Abstract
Circadian clocks are responsible for daily rhythms in a wide array of processes, including gastrointestinal (GI) function. These are vital for normal digestive rhythms and overall health. Previous studies demonstrated circadian clocks within the cells of GI tissue. The present study examines the roles played by the suprachiasmatic nuclei (SCN), master circadian pacemaker for overt circadian rhythms, and the sympathetic nervous system in regulation of circadian GI rhythms in the mouse Mus musculus. Surgical ablation of the SCN abolishes circadian locomotor, feeding, and stool output rhythms when animals are presented with food ad libitum, while restricted feeding reestablishes these rhythms temporarily. In intact mice, chemical sympathectomy with 6-hydroxydopamine has no effect on feeding and locomotor rhythmicity in light-dark cycles or constant darkness but attenuates stool weight and stool number rhythms. Again, however, restricted feeding reestablishes rhythms in locomotor activity, feeding, and stool output rhythms. Ex vivo, intestinal tissue from PER2::LUC transgenic mice expresses circadian rhythms of luciferase bioluminescence. Chemical sympathectomy has little effect on these rhythms, but timed administration of the β-adrenergic agonist isoproterenol causes a phase-dependent shift in PERIOD2 expression rhythms. Collectively, the data suggest that the SCN are required to maintain feeding, locomotor, and stool output rhythms during ad libitum conditions, acting at least in part through daily activation of sympathetic activity. Even so, this input is not necessary for entrainment to timed feeding, which may be the province of oscillators within the intestines themselves or other components of the GI system.
Keywords: colon, sympathetic nervous system, 6-hydroxydopamine, PERIOD2, suprachiasmatic nucleus
biological rhythms and the clocks that regulate them are important features of gastrointestinal (GI) and metabolic physiology that impact human health at several levels (5, 11, 12). Studies in healthy human volunteers have shown circadian changes in many aspects of GI function (12). For example, ambulatory colonic pressure recordings from healthy individuals exhibit maximal colonic activity during the day, especially following awakening and following a meal, and minimal activity during the night. GI disease states also emerge when circadian clocks are disrupted (19, 32, 26). GI symptoms, such as abdominal pain, constipation, and diarrhea, are more common in shift workers and time zone travelers than in the general population, and diagnoses of irritable bowel syndromes (IBS) have been associated with sleep disturbances, occurring in 26–55% of patients. It is unclear whether sleep disturbances are the cause or the effect of IBS, although the severity of bowel symptoms varies with the quality of the preceding night's sleep in IBS patients. Furthermore, IBS, peptic ulcers, and GI cancers are more common in nurses who work rotating shifts than in those who work day-only or night-only shifts (23).
Circadian biological rhythms are fundamental properties of most organisms, ranging from Cyanobacteria to humans, and pervade all aspects of biological organization, from transcriptional regulation to behavior (1, 27). In animals, circadian rhythms are properties of a complex gene network of highly conserved “clock genes” and their outputs, which autonomously feed back to generate an oscillation of transcriptional activity with an ∼24-h period. These genes are expressed rhythmically in many cell types and many tissues throughout the body (1) and are believed to mediate rhythmic processes locally in concert with the circadian system as a whole (1, 27). Briefly, positive elements clock and bmal1 are transcribed and translated and then form dimers that reenter the nucleus. There, the CLOCK/BMAL1 dimers stimulate transcription of genes containing an e-box or e-boxes on their promoters. Among these are the negative elements period (per) 1, 2, and 3 and cryptochrome (cry) 1 and 2. These are transcribed and translated and, in turn, form oligomers that enter the nucleus to interfere with CLOCK/BMAL1 activity (1, 20). Other components of this transcriptional, translational feedback loop modulate and stabilize the core of this loop. These are reviewed extensively elsewhere (17, 28, 37).
In mammals, circadian function is also hierarchically organized, in that the hypothalamic suprachiasmatic nuclei (SCN) serve as the conduits for light-dark (LD) cycles and as central pacemakers for the entire system (22). Entrainment or synchronization of circadian rhythms to LD cycles is mediated through a specific retinohypothalamic tract from the inner retina to the SCN, and interruption of the retinohypothalamic tract results in animals “free-running,” or expressing an endogenous period of approximately, but not exactly, 24 h (18). Surgical destruction or isolation of the SCN results in arrhythmicity in all behavioral and physiological outputs in every mammal studied, and transplantation of SCN tissue or cells into the brains of SCN-lesioned (SCNX) rodents restores behavioral rhythms (21). These studies strongly indicate that the SCN are indeed the master pacemakers for all biological clock function in mammals, except for two details. 1) Although the SCN express all the “clock genes” rhythmically, as one might expect for the master pacemaker, clock genes are expressed in many parts of the brain and peripheral tissues in a rhythmic fashion in vivo and ex vivo. 2) Although SCN lesion abolishes free-running rhythmicity, Comperatore and Stephan (7) showed that rhythmic presentation of food to SCNX rodents can entrain anticipatory wheel running in rats and mice, suggesting a “food-entrainable oscillator” (FEO) semi-independent of the SCN.
One candidate for an FEO is the GI system itself. Early work by Comperatore and Stephan (6, 7) showed that irregular contractions in the duodenum increased 2 h before a timed meal was presented to rats and that this effect was unaffected by subdiaphragm vagotomy, suggesting that the intestine itself may be able to anticipate a meal. Recently published work from our laboratory and others supports the view that the intestine contains a circadian oscillator (14, 16, 30, 31). Transcriptional profiling of the distal colon shows that a large proportion of genes associated with normal and diseased states of the colon, including all the clock genes, are expressed rhythmically (16). Clock genes are rhythmically expressed in the entire GI tract (GIT) at the mRNA and protein levels, and timed feeding shifts the phase of these rhythms in vivo and ex vivo (14, 25). This effect is also unaffected by vagotomy. Finally, knockout of per1 and per2 abolishes circadian rhythms in colonic activity in vivo and ex vivo (15). Thus the GIT contains a circadian oscillator capable of entraining to timed meals.
However, even though the GIT contains a clock that can entrain to a timed meal, this does not mean that, under normal circumstances, the GIT clock acts independently of the SCN. Indeed, intact mice and rats will entrain to a timed food presentation, such that SCN-dependent activity and FEO-dependent activity are in synchrony (9). Therefore, the present study was conducted to determine the role of the SCN in GIT circadian rhythms and to ask whether the known role of SCN regulation of sympathetic tone is responsible for central and peripheral coordination of GIT function.
METHODS
Animals
All in vivo experiments utilized male wild-type Sv/129 mice (Jackson Laboratory, Bar Harbor, ME). All colonic tissue cultured for ex vivo experiments was excised from transgenic PER2::LUC C57/BL6 mice (36) (gift from Dr. Shin Yamazaki, Vanderbilt University, Nashville, TN). Animals were treated in accordance with the guidelines of the University of Kentucky Institutional Animal Care and Use Committee, which approved our protocols.
Procedures
Male mice (n = 18) were placed in polycarbonate cages, and locomotor activity was recorded continuously from running wheels equipped with magnetic switches attached to the wire cage tops. Activity was recorded, compiled, and analyzed using data acquisition and control systems (VitalView version 4.1) from Mini Mitter (Sun River, OR), and data were analyzed with ActiView software.
