Abstract
The orexigenic hormone ghrelin is important in diabetes because it has an inhibitory effect on insulin secretion. Ghrelin ablation in leptin-deficient ob/ob (Ghrelin−/−:ob/ob) mice increases insulin secretion and improves hyperglycemia. The physiologically relevant ghrelin receptor is the growth hormone secretagogue receptor (GHS-R), and GHS-R antagonists are thought to be an effective strategy for treating diabetes. However, since some of ghrelin's effects are independent of GHS-R, we have utilized genetic approaches to determine whether ghrelin's effect on insulin secretion is mediated through GHS-R and whether GHS-R antagonism indeed inhibits insulin secretion. We investigated the effects of GHS-R on glucose homeostasis in Ghsr-ablated ob/ob mice (Ghsr−/−:ob/ob). Ghsr ablation did not rescue the hyperphagia, obesity, or insulin resistance of ob/ob mice. Surprisingly, Ghsr ablation worsened the hyperglycemia, decreased insulin, and impaired glucose tolerance. Consistently, Ghsr ablation in ob/ob mice upregulated negative β-cell regulators (such as UCP-2, SREBP-1c, ChREBP, and MIF-1) and downregulated positive β-cell regulators (such as HIF-1α, FGF-21, and PDX-1) in whole pancreas; this suggests that Ghsr ablation impairs pancreatic β-cell function in leptin deficiency. Of note, Ghsr ablation in ob/ob mice did not affect the islet size; the average islet size of Ghsr−/−:ob/ob mice is similar to that of ob/ob mice. In summary, because Ghsr ablation in leptin deficiency impairs insulin secretion and worsens hyperglycemia, this suggests that GHS-R antagonists may actually aggravate diabetes under certain conditions. The paradoxical effects of ghrelin ablation and Ghsr ablation in ob/ob mice highlight the complexity of the ghrelin-signaling pathway.
Keywords: growth hormone secretagogue receptor, insulin secretion, type 2 diabetes
obesity is one of the most alarming health concerns in Western and developing countries. Obesity causes insulin resistance, often leading to type 2 diabetes. Ghrelin is a multifaceted hormone best known for its orexigenic action; growth hormone secretagogue receptor (GHS-R) is recognized as a physiologically relevant receptor for ghrelin, mediating ghrelin's effects on growth hormone release, food intake, and adiposity (6, 12, 46). Both ghrelin and GHS-R are expressed in pancreatic islets (51, 53). We demonstrated previously that whereas ghrelin gene ablation in wild-type mice has no effect on hyperphagia or obesity, ghrelin ablation in leptin-deficient ob/ob mice ameliorates the diabetic condition (44). Studies have demonstrated that exogenous ghrelin administration inhibits insulin secretion both in vivo and in vitro (4, 16, 41, 44), confirming ghrelin as a negative regulator of insulin secretion and implying that ghrelin has an important role in glucose homeostasis.
We reported previously that circulating ghrelin increases during fasting and that glucose concentrations decrease in calorie-restricted ghrelin- and Ghsr-ablated mice, which suggests that both ghrelin and GHS-R are involved in glucose sensing (45, 46). We have shown that ghrelin's stimulatory effects on growth hormone (GH) release and feeding are mediated through GHS-R (46). However, it is unknown whether ghrelin's effect on insulin secretion is mediated through GHS-R. A widely used GHS-R antagonist, [d-Lys3]-GHRP-6, has been shown to block ghrelin's inhibitory effect on insulin secretion (16). It was thus inferred that ghrelin's effect on insulin secretion is mediated through the GHS-R and that GHS-R antagonists would be a viable approach for treating diabetic hyperglycemia (1, 2, 16). However, a recent report showed that [d-Lys3]-GHRP-6 blocks receptors other than GHS-R; for example, the CCR5 and CXCR4 receptors, which are used by HIV to enter cells, can also be blocked by [d-Lys3]-GHRP-6 (34). In addition, GHS-R is known to have constitutive activity, which is capable of producing its biological response in the absence of a bound ligand (21). Some GHS-R-associated effects may be due to the constitutive activity of GHS-R rather than the activation of ghrelin. We have shown recently that ghrelin- and Ghsr-null mice have differential phenotypes in thermogenic regulation and sleep; others have shown that ghrelin directly stimulates liver glucose output and adipogenesis by mechanisms independent from GHS-R (18, 30, 47, 48, 50). The evidence collectively suggests that some ghrelin functions may be mediated by subtype receptor(s) other than GHS-R. It is unknown whether ghrelin's effect on pancreatic β-cells is mediated exclusively by GHS-R or whether GHS-R antagonists can be used as antidiabetic agents.
The objectives of this study were to 1) assess the effect of GHS-R ablation on glucose homeostasis under obese and diabetic states, 2) determine whether ghrelin deletion and GHS-R deletion have similar effects on glycemic control in leptin-deficient mice, and 3) shed light on whether ghrelin's inhibitory effect on insulin secretion is mediated through GHS-R. To enable these studies, we bred our Ghsr−/− mice with leptin-deficient ob/ob mice to generate a mouse model lacking both GHS-R and leptin (Ghsr−/−:ob/ob).
METHODS
Generation of Ghsr−/−:ob/ob mice.
Our studies were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. The generation of ghrelin−/−, Ghsr−/−, and ghrelin−/−:ob/ob mice has been described by us previously (43, 44, 46). All mice used in the study were on the C57BL/6J background. To generate Ghsr−/−:ob/ob mice, N12 Ghsr−/− mice were bred to ob/+ mice from The Jackson Laboratory, creating compound heterozygotes, i.e., Ghsr+/−:ob/OB mice. In the second cross, these mice were interbred to generate Ghsr−/−:ob/ob mice and Ghsr−/−:OB/OB (Ghsr−/−) mice. In parallel, Ghsr+/+:ob/OB mice were bred to each other to produce Ghsr+/+:ob/ob (ob/ob) and Ghsr+/+:OB/OB [wild-type (WT)] mice. To minimize animal-to-animal variations, only littermate male mice were used. Mice were maintained under controlled temperature (∼75°F) and illumination (12:12-h light-dark cycle, 6 AM to 6 PM), with free access to water and regular chow.
General phenotypical characterization.
Body weight and food intake were measured weekly, and blood glucose was measured biweekly. Measurements were taken at the same time each day (between 9 and 10 AM) from 8 to 16 wk of age. Blood glucose concentrations were determined by a One-Touch Ultra glucometer (Lifescan, Milpitas, CA). Plasma triglycerides, total cholesterol, HDL, LDL, and free fatty acids (FFA) were measured by the lipid core of Hormone Assay & Analytical Services at Vanderbilt University.
