Abstract
Tuberous sclerosis complex (TSC) is a multiorgan hamartomatous disease caused by loss of function mutations of either the TSC1 or TSC2 genes. Neurological symptoms of TSC predominate in younger patients, but renal pathologies are a serious aspect of the disease in older children and adults. To study TSC pathogenesis in the kidney, we inactivated the mouse Tsc1 gene in the distal convoluted tubules (DCT). At young ages, Tsc1 conditional knockout (CKO) mice have enlarged kidneys and mild cystogenesis with increased mammalian target of rapamycin complex (mTORC)1 but decreased mTORC2 signaling. Treatment with the mTORC1 inhibitor rapamycin reduces kidney size and cystogenesis. Rapamycin withdrawal led to massive cystogenesis involving both distal as well as proximal tubules. To assess the contribution of decreased mTORC2 signaling in kidney pathogenesis, we also generated Rictor CKO mice. These animals did not have any detectable kidney pathology. Finally, we examined primary cilia in the DCT. Cilia were longer in Tsc1 CKO mice, and rapamycin treatment returned cilia length to normal. Rictor CKO mice had normal cilia in the DCT. Overall, our findings suggest that loss of the Tsc1 gene in the DCT is sufficient for renal cystogenesis. This cytogenesis appears to be mTORC1 but not mTORC2 dependent. Intriguingly, the mechanism may be cell autonomous as well as non-cell autonomous and possibly involves the length and function of primary cilia.
Keywords: tuberous sclerosis complex, mammalian target of rapamycin complex 1 and 2, rapamycin
tuberous sclerosis complex (TSC) is a multiorgan hamartomatous disease of the brain, skin, and kidney found in ∼1 in 6,000 live births (37). Neurological symptoms of TSC predominate in young patients and include mental retardation, epilepsy, and autism (7). Renal pathology is less common in young children but is usually seen after the second decade of life. The renal pathology is diverse and includes cysts, angiomyolipomas (AML), and, in rare instances, renal cell carcinoma (21, 34). AMLs occur in up to 70% of patients with TSC and cysts are seen in ∼30% of patients (21). While most kidney lesions are asymptomatic in TSC patients, a subset of patients present with severe polycystic kidney disease (PKD) that causes significant morbidity and mortality (24).
TSC results from a loss of function mutations of either the TSC1 or TSC2 genes. In normal tissue, hamartin and tuberin (the protein products of TSC1 and TSC2, respectively) form a complex that regulates mammalian target of rapamycin (mTOR) signaling activity (4, 23). The mTOR kinase regulates many critical cellular processes including control of cell size, proliferation, cell survival, and protein synthesis (14). mTOR carries out these functions with specific protein binding partners that associate into two distinct complexes. mTOR complex 1 (mTORC1) contains Raptor/mLST8/GβL, phosphorylates p70-S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), and controls mRNA translation (29). mTOR complex 2 (mTORC2) contains Rictor/GβL/mSin1, directly phosphorylates and activates Akt and protein kinase Cα, and indirectly activates NDRG1 via SGK1 phosphorylation (10). In contrast to mTORC1, mTORC2 function is much less well defined but appears to be involved with cytoskeletal regulation (25). Raptor and Rictor are mutually exclusive binding partners of mTOR, controlling substrate specificity as well as sensitivity to rapamycin, a selective mTORC1 inhibitor. Current data suggest that the hamartin/tuberin complex is a negative regulator of mTORC1 but a positive regulator of mTORC2 (12). Loss of either hamartin or tuberin then leads to increased mTORC1 activity but decreased mTORC2 activity (12, 35). Current genetic models of TSC support a germline mutation inactivating one allele of either TSC1 or TSC2 for each patient. Loss of heterozygosity at the other allele allows constitutive activation of mTORC1 signaling, resulting in phosphorylation and activation of ribosomal S6K1 and other downstream targets and is thought to lead to hamartoma formation (5, 26). Contributions of decreased mTORC2 activity in TSC have not been well studied but may play an important role in TSC pathogenesis.
Accumulating evidence connects mTOR signaling to kidney development and cystogenesis. Large deletions of both TSC2 and PKD1 in a subset of patients with TSC causes TSC and severe kidney disease. Intriguingly, recent reports show a direct physical interaction between tuberin and polycystin-1. This interaction is required for the proper subcellular localization of tuberin as well as mTORC1 inhibition (8, 27). Additionally, hamartin has been shown to localize to the basal body of the primary cilia, an organelle the dysfunction of which leads to cystogenesis (11). Furthermore, loss of either Tsc1 or Tsc2 in mouse embryonic fibroblasts and knockdown of tsc1a in zebrafish cause increased ciliary length (9, 11, 38).
The first animal models of TSC were generated by conventional inactivation of the Tsc1 or Tsc2 genes and revealed an absolute requirement of either gene during embryonic development, as homozygous mutants died before embryonic day 10 (18, 19). Rodents heterozygous for Tsc1 or Tsc2 develop kidney lesions in adulthood with some kidney lesions progressing to malignancy (18, 19, 36). To define the role of hamartin in specific organs, conditional knockout (CKO) models of Tsc1 have been generated to dissect tissue-specific pathogenesis in TSC (30, 31, 33, 39). Conditional loss of Tsc1 in the proximal convoluted tubule (PCT) resulted in cystic kidneys, although the mechanisms linking dysregulation of mTORC1 and mTORC2 signaling with cystogenesis were not extensively studied (39).
We now show that CKO of the Tsc1 gene in the distal convoluted tubule (DCT) causes cystogenesis. In these kidneys, there is both increased mTORC1 and decreased mTORC2 signaling. Notably, primary cilia in the DCT are longer than those from control mice, suggesting defects in cilia structure and function. Treatment of these CKO mice with the mTORC1 inhibitor rapamycin prevents cystogenesis and normalized cilia length. However, loss of mTORC2 activity alone, through CKO of the Rictor gene in the DCT, did not cause cystogenesis or alter cilia length. Our findings suggest that loss of the Tsc1 gene in the DCT is sufficient for renal cystogenesis in TSC. This appears to require increased mTORC1 activity, possibly through cilia-dependent mechanisms that may be both cell autonomous and non-cell autonomous.
MATERIALS AND METHODS
Mice.