Mice were kept in a 12:12-h LD cycle (150-lux illuminance) for 14–21 days before receiving experimental or control surgeries (see below). The animals were allowed to recover in LD for 14 days; then food and stool collection (see below) was conducted, and data were recorded every 4 h for 3 days. Mice were allowed to recover in constant darkness (DD) for 7 days. They remained in DD for 3 additional days, during which food and stool collections were conducted every 4 h in reference to individual activity onset (circadian time) (12). Mice remained in DD for another 7 days for recovery. All mice were then subjected to a restricted feeding (RF) regimen while they remained in DD for 10 days. Food and stool collections were conducted during the final 3 days of RF. Then animals were allowed to recover for 7 days in DD. Half of the animals subjected to an experimental surgery and half of the mice subjected to control surgery were injected intraperitoneally with 6-hydroxydopamine (6-OHDA) (see below). The remaining mice were injected with ascorbate vehicle. All injections were given prior to each change in light cycle and feeding regimen (LD, DD, RF), which were repeated corresponding to the order specified above. As described above, food and stool measurements were performed for 3 days every 4 h, and animals were allowed to recover for 1 wk in DD following each change in light cycle or feeding regimen. After the second RF regimen, mice were released into DD until they were euthanized for organ harvest. All observations and manipulations of animals in DD were conducted with the aid of Night Owl (Optics Planet) infrared binocular viewers.
Surgery
Mice were anesthetized with ketamine and xylazine (80 and 20 mg/kg body wt, respectively). A stereotaxic instrument (Stoelting, Wood Dale, IL) was used to position a stainless steel electrode (100 μm) within the SCN according to stereotaxic coordinates of the mouse brain (9a). Electrolytic lesions of the SCN (anteroposterior 5.40 ± 9 cm, mediolateral 2.60 ± 3 cm bilaterally, dorsoventral 3.00 ± 4 mm) were produced using the custom-made electrode connected to a stimulator (SD9K square-pulse model, Astro-Med) set to 50 V. Current was applied for 20 s, followed by a 10-s pause and another 10 s of stimulation. Mice that received current to regions of the brain other than the SCN were considered animals that received a sham operation. All sham lesions were located in tissue surrounding the SCN or were incomplete SCN lesions. Sham animals were verified upon recovery from surgery by rhythmic locomotor activity records. The surgical site was held closed using a 9-mm wound-closing kit (Stoelting). The lesion site was later verified by immunohistochemical staining on histological sections.
Food Consumption and Stool Measurement
During periods of GI data collection, the cage bottoms were lined with one layer of Versi-Dry lab soaker material (VWR, Philadelphia, PA). Food (2018 Teklad Global 18% Protein Rodent Diet, Teklad Diets, Madison, WI) weight was measured under infrared light before and after every 4-h time point during the 3-day collections, with the exception of the timed feeding experiment, during which food consumption was measured during the 4-h time point daily over which food was available. At the end of every 4-h time point, stools were collected, counted, and weighed. Stool weight and stool number for each mouse were recorded. Cages were changed and new material was placed in the cage bottom following each time point. Water was available ad libitum throughout all experiments.
Restricted Feeding
The RF protocol was similar to that employed by Gooley et al. (10). Mice were given access to food from zeitgeber time (ZT) 0 (12 PM or time of previous lights on) to ZT4 (4 PM) for a total of 10 days. The initial 7 days served to ensure entrainment to the timed feeding by development of anticipatory activity associated with food availability (FAA); during the following 3 days, food consumption and stool output were measured.
6-OHDA Protocol
6-OHDA (50 mg/kg; Sigma, St. Louis, MO) in 0.2% ascorbate is a neurotoxin that causes the degeneration of sympathetic terminals in the peripheral sympathetic nervous system (24). It is an accepted way to determine whether sympathetic activity mediates downstream processes. In this case, 6-OHDA was injected intraperitoneally 1–2 days prior to the start of each of the final three in vivo experiments (3 injections per mouse in total). Controls received ascorbate only. Half of the animals from the SCNX and sham groups received 6-OHDA treatment. Experiments were performed during LD, DD, and RF, as described above.
Immunohistochemistry
To ascertain the completeness of SCN lesion, we visualized immunoreactivity for VIP in the brain, because VIP marks the presence of SCN tissue. We also visualized tyrosine hydroxylase (TH) immunoreactivity in the brain and intestines. TH immunoreactivity in the brain should not be affected by 6-OHDA, since 6-OHDA typically does not pass the blood-brain barrier (BBB) but should decrease TH immunoreactivity in the intestines. After data collection and recovery in DD, all mice were euthanized by transcardial perfusion with PBS and 4% paraformaldehyde. A separate set of mice (n = 4) were treated with 6-OHDA or ascorbate (n = 2 each), allowed to survive for 3 days, and then euthanized as described above. These mice were treated to gain an estimate of the effect of 6-OHDA at the time at which the actual behavioral and physiological measurements were obtained. Brain tissue was harvested and cryoprotected in a sucrose solution gradient (10%, 20%, and 30%). The brain tissue was kept at −80°C until sectioned. A cryostat (Leica CM 1850) was used to slice the tissue into 40-μm coronal sections, which were placed into PBS. Sections were washed thoroughly with PBS and incubated for 15 min in H2O2 solution [1.2 ml of 30% H2O2 in 100 ml of double-distilled H2O (ddH2O)]; then they were washed again. Sections were left to incubate in PBS-goat serum-Triton X (PBSGT) for 30 min at room temperature and then incubated at 4°C for 48 h in a 1:1,000 dilution of primary antiserum [rabbit anti-VIP (Bachem) and rabbit anti-TH (Santa Cruz Biotechnology)] in PBSGT. For TH immunofluorescence, tissues were incubated in a 1:500 dilution of rabbit anti-TH in PBSGT (EMD Millipore, Billerica, MA). Sections were thoroughly washed with PBS and incubated in a 1:500 dilution of secondary antiserum (goat anti-rabbit; Vector Laboratories, Burlingame, CA) in PBSGT at room temperature for 2 h. For immunofluorescence detection, tissues were incubated in a 1:100 dilution of FITC-goat anti-rabbit (Vector Laboratories). The sections were washed and exposed to avidin-biotin (Vectastain ABC Kit, Vector Laboratories) in PBSGT for 90 min at room temperature and washed again. Finally, sections were incubated in diaminobenzidine-nickel-H2O2 solution until immunohistochemical staining became clearly visible (∼3 min). This solution was composed of 100 mg/50 ml NiCl in Tris buffer added to 100 mg/50 ml diaminobenzidine in Tris buffer and 50 μl of 30% H2O2. The sections were washed and transferred to slides to dry overnight. On the following day, the slides were dehydrated in ethanol and cleared in xylenes before coverslips were applied.
Western Blot
Samples (2 × 3 mm) from proximal colonic tissue were homogenized directly in 0.5 ml of Laemmli buffer and boiled for 30 min. Protein content from each sample was determined by Nanodrop, and 50 μg of individual samples were loaded accordingly. Samples and protein ladder (Precision Plus Protein Dual Color Standards, catalog no. 161-0374, Bio-Rad) were separated on a 10% SDS-polyacrylamide gel by SDS-PAGE using 1× running buffer (30.28 g of Tris base, 144.0 g of glycine, and 10 g of SDS in 10 liters of ddH2O). The gel was then used for protein transfer to a nitrocellulose membrane (Whatman, Dassel, Germany) by electroblotting in transfer buffer (3.03 g of Tris, 14.4 g of glycine, 200 ml of MeOH, and 0.05% SDS in 1 liter of ddH2O). The membrane was rinsed in PBS for 5 min and then blocked in 5% nonfat dry milk in PBS with 0.1% Tween 20 (PBT) for 30 min. Rabbit polyclonal IgG anti-TH antibody (EMD Millipore) was added at a 1:1,000 dilution and left overnight on a rotator at 4°C. The membrane was blocked for 7 min in 5% nonfat dry milk in PBT twice and washed in 1× casein (rabbit IgG Vectastain ABC-Amp Kit, Vector Laboratories) for 7 min. The membrane was incubated in biotinylated anti-rabbit IgG (rabbit IgG Vectastain ABC-Amp Kit) for 40 min. After two 7-min washes in PBT with 5% milk and one 7-min wash in 1× casein solution, the membrane was incubated for 30 min in ABC-Amp solution (rabbit IgG Vectastain ABC-Amp Kit) and then washed three times in 1× casein for 7 min. For equilibration, the membrane was incubated in 0.1 M Tris buffer (pH 9.5) for 10 min. Immunoreactive bands were detected using an alkaline phosphatase substrate staining solution [Alkaline Phosphatase Substrate Kit IV 5-bromo-4-chloro-3′-indoyl phosphate (BCIP/NBT), Vector Laboratories]. Blots were scanned and imported into National Institutes of Health ImageJ, where individual lanes were quantified as density relative to internal standard. Internal standards of separate blots were normalized to each other for averaging purposes.