Body composition and indirect calorimetry.
Whole body composition (fat and lean mass) of mice was measured by an Echo MRI-100 whole body composition analyzer (Echo Medical Systems, Houston, TX). Metabolic parameters were obtained using the Oxymax (Columbus Instruments, Columbus, OH) open-circuit indirect calorimetry system. To minimize the confounding effects of stress, mice were caged individually in metabolic chambers and given free access to regular chow and water for 1 wk. They were then placed in metabolic cages for ≥4 days before the indirect calorimetry testing. Indirect calorimetry studies were carried out for 72 h. The first 24 h were considered the acclimation phase, and average data of the final 48 h were analyzed. Oxygen consumption (V̇o2; ml·kg−1·h−1), carbon dioxide production (V̇co2; ml·kg−1·h−1), and locomotor activity (beam break counts) were measured. Respiratory exchange ratio (RER) and energy expenditure (EE; or heat generation) were calculated as we described previously (27, 33). Locomotor activity (on x-axis) was measured using infrared beams, and the number of beam breaks during the recording period was defined as locomotor activity.
Glucose and insulin tolerance test.
Mice were fasted for 18 h (from 3 PM to 9 AM) prior to testing and then given an intraperitoneal (ip) injection of d-glucose (0.625 g/kg for obese mice, or 2.0 g/kg for lean mice). Blood glucose was measured by tail bleeds at different time points. Fifty micoliters of blood from tails was collected in EDTA-coated tubes, and plasma samples were obtained by low-speed centrifugation. Insulin was analyzed by Hormone Assay & Analytical Services Core at Vanderbilt University using RIA assays. The insulin tolerance test (ITT) was done similarly, except the mice were fasted for only 6 h after lights-on, and Humulin (Eli Lilly, Indianapolis, IN) was administered by ip injection. 2.5 U/kg Humulin was used for obese mice.
Quantitative gene expression.
All mice were euthanized in the morning between 9 and 11 AM. Total RNA from whole pancreas was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Relative quantitative RT-PCR was performed in triplicates, as described previously (44). Primer information is available upon request.
Histological analysis.
Entire pancreases were fixed overnight in 10% formaldehyde solution at room temperature and then dehydrated and embedded in paraffin. Tissue blocks of whole pancreas (showing head, body, and tail of the pancreas) were then sectioned at 5 μm and stained with hematoxylin and eosin (H & E) for morphometric analysis. The H & E staining was carried out according to the standard protocols. The average area of pancreatic islets was measured using Axiophot microscope, and the image was analyzed using the Scion Image for Windows analysis software [National Institutes of Health (NIH), Bethesda, MD]. The sectional area of islets was measured at a magnification of ×10. All islets on each randomly selected section were counted, and ≥120 islets were counted for each mouse.
Statistical analyses.
All data are expressed as means ± SE. We used a two-tailed Student t-test, or one- or two-way ANOVA, to determine significance of differences between genotypes or treatments. P < 0.05 is defined as statistical significance.
RESULTS
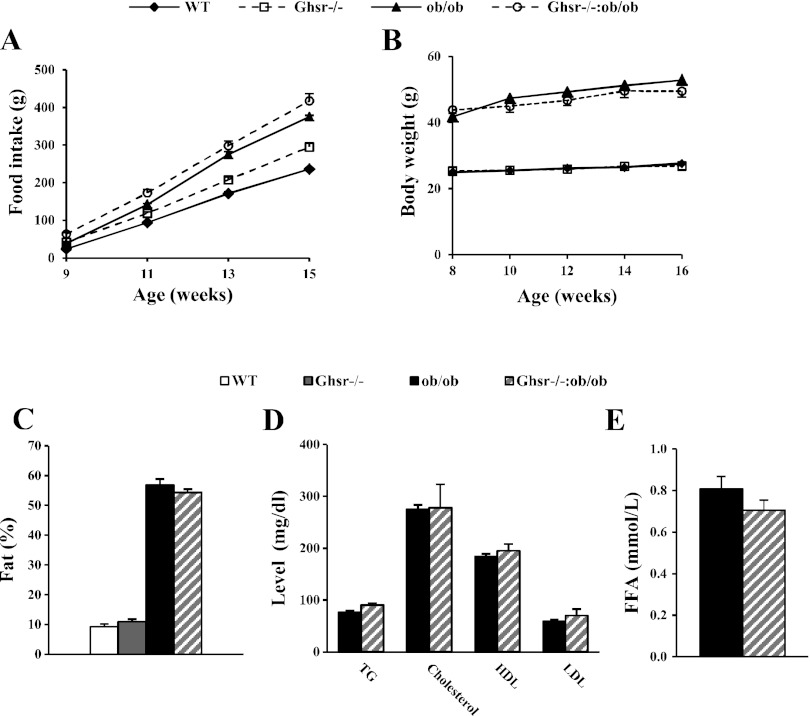
GHS-R deficiency affects neither the body weight nor food intake of ob/ob mice. No differences were observed in food intake or body weight between WT and Ghsr−/− mice (Fig. 1, A and B). Ghsr−/−:ob/ob mice were hyperphagic compared with the lean WT and Ghsr−/− mice, but food intake was similar to that of ob/ob mice (Fig. 1A). Similar to Ghrelin−/−:ob/ob mice, the body weight of Ghsr−/−:ob/ob mice was significantly higher than that of WT and Ghsr−/− mice but similar to that of ob/ob mice (Fig. 1B). These results suggest that ablation of GHS-R in ob/ob mice does not protect mice from hyperphagia or obesity resulting from leptin deficiency.
Fig. 1.
General characterization of growth hormone secretagogue receptor (Ghsr)−/−:ob/ob mice. A and B: food intake and body weight. C: body composition of fat content. D: fed plasma lipid concentrations: triglycerides (TG), cholesterol, HDL, and LDL. E: fed free fatty acid (FFA). The data are presented as means ± SE (n = 8). P < 0.05, obese vs. lean mice. P > 0.05, ob/ob vs. Ghsr−/−:ob/ob mice. WT, wild type.
