Abstract
This article describes and critically appraises clinical trials assessing misoprostol effectiveness in preventing primary postpartum haemorrhage (PPH) in home and community settings in low- and middle-income countries. Of 172 identified studies of misoprostol use in labour only six fulfilled the inclusion criteria. All trials used 600μg misoprostol in the intervention arm; three assessed misoprostol alongside components of active management of the third-stage labour (AMTSL), two used expectant management of labour and one allowed birth attendants to choose management practice. The three AMTSL studies showed no significant differences in PPH incidence or referral to higher centres and only one study showed significant decrease in severe PPH using misoprostol. One expectant management study and the choice of management by birth attendants study found significant decreases in PPH incidence with misoprostol. All studies showed significantly increased risk of shivering with misoprostol. Studies were biased by use of alternative uterotonics in the control arm, confounding management practices, and subjective assessment and, with one exception, exclusion of high-risk women. PPH incidence fell in both the control and intervention groups in both the landmark papers that informed the World Health Organization (WHO) decision to admit misoprostol to the Essential Medicines List. This suggests factors other than misoprostol use are crucial. Current evidence does not support misoprostol use in home and community settings in low- and middle-income countries for PPH prevention. WHO should rethink its recent decision to include misoprostol on the Essential Medicines List.
Introduction and background
The World Health Organization (WHO) estimated that in 2008 there were 342,900 maternal deaths relating to pregnancy and childbirth;1 most of them occurred in developing countries.2 A quarter of maternal deaths are said to be associated with postpartum haemorrhage (PPH),3 defined as blood loss greater than 500 mL following a vaginal delivery.
PPH can be primary or secondary. WHO defines primary PPH as blood loss occurring within 24 hours of delivery; secondary as blood loss occurring from 24 hours to 12 weeks postnatally. This paper will consider only primary PPH.
PPH in anaemic populations is associated with increased morbidity and mortality; relative risks of maternal death for haemoglobin (Hb) levels of 40–80 and <47 g/L are 1.35 and 3.51 respectively.4 There is a high prevalence of anaemia in pregnant women (48.2% and 57% in SE Asia and Africa, respectively, compared with 25.1% in Europe)5 but in the absence of antenatal screening for anaemia, it is not possible to identify women with increased risk of significant morbidity and mortality prior to labour.
In the absence of a public health strategy to tackle maternal anaemia, the current emphasis is on primary prevention of PPH. Since the risk factors for PPH are unknown, the strategies are implemented universally. WHO guidelines6 recommend skilled birth attendants perform active management of the third stage labour (AMTSL). AMTSL consists of three interventions: prophylactic administration of a uterotonic drug, where oxytocin is the drug of choice followed by ergometrine/methylergometrine; early cord clamping and cutting; and controlled cord traction.
Oxytocin and ergometrine preparations are heat sensitive and usually require intravenous or intramuscular administration.7 Misoprostol, another uterotonic, is a synthetic E1 prostaglandin analogue that can be given in oral, sublingual, buccal, vaginal or rectal preparations and is stable. In situations where skilled birth attendants are unavailable, WHO guidelines advocate misoprostol use8 for PPH prevention while the International Confederation of Midwives and the International Federation of Gynaecology and Obstetrics (ICM/FIGO) suggest using misoprostol in all situations where oxytocin is not available.9
A Cochrane review of trials on ‘Prostaglandins for preventing Postpartum haemorrhage’10 found oxytocin to be of greater effectiveness than misoprostol. Early studies of misoprostol compared against placebo were equivocal but three recent trials, published in 2005 and 2006, appeared more promising, although the review noted that differences in trial designs and settings made comparison difficult. These three trials are included in the analysis below.11–13
Some academics and practitioners advocate misoprostol use for the prevention of PPH in home births and community settings for maternal mortality.14 In May 2011, the 18th Expert Committee on the Selection and Use of Essential Medicines approved the inclusion of misoprostol for the prevention of PPH in settings where parenteral uterotonics are not available or feasible. The decision was based on the evidence from four randomized controlled trials (RCTs) submitted to the committee.11–13,15 These four studies are included in our analysis.
This paper will identify, describe and critically appraise clinical trials assessing misoprostol use in home and community settings in low-income countries with respect to study design, intervention and outcomes.