Ex Vivo Tissue Excision and Preparation
Unless otherwise noted, all reagents were purchased from Sigma. Five-week-old transgenic mice were anesthetized with ketamine and xylazine (80 and 20 mg/kg body wt, respectively). Colonic tissues were excised from the cecum to ∼2 mm proximal to the rectum and placed into ice-cold Krebs solution (in mM: 117 NaCl, 5 KCl, 1.2 MgSO4, 25 NaHCO3, 1.2 NaH2PO4, 10 glucose, and 2.5 CaCl2, pH 7.6). The distal colon was rinsed in Krebs solution, bisected longitudinally, and cut into two to three 1- to 3-mm2 sections. Each piece was placed lumen-side-up onto sterile mesh in recording medium consisting of HEPES-buffered DMEM (33) with 0.1 mM luciferin (Biosynth, Staad, Switzerland) in a 35-mm petri dish and sealed with a 40-mm cover glass (Fisher Scientific, Pittsburg, PA). The dishes were placed into a LumiCycle photoluminometer (Actimetrics), which measured mPER2::LUCIFERASE bioluminescence every 10 min. The plates were removed when the oscillations appeared fully damped.
6-OHDA Ex Vivo
At 48 h prior to tissue excision and culture, mice (n = 4 for each treatment) were injected intraperitoneally with 6-OHDA (50 mg/kg) or 0.2% ascorbate vehicle and euthanized as described above. Distal colon pieces were cultured as described above, and bioluminescence data were collected until the tissues showed no discernible rhythmicity.
Isoproterenol Ex Vivo
Isoproterenol is a β-adrenergic agonist that can mimic the effects of endogenous norepinephrine (NE) in a wide array of peripheral tissues. We therefore asked whether isoproterenol affected the circadian rhythm of PER2 expression in explanted intestinal tissue. Tissues (n = 6–8 from 3 mice for each experiment) were excised, cut into sections, and placed into a LumiCycle, as described above. Bioluminescence was recorded for 2–3 days to determine the peak phase of PER2 expression. On the 3rd day in culture, the medium was replaced with fresh medium containing 100 μM isoproterenol or 0.2% ascorbate vehicle at 4, 9, 12, 16, or 21 h following the previous peak expression. As described above, recording continued until tissue rhythms damped to arrhythmicity.
Statistics
In vivo.
Significance between peaks and troughs of rhythmic food consumption and stool production during ad libitum feeding and RF was determined by one- or two-way repeated-measures ANOVA, which was followed by a Student-Newman-Keuls post hoc test using SigmaStat 3.5 software. Rhythmicity (P < 0.05) was determined using CircWave v1.4 (R. A. Hut). Amplitude between sham groups was assessed using raw data and a Student's t-test in SigmaStat 3.5. All tests were performed at a 95% confidence level (α = 0.05).
Ex vivo.
Bioluminescence rhythms were detrended by application of a low-order polynomial to the raw data. The resulting baseline was then subtracted from the data set (LumiCycle Analysis Software, Actimetrics). The period and amplitude were determined from these baseline-subtracted data using LumiCycle Analysis Software. For the 6-OHDA-treated samples, the damping coefficient of each rhythm was calculated using a previously published equation (35): βj = [2/(t − t0)]ln(Ajt/Aj), where the damping coefficient (βj) is expressed as the ratio of amplitude from the 1st day of observation (Ajt) to the average amplitude from t until the oscillation becomes desynchronized (t0). Average period and damping coefficients between groups were compared by one-way ANOVA. For the timed isoproterenol pulse, peak phase of bioluminescence on the day before and on the 2nd and 3rd days after drug administration was determined for each tissue in CircWave by recording each center-of-gravity value. Each individual trace was analyzed to determine if the pulse resulted in a peak phase earlier (advance) or later (delay) than the prepulse phase, and the resulting change in phase was assigned a positive or negative value, respectively. Average change in phase (phase-response curve) was then subjected to one-way ANOVA to determine significant differences pre- and postpulse between treatment groups. Changes in phase, positive for phase advances and negative for phase delays, were plotted against the time of the pulse relative to peak PER2 expression. All tests were performed at a 95% confidence level (α = 0.05).
RESULTS
Locomotor Activity
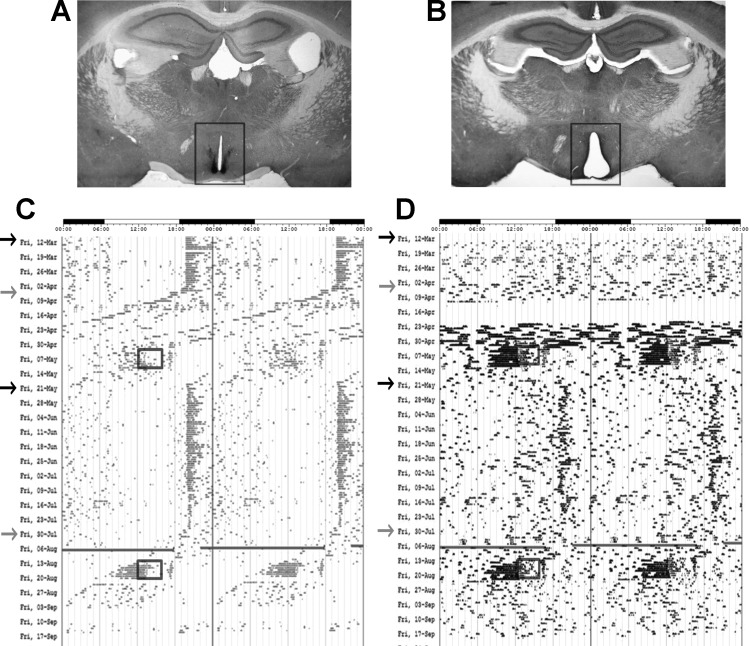
The locomotor activity of representative mice throughout the various light cycles and feeding regimens can be seen in the actograms in Fig. 1. As expected, sham-operated mice in LD (Fig. 1A) retained circadian locomotor rhythms (Fig. 1C, black arrows), whereas ablation of the SCN (Fig. 1B) resulted in locomotor arrhythmicity (Fig. 1D, black arrows). In DD, sham animals exhibited free-running rhythms of wheel-running activity in which they expressed endogenous periods of 23.43 ± 0.27 h (Fig. 1C, gray arrows), and lesioned animals continued to be arrhythmic (Fig. 1D, gray arrows). Sham mice that received 6-OHDA expressed free-running periods of 23.8 ± 0.08 h, while mice receiving 6-OHDA expressed periods of 23.58 ± 0.21 h. No statistical differences among these periods were detected. Again, SCNX mice continued to be arrhythmic. All animals exhibited FAA preceding food presentation during RF (black boxes in Fig. 1, C and D).
Fig. 1.
Light cycles and feeding regimens of operated animals. A: intact bilateral suprachiasmatic nuclei (SCN) immunostained for VIP in a sham-operated animal. B: section from a mouse in which the SCN was electrolytically lesioned (SCNX). VIP staining was not visible in negative controls (data not shown). C and D: actograms showing circadian locomotor activity. C: rhythmic circadian locomotor activity of a sham-operated animal injected with ascorbate. D: arrhythmic locomotor behavior of a SCNX animal injected with ascorbate. Arrows indicate beginning of a new light cycle: black arrows show light-dark (LD) cycles, and gray arrows show constant darkness (DD). Boxes indicate 4 h of midday food availability during restricted feeding (RF).
