Abstract
Human SLC2A9 (GLUT9) is a novel high-capacity urate transporter belonging to the facilitated glucose transporter family. In the present study, heterologous expression in Xenopus oocytes has allowed us to undertake an in-depth radiotracer flux and electrophysiological study of urate transport mediated by both isoforms of SLC2A9 (a and b). Addition of urate to SLC2A9-producing oocytes generated outward currents, indicating electrogenic transport. Urate transport by SLC2A9 was voltage dependent and independent of the Na+ transmembrane gradient. Urate-induced outward currents were affected by the extracellular concentration of Cl−, but there was no evidence for exchange of the two anions. [14C]urate flux studies under non-voltage-clamped conditions demonstrated symmetry of influx and efflux, suggesting that SLC2A9 functions in urate efflux driven primarily by the electrochemical gradient of the cell. Urate uptake in the presence of intracellular hexoses showed marked differences between the two isoforms, suggesting functional differences between the two splice variants. Finally, the permeant selectivity of SLC2A9 was examined by testing the ability to transport a panel of radiolabeled purine and pyrimidine nucleobases. SLC2A9 mediated the uptake of adenine in addition to urate, but did not function as a generalized nucleobase transporter. The differential expression pattern of the two isoforms of SLC2A9 in the human kidney's proximal convoluted tubule and its electrogenic transport of urate suggest that these transporters play key roles in the regulation of plasma urate levels and are therefore potentially important participants in hyperuricemia and hypouricemia.
Keywords: Xenopus oocytes, splice variants, hyperuricemia, hypouricemia, facilitative hexose transporters
urate is an organic anion and the physiologically predominant form of uric acid, the end product of purine metabolism in humans and higher primates. Due to the loss of hepatic uricase activity, humans and higher primates maintain high levels of urate in the blood (180–420 μM) compared with the majority of mammals (30–120 μM), which do express uricase (16). The role for elevated urate in human plasma has not been explained, although one suggestion is that it may function as an antioxidant (33). Human plasma urate levels are regulated within closely defined limits, and even small increases above normal show significant correlation with the incidence of gout, metabolic disease, diabetes, cardiovascular morbidity and mortality, and hypertension (7, 15, 17, 29, 30, 32).
The high circulating levels of plasma urate result from a balance between intake from the diet and production in the liver and muscle, and loss in the urine. About 70% of daily urate production enters the renal filtrate, and 10% is finally excreted in the urine (26). The kidney epithelium is therefore the main regulatory site of plasma urate, where this metabolite's reabsorption and secretion occur. However, the molecular basis for urate handling in the human kidney has not been fully determined because of differences between species and the multitude of urate transport systems involved. The proposed urate transport systems in the human proximal nephron include the electroneutral urate/anion exchanger SLC22A12 (URAT1) (14), the organic anion transporters SLC22A6/8 (OAT1/3) (1, 20), the multidrug resistance protein ABCC4 (MRP4) (36, 41), the breast cancer resistance protein ABCG2 (BCRP) (41), and the sodium/phosphate transporter SLC17A3 (NPT4) (19). SLC22A12's role in urate reabsorption has been confirmed with loss-of-function mutations in this gene being associated with renal hypouricemia (14). In addition, mutations in SLC2A9 (GLUT9) have also been correlated with plasma urate levels in the Dalmatian dog model and in humans. Two putative electrogenic urate transporters have also been identified. Transport of PAH by the voltage-driven organic anion transporter SLC17A1 (NPT1/OATv1) cloned from the pig kidney is electrogenic, and uptake is competitively inhibited by urate (2, 20). There is, however, no evidence that SLC17A1 is expressed in the human kidney. Finally, a soluble protein initially cloned from the rat kidney, the urate transporter UAT1 displays voltage-sensitive channel activity that is highly urate specific (24). Human UAT (also known as galectin) (26), however, is expressed ubiquitously in numerous tissues, undermining claims that it mediates urate secretion specifically in the kidney.
Genome-wide association scans have linked single nucleotide polymorphisms (SNPs) in the gene encoding the facilitative hexose transporter isoform SLC2A9 with abnormal plasma urate concentrations in human population cohorts. SLC2A9 is a member of the facilitative glucose transporter gene family (GLUTs) but is now primarily described as a novel high-capacity urate transporter, which can exchange both glucose and fructose for urate (9, 37). SLC2A9 has two splice variants, SLC2A9a (full length) and SLC2A9b (or ΔN), both of which are present in the human kidney (3). The two splice variants differ in their N-terminal sequence and are expressed differentially in polarized cells: SLC2A9b (512 amino acids) is localized apically, while SLC2A9a (540 amino acids) is expressed in the basolateral membrane. Current evidence suggests that the two isoforms are functionally identical in terms of hexose and urate transport kinetics (3, 9).
We have postulated that negatively charged urate transport by human SLC2A9 is electrogenic. In support of this, Anzai et al. (2) have provided indirect evidence that human SLC2A9 urate transport in Xenopus laevis oocytes is influenced by membrane potential. Depolarizing the oocyte membrane through elevation of extracellular K+ resulted in stimulation of radiolabeled urate uptake. They did not, however, directly measure currents generated in SLC2A9-producing oocytes in response to urate exposure. The predominant basolateral localization and voltage dependence of SLC2A9 led to the conclusion that this protein is mainly involved in urate reabsorption, in concert with apically expressed SLC22A12. Moreover, recent findings by Bibert et al. (5) suggest that urate uptake mediated by mouse SLC2A9 induced an outward current when produced in oocytes.
In the present study, we have undertaken a combined electrophysiological and radiotracer flux analysis of recombinant human SLC2A9 isoforms produced in X. laevis oocytes. Using the two-electrode, voltage-clamp technique, the electrogenic nature of urate transport mediated by SLC2A9a and SLC2A9b was investigated by examining the effect of membrane potential and ion concentrations on membrane currents. We also measured the Km for urate influx under voltage-clamp conditions. Using radiotracer fluxes, we investigated the effect of intracellular hexoses on urate uptake, showing key functional differences in the two isoforms of the transporter. We also demonstrate that extracellular urate accelerates efflux of urate from the oocytes, suggesting that SLC2A9 acts as a nonobligatory urate-urate exchanger. The permeant selectivity of SLC2A9 was examined by testing its ability to transport other radiolabeled purine and pyrimidine nucleobases. Finally, we report kinetic studies of urate efflux, supporting the concept that the direction of urate flux is determined primarily by the electrochemical gradient.
MATERIALS AND METHODS
In vitro transcription and production in X. laevis oocytes.
Both isoforms of human SLC2A9 (SLC2A9a and SLC2A9b) were expressed in the plasma membrane when SLC2A9 transcripts were injected into X. laevis oocytes, with surface expression of both SLC2A9 isoforms determined to be equivalent under the conditions used (Fig. 1). All experiments reported here were undertaken with both isoforms of SLC2A9 and will be referred to as SLC2A9a (full length) and SLC2A9b (ΔN) throughout the remainder of the paper.
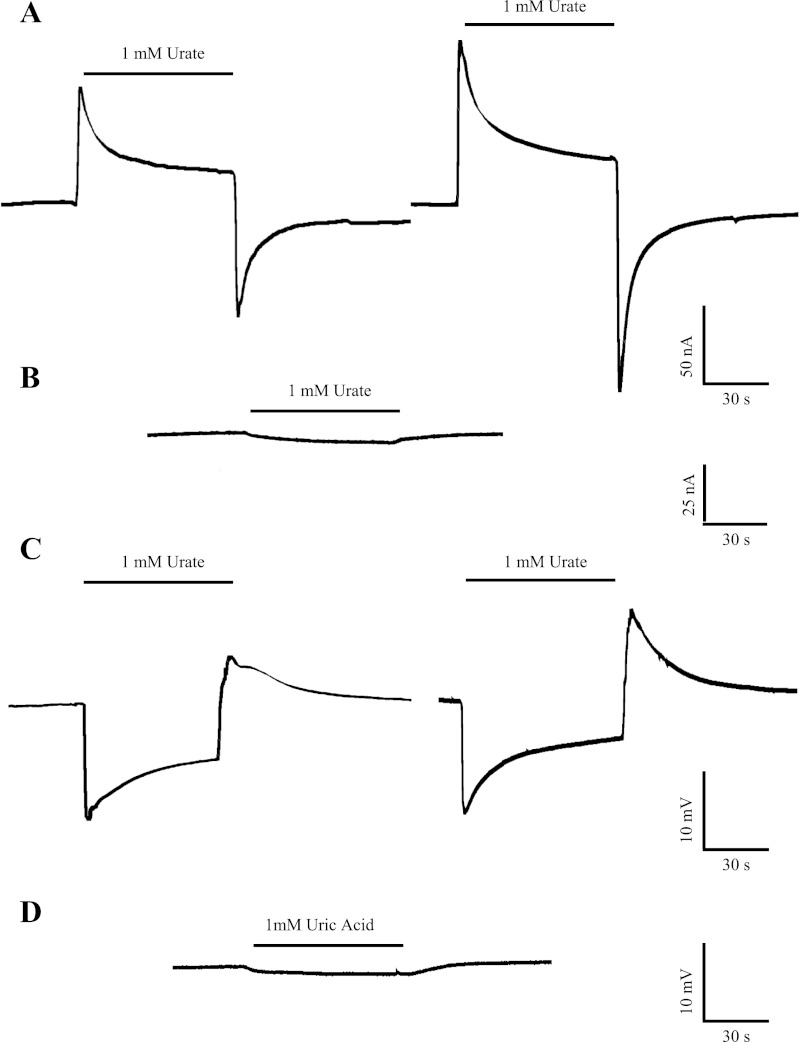
Fig. 1.
Representative current and voltage recordings of SLC2A9 (GLUT9). A: representative current trace in a single SLC2A9a (left)- and SLC2A9b (right)-producing Xenopus laevis oocyte clamped at −30 mV in Na+-containing transport medium (100 mM NaCl, pH 7.5). The horizontal bar denotes addition of urate (1 mM) to the bath. Baseline currents of 10 and −10 nA were measured for the representative SLC2A9a- and SLC2A9b-producing oocytes, respectively, before the addition of urate. B: the same experiment was performed in a representative control water-injected oocyte. The baseline current measured before the addition of urate was −15 nA. C: representative voltage response in a single SLC2A9a (left)- and SLC2A9b (right)-producing oocyte in Na+-containing transport medium (100 mM NaCl, pH 7.5). Oocytes were penetrated with microelectrodes, and membrane voltage was recorded under non-voltage-clamp conditions. Resting membrane potentials were −28 mV for SLC2A9a and −30 mV for SLC2A9b. Voltage changes were measured in response to the addition of urate (1 mM) to the bath. D: the same experiment was performed in a representative control water-injected oocyte. The resting membrane potential was −25 mV. All experiments were performed using eggs from 3 different frogs with similar results.