Emx1-Cre mice were obtained from Jackson Laboratories (Bar Harbor, Maine; line no. 005628) and maintained on a C57Bl/6 background. Mice with a Tsc1 “floxed” allele were obtained from Dr. D. Gutmann (Washington University, St. Louis, MO) and maintained on a mixed SV129J/C57Bl/6 background. Mice with the Rictor floxed allele were a gift of Dr. Mark Magnuson (Vanderbilt University) and were maintained on a C57Bl/6 background (28). Through interbreeding, we generated Tsc1Flox/Flox;Emx1-Cre mice (Tsc1 CKO) and RictorFlox/Flox;Emx1-Cre (Rictor CKO) mice that are homozygous for either the Tsc1 or Rictor floxed allele and heterozygous for Emx1-Cre. Heterozygous Tsc1Flox/wt;Emx1-Cre animals were used as controls and were indistinguishable from other control genotypes including Tsc1Flox/Flox or Tsc1Flox/wt Emx1-Cre negative mice. RictorFlox/Flox;Cre-negative animals were used as controls for Rictor CKO mice. Cre reporter strains included Z/EG transgenic mice (Jackson Laboratories; line no. 004178) and mTomato/mGFP transgenic mice (Jackson Laboratories; line no. 007676). Animal experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Serum chemistries.
Blood samples were collected at euthanasia through cardiac puncture. Initial blood urea nitrogen (BUN) measurements were performed by the laboratory of Dr. Raymond Harris (Vanderbilt University). Additional BUN and serum chemistries performed by Antech (Southaven, MS).
Rapamycin treatment.
Rapamycin (LC Laboratories, Woburn, MA) was dissolved in a stock solution at 30 mg/ml in ethanol. Before use, the stock solution was diluted with vehicle 0.25% Tween 20/0.25% polyethylene glycol in PBS. Tsc1 CKO mice and control littermates received intraperitoneal injections with either rapamycin (3 mg/kg) or vehicle twice a week, starting at postnatal days (P)13–15.
Antibodies.
Arl13b (1:500; gift of T. Caspary, Emory University, Atlanta, GA), aquaporin 1 (AQP1; 1:500; Abcam), AQP2 (1:500; Abcam), calbindin D-28k (1:2,000; Swant), NCC (1:500; Millipore), fluorescein-labeled Lotus Tetragonolobus Lectin (1:2,000; Vector Laboratories), phospho-Akt Serine473 (clone D9E, 1:1,000; Cell Signaling), phospho-S6 Serine235/236 (1:1,000; Cell Signaling), Akt (1:1,000; Cell Signaling), S6 ribosomal protein (clone 5G10, 1:1,000; Cell Signaling), phospho-NDRG1 Thr346 (1:1,000; Cell Signaling), and actin (1:1,000; Cell Signaling).
Immunofluorescence and imaging.
Paraffin sections underwent antigen retrieval in 10 mM citrate buffer. Both frozen and paraffin sections were blocked in 5% normal goat serum, incubated in primary antibodies overnight at 4°C, followed by AlexaFluor conjugated secondary antibodies (Invitrogen) for 1 h. Slides were mounted with Vectashield (Vector Laboratories). Photomicrographs were taken using an Olympus BX UCB epifluorescence microscope and a Hamamatsu ORCA-ER CCD camera. Photomicrographs for cilia were taken on a Nikon spinning disk confocal microscope (Quorum Systems) with Metamorph software. Z-stack images were Z-projected, and cilia length was measured using ImageJ Software (Version 1.43S; National Institutes of Health). Cilia length was measured from calbindin-positive tubules in each photomicrograph using sections obtained from three control and three Tsc1 or Rictor CKO mice. Measurements of cilia length were done while blinded to genotypes or treatment with vehicle or rapamycin. Due to the variability of numbers of visible cilia in each section, between 87 and 150 cilia were measured for each group.
Immunoblotting.
Kidneys were snap frozen in liquid nitrogen and homogenized in RIPA buffer containing phosphatase and protease inhibitor cocktails (Sigma-Aldrich). Western blotting was completed using standard conditions. Blots were probed with either horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) or with fluorescent-tagged secondary antibodies, Alexa 680 (rabbit; Invitrogen), and IRDye 800 fluorochromes (mouse and rat; Licor). For horseradish-peroxidase-conjugated secondary antibodies, signal was developed using ECL Western blotting substrate (Pierce, Rockford IL) and visualized on BioMax film (Kodak). Fluorescent-tagged secondary antibodies were visualized using an Odyssey fluorescence scanner. After visualization, digitized band densities were quantitated using ImageJ (National Institutes of Health).
RESULTS
Loss of Tsc1 in the DCT increases kidney size.
Patients with TSC have multiorgan manifestations, usually with early neurological involvement followed by renal involvement in older children and adults. To address the dual pathologies of kidney and brain in TSC, we generated Tsc1 CKO (Tsc1Flox/Flox;Emx1-Cre, CKO) mice. While usually described as a “brain-specific” gene, the Emx1 promoter also directs Cre expression in the kidney (2, 15). Tsc1 CKO mice are postnatally viable but small with extensive brain abnormalities, seizures, and complete mortality by P25 (3). Because available antibodies are not suitable to determine hamartin levels, we assessed Tsc1 gene inactivation by isolating genomic DNA from kidneys and used PCR to determine recombination status. As expected, DNA from Tsc1 CKO kidneys yielded both recombined and unrecombined bands indicating targeted heterogeneity within the kidney while control kidneys had only the unrecombined Tsc1 gene (data not shown). By P15, kidneys from Tsc1 CKO mice were larger than littermate controls (Fig. 1A). When normalized for total body weight, the difference in kidney size was even more striking (Fig. 1B). Despite size abnormalities in the Tsc1 CKO kidneys at P15, there was no significant change in BUN between Tsc1 CKO mice and littermate controls (Fig. 1C). This suggests that Emx1-Cre-driven loss of Tsc1 in the kidney leads to kidney size abnormalities but not overt renal failure, suggesting the premature death of Tsc1 CKO mice is likely due to their extensive brain pathology (3).