Food Consumption and Stool Measurement
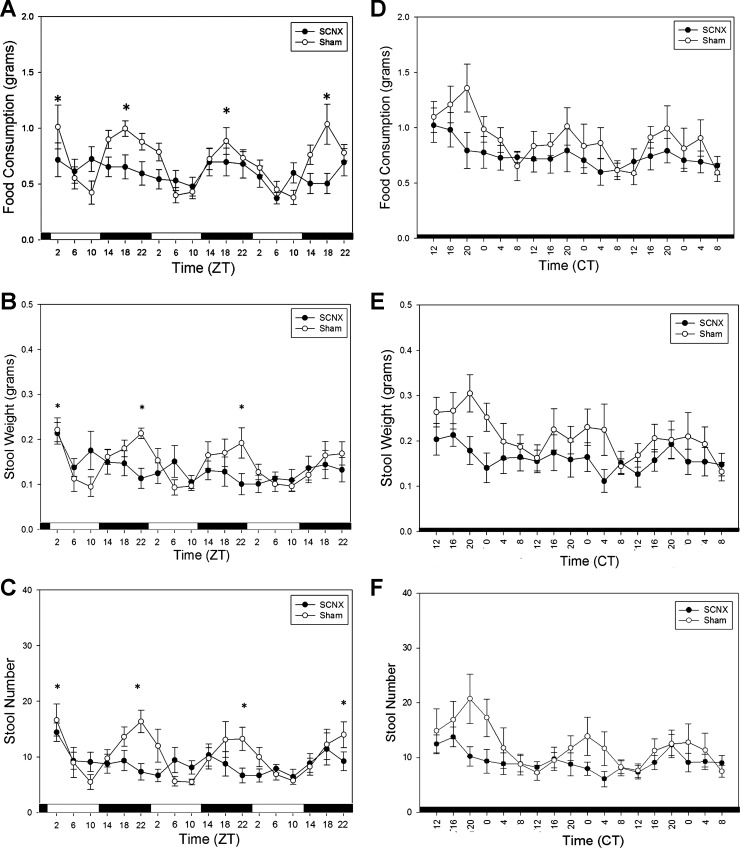
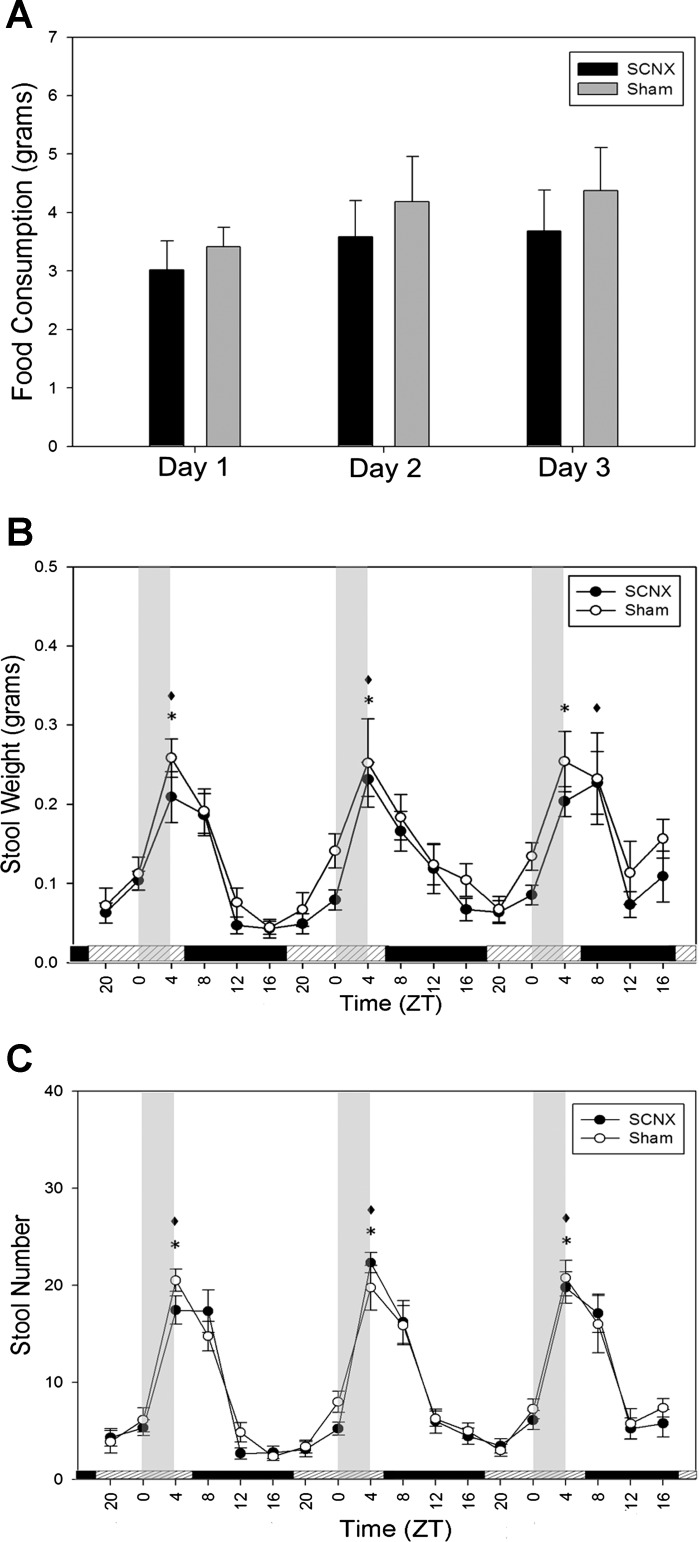
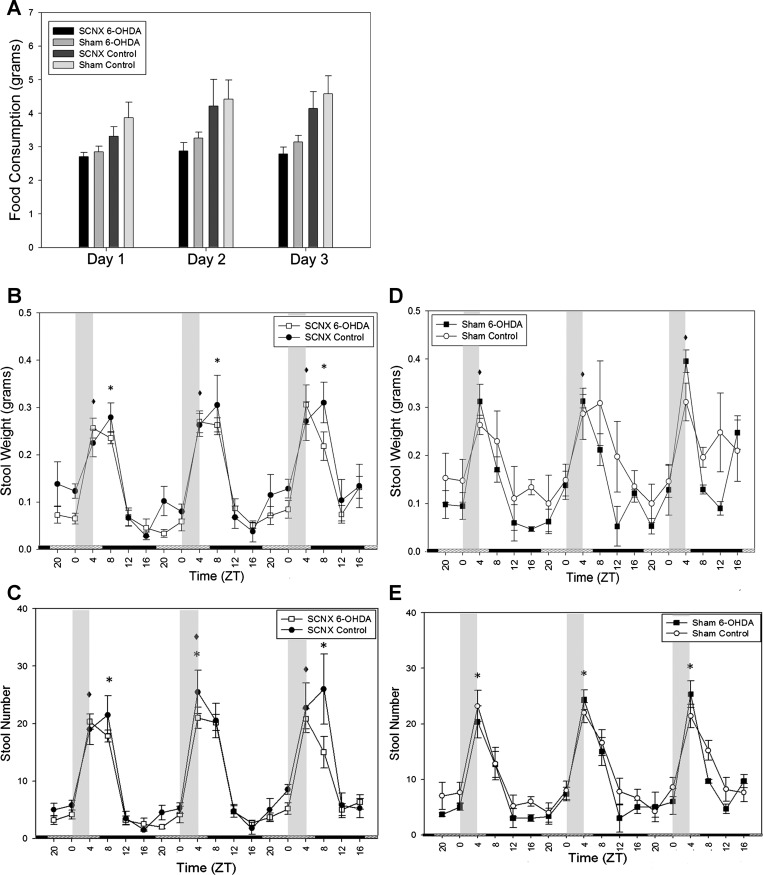
Figure 2 shows feeding and defecation measurements of SCNX and sham mice prior to drug or vehicle treatment. In sham animals in LD, significant differences exist between the peaks and troughs, although the SCNX animals were arrhythmic (Fig. 2, A–C). In DD, sham mice expressed rhythmic locomotor activity, feeding, and stool production. SCNX animals expressed arrhythmic feeding, stool weight, and stool number in LD and DD (Fig. 2, D–F). During RF in DD, two-way ANOVAs reported no significant differences between the food consumption of SCNX and sham groups on any day (Fig. 3A). SCNX and sham animals showed significant rhythms in amplitude of colonic motility (Fig. 3, B and C), which peak just following the window of food availability.
