Abstract
Cachexia, the metabolic dysregulation leading to sustained loss of muscle and adipose tissue, is a devastating complication of cancer and other chronic diseases. Interleukin-6 and related cytokines are associated with muscle wasting in clinical and experimental cachexia, although the mechanisms by which they might induce muscle wasting are unknown. One pathway activated strongly by IL-6 family ligands is the JAK/STAT3 pathway, the function of which has not been evaluated in regulation of skeletal muscle mass. Recently, we showed that skeletal muscle STAT3 phosphorylation, nuclear localization, and target gene expression are activated in C26 cancer cachexia, a model with high IL-6 family ligands. Here, we report that STAT3 activation is a common feature of muscle wasting, activated in muscle by IL-6 in vivo and in vitro and by different types of cancer and sterile sepsis. Moreover, STAT3 activation proved both necessary and sufficient for muscle wasting. In C2C12 myotubes and in mouse muscle, mutant constitutively activated STAT3-induced muscle fiber atrophy and exacerbated wasting in cachexia. Conversely, inhibiting STAT3 pharmacologically with JAK or STAT3 inhibitors or genetically with dominant negative STAT3 and short hairpin STAT3 reduced muscle atrophy downstream of IL-6 or cancer. These results indicate that STAT3 is a primary mediator of muscle wasting in cancer cachexia and other conditions of high IL-6 family signaling. Thus STAT3 could represent a novel therapeutic target for the preservation of skeletal muscle in cachexia.
Keywords: Janus-activated kinase; interleukin-6; T cell protein tyrosine phosphatase/protein tyrosine phosphatase, nonreceptor type 2; suppressors of cytokine signaling 3; electroporation; atrophy; burn; sepsis; lipopolysaccharide; incb018424; signal transducer and activator of transcription 3 inhibitory peptide
cachexia, from “kakos” meaning “bad” and “hexis” meaning “to have (fut.),” is a devastating syndrome that involves the loss of both muscle and adipose tissue and can be associated with many disease states, such as certain types of cancer, congestive heart failure, diabetes, kidney failure, and HIV/AIDS (15, 16). The whole body wasting that occurs in the presence of cancer has been acknowledged clinically since the time of the ancient Greeks, who coined the term. Clinically, cachexia is defined as an unintentional 10% loss of body weight over a 12-mo period that is directly associated with an underlying disease (39). The progressive loss of adipose tissue and skeletal muscle despite adequate feeding results in weakness, reduced ambulation, diminished quality of life, poor response to therapy, and often death due to respiratory failure or infection. In cancer alone, cachexia afflicts some 80% of all patients and is itself responsible for 25–30% of all cancer-related deaths. Currently, there are no approved effective treatments for muscle wasting in cancer. Understanding the molecular mechanisms responsible for muscle wasting is necessary to develop targeted therapies for these most vulnerable patients.
Both host and tumor-derived factors have been shown experimentally to contribute to muscle wasting (4). Members of the TGFβ superfamily, including TGFβ itself, activin, GDF-15, and the muscle-specific family member myostatin can cause weight loss and muscle loss through SMAD pathway activation whether or not their levels are increased in cancer (6, 35, 63). As well, inflammatory cytokines, including tumor necrosis factor (TNF)/cachectin, IL-1α and -β, interferon-γ, and IL-6 and related ligands, have been implicated in cachexia either through experimental manipulation using mouse models or by association of serum levels in patients with cachexia (3). In addition to producing anorexia, certain cytokines are known to directly induce proteolysis or lipolysis in vitro or in vivo. In the case of TGFβ family members, muscle wasting is downstream of SMAD activation (35), whereas TNF-induced muscle wasting is mediated largely through NF-κB (11, 45). The pathways through which other cytokines initiate muscle loss are not described.
IL-6 is a multifunctional cytokine involved in a variety of host defenses and pathological processes (40). IL-6-secreting cells can induce wasting of both muscle and fat stores and ultimately death (23, 55). Serum IL-6 levels are elevated in most experimental models of cachexia, and IL-6 is responsible at least in part for the muscle wasting seen in mice with colon-26 cancer cachexia as well as in Yomoto uterine cancer cachexia (49–52). Suramin, an antagonist of IL-6 binding to its receptor, markedly decreased the rate of cachexia in C26 tumor bearers (50), whereas IL-6-neutralizing antibodies have been shown to slow cachexia in Yomoto-bearing mice (52). Inhibition of IL-6-using antibody against the soluble receptor slows muscle wasting in ApcMin mice (57). Moreover, IL-6 has been demonstrated to be a very sensitive predictor of weight loss in a number of series, including patients with advanced small-cell lung cancer (46) and colon cancer (12, 17), and elevated IL-6 levels are associated with reduced survival in a variety of cancer types (48). In addition to IL-6 itself, other IL-6 family cytokines have also been implicated in muscle wasting, including ciliary neutrophic factor (CNTF) (14, 27) and leukemia inhibitor factor (LIF) (1, 38).
IL-6 and related ligands activate signaling by binding ligand-specific α-receptors [IL-6 receptor-α, a.k.a. gp80 in the case of IL-6 and CNTF-R and oncostatin M (OSM)-R in the case of CNTF and OSM] in either membrane-bound or soluble forms (7, 29). These ligand receptor complexes bind ubiquitously expressed type I cytokine receptor IL-6 signal transducer/gp130, inducing activation of three major pathways: the signal transducers and activators of transcription 1 and 3 (STAT1/3), ERK, and phosphatidylinositol 3-kinase/Akt pathways (19). For the former, binding of gp130 induces activation of associated Janus kinases (JAKs), which subsequently recruit SH2-containing proteins, including STAT3. Phosphorylation of STAT3 on tyrosine 705 results in dimerization, nuclear localization, DNA binding, and target gene regulation. Signaling can be antagonized at the level of JAK or STAT3 by feedback inhibition through STAT3 target genes, including suppressors of cytokine signaling (SOCS) proteins, particularly SOCS3 (26). Dephosphorylation of STAT3 is mediated by T cell protein tyrosine phosphatase/protein tyrosine phosphatase, non receptor type 2 (TC-PTP/PTPN2) in the nucleus and cytoplasm (58).
Of pathways stimulated by IL-6 ligands, the ERK pathway has been implicated previously in cancer cachexia and muscle wasting (44), and Akt is the primary anabolic pathway for skeletal muscle (22). Although STAT3 activation has been reported in muscle of mice with cachexia (5, 8), no functional data on STAT3 on modulating muscle size yet exist. Thus here we sought to determine the role of IL-6-induced and cancer-induced JAK/STAT3 pathway activation on skeletal muscle size and wasting.
MATERIALS AND METHODS
Cachexia models.