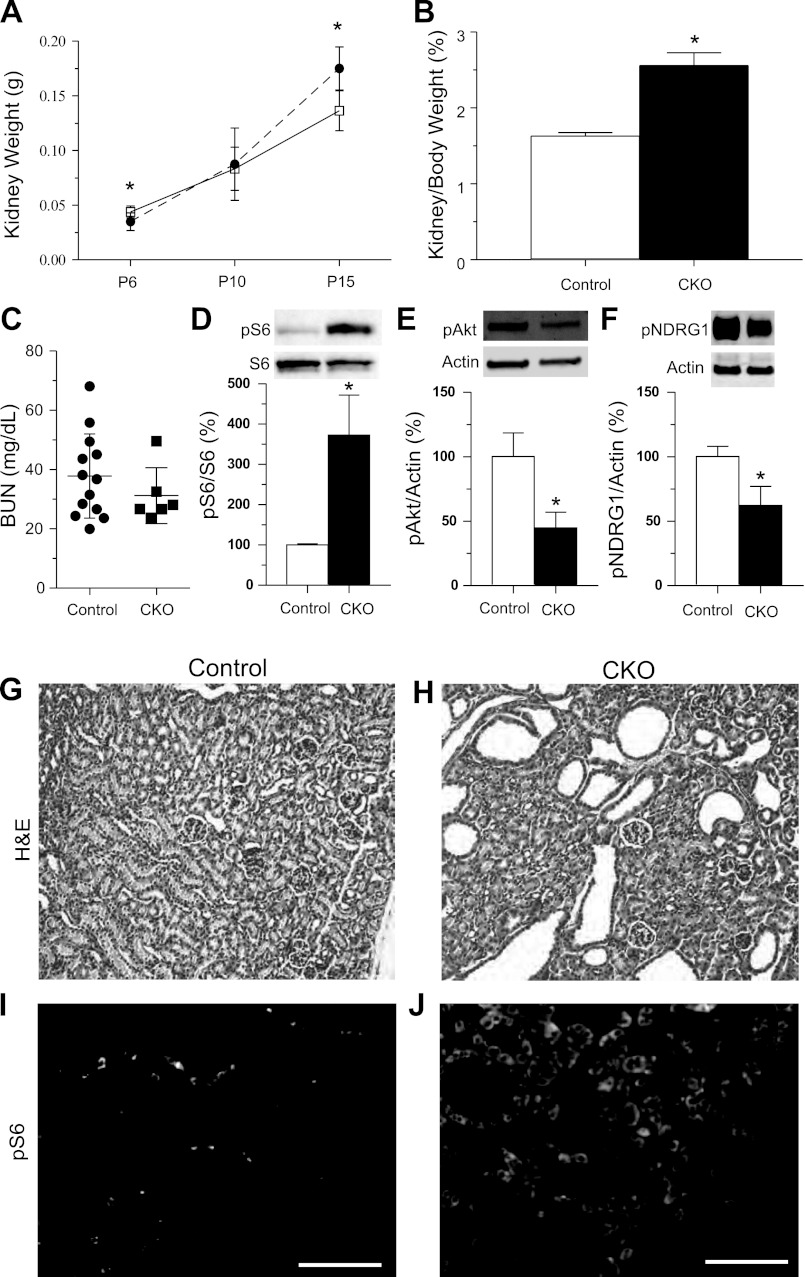
Fig. 1.
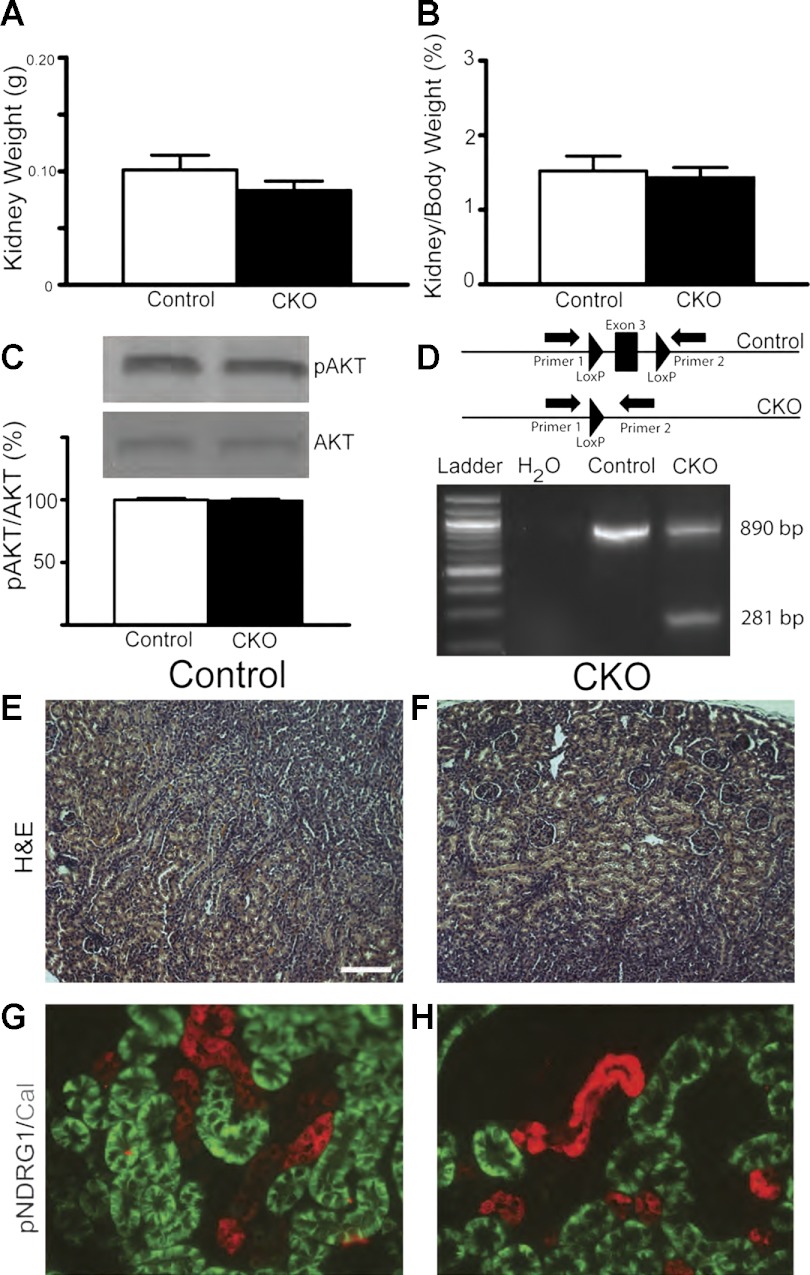
Postnatal day (P)15 Tsc1 conditional knockout (CKO) mice have large kidneys, renal cysts and increased mammalian target of rapamycin complex 1 (mTORC1) signaling. A: kidney weights from control (solid line) or Tsc1 CKO mice (dashed line) at P6 to P15; n ≥ 3 for each group. *P < 0.03. B: kidney weight normalized to body weight from P15 control and Tsc1 CKO mice. (control n = 9; CKO n = 3). *P < 0.03. C: no differences in blood urea nitrogen (BUN) levels from control and Tsc1 CKO mice. *P > 0.05, Student's t-test (control n = 13; CKO n = 6). D: immunoblotting for phospho-S6 reveals increased mTORC1 activity. Blots were stripped and reprobed for total S6 levels. E: immunoblotting for phospho-Akt (Ser473) reveals decreased mTORC2 signaling. F: decreased phospho-NDRG1 (Thr346) further reveals decreased mTORC2 signaling in P15 Tsc1 CKO kidney compared with littermate controls. Data were analyzed with Student's t-test. *P < 0.001 for phospho-S6, P < 0.05 for phospho-Akt and *P < 0.005 for phospho-NDRG1. All graphs are plotted as means ± SD, control extract expression levels were set to 100%. G and H: hematoxylin and eosin (H&E) staining of kidney sections from P15 control and Tsc1 CKO mice. Moderate cystic dilations are seen in kidneys from Tsc1 CKO mice. I and J: immunofluorescence for phospho-S6 in control and Tsc1 CKO mice. Phospho-S6 is diffusely expressed in the majority of the Tsc1 CKO kidney but found only in isolated cells in kidney from control littermates. Scale bars = 100 μm.
Increased mTORC1 signaling and mild cystogenesis.
To determine if abnormalities of kidney size were associated with increased mTORC1 signaling, we measured levels of phospho-S6 in kidney extracts from control and Tsc1 CKO kidneys. P15 CKO kidneys had increased ratios of phospho-S6 to total S6, indicating increased mTORC1 signaling (Fig. 1D). We also examined mTORC2 signaling using phosphorylation of Akt at Ser473, a sensitive and specific readout of mTORC2 activity (16, 28). We found a significant reduction of phospho-Akt in these