Abstract
Maternal alcohol consumption during pregnancy is a significant field of scientific exploration primarily because of its negative effects on the developing fetus, which is specifically defined as fetal alcohol spectrum disorders. Though the effects on the mother are less explored compared with those on the fetus, alcohol produces multiple effects on the maternal vascular system. Alcohol has major effects on systemic hemodynamic variables, endocrine axes, and paracrine factors regulating vascular resistance, as well as vascular reactivity. Alcohol is also reported to have significant effects on the reproductive vasculature including alterations in blood flow, vessel remodeling, and angiogenesis. Data presented in this review will illustrate the importance of the maternal vasculature in the pathogenesis of fetal alcohol spectrum disorders and that more studies are warranted in this field.
Keywords: fetal alcohol spectrum disorders, pregnancy, uterine, endothelium
this article is part of a collection on Cardiovascular Regulation in Pregnancy. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Maternal alcohol consumption during pregnancy is a significant field of scientific exploration primarily because of its negative effects on the developing fetus, specifically defined as fetal alcohol spectrum disorders (FASD) (82). These deficits span numerous organ systems, but the most studied effects are on the developing brain neuronal losses and its related behavioral abnormalities with learning and memory impairment (74). The Surgeon General of the United States released a warning in 1981 and again in 2005 urging women who are or may become pregnant to abstain from drinking (86). Despite these specific and clear public health initiatives, it is currently estimated that about 2–5% of young school children in the United States and some West European countries may be affected by FASD (53), showing that a large number of pregnant women consume alcohol during pregnancy. A main reason to be concerned about these statistics is that fetal alcohol deficits are considered to last for a lifetime, and there are no approved drug therapies for treatment of FASD, nor are there established diagnostic or therapeutic tools to be able to prevent and/or ameliorate some of these deficits.
The focus of research on the effects of maternal alcohol consumption has historically been on the developing fetal brain due to the obvious manifestations of craniofacial and behavioral impairments in fetal alcohol syndrome (FAS, an extreme manifestation of FASD including craniofacial deficits, central nervous system dysfunction, and growth restriction). Although alcohol (ethanol) is a simple molecule (C2H5OH), it has posed considerable difficulties to scientists in identifying converging candidate mechanisms underlying alcohol-mediated deficits because of diverse effects of alcohol based on the timing (first, second, or last third or throughout pregnancy), pattern (acute, chronic, or binge), and dose of exposure (50). Furthermore, there are organ/region-specific developmental differences in vulnerability (44, 49–51, 96); for example, alcohol produces differential effects on brain regions depending on whether the cells are proliferating or differentiating (52). In addition, it has been noted in animal model studies that binge drinking, wherein a lower dose of alcohol is consumed in a short intermittent pattern to generate a higher-peak blood alcohol concentration (BAC), produces significantly greater deficits than a higher dose of alcohol continuously administered that generates a lower-peak BAC (8, 50). Finally, one should also carefully address the animal model system that is used to understand the mechanistic perspectives underlying alcohol-induced deficits (15, 16); for instance, the developing brain in the humans has its peak growth velocity (first derivative of brain weight with reference to time) peak at parturition, whereas in rats or mice, this event occurs shortly after parturition (15).
Alcohol freely permeates through all body fluid compartments including the vasculature, interstitial fluid, and intracellular space; thus the alcohol concentration is the same in any body fluid compartment in the mother and the fetus. In contrast to numerous fetal studies, very few studies have focused on the effects of alcohol on the mother during pregnancy. This is important from the perspective of both the mother's and the offspring's health since it has been suggested that alcohol may lead to FASD partly via its effects on the mother, maternal-fetal interface, as well as events like parturition (15). In this review, we will specifically focus on the effects of maternal alcohol consumption during pregnancy on the maternal vascular system.
