phenol red, pyruvate, and glucose) was purchased from MediaTech Cellgro (Herndon, VA). Phospho-PKC-α (Ser 657) and PKC-α antibodies were purchased from Upstate Biotechnology (Lake Placid, NY) and BD Transduction Laboratory (San Diego, CA), respectively. Antibodies against the β-subunit of F0F1-ATPase, MitoTracker Red 580, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), and 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) were supplied by Life Technologies-Molecular Probes (Eugene, OR). The sources of other reagents have been described previously (16, 20, 22, 25, 26).
Isolation and culture of RPTC.
Renal proximal tubules were isolated from rabbit kidneys by the iron oxide perfusion method and cultured in 35-mm culture dishes in improved conditions as previously described (25, 26). The culture medium was a 50:50 mixture of DMEM and Ham's F-12 nutrient mix without phenol red, pyruvate, and glucose, supplemented with 15 mM NaHCO3, 15 mM HEPES, and 6 mM lactate (pH 7.4, 295 mosmol/kgH2O). Human transferrin (5 μg/ml), selenium (5 ng/ml), hydrocortisone (50 nM), bovine insulin (10 nM), and l-ascorbic acid-2-phosphate (50 μM) were added to the media immediately before daily media change.
Adenoviral constructs and amplification.
An adenoviral vector encoding the dominant negative (inactive) PKC-α (dnPKC-α) was constructed by Dr. Trevor Biden (Garvan Institute of Medical Research, St. Vincent's Hospital, Sydney, Australia) as described previously (5). A dominant negative (kinase dead, inactive) PKC-α mutant was constructed by mutation at the ATP-binding site (replacement of lysine with arginine at position 368) (5). This mutation destroyed the construct's catalytic activity. Overexpression of kinase-dead forms of PKC isozymes has been shown to act in an isoenzyme-specific dominant negative fashion (5). An aliquot of the adenovirus encoding dnPKC-α was obtained from Dr. Biden and was amplified in our laboratory. Adenoviral vectors encoding the wild-type PKC-α (wtPKC-α), the green fluorescent protein (GFP)-PKC-α mutant, and the shuttle plasmid were constructed as previously described (31, 31, 40). Aliquots of these adenoviral vectors were a generous gift from Dr. Allen M. Samarel (Loyola University Medical Center, Chicago, IL).
Adenoviruses were amplified in AD293 and HEK293 cells as we described previously (38). Adenoviral particles were isolated and purified from HEK293 lysates by centrifugation in CsCl density gradient (7.5 and 8.3 M) at 175,500 g for 1 h. The multiplicity of infection (MOI) was determined by a viral dilution assay in HEK-293 cells grown in 96-well plates.
Overexpression of wild-type and inactive PKC-α.
All transfections were carried out in confluent quiescent cultures of RPTC. Selective overexpression of wild-type and inactive PKC-α was achieved by transfecting RPTC using adenoviral vectors encoding wtPKC-α (multiplicity of infection, MOI = 75) and dnPKC-α (MOI = 100). Infection with adenoviral particles encoding an empty pShuttle vector (MOI = 50) was used as a control. Culture media were changed 24 and 48 h following transfections of RPTC with respective PKC-α mutants or the empty pShuttle vector.
Oxidant treatment of RPTC monolayer.
Confluent monolayers of RPTC were treated with a model oxidant, tert-butylhydroperoxide (TBHP; 350 μM), for 45 min. Controls were treated with the diluent (DMSO, 0.1%). Following TBHP exposure, the monolayer was washed with fresh warm (37°C) medium and cultured for an additional 4, 24, or 96 h. To assess the role of PKC-α in RPTC survival and recovery, RPTC were transfected with wtPKC-α, dnPKC-α, and the empty pShuttle vector at 48 h before injury, and exposed to TBHP for 45 min.
ROS/nitrogen species generation.
The carboxy-derivative of fluorescein, carboxy-H2DCFDA, was used to assess oxidant generation in RPTC as described previously (27). RPTC were loaded with 5 μM carboxy-H2DCFDA followed by treatment with TBHP for 50 min. Following incubation, RPTC were suspended in the media, transferred to 48-well plates, and fluorescence was analyzed by fluorometry. Negative controls (unstained RPTC) were included in each experiment. All results were corrected for autofluorescence and expressed as arbitrary fluorescence units per milligram cellular protein.
State 3 respiration.
Basal and uncoupled oxygen consumption were measured polarographically using a Clark-type electrode as described previously (20, 24, 26). State 3 respiration in mitochondria energized by electron donors to respiratory complexes I and II was measured in the assay buffer containing digitonin (0.1 mg/ml) and respiratory substrates as described previously (23, 37). State 3 respiration was initiated by addition of 0.4 mM ADP. State 4 respiration was measured following addition of oligomycin (0.6 μg/ml) to RPTC respiring at state 3.
Intracellular ATP content.
Intracellular ATP content in RPTC was measured by the luciferase method in freshly prepared RPTC lysates using an ATP Bioluminescence Assay Kit HS II (Roche, Mannheim, Germany) and the manufacturer's protocol as described previously (21–23).
Mitochondrial membrane potential.
Mitochondrial membrane potential (ΔΨm) was assessed as described previously (21, 28) using JC-1, a cationic dye that exhibits potential-dependent accumulation and formation of red fluorescent J-aggregates in mitochondria. Each set of samples included a positive control for mitochondrial depolarization (RPTC treated with FCCP, 2 μM) and hyperpolarization (RPTC treated with 0.6 μg/ml oligomycin). Fluorescence was determined by flow cytometry (FACSCalibur, BD Biosciences) using excitation by a 488-nm argon-ion laser. The JC-1 monomer (green) and the J-aggregates (red) were detected separately in FL1 (emission, 525 nm) and FL2 (emission, 590 nm) channels, respectively. ΔΨm is presented as the red-to-green fluorescence intensity ratio.
Isolation of RPTC mitochondria.
RPTC were homogenized, and mitochondria were isolated as described previously (23, 37). Purity of mitochondrial fractions was tested by measuring protein levels of the endoplasmic reticulum marker calreticulin, lysosomal protein LAMP-1, and the peroxisomal marker catalase. Mitochondria resulting from this isolation were free of lysosomal and endoplasmic reticular contaminations (28, 37). The mitochondrial fraction contained some peroxisomes that sedimented at the g force similar to that used to sediment mitochondria (28). The final mitochondrial pellet was resuspended in assay buffers used for measuring activities of respiratory complexes and F0F1-ATPase or in Laemmli sample buffer for immunoblot analysis.
Activity of respiratory complexes.
Isolated mitochondria were suspended in the hypotonic assay buffer (25 mM potassium phosphate buffer containing 5 mM MgCl2, pH 7.2) and freeze-thawed in liquid nitrogen. Activities of complexes I, II, III, and IV were measured, and their activity was calculated as described previously (37). In all assays, the results were corrected for changes in the absorbance in blank samples containing no mitochondria.
F0F1-ATPase activity.
ATPase activity of ATP synthase was determined in freshly isolated mitochondria by measuring the release of Pi from ATP by the method of Law et al. (15) as described previously (28, 38). Each sample was run in the absence and presence of oligomycin (10 μg/ml), and the oligomycin-sensitive ATPase activity of F0F1-ATPase was calculated.
Immunoblotting.
Phosphorylation and protein levels of PKC-α in RPTC lysates and in isolated mitochondria were assessed by immunoblot analysis as described previously (22).
Mitochondrial morphology.
RPTC monolayers were loaded with 100 nM MitoTracker Red 580 for 30 min at 37°C. The live monolayers were examined under a Zeiss fluorescent microscope (Axioscop) using a water-immersion objective (×63).
Assessment of RPTC death.
RPTC apoptosis was evaluated by measuring phosphatidylserine externalization on the plasma membrane using an annexin V/propidium iodide-binding assay as previously described (22, 28, 38). Cells positive for annexin V and negative for propidium iodide were considered apoptotic. Cells positive for propidium iodide and negative for annexin V were considered necrotic.
LDH release.
Release of lactate dehydrogenase (LDH) from RPTC into the culture medium was used as another marker of RPTC plasma membrane permeabilization and cell lysis and was determined as described previously (28).
All results were normalized to cellular protein, which was measured by a bicinchoninic acid (BCA) assay using BSA as the standard.