All animal studies were approved by the University of Miami and Thomas Jefferson University Institutional Animal Care and Use Committees. For delivery of sustained high levels of IL-6, athymic nude mice (Harlan Laboratories, Indianapolis, IN) were injected intramuscularly with Chinese hamster ovary (CHO) cells expressing recombinant human IL-6 or with similarly selected cells expressing no recombinant protein. Mice were then euthanized at different time points (4, 8, 12, and 16 days) after injection of CHO cells. C57BL/6J male mice were implanted s.c. with Alzet osmotic minipumps delivering 1 μg/h recombinant murine IL-6 for 7 days. For cancer cachexia, CD2F1 female mice (Harlan) were injected intrascapularly with 106 C26 (colon-26) adenocarcinoma cells (Donna McCarthy). C57BL/6J female mice were injected with 106 B16F10 melanoma cells (ATCC, Manassas, VA) or 106 Lewis lung carcinoma cells (Denis Guttridge). ApcMin mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in our colony. All tumor-bearing mice were euthanized when weight loss was ≥10%. For sterile sepsis, C57BL/6J wild-type or IL-6-null (B6.129S2-IL6tm1Kopf/J) male mice (Jackson) were given LPS (1 μg/h for 7 days) by osmotic pump implanted sucutaneously. IL-6 serum levels were measured by IL-6 Quantikine M ELISA (R & D Systems).
Gene transfer.
Gene transfer in skeletal muscle was performed as described (43). Briefly, on day 0, legs were shaved and tibialis muscles were pretreated by injection of 25 μl of 0.5 U/μl hyaluronidase through the skin. Two hours later, 50 μg of plasmid DNA in PBS was injected in the tibialis anterior muscle using a Hamilton syringe. Next, three pulses (20 ms each at 75 V/cm, 1 pulse/s) were applied through the skin to the dorsal aspect of the lower limb using a square pulse generator (ECM-830) and stainless-steel paddle electrodes (BTX-Harvard Apparatus). The polarity was then inverted, and three more pulses were delivered to the muscle. Cotransfection of an expression vector carrying a green fluorescent protein under the control of the cytomegalovirus promoter (pCMV-GFP) at 1/10 the molarity of experimental construct or empty vector was performed to identify transfected fibers. Transfections were performed the same day as tumor inoculation for the CHO cell and C26 tumor-bearing experiments, and mice were euthanized 9 days later for CHO/nude mouse experiments and 12 days later for C26 tumor-bearing experiments.
Cell culture.
C2C12 skeletal myoblasts (ATCC) were grown in high-glucose DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, 100 mg/ml sodium pyruvate, and 2 mM l-glutamine and maintained at 37°C in 5% CO2. Differentiation to myotubes was induced by shifting confluent cultures to DMEM supplemented with 2% horse serum and replacing the medium every 2nd day for 5 days. At 5 days, myotubes were treated with recombinant murine IL-6 (R & D Systems) 10 or 100 ng/ml in the presence or absence of 1 nM Velcade (Selleck Chemicals), 50 μM STAT3 inhibitor peptide (Calbiochem) (62), 400 nM JAK1/2 inhibitor (INCB018424; ChemieTek) (56), or specific adenoviral constructs. In the case of adenovirus, 24 h after infection the cells were washed and challenged with IL-6 for 48 h, and then cells were fixed and measured.
Plasmid and adenoviral constructs.
Plasmids were STAT3 Y705F Flag pRC/CMV (dnSTAT3; plasmid 8709), Stat3-C Flag pRc/CMV (cSTAT3; plasmid 8,722), and pCMV-GFP [plasmid 11,153 (37)] from Addgene, short hairpin (sh)STAT3 plasmid RMM3981-97059840 from Open Biosystems, and pcDNA3.1 (pCMV-empty) from Invitrogen. Replication defective Ad-CMV-GFP (no. 1060) was purchased (Vector Biolabs, Philadelphia, PA). Ad-cSTAT3-GFP, Ad-dnSTAT3, Ad-shSTAT3, Ad-PTPN2-GFP, and Ad-dnSTAT3-GFP were made using human recombinant adenovirus serotype 5 (D1/E3) construct, in which GFP and genes of interest are expressed from separate promoters (Vector Biolabs).
RNA extraction and quantitative real-time PCR.
Total RNA was extracted from flash-frozen quadriceps using TRIzol (Sigma-Aldrich), as described previously (64). cDNA was used as a template in real-time PCR reactions with QuantiTect SYBR-Green PCR Master Mix (BioRad) and run on a Bio-Rad MyIQ machine. Relative gene expression was normalized by dividing the specific expression value by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression value and calculated using the 2−ΔΔCT method (45a). Primer sequences are as reported (8).
Western blotting analysis and antibodies.
Proteins in muscle and C2C12 myotubes were assayed by Western blotting of total lysates, as reported (8). Antibodies were rabbit monoclonal phospho-STAT3 (Tyr705), GFP and GAPDH, and rabbit polyclonal STAT3 from Cell Signaling, mouse monoclonal gp130 from R & D Systems, mouse monoclonal Flag and β-actin from Sigma, and rabbit polyclonal fibrinogen from Dako.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared from flash-frozen muscle as reported (8, 9), using 3′-biotinylated-STAT3 oligonucleotides (5′-GATCCTTCTGGGAATTCCTAGATC-3′, 3′-CTAGGAAGACCCTTAAGGATCTAG-5′) from IDT and the Light Shift Chemiluminescent electrophoretic mobility shift assay (EMSA) kit (Pierce).
Morphological studies (fiber size, immunofluorescence, immunohistochemistry).
For morphometric studies in Fig. 1, images were obtained in brightfield. For studies involving adenovirus, direct GFP fluorescence was used. For inhibitor studies, C2C12 myotubes were fixed in ice-cold acetone-methanol and incubated with an anti-myosin heavy chain antibody (1:1,000; Millipore) and an AlexaFluor 488-labeled secondary antibody (Invitrogen). In all cases, analysis of GFP-positive fiber size was by measuring average fiber diameter of long multinucleate fibers, avoiding regions of clustered nuclei on a calibrated image using National Institutes of Health (NIH) Image J 1.43 software. For histology and morphometry of muscle, 8-μm-thick cryosections of tibialis muscles were fixed in formaldehyde vapor. For immunofluorescence analysis, cryosections of either gastrocnemius or tibialis muscles were fixed in 3% formaldehyde and then incubated with pSTAT3 primary antibody (1:50) and AlexaFluor 594-conjugated secondary antibody (Invitrogen). For immunohistochemistry (IHC) analysis, muscle sections were fixed in cold acetone and then in 4% BSA-PBS containing 3% H2O2 and incubated with pSTAT3 primary antibody (1:50) and then Real Envision Detection System using DAB+ chromogen (Dako). All samples were observed under an Olympus IX70 fluorescence microscope, and calibrated images were recorded for morphometric examination. Morphometry was determined from a section taken at the midbelly. All GFP-positive fibers were measured by tracing the perimeter of each individual fiber using a Cintiq pen tablet input device (Wacom) and Image J for calculation of the cross-sectional area.