Methods
Medline and Embase databases were searched for clinical studies assessing misoprostol use in community and home birth settings in low- and middle-income countries (defined by World Bank classification) published before November 2011 using search terms ‘PPH’; bleeding in TSL; misoprostol; RCTs; and ‘prevention’. The database search revealed two systematic reviews10,16 and further studies were also identified from the sources. Studies were excluded if duplicate, considering injectable prostaglandins, non-RCTs, not reported in English, in high-income and hospital settings.
Studies were appraised using a framework adapted from Fowkes and Fulton, Critical Appraisal Skills Programme and Cochrane guidelines for systematic review. These addressed study design (setting, number of participants, level of blinding, risk status of women, methods to measure outcomes), intervention (route and dose of misoprostol, the control arm, attendance at birth, management used in TSL) and the outcomes of the studies.
Results
Result of literature search
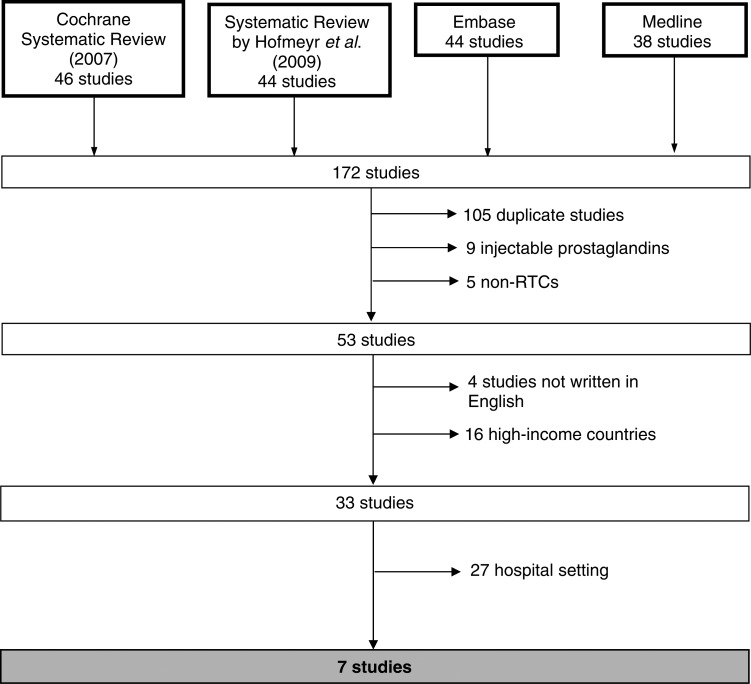
The literature search elicited 172 studies. After exclusion criteria were applied, seven studies remained (Figure 1), of which two were duplicate. Derman13 and Patted17 reported the results of their study in two separate papers: the former reporting effectiveness of misoprostol and the latter reporting side-effects experienced. Prata's study lacks clarity with respect to randomization. Its design is similar to Chandhiok's cluster randomized trial. We gave Prata's study the benefit of the doubt and included it in the analysis. Literature search identified all relevant studies considered in the Cochrane review. In addition we reviewed the references underpinning the WHO decision to add misoprostol to the WHO Essential Medicines List.
Figure 1.
Schematic of literature search
Results of studies against framework
All six studies were RCTs and used misoprostol in the intervention arm; three studies included misoprostol use alongside all or some components of AMTSL; two investigated use with expectant management, which is defined as no early cord clamping and cutting and no cord traction; one study allowed traditional birth attendants (TBAs) to manage and document how TSL was managed for each participant regardless of group.
Study design (Table 1)
Table 1.