same kidney samples (Fig. 1E). Additional evidence for decreased mTORC2 signaling is seen by reduced phosphorylation of NDRG1 at Thr346 (Fig. 1F). Histological examination of kidneys from Tsc1 CKO mice at P15 showed cystic changes when compared with littermate controls (Fig. 1, G and H). Immunofluorescence for phospho-S6 again showed increased mTORC1 signaling throughout the kidney in Tsc1 CKO mice compared with controls (Fig. 1, I and J). Interestingly, both cystic and morphologically normal appearing tubules had increased mTORC1 signaling.
Emx1-Cre expression in the DCT.
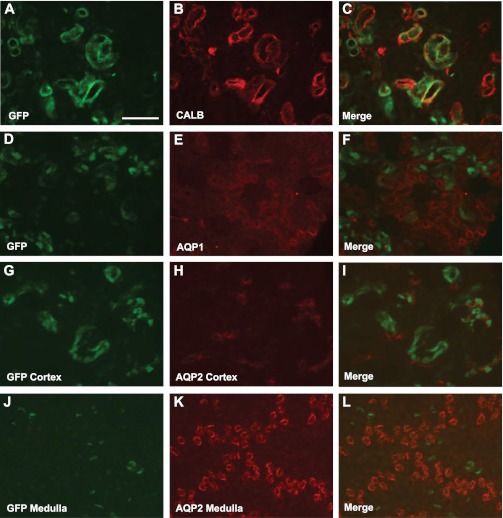
To determine expression within the kidney, we crossed Emx1-Cre mice to reporter animals that express GFP only after exposure to Cre recombinase. Animals transgenic for both Emx1-Cre and Z/EG revealed GFP signal within the cortex but not medulla. GFP coexpression was determined within the kidney using markers for the DCT (calbindin and NCC), the PCT (Aqp1), and collecting duct (Aqp2). Abundant GFP coexpression was seen with calbindin (Fig. 2, A-C), and similar results were seen with NCC (data not shown). While most cells in the DCT were double labeled with GFP, a few calbindin- and NCC-positive cells did not double label, suggesting that a subset of DCT cells did not express Emx1-Cre and were not targeted. GFP did not coexpress with either Aqp1 or Aqp2 in the kidney (Fig. 2, D–L) indicating that Emx1-Cre expression was restricted to the DCT.
Fig. 2.
Emx1-Cre mediates gene recombination in the distal convoluted tubule. A–C: GFP expression from Cre recombinase fate mapping (A) and calbindin (CALB), a marker of the distal convoluted tubules (DCT; B), colocalize in the cortex of the kidney, merged image (C). Neither AQP1, a marker of the proximal tubule, nor aquaporin 2 (AQP2), a marker of collecting ducts, colocalize in the cortex with GFP (D–F and G–I, respectively). Only faint reporter GFP was observed in the medulla, and this signal did not colocalize with AQP2 (J–L). Scale bar = 100 μm.
Rapamycin withdrawal from Tsc1 CKO mice leads to massive kidney enlargement and severe cystogenesis.