Effects of Alcohol on Maternal Systemic Hemodynamics
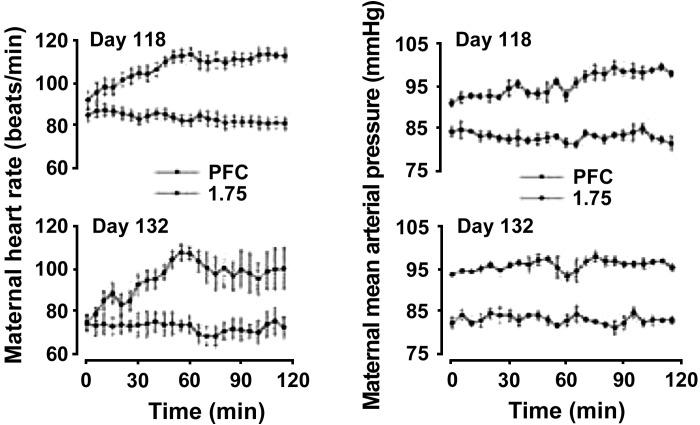
In men and nonpregnant women, different alcohol-dose and blood pressure relationships have been described including linear (with and without threshold), J shaped, U shaped, etc., as a function of age, sex, smoking habits, race, and drinking pattern/frequency of drinking, with high levels of alcohol consumption being a risk factor for hypertension (24, 76, 78, 90, 99). Since most alcohol studies have been traditionally conducted mainly in men (25), it is important to note that women metabolize alcohol distinctly different from men because the first pass metabolism and gastric alcohol dehydrogenase activity are both as low as 23 and 59% of those of men, respectively resulting in much higher BACs (23). In an early study, Barker had demonstrated that moderate (men ≤ 280 g, and women ≤ 168 g) and heavy (men > 280 g, and women > 168 g) alcohol consumption is reported to produce an increase in mean systolic and diastolic pressures by 4 mmHg compared with those with low intake (men ≤ 168 g, women ≤ 112 g) (4). However, there are no reports of maternal alcohol effects during pregnancy on systemic hemodynamic parameters in humans. In sheep, binge alcohol exposure during the third trimester equivalent of human brain development showed dose-dependent increases in the maternal heart rate and blood pressure with 0.75, 1.25, 1.5, and 1.75 g/kg of alcohol, generating peak BACs of about 80.8 ± 6.5, 182.5 ± 13.5, 224.4 ± 13.9, and 260.6 ± 20.0 mg/dl respectively (Fig. 1) (17). These alterations were attributed to direct alcohol effects on peripheral vascular resistance that will be discussed in the subsequent sections as well as from reflexes such as chemoreflex arising from maternal hypoxia and acidosis (17, 33). However, studies on the effects of alcohol on systolic and diastolic pressures in women as well as in animal models like rats, the most widely used model to study FASD, are highly warranted.
Fig. 1.
Maternal heart rate (in beats/min, left) and mean arterial pressure (in mmHg, right) on gestational day (GD) 118 (top) and 132 (bottom) experimental days in response to saline [pair-fed control (PFC)] and 1.75 g/kg ethanol doses in sheep. Alcohol was intravenously delivered to ewes beginning on GD 109 for 1 h on 3 consecutive days each week followed by 4 days without exposure, and the pattern was repeated till GD 132. For clarity, the 0.75-, 1.25-, and 1.5-dose plots were not presented but fell between the PFC and 1.75-dose groups in a dose-dependent manner. Maternal heart rate on GD 118 and GD 132 were significantly higher in all ethanol treatment groups compared with PFC. Maternal mean arterial pressure was progressively higher with increasing doses of ethanol on both GD 118 and 132. All values are means + SE. Adapted with permission from Cudd et al. (17).
Effects of Alcohol on Maternal Endocrine and Paracrine Systems Regulating Vascular Resistance
Numerous studies have investigated alcohol effects on maternal hormones that are known to have substantial effects on maternal resistance vessels. Herein, we will review what is known about alcohol effects on the major endocrine axes that regulate total peripheral resistance. The hypothalamic pituitary adrenal (HPA) axis has immense effects on vascular system accompanied by sodium retention and enhanced vascular reactivity, leading to vasoconstriction via glucocorticoid receptors mediated by suppression of nitric oxide (NO) system (41). In humans, it has been reported that alcohol exposure during pregnancy increases basal cortisol levels in the offspring (36); however, no information is available on the mother. Even animal studies have predominantly concentrated on the fetus or the neonate with only few studies on the maternal HPA axis wherein alcohol is reported to increase maternal basal and stress HPA activity (18, 94). Furthermore, these alcohol-induced increases in HPA signaling may be important not only for normal parturition but also for maturation of