Characteristics of misoprostol clinical studies conducted in community and home settings
| Walraven et al. (2005) | Høj et al. (2005) | Chandhiok et al. (2006) | Derman et al. (2006) & Patted et al. (2009) | Prata et al. (2009) | Mobeen et al. (2011) | |
|---|---|---|---|---|---|---|
| Gambia | Guinea-Bissau | India | India | Ethiopia | Pakistan | |
| Study design | ||||||
| Setting | 26 primary healthcare (PHC) villages | Local primary health centre | Rural primary health centres | 4 primary healthcare areas | Home | Home |
| Number of participants | 1229 (Control n = 599; Intervention n = 630) | 661 (Control n = 331; Intervention n = 330) | 1200 (Controls n = 600; Misoprostol n = 600) | 1616 (Control n = 807; Intervention n = 809) | 966 (Control n = 481; Intervention n = 485) | 1116 (Control n = 583; Intervention n = 533) |
| Blinding | Double blind | Double blind | No blinding for participant or attendant | Double blind | No blinding for participant or attendant | Double blind |
| Risk status of women | Low risk (exclusion of nullipara, grand multipara, complications requiring hospital referral) | Low and high risk | Low risk (exclusion of systemic disease, those at ‘high risk’, previous uterine surgery, multiple pregnancy) | Low risk (exclusion of those unsuitable for home or subcentre births per India guidelines) | Low risk (exclusion chronic diseases; high-risk pregnancies as identified by the provider; previous caesarean section) | Low risk (exclusion of those with pregnancy complications including hypertension, Hb <8 g/dL, non-cephalic presentation) |
| Method of blood loss measurement | Objective: for a minimum of one hour | Objective: for one hour after delivery | Objective: at one hour, and if persistent for a further hour | Objective: at one hour, and if persistent for a further hour | Subjective: visually estimated | Objective: for a minimum of one hour or until active bleeding stopped |
| Method of Hb measurement | Antenatal visit and 3–5 days after delivery | Finger prick before and 24 hours after delivery | None done | None done | None done | Finger prick in third trimester and 3–5 days after delivery |
| Intervention | ||||||
| Misoprostol | Oral 600 μg | Sublingual 600 μg | Oral 600 μg | Oral 600 μg | Oral 600 μg | Oral 600 μg |
| Comparison | 2 mg ergometrine orally | Placebo | Normal practice 88.5% 0.2 mg methergine IM, 9.7% 0.125 mg methergine oral, 1.8% no uterotonic | Placebo | Placebo | Placebo |
| Attendant at birth | Trained traditional birth attendant | Auxiliary nurse midwife | Paramedical worker | Auxiliary nurse midwife | Traditional birth attendant | Trained traditional birth attendant |
| Management in TSL | Controlled cord traction but delayed cord cutting | Controlled cord traction and cord clamp and cut at delivery | Varied between intervention and comparison group | Expectant management | Expectant management | Undetermined; Controlled cord traction in 28.3% controls and 28.8% intervention |
Hb, haemoglobin; IM, intramuscular
Setting and size
Numbers of women recruited to each study ranged from 661 to 1616; all six studies were conducted in home or community primary healthcare settings of India, Gambia, Guinea-Bissau, Ethiopia and Pakistan.
Blinding
Four studies were double-blind trials (two AMTSL, one expectant management and one where management could be chosen by the birth attendant). In both Prata's18 and Chandhiok's19 studies, there were designated intervention and control sites, and therefore blinding of participants and birth attendants was not possible.
Risk status of women
Five of the six studies excluded women believed to be at high risk of developing PPH or at risk of a poor outcome if complications were to occur. Høj was the only study that included all women giving birth at the health centre. The definition of ‘high risk’ varied across studies and included previous uterine surgery, multiple pregnancy, grand multipara and haemoglobin levels <80 g/L.
Method of blood loss measurement
Five studies used drapes and weights, and period of blood loss ranging from one hour after delivery or until active bleeding ceased. Prata's study stated that objective measurement was unfeasible and therefore blood loss was visually estimated.
Intervention and controls (Table 1)
Intervention arm
All studies used 600 μg misoprostol in the intervention group, with five studies administering it orally and one sublingually.
Control arm
Four studies used placebo and two studies used alternative uterotonics in the control arm. Walraven used 2 mg oral ergometrine and Chandhiok followed ‘normal practice’ where 88.5% used 0.2 mg methergine intramuscular, 9.7% took 0.125 mg oral methergine and 1.8% no uterotonic.
Attendant at birth
The six studies reported attendants at birth as TBAs, trained TBAs, auxillary nurse midwives and paramedical workers. The proficiency of the attendants was hard to ascertain, as there was limited and varying information provided regarding ability or duration and scope of training in the papers.
Management of TSL
Walraven's study trained TBAs to use controlled cord traction, one component of AMTSL, but no early cord cutting. Attendants in Høj's study were auxillary nurse midwives who used both traction and early cord clamping and cutting. Paramedical workers in Chandhiok's study had different management practices for the intervention group where mothers experienced AMTSL, and the control group where most mothers (94.2%) underwent expectant management. Derman and Prata studied misoprostol use alongside expectant management. Mobeen claimed that TBAs assisting deliveries were trained in management of TSL including uterine massage, cord traction, delayed cutting of cord and immediate suckling of breast. TBAs were allowed to choose management practice. The intervention and control groups were comparable with cord traction performed in 28.3% and 28.8% of cases, respectively, with similar results for the other third-stage management techniques.