GHS-R ablation affects neither body composition nor lipid profile of ob/ob mice. The body fat contents of ob/ob and Ghsr−/−:ob/ob mice were significantly higher than that of WT and Ghsr−/− mice, but there was no difference between ob/ob and Ghsr−/−:ob/ob mice (Fig. 1C). Furthermore, compared with ob/ob mice, the Ghsr−/−:ob/ob mice showed similar plasma lipid profiles in triglyceride, cholesterol, HDL, and LDL (Fig. 1D) as well as FFA (Fig. 1E). Thus, GHS-R ablation affects neither body composition nor lipid profiles of ob/ob mice.
Metabolic profile of Ghsr−/−:ob/ob mice during indirect calorimetry.
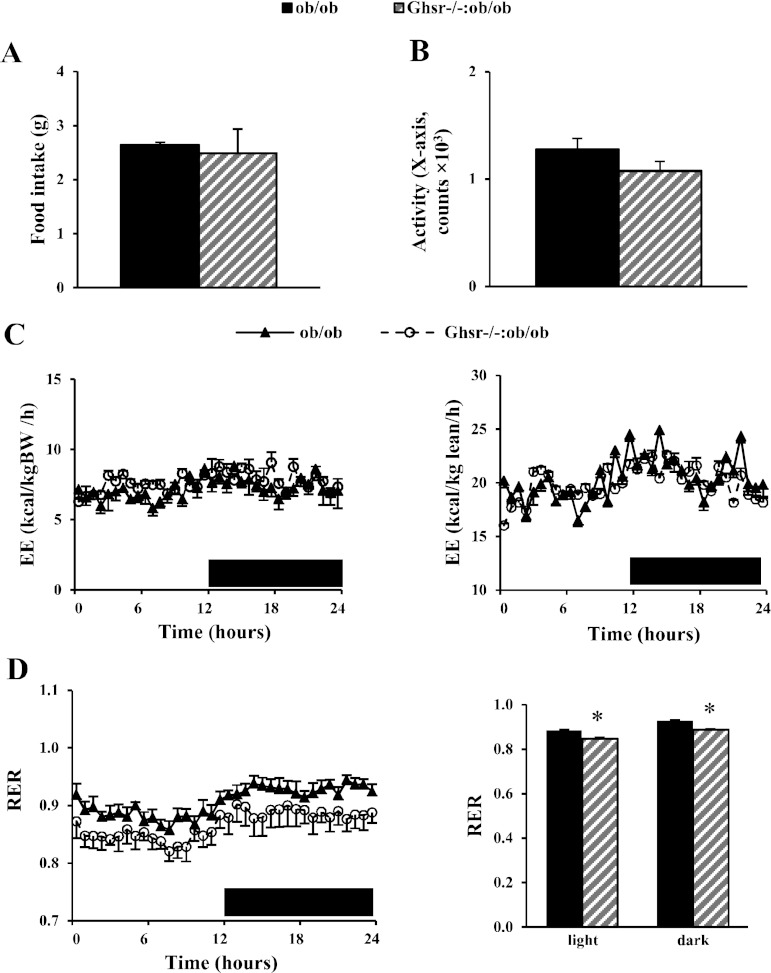
Under the ad libitum-fed condition, there was no difference in total food intake (Fig. 2A) or locomotor activity (Fig. 2B) between ob/ob and Ghsr−/−:ob/ob mice during calorimetry study. The characteristics of light and dark circadian rhythm were totally lost in mice on ob/ob the background (Fig. 2, C and D). No differences were observed in total V̇o2, V̇co2, or energy expenditure between ob/ob and Ghsr−/−:ob/ob mice whether normalized by either body weight or lean mass (Fig. 2C). The Ghsr−/−:ob/ob mice showed a lower RER compared with ob/ob mice, and the difference persisted throughout the light and dark phases (Fig. 2D). There were no differences in energy expenditure or RER between ob/ob and Ghrelin−/−:ob/ob mice (data not shown).
Fig. 2.
Metabolic profiles of Ghsr−/−:ob/ob mice. Food intake (A), activity (B), energy expenditure (EE) normalized by body weight (BW) or lean mass (C), and respiratory exchange ratio (RER; D) were analyzed using the Comprehensive Laboratory Animal Monitoring System. The data are presented as means ± SE (n = 8). *P < 0.05, ob/ob vs. Ghsr−/−:ob/ob mice.
GHS-R ablation aggravates hyperglycemia of ob/ob mice.
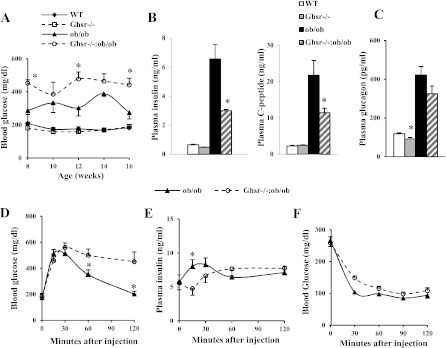
As expected, under the ad libitum-fed condition, the lean mice were euglycemic, and the ob/ob mice developed hyperglycemia when compared with lean mice. Surprisingly, in contrast to the improved glycemic condition observed in Ghrelin−/−:ob/ob mice, the Ghsr−/−:ob/ob mice exhibited worsened hyperglycemia (Fig. 3A) and reduced plasma insulin and C-peptide (Fig. 3B) compared with ob/ob mice. Whereas plasma glucagon levels of Ghsr−/− mice were significantly lower than those of WT mice, the glucagon levels in Ghsr−/−:ob/ob mice showed a trend of decrease compared with that of ob/ob mice, but this trend failed to reach statistical significance (Fig. 3C). These results suggest that ablation of GHS-R further exacerbates hyperglycemia of ob/ob mice by inhibiting insulin secretion.
Fig. 3.
Ghsr−/−:ob/ob mice have more severe hyperglycemia than ob/ob mice. A: fed blood glucose concentrations at various ages. B and C: plasma insulin and C-peptide and glucagon concentrations at 16 wk of age. D and E: blood glucose and plasma insulin concentrations during glucose tolerance tests after 18 h of fasting. F: blood glucose concentrations during insulin tolerance tests after 6 h of fasting. The data are presented as means ± SE (n = 8). *P < 0.05, ob/ob vs. Ghsr−/−:ob/ob mice.