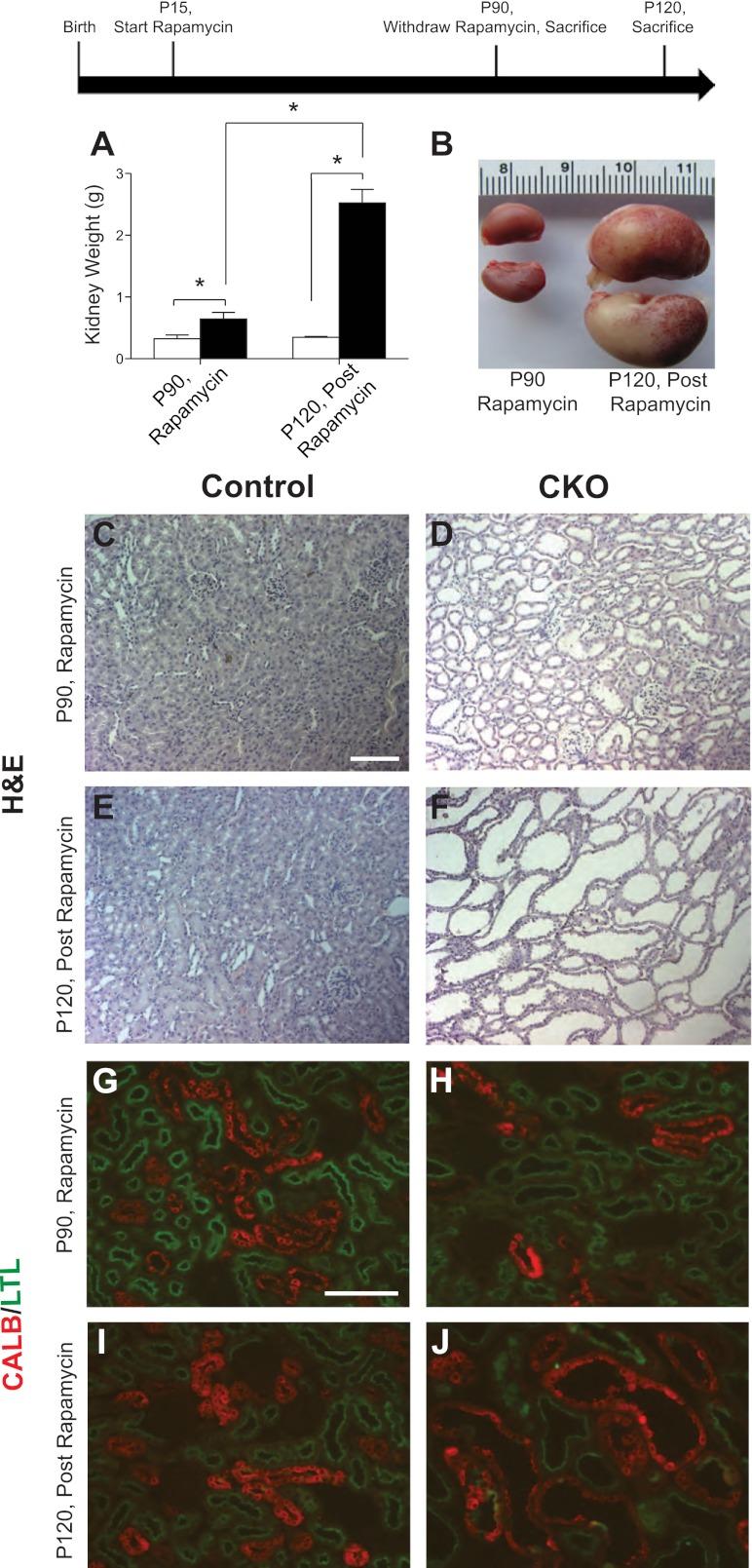
To see if kidney abnormalities in Tsc1 CKO mice were dependent on increased mTORC1 signaling, we treated Tsc1 CKO mice twice weekly with rapamycin starting at P14 until P90. Using this regimen, we achieved nearly 100% survival of CKO animals at P90 whereas prior natural history studies (3) demonstrated complete mortality by P25. A group of control and Tsc1 CKO animals were euthanized at P90 while on rapamycin with another group of control and Tsc1 CKO animals euthanized on P120, 30 days after stopping rapamycin. On rapamycin, kidneys from P90 Tsc1 CKO mice were slightly larger than kidneys from littermate controls (Fig. 3A). However, in the postrapamycin group, kidneys from Tsc1 CKO mice were greatly increased in size compared with control littermates (Fig. 3, A and B). While some small cysts were seen in P90 Tsc1 CKO kidneys on rapamycin (Fig. 3, C and D), P120 Tsc1 CKO mice postrapamycin exhibited severely dilated cystic-appearing structures (Fig. 3, E and F). While Emx1-Cre expression appeared to be restricted to the DCT (Fig. 2), cystic dilatations were seen in Tsc1 CKO in both the PCT and DCT (Fig. 3, G–J), suggesting both cell autonomous and non-cell autonomous mechanisms.
Fig. 3.
Rapamycin treatment partially inhibits cyst formation in Tsc1 CKO mice and upon withdrawal causes giant cystic kidneys. Two groups of mice were treated with rapamycin from P15 to P90. At P90, kidneys from one group of animals euthanized on rapamycin were analyzed. For the second group of animals, rapamycin treatment was discontinued at P90 and mice were euthanized 30 days postrapamycin treatment at P120. A: rapamycin treatment reduces cystogenesis in Tsc1 CKO kidneys. After rapamycin withdrawal Tsc1 CKO kidneys become much larger than those from control littermates (n ≥ 3 for each group of control and Tsc1 CKO mice). B: gross images of representative Tsc1 CKO kidneys on rapamycin at P90 (left) or 30 days postrapamycin treatment at P120 (right). C–F: H&E staining of paraffin sections from Tsc1 CKO and control littermate kidneys on or off of rapamycin. Rapamycin-treated Tsc1 CKO mice have slightly dilated tubules at P90 (D) compared with controls (C). At P120, 30 days postrapamycin treatment, kidneys from Tsc1 CKO show large cystic dilations of all tubules (F) compared with littermate controls (E). G–J: immunofluorescence for LTL [proximal convoluted tubule (PCT) marker, green] and the calbindin (DCT marker, red). Dilations of both the PCT and DCT are observed while on rapamycin (H) and postrapamycin treatment (J) compared with controls (G and I, respectively). Graphs are plotted with means ± SD. Data were analyzed with Student's t-test. *P < 0.001. Scale bar = 100 μm.