Statistical analysis.
Data are presented as means ± SE and were analyzed for significance by ANOVA. Multiple means were compared using Fisher's protected least significance difference test with a level of significance of P < 0.05. RPTC isolated from an individual rabbit represented one experiment (n = 1) consisting of data obtained from 2–10 culture plates.
RESULTS
Oxidant induces activation of PKC-α in RPTC.
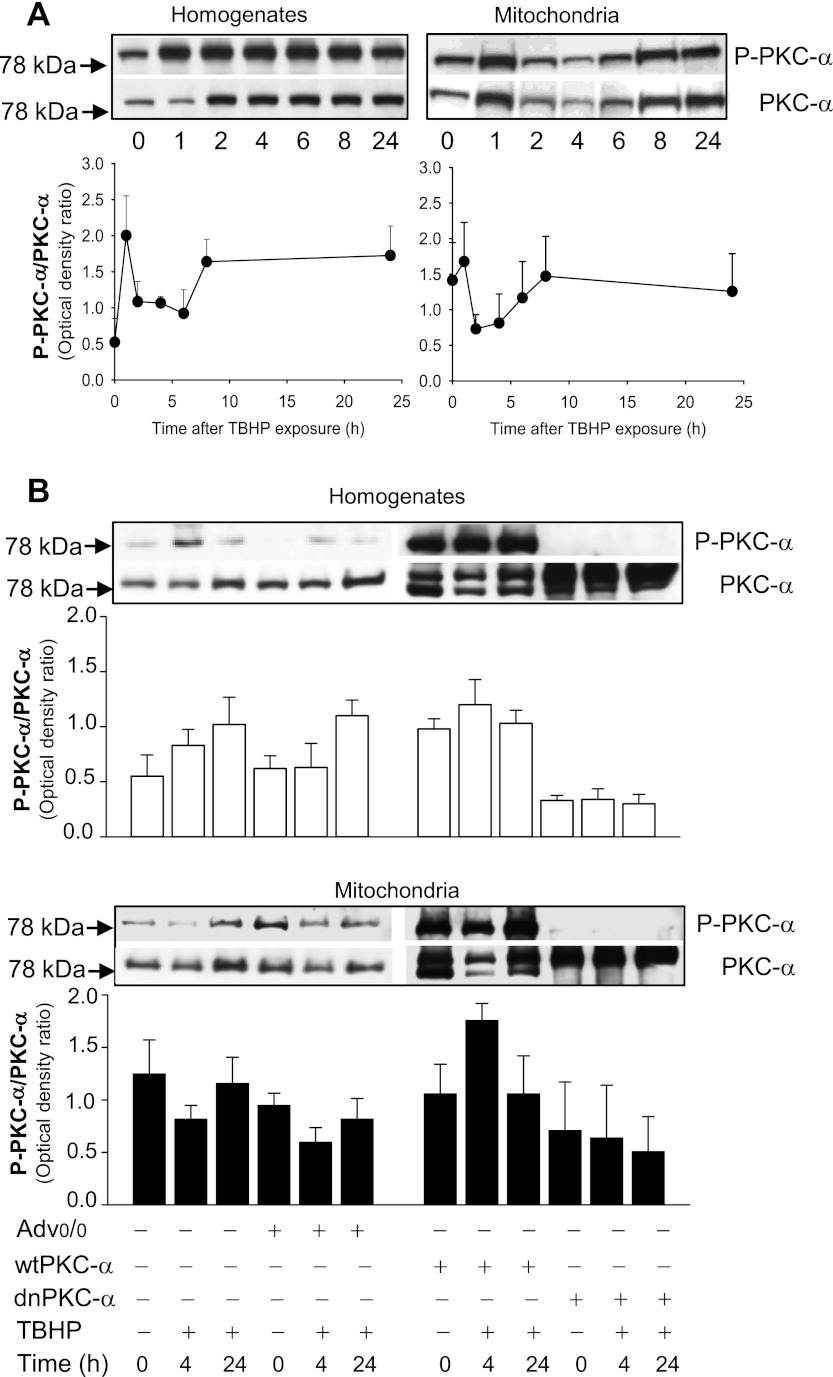
The protein levels of PKC-α increased during TBHP exposure in RPTC and remained elevated during 24 h after the exposure. The ratio of phosphorylated and total protein levels of PKC-α were increased two- to fourfold compared with control RPTC, which suggested that TBHP induces PKC-α activation (Fig. 1A). Expressing wtPKC-α in RPTC cultures resulted in increases in protein levels of total and phosphorylated (active) PKC-α and maintained the levels of active PKC-α following TBHP-induced injury (Fig. 1B). Transfection of the inactive PKC-α mutant (dnPKC-α) into RPTC blocked PKC-α phosphorylation in noninjured RPTC and abrogated increases in PKC-α phosphorylation in TBHP-injured RPTC (Fig. 1B). The adenovirus coding the empty pShuttle vector had no effect on the levels of PKC-α in RPTC (Fig. 1B). These data suggest that the oxidant exposure activates PKC-α in RPTC during the early recovery period following the exposure.
Fig. 1.
A: protein levels of phosphorylated PKC-α (P-PKC-α) and total PKC-α (PKC-α) in renal proximal tubule epithelial cell (RPTC) homogenates (left) and mitochondria (right) at different times after tert-butyl hydroperoxidel (TBHP) exposure. Shown is the ratio of phosphorylated PKC-α to total PKC-α in homogenates (left) and mitochondria (right) at different time points following TBHP exposure in RPTC. The results (quantified by densitometry) are means ± SE (n = 5). B: protein levels of P-PKC-α and total PKC-α in RPTC infected with the adenovirus carrying the empty vector (Advo/o), wild-type PKC-α (wtPKC-α), and the inactive mutant of PKC-α (dnPKC-α) in noninjured and TBHP-injured RPTC. The samples were taken at 4 and 24 h after TBHP exposure. The blots are representative of 5 independent experiments.
Oxidant decreases mitochondrial levels of PKC-α in RPTC.
Protein levels of phosphorylated PKC-α in RPTC mitochondria increased at 1 h after oxidant exposure (Fig. 1A). This increase was due to the translocation of PKC-α to mitochondria, as the total levels of PKC-α in this organelle increased at 1 h of the recovery period and the average ratio of phosphorylated and total protein levels of PKC-α did not change. The initial increase in PKC-α levels in mitochondria was transient and was followed by significant decreases in protein levels of phosphorylated and total PKC-α between 2 and 6 h after TBHP exposure (Fig. 1A). The levels of PKC-α protein were restored at 8 h after TBHP treatment (Fig. 1A). Expressing wtPKC-α increased mitochondrial levels of total and phosphorylated (active) PKC-α in noninjured and TBHP-injured RPTC (Fig. 1B). In addition, at 4 h following injury in RPTC overexpressing wtPKC-α, the mitochondrial p-PKC-α/PKC-α ratio (indicative of activation) was increased 66% compared with noninjured controls (Fig. 1B). As a result, the p-PKC-α/PKC-α ratio in mitochondria of injured RPTC overexpressing wtPKC-α was twofold higher than this ratio in nontransfected RPTC injured by TBHP (Fig. 1B). In contrast, transfection of dnPKC-α into RPTC blocked PKC-α phosphorylation in mitochondria of noninjured RPTC and abrogated increases in PKC-α phosphorylation in mitochondria of TBHP-injured RPTC (Fig. 1B). The adenovirus coding the empty pShuttle vector had no effect on mitochondrial levels of PKC-α in noninjured and TBHP-injured RPTC (Fig. 1B). These data demonstrate that the levels of active PKC-α in RPTC mitochondria are decreased during the early recovery after oxidant exposure and that overexpressing wtPKC-α maintains the mitochondrial levels of active PKC-α in injured RPTC.