fetal organ systems in preparation for birth (10, 12, 13, 18, 57, 81, 100). The hypothalamic pituitary thyroid axis modulates HPA activity and has been implicated in FAS because of the similarities in developmental behavioral and neuroanatomical deficits in maternal thyroid deficient disorders and FAS (31, 74). Numerous reports exist on maternal alcohol effects on fetal, neonatal, and young offspring hypothalamic pituitary thyroid axis (19, 65, 97, 98). Alcohol-exposed rat dams have suppressed levels of T3 and T4, and maternal T4 supplementation ameliorates certain learning deficits in FASD offspring (97, 98). In ewes, alcohol exposure results in decreased maternal T3, but not T4, toward the end of gestation (19). More work has been performed on the hypothalamic pituitary gonadal axis. The androgens and estrogens have profound effects on the vascular system (46, 77). Alcohol consumption in women during pregnancy that has led to FAS has been noted to lead to lower levels of sex hormone-binding globulin throughout pregnancy and total testosterone concentrations at 16–20 wk of gestation (term is roughly 285 days) (25, 102). These early studies also showed that free testosterone was higher between 16 and 20 wk of gestation, whereas a lower level of dehydroepiandrosterone sulfate was observed between 16 and 32 wk of gestation (102). Furthermore, recent studies have shown a nearly 25% decrease in serum testosterone levels in women who drink a median of one glass a day (85). Furthermore, in rats, alcohol exposure in utero has been reported to lead to decreased testosterone levels in the male fetuses and neonates that may be attributed to increased aromatase activity in the whole hypothalamus in the fetus and the hypothalamic preoptic area tissue postnatally (54, 55, 92, 93). In the field of estrogens, although earlier studies have reported decreases in 17β-estradiol and estriol levels in mothers with FAS infants (32), more recent studies focusing on lower alcohol consumption (median of 0.2, 1.1, and 4.4 oz/wk) showed that estradiol levels are not associated with occasional/light/moderate alcohol consumption (85). Another hormone critical in maintaining the vascular tone is the potent vasoconstrictor arginine vasopressin (AVP) that also has antidiuretic properties. Virtually nothing has been reported about AVP levels in mothers consuming alcohol during pregnancy; however, in the offspring, one study showed that alcohol [35% calories derived from alcohol, gestational day (GD) 6–20] leads to increased neurohypophysis activity and higher AVP levels, a reason cited for alcohol-induced increased HPA activity, whereas another study using 36% alcohol-derived calories throughout gestation showed no difference in offspring AVP mRNA levels compared with the controls, though males exhibited higher mRNA levels than females (20, 43, 103). Another study again using 35% calories derived from alcohol (GD 7–21) demonstrated 30% decrease in AVP mRNA and decreases in plasma AVP levels in response to 20% hemorrhage in rats by adulthood (5). Another system that has significant effects on the systemic vascular resistance is the renin angiotensin system for which there are no studies on the maternal effects of alcohol in either the mother or the fetus. The last system that we will discuss herein is the effects of alcohol on the catecholamines produced by the sympathetic division of the autonomic nervous system and the adrenal medulla. Early studies have shown acute alcohol (4 g/kg, GD 21)-induced increases in plasma norepinephrine concentrations in pregnant mothers in the rat (56). In men, acute alcohol consumption (0.5 ml/kg) increased plasma catecholamine levels and chronic alcohol exposure (BAC, ∼192 mg/dl) increased their excretion (35, 58), and in rats, chronic alcohol (80 mM for 15–18 days) potentiates α1-adrenergic receptor-mediated vasoconstriction (64). Despite these observations, studies that directly test the effects of alcohol-induced endocrine changes on the maternal vascular system are still warranted.