Outcomes (Table 2)
Table 2.
Outcomes and results of misoprostol clinical studies conducted in community and home settings
| Outcome measures | Walraven et al. (2005) | Høj et al. (2005) | Chandhiok et al. (2006) | Derman et al. (2006) & Patted et al. (2009) | Prata et al. (2009) | Mobeen et al. (2011) |
|---|---|---|---|---|---|---|
| Gambia | Guinea-Bissau | India | India | Ethiopia | Pakistan | |
| PPH (blood loss >500 mL) | Not significant | Not significant | Not significant | |||
| RR 0.91 (0.67–1.24) | RR 0.89 (0.76–1.04) | Miso 0.7% vs Con 0.8% | RR 0.53 (0.39–0.74) | RR 0.76 (0.59–0.97) | ||
| Severe PPH (blood loss >1000 mL) | Not significant | Not significant | ||||
| RR 0.48 (0.09–2.59) | RR 0.66 (0.45–0.98) | RR 0.20 (0.04–0.91) | RR 0.57 (0.27–1.22) | |||
| Mean blood loss | Not significant | Not significant | Not significant | P < 0.0001 | Not significant | |
| Mean difference −8 to 31 | Mean difference −0.5% to 20.4% | Miso 139.7 ± 110.4 vs Con 211.0 ± 83.4 | Miso 214.3 (SD 144.6) vs Con 262.3 (SD 203.2) | Miso 337 (SD 226) vs Con 366 (SD 262) | ||
| Duration of TSL (mins) | Miso 7.9 ± 4.2 vs Con 10.9 ± 4.3 | |||||
| Drop in Hb >2 g/dL | RR 0.77 (0.6–0.98) | Not significant, RR 0.79 (0.62–1.02) | ||||
| Mean Hb conc. change | RR 0.17 (0.06–0.29) | Not significant Mean difference mmol/l RR 0.16 (−0.01 to 0.32) | ||||
| Additional uterotonic | Not significant | Not significant (0.4% vs 0.7% P = 0.3413) | OR 0.42 (0.28–0.61) | |||
| Miso n = 4 vs Con n = 3 | ||||||
| Blood transfusion | Not significant | Miso 0.1% vs Con 0.9% (P = 0.0382) | OR 0.13 (0.04–0.36) | |||
| Miso n = 1 vs Cont n = 0 | ||||||
| Side-effects | Shivering, RR 2.74 (2.14–3.52) | Shivering, RR 2.43 (1.96–3.01) | Mild shivering, Miso 36% vs Con 18.7% | Shivering, Miso 52.2% vs Con 17.3% | Shivering, OR 2.56 (1.59–4.11) | Shivering, Miso 9.4% vs Con 3.9% |
| Vomiting, RR 0.5 (0.29–0.88) | Fever RR, 7.09 (3.84–13.1) | Nausea Miso, 10.2% vs Con 20.2% | Fever Miso, 4.2% vs Con 1.1% | Sweating, OR 2.24 (1.62–3.12) | Chills/cold, Miso 9.9% vs Con 5% | |
| Referral to higher health facility | Not significant | Miso 0.5% vs Con 1.5% (P = 0.0475) | OR 0.42 (0.28–0.61) | Not significant (PPH P = 0.542, retained placenta P = 0.538, Multiple cause P = 0.579) | ||
| Miso 0.3% vs Con 0.3% | ||||||
| Other outcomes | Postpartum anaemia <8 g/dL – discrepancy in results reported | 10% fall in Hb conc., RR 0.92 (0.74–1.14) | Surgery intervention | IV fluids | Drop in Hb >3 g/dL | |
| Blood loss >1500 mL RR 0.28 (0.12–0.64) | Miso 0.1% vs Con 1.0% (P = 0.0209) | OR 0.18 (0.07–0.44) | RR 0.53 (0.34–0.83) |
All results significant unless stated otherwise
IV, intravenous
No study used maternal mortality as a primary outcome measure. The primary outcome for Høj, Derman, and Mobeen was incidence of PPH; the remaining studies documented PPH incidence, blood loss, duration of TSL, postpartum Hb <8 g/dL, referrals to higher health facilities and need for additional interventions. All six reported a significantly increased risk of shivering when using misoprostol. Increased fever was reported in two studies, and both sweating and vomiting were reported as significant in one study.