Fig. 2.
Food consumption and stool output are rhythmic in sham (n = 8) and arrhythmic in SCNX (n = 10) animals during ad libitum food availability. A–C: food consumption, stool weight, and stool number in SCNX and sham animals during 3 days in LD. ZT, zeitgeber time. D–F: food consumption, stool weight, and stool number in SCNX and sham animals during 3 days in DD. CT, circadian time. For determination of circadian rhythmicity of a data set, peak values were compared with the corresponding following and/or preceding trough by 1-way ANOVA. *Significance in sham animals (P < 0.05). No significance was reported in SCNX animals. Values are means ± SE.
Fig. 3.
Colonic motility in SCNX and sham animals directly entrains to food availability. Food was available for 4 h daily (from 12 PM to 4 PM) during ZT0–ZT4 (vertical gray bars in B and C). Bars along the x-axis in B and C represent the previous LD cycle. A: food consumption during each day of RF in SCNX and sham mice. B: stool weight during RF. C: stool number during RF. *P < 0.05. No significant difference was shown by 2-way ANOVA between groups in A. Values are means ± SE.
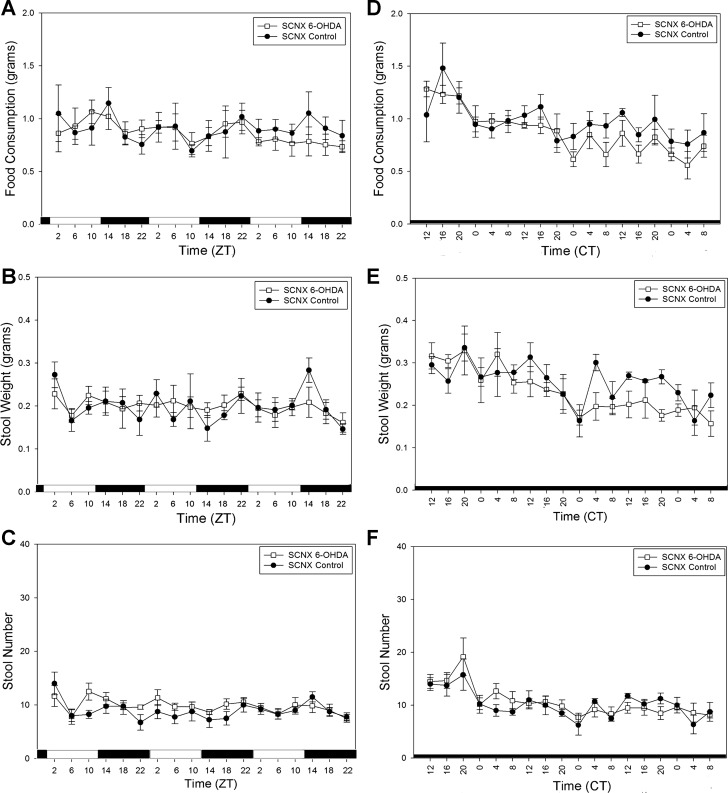
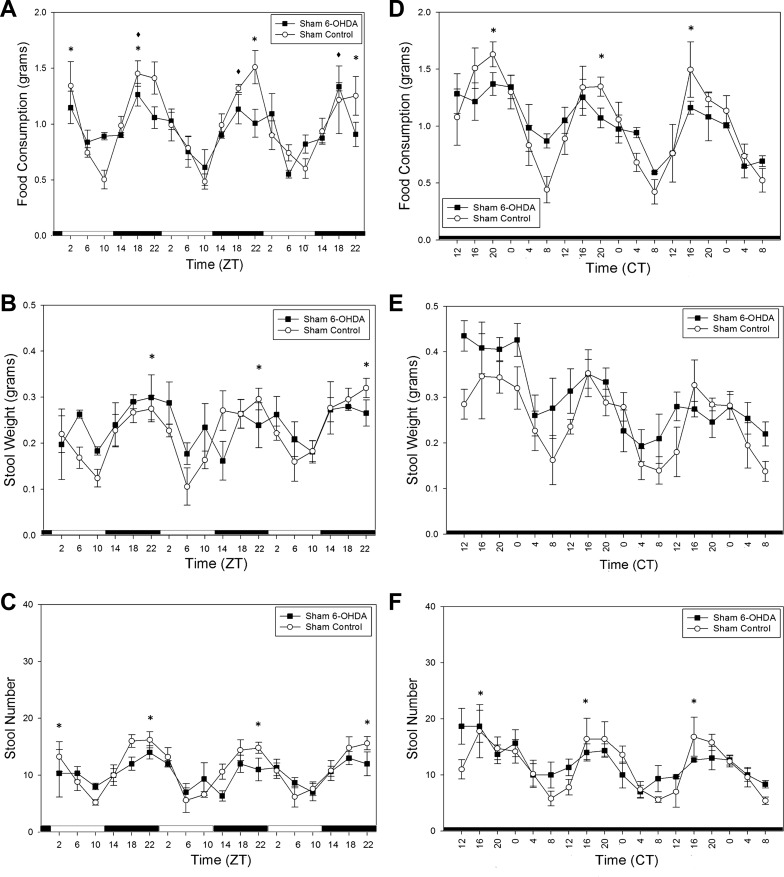
After intraperitoneal injection of 6-OHDA or ascorbate, food consumption and stool output rhythms of the SCNX animals were still abolished in LD (Fig. 4, A–C) and DD (Fig. 4, D–F). Importantly, the sham control animals maintained clear rhythmicity in locomotor activity, feeding (Fig. 5A), and stool output in LD, while the sham 6-OHDA-treated animals only retained rhythmicity in locomotor activity and feeding activity (Fig. 5A) but showed attenuated stool output rhythms (Fig. 5, B and C). This persisted in DD (Fig. 5, D–F), as determined by a one-way ANOVA, and there was a significant difference (P = 0.032) in rhythm amplitude of stool number output between the two groups as determined by a t-test. CircWave analysis was also employed for further quantitative information about the rhythms of the 6-OHDA-treated sham animals in Fig. 5. All sham control rhythms passed CircWave analysis for all 3 days (Fig. 5, A–F). All 3 days of food consumption in the sham 6-OHDA-treated animals were considered rhythmic by CircWave in LD, but stool weight and stool number rhythms were not (Fig. 5, A–C). Thus it may be concluded that 6-OHDA-induced sympathectomy specifically attenuates, but does not abolish, circadian colonic rhythmicity. During the second exposure to a RF regimen, there were no significant differences in food consumption between any of the four groups according to two-way ANOVA (Fig. 6A). Similar to the entrainment during RF in Fig. 3, all animals show significant rhythmicity in all stool output parameters (Fig. 6, B–E).
Fig. 4.
SCN lesion yields arrhythmic food consumption and stool output during ad libitum food availability in 6-hydroxydopamine (6-OHDA)-treated (n = 6) and vehicle-treated (n = 4) mice. A–C: food consumption, stool weight, and stool number in SCNX animals during LD. D–F: food consumption, stool weight, and stool number in SCNX animals during DD. Values are means ± SE.
Fig. 5.
Differences in stool output rhythms between 6-OHDA-treated (n = 3) and vehicle-treated (control, n = 5) sham animals during ad libitum conditions. A–C: food consumption, stool weight, and stool number in 6-OHDA- and vehicle-treated sham animals in LD. D–F: food consumption, stool weight, and stool number in 6-OHDA- and vehicle-treated sham animals during DD. *P < 0.05 for sham control. *P < 0.05 for sham 6-OHDA. Values are means ± SE.