The role of major paracrine factors that regulate vascular resistance is also of importance to the effects of alcohol on changes in gestational physiology. We will first review the effects of alcohol on the prostanoid system that is activated during pregnancy. In one study, pregnant women who were consuming ≥48 g alcohol per day and those who abstained were studied and no significant differences in levels of urine 8-isoprostane F2α (oxidative stress marker), 2,3-dinor-6-keto-prostaglandin F1α (a vasodilator metabolite of prostaglandin I2), and 11-dehydro-thromboxane B2 (a vasoconstrictor metabolite of thromboxane A2) were detected, and the authors concluded against a significant role for eicosanoid markers of oxidative stress (79). In contrast, an earlier study conducted in drinking pregnant women who consumed higher amounts of alcohol (5- to 20-yr history of alcohol abuse; consumption, ∼140–840 g/wk during first half of gestation or beyond) showed the ratio of the urinary combined prostacyclin metabolites (vasodilator) and thromboxane B2 (vasoconstrictor) to be significantly lower compared with that in the controls (101). Similar observations were found in the fetoplacental compartment; alcohol perfusion (240 min or 60 min; 200 or 300 mg%) of single human cotyledons increased the ratio of thromboxane to prostacyclin at few time points (80). Randall and Saulnier (72) have demonstrated that perfusion (100 mM) in human umbilical veins showed an increased thromboxane-to-prostacyclin ratio at all time points. Next, we will discuss oxygen free radicals as redox balance impairment produces vasoconstriction. Substantial evidence exists to demonstrate maternal alcohol exposure increases oxidative stress markers in the fetus (11), fetoplacental compartment (40), maternal ovine uterine artery endothelial cells (69), etc., via multiple mechanisms that are discussed in other excellent review articles (9, 11, 27), whereas there is a paucity of human clinical studies especially in pregnant women (79). Another paracrine factor that is critical in gestational adaptations is NO. More is known about alcohol effects in adult nonpregnant humans and animals; one report showed that in vitro acute treatment of alcohol (0.1%, 3 h) increased basal and stimulated bovine aortic endothelial cell NO release (91), whereas alcohol (4 g/kg for 12 wk) given to male rats decreased aortic endothelial NO synthase expression, NO levels, and impaired vasorelaxation (34). However, Baraona et al. (3) showed that in male rats, chronic alcohol (increasing alcohol levels in diet from 30–50 g/l over 5 days) increased hepatic inducible NO synthase activity and NO levels. Again, in male rats, alcohol (BAC, ∼41 mM) produced vessel-specific increases in blood flow only in the coronary, mesenteric, and renal arteries, and the authors have attributed this to increased NO and inducible NO synthase (2). In contrast, intravenous infusion of alcohol in nonpregnant male and female rabbits (3–30 mmol/kg) (62) as well as in healthy human volunteers (0.25 and 1.0 g/kg) (61) dose-dependently decreased exhaled NO. Similarly, extensive human, animal, as well as cell culture studies that show increased or decreased NO, depending on the dose, duration, and pattern of exposure in nonpregnant adults (88), are extensively reviewed elsewhere, which are beyond the focus of the current review on pregnant mothers. In one study, alcohol administered in the diet (GD 6–18, peak BAC, 0.11 g/dl) to pregnant C57BL/6J mice showed reduced NO modulation of the mesenteric artery vascular response (14). In vitro chronic binge-like alcohol treatment (300 and 600 mg/dl) to uterine artery endothelial cells derived from third trimester pregnant ewes decreased excitatory serine-635 endothelial NO synthase phosphorylation levels at both concentrations (67). In placental villi, in vitro acute alcohol exposure for 2 h (100 and 200 mM) decreased tissue NO concentrations and cGMP levels and increased superoxide dismutase activity (39). In the fetus, similarly, it is reported that alcohol induces impairments in neuronal NO system as well as decreases in NO synthase-positive neurons, possibly leading to teratogensis (6, 7, 63). Thus it appears that alcohol effects on these paracrine factors during pregnancy involve multiple variables including alcohol dose, sex, duration, enzyme isoforms, pattern of alcohol exposure, gestational programming of enzyme expression/activity, as well as regional differences among the organ vascular beds. However, despite these advances, studies that test the cause and effect relationship between alcohol-induced alterations in these paracrine/intrinsic factors and vascular function and/or uterine vascular development during pregnancy are highly warranted.
Maternal Alcohol Effects on Vascular Reactivity
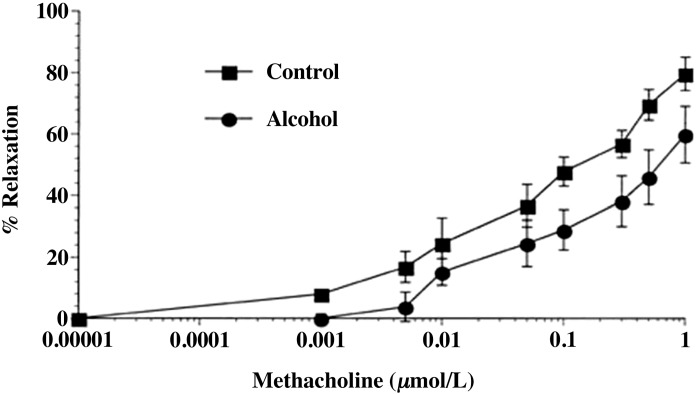