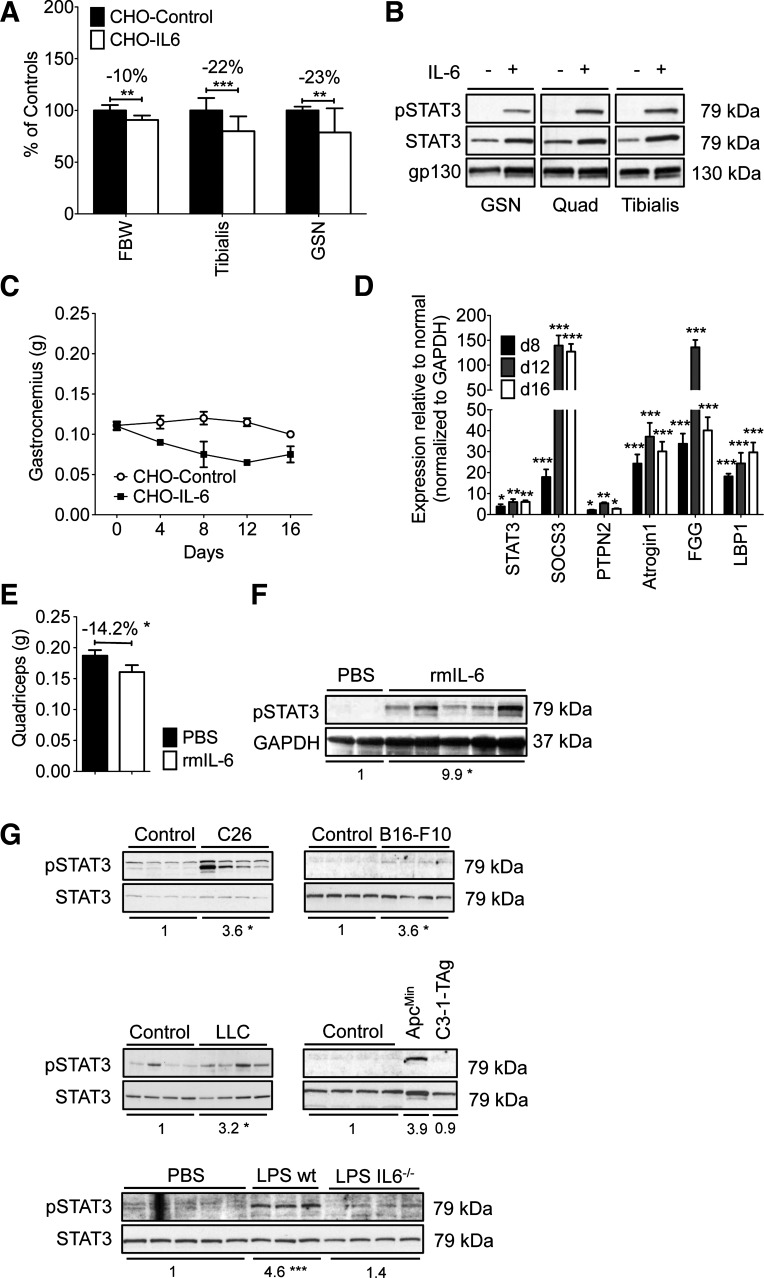
Fig. 1.
IL-6-induced and tumor-induced muscle wasting were associated with STAT3 activation in vivo and in vitro. A: Chinese hamster ovary (CHO)/IL-6 cells injected in athymic nude mice caused loss of body weight [final body weight (FBW)] and muscle weight, including tibialis anterior and gastrocnemius vs. CHO/control mice (n = 8/group, euthanized on day 12). Data are representative of >10 experiments. B: Western blotting analysis of muscle from mice in A reveals increased Y705-STAT3 in the gastrocnemius (GSN), quadriceps (Quad), and tibialis anterior of mice treated with CHO/IL-6 cells (+IL-6) vs. CHO/controls (−IL-6). Blot is representative of 4 independently assayed samples from each group on day 8, but virtually identical results were observed on days 4 and 12 (not shown). C: muscle wasting was progressive over time with CHO/IL-6 cells vs. CHO/controls. Gastronemius is shown (n = 4–6/point). D: quantitative real-time RT-PCR (qPCR) shows elevated expression of STAT3 target genes and atrogin-1 in quadriceps from mice with cachexia and CHO/IL-6 from days 8, 12, and 16 normalized to CHO/control samples (n = 3/condition, sampled in triplicate). E and F: recombinant IL-6 administered by osmotic pumps (1 μg/h for 7 days) induced a marked reduction in quadriceps weight (n = 4–6/group; E) and increased pY705-STAT3 by Western blotting analysis of mice euthanized after 7 days of treatment (F). This experiment is 1 of 2. Fold change vs. control group (PBS) for the pSTAT3/GAPDH ratio is shown under the blots (*P < 0.05). G: quadriceps pY705-STAT3 was increased in experimental models of cancer cachexia when weight loss in the tumor-bearing group was equal to 10% of starting body weight for C26, B16-F10, and Lewis lung carcinoma (LLC)-tumor bearing mice and ApcMin mice or after 7 days of chronic LPS administration by osmotic pump. Note that IL-6-null (IL6−/−) mice showed less pSTAT3 with LPS pumps. Fold change vs. control group for the pSTAT3/STAT3 ratio is reported under each of the blots. *P < 0.05; **P < 0.01; ***P < 0.001.
Statistical analysis.
All results are expressed as means ± SE except where noted. Western blots show independent samples and are representative of at least two trials. Significance of the differences was evaluated by analysis of variance, followed by Tukey's posttest.
RESULTS
IL-6 induced muscle wasting and STAT3 activation in mice.
To model the sustained high levels of IL-6 observed in cancer, sepsis, burn, and other conditions associated with muscle wasting, we administered IL-6 to mice by using two approaches. In the first, we injected athymic nude mice with CHO cells expressing human IL-6 vs. control CHO cells expressing no recombinant protein (65). In the second, we implanted osmotic pumps delivering recombinant murine IL-6 in C57BL/6J mice. CHO/IL-6 treatment led to blood levels of 80–100 ng/ml IL-6, as reported previously (65). Compared with CHO/control mice, which maintained tumor-free body mass vs. starting body mass over the course of the experiment, CHO/IL-6 injected mice grew markedly wasted, with a significant loss of body mass and proportionately greater loss of muscle mass (Fig. 1A). All muscles were affected. Consistent with direct signaling of IL-6 on muscle, pY705-STAT3 was markedly elevated in gastrocnemius, quadriceps, and tibialis anterior muscles of CHO/IL-6 mice (Fig. 1B). Muscle weight loss was progressive with longer exposure to IL-6 (Fig. 1C). Expression of the RNA for STAT3 was elevated along with expression of the STAT3 target genes SOCS3, fibrinogen, and LBP1, the latter two of which are acute-phase reactants (Fig. 1D). Consistent with muscle wasting, we also observed markedly elevated expression of Atrogin-1, a muscle-specific ubiquitin ligase expressed in virtually all conditions of muscle wasting. Administration of recombinant murine IL-6 by pump over 7 days also led to weight loss, systemic muscle wasting (quadriceps is shown; Fig. 1E), and increased pY705-STAT3 in skeletal muscle (Fig. 1F). Thus IL-6 alone is sufficient to induce systemic muscle STAT3 activation and wasting.