Recombinant human SLC2A9 isoforms were produced in oocytes by standard procedures (9, 42). SLC2A9a cDNA (GenBank accession number BC110414) and SLC2A9b cDNA (GenBank accession number BC018897) in the enhanced X. laevis expression vector pGEM-HE (25) were linearized with Nhe1 and transcribed in vitro with T7 polymerase using the mMESSAGE mMACHINE (Ambion, Austin, TX) transcription system. The remaining template was removed by digestion with RNase-free DNase1. Stage V-VI oocytes were isolated by collagenase treatment (2 mg/ml for 2 h) of ovarian lobes from female X. laevis (Biological Sciences Vivarium, University of Alberta, and Nasco, Fort Atkinson, WI) that had been anesthetized by immersion in 0.3% (wt/vol) tricaine methanesulfonate (pH 7.4). The remaining follicular layers were removed by phosphate treatment (100 mM K2PO4) and manual defolliculation. The animal protocols employed in this study were approved by the University of Alberta Animal Policy and Welfare Committee. Oocytes were injected with either 20 nl of water containing 20 ng of RNA transcript (1 ng/nl) encoding SLC2A9a or SLC2A9b or 20 nl of water alone (control). Injected oocytes were then incubated for 4 days at 18°C in modified Barth's medium [MBM; 88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES, 2.5 mM sodium pyruvate, 0.1 mg/ml penicillin, and 0.05 mg/ml gentamycin sulfate, pH 7.5] changed daily before the transport activity assays.
Transport media.
Nucleobase uptake and urate electrophysiology studies were performed in Na+-containing transport medium composed of 100 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2 and 10 mM HEPES (pH 7.5). In electrophysiology experiments examining the Na+ dependence of transport, Na+ in the transport medium was replaced by equimolar choline chloride (100 mM ChCl) to maintain isomolarity. To examine the effect of Cl− on SLC2A9 currents, the Cl− concentration in the transport medium was substituted with gluconate (i.e., the gluconate salt of Na+). Cl− concentrations tested were 25, 50, 75, and 100 mM. To promote membrane resealing following injection of [14C]urate or other unlabeled substances, radiotracer efflux experiments and uptake experiments with varied intracellular milieu were performed in Ca2+-containing MBM (pH 7.5). Similarly, the effect of Cl− on SLC2A9-mediated [14C]urate influx was measured in MBM in which the concentration of Cl− was reduced by replacement with gluconate. Cl− concentrations tested were 2, 25, 50, 75, and 90 mM. All chemicals were obtained from Sigma-Aldrich (Oakville, ON), unless otherwise noted.
Nucleobase uptake studies.
Transport assays to measure initial rates of SLC2A9 uptake (influx) were performed as described previously (40) on groups of 10–12 oocytes at room temperature (20°C) in 200 μl of Na+-containing transport medium containing the appropriate 14C- or 3H-labeled permeant (1 and 2 μCi/ml, respectively; Moravek, Brea, CA). Unless otherwise indicated, the concentration of radiolabeled urate, nucleobase, or uridine was 20 μM. At the end of the incubation period (20 min), extracellular label was removed by six rapid washes in ice-cold transport medium, and individual oocytes were dissolved in 1% (wt/vol) SDS for quantification of oocyte-associated radioactivity by liquid scintillation counting (LS 6000 IC, Beckman, Fullerton, CA).
Results in units of picomoles per ooctye per minute are given as means ± SE for 10–12 individual oocytes. Each experiment was performed at least twice using different batches of cells from different animals. Statistical significance of the reported data sets was evaluated using Student's t-test.
Radioisotope efflux studies.
Efflux experiments were modified from a previously published protocol (9). Briefly, efflux assays were performed in groups of 20 oocytes for each experimental and control condition. SLC2A9a or SLC2A9b isoform or control oocytes were injected with varying volumes of [14C]urate (5–50 nl; Moravek) and stored in cold MBM (4°C) before the assay of transport activity. To initiate efflux, oocytes were transferred to clean glass tubes and excess cold MBM was removed and replaced with MBM at room temperature (20°C; 1 ml). Simultaneously, 20 μl of medium was sampled to obtain the zero time point. The incubation volume was kept constant by sequential addition of MBM (20 μl) after removal of each sample (20 μl). Sampling was carried out over a period of 14 min. Following the last time point, MBM was removed from the tubes and the oocytes were dissolved in 1 ml of 5% (wt/vol) SDS. The lysate (50 μl) was sampled in triplicate to obtain the total amount of radioactivity remaining within the cells. The counts obtained were corrected for background and dilution and expressed as the percentage of total [14C]urate remaining within oocytes at a given time. As shown previously (9), urate efflux can be modeled by a single exponential curve when measured over a 60-min time period. In the present study, all efflux experiments were carried out over a 14-min time period, which coincides with the linear portion of the curve, and represents the initial rate of efflux. As such, the results were plotted against time and fitted with a straight line y = ax + b, where a represents the slope of the straight line and b represents the y-intercept (Prism GraphPad v.5.00, GraphPad Software, La Jolla, CA). The magnitude of the slope (in % urate efflux/min) represented the initial rate of efflux for a given concentration and was converted to picomoles per oocyte per minute. Starting intracellular concentrations of injected [14C]urate (specific activity 58.1 mCi/mmol) were calculated from the total amounts of radioactivity in each batch of 20 cells, assuming a water content of 0.5 μl/oocyte (25). A series of four to six experiments was carried out resulting in a range of [14C]urate intracellular concentrations (S) between 17 and 624 μM for SLC2A9a and between 39 and 495 μM for SLC2A9b, the low solubility of urate and maximum injectable volume setting an upper limit to the intracellular levels that could be achieved. Initial rates (v0) of efflux were plotted against individual values of S and analyzed by least squares fits to the Michaelis-Menten equation (Sigmaplot 11, Systat Software) to estimate values (±SE) for Vmax, the maximal rate of efflux, and Km, the SLC2A9's internal apparent affinity for urate. Thin-layer chromatography (9) has demonstrated that the 14C activity collected from the oocytes' incubation media represents that of the injected [14C]urate. Furthermore, Woodward et al. (41) found no uricase mRNA in X. laevis oocytes, further strengthening the argument that injected urate is not broken down inside these cells.
A similar approach was used to investigate the effect of extracellular substances on SLC2A9-mediated urate efflux. Polyethylene glycol (PEG), d-glucose, d-fructose, each at a concentration of 5 mM, and urate (1 mM) were dissolved in MBM, and urate efflux for both SLC2A9 isoforms was obtained. The rates of efflux under each condition were obtained from the slopes of the fitted lines and normalized against the efflux in the presence of extracellular PEG (5 mM) as the osmotic control substance. Control experiments verified that PEG gave the same results as l-glucose, the nonmetabolizable enantiomer of d-glucose (data not shown). The experiment was repeated six times, using oocytes from different frogs.
Effect of chloride on [14C]urate influx.
Transport assays were performed as described previously (40) in groups of SLC2A9a- or SLC2A9b-producing oocytes at room temperature (20°C) in 200 μl of MBM containing [14C]urate. The total concentration of urate used at each Cl− concentration tested was 100 μM. At the end of the incubation period (20 min), oocytes were processed for liquid scintillation counting as described for nucleobase uptake studies. The mean urate uptake at each Cl− concentration was averaged from 12 oocytes, and the experiment was repeated three times using oocytes from different frogs. Corrected for uptake in water-injected oocytes, urate influx (means ± SE) is presented as % uptake relative to that in normal MBM.
Effect of intracellular hexoses on [14C]urate influx.
Intracellular substances urate, d-glucose, d-fructose, 2-deoxy-d-glucose (2DOG), and 3-O-methyl-d-glucopyranose (3OMG) were microinjected into SLC2A9a- or SLC2A9b-producing oocytes before transport assays. Fifty nanoliters of 50 mM hexose and 50 nl of 1 mM urate were injected, to give final intracellular concentrations of 5 and 0.2 mM, respectively. The influx of 100 μM urate was then measured over 20 min, as described above. Given that oocytes injected with 5 or 0.2 mM PEG as osmotic controls showed comparable urate uptake at 100 μM, only 5-mM PEG controls are shown for clarity. Mean uptake values were derived from four independent experiments.
Electrophysiological studies.
Urate-evoked membrane currents were measured in SLC2A9a- or SLC2A9b-producing oocytes at room temperature (20°C) using a GeneClamp 500B oocyte clamp (Molecular Devices, Sunnyvale, CA) in the two-electrode, voltage-clamp mode. The GeneClamp 500B was interfaced to an IBM-compatible PC via a Digidata 1322A A/D converter and controlled by pCLAMP software (Version 9.0, Molecular Devices). The microelectrodes were filled with 3 M KCl and had resistances that ranged from 0.5 to 2.5 MΩ. Oocytes were penetrated with the microelectrodes, and their membrane potentials were monitored for periods of 10–15 min. Oocytes were discarded when membrane potentials were unstable, or more positive than −30 mV. Current signals were filtered at 20 Hz (4-pole Bessel filter) and sampled at a sampling interval of 20 ms. The effect of urate transport on membrane potential was also recorded in unclamped oocytes expressing SLC2A9a or SLC2A9b in the presence of varying Cl− concentrations. Current-voltage (I-V) curves were determined from differences between steady-state currents generated in the presence and absence of the permeant during 250-ms voltage pulses to potentials between −100 and +60 mV (10-mV increments). I-V curves were measured before and 30 s after the addition of the permeant. For I-V relationships, the voltage rise time of the clamp was adjusted by use of an oscilloscope such that it varied between 200 and 500 μs. Currents were filtered at 2 kHz (4-pole Bessel filter) and sampled at a rate of 200 μs/point (corresponding to a sampling frequency of 5 kHz). Urate kinetic parameters (Km, apparent affinity for urate, and Imax, predicted current maximum) calculated from electrophysiology experiments were determined by current measurements at different urate concentrations (0–5 mM) and analyzed by least squares fits to the Michaelis-Menten equation (Sigmaplot 11, Systat Software). The Km for urate was determined from fits to data averaged from individual oocytes normalized to the Imax value obtained for that oocyte and is presented as means ± SE of seven or more cells in three independent experiments. In anion substitution experiments, the bath electrode was grounded with a 5% agar bridge containing 3 M KCl to minimize voltage offsets when the concentration of Cl− ions is reduced.
RESULTS
Urate currents of SLC2A9.