Few studies have been conducted on the effects of alcohol during pregnancy on the systemic vascular reactivity to different vasoconstrictive and dilatory agonists. Assessment of vessel function and their mechanical properties using myography (84) in various maternal vascular beds provide the basis for understanding gestational programming characterized by dramatic vascular adaptations including increases in heart rate and cardiac output as well as decreases in mean arterial pressure (45). Furthermore, the most substantial adaptation takes place in the uterine artery; uterine artery diameter is reported to increase from about 1.4 mm in nonpregnant state to 2.8 mm by week 21 and to 3.4 mm in the third trimester, and the mean blood velocity increases from 8.4 cm/s in the nonpregnant state to 61.4 cm/s in the third trimester (59). Similarly, in the sheep, the uterine blood flow during the third trimester increases between 30- to 50-fold compared with the nonpregnant state (45, 75). Such organ-specific vascular effects are further substantiated by vessel functional studies using myography; in one study uterine artery had decreased sensitivity to thromboxane compared with the carotid artery during pregnancy (95), whereas in another study myometrial vessels were less responsive to bradykinin compared with omental vessels (1). With reference to maternal alcohol consumption, in one study alcohol was administered in the diet between GD 6 and 18 in C57BL/6J mice to generate a peak BAC of around 110 mg/dl (14). The authors report reduced maximal relaxation response to methacholine in maternal mesenteric artery and found that alcohol reduced the NO component of modulation of the vascular response (Fig. 2) (14). These effects were further specific to pregnancy, an expected finding as the endothelium is programmed during pregnancy. To date, however, there is only one study examining alcohol-induced maternal vascular reactivity and that too on a systemic vascular bed. Thus it is highly warranted that more functional studies be conducted to assess effects of gestational alcohol exposure on vascular responses to vasoconstrictors and dilators on reproductive vasculature, especially the uterine and the placental resistance arteries, since these data would give important insights into the effects of alcohol on nutrient and gas delivery from the mother to the fetus.
Fig. 2.
Concentration response curves to methacholine. The effect of chronic alcohol consumption on mesenteric artery vascular response to methacholine in pregnant mice are shown (see text for details). Values are expressed as means ± SE. Adapted with permission from Cook et al. (14).
Maternal Alcohol Effects on Reproductive Vasculature
Major adaptations occur in the uteroplacental circulation during pregnancy. For instance, in animal model systems, it has been shown that the uterine vascular resistance drops significantly from 4.91 mmHg/min·ml in the nonpregnant state to 0.198 mmHg/min·ml in the second trimester and 0.07 mmHg/min·ml in the third trimester of gestation (75). The percentage of cardiac output perfusing the uterus increases from 0.5% in the nonpregnant state to around 7.65 and 15.7% in the second and third trimesters of gestation, and the blood flows to the uterus and the mammary gland alone account for nearly one fifth of the cardiac output by term (75). These changes are critical to meet the growing requirements of the developing fetus. In pregnant sheep, intravenous infusion of 1 g alcohol/min over 1 h decreased uterine as well as placental blood flow, and the reductions were maintained for at least 2 h after the end of alcohol treatment; uterine blood flow significantly decreased from 1,477 ± 169 to 1,180 ± 195 ml/min, whereas the umbilical blood flow significantly decreased from 572 ± 74 to 391 ± 74 ml/min (21). Another pattern of alcohol administration was followed in a subsequent study where four intermittent 2 or 4 g/kg body wt doses were administered over 28 min with a 56-min interval between doses, leading to a progressive increase in the BAC to around 332 and 538 mg/dl, respectively (73). Although absolute uterine blood flows were not reported in this study, it was observed that with time, an increase in the uterine blood flow was observed (73). This contrast could be attributed to this intermittent pattern of infusing alcohol over more than 4 h, generating higher BACs or a compensatory change in uterine resistance or perfusion pressure. In another study in rats, progressively increasing concentrations of alcohol, 10% and 20% vol/vol, was fed via diet for a month before pregnancy followed by 30% vol/vol during gestation (38). Microsphere analysis was then used to assess placental blood flow which decreased by around 52% in the alcohol treatment group compared with the controls (38). More studies are needed to understand the effects of alcohol on chronic exposure that mimics human binge drinking, for example during all three trimesters of gestation compared with for instance the third trimester of gestation.