GHS-R ablation inhibits insulin secretion of ob/ob mice but has no effect on insulin sensitivity. A low-dose (0.625 g/kg) ip glucose tolerance test (GTT) was selected to study the glucose and insulin responses of Ghsr−/−:ob/ob mice, because mice on ob/ob background are glucose intolerant (44). Compared with ob/ob mice, Ghsr−/−:ob/ob mice displayed increased blood glucose excursions at 30, 60, and 120 min following an ip glucose bolus (Fig. 3D); first-phase (15 min) plasma insulin concentrations were decreased in Ghsr−/−:ob/ob mice compared with ob/ob mice (Fig. 3E). To elucidate whether the worsened hyperglycemia of Ghsr−/−:ob/ob mice was also attributable to insulin sensitivity, ITTs were performed. No statistically significant differences were observed in blood glucose concentrations between ob/ob and Ghsr−/−:ob/ob mice (Fig. 3F). These data suggest that GHS-R ablation in leptin-deficient mice further impairs β-cell insulin secretory function but has no effect on insulin sensitivity.
Morphology of pancreatic islets in Ghsr−/−:ob/ob mice.
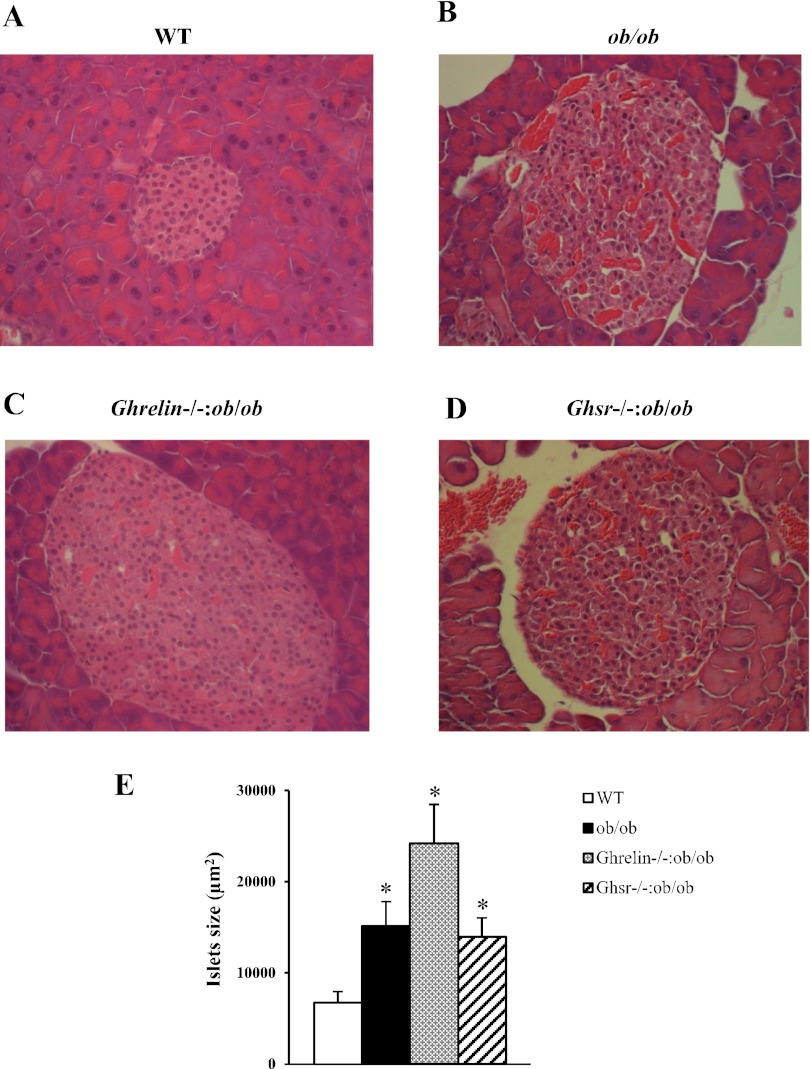
Although obese mice (ob/ob, Ghrelin−/−:ob/ob, and Ghsr−/−:ob/ob) had significantly enlarged islets compared with lean WT mice (Fig. 4, A–D), there was no difference in islet size between ob/ob and Ghsr−/−:ob/ob mice. On the other hand, Ghrelin−/−:ob/ob mice appeared to have larger islets than ob/ob and Ghsr−/−:ob/ob mice, but the difference failed to reach statistical significance (P = 0.08) due to the wide variation in islet size (Fig. 4E). We noted that ob/ob and Ghsr−/−:ob/ob mice appeared to have more islet vascularization than WT and Ghrelin−/−:ob/ob mice (Fig. 4, A–D). Ghsr−/−:ob/ob mice had pancreatic islet morphology similar to that of ob/ob mice but were different from that of Ghrelin−/−:ob/ob mice.
Fig. 4.
Histological analysis of pancreatic islets. Pancreas sections from age-matched WT, ob/ob, Ghrelin−/−:ob/ob, and Ghsr−/−:ob/ob mice were stained with hematoxylin and eosin for morphological characterization. The data are presented as means ± SE (n = 3). *P < 0.05, WT vs. ob/ob, Ghrelin−/−:ob/ob, and Ghsr−/−:ob/ob mice. P = 0.08, ob/ob vs. Ghsr−/−:ob/ob mice.
Expression profiles of β-cell regulatory genes in whole pancreas of ob/ob, Ghrelin−/−:ob/ob, and Ghsr−/−:ob/ob mice.