Fig. 6.
Colonic motility in all treated animals directly entrains to food availability. Food was available for 4 h daily (from 12 PM to 4 PM) during ZT0–ZT4 (vertical gray bars in B–E). A: food consumption of each surgery/treatment group. B and C: stool weight and stool number of SCNX animals during RF. D and E: stool weight and stool number of sham animals during RF. *P < 0.05 for sham control. *P < 0.05 for sham 6-OHDA. No significant difference (by 2-way ANOVA) in food consumption was shown between groups in A. Values are means ± SE.
Immunohistochemistry
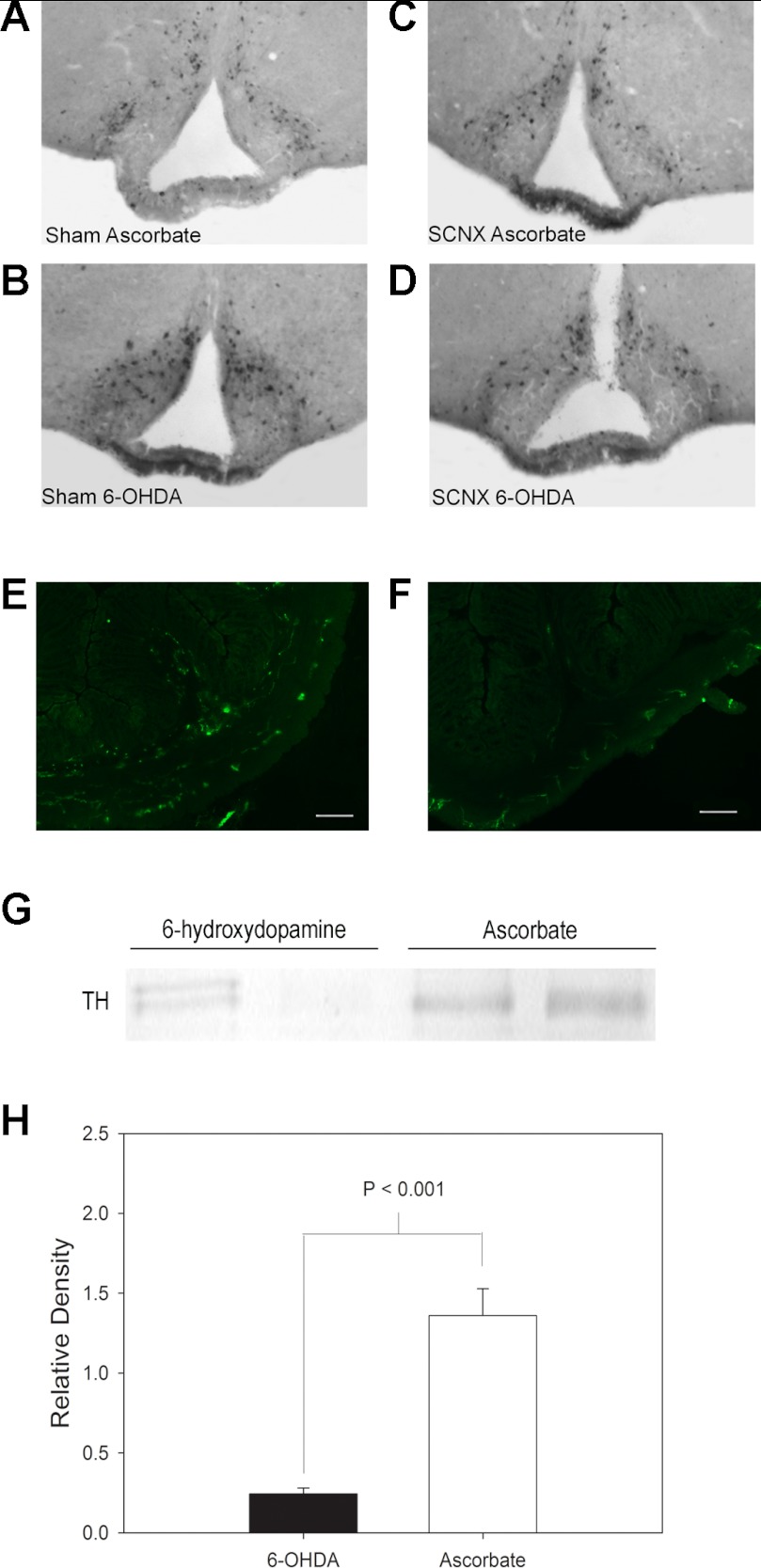
VIP is a protein that is expressed prominently in the SCN, making the bilateral nuclei clearly visible following immunohistochemical staining with VIP antiserum. Complete SCN lesions showed no VIP immunoreactivity in the SCN region of the hypothalamic tissues. TH, involved in dopamine synthesis, is present in dopaminergic neurons. Neurons that have undergone a sympathectomy (such as by 6-OHDA) will no longer show TH expression. When injected peripherally, 6-OHDA cannot cross the BBB and enter the brain. In sham and SCNX animals, there was no significant decrease in TH-expressing neurons in the brain, indicating that SCN ablation surgery did not compromise the structural or functional integrity of the BBB (Fig. 7, A–D). Western blot analysis (see below) and immunofluorescent visualization of TH-immunoreactive fibers (Fig. 7, E and F) show that 6-OHDA decreased or abolished intestinal TH immunoreactivity.
Fig. 7.
SCN lesion and 6-OHDA treatment do not decrease the number of tyrosine hydroxylase (TH)-expressing neurons within the arcuate nucleus, but 6-OHDA treatment does decrease TH immunoreactivity in the colon. A–D: representative histological sections showing TH immunohistochemical staining of the arcuate nucleus in the brain of an ascorbate-treated sham animal, a 6-OHDA-treated sham animal, an ascorbate-treated SCNX animal, and a 6-OHDA-treated SCNX animal. E and F: representative distal colonic sections showing immunofluorescent detection of TH-expressing neurons in ascorbate-treated (E) and 6-OHDA-treated (F) animals. Scale bars, 100 μm. G: representative Western blot showing immunoreactive TH. Left to right, 6-OHDA-treated tissues (lanes 1 and 2) and ascorbate-treated tissues (lanes 3 and 4). H: average composite of relative density of experimental and control immunoreactive TH bands of 4 independent Western blots.
Western Blot
TH-immunoreactive bands were detected on the nitrocellulose membrane at 60 kDa (Fig. 7G). Bands from 6-OHDA-treated tissues showed a significant decrease in relative density compared with vehicle-treated tissues as analyzed by t-test (P < 0.001, n = 4 tissues per treatment; Fig. 7H).
6-OHDA Ex Vivo
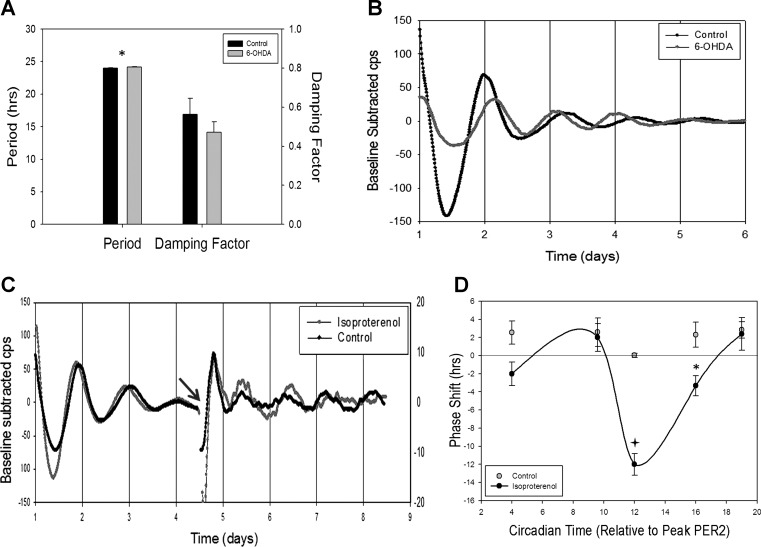
To determine whether 6-OHDA-induced peripheral sympathectomy affected the endogenous clock within the GIT, mPER2 bioluminescence was measured ex vivo from mice that had been subjected to this treatment. Figure 8A summarizes the data from this experiment. Control tissues had an average period of 24.00 ± 0.02 h and damping coefficient of 0.56 ± 0.08, while 6-OHDA-treated tissues oscillated with an average period of 24.14 ± 0.064 h with a damping coefficient of 0.47 ± 0.05. Student's t-test analysis showed significant difference in period (t = 2.052, P < 0.05) but no difference in damping coefficient. Representative traces of PER2 expression rhythms from 6-OHDA-treated and control tissues in Fig. 8B highlight the difference in period length and the similar rate of damping.