Because angiogenesis and vessel remodeling are major contributing mechanisms defining the uteroplacental circulation during gestation, we will now review what is known about the effects of alcohol on these adaptations. More is known about the effects of alcohol in the nonpregnant state; for instance, alcohol (1%, every night for 4 wk) increased tumor angiogenesis in male mice (87), whereas Radek at al. (66) demonstrated in nonpregnant female mice that acute alcohol exposure (100 mg/dl) impaired wound angiogenesis. Again, during gestation, more is known about the effects of alcohol on fetal angiogenesis compared with the mother; for example, angiogenic measures such as vessel perimeter and absolute cross-sectional area are decreased in human embryonic and fetal brain (83), and region-specific microvascularity is altered in the rat fetal brain (42). In the mother, chronic alcohol (37% of caloric content, GD 6–16) impairs the physiological conversion of the maternal uterine vasculature within the mesenteric triangle in rats, a necessary step for delivery of nutrients and gas to the fetus; for instance, the spiral artery vascular muscular layer was disrupted in the control dams, whereas the thickness was 12–15 μm in the alcohol-exposed mothers (30). In a chick extraembryonic model, moderate and heavy alcohol doses (30% or 50%, 24 or 48 h) impaired vascular development, and the authors (89) have attributed these deficits to angiogenic growth factors, associated receptors, and oxidative stress, whereas Gu et al. (29), again using the chick extraembryonic model, showed that administration of alcohol (0.25 g·kg−1·day−1, 7 days) induced greater branching of blood vessels (29). In uterine artery endothelial cells derived from pregnant ewes, in vitro chronic binge-like alcohol exposure (300 or 600 mg/dl) inhibits estradiol 17β-induced endothelial proliferation (67). In a more recent study, biotinylated capture and color-coded reporter probes were used to digitally characterize 85 angiogenesis-associated genes in the uterine vasculature following chronic binge alcohol exposure (22, 26). In this study, 20 angiogenesis-associated genes were downregulated and two upregulated (71). The downregulated genes included angiogenic growth factors/receptors (placental growth factor), adhesion molecules (angiopoietin-like-3, collagen-18A1, endoglin), proteases/matrix proteins/inhibitors (alanyl aminopeptidase, collagen-4A3, heparanase, plasminogen, platelet factor-4, plexin domain containing-1, tissue inhibitor of metalloproteinases-3), transcription/signaling molecules (heart and neural crest derivatives-2, DNA-binding protein inhibitor-1, NOTCH4, ribosomal protein-L13a, ribosomal protein large-P1), cytokines/chemokines (interleukin-1B), and miscellaneous growth factors [leptin, platelet-derived growth factor-α, transforming growth factor-α], whereas plasminogen activator urokinase and transforming growth factor-β receptor-1 were upregulated (71). In summary, these studies show that alcohol affects vascular adaptations during pregnancy by altering angiogenic indexes.
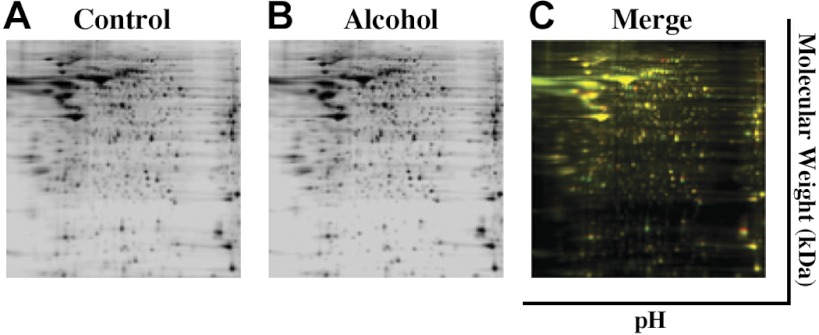
Now, we will review the mechanistic perspectives underlying alcohol-induced angiogenesis and vascular remodeling via its effects on the endothelial proteome. This is an important area of exploration since the vascular endothelium is programmed during pregnancy (47, 48); for example, we reported that 17β-estradiol and its metabolites induce proliferation of uterine endothelial cells only during pregnancy and not in the nonpregnant state (37). In one study, uterine artery endothelial cells were isolated from pregnant ewes, fluorescence-activated cell sorted, validated, and maintained in culture and treated with chronic in vitro binge-like alcohol (300 mg/dl, 3-h treatment on 3 consecutive days followed by 4 days without exposure for 2 wk) (69). Two-dimensional differential in gel electrophoresis separation followed matrix-assisted laser desorption time-of-flight mass spectrometry tandem mass spectrometry was then used to demonstrate that alcohol significantly decreased 30 proteins and increased 19 others in the uterine endothelium (Fig. 3). Gene enrichment and functional annotation cluster analysis was then used to show significant enrichment in three categories: oxidative stress-associated glutathione S-transferase and thioredoxin, as well as vesicle transport-related pathways (69). It has also been demonstrated that alcohol significantly alters the protein expression in the uterine artery endothelial caveolar lipid rafts where numerous receptors, signaling molecules, ion channels, etc., reside (28, 60, 68). Subsequently, using a similar binge-like alcohol exposure paradigm, a study using isobaric tags for relative and absolute quantitation-labeled proteomic approach reported that 14 maternal uterine endothelial proteins were significantly upregulated and 17 significantly downregulated by alcohol, including those related to cell structure, transcription and translation regulation, histones, Ca2+/NO, and redox balance (J. Ramadoss and R. R. Magness, in review). Alcohol altered processes at multiple levels including epigenetic, transcriptional, and translational (J. Ramadoss and R. R. Magness, in review). Thus these reports demonstrate that alcohol has specific and possibly detrimental effects on the uteroplacental compartment at the level of the vasculature.