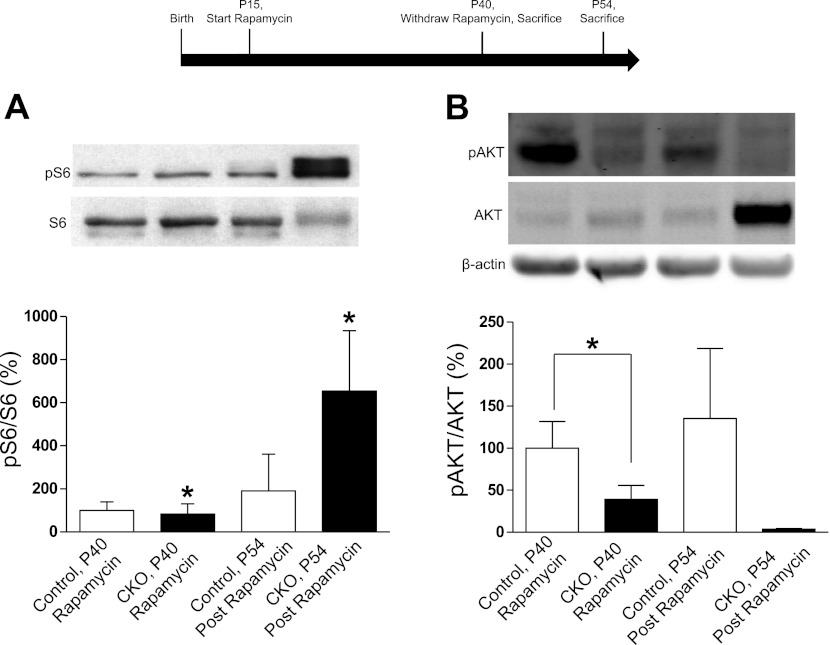
To further explore mTORC1 and mTORC2 signaling in the kidney, Tsc1 CKO and control mice were treated with rapamycin from P14 to P40. Kidneys were removed within 24 h of the last dose of rapamycin or 2 wk following rapamycin cessation. Immunoblots revealed increased mTORC1 signaling as indicated by pS6 levels in Tsc1 CKO kidneys 2 wk after rapamycin cessation, while levels in Tsc1 CKO and control kidneys on rapamycin were suppressed to levels below those seen in control mice off rapamycin (Fig. 4). As the Tsc1/Tsc2 genes have been reported to activate mTORC2 signaling, we assessed Akt phosphorylation at Serine473. Using this same group of control and Tsc1 CKO mice, we found significantly decreased levels of mTORC2 signaling in Tsc1 CKO mice that were on rapamycin as well those examined after rapamycin withdrawal (Fig. 4). These overall results suggest that renal abnormalities in Tsc1 CKO mice are mainly due to mTORC1-dependent signaling. However, rapamycin treatment did not completely reverse the pathology, with P90 animals on rapamycin still displaying cystic changes, suggesting other mechanisms including decreased mTORC2 signaling may contribute. Alternatively, rapamycin treatment starting at P14 may simply be too late to reverse kidney pathology. Rapamycin injections at P6 were attempted but hampered by toxicity and mortality in both Tsc1 CKO and control animals.
Fig. 4.
Rapamycin treatment normalizes mTORC1 but not mTORC2 signaling in Tsc1 CKO kidneys. Two groups of animals were treated with rapamycin from P15 to P40. Kidneys were taken from the first group of euthanized animals at P40 (n = 4 for control mice and n = 3 for CKO). Rapamycin treatment was discontinued in the second group of animals for 14 days, from P40 to P54 and protein extracts made from these kidneys at P54 (n = 3 for control and n = 2 for CKO). A: mTORC1 signaling, as shown by S6 protein phosphorylation, in Tsc1 CKO kidneys was decreased to that seen in control animals with rapamycin treatment. Postrapamycin phospho-S6 levels were greatly increased. *P < 0.05. B: mTORC2 signaling, as shown by Akt phosphorylation at Serine473, in P40 Tsc1 CKO kidneys was significantly decreased while on rapamycin. *P = 0.039. While trending towards significance, CKO extracts postrapamycin treatment did not have statistically significant decreases in phospho-Akt (Serine473); P = 0.12.
Loss of rictor in the DCT is not sufficient to cause overt kidney abnormalities.
These findings with rapamycin suggest that reversal of mTORC1 signaling was largely responsible for the kidney pathology in Tsc1 CKO mice. To isolate the contribution of decreased mTORC2 signaling during kidney development, we developed a Rictor CKO mouse model using the same breeding strategy used for Tsc1 CKO mice. These homozygous Rictor floxed mice with a single copy of Emx1-Cre (Rictor CKO) are viable with no overt brain abnormalities (R. P. Carson and K. Ess, unpublished data). At P15, kidneys from Rictor CKO mice also had no noticeable differences in weight compared with control littermates (Fig. 5, A and B). As antibodies against Rictor did not give reproducible results, mTORC2 signaling was assessed using levels of phospho-Akt (Serine473). No differences were seen in Rictor CKO compared with control mice (Fig. 5C), likely due to decreased mTORC2 signaling being restricted to the DCT. To determine if the Rictor gene was inactivated, we extracted genomic DNA from kidneys and used PCR to measure recombination. DNA from Rictor CKO mice had unrecombined and prominent recombined bands, indicating Rictor inactivation in a subset of the kidney (Fig. 5D). Kidney DNA extracted from a control littermate only showed the unrecombined gene. Rictor CKO kidneys appeared normal and indistinguishable from littermate controls (Fig. 5, E and F). Coimmunofluorescence for phosphorylated NDRG1, a downstream indicator of mTORC2 activity, and calbindin, a marker of the DCT, showed that even in kidneys from control animals the DCT has undetectable levels of endogenous mTORC2 activity (Fig. 5G). Therefore, further decreases of mTORC2 activity in the DCT of Rictor CKO animals were not measurable with immunofluorescence (Fig. 5H). These findings suggest that kidney abnormalities seen after the loss of the Tsc1 gene in the DCT are largely due to increased mTORC1 signaling.
Fig. 5.
Rictor CKO kidneys at P15 do not develop cysts. A and B: kidneys from P15 Rictor CKO mice did not show alterations in kidney weight nor in kidney-to-body weight ratios compared with control littermates (control n = 15; CKO n = 7). C: no changes in AKT phosphorylation at Serine 473 were detected through western blot analyses of protein extracts from P9-P15 Rictor CKO mice. D: PCR reveals recombination of exon three of the Rictor floxed allele in the kidney of Rictor CKO mice. E and F: H&E staining do not show any appreciable kidney abnormalities. G and H: coimmunofluorescence for pNDRG1, a marker of mTORC2 activity, and calbindin, a marker of the DCT, revealed undetectable levels of mTORC2 activity in the DCT in both control and CKO animals.