STAT3 is activated in muscle in cancer cachexia and sepsis.
High IL-6 levels have been reported to correlate with the degree of muscle wasting in cancer patients and mouse models of cachexia. Previously, we have reported that C26 tumor-dependent cachexia is associated with high serum levels of several IL-6 family ligands (IL-6, IL-11, LIF, and OSM) along with robust activation of the STAT3 pathway in skeletal muscle (Ref. 8 and Fig. 1G). Baltgalvis et. al. (5) have reported elevated pY705-STAT3 in ApcMin mice with cachexia due to intestinal cancer. To survey the potential universality of STAT3 activation in muscle wasting, we assayed muscle from mice with ≥10% weight loss due to B16.F10 melanoma (31), Lewis lung adenocarcinoma (LLC) (60), and, to compare with prior data, ApcMin and C3-1-TAg prostate cancer (36). All but the last showed increased pY705-STAT3 in skeletal muscle, suggesting that STAT3 activation is a common feature in many types of experimental cancer cachexia (Fig. 1G). Sepsis is also associated with high serum IL-6, and here we show that chronic administration of LPS in mice by osmotic minipump for 7 days led to 10% weight loss (data not shown) and increased levels of pY705-STAT3 in quadriceps. STAT3 activation was reduced in LPS pump-treated Il6-null mice, suggesting that IL-6 contributes to but is not solely responsible for STAT3 activation in cachexia of sepsis (Fig. 1G). It should be noted that the degree of STAT3 phosphorylation was not consistent across the cachexia models. Generally, those known to be associated with high IL-6 and more aggressive wasting, including CHO/IL-6, C26, ApcMin, and sepsis, showed reproducibly higher pSTAT3 levels than did those that are not classically associated with IL-6, namely LLC and B16.F10 melanoma, as well as sepsis in the absence of IL-6.
IL-6 induced wasting and STAT3 activation in muscle cultures.
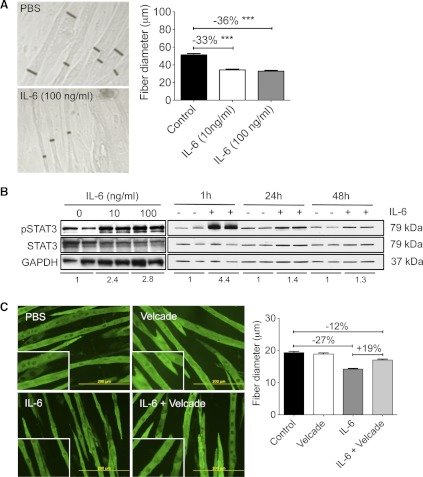
We next sought to determine the direct effects of IL-6 on muscle using the C2C12 myotube model. To study effects on myofiber size per se vs. differentiation and fusion, this and all subsequent experiments were performed on C2C12 myotubes at 4 or 5 days after the onset of differentiation, when fibers cover >80% of the culture area. Under these conditions, 48 h of recombinant murine IL-6 (10 or 100 ng/ml) exposure resulted in a significant reduction in fiber diameter (−36%, P < 0.001) compared with controls (Fig. 2A). C2C12 myofiber wasting was accompanied by increased pY705-STAT3 at both 10 and 100 ng/ml and at 1, 24, and 48 h after the initial stimulation with IL-6. The difference between control and IL-6-induced pSTAT3 levels lessened over time, consistent with the induction of feedback inhibitory pathways, including expression of SOCS3 (Ref. 26 and Fig. 2B).
Fig. 2.
IL-6 induced loss of C2C12 myofiber mass and activated STAT3 in a proteasome-dependent manner. A: IL-6 treatment resulted in loss of C2C12 myofiber diameter after 48 h. Black bars show representative myotube diameter (n = 158–200 fibers/condition from 3 independent wells). Data are representative of >8 experiments. ***P < 0.001. B: in C2C12 myotubes, increased pSTAT3 was observed after 1 h of treatment with 10 or 100 ng/ml IL-6 (left) and after 1, 24, and 48 h of 100 ng/ml IL-6. Fold change vs. respective control group for the pSTAT3/STAT3 ratio is reported under each set of blots. Data are representative of >5 trials. C: Velcade/bortezomib (1 nM) cotreatment for 48 h prevented IL-6-induced muscle atrophy (n = 170–210 fibers/well from 3 independent wells for each condition).
IL-6 has been shown conflictingly to both induce proteolysis and also induce protein synthesis and protein accumulation in the C2C12 myotube model (2, 13). To determine whether IL-6 induced C2C12 fiber atrophy results from activation of the ubiquitin-proteasome pathway, we incubated myotubes in the presence of 1 nM Velcade and IL-6. In fact, IL-6-induced atrophy was reduced but not abolished in the presence of the proteasome inhibitor (Fig. 2C), suggesting that part of the IL-6 effect is likely through this pathway.
STAT3 activation is sufficient to induce muscle wasting in vitro and in vivo.
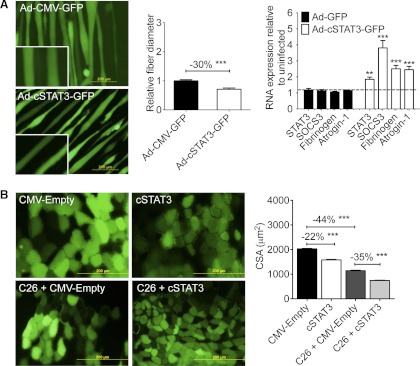
Given the positive relationship between elevated IL-6, STAT3 phosphorylation, and muscle wasting, we next sought to determine whether STAT3 activation was sufficient to induce muscle wasting. Thus we infected C2C12 myofibers with a recombinant adenovirus expressing a mutant, constitutively activated STAT3 (cSTAT3) known to possess increased DNA binding/transcriptional activity (10). Under conditions in which total STAT3 RNA was increased approximately twofold by quantitative real-time PCR, Ad-cSTAT3-GFP infection resulted in a 30% reduction in fiber diameter vs. Ad-GFP and induced the expression of known STAT3 target genes, including SOCS3 and the STAT3-mediated acute-phase response gene fibrinogen. Consistent with wasting, atrogin-1 expression was also markedly increased (Fig. 3A). Thus STAT3 activation is by itself sufficient to induce muscle fiber wasting in cell culture.