Fig. 3.
Representative two-dimensional (2-D) differential in gel electrophoresis (DIGE) images of paired control and alcohol uterine endothelial samples depicting a rich array of fluorescent spots. Equal amounts of Cy2 (standard with equally mixed samples), Cy3 (control), and Cy5 (alcohol)-labeled samples were mixed and then separated on analytical 2-D DIGE. A and B: black and white images depict fluorescent signals derived from the red and green channels. C: green spots on the 2-D gel depict maternal uterine endothelial proteins that were downregulated by alcohol, whereas red spots were proteins that were upregulated and yellow spots were unaltered by binge alcohol exposure. Adapted with permission from Ramadoss and Magness (69).
Future Directions and Perspectives
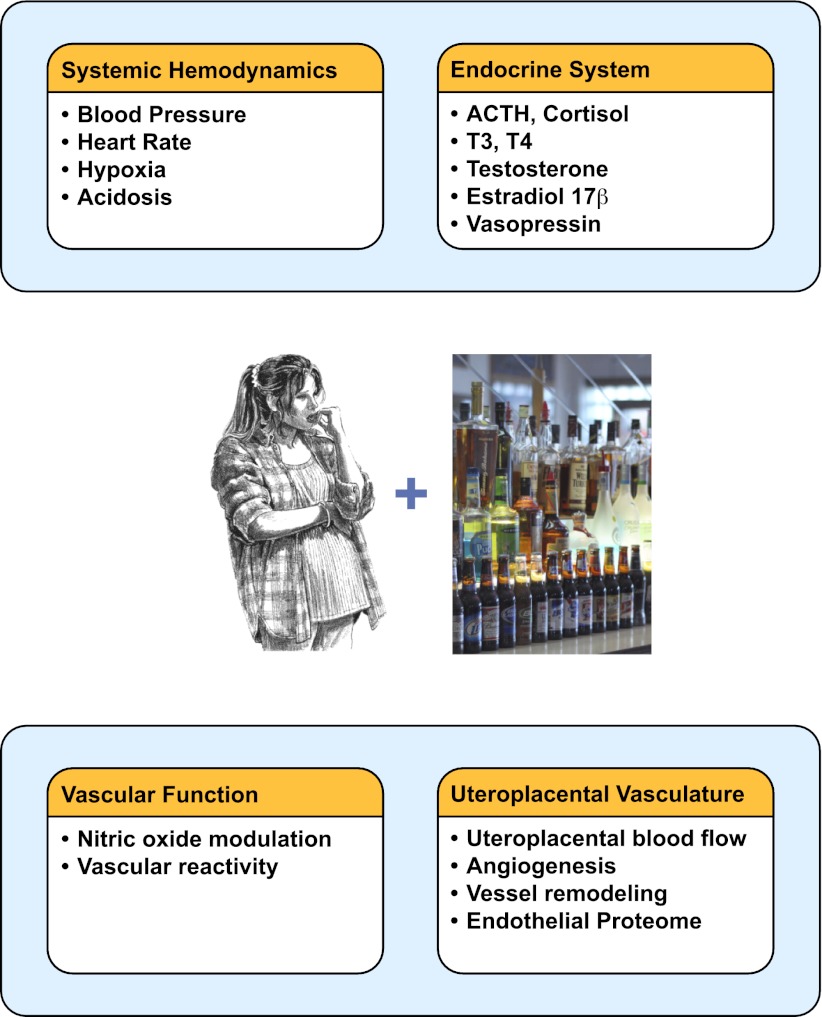
It is evident that alcohol alters maternal systemic and reproductive circulatory adaptations during pregnancy (Fig. 4). Furthermore, studies are warranted to investigate how varying patterns of alcohol consumption (chronic, binge, etc.) during different temporal periods of pregnancy affect systemic hemodynamic variables such as blood pressure and total peripheral resistance and how these variables are altered by baro- and chemoreflexes. Further studies are warranted evaluating focused interaction between alcohol and reproductive hormones such as testosterone and 17β-estradiol. An area where hardly any work has been performed is on the vascular reactivity of the uteroplacental vasculature in response to vasoconstrictors and dilators following maternal alcohol exposure during pregnancy. These studies will provide immense insights on the role of the maternal uteroplacental vasculature in the pathogenesis of FASD. These studies will also demonstrate the importance of the mother, the maternal vasculature, and the maternal-fetal interface in addition to the direct effects of alcohol on the developing fetal brain in the pathogenesis of FASD.