Results of the three AMTSL studies
There were no significant differences between control and misoprostol groups with regard to PPH incidence (blood loss >500 mL) in the three studies.
The Chandhiok study was problematic in that management of TSL differed between the intervention and control group and varied within the control group. The Walraven study presents conflicting results claiming significantly lower rates of postpartum anaemia in the text presented as odds ratio, whereas the table of results shows statistically non-significant relative risk results. Hb results in this study are hard to interpret since the baseline between the two groups were different (0.01–0.33, P = 0.04), and therefore had to be adjusted. Høj was the only study to use placebo in the control group and found significant results for severe blood loss only (1000–1500 and >1500 mL).
Expectant management study results
Both studies compared misoprostol against a placebo and found significant differences in outcomes. Derman found significant reductions in blood loss, need for referral and use of transfusion or surgical intervention in women receiving misoprostol. A temporal trend, with a decreasing incidence of PPH in both misoprostol and control groups over time, was also noted. Non-significant results were found for need for additional uterotonics and maternal mortality measures. However, the study is biased by extensive criteria used to exclude high-risk women. All outcome measurements of referral and additional interventions in Prata's study were significantly lower in the intervention group, but the lack of blinding of attendant and subjective visual estimation of blood loss open the study to bias.
No predetermined management results
In contrast to Høj's findings, in the Mobeen study misoprostol was only found to cause significant reduction in PPH incidence of over 500 mL and no significant reduction for blood loss over 750 or 1000 mL. In the same study, non-significant differences were found between intervention and control groups for a decrease of Hb >2 g/dL, but statistical significance was found for Hb decrease of >3 g/dL. These Hb results seemingly contradict the blood loss results. Interestingly, a trend over time was also noted, with both PPH and Hb outcomes only reaching statistical significance in the second year of the study.
DISCUSSION
Evidence for misoprostol use
All six studies concluded that misoprostol holds beneficial effects; however, the results of the studies do not fully support the conclusions. Moreover, all studies show limitations with regard to study design, exclusion criteria, intervention and controls, and use of outcomes.
Study design
Only four of the six studies were double-blind trials. Randomisation in Prata's study is not known and other issues included lack of blinding as the outcome measures of referral relied on subjective visual estimation of blood loss. Visual estimation is notoriously unreliable and usually underestimated.20 Therefore, the study may underestimate blood loss in control groups and promote referral and administration of additional interventions for women in the known control group. The lack of blinding in Chandhiok's study was not as problematic, as blood loss was measured objectively; however, reporting of side-effects may have been influenced. It is also unreported as to whether referral to higher health facilities or administration of additional interventions were based on blood loss measures or solely based on the attendant's subjective decision. Another major limitation in Chandhiok's study is additional confounding factors. The intervention group received misoprostol with AMTSL; the control group followed ‘routine practice’ that varied with regard to the pharmaceutical intervention where some women received alternative uterotonics while others took no uterotonics and also TSL was predominantly managed expectantly (94.2%). The differences in the management of the third stage confound the interpretation and misoprostol cannot be attributed as the sole causative agent of the significant results found. These two trials have not been used by the WHO in their recent assessment of misoprostol efficacy for addition to the Essential Medicines List.
The use of alternative uterotonics in the control group also poses problems with interpreting results, as it may show equivalence of the two drugs rather than any definite benefit of misoprostol. Walraven study used 2 mg oral ergometrine tablets for the control arm. Clinical trials and subsequent recommendations show oral ergometrine having no clinical effect on reducing blood loss in the postpartum period.21,22 Additionally, the control group experienced a PPH incidence of 12% compared with 11% in the misoprostol group; a non-significant result was found for PPH (blood loss >500 mL) when used as an outcome. The literature suggests that PPH incidence rates for women receiving no prophylactic treatment is around 10–15%;11–13,19 therefore, it is difficult to conclude whether misoprostol had any effect.
Exclusion criteria
Five of the six studies excluded high-risk women, i.e. those deemed to be at high risk of developing PPH or those prone to suffer poor outcomes. Results from both Derman and Mobeen have been cited by the WHO and used as evidence of misoprostol effectiveness in home births with unskilled attendants at birth. However, both studies have extensive exclusion criteria.
In the Derman study, all women unsuitable for home or subcentre births according to India's guidelines were excluded. Of the 4248 women assessed for eligibility into the study, 2628 were not randomized into the study for the following reasons: ineligible (n = 2066) due to plans not to deliver at home or at the subcentre, being high risk, or normal vaginal delivery was unlikely; refusal to participate (n = 185); birth attendant not present at birth (n = 324) and medication unavailable (n = 53). This meant only 38% of women assessed were finally included in the trial.