Fig. 3.
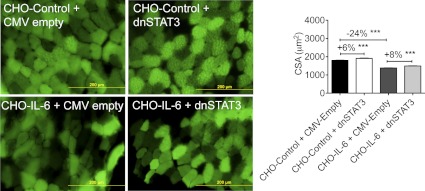
STAT3 was sufficient to induce muscle fiber atrophy both in vitro and in vivo. A: constitutively active STAT3 [Ad-cSTAT3-green fluorescent protein (GFP)] resulted in decreased C2C12 myofiber diameter 48 h after infection (n = 150–200 fibers/condition from 3 independent wells) and increased transcription of known STAT3 target genes as well as atrogin-1 (n = 3 wells/group in triplicate). B: cytomegalovirus (CMV)-cSTAT3 transfection reduced cross-sectional area (CSA) in the tibialis anterior muscle of non-tumor-bearing and C26-bearing CD2F1 mice. Mice were transfected on the same day as PBS, or tumor cells were injected and then euthanized 12 days later. Only positively transfected fibers (i.e., green fibers coexpressing pCMV-GFP) were measured (n = 650–1,900 fibers/condition; n = 8 tumor-bearing and non-tumor-bearing mice). Both experiments have been performed >3 times. **P < 0.01; ***P < 0.001.
To assay STAT3 activity in vivo, we used direct injection and electroporation of a CMV-cSTAT3 plasmid into the tibialis anterior of CD2F1 mice. CMV/empty vector was electroporated into the contralateral leg as an internal control. Coinjection of CMV/GFP was used to mark transfected fibers. Transfection of cSTAT3 was sufficient to induce a marked reduction in fiber cross-sectional area in non-tumor-bearing mice (−22% vs. empty vector controls, P < 0.001). Furthermore, cSTAT3 transfection exacerbated muscle fiber atrophy in the presence of the C26 tumor, reducing cross-sectional area an additional 35% compared with C26 plus empty vector alone (P < 0.001; Fig. 3B). These results indicate that STAT3 activation is sufficient for muscle fiber atrophy in vivo as well.
Inhibition of STAT3 prevents IL-6-induced myofiber atrophy in vitro.
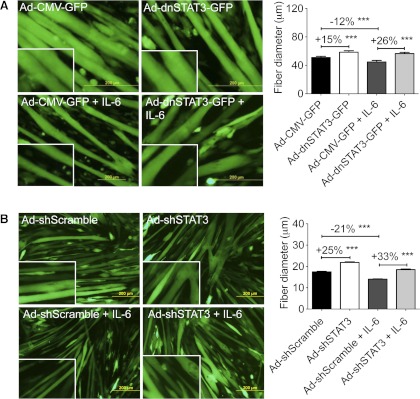
Next, we sought to determine whether STAT3 is not only sufficient but also necessary to induce fiber atrophy. The dominant negative STAT3 (dnSTAT3) mutation results in a phenylalanine substitution at Tyr705, thus preventing IL-6-induced STAT3 dimerization and nuclear translocation (30). Infection of C2C12 myotubes with Ad-dnSTAT3-GFP resulted in myofiber hypertrophy vs. Ad-GFP alone in the absence of exogenous IL-6 (+15%, P < 0.001) and completely blocked myofiber atrophy induced by IL-6 (+26% vs. IL-6 Ad-GFP, P < 0.001) (Fig. 4A). The hypertrophic effect of dnSTAT3 at baseline was likely due to inhibition of endogenous IL-6 signaling because IL-6 is expressed by C2C12 myotubes (20). Ad-shSTAT3-GFP induced baseline hypertrophy more robustly than dnSTAT3 (+25%, P < 0.001) and prevented IL-6-mediated wasting (+33% vs. IL-6 Ad-shScramble, P < 0.001) (Fig. 4B).
Fig. 4.
STAT3 activity was necessary for muscle atrophy. A: infection of adenovirus expressing dominant negative STAT3 (Ad-dnSTAT3-GFP) resulted in increased fiber diameter in control and IL-6-treated C2C12 myotubes (n = 200–300 fibers from 3 independent wells/condition). B: Ad-shSTAT3-GFP also increased baseline myofiber size and inhibited IL-6-dependent fiber atrophy (n = 200–300 fibers from 3 independent wells/condition). Data represent several independent experiments in which adenovirus was applied for 24 h and then washed out, and IL-6 was applied for 48 h and cells were fixed and measured. ***P < 0.001.
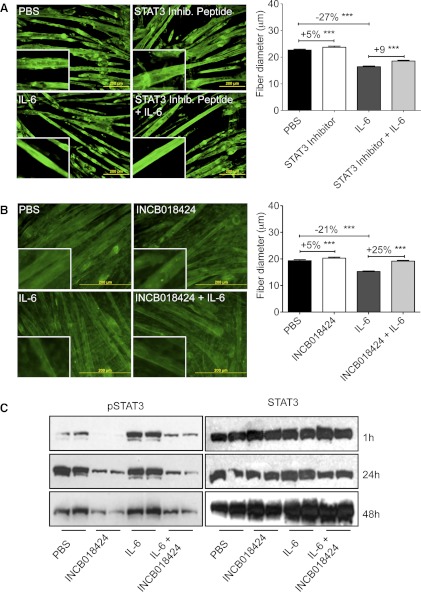
We next sought to inhibit STAT3 pharmacologically. C2C12 myotubes were treated with a cell-permeable STAT3 SH2 domain mimetic peptide (SIP) (62). SIP is a potent and selective inhibitor of STAT3 SH2 domain/phosphotyrosine interactions in cancer cells (62). The 29-mer cell-permeable peptide is derived from the STAT3 SH2 domain, can replicate STAT3 biochemical properties, binds with high affinity to known STAT3-binding phosphotyrosine peptide motifs, and prevents activation of endogenous STAT3. C2C12 myotubes were treated for 48 h with 50 μM STAT3 inhibitory peptide in the presence or absence of 100 ng/ml IL-6. STAT3 inhibitory peptide resulted in mild hypertrophy at baseline (+5% vs. PBS, P < 0.001) and a partial reduction in loss of fiber diameter with IL-6 treatment (Fig. 5A).
Fig. 5.
Pharmacological inhibition of JAK/STAT3 prevented IL-6-dependent myofiber atrophy. A: STAT3 inhibitor peptide, 50 μM, induced modest myofiber hypertrophy and prevented IL-6-induced fiber atrophy after 48 h of cotreatment (n = 200–300 fibers from 3 independent wells/condition). B: the JAK inhibitor INCB018424 (400 nM) induced modest hypertrophy in control myotubes and completely blocked IL-6-mediated myofiber atrophy after 48 h of cotreatment (n = 200–300 fibers from 3 independent wells/condition). Data represent 3 separate experiments. C: Western blotting analysis of whole C2C12 culture extracts show markedly reduced pSTAT3 with INCB018424 treatment at 1, 24, and 48 h. A and B are representative of at least 2 experiments each in which inhibitor and IL-6 were added at the same time and cells were incubated for 48 h and then fixed and measured. ***P < 0.001.