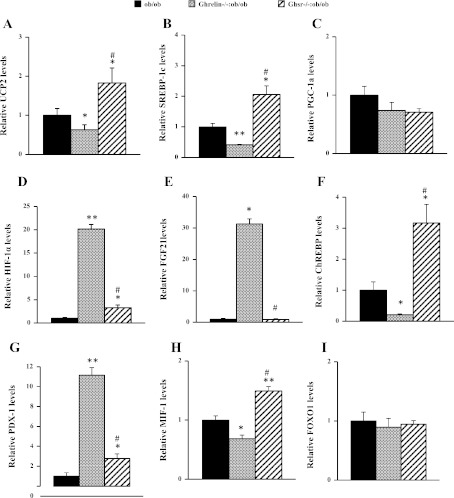
We studied the expression of regulators involved in insulin secretion and pancreatic β-cell mass. We showed previously that pancreatic uncoupling protein 2 (UCP2) mRNA expression is decreased in whole pancreas of Ghrelin−/−:ob/ob mice (44). In contrast, the Ghsr−/−:ob/ob mice have increased UCP2 expression in whole pancreas compared with ob/ob mice (Fig. 5A). The sterol regulatory element-binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) are positive transcriptional regulators of UCP2, targeting the E-box and TRE regions of UCP2 promoter, respectively (32). Indeed, SREBP-1c levels in whole pancreas were decreased in Ghrelin−/−:ob/ob mice but increased in that of Ghsr−/−:ob/ob mice (Fig. 5B), which is in line with UCP2 levels shown in these mouse models; however, there is no difference in PGC-1α levels (Fig. 5C). Whereas UCP2 decreases ATP levels in β-cells, hypoxia-inducible factor-1α (HIF-1α) exerts its effect on β-cell function by stimulating ATP (9). Fibroblast growth factor-21 (FGF-21) has been shown to improve β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and the Akt signaling pathway (52). Remarkably, although we detected significant increases in the expression of both HIF-1α and FGF-21 in whole pancreases of Ghrelin−/−:ob/ob mice, we found much lower levels of HIF-1α and FGF-21 expression in those of Ghsr−/−:ob/ob mice (Fig. 5, D and E). Carbohydrate response element-binding protein (ChREBP) is a key regulator of glucose metabolism and fat storage (15). Pancreatic and duodenal homeobox-1 (PDX-1) is a transcription factor necessary for pancreatic development and β-cell maturation. It has been shown that PDX-1 is negatively regulated by ChREBP (13). Our results showed that ChREBP expression was decreased in whole pancreases of Ghrelin−/−:ob/ob mice but increased in thos of Ghsr−/−:ob/ob mice (Fig. 5F). In contrast, PDX-1 showed an opposite expression pattern in whole pancreas of both double-null mice compared with ChREBP (Fig. 5G). Deficiency of macrophage migration inhibitory factor-1 (MIF-1) has been shown to protect pancreatic islets from palmitic acid-induced apoptosis (40). Yet whereas we detected lower levels of MIF-1 in whole pancreases of Ghrelin−/−:ob/ob mice, we observed higher levels of ChREBP in Ghsr−/−:ob/ob mice (Fig. 5H). In addition, we have studied expression of forkhead box protein O1 (FOXO1). FOXO1 has been shown to protect pancreatic β-cells from fatty acid insult (31), but FOXO1 levels did not change in our mouse models (Fig. 5I). Collectively, these data exemplify the dramatic differences between the pancreatic gene expression profiles of Ghrelin−/−:ob/ob and Ghsr−/−:ob/ob mice, suggesting that whereas ablation of ghrelin in ob/ob mice improves β-cell function, ablation of Ghsr in ob/ob mice may worsen β-cell function.
Fig. 5.
The mRNA expression of negative and positive β-cell regulatory genes in pancreata of obese mice. Uncoupling protein 2 (UCP2; A), sterol regulatory element-binding protein-1c (SREBP-1c; B), perxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; C), hypoxia-inducible factor-1α (HIF-1α; D), fibroblast growth factor-21 (FGF21; E), carbohydrate response element-binding protein (ChREBP; F), pancreatic and duodenal homeobox-1 (PDX-1; G), macrophage migration inhibitory factor-1 (MIF-1; H), and forkhead box protein O1 (FOXO1; I). The data are presented as means ± SE (n = 9–12). *P < 0.05 and **P < 0.001, ob/ob vs. Ghrelin−/−:ob/ob mice or ob/ob vs. Ghsr−/−:ob/ob mice. #P < 0.05, Ghrelin −/−:ob/ob vs. Ghsr−/−:ob/ob mice.
DISCUSSION
The best-known functions of ghrelin are its roles in stimulating GH release and promoting appetite and adiposity. We and others have shown that ghrelin is a negative regulator of insulin secretion (16, 44, 45). We reported that ablation of ghrelin expression augments insulin secretion and improves insulin sensitivity, resulting in the improvement of hyperglycemia and glucose intolerance in diabetic ob/ob mice (44). Blockade of ghrelin has also been shown to increase insulin secretion and prevent glucose intolerance induced by a high-fat diet (16). These findings suggest that suppressing ghrelin signaling prevents both genetically and environmentally induced β-cell impairments. It has thus been speculated that GHS-R antagonists may serve as antidiabetic agents. Although the effects of deletion and/or pharmacological blockade of GHS-R on glucose homeostasis have been examined in normal lean mice (11, 29, 60), the effect of GHS-R in obese and diabetic subjects is unknown. Most diabetic patients are obese, so it is important to understand the role of GHS-R on glucose homoeostasis under an obese diabetic state.
Leptin and ghrelin have opposite effects on food intake and body weight regulation. It is thus important to understand the interplay between leptin signaling and ghrelin signaling. Both the leptin receptor and the ghrelin receptor are expressed within the same nuclei of the hypothalamic arcuate nucleus, which regulate appetite and satiety (35). One report showed that the effects of ghrelin on GH secretion and food intake are suppressed in leptin receptor-knockout db/db mice, suggesting that cross-talk exists between the ghrelin- and leptin-signaling pathways (24). Another report showed that ghrelin receptor deficiency does not impact the anorexigenic effect of leptin, suggesting that ghrelin and leptin may act on separate and distinct neuronal populations (35). In diet-induced obese mice, the circulating ghrelin level is lower, and the role of ghrelin on food intake is suppressed (5, 24). In obese leptin-resistant Zucker rats, GHRP-6-induced Fos response is increased, but central infusion of leptin suppresses this GHRP-6-induced Fos response (22). These studies suggest that ghrelin-regulatory circuits in the hypothalamus are dynamically regulated, and the regulation may vary based on nutritional states.
To study the role of GHS-R on glucose homeostasis under obese and diabetic conditions, we bred the Ghsr−/− mice with leptin-deficient ob/ob mice to generate Ghsr−/−:ob/ob mice. Similarly to Ghrelin−/−:ob/ob mice, we observed that GHS-R ablation in ob/ob mice failed to rescue the hyperphagic or obese phenotypes of ob/ob mice (Fig. 1, A–C). Plasma lipids, such as plasma levels of triglyceride, cholesterol, HDL, LDL, and FFA, play important roles in insulin resistance and glucose homeostasis. Here, we found that Ghsr−/−:ob/ob mice had lipid profiles similar to those of ob/ob mice (Fig. 1, D and E). These findings indicate that unopposed action of ghrelin or GHS-R in ob/ob mice is not the underlying cause of leptin-dependent obesity and that leptin has a dominant effect on energy homeostasis.