Mobeen excluded women presenting with a pregnancy complication including but not limited to hypertension, Hb <8 g/dL, and previous C-section so that of the 1384 women screened for eligibility, only 1119 (81%) were included in the study. The main reasons for exclusion were ineligible at antenatal screening or referral to higher care by TBA (n = 92), delivery outside the study area (n = 55), and TBA or supplies not available during birth (n = 53).
The Walraven study excluded high-risk women (44%) during initial assessment at the mobile antenatal clinic. However, these women were allowed to re-enter the study if a TBA was called to attend the delivery and transfer to a health facility was not possible at the time (n = 492; 40% of the final sample). Høj's study included all the 661 women assessed.
To conclude that misoprostol is both safe and effective would be premature in light of the number of women being excluded from these trials. It should be noted that these studies also required the birth attendant to be present; 377, 53 and 120 women delivered without attendant in Derman, Mobeen and Walraven studies, respectively, and all were excluded. In all four studies included in the WHO assessment of misoprostol for the Essential Medicines List, the attendants were able to assess antenatal complications, manage uncomplicated labour and detect obstetric complications. It is therefore important to consider that the outcomes of the studies may have been influenced by the skill of the birth attendants. In many areas there is limited access to personnel with these skills, and therefore women cannot be assessed for their suitability for misoprostol.
Høj's study did include high-risk women, placebo was used in control group and blood loss was measured objectively. This study may suggest misoprostol being increasingly effective against severe levels of blood loss. However, these were women delivering at the local health centre with trained midwives performing AMTSL.
Temporal trends
The Derman study showed that PPH rates for sequential subgroups of women recruited in the three-year study period in the placebo group fell from around 17% to 7% over the course of the study, i.e. to levels similar to the first three subgroups for misoprostol (Figure 2). There is no more information provided on how sequential subgroups were allocated.
Figure 2.
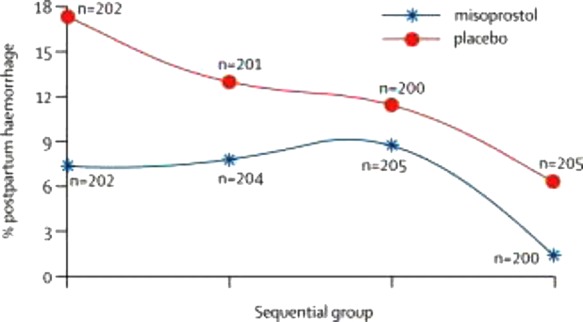
From Derman study showing temporal trends over the course of the trial
A further post hoc analysis revealed that midwives recruited later in the study were more experienced, or that cumulative training and monitoring over the three-year study period improved the attendants’ skills. Enhanced skills were associated with a shorter second stage of labour and resulted in a lower rate of PPH.23
In the Mobeen study there was no differences found between the misoprostol and control group with regard to PPH outcomes (>500 or >1000 mL) or Hb difference outcomes (>2 or >3 g/dL) in the first year of study from June 2006 to May 2007. However, during the second year, a statistical difference was found between the two groups across all outcomes. It was noted in the discussion that ‘analysis of other outcome variables shows significant improvements in both antenatal and delivery care in the last year of the study, in comparison with the first year’; in a later reply to correspondence it was noted that compared with the first year of study, there were significantly more antenatal visits (P < 0.0001), decreased occurrence of prolonged or obstructed labour leading to fewer referrals (P = 0.0002) and fewer neonatal deaths (P = 0.043) in the second year of study. The study team concluded that these findings were a result of continual support, skill building and training given to TBAs.
These findings highlight the importance of factors other than pharmacological intervention, namely training of birth attendants. However, it is difficult to attribute the improvements of outcome due to limited data. AMTSL techniques are recognized as the most effective method in reducing PPH incidence and a recent systematic review concluded its practice reduces bleeding in the postpartum period significantly.24 The WHO technical report detailing reasoning behind the addition of misoprostol to the Essential Medicines List refers to a study by Walraven et al.,25 which lists five key barriers to reduction in PPH associated deaths: socioeconomic and cultural barriers preventing access to healthcare; lack of community awareness of poor maternal health; lack of skilled health providers with midwifery skills at every birth; lack of health facilities with adequate transportation, equipment and referral structures; and lack of specialist obstetricians to contribute to training. It is surprising that the distribution of misoprostol is detailed as a method to raise community awareness and improve birth preparedness in the absence of skilled attendance.