Fig. 8.
6-OHDA has little effect and isoproterenol causes a phase shift in PER2 expression ex vivo. A: period and damping factor in tissues from vehicle-treated (n = 4) and 6-OHDA-treated (n = 4) animals. Values are means ± SE. *P < 0.05 (Student's t-test). B: representative traces of baseline-subtracted luciferase bioluminescence [in counts per second (cps)] from tissues of vehicle-treated (control) and 6-OHDA-treated animals. C: representative traces of baseline-subtracted luciferase bioluminescence from tissues treated with a pulse of isoproterenol or vehicle solutions. Phase resetting occurs after treatment (gray arrow) in isoproterenol-treated, but not control, tissues. D: phase-response curve generated using PER2 expression data from isoproterenol- and vehicle-treated tissues. *P < 0.005; +P < 0.001 (by 2-way ANOVA). Phase shifting is dependent on time of isoproterenol treatment in relation to peak PER2 expression.
Isoproterenol Ex Vivo
To determine if isoproterenol was able to affect PER2 expression rhythms in GI tissues, medium of colonic tissue in culture for several days was replaced with medium containing isoproterenol or vehicle at different times relative to peak PER2 bioluminescence. Representative traces of PER2 expression in tissues treated with isoproterenol or ascorbate (control) can be seen in Fig. 8C, with the gap between day 4 and day 5 denoting media exchange. Isoproterenol administration resulted in a phase shift that was dependent on the time of drug administration. Figure 8D shows the composite data from five differently timed isoproterenol pulses at different phases relative to the previous peak of PER2::LUC bioluminescence. At 4 h after peak expression, as PER2 levels were declining, isoproterenol administration caused a phase delay of 2 h. After 5 h, as PER2 approached nadir, the pulse resulted in a 2-h advance in phase. The most dramatic phase shift of 12-h delay (or advance) was produced when isoproterenol was administered at the trough of bioluminescence. The phase shifts became less dramatic when the drug was administered during the rising phases, with a 3-h delay 16 h after peak expression and a 2-h advance at 19 h after peak expression. Vehicle-treated samples showed no phase shift at any time point (Fig. 8D).
DISCUSSION
There is little doubt that the mammalian GI system contains a circadian clock capable of entraining to and anticipating the availability of food, and this clock may bear direct relevance to GI health and disease (13). First, 0.6–3.7% of the colonic transcriptome is expressed rhythmically on a circadian basis. Rhythmic transcripts include known signals involved in intestinal motility, inflammation, cell signaling, and cell cycle, including several linked to colorectal cancer (16). Second, the myenteric plexus and many cell types of the endothelium express circadian patterns of mRNA and protein levels corresponding to known “canonical” clock genes in vivo and ex vivo. In vivo, intestinal clock gene rhythms phase shift in response to a shift in a timed food presentation, even though rhythms of clock genes within the SCN do not respond to this cue (14, 30). Furthermore, these rhythms correspond to congruent rhythms of intestinal motility in vivo, in terms of rhythmic defecation and intracolonic pressure, and ex vivo, in terms of rhythms of spontaneous contractility and in response to exogenous acetylcholine (15).
The circadian molecular clockworks are necessary for the expression of circadian rhythms of intestinal function. Knockout of per1 or per2 alters the period and decreases the amplitude of many measures of colonic motility, but double knockout of per1 and per2 abolishes these rhythms. Circadian rhythms of stool number and weight in LD and DD and intracolonic pressure changes in vivo are absent in per1/per2 double-knockout mice. Ex vivo, while colonic circular muscle contractility is expressed on a circadian basis in wild-type mice, the contractility is arrhythmic on a circadian basis in per1/per2 mice as well. Thus, at least the per1/per2 arms of the molecular feedback loop are required for circadian patterns of intestinal function, and it is likely that the entire gene network is involved (15).
However, the overwhelming evidence for a GI clock does not necessarily mean that circadian patterns of intestinal function are independent of central nervous pacemakers in the SCN. In the present study, we have shown that the SCN are critical for self-sustaining rhythms in the GIT. Circadian rhythms of locomotor activity, food consumption, stool number, and stool weight are abolished by SCN lesion in LD and DD. Similarly, Polidarová et al. (25) recently found that rats made arrhythmic with constant bright light (LL) also expressed disrupted or arrhythmic patterns of clock gene mRNA in the colon, duodenum, and liver. These authors inferred that the SCN was made arrhythmic with exposure to LL, whereas the present study explicitly addresses it.
Even so, the present study also corroborates the view that some circadian functions are retained in SCNX mice. First, SCNX mice anticipate the presence of a timed meal by anticipatory wheel-running behavior. Second, timed feeding reinstates circadian patterns of feeding and defecation. These measures of GIT activity also anticipate the presence of a meal. Similarly, Polidarová et al. (25) also showed that rhythms of GIT clock gene expression in rats are restored with timed feeding. Finally, colonic tissue retains rhythmic PER2 expression ex vivo.
How can we reconcile the fact that SCN lesion completely abolishes behavioral and intestinal circadian rhythms and the fact that feeding and intestinal rhythms can still anticipate a meal? While it is true that the GIT retains circadian patterns of clock gene expression and motility ex vivo, the amplitude of these rhythms is reduced, and the rhythms damp over ∼10 days. It is likely that restoration of rhythmicity by the GIT requires stimulation from a meal itself and/or circadian signals from the SCN.
One pathway by which the SCN is known to influence downstream processes is through the regulation of sympathetic tone. In rats, at least, sympathetic tone is rhythmic in sympathetic ganglia, the heart, and pineal and adrenal glands, such that maximal turnover occurs during the subjective night, when locomotor activity is highest (2), and these rhythms are abolished by SCN lesion (34). Sympathetic activity is required for the expression of several peripheral circadian rhythms, most notably circadian patterns of heart rate and liver function. In the case of circadian regulation of heart rate in rats, SCN lesion abolishes circadian patterns of heart rate and heart rate variability (34). This effect is simulated by chemical sympathectomy through previous treatment with guanethidine. Similarly, SCN lesion and surgical denervation of sympathetic afferents to the liver abolish circadian patterns of hepatic gluconeogenesis and several hepatic enzymes and glucocorticoid rhythms (3, 4, 29).