Since urate is predominately a weak acid at pH 7.5 (pKa = 5.75) (16), we examined the electrogenic nature of SLC2A9-mediated transport of urate in X. laevis oocytes using the two-electrode, voltage-clamp technique. In the representative experiment shown in Fig. 1A, an oocyte producing SLC2A9a or SLC2A9b was voltage clamped at −30 mV and a transient outward current was generated when the extracellular medium (100 mM NaCl, pH 7.5) was changed to one containing 1 mM urate. This current decayed to a new steady state, and, upon removal of extracellular urate, a corresponding inward transient current, which returned to baseline, developed. Current magnitudes were similar in SLC2A9a- and SLC2A9b-producing oocytes and typically ranged in different experiments from 40 to 200 nA for SLC2A9a and 60 to 250 nA for SLC2A9b. In contrast, these currents were not observed in control water-injected oocytes (Fig. 1B). Following the addition of 1 mM urate to water-injected oocytes, small inward currents which slowly increased in amplitude were observed, possibly due to interactions of urate with endogenous oocyte transporters and channels (34). The much larger currents in SLC2A9-producing oocytes are consistent with the movement of negatively charged urate through the transporter, the large outward spike in the current record observed following the addition of urate decaying to a new steady state as the inwardly directed urate gradient is reduced. Upon removal of extracellular urate, transient inward currents are observed as a result of the efflux of cytoplasmic urate. These transient currents also decay as the urate gradient is once again depleted. The current record is biphasic, likely due to the bidirectional movement of urate mediated by SLC2A9. SLC2A9 therefore mediates the electrogenic transport of urate. Figure 1C depicts a typical membrane voltage response in oocytes expressing SLC2A9a or SLC2A9b with exposure to urate (1 mM; 100 mM NaCl, pH 7.5) under non-voltage-clamped conditions. Following the addition of urate, an initial rapid and transient hyperpolarization is observed, followed by a slow decay to a new steady state. Upon removal of urate, a rapid and transient depolarization is seen, which also decays to a new steady state. Voltage changes were similar in SLC2A9a- and SLC2A9b-producing oocytes, and in the range of 10–20 mV for all oocytes tested (data not shown). Finally, under the same conditions, in response to the addition of urate (1 mM), water-injected oocytes showed a small hyperpolarization (<5 mV) that returned to baseline upon removal of urate (Fig. 1D).
Voltage dependence of SLC2A9-mediated transport.
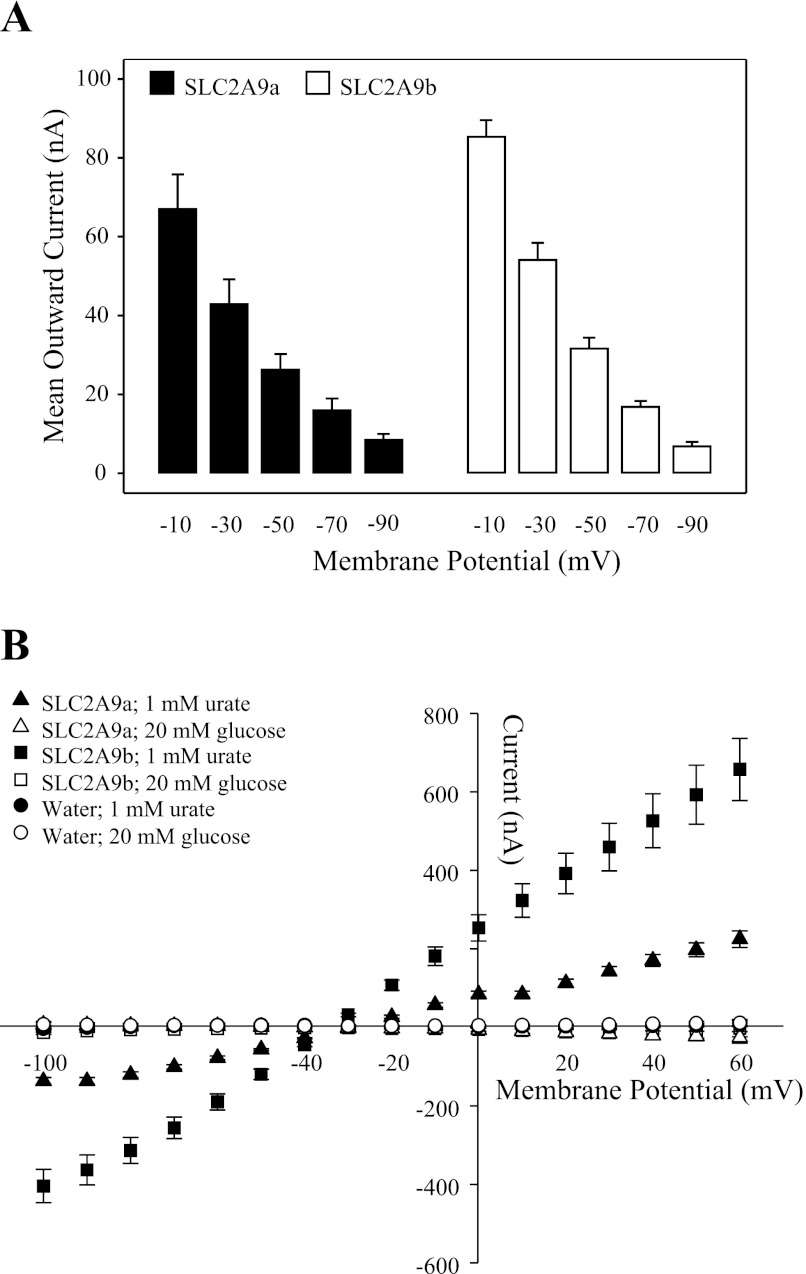
To obtain the current magnitudes in response to the addition of urate, two methods were used. In the first, current was calculated as the difference between baseline current and the transient spike observed following urate addition. In the second method, the steady-state urate current that followed the transient spike was calculated by integrating the area under the current record from baseline using fixed time points. The two methods showed similar voltage dependence (data not shown), and in voltage dependence and subsequent experiments reported here, current was calculated as the difference from baseline to transient spike. To examine the voltage dependence of transport mediated by SLC2A9, steady-state currents were measured sequentially at five different holding potentials (−10, −30, −50, −70, and −90 mV) in response to the addition of urate (1 mM urate; 100 mM NaCl, pH 7.5) (Fig. 2A). Additionally, the I-V relationship of the two isoforms of SLC2A9-mediated urate transport was compared with that of glucose (Fig. 2B). Currents evoked by urate (1 mM; 100 mM NaCl, pH 7.5) at potentials between −100 and +60 mV were voltage dependent, with the magnitude of the outward current increasing as the membrane potential became more positive. The I-V curves observed with urate showed a similar voltage dependence to those observed by Bibert et al. (5) for mouse GLUT9a. Measured in the same oocyte, glucose-induced currents (20 mM; 100 mM NaCl, pH 7.5) were minor compared with those of urate and exhibited a slight voltage dependence (Fig. 2B). Since glucose is electrically neutral and transport of glucose mediated by SLC2A9a or SLC2A9b is not coupled to the movement of ions (3), the currents observed in the I-V relationship may be the result of glucose transport mediated by endogenous sodium glucose transporters (39, 40). Urate- and glucose-induced currents were negligible in water-injected oocytes (Fig. 2B), suggesting low levels of endogenous transport activity. These results contrast with those of Bibert et al. (5), who found significant urate-induced currents in water-injected oocytes. Although d-glucose and d-fructose are high-affinity permeants of SLC2A9a, neither inhibits [14C]urate uptake (9). Consistent with this, SLC2A9a and SLC2A9b urate currents were unaffected by high concentrations (5–20 mM) of extracellular d-glucose (data not shown).
Fig. 2.
Voltage dependence of SLC2A9-mediated transport of urate. A: SLC2A9a (filled bars)- or SLC2A9b (open bars)-producing oocytes were voltage clamped sequentially at 5 different holding potentials (−10, −30, −50, −70, and −90 mV), and the maximum current generated in response to the addition of 1 mM urate (100 mM NaCl, pH 7.5) was measured at each potential. Values are means ± SE of 8–12 oocytes from 2 different batches of cells. B: current-voltage (I-V) curves for SLC2A9a (triangles) and SLC2A9b (squares) were generated from the difference between steady-state currents recorded in the presence and absence of 1 mM urate (solid symbols) or in the presence and absence of 20 mM glucose (open symbols) in Na+-containing medium (100 mM NaCl, pH 7.5) upon voltage pulses from holding potentials of −30 mV to final potentials ranging between −100 and +60 mV in 10-mV steps. Urate- and glucose-induced I-V curves were measured in the same SLC2A9-producing oocytes, and data were averaged from 4–5 oocytes from the same batch of cells used on the same day. Urate- and glucose-induced I-V curves were also measured in water-injected oocytes (circles), and data were averaged from 6 oocytes from the same batch of cells used on the same day. Data are representative of those obtained with eggs from 3 different frogs.
Ion dependence of SLC2A9-mediated transport.
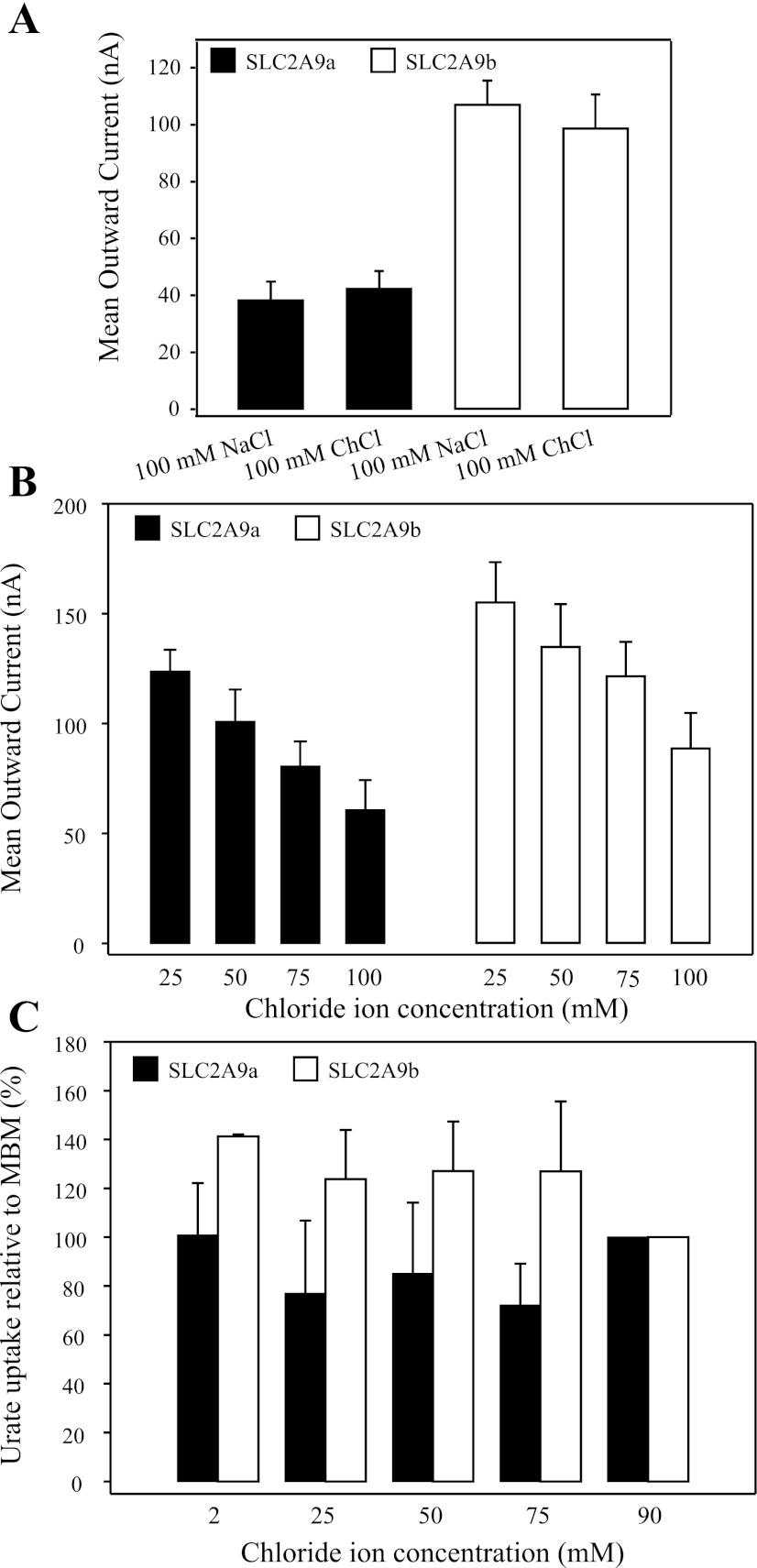
The dependence of SLC2A9-mediated transport of urate on the presence of extracellular Na+ was examined at a holding potential of −30 mV (Fig. 3A). Currents in response to the addition of 1 mM urate were measured in oocytes producing SLC2A9a or SLC2A9b, in the presence (100 mM NaCl, pH 7.5) and absence (100 mM ChCl, pH 7.5) of extracellular Na+. Urate-induced currents were not significantly different in the presence and absence of Na+, demonstrating that the transport of urate by SLC2A9 was not Na+ dependent.