Testing one step upstream in the pathway, we treated C2C12 cells with the JAK1/2 inhibitor INCB018424, which has been shown to reduce pSTAT3 in peripheral blood of patients with myelofibrosis. INCB018424 (400 nM) completely blocked IL-6-induced wasting, whereas it induced only a mild hypertrophy at baseline (Fig. 5B). This activity was associated with significant reductions in pY705-STAT3 levels at every time point (Fig. 5C). Taken together, these data using genetic and pharmacological inhibition of STAT3 indicate that STAT3 activation is necessary for IL-6-mediated myofiber wasting in vitro.
STAT3 inhibition reduced muscle wasting in vivo downstream of IL-6.
We next sought to determine whether blocking STAT3 activity could reduce IL-6-induced muscle wasting in vivo. First, the tibialis anterior muscles of athymic nude mice were subjected to electroporation with CMV/dnSTAT3 and CMV/empty vector in opposite legs, and then mice were injected with either CHO/control or CHO/IL-6 cells. As described previously, 14 days later CHO/control mice had gained body weight compared with initial body weight, whereas CHO/IL-6 mice had lost body weight and muscle mass (data not shown) along with muscle fiber diameter (Fig. 6). CMV-dnSTAT3 transgenesis resulted in an increase in muscle fiber diameter vs. CMV/empty vector in CHO/control mice (+6%, P < 0.001). In the setting of CHO/IL-6 treatment, where muscle diameter was reduced 24% vs. CHO/control;CMV/empty vector, CMV-dnSTAT3 partially blocked IL-6 induced wasting (8% vs. CHO/IL-6;CMV/empty vector, P < 0.001).
Fig. 6.
STAT3 inhibition prevented IL-6-dependent muscle wasting in mice. Expression of dnSTAT3 through CMV-dnSTAT3 transfection of tibialis anterior muscle on the day of CHO injection resulted in basal hypertrophy in CHO/control mice and reduced muscle wasting in CHO/IL-6 mice vs. the CMV-empty vector transfected controls 9 days later (n = 450–1,200 fibers from n = 8 mice/condition, repeated twice). ***P < 0.001.
STAT3 inhibition reduced muscle wasting in vivo downstream of C26 cachexia.
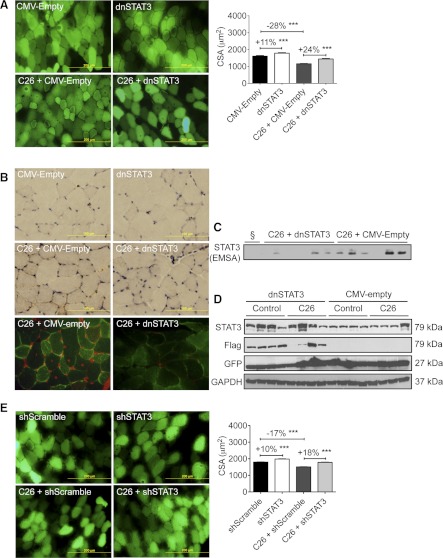
In the more physiological model of cancer cachexia, C26 adenocarcinoma, dnSTAT3 resulted in more robust inhibition of wasting (Fig. 7A). In non-tumor-bearing mice, CMV-dnSTAT3 induced an 11% increase in fiber diameter (P < 0.001). C26 tumors induced a 28% loss in muscle fiber diameter (P < 0.001) vs. non-tumor-bearing CMV/empty vector controls, whereas CMV-dnSTAT3-expressing fibers were 24% larger. These results on fiber size were associated with decreased STAT3 activity. Immunohistochemical and immunofluorescent staining for pSTAT3 were reduced by dnSTAT3 transfection in both non-tumor-bearing and tumor-bearing mice (Fig. 7B). Moreover, EMSA of nuclear extracts from tibialis anterior of dnSTAT3-transfected C26 tumor-bearing mice showed markedly reduced STAT3 DNA binding activity vs. empty vector tumor-bearing controls (Fig. 7C). Western blotting analysis of total lysates from transfected muscles confirmed an overall increased level of total STAT3 and a comigrating protein detected with anti-Flag antibody, both of which are consistent with the expression of the Flag-tagged dnSTAT3 protein in CMV-dnSTAT3 transfected mice, along with expression of GFP from the reporter plasmid (Fig. 7D).
Fig. 7.
STAT3 inhibition using dnSTAT3 or short hairpin (sh)STAT3 prevented C26 tumor-induced muscle atrophy in mice. Mice were transfected on the day of tumor inoculation and euthanized 12 days later. A: CMV-dnSTAT3 transfection of tibialis anterior muscle resulted in basal hypertrophy in non-tumor-bearing mice and reduced muscle wasting in C26 tumor-bearing mice vs. CMV-empty vector transfected controls (n = 250–500 fibers from n = 8 mice/condition, repeated twice). B: immunohistochemistry (brown staining; top) and immunofluorescence (bottom) for pY705-STAT3 in tibialis anterior muscles electroporated with CMV-dnSTAT3. Note pSTAT3 nuclear localization is reduced in the presence of dnSTAT3 (brown staining in immunohistochemistry, red staining in immunofluorescence analyses). C: CMV-dnSTAT3 transfection decreased STAT3 DNA-binding activity in nuclear extracts derived from transfected tibialis muscle in mice with C26 cachexia, as shown by the electrophoretic mobility shift assay (EMSA; n = 6/condition). §Unlabeled probe control. D: Western blotting analysis of total lysate from tibialis of control (non-tumor-bearing) and C26 tumor-bearing mice transfected with CMV-dnSTAT3 or empty vector shows expression of the Flag-taged-dnSTAT3 and GFP, with GAPDH as loading control (n = 4). E: CMV-shSTAT3 increased muscle CSA and prevented C26-induced fiber wasting in the tibialis muscle vs. CMV-shScramble (n = 1,650–2,750 fibers from n = 8 mice/condition, repeated twice). ***P < 0.001.
As in vitro, we also assayed the efficacy of shSTAT3 in blocking wasting (Fig. 7E). In this experiment, a CMV-shSTAT3 plasmid or, as a control, CMV-shScramble plasmid was transfected into contralateral tibialis anterior muscles of mice that were then injected with C26 cells or an equivalent volume of saline. CMV-shSTAT3 resulted in mild muscle hypertrophy in the absence of a C26 tumor (+10%, P < 0.001) and complete inhibition of muscle fiber wasting in the setting of C26 cachexia (+18%, P < 0.001). Thus inhibition of STAT3 locally in skeletal muscle reduces or blocks muscle wasting due to IL-6 treatment and cancer cachexia, indicating that STAT3 activation in skeletal muscle is largely or entirely responsible for muscle wasting downstream of IL-6 or cancer.