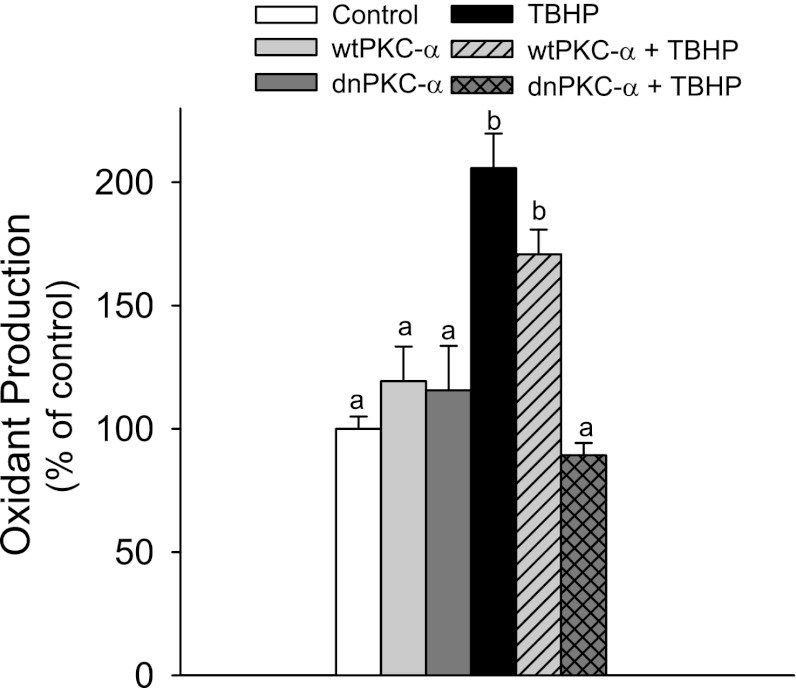
PKC-α activation mediates oxidant generation.
Carboxy-H2DCFDA can be oxidized by both ROS and reactive nitrogen species (RNS). Exposure to TBHP resulted in a 210% increase in ROS/RNS levels in RPTC (Fig. 2). Overexpressing wtPKC-α had no effect on ROS/RNS levels in noninjured and TBHP-injured RPTC (Fig. 2). Overexpressing dnPKC-α decreased basal levels of ROS/RNS in noninjured RPTC and blocked the increase in ROS/RNS levels in cells exposed to TBHP (Fig. 2). These data show that blocking PKC-α inhibits TBHP-induced ROS/RNS generation and that PKC-α activation mediates increases in oxidant production in injured RPTC.
Fig. 2.
Oxidant production in confluent noninjured and TBHP-injured RPTC overexpressing wtPKC-α and dnPKC-α. The data are expressed as % of control. Oxidant generation in controls was 63,967 ± 8,499 arbitrary fluorescence units/mg protein.
Activation of PKC-α promotes recovery of the electron transport chain after oxidant injury.
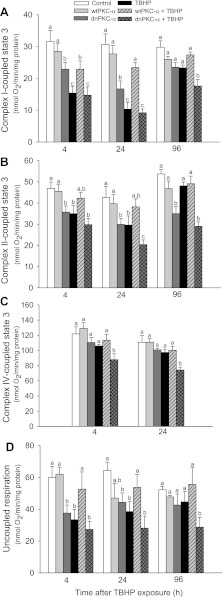
We hypothesized that translocation of the active PKC-α to mitochondria of oxidant-injured RPTC plays a role in recovery of mitochondrial function following oxidant exposure. To test this hypothesis, we determined mitochondrial respiration in RPTC overexpressing wtPKC-α and dnPKC-α during recovery after TBHP exposure. State 3 respiration (the maximum respiration capacity) in mitochondria energized with glutamate+malate decreased to 48% of control level at 4 h after TBHP injury and had not recovered by day 4 following injury (Fig. 3A). Overexpressing wtPKC-α reduced the decreases and promoted full recovery of complex I-coupled state 3 respiration by day 4 following TBHP injury. In contrast, overexpressing dnPKC-α blocked recovery of complex I-coupled state 3 respiration in TBHP-injured RPTC (Fig. 3A).
Fig. 3.
Recovery of state 3 respiration coupled to the oxidation of electron donors to complex I (glutamate+malate; A), complex II (succinate+rotenone; B), and complex IV [ascorbate+N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD); C] following TBHP-induced injury in RPTC overexpressing wtPKC-α and dnPKC-α. D: recovery of uncoupled respiration following TBHP injury in RPTC expressing wtPKC-α and dnPKC-α. Results are the average ± SE of 5–7 independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
State 3 respiration in mitochondria energized with succinate+rotenone decreased to 75% of controls after TBHP injury and recovered by day 4 following injury (Fig. 3B). Overexpressing wtPKC-α had no effect on complex II-coupled state 3 respiration in noninjured RPTC and prevented its decreases in TBHP-injured RPTC (Fig. 3B). Overexpressing the inactive mutant of PKC-α decreased complex II-coupled state 3 respiration in noninjured RPTC and blocked the recovery of this function following TBHP-induced injury (Fig. 3B). State 3 respiration in mitochondria energized with ascorbate+N,N,N′,N′-tetramethyl-p-phenylenediamine decreased (substrates for complex IV) decreased by 17% at 4 h after TBHP exposure. Overexpressing wtPKC-α maintained complex IV-coupled respiration in TBHP-injured RPTC whereas overexpressing the inactive mutant of PKC-α exacerbated TBHP-induced decreases and blocked recovery of this function following oxidant-induced injury (Fig. 3C).
In the next series of experiments, we tested whether increased activation or blocking of PKC-α alters the integrity of the electron transport chain and the electron transport rate in oxidant-injured RPTC (using uncoupled oxygen consumption as a marker). TBHP exposure decreased uncoupled respiration to 55% of the control RPTC (Fig. 3D). Overexpressing wtPKC-α had no effect on uncoupled respiration in noninjured RPTC and prevented the decreases in uncoupled respiration in TBHP-injured RPTC (Fig. 3D). TBHP-injured RPTC overexpressing wtPKC-α maintained uncoupled respiration at the control levels during the recovery period. In contrast, overexpressing dnPKC-α decreased uncoupled respiration in noninjured RPTC to 63% of controls and blocked recovery of uncoupled respiration in TBHP-injured cells (Fig. 3D).
Infecting RPTC with an adenovirus carrying the empty vector had no effect on state 3 and uncoupled respirations (state 3 respiration using glutamate+malate: 34.8 ± 6.1 vs. 30.6 ± 3.5 nmol O2·min−1·mg protein−1 in noninfected and empty vector-infected RPTC, respectively; uncoupled respiration: 60.0 ± 8.2 vs. 49.5 ± 2.7 nmol O2·min−1·mg protein−1 in noninfected and empty vector-infected RPTC, respectively). Thus our data show that blocking PKC-α in RPTC decreases the electron transport rate through the respiratory chain and reduces maximum mitochondrial respiration coupled to oxidation of substrates through complexes I, II and IV. Furthermore, the inactive PKC-α blocks recovery of mitochondrial respiration following oxidant injury. Our results suggest that active PKC-α 1) maintains integrity of the electron transport chain and electron transport rate and 2) promotes recovery of mitochondrial respiration following oxidant injury in RPTC.
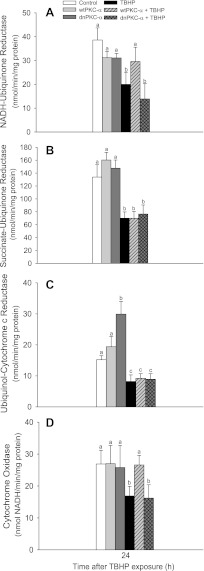
Activation of PKC-α prevents dysfunction of complexes I and IV following oxidant injury.
Because our data showed that the respiratory chain is a target for PKC-α, we examined whether the activities of respiratory complexes are regulated by PKC-α. Overexpressing wtPKC-α had no significant effect on activities of complexes I, II, III, and IV in mitochondria isolated from noninjured RPTC (Fig. 4). Overexpressing the inactive mutant of PKC-α had no effect on activities of complexes I, II, and IV but increased the activity of complex III twofold in noninjured RPTC (Fig. 4). TBHP exposure decreased activities of all four complexes of the respiratory chain (Fig. 4). Overexpressing wtPKC-α significantly improved the activity of complex I and prevented the decreases in activity of complex IV in TBHP-injured RPTC (Fig. 4, A and D). Activities of complexes II and III were not affected by PKC-α activation status in TBHP-injured RPTC (Fig. 4, B and C). Activities of the four complexes of the respiratory chain following TBHP injury were not affected by overexpressing the inactive mutant of PKC-α (Fig. 4). These results show that complexes I and IV are specific targets for active PKC-α and that the activation of PKC-α improves the function of these complexes in oxidant-injured RPTC.