Fig. 3.
Effect of extracellular ions on the transport activity of SLC2A9. A: mean urate (1 mM)-induced currents in oocytes producing SLC2A9a (filled bars) or SLC2A9b (open bars) were measured in transport media containing Na+ (100 mM NaCl, pH 7.5) or choline (100 mM ChCl, pH 7.5) at a membrane potential of −30 mV. Values are means ± SE of 3 different oocytes from the same batch of cells used on the same day. B: mean urate (1 mM)-induced currents in oocytes producing SLC2A9a (filled bars) and SLC2A9b (open bars) were measured in transport media containing a range of Cl− concentrations (25, 50, 75, and 100 mM, pH 7.5). Cl− was replaced with Na+ gluconate to maintain osmotic conditions. Membrane potential was held at −30 mV. Values are means ± SE of 12–15 oocytes from 2 different batches of cells. The currents were not corrected for those in control water-injected cells. C: uptake of 100 μM radiolabeled urate was measured in oocytes producing SLC2A9a (filled bars) and SLC2A9b (open bars) in the presence of varying concentrations of Cl− (2, 25, 50, 75, and 90 mM). Uptake at each Cl− concentration was measured in 12 oocytes, and the experiments were repeated in 3 batches of cells from different frogs. The data are averaged and presented as the % uptake relative to that in normal modified Barth's medium (MBM). Values are corrected for basal nonmediated uptake in control water-injected oocytes.
The effect of extracellular Cl− on SLC2A9-mediated transport of urate was also examined at a holding potential of −30 mV (Fig. 3B). Currents were measured in SLC2A9a- or SLC2A9b-producing oocytes in the presence of a high extracellular Cl− concentration (100 mM NaCl, pH 7.5), intermediate Cl− concentrations (75 mM NaCl+25 mM Na+ gluconate and 50 mM NaCl+50 mM Na+ gluconate, pH 7.5), and with the extracellular Cl− concentration reduced by 75% (25 mM NaCl+75 mM Na+ gluconate, pH 7.5). Decreasing the external Cl− concentration markedly increased the magnitude of the outward currents associated with urate uptake, in both isoforms of SLC2A9, demonstrating that SLC2A9-mediated transport is affected by the extracellular concentration of Cl−. In contrast, Anzai et al. (2) and Bibert et al. (5) found no effect of lowering the external Cl− concentration on the transport of radiolabeled urate by SLC2A9a or its mouse homolog, although uptake was measured over an extended period and, unlike the present electrophysiological study, was not performed under voltage-clamp conditions. Our [14C]urate influx studies, performed on SLC2A9a and SLC2A9b under initial rate conditions, also found no difference in SLC2A9-mediated uptake of urate as the extracellular concentration of Cl− was varied between 2 and 90 mM (Fig. 3C).
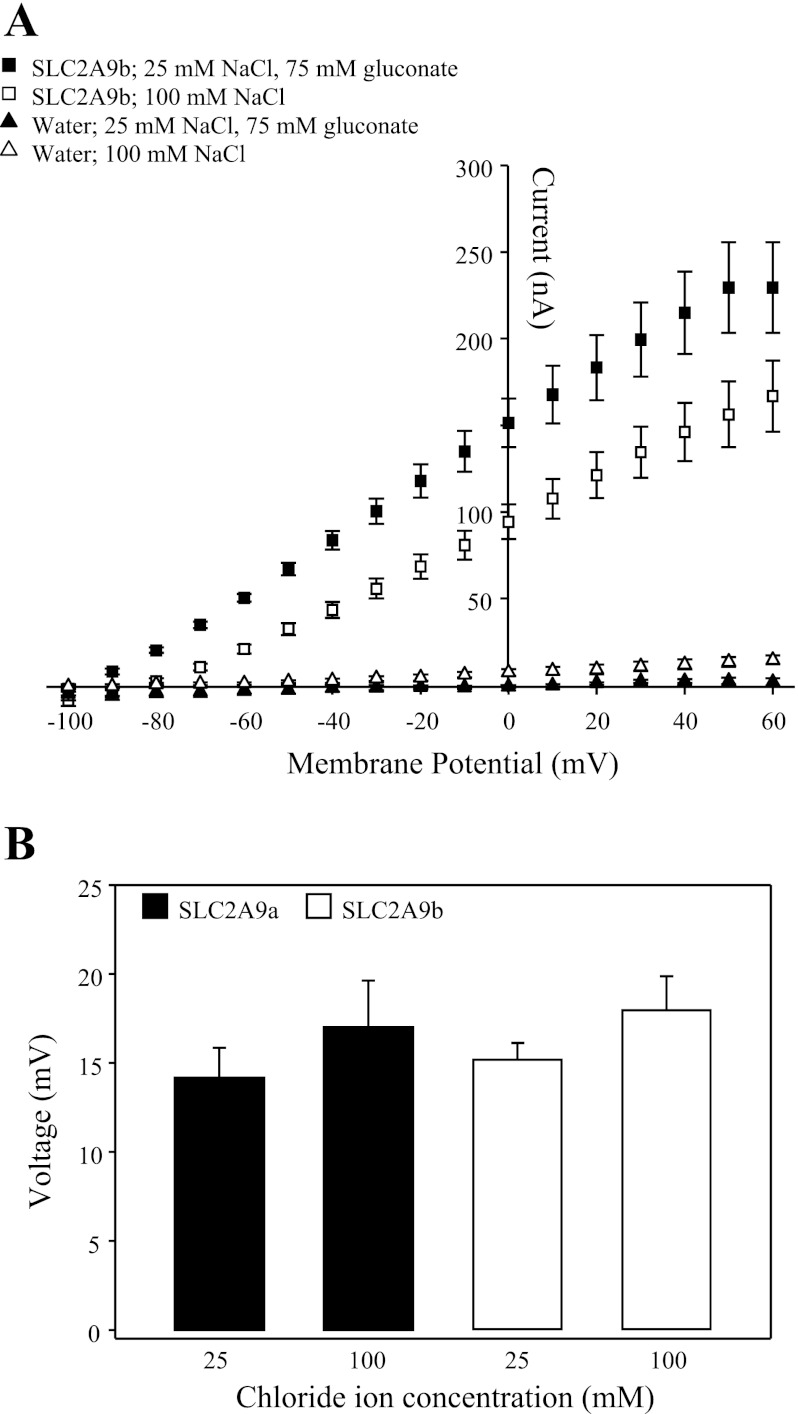
Additionally, the I-V relationship of SLC2A9b-mediated urate transport was measured in the presence of a high extracellular Cl− concentration (100 mM NaCl, pH 7.5) and with the extracellular Cl− concentration reduced (25 mM NaCl+75 mM Na+ gluconate, pH 7.5) (Fig. 4A). Oocytes were clamped at a holding potential of −90 mV to maximize the current difference between the high and reduced Cl− concentrations. Currents evoked by urate (1 mM; pH 7.5) at potentials between −100 and +60 mV were voltage dependent, with the magnitude of the outward current increasing as the membrane potential became more positive. The magnitude of the currents was greater in the presence of a reduced extracellular Cl− concentration (25 mM NaCl+75 mM Na+ gluconate, pH 7.5) than with a high extracellular Cl− concentration present (100 mM NaCl, pH 7.5), consistent with results seen in Fig. 3B. Similar results were seen with SLC2A9a (data not shown). The effect of extracellular Cl− on SLC2A9 membrane voltage changes was also examined for the two isoforms of SLC2A9 (Fig. 4B). Voltage changes were measured in the presence of a high (100 mM NaCl, pH 7.5) and a reduced Cl− concentration (25 mM NaCl+75 mM Na+ gluconate, pH 7.5) in response to the addition of 1 mM urate. No significant difference in membrane voltage was observed with either Cl− condition tested in oocytes producing SLC2A9a or SLC2A9b.
Fig. 4.
Effect of chloride on SLC2A9-mediated transport of urate. A: I-V curves for SLC2A9b were generated from the difference between steady-state currents recorded in the presence and absence of 1 mM urate in medium containing a high Cl− concentration (100 mM NaCl, pH 7.5; □) or in medium in which the Cl− concentration was reduced (25 mM NaCl+75 mM Na+ gluconate, pH 7.5; ■). Membrane voltage was stepped from a holding potential of −90 mV to final potentials ranging between −100 and +60 mV, in 10-mV steps. Urate-induced I-V curves generated in the presence of high and reduced Cl− concentrations were measured in the same SLC2A9b-producing oocytes, and data are averaged from 7 oocytes from the same batch of cells used on the same day. Similar results were seen with SLC2A9a-producing oocytes (data not shown). I-V curves were also measured in water-injected oocytes (triangles), and data are averaged from 7 cells used on the same day. B: mean urate (1 mM)-induced voltage changes in oocytes producing SLC2A9a (filled bars) or SLC2A9b (open bars) were measured in medium containing a high (100 mM NaCl, pH 7.5) or reduced (25 mM NaCl+75 mM Na+ gluconate, pH 7.5) Cl− concentration. Values are means ± SE of 7–9 different oocytes. The data are representative of 2 separate experiments. Voltage changes were not observed in water-injected oocytes (data not shown).
Further studies examined the effect of other anion substitutions on SLC2A9-mediated currents. Currents produced following the addition of urate (1 mM) to SLC2A9a- and SLC2A9b-producing oocytes were measured in media in which the Cl− concentration was reduced by 90% and replaced by I− (90 mM NaI+10 mM NaCl, pH 7.5), and in media in which Cl− was completely removed and replaced by methyl sulfate (100 mM Na+ methyl sulfate, pH 7.5). Significant currents were observed with SLC2A9a and SLC2A9b in the NaI and Na+ methyl sulfate media but were less than those seen in the presence of high (100 mM NaCl, pH 7.5) and gluconate-reduced Cl− concentrations (25 mM NaCl+75 mM Na+ gluconate, pH 7.5).