DISCUSSION
Given the preponderance of data implicating IL-6 and related ligands in muscle wasting of cancer cachexia, we sought to determine the signaling mechanism in skeletal muscle responsible for that activity. Specifically, we hypothesized that STAT3 activation in skeletal muscle would lead to loss of muscle mass and that STAT3 inhibition would abolish IL-6 and cancer-induced wasting. Indeed, our results in cell culture and in mice show that STAT3 activation is itself both necessary and sufficient for muscle wasting. This is true both for muscle wasting downstream of IL-6 as well as in a mixed cytokine model of C26 tumor-induced cachexia. Moreover, STAT3 was widely activated in other cancers, including Lewis lung carcinoma, ApcMin colon cancer, B16 melanoma, and in our model of sterile sepsis.
Indeed, although we focus in large part on IL-6-mediated wasting in this study, the C26 model of cachexia is also characterized by high levels of three other ligands that activate the gp130/JAK/STAT3 axis, namely CNTF, OSM, and LIF (8). Each of these binds a specific α-receptor before activating gp130, and all are likely responsible in part for the induction of STAT3 phosphorylation. The integration of these ligands upon STAT3 suggests that STAT3 inhibition per se might be a more powerful approach than antibody inhibition of individual cytokines to reducing muscle wasting in cancers where several cytokines are elevated. Moreover, given the similar activation in other cancers, our results might also be generalizable to any condition characterized by high levels of IL-6 family cytokines, including, for example, burn injury and sepsis.
This causative role of STAT3 in muscle wasting is less obvious than it might seem upon casual inspection. IL-6 and related ligands activate several signaling pathways, including two major pathways, ERK and STAT1/3 (33). ERK has been implicated previously in C26 cachexia (44) and might have been entirely responsible for muscle loss. Inhibition of STAT1 blocks cathepsin induction by interferon-γ in cultures (21); however, the role of STAT1 in the setting of cachexia has not been investigated. Given that STAT3 inhibition blocks C26 tumor-induced wasting entirely, the STAT3 pathway apparently takes precedence over ERK or STAT1 activation. However, STAT3 inhibition does not completely abolish IL-6-induced wasting in vivo, suggesting that either the inhibition was incomplete, these other pathways might also be important drivers of muscle wasting, or both. Paradoxically, IL-6 and other ligands have also been shown to result in activation of Akt, the primary anabolic pathway in skeletal muscle. Whether and how this might contribute to muscle wasting is currently unclear but is consistent with prior reports of increased protein synthesis in IL-6-treated C2C12 cells (2) and in muscle of mice (43) and patients with cachexia (28).
Our results are also somewhat surprising in light of other known roles for IL-6 and STAT3 in skeletal and cardiac muscle. In humans, acute muscle damage elicits IL-6-mediated STAT3 localization to satellite cell nuclei (54), whereas in mice IL-6/STAT3 is necessary for satellite cell-mediated muscle hypertrophy (47). It is not immediately clear how effects on satellite cells might contribute to wasting. However, these roles of IL-6 on satellite cells do not exclude a role for IL-6/STAT3 in mature muscle fibers. Speculatively, the coupling of satellite cell proliferation and fiber wasting locally might serve to promote the fusion of myoblasts and repair of muscle by the opening of space in the syncytium, a process that might become pathogenic when activated systemically. Also surprising is that our observations in striated muscle are counter to those in cardiac muscle. Whereas the roles of IL-6 in promoting heart failure and preserving heart function after injury are complex, context, and species specific, STAT3 activation in the heart promotes both hypertrophy and cell survival (32, 33, 41, 59). Indeed, STAT3 is required for specification of cardiomyocytes from stem cells, murine germline deletion of STAT3 results in embryonic lethality at the time of heart development, and cardiomyocyte-specific ablation of STAT3 results in heart failure (7, 18). The mechanisms responsible for these apparently opposite effects of STAT3 in cardiac vs. skeletal muscle have not yet been explored.
Although our results firmly place STAT3 downstream of IL-6 and cancer in causing muscle wasting, the mechanisms by which STAT3 induces atrophy are still unknown. We hypothesize that STAT3 activation of transcription is responsible, given that dnSTAT3 largely inhibits nuclear but not mitochondrial effects of STAT3 (24). The number, identity, and activity of the muscle-specific STAT3 target genes mediating wasting are under active investigation in our laboratory. Among these might be members of the ubiquitin-proteasome pathway, because our data indicate that IL-6-mediated wasting can be reduced in the presence of proteasome inhibitor. A second distinct mechanism potentially leading to muscle wasting is STAT3-mediated induction of the acute-phase response in muscle, which we observed previously in C26 tumor-induced cachexia (8). With the robust induction of acute-phase transcripts, including fibrinogen, haptoglobin, and many other acute-phase response genes, freed amino acids derived from proteolysis of structural proteins would be synthesized into acute-phase response proteins that are subsequently secreted. Ultimately, this would drain protein reserves in skeletal muscle and thus represents a causal mechanism for muscle wasting in cancer. Indeed, Tisdale (53) and Fearon et al. (17) proposed that, in cancer cachexia, persistent elevations in serum acute-phase response proteins could result in muscle atrophy.
In the broader context, IL-6 family ligands represent a subset of all factors associated with or known to induce muscle wasting. Among the other factors is TNF, which might induce cachexia partly through inducing IL-6 expression (2) and perhaps as well through directly activating STAT3 (25). TNF-induced NF-κB might also promote nuclear retention of STAT3 and vice versa (34), although none of these proposed relationships has been examined explicitly in cancer cachexia. The TGFβ/myostatin pathway has been shown to induce expression of IL-6 (61), suggesting that myostatin might also work indirectly through IL-6/STAT3, although this must be experimentally confirmed. Given such interactions, STAT3 inhibition could be additive or synergistic with the targeting of those other pathways.
In conclusion, our findings identify STAT3 as a causative factor and potential therapeutic target in muscle wasting during conditions of high IL-6 family ligands. Currently, there is much effort directed at developing STAT3 inhibitors for the purpose of targeting its oncogenic effects (42). An opportune side effect of such strategies might be the preservation of skeletal muscle. Given the large number of preclinical and clinical studies using such approaches, investigators should consider quantifying the effects of their JAK and STAT3 inhibitors on muscle mass and function.