In the present study, treatment with 6-OHDA, a neurotoxin that destroys sympathetic terminals, reduced the amplitude or abolished circadian patterns of stool number and weight but had little effect on the rhythms of food consumption of sham mice, suggesting that sympathetic activity is required for sustained circadian patterns of intestinal motility but has relatively little effect on feeding. In SCNX mice, 6-OHDA had no additional effect on the already arrhythmic pattern of food consumption, stool number, and stool weight. Nonetheless, timed feeding restored the rhythms of locomotor activity, stool number, stool weight, and anticipatory locomotor activity of sham and SCNX mice. The most likely scenario is that the GIT does indeed contain a circadian clock capable of responding to and anticipating the regular presence or absence of food. However, it is coordinated on a daily basis with the light-sensitive circadian clock in the SCN via the circadian changes in sympathetic tone under the control of SCN efferents. The SCN pacemaker interacts with GIT oscillators through entrainment of circadian clocks residing in the GIT. This is evident in the fact that the β-adrenergic agonist isoproterenol phase shifts circadian rhythms of PER2 expression in colonic tissues ex vivo in a phase-dependent fashion, indicating that the GIT clock is only sensitive to sympathetic input at certain times of day. PER2 expression rhythms are phase-delayed some 12 h when presented 12 circadian hours following the last PER2 peak but are either not affected or phase-advanced at other circadian phases. This is not due to effects on myenteric plexus catecholaminergic neurons, because pretreatment of PER2::LUC mice with 6-OHDA has relatively little effect on rhythms of luciferase bioluminescence. It is also not likely due to effects of 6-OHDA on the central nervous appetitive apparatus, since no effect of 6-OHDA can be observed in immunohistochemical detection of TH in cell bodies or known projection areas of catecholamine neurons. Furthermore, there is no effect of 6-OHDA on free-running locomotor rhythms, punctuating the view that the effects of 6-OHDA are peripheral.
The most likely scenario is that circadian oscillators within the SCN, entrained to the LD cycle, influence a circadian change in sympathetic tone by increasing release of postganglionic NE primarily during the subjective night. Sympathetic NE entrains circadian oscillators within the myenteric plexus of the GIT, which in turn regulate downstream processes such as motility, enzymatic activity, cell signaling, and even inflammation. However, as is the case with any sympathetically regulated process and other regulators, such as stress, changes in blood pressure, and temperature fluctuations, sympathetic output can change outside the purview of the clock. Furthermore, the GIT itself can detect the regular presence or absence of food, even in the absence of SCN sympathetic input. The mechanisms by which the GIT might anticipate food are unknown but may involve GIT hormones and/or hepatic input, which is also known to contain a clock (12, 13). Davidson and colleagues (8) investigated the role of peripheral tissues, including liver and GIT tissues, in FAA during RF and concluded that the FEO that drives FAA is not located in the periphery, suggesting that further inquiries should focus instead on the central nervous system (CNS). The results presented here, while incapable of providing an anatomic location of the FEO, are in agreement that GIT tissues act independently of the CNS and implicate sympathetic signaling as a possible mechanism through which the CNS communicates time of day to the GIT. The presence of a circadian clock within the GIT serves as an adaptive mechanism by which the repeated, predictable presence of food can entrain the GI apparatus for efficient metabolism of foodstuffs. Among the questions that remain are, of course, the mechanisms by which the GIT anticipates food and the pathways by which peripheral detection of a meal influences behavior such as anticipatory wheel running.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK-074477 and by the University of Kentucky.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N.M. and V.M.C. are responsible for conception and design of the research; J.N.M., J.K.P., and Y.L. performed the experiments; J.N.M., J.K.P., Y.L., and V.M.C. analyzed the data; J.N.M., J.K.P., Y.L., and V.M.C. interpreted the results of the experiments; J.N.M. and J.K.P. prepared the figures; J.N.M., J.K.P., and V.M.C. drafted the manuscript; J.N.M., J.K.P., and V.M.C. edited and revised the manuscript; J.N.M., J.K.P., Y.L., and V.M.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Melissa Whitfield-Rucker for administrative support and Dr. Willemintje Hoogerwerf, Dr. Gang Wang, Dr. Amit Trivedi, Clifford Harpole, and Greg Artiushin for helpful discussions.
REFERENCES
- 1. Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544– 556, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brusco LI, García-Bonacho M, Esquifino AI, Cardinali DP. Diurnal rhythms in norepinephrine and acetylcholine synthesis of sympathetic ganglia, heart and adrenals of aging rats: effect of melatonin. J Auton Nerv Syst 74: 49– 61, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pévet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci 22: 2531– 2540, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cailotto C, van Heijningen C, van der Vliet J, van der Plasse G, Habold C, Kalsbeek A, Pévet P, Buijs RM. Daily rhythms in metabolic liver enzymes and plasma glucose require a balance in the autonomic output to the liver. Endocrinology 149: 1914– 1925, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Cassone VM, Stephan FK. Central and peripheral regulation of feeding and nutrition by the mammalian circadian clock: implications for nutrition during manned space flight. Nutrition 18: 814– 819, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Comperatore CA, Stephan FK. Entrainment of duodenal activity to periodic feeding. J Biol Rhythms 2: 227– 242, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Comperatore CA, Stephan FK. Effects of vagotomy on entrainment of activity rhythms to food access. Physiol Behav 47: 671– 678, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2: 32– 39, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Davidson AJ, Stephan FK. Plasma glucagon, glucose, insulin, and motilin in rats anticipating daily meals. Physiol Behav 66: 309– 315, 1999 [DOI] [PubMed] [Google Scholar]
- 9a. Franklin BJ, Paxinos G. The Rat Brain in Stereotaxic Coordinates (3rd ed.). New York: Academic, 2007 [Google Scholar]
- 10. Gooley JJ, Schmoer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 9: 398– 407 2006 [DOI] [PubMed] [Google Scholar]
- 11. Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell 134: 728– 742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoogerwerf WA. Biologic clocks and the gut. Curr Gastroenterol Rep 8: 353– 359, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord 10: 293– 300, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hoogerwerf WA, Hellmich HL, Cornelisson G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250– 1260, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hoogerwerf WA, Shahinian VB, Cornelisson G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol 298: G143– G150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135: 2019– 2029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isojima Y, Okumura N, Nagai K. Molecular mechanism of mammalian circadian clock. J Biochem 134: 777– 784, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res 460: 297– 313, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Kanaly T, Shaheen NJ, Vaughn BV. Gastrointestinal physiology and digestive disorders in sleep. Curr Opin Pulm Med 15: 571– 577, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15: R271– R277, 2006 [DOI] [PubMed] [Google Scholar]
- 21. LeSauter J, Lehman MN, Silver R. Restoration of circadian rhythmicity by transplants of SCN “micropunches.” J Biol Rhythms 11: 163– 171, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Mistlberger RE, Antle MC. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem 49: 119– 136, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol 105: 842– 847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pawlik MW, Obuchowicz R, Biernat J, Szczepanski W, Pajdo R, Kwiecien SS, Brzozowski T, Konturek SJ, Pawlik WW. Effects of peripherally and centrally applied ghrelin in the pathogenesis of ischemia-reperfusion induced injury of the small intestine. J Physiol Pharmacol 62: 429– 439, 2011 [PubMed] [Google Scholar]
- 25. Polidarová L, Sládek M, Soták M, Pácha J, Sumová A. Hepatic, duodenal, and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chronobiol Int 28: 204– 215, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol 295: R2034– R2040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 418: 935– 941, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647– 676, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Shibata S. Neural regulation of the hepatic circadian rhythm. Anat Rec A Discov Mol Cell Evol Biol 280: 901– 909, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Sládek M, Rybová M, Jindráková Z, Zemanová Z, Polidarová L, Mrnka L, O'Neill J, Pácha J, Sumová A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 133: 1240– 1249, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Soták M, Polidarová L, Musílková J, Hock M, Sumová A, Pácha J. Circadian regulation of electrolyte absorption in the rat colon. Am J Physiol Gastrointest Liver Physiol 301: G1066– G1074, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol 7: 29– 36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vujovic N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol 295: R355– R3560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warren WS, Champney TH, Cassone VM. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol Behav 55: 1091– 1099, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Wu H, Lu J, Li B. A coupled oscillatory model mimicking avian circadian regulatory systems. J Biol Physics 26: 261– 272, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoo SH, Yamazaki S, Lowry PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Neuroscience 101: 5339– 5346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet 2: 702– 715, 2001 [DOI] [PubMed] [Google Scholar]