Anzai et al. (2) and Bibert et al. (5) concluded that SLC2A9 does not have an exchange mechanism for inorganic Cl−. Our observations support the conclusion that Cl− is not required for SLC2A9-mediated urate transport and instead suggest that Cl−, and other anions, are inhibitory, possibly through negative-charge competition with urate for binding to the transporter. The lack of effect of Cl− observed with [14C]urate influx vs. the inhibition seen with electrophysiology studies is due to differences in the experimental techniques. In voltage-clamped oocytes, urate-induced currents decrease when the membrane potential is hyperpolarized (Fig. 2A). In [14C]urate influx experiments, the membrane potential is hyperpolarized as [14C]urate enters the cell (data not shown). The effect of Cl− on [14C]urate uptake is therefore the combination of both an increased influx of urate due to decreased Cl− concentration and a decreased influx of urate due to membrane hyperpolarization. As a result, decreasing the external Cl− concentration (Fig. 3C) shows no net effect on extracellular [14C]urate uptake. In anion substitution experiments, reduced but significant currents were observed in solutions containing I− (90 mM NaI+10 mM NaCl) and when the Cl− concentration was reduced to 0 mM (100 mM Na+ methyl sulfate), further confirming that transport mediated by SLC2A9 does not require Cl−. This finding, combined with the observation that transport mediated by SLC2A9 is stimulated when the Cl− ion concentration is reduced (25 mM NaCl+75 mM Na+ gluconate), confirms that anions exhibit charge competition with urate for binding to the transporter.
Urate influx kinetics under voltage-clamp conditions.
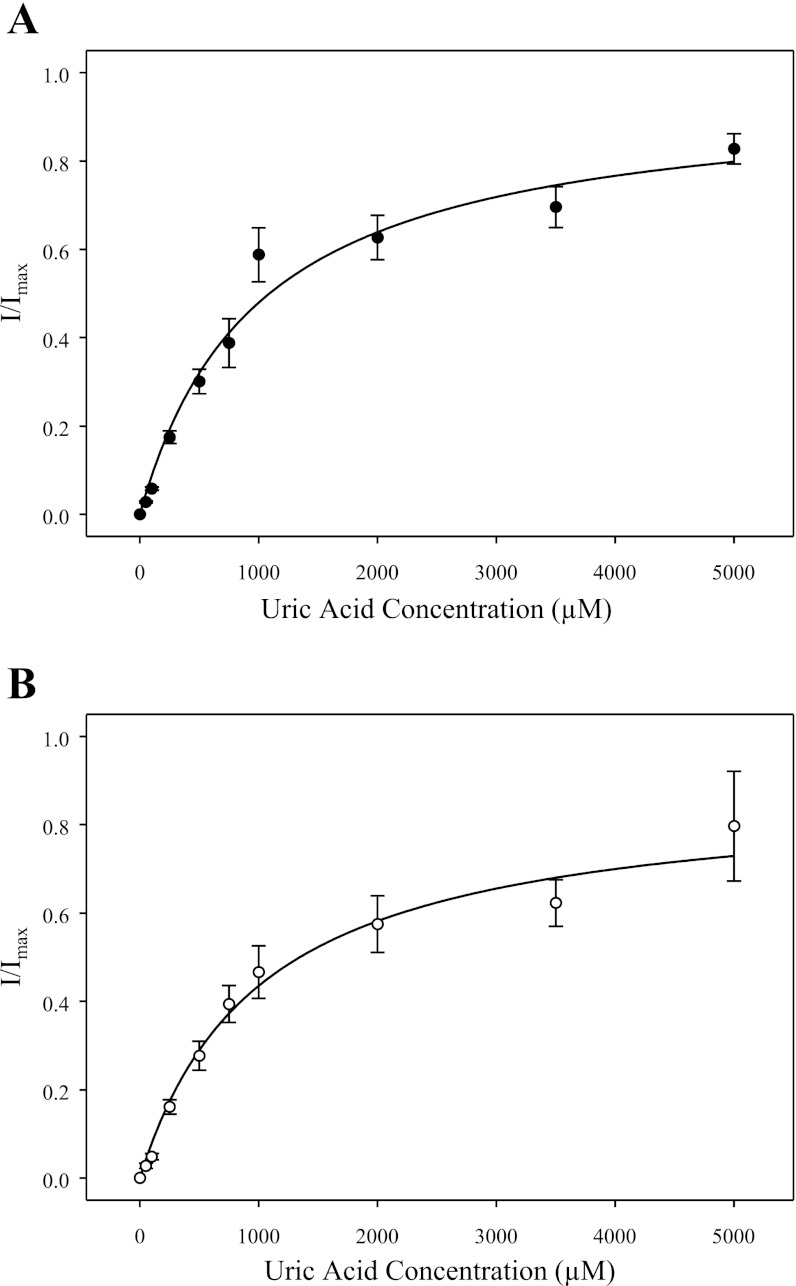
Figure 5 shows the dependence of SLC2A9-mediated currents on the concentration of extracellular urate (0–5 mM; 100 mM NaCl, pH 7.5). Oocytes producing SLC2A9a (Fig. 5A) or SLC2A9b (Fig. 5B) were voltage clamped at a holding potential of −30 mV, and the currents generated in response to increasing urate concentrations were measured. For both isoforms, currents were saturable and consistent with simple Michaelis-Menten kinetics. For SLC2A9a, the apparent Km value was 1.0 ± 0.15 mM (Fig. 5A), while for SLC2A9b the apparent Km value was 1.0 ± 0.20 mM (Fig. 5B). At 5 mM urate, the corresponding small inward current in control water-injected oocytes was <25 nA. These Km values are similar to those determined by radiotracer flux experiments in unclamped oocytes (∼1 mM for both isoforms) (Ref. 20; data not shown). The large Imax observed for both isoforms (150–200 nA for SLC2A9a and 175–250 nA for SLC2A9b) confirms by electrophysiology that SLC2A9 is a high-capacity urate transporter.
Fig. 5.
Urate influx kinetics of SLC2A9. The dependence of SLC2A9a- and SLC2A9b-mediated currents on the external concentration of urate (0–5 mM) was measured at a membrane potential of −30 mV (100 mM NaCl, pH 7.5). SLC2A9a and SLC2A9b currents are averaged from concentration-dependent relationships for 7 different oocytes, where currents at each urate concentration were normalized to the fitted predicted current maximum (Imax) for that oocyte. The currents were not corrected for those in control water-injected cells. A: SLC2A9a-producing oocytes: Km = 1.0 ± 0.15 mM. B: SLC2A9b-producing oocytes: Km = 1.0 ± 0.20 mM. Individual Imax values were in the range 150–200 nA for SLC2A9a-producing oocytes and 175–250 nA for SLC2A9b- producing oocytes. Data are representative of 3 experiments.
SLC2A9 has symmetrical affinity and capacity for urate efflux.
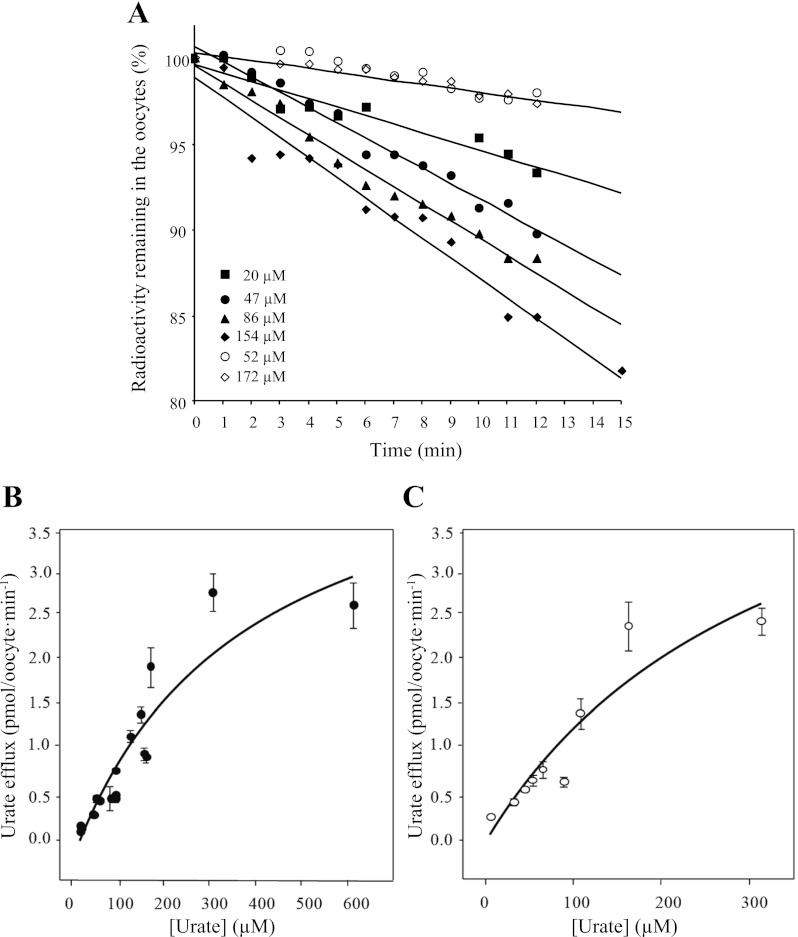
To determine whether SLC2A9-mediated fluxes of urate are symmetrical or vectorial in nature, we compared the kinetics for [14C]urate efflux to those of influx. Since the apparent affinity (Km ∼1 mM) and capacity (Vmax ∼15 pmol·oocyte−1·min−1) of the transporter for [14C]urate influx have previously been established (9), we determined the corresponding kinetics of urate efflux. Following injection of oocytes with varying amounts of [14C]urate, individual concentrations of intracellular urate at time 0 ranged between 17 and 624 μM for SLC2A9a and 39 and 495 μM for SLC2A9b. The low solubility of urate and maximum injectable volume set an upper limit to the intracellular concentrations of urate that could be achieved. As shown for SLC2A9a in Fig. 6A, efflux at each urate concentration was plotted against time and fitted with a straight line to determine the initial rate of efflux. The experiments were also repeated in control water-injected oocytes (Fig. 6A). Figure 7B shows the Michaelis-Menten fit for the initial rates of SLC2A9a-mediated urate efflux plotted against the starting intracellular urate concentration, and Fig. 6C shows the corresponding data for SLC2A9b. Efflux rates for the two isoforms are similar and appear to be saturable. Projected apparent Km values were outside the concentration studied, but were ∼1 mM for both transporter isoforms and of the same magnitude as those seen for SLC2A9a and SLC2A9b urate influx measured either electrophysiologically (Fig. 5) or using [14C]urate (Ref. 9; data not shown). Efflux of [14C]urate from control water-injected oocytes was minimal, suggesting that endogenous transporters did not contribute to urate efflux.
Fig. 6.