Fig. 4.
Activities of NADH:ubiquinone oxidoreductase (complex I; A), succinate:ubiquinone oxidoreductase (complex II; B), ubiquinol:cytochrome c oxidoreductase (C), and cytochrome oxidase (complex IV; D) in mitochondria isolated from RPTC overexpressing wtPKC-α and dnPKC-α. RPTC were subjected to TBHP exposure, and mitochondria were isolated at 24 h following the exposure. Activities are the average ± SE of 5–10 independent experiments (RPTC isolations). Values with dissimilar superscripts are significantly different (P < 0.05) from each other.
Activation of PKC-α prevents mitochondrial hyperpolarization following oxidant injury.
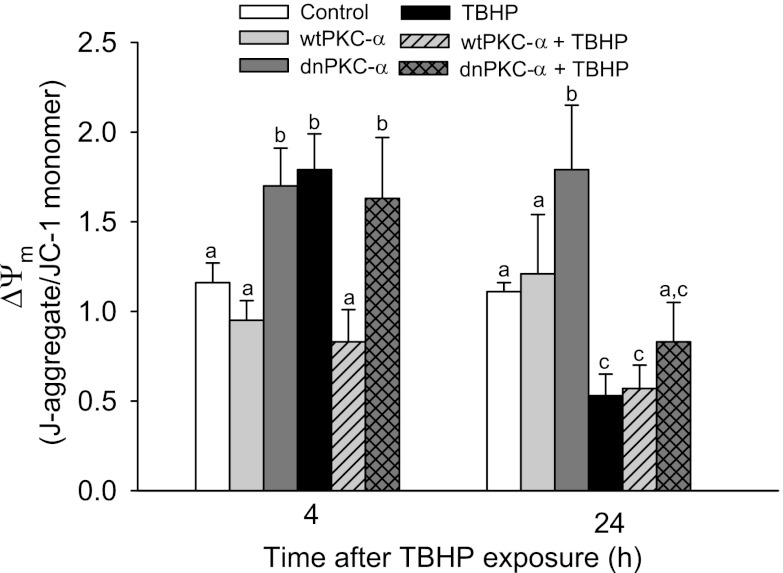
To test whether the disruption of the electron transfer chain was associated with changes in ΔΨm, we assessed ΔΨm in injured and noninjured RPTC using the J-aggregate/JC-1 ratio as a marker. TBHP exposure initially resulted in a 1.7-fold increase in ΔΨm (4 h after the exposure) followed by mitochondrial depolarization at 24 h after injury (Fig. 5). Overexpressing wtPKC-α had no effect on ΔΨm in noninjured RPTC, but it prevented mitochondrial hyperpolarization at 4 h after TBHP-induced injury (Fig. 5). In contrast, overexpressing dnPKC-α resulted in a 1.7-fold increase in ΔΨm in noninjured and TBHP-injured RPTC (Fig. 5). A similar (1.5-fold) increase in ΔΨm was caused by exposing RPTC to oligomycin (F0F1-ATPase inhibitor). These data demonstrate that inhibition of PKC-α in RPTC leads to mitochondrial membrane hyperpolarization and that PKC-α activation in oxidant-injured RPTC prevents this hyperpolarization.
Fig. 5.
Effect of overexpressing wtPKC-α and dnPKC-α on the mitochondrial membrane potential (ΔΨm) in RPTC following TBHP injury. The results are expressed as the ratio of the aggregate to monomeric forms of JC-1. Results are the average ± SE of 6–9 independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
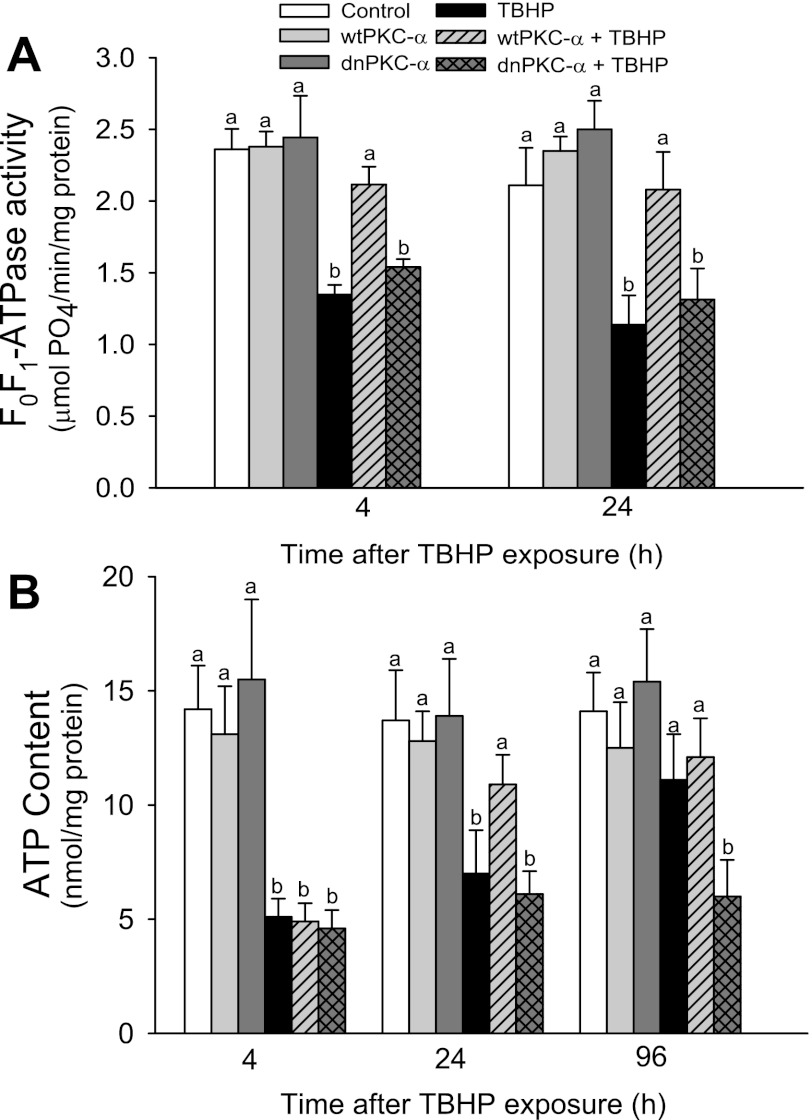
Activation of PKC-α maintains F0F1-ATPase activity following oxidant injury.
Because our data demonstrated that inhibition of F0F1-ATPase by oligomycin leads to mitochondrial hyperpolarization, we hypothesized that the increases in ΔΨm resulting from overexpressing dnPKC-α may also be due to an effect of PKC-α on F0F1-ATPase activity. TBHP-induced injury decreased F0F1-ATPase activity to 57 and 54% of control levels at 4 and 24 h, respectively, after the exposure (Fig. 6A). Overexpressing wtPKC-α or dnPKC-α had no effect on F0F1-ATPase activity in noninjured RPTC. However, overexpressing wtPKC-α, but not dnPKC-α, in RPTC blocked the decreases in F0F1-ATPase activity following TBHP-induced injury (Fig. 6A). TBHP exposure and overexpressing dnPKC-α or wtPKC-α had no effect on protein levels of the catalytic β-subunit of F0F1-ATPase (Fig. 6B). These data demonstrate that the activation of PKC-α is crucial for maintaining F0F1-ATPase activity in oxidant-injured RPTC. Furthermore, these results demonstrate that the decreases in F0F1-ATPase activity induced by inhibition of PKC-α are not due to reduced protein levels of this enzyme.
Fig. 6.
Effect of overexpressing wtPKC-α and dnPKC-α on: A: activity of the F0F1-ATPase in isolated mitochondria. B. intracellular ATP content in RPTC following TBHP-induced injury. The results are the average ± SE of 6–9 independent experiments (RPTC isolations). Values with dissimilar superscripts at a given time point are significantly different (P < 0.05) from each other.
Activation of PKC-α promotes recovery of ATP content following oxidant injury.