We reported recently that ghrelin ablation and Ghsr ablation have distinct effects on energy expenditure in older mice; older Ghsr−/− mice (but not older Ghrelin−/− mice) have an elevated energy expenditure (30) due to increased thermogenesis in brown adipose tissue (27). Characterization of the metabolic state of Ghsr−/−:ob/ob mice revealed no differences in energy intake or locomotor activity (Fig. 2, A and B). Regardless of normalizing by body weight or lean mass, the energy expenditure of Ghsr−/−:ob/ob mice was no different from that of ob/ob mice (Fig. 2C). It is known that ob/ob mice have almost no brown adipose tissue, which makes them severely thermogenetically impaired (23). The lack of brown adipose tissue in Ghsr−/−:ob/ob may thus obscure the effect of the GHS-R on energy expenditure. Interestingly, Ghsr−/−:ob/ob mice had a significant reduction in RER during both the light and the dark periods (Fig. 2D), indicating that the Ghsr−/−:ob/ob mice preferentially utilize fat as an energy source under the leptin-deficient background. Our data are consistent with previous reports that Ghrelin−/− and Ghsr−/− mice have decreased RER in a diet-induced obese state (54, 60). However, the body composition analysis (Fig. 1C) showed that the preferential fat consumption associated with GHS-R ablation is insufficient to rescue the obesity of ob/ob mice. This again supports the conclusion that leptin plays a dominant role in regulating energy metabolism and body composition.
In surprising contrast to Ghrelin−/−:ob/ob mice, we found that Ghsr−/−:ob/ob mice exhibited higher basal glucose and lower insulin and C-peptide levels than ob/ob mice (Fig. 3, A and B). C-peptide, generated during proinsulin processing and secreted along with insulin, is a more accurate measurement for insulin secretion than plasma insulin itself, because plasma insulin concentrations can also be affected by degradation (55). The decreased C-peptide levels along with lower insulin concentrations confirm that insulin secretion is indeed reduced in Ghsr−/−:ob/ob mice. GHS-R is reported to be expressed in both α- and β-cells of pancreatic islets (14, 25). Chuang et al. (11) showed that ghrelin injections increase blood glucose and plasma glucagon in wild-type mice; consistently, they showed that GHS-R knockout mice have lower glucagon and fasting blood glucose. This suggests that ghrelin's regulation of blood glucose may also involve stimulation of glucagon secretion from α-cells. Similar to the observation made by Chuang et al. (11), we detected lower glucagon levels in Ghsr−/− mice compared with those of WT mice (Fig. 3C). As expected, both obese ob/ob and Ghsr−/−:ob/ob mice were hyperglucagonemic compared with lean WT and Ghsr−/− mice. Although there was a trend toward decreasing glucagon in Ghsr−/−:ob/ob mice compared with that of ob/ob mice, it did not reach statistical significance (Fig. 3C). The discrepancy between the glucagon phenotype observed in Ghsr−/−:ob/ob mice vs. that of Ghsr−/− mice might be due to the nutritional state of the double-null mice and/or the leptin-deficient background. Since we did not detect significant elevation of glucagon in Ghsr−/−:ob/ob mice, the worsened hyperglycemia of the double-mutant mice cannot be explained by elevated glucagon secreted by α-cells.
Our hormonal analysis data suggest that the Ghsr ablation in ob/ob mice decreases insulin secretion. In agreement, glucose tolerance tests revealed that Ghsr−/−:ob/ob mice have worsened glucose tolerance, showing increased glucose but reduced insulin when compared with ob/ob mice (Fig. 3, D and E). First-phase insulin secretion is the earliest detectable sign of prediabetes (36). In line with the attenuated glucose response in Ghsr−/−:ob/ob mice, first-phase (15 min) plasma insulin concentrations were decreased in Ghsr−/−:ob/ob mice compared with ob/ob mice, supporting that Ghsr ablation attenuates insulin secretion in the leptin-deficient background (Fig. 3E). GHS-R ablation in ob/ob mice does not have a significant impact on insulin sensitivity (Fig. 3F), suggesting that insulin sensitivity does not contribute to the worsened hyperglycemia and glucose intolerance of Ghsr−/−:ob/ob mice. Collectively, our data suggest that Ghsr ablation in ob/ob mice does not affect glucose counterregulation or insulin sensitivity but further diminishes insulin secretion and aggravates hyperglycemia. This surprising outcome reveals that ghrelin and GHS-R have distinct effects on insulin secretion in ob/ob mice.
Pancreatic function can be affected by islet function or islet cell mass. The ob/ob mice have increased islet cell mass, due primarily to islet cell hyperplasia and hypertrophy (26, 49). To exclude the possibility that the reduced pancreatic function of Ghsr−/−:ob/ob mice was due to reduced islet cell mass, we performed histological examinations by H & E staining to evaluate islet size. As expected, we found that islet size of obese (Ghrelin−/−:ob/ob, Ghsr−/−:ob/ob, and ob/ob) mice was significantly greater than that of lean WT mice, but there was no difference in islet size between ob/ob and Ghsr−/−:ob/ob mice (Fig. 4E). This suggests that the islet impairment in Ghsr−/−:ob/ob mice is likely due to the effect of GHS-R on β-cell function but not on islet cell mass. It is intriguing that the islet blood vessel distribution of Ghrelin−/−:ob/ob mice is similar to normal WT lean mice, whereas the islet vasculature morphology of Ghsr−/−:ob/ob mice closely resembles that of ob/ob mice (Fig. 4, A–D). The ob/ob mouse islets are more sensitive to sympathetic inhibition of catecholamines in the circulation (39) and have reduced capacity for blood flow (28). This vascular feature of ob/ob mice increases β-cell stress and causes more islet cell damage. These morphology data suggest that ghrelin ablation may improve islet vasculature of ob/ob mice, but Ghsr ablation does not. Again, this is in line with our observation that ghrelin−/−:ob/ob and Ghsr−/−:ob/ob mice have differential diabetic phenotypes. Ghsr ablation-associated pancreatic impairment is likely attributable to the effect of GHS-R on pancreatic function but not islet cell mass.