Urate efflux kinetics of SLC2A9. A: representative results demonstrating [14C]urate efflux from [14C]urate- preloaded SLC2A9-producing oocytes (filled symbols) and from water-injected oocytes (open symbols). Magnitudes of the slopes of the lines (±SE), indicating the rates of intracellular urate efflux (% efflux/min) for each concentration, were water-injected oocytes, 0.26 ± 0.06 (52 μM) and 0.21 ± 0.06 (172 μM); SLC2A9a-producing oocytes, 0.46 ± 0.06 (20 μM), 0.89 ± 0.06 (47 μM), 0.92 ± 0.06 (86 μM), and 1.10 ± 0.06 (154 μM). Only low and high intracellular urate concentrations for water-injected oocytes are shown for clarity. B: initial rates of [14C]urate efflux for SLC2A9a-producing oocytes in units of pmol·oocyte−1·min−1 are plotted against the starting internal urate concentration. C: corresponding data for [14C]urate efflux in SLC2A9b-producing oocytes. Fitted apparent Km values were ∼1 mM for both transporter isoforms. Data are representative of 4–6 independent experiments.
Fig. 7.
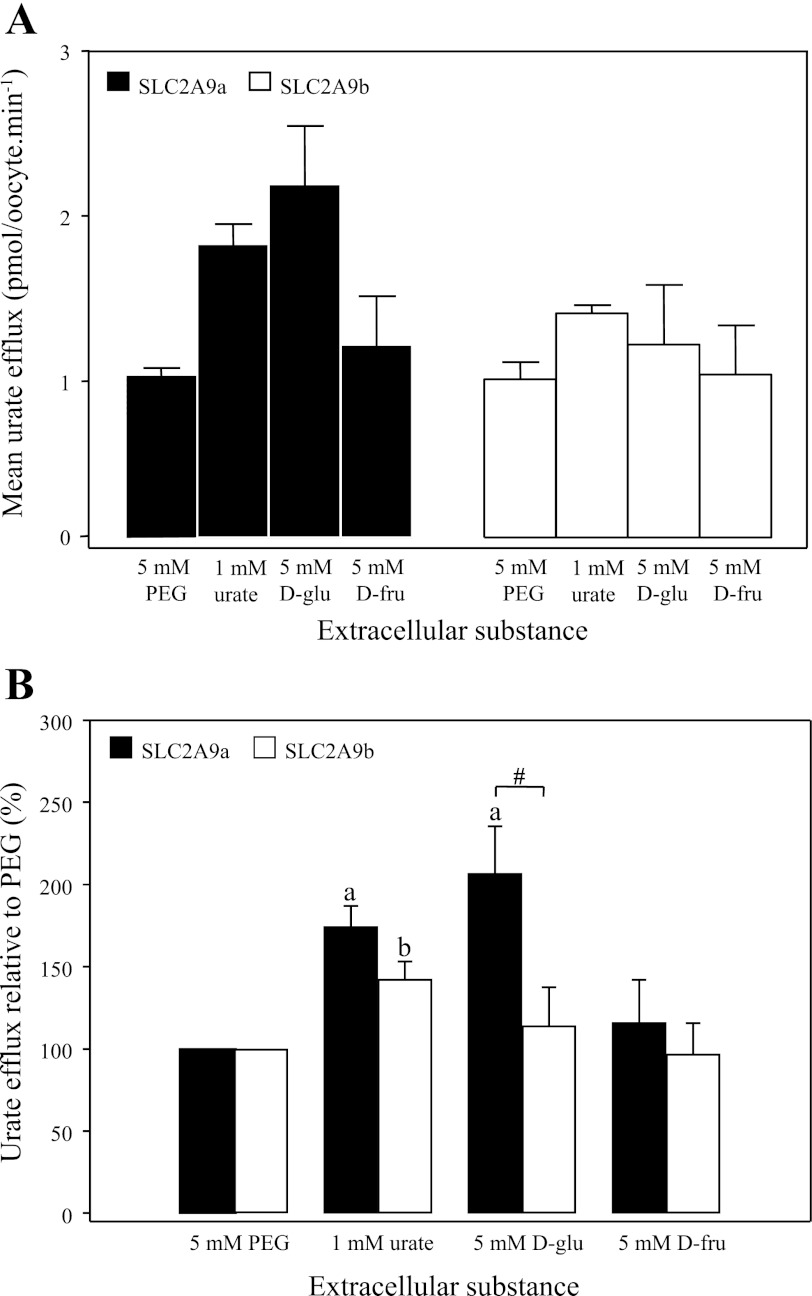
Effect of extracellular substances on SLC2A9-mediated urate efflux. SLC2A9a (filled bars)- and SLC2A9b (open bars)-producing oocytes were preloaded with [14C]urate, and the rate of urate efflux was measured in the presence of different substances added to MBM. Values are means of urate efflux ± SE of 20 oocytes/condition from 3–6 batches of cells used on the same day. Efflux rates were corrected for variations in intracellular [14C]urate concentrations. A: mean [14C]urate efflux rates in pmol·oocyte−1·min−1. B: mean [14C]urate efflux rates expressed as % efflux compared with osmotic control (5 mM PEG). Data are averaged from 6 experiments. a and b: Statistically significant urate efflux values from the control (5 mM PEG) for SLC2A9a- and SLC2A9b-producing oocytes, respectively (P ≤ 0.01). #Statistical significance (P ≤ 0.05) between SLC2A9a and SLC2A9b isoforms in response to extracellular 5 mM d-glucose.
Effect of extracellular substances on SLC2A9-mediated urate efflux.
We have demonstrated previously that both d-glucose and d-fructose, which have been shown to be high-affinity permeants for SLC2A9a (11), are not competitive inhibitors of urate uptake. However, both of these hexoses can accelerate urate efflux mediated by SLC2A9a when placed in the extracellular medium, suggesting that facilitated movement of both urate and hexoses is likely mediated by the same carrier (9). In the present study, we extended this experiment to include the effects of extracellular hexoses on both SLC2A9a- and SLC2A9b-mediated efflux to compare the two isoforms, as well as tested the effect of extracellular urate on urate efflux mediated by the two isoforms. The next section also presents complementary new findings for the trans effects of urate and sugars on [14C]urate influx.
For each condition, SLC2A9a- or SLC2A9b-producing oocytes were preloaded with [14C]urate to achieve final, intracellular permeant concentrations in the micromolar range. Urate efflux was measured over 14 min, at 2-min intervals. Initial rates of urate efflux obtained from the linear fits of efflux data were corrected for variations in intracellular urate concentrations and normalized to urate efflux under osmotic control conditions (5 mM PEG).
SLC2A9a-mediated urate efflux (Fig. 7B) was significantly accelerated in the presence of excess extracellular urate (1 mM) and d-glucose (5 mM), while d-fructose produced small, but insignificant acceleration. Figure 7A provides average urate efflux rates for both isoforms, while Fig. 7B expresses the same data as a percentage of control to allow for comparison of effects of extracellular solvents on SLC2A9a and SLC2A9b. These results are consistent with the effect of extracellular hexoses previously reported by our group (9). SLC2A9b-mediated urate efflux (Fig. 7B), on the other hand, was significantly accelerated only in the presence of 1 mM extracellular urate. Although SLC2A9b repeatedly showed decreased acceleration of urate efflux in the presence of excess trans urate, compared with SLC2A9a, this difference did not reach statistical significance. Both SLC2A9 isoforms therefore function as nonobligatory urate-urate exchangers, SLC2A9a, but not SLC2A9b, also exhibiting trans-acceleration by physiological levels of extracellular d-glucose.
Effect of intracellular substances on SLC2A9-mediated urate influx.
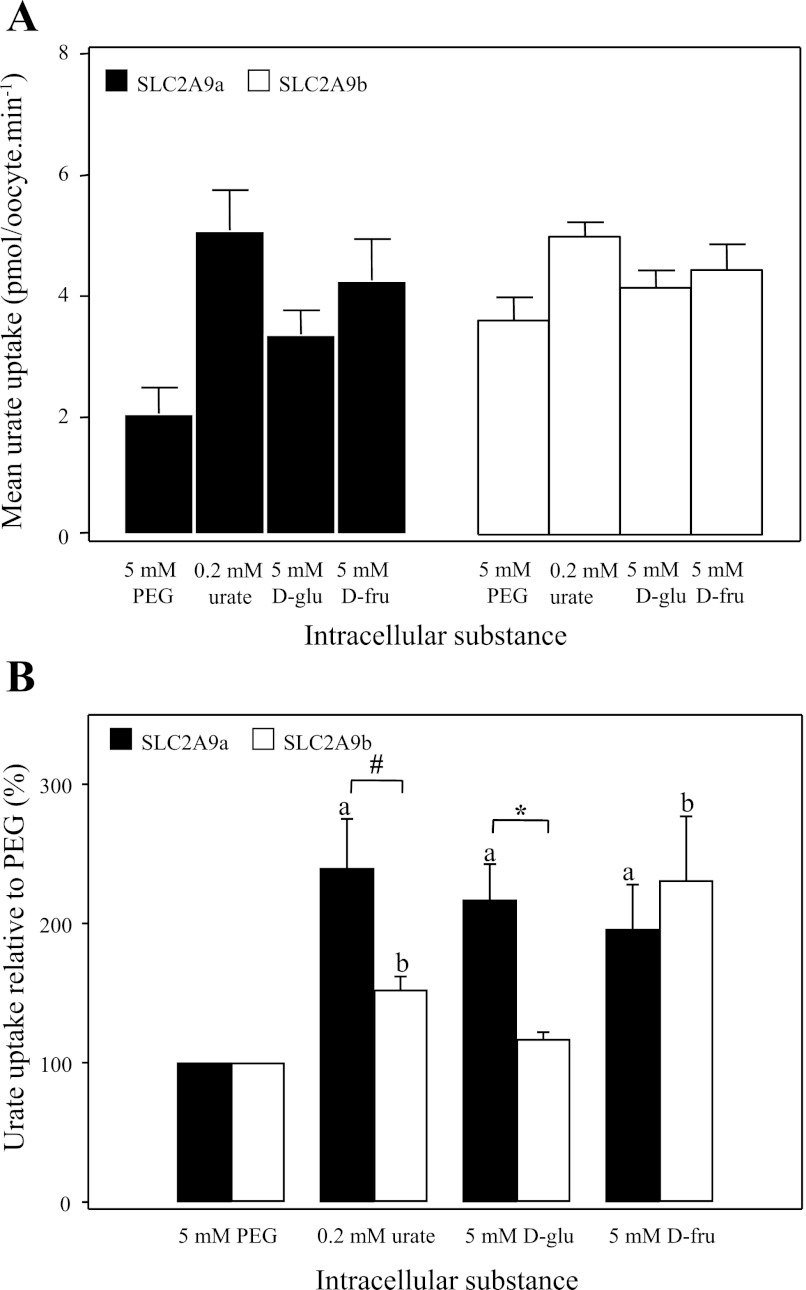
To further investigate the possible effects of permeant hexoses on SLC2A9-mediated urate uptake, we preloaded SLC2A9a- or SLC2A9b-producing oocytes with various substances at a final intracellular concentration of 5 mM (unless otherwise stated) and measured [14C]urate influx (Fig. 8). Similar to Fig. 7, Fig. 8A provides average urate uptake rates for both isoforms, while Fig. 8B expresses the same data as the percentage of control to allow for comparison of effects of intracellular permeants on SLC2A9a- and SLC2A9b-mediated transport. Uptake data were normalized to the osmotic control (5 mM PEG) for each experiment. All substances tested produced a significant increase in urate uptake in the case of SLC2A9a-producing oocytes. SLC2A9b-producing oocytes showed similar responses except that intracellular d-glucose was without effect. In other experiments, the nonmetabolized d-glucose analogs 2DOG and 3OMG increased urate uptake into oocytes producing SLC2A9a but not SLC2A9b (data not shown). Significant differences between the two isoforms were also observed in response to intracellular urate and d-glucose, with SLC2A9a's response to these intracellular substances being significantly greater than that of SLC2A9b. The results also reveal interesting differences in permeant specificity between the exofacial and endofacial binding sites for hexoses. Specifically, while extracellular d-fructose was a poor accelerator of urate efflux in both SLC2A9a- and SLC2A9b-producing oocytes (Fig. 7B), it significantly increased urate uptake by both SLC2A9 isoforms when placed inside the cell (Fig. 8B).