We have tested whether PKC-α-mediated changes in respiratory capacity and activities of complex I, complex IV, and F0F1-ATPase result in changes in ATP content in RPTC. ATP levels in TBHP-injured RPTC decreased to 36% of controls and recovered to 82% of controls 4 days after injury (Fig. 6B). Overexpressing wtPKC-α improved ATP levels in TBHP-injured RPTC and maintained intracellular ATP content during the recovery period (Fig. 6B). In contrast, overexpressing dnPKC-α blocked the recovery of intracellular ATP levels following TBHP exposure (Fig. 6B). These results demonstrate that PKC-α activation reduces the decreases in ATP content and promotes recovery of ATP content following TBHP-induced injury in RPTC.
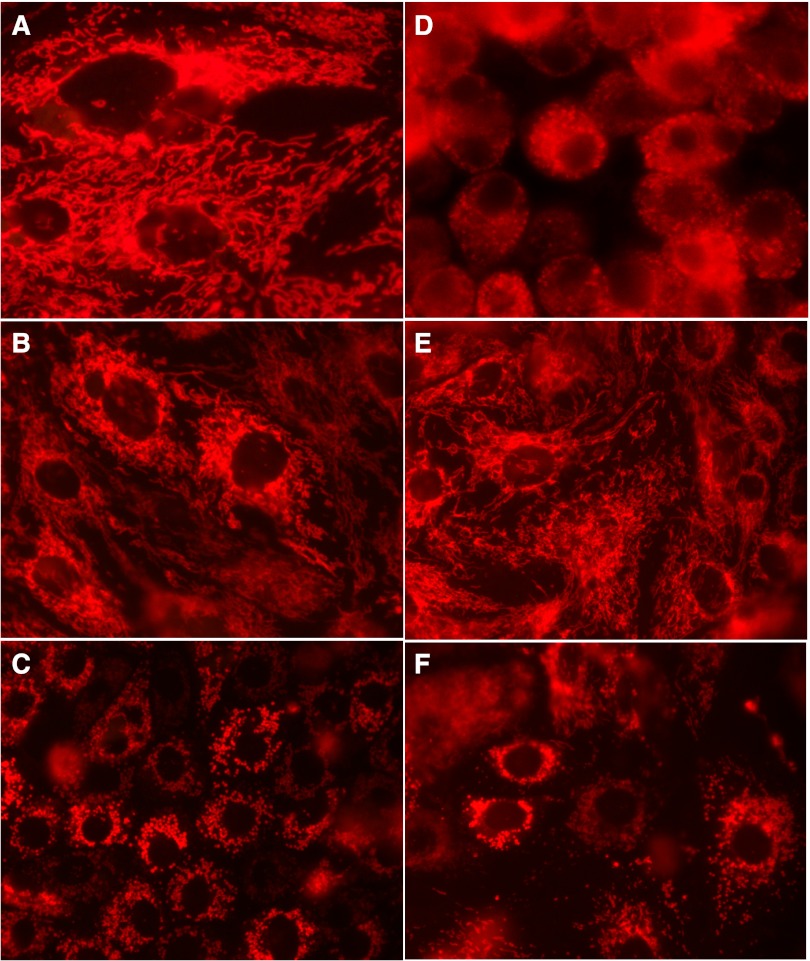
Inhibition of PKC-α induces mitochondrial fragmentation.
Morphological examination of noninjured RPTC revealed dense and elongated mitochondria distributed throughout the cytoplasm (Fig. 7A). Similar morphology and elongated mitochondria forming a dense network were found in noninjured RPTC overexpressing wtPKC-α (Fig. 7B). In contrast, the mitochondria were fragmented into small spheroids localized around the nucleus in noninjured RPTC overexpressing dnPKC-α (Fig. 7C). Mitochondrial fragmentation was found in some cells at 24 h following infection of RPTC with the adenoviral vector coding dnPKC-α (data not shown). At 48 h after infection, the majority of RPTC present in the monolayer contained fragmented mitochondria (Fig. 7C).
Fig. 7.
Effect of overexpressing wtPKC-α and dnPKC-α on mitochondrial morphology in live RPTC at 4 h following TBHP exposure. A: controls. B: wtPKC-α. C: dnPKC-α. D: TBHP. E: wtPKC-α+TBHP. F: dnPKC-α+TBHP. Representative images of live cells from 5 independent experiments (RPTC isolations) are shown. Original magnification, × 630.
The exposure of RPTC to TBHP induced morphological changes in mitochondria resembling mitochondrial fragmentation present in RPTC expressing dnPKC-α (Fig. 7D) although not all cells present in a monolayer contained fragmented mitochondria (data not shown). Overexpressing wtPKC-α in RPTC improved mitochondrial morphology and prevented mitochondrial fragmentation following TBHP exposure (Fig. 7E). Overexpressing dnPKC-α was associated with mitochondrial fragmentation and accumulation of mitochondrial fragments into larger clusters (Fig. 7F). Once fragmented, these mitochondria never regained their original morphology and the mitochondrial clusters increased in size during 9 days following TBHP exposure (data not shown). These changes were associated with the gradual loss of injured RPTC from the monolayer. These data demonstrate that TBHP induces mitochondrial fragmentation and that PKC-α activation prevents this event in TBHP-injured RPTC. Blocking PKC-α in noninjured RPTC leads to mitochondrial fragmentation similar to that observed in oxidant-injured RPTC.
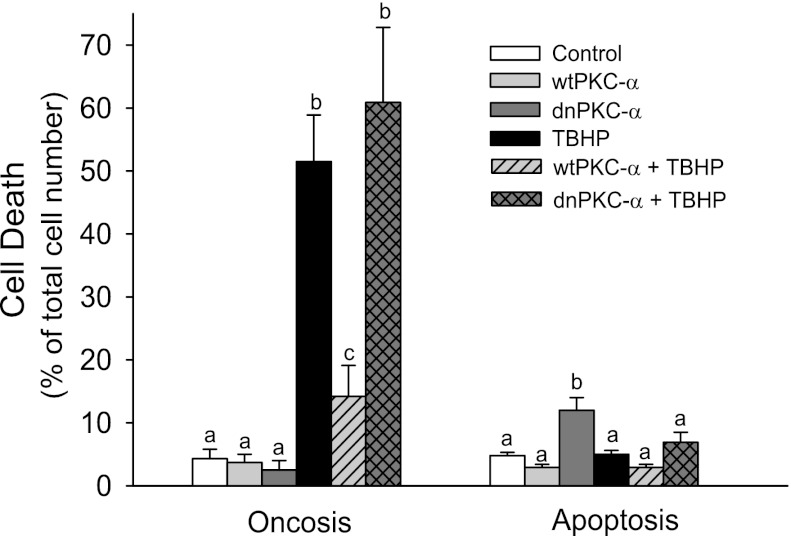
Inhibition of PKC-α mediates oxidant-induced RPTC death.
Exposure to TBHP resulted in the release of LDH from injured RPTC. Figure 8 shows that LDH release increased from 4% in controls to 51% in TBHP-injured RPTC. Overexpressing wtPKC-α had no effect on LDH release in noninjured RPTC and decreased LDH release to 14% in TBHP-injured RPTC (Fig. 8). In contrast, overexpressing dnPKC-α exacerbated LDH release in oxidant-injured RPTC (Fig. 8). No changes in apoptosis were found following TBHP-induced injury regardless of the absence or presence of increased protein levels of wtPKC-α and dnPKC-α (Fig. 8). However, overexpressing dnPKC-α in noninjured RPTC increased apoptosis 2.5-fold compared with controls (Fig. 8). The adenovirus carrying the empty vector had no effect on LDH release in RPTC (data not shown). These data show cell death upon PKC-α inhibition and in oxidant-injured RPTC, and demonstrate the protective effects of PKC-α activation against RPTC death induced by the oxidant.
Fig. 8.
Cell death following TBHP-induced injury in RPTC overexpressing wtPKC-α and dnPKC-α. Oncosis (left) was measured using the release of lactate dehydrogenase as a marker. Apoptosis (right) was assessed using an annexin V/propidium iodide-binding assay. Results are the average ± SE of 4–7 independent experiments (RPTC isolations). Values with dissimilar superscripts are significantly different (P < 0.05) from each other.
Translocation of PKC-α to plasma membrane of oxidant-injured RPTC is associated with cell death.