Considerations regarding availability and safety
Misoprostol is available in 63 countries,26 obtainable through pharmacies, drug shops or the informal sector without a prescription in some areas.27 It is currently licensed for PPH prevention in India, Bangladesh, Nepal, Ghana, Kenya, Mozambique, Nigeria, Sudan, Tanzania, Uganda, Zambia, Somaliland, Pakistan, and Sierra Leone.26,28
The scope of registration and availability of misoprostol is often limited based on its perceived misuse.29 In countries where abortion is illegal or strongly controlled, there is widespread self-medicated use of misoprostol by women to terminate unwanted pregnancies.27 Misoprostol is also dangerous if used inappropriately for labour induction.30 It would therefore be essential to ensure adequate mechanisms are in place to ensure safe usage of misoprostol, if there was consistent evidence to support its use. Antenatal services would need to be in place to target women for whom misoprostol is a safe option as would adequate monitoring of adverse events and reactions.
A 2011 WHO unedited report31 cautions that ‘[to] recommend misoprostol for prevention and treatment of PPH could divert the attention or reduce attempts to implement oxytocin availability, a superior treatment.’ This concern is supported by current developments. Uganda introduced misoprostol for prevention of PPH in 10 districts,32 and National Medical Stores and health facilities are stocking more misoprostol than oxytocin (personal communication). There are various partnerships and ongoing projects aiming not only to advocate misoprostol use in developing countries and disseminate evidence-based recommendations (collaboration between FIGO and Gynuity Projects funded by the Gates, misoprostol.org), but also to assess and improve misoprostol distribution (Venture Strategies for Health and Development working closely with UC Berkeley and DKT International, POPPHI).
It is important for governments and policy-makers to take a long-term view by weighing up the potential health benefits, feasibility and costs between implementing a PPH prevention programme involving misoprostol and other interventions, such as horizontal health system strengthening and prevention of anaemia and other risk factors associated with poor maternal outcomes.
Conclusion
The current evidence used to support the use of misoprostol in home and community settings in low-and middle-income countries for prevention of PPH, where parenteral uterotonics are not available, is at best weak and inconclusive. There are a limited number of studies, the majority of which have significant biases in the study design. The exclusion of high-risk women and the finding in two studies of reductions in PPH incidence in both intervention and control arms over time suggest that other factors are important. The evidence to support a change in WHO policy with respect to adding misoprostol to its essential drugs list for prevention of PPH is weak. Governments and policy-makers in low- and middle-income countries should focus strategies for maternal health on prevention, risk factors and health systems including the training of birth attendants.
DECLARATION
Competing interests
None declared
Funding
None
Ethical approval
No ethical approval required
Guarantor
PB and AP
Contributorship
PB conceived the study, CC performed literature review and drafted the manuscript. All authors were involved in analysis and drafting and editing
Acknowledgements
This paper emerged from the collaborative research project Accessing Medicines in Africa and South Asia (AMASA, 2010–2013) funded by the European Union's Framework Programme 7. The EU is not responsible for views advanced here
References
- 1.Hogan M, Foreman K, Naghavi M, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010;375:1609–23 [DOI] [PubMed] [Google Scholar]
- 2.Hill K, Thomas K, Abou Zahr C, et al. Estimates of maternal mortality worldwide between 1990 and 2005: an assessment of available data. Lancet 2007;370:1311–9 [DOI] [PubMed] [Google Scholar]
- 3.WHO The World Health Report 2005. Make every mother and child count 2005 See http://www.who.int/whr/2005/whr2005_en.pdf (last accessed 3 July 2012)
- 4.Brabin B, Hakimi M, Pelletier D An analysis of anemia and pregnancy-related maternal mortality. J Nutr 2001;131:604S–14S [DOI] [PubMed] [Google Scholar]
- 5.WHO Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organisation, 2008 [Google Scholar]
- 6.WHO WHO Recommendations for the Prevention of Postpartum Haemorrhage. Geneva: WHO, 2007 [Google Scholar]