Fig. 8.
Effect of intracellular substances on SLC2A9-mediated urate influx. SLC2A9a (filled bars)- and SLC2A9b (open bars)-producing oocytes were preloaded with different cold substances, at a final intracellular concentration of 5 mM, unless otherwise stated, and 100 μM [14C]urate uptake was measured. Values are means of urate influx± SE of 10 oocytes/condition from 3–6 batches of cells used on the same day. A: mean [14C]urate uptake rates in pmol·oocyte−1·min−1. B: mean [14C]urate uptake expressed as % urate uptake compared with osmotic control (5 mM PEG). Means represent averaged data from 4 experiments. a and b: Statistically significant urate influx values from the control (5 mM PEG) for SLC2A9a- and SLC2A9b-producing oocytes, respectively (P ≤ 0.01). #Statistical significance (P ≤ 0.05) between SLC2A9a and SLC2A9b isoforms in response to intracellular urate. *Statistical significance (P ≤ 0.01) between the two isoforms in response to intracellular d-glucose.
Nucleobase transport mediated by SLC2A9.
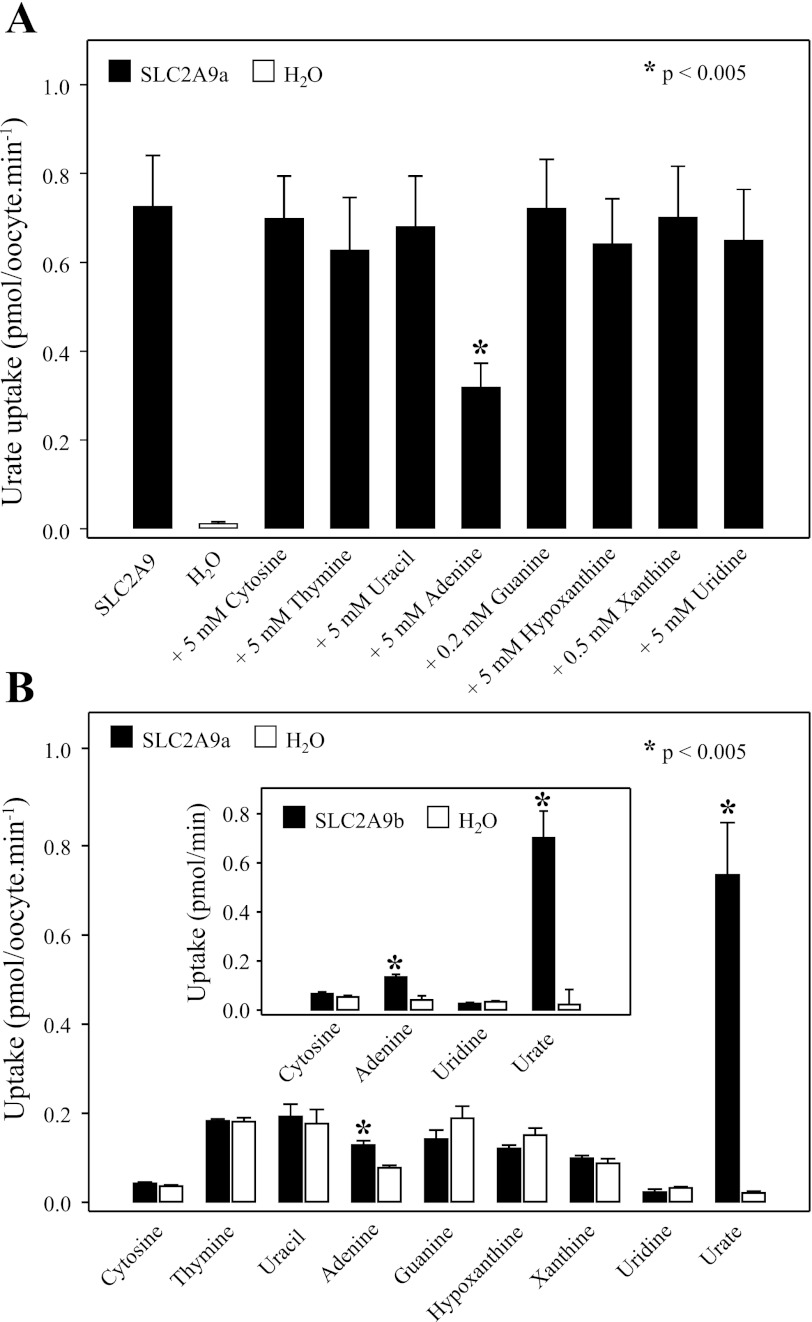
The pathways for nucleobase transport in mammalian cells have not been fully characterized, and no human transporter specific for nucleobases has been cloned. Since SLC2A9 has been shown to be a high-capacity urate transporter (9), and since urate is a negatively charged purine nucleobase, we have tested the hypothesis that SLC2A9 may also transport other nucleobases. In the cross-inhibition experiment in X. laevis oocytes shown in Fig. 9A, SLC2A9a-mediated urate transport (20 μM; 100 mM NaCl, pH 7.5; 20-min flux) was measured in the absence (control) or presence of excess (0.2–5 mM) pyrimidine (uracil, thymine, and cytosine) or purine (adenine, guanine, hypoxanthine, and xanthine) nucleobases or the pyrimidine nucleoside uridine (5 mM). Mediated transport of urate (uptake in RNA transcript-injected oocytes minus uptake in water-injected oocytes) was inhibited 55% by excess (5 mM) unlabeled adenine but was unaffected by any other nucleobases tested, or by uridine. The finding that SLC2A9a-mediated urate transport was inhibited by adenine suggests that adenine may be an SLC2A9 permeant.
Fig. 9.
Nucleobase transport mediated by SLC2A9. A: inhibition of SLC2A9a-mediated urate influx by pyrimidine and purine nucleobases and the pyrimidine nucleoside uridine. Urate (20 μM; 100 mM NaCl, pH 7.5) influx was measured in SLC2A9a-producing oocytes in the absence (control) or presence of excess nonradioactive nucleobases or uridine. Because of their low solubility, guanine and xanthine were added at concentrations of 0.2 and 0.5 mM, respectively. Other nucleobases and uridine were tested at a concentration of 5 mM. Values were corrected for basal nonmediated uptake in control water-injected oocytes and are means ± SE of 10–12 oocytes. H2O, water-injected oocytes. B: uptake of 20 μM radiolabeled pyrimidine and purine nucleobases (20 μM) and the pyrimidine nucleoside uridine (20 μM) were measured in oocytes producing SLC2A9a (filled bars) or in control water-injected oocytes (open bars) and compared with the uptake of urate (20 μM). Values are means ± SE of 10–12 oocytes. Inset: the same experiment repeated in oocytes producing SLC2A9b. Data are representative of those obtained in 2 or more experiments.
Transport inhibition can occur in the absence of translocation of the inhibiting substance, and low-affinity permeants may not cause detectable cross-inhibition. The ability of SLC2A9a or SLC2A9b to transport nucleobases and uridine was therefore also measured directly with a panel of radiolabeled nucleobases and uridine. Figure 9B shows the uptake of nucleobases (20 μM) and uridine (20 μM) compared with urate in SLC2A9a-producing oocytes and in control water-injected cells. SLC2A9a transported urate at high levels, and, consistent with the inhibition profile shown in Fig. 9A, there was also a small but significant amount of adenine transport (mediated fluxes of 0.71 ± 0.11 and 0.051 ± 0.010 pmol·oocyte−1·min−1 for urate and adenine, respectively). No uptake was observed with any other nucleobases tested, or with uridine. Corresponding data for adenine, uridine, and cytosine transport by SLC2A9b in Fig. 9B (inset) showed a similar pattern. Therefore, both SLC2A9 isoforms are not entirely specific for urate and mediate a small but significant amount of adenine uptake. However, SLC2A9 does not transport other purine (or pyrimidine) nucleobases. Functioning as a high-capacity urate transporter, SLC2A9 is not a major route for the entry of other nucleobases into cells.
DISCUSSION
In this paper, we have examined the membrane currents associated with urate fluxes mediated by human SLC2A9a and SLC2A9b produced in X. laevis oocytes. The uptake of the organic anion urate generated a membrane potential-sensitive outward current, the simplest interpretation of which is that urate enters the cell in its charged form with no coupled cation or anion movement, which confirms previous observations of mouse full-length SLC2A9 being an electrogenic uniporter of urate (5, 31). In corresponding radiotracer flux studies under non-voltage-clamped conditions, we have also found that the apparent Km for urate efflux is equal to or greater than that for uptake (∼1 mM). Rates of urate influx and efflux are also similar, which suggests that the driving force of the membrane potential would favor urate efflux over influx under physiological conditions. Uptake of urate mediated by SLC2A9 appears to be affected by the extracellular Cl− concentration, but there is no evidence for the transporter to be exchanging these two anions. The ability for this transporter to also mediate the uptake of adenine suggests that this may provide an additional route of uptake for this nucleobase. However, SLC2A9 does not function as a generalized nucleobase transporter, and the fluxes of adenine relative to urate are small. SLC2A9a is expressed in the basolateral membrane of hepatocytes, where the majority of urate is generated, and in the basolateral membrane of the kidney proximal convoluted tubule. In both sites, the membrane potential would promote the efflux of the organic anion into the plasma, helping to maintain the high circulating levels of this metabolite and providing a plausible mechanism for the contribution of SLC2A9 SNPs to hyperuricemia (2, 8, 11, 21, 22, 27, 35, 37, 38).
In other species, which have an active uricase in the liver, SLC2A9 would also appear to play a major role in urate metabolism. The constant removal of urate from the liver cytoplasm will normally result in a net electrochemical gradient from the blood plasma into the hepatocytes. Thus, in the case of the Dalmatian, which has a mutation in SLC2A9 but a functional uricase in the liver (4), the hyperuricemia seen in this breed could be explained partially by a reduced uptake of urate into the hepatocytes; however, the complex interactions involving renal secretion mediated by SLC2A9b and the uptake across the BLM via SLC2A9a would also have to be taken into consideration. Consequently, for each species the ultimate direction of flux facilitated by basolateral SLC2A9 in both the liver and the kidneys will be determined by local electrochemical gradients across the cell membranes in question.