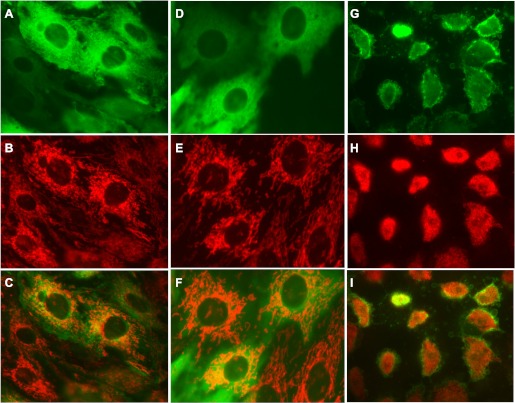
The ability to visualize localization of PKC-α in live cells using a GFP-linked mutant of wtPKC-α provided an opportunity to assess concomitant changes in mitochondrial morphology and subcellular localization of PKC-α in oxidant-injured RPTC. In noninjured RPTC, wtPKC-α was distributed throughout the cell in a pattern suggesting the presence of PKC-α in the cytoplasm, cytoskeletal structures, and mitochondria. In addition, PKC-α accumulated in the perinuclear area that was rich in mitochondria (Fig. 9. A–C). None or very little PKC-α was present in RPTC nuclei and in association with the plasma membrane (Fig. 9A). Following TBHP exposure, the pattern of subcellular distribution of PKC-α in RPTC overexpressing wtPKC-α was similar as in controls and mitochondrial morphology was not different from controls (Fig. 9, D–F). However, some minor areas in TBHP-injured monolayers overexpressing wtPKC-α contained cells that had a different PKC-α distribution. In these cells, the majority of PKC-α was associated with the plasma membrane and very little PKC-α was present in the cytoplasm or in the perinuclear area (Fig. 9G). The subcellular organization in these RPTC was disrupted, with shorter and tightly packed mitochondria clustered in the central part of the cell and wtPKC-α dissociated from mitochondria (Fig. 9H). These cells appeared to have disrupted plasma membranes, with numerous swollen vesicles budding from the plasma membrane or already ruptured (Fig. 9, G and I). Cells that displayed this morphology never recovered after TBHP exposure (as assessed over the period of up to 9 days after injury) and remained on the dish unchanged or detached from the monolayer, suggesting that they were not viable. These observations suggest that PKC-α translocation to the plasma membrane is accompanied by the loss of normal mitochondrial morphology and RPTC death.
Fig. 9.
Effect of subcellular localization of wtPKC-α on mitochondrial morphology in live TBHP-injured RPTC. RPTC were infected with an adenoviral vector encoding a green fluorescent protein (GFP)-wtPKC-α construct at 48 h before TBHP exposure (0.35 mM; 45 min). Four hours after TBHP exposure, the live monolayers were stained with MitoTracker Red 580 and examined under a Zeiss fluorescent microscope (Axioscop). A–C: noninjured RPTC overexpressing GFP-wtPKC-α. D–F and G–I: TBHP-injured RPTC overexpressing GFP-wtPKC-α. Representative images of live cells from 3 independent experiments (RPTC isolations) are shown. Original magnification, ×630.
DISCUSSION
Renal regeneration following acute renal injury is associated with differential changes in the activity and/or expression of PKC isozymes. Activation of PKC-δ, PKC-ε, and PKC-ζ, but not PKC-α has been reported during compensatory renal hypertrophy (8). In contrast, PKC-α expression in the renal cortex is increased by ischemia-reperfusion injury and during subsequent regeneration (29). This suggests that renal PKC isozymes respond differentially to different types of injury. Toxicant-induced kidney injury has even more complex effects on PKC isozymes. PKC-α is downregulated by folic acid-induced injury (8) but upregulated by two dissimilar nephrotoxicants, cisplatin and DCVC, in RPTC (16, 21). Our previous study in RPTC demonstrated that total PKC activity decreases following injury caused by a model oxidant, TBHP, and recovers before the return of mitochondrial and transport functions (20). A general and isozyme-nonselective inhibition of PKC decreases recovery of mitochondrial function whereas nonselective PKC activation (phorbol ester) promotes recovery of mitochondrial function in RPTC following oxidant-induced injury (20). This study focused on the role of a specific PKC isozyme, PKC-α, in 1) mitochondrial dysfunction that occurs following injury induced by an oxidant and 2) the repair of mitochondrial function and energy deficits following oxidant-induced injury in RPTC. In addition, we aimed to identify specific mitochondrial targets of PKC-α in oxidant-injured and recovering RPTC.
PKC-α was activated in RPTC injured by TBHP. Despite inducing an overall increase in phosphorylation (indicative of activation) of PKC-α, TBHP exposure decreased the levels of phosphorylated and total PKC-α in RPTC mitochondria between 2 and 6 h after the exposure. Decreases in mitochondrial levels of PKC-α coincided with the decreases in respiration, activities of respiratory complexes and F0F1-ATPase, and ATP content, as well as hyperpolarization of the inner membrane followed by the decreases in ΔΨm at 24 h. Previously, we found a similar pattern of PKC-α activation with concomitant loss of PKC-α protein from mitochondria, decreases in mitochondrial function, mitochondrial hyperpolarization, and energy deficits in RPTC injured by cisplatin (21). Interestingly, inhibition of PKC-α in cisplatin-injured RPTC using an inhibitor of classic isozymes of PKC (Go6976) decreased the overall levels of phosphorylated PKC-α in RPTC, but not in mitochondria, which suggested that the inhibitor prevented the loss of phosphorylated PKC-α from this organelle (21). Therefore, we hypothesized that the association of PKC-α with mitochondria is necessary to prevent or reduce mitochondrial dysfunction induced by TBHP. Consequently, our present study explored the role of increased levels of wild-type and inactive forms of PKC-α in mitochondrial functions of TBHP-injured RPTC.
Our data show that while the ratio of phosphorylated to total PKC-α protein (indicative of PKC-α activation) in mitochondria of injured RPTC decreased after oxidant injury in nontransfected cells, this ratio was twofold higher in mitochondria of injured RPTC transfected with wtPKC-α. Thus the overexpression of wtPKC-α improved activation of PKC-α in mitochondria in response to TBHP-induced injury. In contrast, RPTC overexpressing dnPKC-α had increased levels of PKC-α protein in mitochondria, but PKC-α was negligible in both noninjured and oxidant-injured RPTC. Overexpressing wtPKC-α had no effect on mitochondrial function in noninjured RPTC. In injured RPTC, overexpressing wtPKC-α did not alter TBHP-induced increases in ROS generation, but diminished mitochondrial dysfunction and accelerated subsequent recovery of mitochondrial functions and ATP content. Overexpressing wtPKC-α improved NADH-ubiquinone oxidoreductase (complex I) activity and state 3 respiration coupled to complex I and prevented TBHP-induced decreases in the activity of cytochrome oxidase (complex IV) and state 3 respiration coupled to this complex. The protective effects of wtPKC-α were present despite the lack of effect on oxidant generation in TBHP-exposed RPTC. Thus our data show that the improvement in the activity of complex I due to increased levels of PKC-α does not block ROS generation stimulated by TBHP and suggest that complex I is not the main source of ROS in this model of injury. Activities of complexes II and III remained reduced in TBHP-injured RPTC regardless of the activity status of PKC-α, demonstrating that PKC-α does not mediate oxidant-induced decreases in activities of complex II and/or complex III. The decreases in the activities of respiratory complexes, state 3 respiration, F0F1-ATPase activity, and ATP content at 4 h after TBHP exposure coincided with hyperpolarization of the mitochondrial membrane and the lowest levels of PKC-α in mitochondria. ΔΨm is dependent on the activity of complexes I, III, and IV, which pump protons from the mitochondrial matrix into the intermembrane space; the activity of F0F1-ATPase, which allows protons to return to the mitochondrial matrix; and the conformation of the mitochondrial permeability transition pore (MPTP). F0F1-ATPase activity was decreased in RPTC injured by TBHP at 4 and 24 h after the exposure, and the resulting accumulation of protons in the intermembrane space in TBHP-injured RPTC could explain increases in ΔΨm at 4 h. However, reduced F0F1-ATPase activity could not explain decreases in ΔΨm at 24 h after TBHP treatment. Overexpressing the inactive PKC-α in noninjured RPTC increased ΔΨm without producing any changes in F0F1-ATPase activity, which suggests that the changes in F0F1-ATPase function were not a major mechanism regulating ΔΨm in TBHP-injured RPTC.