Abnormalities of the primary cilia in the DCT from Tsc1 but not Rictor CKO mice.
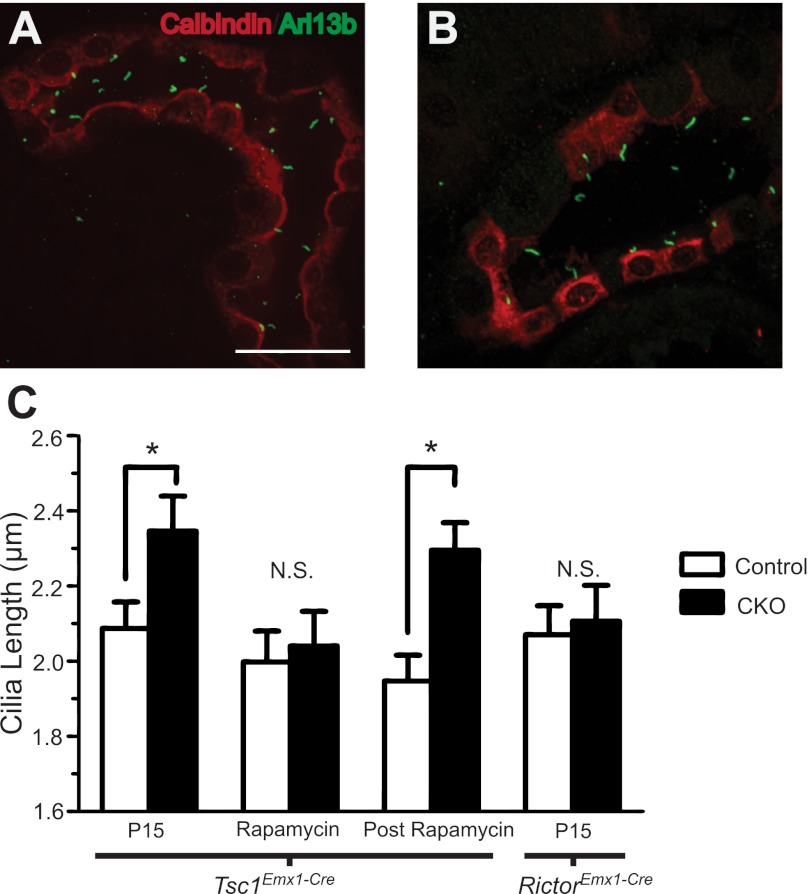
The precise pathological mechanisms causing cystogenesis or brain malformations in TSC are poorly understood. As other diseases such as Bardet-Biedl Syndrome and Joubert Syndrome also have shared brain and kidney pathology, we hypothesized that abnormalities of the primary cilia may connect TSC to “ciliopathies” (1). This hypothesis was bolstered by a recent report (11) that hamartin is expressed in the basal body and Tsc1-deficient fibroblasts have abnormalities of cilia length. In addition, zebrafish with Morpholino knockdown of tsc1a also have elongated cilia and rapamycin treatment shortened cilia (38). We measured the length of primary cilia in the DCT from P15 Tsc1 CKO and control mice and found longer primary cilia in the DCT from mutant mice (Fig. 6B). In contrast, P90 mice on rapamycin did not have changes in cilia length. However, the P120 Tsc1 CKO mice (30 days after stopping rapamycin injections) again had increased cilia length compared with controls (Fig. 6). To assess the potential contribution of decreased mTORC2 signaling to this phenotype, cilia from P15 Rictor CKO and control littermates were measured with no alterations in their length seen. Our pharmacologic and genetic data suggest that cilia length abnormalities in the DCT are due to increased mTORC1 but not decreased mTORC2 signaling.
Fig. 6.
Primary cilia are longer in Tsc1-deficient but not Rictor-deficient tubules. A and B: representative photomicrographs of cilia in the DCT of Tsc1 CKO mice on rapamycin at P90 (A) or 30 days after rapamycin withdrawal at P120 (B). Coimmunofluorescence for Arl13B (a marker of primary cilia) and calbindin (marker of the DCT). C: cilia length in untreated mice at P15, during rapamycin treatment at P90 and 30 days postrapamycin withdrawal at P120. Primary cilia in the DCT of kidneys from Tsc1 CKO mice were longer 30 days after rapamycin cessation. No changes in cilia length were noted between control mice and Rictor CKO mice. *P < 0.05. Scale bar = 25 μm. N.S, not statistically significant; n = 3 for each group of control, Tsc1 CKO and RictorEmx1−Cre CKO mice.
DISCUSSION
Our results demonstrate the formation of dilated cystic tubules from selective loss of Tsc1 in the DCT. This extends previous studies (30, 39) that have shown cystogenesis from Tsc1 inactivation in the PCT or from both the PCT and DCT. Additionally, we demonstrate an integral role for abnormal mTORC1 but not mTORC2 signaling in cystogenesis. This is shown by both pharmacological experiments using rapamycin and from the selective inactivation of the Rictor gene in the DCT. Furthermore, we found abnormally long primary cilia in Tsc1-deficient but not Rictor-deficient DCT. The abnormal cilia length in the DCT of Tsc1 CKO mice is also reversible by treatment with rapamycin. These findings strongly suggest that increased mTORC1 but not decreased mTORC2 is the main contributor to cyst formation in TSC. Kidney disease initiation and progression in TSC may be due to defects in the structure or function of the primary cilia, linking TSC to other disorders that have dual kidney and the brain pathologies.
Intriguingly, cystic changes were not restricted to the DCT. This is shown by increased mTORC1 activity at P15 throughout the kidney and cystic dilations within both the PCT and DCT in the kidneys at P120 postrapamycin withdrawal (Fig. 3). The presence of cysts in compartments other than Emx1-Cre expression domains suggests that non-cell-autonomous processes may be contributing to this cystic phenotype. This may also explain the decrease in mTORC2 signaling seen in Tsc1 CKO but not Rictor CKO kidney. Non-cell autonomous mechanisms have recently been reported in studies (5, 17) using human samples and from a zebrafish model of TSC. Newer therapies for patients with TSC need to consider potential non-cell autonomous mechanisms when designing therapies to alter the course of renal disease. An alternative explanation is that loss of the Tsc1 gene may cause altered cell fate, with DCT cells inappropriately expressing markers of the PCT.