Mitochondrial UCP2 is a negative regulator of pancreatic β-cell function. UCP2 decreases ATP production and results in a reduced ATP/ADP ratio, thereby inhibiting insulin secretion in pancreatic β-cells (17, 56). Overexpression of UCP2 leads to reduced insulin secretion (7); in contrast, UCP2-deficient mice have increased insulin secretion (56). UCP2-ablated ob/ob mice have restored first-phase insulin secretion and improved glycemia (56). Previously, we showed that Ghrelin−/−:ob/ob mice have attenuated hyperglycemia and improved β-cell function, compared with that of ob/ob mice, resulting from downregulation of UCP2 (44). Our current study shows that pancreatic UCP2 mRNA was increased significantly in whole pancreas of Ghsr−/−:ob/ob mice compared with that of ob/ob mice (Fig. 5A). Similarly, Ghsr ablation in ob/ob mice upregulated negative β-cell regulators (such as SREBP-1c, ChREBP, and MIF-1) and downregulated positive β-cell regulators (such as HIF-1α, FGF-21, and PDX-1) (Fig. 5, B–I) in whole pancreas. The differential gene expression profiles are in line with the worsened hyperglycemia in Ghsr−/−:ob/ob mice and the improved glycemic control in Ghrelin−/−:ob/ob mice. This supports our conclusion that ghrelin ablation in ob/ob mice improves pancreatic β-cell function, whereas Ghsr ablation in ob/ob mice impairs pancreatic β-cell function. It is worth noting that the pancreatic phenotypes may not be a reflection of changes solely in β-cells, since the gene expression studies were carried out in whole pancreas. Pancreatic cell types other than β-cells may contribute to the pancreatic phenotypes via endocrine and/or exocrine mechanisms.
It is important to emphasize that the glycemic phenotypes of the double-null mice were observed on the leptin-deficient obese background, which are different from that observed in either ghrelin ablation or Ghsr ablation on the wild-type lean background. The glycemic phenotypes of the double-null mice likely result from the interplay between leptin signaling and ghrelin/GHS-R signaling. Our data showed that ghrelin and GHS-R in a leptin-deficient background have differential roles in glucose homeostasis. It is possible that ghrelin's inhibitory effect on insulin secretion is mediated through subtype receptor(s) other than GHS-R. Culture studies of the direct effect of ghrelin in GHS-R knockdown pancreatic β-cells or GHS-R-null islets may help to further address this question.
It is also noteworthy that three peptides, ghrelin (acylated ghrelin), des-acyl ghrelin, and obestatin, are derived from the preproghrelin gene, and all are expressed in the pancreas (8, 42). Whereas ghrelin is known to activate GHS-R, des-acyl ghrelin and obestatin do not, and their receptors are either unknown or debatable. Des-acyl ghrelin is shown to increase food intake and promote obesity (38, 48, 50, 58), whereas obestatin inhibits food intake and enhances energy expenditure (3, 57). More intriguingly, des-acyl ghrelin and obestatin have been shown to have opposing glucoregulatory effects compared with ghrelin; des-acyl ghrelin functions as a potent insulin secretagogue (19, 58), and des-acyl ghrelin and obestatin increase islet cell mass and prevent streptozocin-induced diabetes (10, 20, 37). In Ghrelin−/− mice, the signaling of ghrelin, des-acyl ghrelin, and obestatin are abolished. In Ghsr−/− mice, the signaling pathway of ghrelin is abolished, but the signaling pathways of des-acyl ghrelin and obestatin remain intact. The glycemic phenotype of Ghsr−/− mice may be a result of unopposed des-acyl ghrelin signaling and/or obestatin signaling.
Ghrelin O-acyltransferase (GOAT) is an acyltransferase that catalyzes ghrelin octanoylation. Ablation of GOAT in mice has no phenotype under a regular diet or high-fat diet. Intriguingly, at 60% calorie restriction, the GOAT-null mice show severe hypoglycemia and low circulating GH (59). This result indicates that ghrelin is essential for maintaining GH levels during severe calorie restriction to prevent hypoglycemia and death. In contrast, our studies were in overnourished obese mice, wherein ablation of ghrelin in ob/ob mice appeared to be beneficial, but ablation of GHS-R in ob/ob mice appeared to be detrimental. The differential effects of ghrelin deficiency in leptin-deficient background mice vs. the GOAT-ablated mice may be due to the difference in nutritional states of the mice and/or the effects of des-acyl ghrelin and obestatin present in GOAT-null mice. Our findings in obese and diabetic leptin-deficient mice suggest that GHS-R antagonists may be harmful when used in treating diabetes in certain obese conditions. However, since most obese humans are leptin resistant rather than leptin deficient (having a metabolic state more like leptin receptor-deficient db/db mice), further studies of the phenotype of GHS-R ablation in db/db mice may provide additional insight.
In conclusion, our data show that ghrelin ablation and GHS-R ablation have opposite effects on glycemic control of leptin-deficient ob/ob mice; ghrelin ablation improves it, and GHS-R ablation worsens it. Ghrelin and Ghsr ablation have differential effects on glucose-induced pancreatic insulin secretion in the leptin-deficient background. In contrast to ghrelin neutralization, GHS-R antagonism may inhibit pancreatic insulin secretion and has deleterious effects on pancreatic function. Ghrelin's inhibitory effect on insulin secretion may be mediated by receptor(s) other than GHS-R. Our new findings highlight the extreme complexity of the ghrelin-signaling pathway in the pancreas and demonstrate that it is critically important to distinguish the effects of ghrelin neutralization from that of GHS-R antagonism. Further studies are needed to fully understand the molecular mechanisms by which ghrelin and GHS-R regulate pancreatic β-cell function and to determine the proper therapeutic applications for ghrelin neutralization and GHS-R antagonists.
GRANTS
This work is a publication of the US Department of Agriculture/Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, and has been funded in part with federal funds from the USDA/ARS under Cooperative Agreement No. 58-6250-0-008. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement from the US government. This work was also supported by NIH/NIA Grant 1-R03-AG-029641-01 (Y. Sun), the American Heart Association 12IRG9230004 (Y. Sun), an NIH-Diabetes and Endocrinology Research Center grant at Baylor College of Medicine (P30-DK-079638), and the Lipid Core of Mouse Metabolic Phenotyping Center at Vanderbilt University (U24-DK-59637).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.M., Y.L., and Y.S. did the conception and design of the research; X.M., Y.L., and Y.S. performed the experiments; X.M. and Y.L. analyzed the data; X.M., L.L., N.F.B., and Y.S. interpreted the results of the experiments; X.M. and Y.L. prepared the figures; X.M. drafted the manuscript; X.M., Y.L., L.L., G.Q., F.A.P., M.W.H., N.F.B., and Y.S. edited and revised the manuscript; X.M., G.Q., F.A.P., M.W.H., N.F.B., and Y.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Monique Rijnkels and Marta L. Fiorotto at the Children's Nutrition Research Center and the Department of Pediatrics at Baylor College of Medicine for their insightful advice and input in real-time PCR analysis and calorimetry studies, respectively. We thank Geetali Pradhan and Michael R. Honig for their editorial assistance.