Overexpressing wtPKC-α ameliorated TBHP-induced early increases in ΔΨm, which demonstrates that active PKC-α plays a role in maintaining ΔΨm during the early period after oxidant injury. Taken together, our data suggest that PKC-α inhibition is responsible for TBHP-induced increases in ΔΨm. Similarly, Lu and colleagues (17) reported that inhibiting PKC elevates ΔΨm in adenocarcinomic human alveolar basal epithelial cells. However, our present study shows that wtPKC-α did not prevent decreases in ΔΨm at later time points (24 h) after TBHP exposure. Thus this study and the previous report from our laboratory (21) show that decreased activity or association of PKC-α with mitochondria is involved in the mechanisms leading to the increases of ΔΨm but is not directly involved in the events resulting in the collapse of ΔΨm in RPTC. Therefore, we speculate that the active PKC-α is involved in the regulation of MPTP conformation. Agudo-Lopez and collaborators (1) previously demonstrated that PKC activation mediates the protective actions of ceramide during ischemia through increased formation of ROS, which leads to the opening of MPTP. Opening of MPTP prevents mitochondrial Ca2+ overload, mitochondrial swelling, and cell death (1). PKC-ε is one of PKC isozymes involved in stabilizing mitochondria by modifying MPTP opening (7). We hypothesize that PKC-α activation plays a similar role in TBHP-injured RPTC. Our results show that PKC-α inhibition reduces ROS production and increases ΔΨm (possibly by inducing MPTP closing) whereas PKC-α activation increases ROS production and prevents increases in ΔΨm (possibly by MPTP opening).
The protective effects of wtPKC-α on mitochondrial function in oxidant-injured RPTC did not prevent initial decreases in ATP content during the early phase of injury (4 h), but accelerated ATP recovery during the repair following injury. The improvements in mitochondrial function in injured RPTC overexpressing wtPKC-α were accompanied by reduced cell death. This protection occurred despite increased ROS generation in TBHP-treated RPTC overexpressing wtPKC-α, which suggests that ROS generated in these conditions were not a primary mechanism responsible for RPTC injury. This notion is also supported by the fact that overexpressing dnPKC-α blocked TBHP-induced ROS generation but did not reduce mitochondrial dysfunction and RPTC death. In contrast to the protective effects of wtPKC-α, inhibition of PKC-α in noninjured RPTC decreased maximum mitochondrial respiration (state 3 and uncoupled respirations) and markedly increased ΔΨm and the activity of complex III. This demonstrates that the inhibition of PKC-α in noninjured RPTC is sufficient to increase accumulation of protons in the intermembrane space, possibly by increased translocation of protons through complex III and/or by the closure of MPTP. Changes in mitochondrial functions induced by dnPKC-α were not associated with alterations in ROS/RNS production. PKC-α inhibition in RPTC had no effect on TBHP-induced decreases in mitochondrial respiration, activities of complexes of the electron transfer chain and F0F1-ATPase, ATP content, and increases in cell death shortly after TBHP exposure. However, inhibition of PKC-α blocked the recovery of activities of complex I, complex IV, and F0F1-ATPase and state 3 respiration, and prevented the return of ATP content following TBHP injury. Unlike wtPKC-α, inhibition of PKC-α blocked oxidant generation in TBHP-injured RPTC, which suggests that PKC-α activation mediated ROS/RNS production in injured RPTC. Therefore, our data demonstrate that active PKC-α decreases RPTC injury and death in oxidant-injured RPTC and stimulates the recovery of mitochondrial function and ATP content after oxidant injury. The protective effects of PKC-α activation are not through blocking ROS production in injured RPTC. These results are consistent with our previous report demonstrating that antioxidants do not diminish RPTC death induced by persistent PKC-ε activation despite blocking ROS generation (28). Taken together, these studies show that ROS are not the primary mechanism of RPTC injury and death in this model of injury.
The question remains how PKC-α maintains the activities of complex I, complex IV, and F0F1-ATPase in TBHP-injured RPTC. How PKC-α affects mitochondrial function to regulate energy production has not been established. The regulation may be direct (phosphorylation of one or more subunits of these complexes by PKC-α) or indirect (dephosphorylation through activating a protein phosphatase). Direct phosphorylation of complex I or complex IV by PKC-α has not been shown. PKC-α may also mediate its effects through a modulation of downstream kinases known to phosphorylate respiratory complexes (protein kinase A) and components of the voltage-dependent anion channel (VDAC; GSK-3β) (9, 18, 32, 33, 39). We have shown that the catalytic β-subunit of F0F1-ATPase is a substrate for PKC-α in RPTC and that PKC-α can phosphorylate F0F1-ATPase, leading to changes in its activity (16). It is also possible that PKC-α phosphorylates one of the components of VDAC and controls MPTP opening/closure, thus regulating ΔΨm. The hyperpolarization of the mitochondrial membrane and proton accumulation in the intermembrane space have damaging effects on complexes I and IV as the energy necessary for uphill transport of protons across the inner membrane exceeds the energy released by oxidation/reduction reactions taking place within these complexes. Thus the regulation of MPTP opening may have indirect effect on the function of respiratory complexes.
Mitochondrial function is dependent on overall mitochondrial morphology and structural integrity. Conditions that disrupt mitochondrial integrity, particularly the integrity of the inner membrane, decrease phosphorylation capacity of mitochondria, disturb mitochondrial ion homeostasis, and release factors that induce cell death. Therefore, we examined whether the protective effects of wtPKC-α on mitochondrial functions are associated with the alterations of mitochondrial morphology in oxidant-injured RPTC. Increases in ΔΨm in noninjured RPTC overexpressing inactive PKC-α were accompanied by mitochondrial fragmentation. Staining of live RPTC mitochondria demonstrated that PKC-α inhibition in noninjured RPTC leads to dramatic changes in mitochondrial morphology and organization similar to those observed in RPTC expressing the constitutively active PKC-ε (28). This effect was specific to the dnPKC-α but not the active form of this kinase. Thus this and previous studies show that inhibition of PKC-α or activation of PKC-ε are detrimental to mitochondrial function and cause mitochondrial hyperpolarization and fission in RPTC. Overall, mitochondrial organization in RPTC overexpressing dnPKC-α appeared to be altered as the fragmented mitochondria were not distributed throughout the cell but rather clustered in the perinuclear area. These changes coincided with decreased respiration and oxidant generation and increased ΔΨm. Similar changes were found in RPTC injured by TBHP but not in TBHP-injured RPTC overexpressing wtPKC-α, which demonstrates that active PKC-α preserves mitochondrial morphology in injured RPTC. It has been shown that mitochondrial fragmentation is caused by the loss of phosphatidylinositol(4,5)bisphosphate (PIP2) from the outer mitochondrial membrane through dephosphorylation by phosphatases or due to masking PIP2 with PH domains (34). Fragmentation of mitochondria that lost PIP2 does not involve apoptosis (34). PKC-α inhibition or depletion mimics the loss of PIP2 from mitochondria and induces mitochondrial fragmentation whereas PKC-α activation prevents the loss or masking of PIP2 in the outer membrane and blocks mitochondrial fragmentation (34).
In conclusion, our study demonstrates that PKC-α activation in RPTC diminishes mitochondrial dysfunction and fragmentation, energy deficits, and cell death following oxidant injury in RPTC. In addition, PKC-α activation promotes the recovery of mitochondrial function and energy status in oxidant-injured RPTC. Inhibition of PKC-α results in mitochondrial hyperpolarization and fragmentation in RPTC and inhibits recovery of mitochondrial function and energy deficits following oxidant injury. F0F1-ATPase, complexes I and IV, and ΔΨm are the major mitochondrial targets of PKC-α in RPTC. Finally, PKC-α mediates TBHP-stimulated oxidant production in RPTC.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 2R01DK59558 (to G. Nowak). The University of Arkansas for Medical Sciences (UAMS) Translational Research Institute, supported by National Institutes of Health National Center for Research Resources Grant UL1 RR029884, provided partial funding for the Flow Cytometry Core at UAMS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.N. and D.B. provided conception and design of research; G.N. analyzed data; G.N. interpreted results of experiments; G.N. prepared figures; G.N. drafted manuscript; G.N. edited and revised manuscript; G.N. and D.B. approved final version of manuscript; D.B. performed experiments.