In contrast, the renal secretory route for urate in humans is still under debate. Recently, a sodium-phosphate transporter 4 (SLC17A3/NTP4) has been implicated in urate secretion. According to Jutabha et al. (19), two missense mutations of this protein decreased urate transport in oocytes and were identified to cause hyperuricemia in Japanese patients. They have demonstrated SLC17A3 to be voltage dependent and capable of mediating urate movement across the membrane, along with many other organic anions. Moreover, a number of diuretics, which have been shown to cause hyperuricemia, were shown to interact with SLC17A3 by inhibiting PAH uptake. Given the above observations, the authors proposed that the urate secretory route is defined by a host of multispecific and drug-sensitive transporters, wherein apical SLC17A3 is thought to be one of the key players. They did not discuss the potential contribution of SLC2A9b (ΔN), which is suggested to be expressed in the apical membrane of human proximal convoluted tubule epithelium (3), has high specificity for urate, is driven by membrane potential, and, as such, is the likely apical component of the urate secretory route. A thorough comparison of SLC2A9a and SLC2A9b in the present study has revealed that although both isoforms act as functionally symmetric uniporters of urate, where the membrane potential drives urate efflux across both poles of the cell, there are fundamental differences in the way these two splice variants respond to intracellular and extracellular monosaccharides. The effect of physiological levels of d-glucose on urate efflux seems to be more pronounced than that of d-fructose, which may explain the frequently observed association between hyperuricemia, hyperfructosemia, and metabolic syndrome (27). Interestingly, there are also marked differences in the SLC2A9's isoform sensitivity to extracellular d-glucose, with the effect of trans-stimulation of urate efflux by d-glucose being lost in SLC2A9b, the apical isoform. This observation has two implications. First, this differential response, combined with the uniform presence of extracellular d-glucose in the lumen and in the bloodstream, suggests that functional symmetry of the two isoforms is lost, favoring the basolateral efflux of urate via SLC2A9a. Second, these results point to the potential, novel role of the N terminus of SLC2A9 in modulating the handling of urate and/or hexoses. How the presence or absence of the first 28 amino acids of the protein confers this difference is unclear; however, Ser-9 on SLC2A9a has been identified as a high-probability residue for phosphorylation by PKA (Ref. 6, K. Witkowska et al., unpublished observations). Whether kinase activity at this site changes the hexose binding profile of the transporter or its interaction with other intracellular binding proteins is yet to be determined. d-Fructose also produced a differential response in urate handling, stimulating urate uptake when placed inside the cell, and failing to significantly affect urate efflux when placed outside of the cell. In this case, both isoforms behaved in a similar manner, suggesting that there is asymmetry in permeant interaction between the exofacial and endofacial binding sites of the transporter, which is independent of the N terminus. Finally, these subtle changes in SLC2A9 isoform-specific responses to hexoses may be cell specific and may also involve the interaction of the transporter with other accessory proteins. For example, SLC2A9/GLUT9 knockout mice (31) do not display any abnormalities in glucose or fructose metabolism perhaps because of the distal expression of the transporter in the mouse renal tubule epithelium, in contrast to the more proximal distribution in humans. Moreover, hyperuricemia has also been correlated with SNPs in PDZK1, a scaffold protein capable of direct and indirect interaction with membrane proteins. Although SLC2A9 does not possess PDZ binding motifs (K. Witkowska et al., unpublished observations), it may interact with transcription factors or signaling messengers, which are modulated by PDZK1.
In summary, the unique expression pattern of SLC2A9 in the human kidney proximal convoluted tubule and its electrogenic transport of urate strongly suggest that this transporter plays a key role in the maintenance of elevated plasma urate levels in humans and is therefore a potentially important transport protein in an investigation of diseases associated with hyperuricemia and hypouricemia. Given the novel finding that the two isoforms of SLC2A9 display differential urate handling profiles in response to hexoses, new light is shed on potential mechanisms of urate handling in the human kidney epithelium and possible new avenues for drug targeting in treatment of hyperuricemia and hypouricemia.
GRANTS
This work was supported by the Canadian Institutes of Health Research. K. Witkowsi was funded by a Queen Elizabeth II PhD Scholarship and an Alberta Diabetes Institute Studentship. J. D. Young is a Senior Investigator of the Alberta Heritage Foundation for Medical Research.
AUTHOR CONTRIBUTIONS
Author contributions: K.W., K.M.S., S.Y.M.Y., E.K., J.D.Y., and C.I.C. provided conception and design of research; K.W., K.M.S., S.Y.M.Y., A.M.N., and D.O. performed experiments; K.W., K.M.S., S.Y.M.Y., and D.O. analyzed data; K.W., K.M.S., S.Y.M.Y., E.K., J.D.Y., and C.I.C. interpreted results of experiments; K.W., K.M.S., and S.Y.M.Y. prepared figures; K.W. drafted manuscript; K.W., E.K., J.D.Y., and C.I.C. edited and revised manuscript; J.D.Y. and C.I.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kelle Moley for the generous gift of SLC2A9a and SLC2A9b cDNA.
REFERENCES
- 1. Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol 19: 151–157, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem 283: 26834–26838, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem 279: 16229–16236, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bannasch D, Safra N, Young A, Karmi N, Schaible RS, Ling GV. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet 4: e1000246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bibert S, Hess SK, Firsov D, Thorens B, Geering K, Horisberger JD, Bonny O. Mouse GLUT9: evidences for a urate uniporter. Am J Physiol Renal Physiol 297: F612–F619, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31: 318–321, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brandstatter A, Kiechl S, Kollerits B, Hunt SC, Heid IM, Coassin S, Willeit J, Adams TD, Illig T, Hopkins PN, Kronenberg F. Sex-specific association of the putative fructose transporter SLC2A9 variants with uric acid levels is modified by BMI. Diabetes Care 31: 1662–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caulfield MJ, Munroe PB, O'Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 5: e197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dehghan A, Kottgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet 372: 1953–1961, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab 297: E831–E835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, Paulweber B, Pfeufer A, Rosskopf D, Volzke H, Illig T, Meitinger T, Wichmann HE, Meisinger C. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40: 430–436, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Endou H, Anzai N. Urate transport across the apical membrane of renal proximal tubules. Nucleosides Nucleotides Nucleic Acids 27: 578–584, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Feig DI, Kang DH, Nakagawa T, Mazzali M, Johnson RJ. Uric acid and hypertension. Curr Hypertens Rep 8: 111–115, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology (Bethesda) 20: 125–133, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Hjortnaes J, Algra A, Olijhoek J, Huisman M, Jacobs J, van der Graaf Y, Visseren F. Serum uric acid levels and risk for vascular diseases in patients with metabolic syndrome. J Rheumatol 34: 1882–1887, 2007 [PubMed] [Google Scholar]
- 18. Johnson RJ, Sautin YY, Oliver WJ, Roncal C, Mu W, Gabriela Sanchez-Lozada L, Rodriguez-Iturbe B, Nakagawa T, Benner SA. Lessons from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in Western society? J Comp Physiol B 179: 67–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jutabha P, Anzai N, Kimura T, Taniguchi A, Urano W, Yamanaka H, Endou H, Sakurai H. Functional analysis of human sodium-phosphate transporter 4 (NPT4/SLC17A3) polymorphisms. J Pharmacol Sci 115: 249–253, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Jutabha P, Kanai Y, Hosoyamada M, Chairoungdua A, Kim DK, Iribe Y, Babu E, Kim JY, Anzai N, Chatsudthipong V, Endou H. Identification of a novel voltage driven organic anion transporter present at apical membrane of renal proximal tubule. J Biol Chem 278: 27930–27938, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H; Consortium EUROSPAN. Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI; Consortium ENGAGE. Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Völker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H; Consortium PROCARDIS. Doering A, Wichmann HE; Study KORA. Spector TD, Peltonen L, Völzke H, Nagaraja R, Vollenweider P, Caulfield M; WTCCC. Illig T, Gieger C. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5: e1000504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kusuhara H, Sugiyama Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokinet 24: 37–52, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Le MT, Shafiu M, Mu W, Johnson RJ. SLC2A9—a fructose transporter identified as a novel uric acid transporter. Nephrol Dial Transplant 23: 2746–2749, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leal-Pinto E, Tao W, Rappaport J, Richardson M, Knorr BA, Abramson RG. Molecular cloning and functional reconstitution of a urate transporter/channel. J Biol Chem 272: 617–625, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861–871, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Marangella M. Uric acid elimination in the urine. Pathophysiological implications. Contrib Nephrol 147: 132–148, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, Nakamura T, Morimoto Y, Kamakura K, Sakurai Y, Nonoyama S, Kanai Y, Shinomiya N. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 83: 744–751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller A, Adeli K. Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol 24: 204–209, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Onat A, Uyarel H, Hergenc G, Karabulut A, Albayrak S, Sari I, Yazici M, Keles I. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am J Hypertens 19: 1055–1062, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Panoulas VF, Milionis HJ, Douglas KM, Nightingale P, Kita MD, Klocke R, Elisaf MS, Kitas GD. Association of serum uric acid with cardiovascular disease in rheumatoid arthritis. Rheumatology (Oxford) 46: 1466–1470, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Preitner F, Bonny O, Laverriere A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA 106: 15501–15506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rathmann W, Haastert B, Icks A, Giani G, Roseman JM. Ten-year change in serum uric acid and its relation to changes in other metabolic risk factors in young black and white adults: the CARDIA study. Eur J Epidemiol 22: 439–445, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27: 608–619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shayeghi M, Akerman R, Jarvis SM. Nucleobase transport in opossum kidney epithelial cells and Xenopus laevis oocytes: the characterisation, structure-activity relationship of uracil analogues and oocyte expression studies of sodium-dependent and -independent hypoxanthine uptake. Biochim Biophys Acta 1416: 109–118, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Stark K, Reinhard W, Neureuther K, Wiedmann S, Sedlacek K, Baessler A, Fischer M, Weber S, Kaess B, Erdmann J, Schunkert H, Hengstenberg C. Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS One 3: e1948, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol 288: F327–F333, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Klocic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Goroni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40: 437–442, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marcano AC, Lathrop GM, Webster J, Farrall M, Spector T, Samani NJ, Caulfield MJ, Munroe PB. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82: 139–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber WM, Schwarz W, Passow H. Endogenous D-glucose transport in oocytes of Xenopus laevis. J Membr Biol 111: 93–102, 1989 [DOI] [PubMed] [Google Scholar]
- 40. Weber WM, Puschel B, Steffgen J, Koepsell H, Schwarz W. Comparison of a Na+/D-glucose cotransporter from rat intestine expressed in oocytes of Xenopus laevis with the endogenous cotransporter. Biochim Biophys Acta 1063: 73–80, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Woodward OM, Kötgen A, Coresh J, Boerwinkle E, Guggino WB, Kögen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. PNAS 106: 10338–10342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yao SYM, Cass CE, Young JD. The Xenopus oocyte expression system for the cDNA cloning and characterization of plasma membrane transport proteins. In: Membrane Transport: A Practical Approach, edited by Baldwin SA. New York: Oxford University Press, p. 47–78, 2000 [Google Scholar]
- 43. Yu XQ, Xue CC, Wang G, Zhou SF. Multidrug resistance associated proteins as determining factors of pharmacokinetics and pharmacodynamics of drugs. Curr Drug Metab 8: 787–802, 2007 [DOI] [PubMed] [Google Scholar]