The primary cilium is an important mechanistic link between PKD and the mTOR pathway (13). PKD is one of several disorders described as a “ciliopathy.” Links between cystogenesis and defects in primary cilium were first identified in a mouse model of PKD that has a mutation of Ift88, a gene required for normal primary cilia (22). Other primary cilium related proteins have been shown to also play a role in cystogenesis (20, 40). Previously, longer cilia have also been found after kidney injury (32). Here, our findings of altered cilia length after loss of Tsc1 in the DCT and normalization with rapamycin treatment link mTORC1 signaling to the primary cilia. Hamartin expression at the basal body (11) coupled with elongated primary cilia in Tsc1- or Tsc2-deficient mouse embryonic fibroblasts further supports our findings. In addition to these results with cultured cells, the knockdown of tsc1a in zebrafish also increased cilia length and caused left-right patterning defects and cystic appearing kidneys (9, 38). The further use of complementary zebrafish and mouse models will greatly enhance our understanding of the role of the TSC genes and mTORC1 in the regulation of primary cilia length and function. Shortened or loss of primary cilia has a clear functional consequence, including altered calcium flux and cell size control in response to flow. However, the functional impact of elongated primary cilia as we have seen here remains to be elucidated. This insight will be particularly useful in studying kidney phenotypes in animal models of TSC and understanding the pathogenesis of PKD and other kidney diseases that have altered mTORC1 signaling.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant 5K08-NS-050484 (to K. C. Ess) and National Institute of General Medical Sciences Grant T32-GM-07347 awarded to the Vanderbilt Physician Scientist Training Program (to E. A. Armour).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.A.A. and R.P.C. performed experiments; E.A.A., R.P.C., and K.C.E. analyzed data; E.A.A., R.P.C., and K.C.E. interpreted results of experiments; E.A.A., R.P.C., and K.C.E. prepared figures; E.A.A., R.P.C., and K.C.E. drafted manuscript; E.A.A., R.P.C., and K.C.E. edited and revised manuscript; K.C.E. conception and design of research; K.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Peggy Winzenburger and Thabiso Chirwa for technical assistance. We also thank Drs. Ray Harris and Mark deCaestecker for very helpful discussions and Dr. Suwan Wang who performed BUN assays.
REFERENCES
- 1. Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7: 125– 148, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev 57: 169– 180, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Carson RP, Van Nielen DL, Winzenburger PA, Ess KC. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis 45: 369– 380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Consortium of European Chromosome 16 Tuberous Sclerosis Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75: 1305– 1315, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Crino PB, Aronica E, Baltuch G, Nathanson KL. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology 74: 1716– 1723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 355: 1345– 1356, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dere R, Wilson PD, Sandford RN, Walker CL. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLos One 5: e9239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiBella LM, Park A, Sun Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum Mol Genet 18: 595– 606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416: 375– 385, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 18: 151– 163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J, Wu S, Wu CL, Manning BD. Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res 69: 6107– 6114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibraghimov-Beskrovnaya O, Natoli TA. mTOR signaling in polycystic kidney disease. Trends Mol Med 17: 625– 633, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Inoki K, Corradetti M, Guan K. Dysregulation of the TSC-mTOR pathway in human disease. Nature Genetics 2004. [DOI] [PubMed] [Google Scholar]
- 15. Inoue K, Terashima T, Nishikawa T, Takumi T. Fez1 is layer-specifically expressed in the adult mouse neocortex. Eur J Neurosci 20: 2909– 2916, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125– 137, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kim SH, Speirs CK, Solnica-Krezel L, Ess KC. Zebrafish model of tuberous sclerosis complex reveals cell-autonomous and non-cell-autonomous functions of mutant tuberin. Dis Model Mech 4: 255– 267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi T, Minowa O, Kuno J, Mitani K, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 59: 1206– 1211, 1999 [PubMed] [Google Scholar]
- 19. Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 11: 525– 534, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129– 137, 2003 [DOI] [PubMed] [Google Scholar]
- 21. O'Callaghan FJ, Noakes MJ, Martyn CN, Osborne JP. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int 94: 853– 857, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709– 718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Povey S, Burley MW, Attwood J, Benham F, Hunt D, Jeremiah SJ, Franklin D, Gillett G, Malas S, Robson EB. Two loci for tuberous sclerosis: one on 9q34 and one on 16p13. Ann Hum Genet 58: 107– 127, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Sampson JR, Maheshwar MM, Aspinwall R, Thompson P, Cheadle JP, Ravine D, Roy S, Haan E, Bernstein J, Harris PC. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am J Hum Genet 61: 843– 851, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296– 1302, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Sepp T, Yates JR, Green AJ. Loss of heterozygosity in tuberous sclerosis hamartomas. J Med Genet 33: 962– 964, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466– 5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell 11: 583– 589, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol 16: 29– 37, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M., Niggli FK, Kriz W, Gröne H-J, Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979– 984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 52: 285– 296, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 20: 2147– 2153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Greenwood JSF, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol 61: 139– 152, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Washecka R, Hanna M. Malignant renal tumors in tuberous sclerosis. Urology 37: 340– 343, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471– 484, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 91: 11413– 11416, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young J, Povey S. The genetic basis of tuberous sclerosis. Mol Med Today 4: 313– 319, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Yuan SAL, Li JD, Diener DR, Choma MA, Rosenbaum JL, Sun ZX. Target-of-rapamycin complex 1 (Torc1) signaling modulates cilia size and function through protein synthesis regulation. Proc Natl Acad Sci USA 109: 2021– 2026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou J, Brugarolas J, Parada L. Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Hum Mol Genet 18: 4428– 4441, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Zullo A, Iaconis D, Barra A, Cantone A, Messaddeq N, Capasso G, Dollé P, Igarashi P, Franco B. Kidney-specific inactivation of Ofd1 leads to renal cystic disease associated to upregulation of the mTOR pathway. Hum Mol Genet 19: 2792– 2803, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]