ACKNOWLEDGMENTS
We thank Dr. Allen M. Samarel (Loyola University Medical Center; Maywood, IL) for providing an aliquot of adenoviral vectors coding wild-type PKC-α and the GFP-wild-type PKC-α construct and Dr. Trevor Biden (Garvan Institute of Medical Research, Sydney, Australia) for providing an aliquot of adenoviral vector coding the dominant negative (inactive) mutant of PKC-α.
REFERENCES
- 1. Agudo-López A, Miguel BG, Fernández I, Martínez AM. Role of protein kinase C and mitochondrial permeability transition pore in the neuroprotective effect of ceramide in ischemia-induced cell death. FEBS Lett 585: 99–103, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Bouwman RA, Musters RJP, van Beek-Harmsen BJ, de Lange JJ, Lamberts RR, Loer SA, Boer C. Sevoflurane-induced cardioprotection depends on PKC-α activation via production of reactive oxygen species. Br J Anaesth 99: 639–645, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bradshaw D, Hill CH, Nixon JS, Wilkinson SE. Therapeutic potential of protein kinase C inhibitors. Agents Actions 38: 135–147, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Breitkreutz D, Braiman-Wiksman L, Daum N, Denning M, Tennenbaum T. Protein kinase C family: on the crossroads of cell signaling in skin and tumor epithelium. J Cancer Res Clin Oncol 133: 793–808, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Carpenter L, Cordery D, Biden TJ. Protein kinase Cδ activation by interleukin-1β stabilizes inducible nitric-oxide synthase mRNA in pancreatic β-cells. J Biol Chem 276: 5368–5374, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cerioni L, Palomba L, Brüne B, Cantoni O. Peroxynitrite-induced mitochondrial translocation of PKCα causes U937 cell survival. Biochem Biophys Res Commun 339: 126–131, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Costa ADT, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem 281: 20801–20808, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dong L, Stevens JL, Fabbro D, Jaken S. Regulation of protein kinase C isozymes in kidney regeneration. Cancer Res 53: 4542–4549, 1993 [PubMed] [Google Scholar]
- 9. Fang J, Prabu SK, Sepuri NB, Raza H, Anandatheerthavarada HK, Galati D, Spear J, Avadhani NG. Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett 581: 1302–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández E, Cuenca N, García M, De Juan J. Two types of mitochondria are evidenced by protein kinase C immunoreactivity in the Müller cells of the carp retina. Neurosci Lett 183: 202–205, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Gopalakrishna R, Chen ZH, Gundimeda U. Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proc Natl Acad Sci USA 91: 12233–12237, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28: 1349–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Gopalakrishna R, Gundimeda U. Antioxidant regulation of protein kinase C in cancer prevention. J Nutr 132: 3819S–3823S, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Kuo T, Huang W, Guh J. WJ9708012 exerts anticancer activity through PKC-α related crosstalk of mitochondrial and endoplasmic reticulum stresses in human hormone-refractory prostate cancer cells. Acta Pharmacol Sinica 32: 89–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Law RH, Manon S, Devenish RJ, Nagley P. ATP synthase from Saccharomyces cerevisiae. Methods Enzymol 260: 133–163, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Godwin ML, Nowak G. Protein kinase C-α inhibits the repair of oxidative phosphorylation after S-(1,2-dichlorovinyl)-l-cysteine injury in renal cells. Am J Physiol Renal Physiol 287: F64–F73, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lu N, Wang W, Liu J, Wong C. Protein kinase C epsilon affects mitochondrial function through estrogen-related receptor alpha. Cell Signal 23: 1473–1478, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Nishihara M, Miura T, Ohori K, Miki T, Shimamoto K. GSK-3β phosphorylated by PKC and Akt inhibits ANT-cyclophilin-D interaction: a possible mechanism of cardiomyocyte protection. J Mol Cell Cardiol 44: 441–442, 2008 [Google Scholar]
- 19. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Nowak G. Protein kinase C mediates repair of mitochondrial and transport functions after toxicant-induced injury in renal cells. J Pharmacol Exp Ther 306: 157–165, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Nowak G. Protein kinase C-α and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem 277: 43377–43388, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nowak G, Bakajsova D, Clifton GL. Protein kinase C-ε modulates mitochondrial function and active Na+ transport after oxidant injury in renal cells. Am J Physiol Renal Physiol 286: F307–F316, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol 291: F840–F855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nowak G, Schnellmann RG. Autocrine production and TGF-β1-mediated effects on metabolism and viability in renal cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F689–F697, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Nowak G, Schnellmann RG. l-Ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol Cell Physiol 271: C2072–C2080, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol Cell Physiol 268: C1053–C1061, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Nowak G, Bakajsova D, Hayes C, Hauer-Jensen M, Compadre CM. γ-Tocotrienol protects against mitochondrial dysfunction and renal cell death. J Pharmacol Exp Ther 340: 330–338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowak G, Bakajsova D, Samarel AM. Protein kinase C-ε activation induces mitochondrial dysfunction and fragmentation in renal proximal tubules. Am J Physiol Renal Physiol 301: F197–F208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int 59: 1789–1797, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Perletti GP, Smeraldi C, Porro D, Piccinini F. Involvement of the α isoenzyme of protein kinase C in the growth inhibition induced by phorbol esters in MH1C1 hepatoma cells. Biochem Biophys Res Commun 205: 1589–1594, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Porter MJ, Heidkamp MC, Scully BT, Patel N, Martin JL, Samarel AM. Isoenzyme-selective regulation of SERCA2 gene expression by protein kinase C in neonatal rat ventricular myocytes. Am J Physiol Cell Physiol 285: C39–C47, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 281: 2061–2070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rasmo DD, Palmisano G, Scacco S, Technikova-Dobrova Z, Panelli D, Cocco T, Sardanelli AM, Gnoni A, Micelli L, Trani A, Luccia AD, Papa S. Phosphorylation pattern of the NDUFS4 subunit of complex I of the mammalian respiratory chain. Mitochondrion 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Rosivatz E, Woscholski R. Removal or masking of phosphatidylinositol(4,5)bisphosphate from the outer mitochondrial membrane causes mitochondrial fragmentation. Cell Signal 23: 478–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem 273: 25436–25442, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Serlachius E, Svennilson J, Schalling M, Aperia A. Protein kinase C in the developing kidney: Isoform expression and effects of ceramide and PKC inhibitors. Kidney Int 52: 901–910, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Shaik ZP, Fifer EK, Nowak G. Akt activation improves oxidative phosphorylation in renal proximal tubular cells following nephrotoxicant injury. Am J Physiol Renal Physiol 294: F423–F432, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaik ZP, Fifer EK, Nowak G. Protein kinase B/Akt modulates nephrotoxicant-induced necrosis in renal cells. Am J Physiol Renal Physiol 292: F292–F303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Technikova-Dobrova Z, Sardanelli AM, Speranza F, Scacco S, Signorile A, Lorusso V, Papa S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: enzyme and substrate characterization and functional role. Biochemistry 40: 13941–13947, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Vijayan K, Szotek EL, Martin JL, Samarel AM. Protein kinase C-α-induced hypertrophy of neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H2777–H2789, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Yeh S, Yi-Kung Huang E, Lu Y, Wang C, Huang CF, Lin W. Mitochondrial anchoring of PKCα by PICK1 confers resistance to etoposide-induced apoptosis. Apoptosis 12: 1857–1871, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wang W, Yeh S, Chang Y, Hsiao S, Lian W, Lin C, Huang CF, Lin W. PICK1, an anchoring protein that specifically targets protein kinase Cα to mitochondria selectively upon serum stimulation in NIH 3T3 Cells. J Biol Chem 278: 37705–37712, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Biswas G, Prabu SK, Avadhani NG. Modulation of mitochondrial metabolic function by phorbol 12-myristate 13-acetate through increased mitochondrial translocation of protein kinase Cα in C2C12 myocytes. Biochem Pharmacol 72: 881–892, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Wu Q, Liu S, Ding L, Ye X, Su W. PKCα translocation from mitochondria to nucleus is closely related to induction of apoptosis in gastric cancer cells. Sci China C Life Sci 45: 237–244, 2002 [DOI] [PubMed] [Google Scholar]