GRANTS
This work was supported by American Cancer Society Research Scholar Grant TBE-111831 to T. A. Zimmers, with support from the Wendy Will Case Cancer Fund and the Amit family, and by NIH Grants R01-CA-122596 and R01-GM-092758 and Pennsylvania Department of Health CURE Grant TJU No. 080-37038-AI0801. This work was conducted using the resources of the Kimmel Cancer Center Bioimaging and Laboratory Animal facilities.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B., L.G.K., and T.A.Z. did the conception and design of the research; A.B., T.A., X.J., Z.Z., R.Z., L.P., and T.A.Z. performed the experiments; A.B., T.A., and T.A.Z. analyzed the data; A.B., T.A., L.G.K., and T.A.Z. interpreted the results of the experiments; A.B., T.A., and T.A.Z. prepared the figures; A.B. and T.A.Z. drafted the manuscript; A.B. and T.A.Z. edited and revised the manuscript; A.B., T.A., X.J., Z.Z., R.Z., L.P., L.G.K., and T.A.Z. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Donna O. McCarthy and Denis Guttridge for cell lines, Dr. Connie Cepko for the pCMV-GFP plasmid, and Dr. James Darnell for the STAT3 plasmids.
Present address of T. Aydogdu: Sanford Burnham Research Institute, La Jolla, CA 92037.
Present address of L. Puzis: Department of Anesthesiology, Stony Brook University, Stony Brook, NY 11794.
REFERENCES
- 1.Akiyama Y, Kajimura N, Matsuzaki J, Kikuchi Y, Imai N, Tanigawa M, Yamaguchi K. In vivo effect of recombinant human leukemia inhibitory factor in primates. Jpn J Cancer Res 88: 578–583, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez B, Quinn LS, Busquets S, Quiles MT, Lopez-Soriano FJ, Argiles JM. Tumor necrosis factor-alpha exerts interleukin-6-dependent and -independent effects on cultured skeletal muscle cells. Biochim Biophys Acta 1542: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care 6: 401–406, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Argilés JM, Busquets S, Toledo M, López-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3: 263–268, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun 391: 1548–1554, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120: 172–185, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 6: e22538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonetto A, Penna F, Minero VG, Reffo P, Costamagna D, Bonelli G, Baccino FM, Costelli P. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-α. Amino Acids 40: 585–594, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell 98: 295–303, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 38: 168–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 89: 431–439, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Espat NJ, Auffenberg T, Rosenberg JJ, Rogy M, Martin D, Fang CH, Hasselgren PO, Copeland EM, Moldawer LL. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am J Physiol Regul Integr Comp Physiol 271: R185–R190, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 27: 793–799, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Fearon KC, McMillan DC, Preston T, Winstanley FP, Cruickshank AM, Shenkin A. Elevated circulating interleukin-6 is associated with an acute-phase response but reduced fixed hepatic protein synthesis in patients with cancer. Ann Surg 213: 26–31, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol 102: 279–297, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol 102: 393–411, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol 285: R1153–R1164, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gallardo E, de Andrés I, Illa I. Cathepsins are upregulated by IFN-gamma/STAT1 in human muscle culture: a possible active factor in dermatomyositis. J Neuropathol Exp Neurol 60: 847–855, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13: 225–229, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Goodman L, Stein GH. Basal and induced amounts of interleukin-6 mRNA decline progressively with age in human fibroblasts. J Biol Chem 269: 19250–19255, 1994 [PubMed] [Google Scholar]
- 24.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324: 1713–1716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol 160: 2742–2750, 1998 [PubMed] [Google Scholar]
- 26.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334: 297–314, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson JT, Seniuk NA, Richardson PM, Gauldie J, Roder JC. Systemic administration of ciliary neurotrophic factor induces cachexia in rodents. J Clin Invest 93: 2632–2638, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jespersen JG, Nedergaard A, Reitelseder S, Mikkelsen UR, Dideriksen KJ, Agergaard J, Kreiner F, Pott FC, Schjerling P, Kjaer M. Activated protein synthesis and suppressed protein breakdown signaling in skeletal muscle of critically ill patients. PLoS One 6: e18090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 121: 3375–3383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem 271: 5961–5964, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Kawamura I, Yamamoto N, Sakai F, Yamazaki H, Naoe Y, Inami M, Manda T, Shimomura K. Activation of lipoprotein lipase and inhibition of B16 melanoma-induced cachexia in mice by ponalrestat, an aldose reductase inhibitor. Anticancer Res 19: 341–348, 1999 [PubMed] [Google Scholar]
- 32.Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation 98: 346–352, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J 32: 680–685, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 15: 283–293, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, Glass DJ. Treating cancer cachexia to treat cancer. Skelet Muscle 1: 2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA 91: 11236–11240, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA 101: 16–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalf D, Gearing DP. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci USA 86: 5948–5952, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 29: 154–159, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res 4, Suppl 3: S233–S242, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, Yamauchi-Takihara K. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res 47: 797–805, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert Opin Ther Pat 21: 65–83, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Penna F, Bonetto A, Muscaritoli M, Costamagna D, Minero VG, Bonelli G, Rossi Fanelli F, Baccino FM, Costelli P. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int J Cancer 127: 1706–1717, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One 5: e13604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed SA, Senf SM, Cornwell EW, Kandarian SC, Judge AR. Inhibition of IkappaB kinase alpha (IKKalpha) or IKKbeta (IKKbeta) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem Biophys Res Commun 405: 491–496, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer 73: 1560–1562, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8: 887–899, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Soda K, Kawakami M, Kashii A, Miyata M. Manifestations of cancer cachexia induced by colon 26 adenocarcinoma are not fully ascribable to interleukin-6. Int J Cancer 62: 332–336, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Strassmann G, Fong M, Freter CE, Windsor S, D'Alessandro F, Nordan RP. Suramin interferes with interleukin-6 receptor binding in vitro and inhibits colon-26-mediated experimental cancer cachexia in vivo. J Clin Invest 92: 2152–2159, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 89: 1681–1684, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura S, Ouchi KF, Mori K, Endo M, Matsumoto T, Eda H, Tanaka Y, Ishitsuka H, Tokita H, Yamaguchi K. Involvement of human interleukin 6 in experimental cachexia induced by a human uterine cervical carcinoma xenograft. Clin Cancer Res 1: 1353–1358, 1995 [PubMed] [Google Scholar]
- 53.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2: 862–871, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One 6: e17392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, Monden M. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest 97: 244–249, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 363: 1117–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLos One 6: e24650, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N, Matsuda T. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun 297: 811–817, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Yamauchi-Takihara K. gp130-mediated pathway and heart failure. Future Cardiol 4: 427–437, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Zhang G, Jin B, Li YP. C/EBPbeta mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J 30: 4323–4335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 25: 1653–1663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao W, Jaganathan S, Turkson J. A cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro. J Biol Chem 285: 35855–35865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock 23: 543–548, 2005 [PubMed] [Google Scholar]
- 65.Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology 38: 326–334, 2003 [DOI] [PubMed] [Google Scholar]