- 7.Hogerzeil H, Walker G, De Goeje M Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin – EDM Research Series No. 008. Geneva: World Health Organisation, 1993 [Google Scholar]
- 8.WHO WHO statement regarding the use of misoprostol for postpartum haemorrhage prevention and treatment 2009. See http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/misoprostol/en/ (last accessed 3 July 2012)
- 9.ICM, FIGO Prevention and Treatment of Post-partum Haemorrhage. New Advances for Low Resource Settings. Joint Statement. Hague and London: ICM and FIGO, 2006 [Google Scholar]
- 10.Gulmezoglu A, Forna F, Villar J, Hofmeyr G Prostaglandins for preventing postpartum harmorrhage. Cochrane Database Syst Rev 2007. Issue 3, Art No.: CD000494 [DOI] [PubMed] [Google Scholar]
- 11.Walraven G, Blum J, Dampha Y, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG 2005;112:1277–83 [DOI] [PubMed] [Google Scholar]
- 12.Høj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomised double blind clinical trial. BMJ 2005;331:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derman RJ, Kodkany BS, Goudar SS, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368:1248–53 [DOI] [PubMed] [Google Scholar]
- 14.Potts M, Prata N, El Refaey H, et al. Empowering women to control post-partum haemorrhage. Lancet 2010;375:459–60 [DOI] [PubMed] [Google Scholar]
- 15.Mobeen N, Durocher J, Zuberi N, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG 2011;118:353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmeyr GJ, Gülmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ 2009;87:666–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patted SS, Goudar SS, Naik VA, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community-based randomised controlled trial in rural India. J Matern Fetal Neonatal Med 2009;22:24–8 [DOI] [PubMed] [Google Scholar]
- 18.Prata N, Gessessew A, Abraha AK, et al. Prevention of Postpartum Hemorrhage: Options for Home Births in Rural Ethiopia. Afr J Reprod Health 2009;13:87–95 [PubMed] [Google Scholar]
- 19.Chandhiok N, Dhillon B, Datey S, Mathur A, Saxena N Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. Int J Gynaecol Obstet 2006;92:170–5 [DOI] [PubMed] [Google Scholar]
- 20.Schorn MN Measurement of blood loss: review of the literature. J Midwifery Womens Health 2010;55:20–7 [DOI] [PubMed] [Google Scholar]
- 21.de Groot AN, van Roosmalen J, van Dongen PW, Borm GF A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstet Gynecol Scand 1996;75:464–8 [DOI] [PubMed] [Google Scholar]
- 22.Mathai M, Gülmezoglu AM, Hill S Saving womens lives: evidence-based recommendations for the prevention of postpartum haemorrhage. Bull World Health Organ 2007;85:322–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudar S, Chakraborty H, Edlavitch S, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. J Matern Fetal Neonatal Med 2008;21:559–64 [DOI] [PubMed] [Google Scholar]
- 24.Begley CM, Gyte GML, Devane D, McGuire W, Weeks A Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2011, Issue 11. Art. No.: CD007412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walraven G, Wanyonyi S, Stones W Management of post-partum hemorrhage in low-income countries. Best Pract Res Clin Obstet Gynaecol 2008;22:1013–23 [DOI] [PubMed] [Google Scholar]
- 26.Fernandez MM, Coeytaux F, de Leon RG, Harrison DL Assessing the global availability of misoprostol. Int J Gynaecol Obstet 2009;105:180–6 [DOI] [PubMed] [Google Scholar]
- 27.Sherris J, Bingham A, Burns M, Girvin S, Westley E, Gomez P Misoprostol use in developing countries: results from a multicountry study. Int J Gynaecol Obstet 2005;88:76–81 [DOI] [PubMed] [Google Scholar]
- 28.Holden M Misoprostol: Strategies, Successes & Challenges. 2009. See http://www.pphprevention.org/files/VSIHoldenPOPPHI20091120FINAL.pdf (last accessed 1 August 2012) [Google Scholar]
- 29.Starrs A, Winikoff B Misoprostol for postpartum hemorrhage: Moving from evidence to practice. International Journal of Gynecology and Obstetrics 2012;116:1–3 [DOI] [PubMed] [Google Scholar]
- 30.Wagner M Born in the USA: how a broken maternity system must be fixed to put mothers and infants first University of California Press: Berkeley: 2006 [Google Scholar]
- 31.WHO Unedited report of the 18th expert committee on the selection and use of essential medicines. Technical Report Series. Accra, Ghana World Health Organization; March 2011
- 32.Annual Health Sector Performance Report Financial Year 2009/2010 Republic of Uganda. See http://www.health.go.ug/docs/AHSPR09.pdf (last accessed 1 August 2012)