REFERENCES
- 1.Ahima RS. Antagonism of ghrelin for glycemic control in type 2 diabetes mellitus? Endocrinology 148: 5173–5174, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52: 947–952, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, Torsello A. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest 29: RC16–RC18, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Briggs DI, Andrews ZB. A recent update on the role of ghrelin in glucose homeostasis. Curr Diabetes Rev 7: 201–207, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151: 4745–4755, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol 31: 44–60, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes 48: 1482–1486, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chanoine JP, Wong AC, Barrios V. Obestatin, acylated and total ghrelin concentrations in the perinatal rat pancreas. Horm Res 66: 81–88, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cheng K, Ho K, Stokes R, Scott C, Lau SM, Hawthorne WJ, O'Connell PJ, Loudovaris T, Kay TW, Kulkarni RN, Okada T, Wang XL, Yim SH, Shah Y, Grey ST, Biankin AV, Kench JG, Laybutt DR, Gonzalez FJ, Kahn CR, Gunton JE. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest 120: 2171–2183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielewska J, Szczepankiewicz D, Skrzypski M, Kregielska D, Strowski MZ, Nowak KW. Ghrelin but not obestatin regulates insulin secretion from INS1 beta cell line via UCP2-dependent mechanism. J Biol Regul Homeost Agents 24: 397–402, 2010 [PubMed] [Google Scholar]
- 11.Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol 25: 1600–1611, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 89: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- 13.da Silva Xavier G, Sun G, Qian Q, Rutter GA, Leclerc I. ChREBP regulates Pdx-1 and other glucose-sensitive genes in pancreatic β-cells. Biochem Biophys Res Commun 402: 252–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H, Yada T, Matsukura S. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 51: 124–129, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Dentin R, Denechaud PD, Benhamed F, Girard J, Postic C. Hepatic gene regulation by glucose and polyunsaturated fatty acids: a role for ChREBP. J Nutr 136: 1145–1149, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 55: 3486–3493, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15: 269–272, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 90: 1055–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gauna C, Kiewiet RM, Janssen JA, van de Zande B, Delhanty PJ, Ghigo E, Hofland LJ, Themmen AP, van der Lely AJ. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab 293: E697–E704, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P, Arnoletti E, Ghe C, Volante M, Papotti M, Muccioli G, Ghigo E. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes 57: 967–979, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Heppner KM, Tong J, Kirchner H, Nass R, Tschöp MH. The ghrelin O-acyltransferase-ghrelin system: a novel regulator of glucose metabolism. Curr Opin Endocrinol Diabetes Obes 18: 50–55, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes 51: 3412–3419, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hukshorn CJ, Saris WH. Leptin and energy expenditure. Curr Opin Clin Nutr Metab Care 7: 629–633, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Iwakura H, Akamizu T, Ariyasu H, Irako T, Hosoda K, Nakao K, Kangawa K. Effects of ghrelin administration on decreased growth hormone status in obese animals. Am J Physiol Endocrinol Metab 293: E819–E825, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kageyama H, Funahashi H, Hirayama M, Takenoya F, Kita T, Kato S, Sakurai J, Lee EY, Inoue S, Date Y, Nakazato M, Kangawa K, Shioda S. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul Pept 126: 67–71, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lavine RL, Voyles N, Perrino PV, Recant L. Functional abnormalities of islets of Langerhans of obese hyperglycemic mouse. Am J Physiol Endocrinol Metab Gastrointest Physiol 233: E86–E90, 1977 [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Saha PK, Ma X, Henshaw IO, Shao L, Chang BH, Buras ED, Tong Q, Chan L, McGuinness OP, Sun Y. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 10: 996–1010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindström P. beta-cell function in obese-hyperglycemic mice [ob/ob Mice]. Adv Exp Med Biol 654: 463–477, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Qi Y, DiStefano PS, Geddes BJ. Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul Pept 150: 55–61, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, Sun Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One 6: e16391, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 57: 846–859, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Oberkofler H, Klein K, Felder TK, Krempler F, Patsch W. Role of peroxisome proliferator-activated receptor-gamma coactivator-1alpha in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology 147: 966–976, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J Clin Invest 109: 1599–1605, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel K, Dixit VD, Lee JH, Kim JW, Schaffer EM, Nguyen D, Taub DD. The GHS-R blocker d-[Lys3] GHRP-6 serves as CCR5 chemokine receptor antagonist. Int J Med Sci 9: 51–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol 520: 281–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poitout V, Robertson RP. An integrated view of beta-cell dysfunction in type-II diabetes. Annu Rev Med 47: 69–83, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Qader SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regul Pept 146: 230–237, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez A, Gómez-Ambrosi J, Catalán V, Gil MJ, Becerril S, Sáinz N, Silva C, Salvador J, Colina I, Frühbeck G. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 33: 541–552, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Rooth P, Taljedal IB. Vital microscopy of islet blood flow: catecholamine effects in normal and ob/ob mice. Am J Physiol Endocrinol Metab 252: E130–E135, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Saksida T, Stosic-Grujicic S, Timotijevic G, Sandler S, Stojanovic I. Macrophage migration inhibitory factor deficiency protects pancreatic islets from palmitic acid-induced apoptosis. Immunol Cell Biol. In press [DOI] [PubMed] [Google Scholar]
- 41.Salehi A, Dornonville de la Cour C, Håkanson R, Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul Pept 118: 143–150, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Soares JB, Leite-Moreira AF. Ghrelin, des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides 29: 1255–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23: 7973–7981, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3: 379–386, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 149: 843–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101: 4679–4684, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szentirmai E, Kapas L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci USA 106: 14069–14074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145: 234–242, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Tomita T, Doull V, Kimmel JR, Pollock HG. Pancreatic polypeptide and other hormones in pancreas of obese (ob/ob) mice. Diabetologia 27: 454–459, 1984 [DOI] [PubMed] [Google Scholar]
- 50.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147: 2306–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 87: 1300–1308, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55: 2470–2478, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 52: 301–310, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest 115: 3573–3578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavaroni I, Deferrari G, Lugari R, Bonora E, Garibotto G, Dall'Aglio E, Robaudo C, Gnudi A. Renal metabolism of C-peptide in man. J Clin Endocrinol Metab 65: 494–498, 1987 [DOI] [PubMed] [Google Scholar]
- 56.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105: 745–755, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science 310: 996–999, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Zhang W, Chai B, Li JY, Wang H, Mulholland MW. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 149: 4710–4716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 107: 7467–7472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115: 3564–3572, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]