Fig. 4.
Maternal alcohol exposure during pregnancy affects systemic hemodynamic variables, endocrine axes regulating vascular resistance, systemic vascular reactivity, uteroplacental hemodynamics, angiogenesis, and vessel remodeling. See text for details.
GRANTS
This study was supported by National Institutes of Health Grants AA-19446 (J. Ramadoss) and HD-38843, HL-49210, and HL-89144 (to R. R. Magness).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R. and R.R.M. drafted manuscript; J.R. and R.R.M. edited and revised manuscript; J.R. and R.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Robert J. Gordon at University of Wisconsin-Madison for figure construction and Dr. Chandra Yallampalli, Dr. Kunju Sathishkumar, and Dr. Madhu Chauhan at the University of Texas Medical Branch-Galveston for valuable suggestions for the manuscript.
REFERENCES
- 1. Ashworth JR, Warren AY, Baker PN, Johnson IR. A comparison of endothelium-dependent relaxation in omental and myometrial resistance arteries in pregnant and nonpregnant women. Am J Obstet Gynecol 175: 1307– 1312, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Baraona E, Shoichet L, Navder K, Lieber CS. Mediation by nitric oxide of the stimulatory effects of ethanol on blood flow. Life Sci 70: 2987– 2995, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS. Ethanol consumption increases nitric oxide production in rats, and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin Exp Res 26: 883– 889, 2002 [PubMed] [Google Scholar]
- 4. Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301: 259– 262, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bird DN, Sato AK, Knee DS, Uyehara CF, Person DA, Claybaugh JR. Effects of prenatal ethanol exposure and sex on the arginine vasopressin response to hemorrhage in the rat. Am J Physiol Regul Integr Comp Physiol 291: R77– R82, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonthius DJ, Bonthius NE, Li S, Karacay B. The protective effect of neuronal nitric oxide synthase (nNOS) against alcohol toxicity depends upon the NO-cGMP-PKG pathway and NF-kappaB. Neurotoxicology 29: 1080– 1091, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bonthius DJ, Luong T, Bonthius NE, Hostager BS, Karacay B. Nitric oxide utilizes NF-kappaB to signal its neuroprotective effect against alcohol toxicity. Neuropharmacology 56: 716– 731, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res 14: 107– 118, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Brocardo PS, Gil-Mohapel J, Christie BR. The role of oxidative stress in fetal alcohol spectrum disorders. Brain Res Rev 67: 209– 225, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Challis J, Sloboda D, Matthews S, Holloway A, Alfaidy N, Howe D, Fraser M, Newnham J. Fetal hypothalamic-pituitary adrenal (HPA) development and activation as a determinant of the timing of birth, and of postnatal disease. Endocr Res 26: 489– 504, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Cohen-Kerem R, Koren G. Antioxidants and fetal protection against ethanol teratogenicity. I Review of the experimental data and implications to humans. Neurotoxicol Teratol 25: 1– 9, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Conley AJ, Assis Neto AC. The ontogeny of fetal adrenal steroidogenesis as a prerequisite for the initiation of parturition. Exp Clin Endocrinol Diabetes 116: 385– 392, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Cook JL, Randall CL. Early onset of parturition induced by acute alcohol exposure in C57BL/6J mice: role of uterine PGE and PGF2alpha. Reprod Fertil Dev 9: 815– 823, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Cook JL, Zhang Y, Davidge ST. Vascular function in alcohol-treated pregnant and nonpregnant mice. Am J Physiol Regul Integr Comp Physiol 281: R1449– R1455, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 230: 389– 393, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Cudd TA. Animal models for the study of prenatal alcohol exposure, fetal alcohol syndrome, alcohol-related birth defects, and alcohol-related neurodevelopmental disorder In: Source Book of Models for Biomedical Research, edited by Conn PM. Totowa, NJ: Humana, 2008, p. 603– 614 [Google Scholar]
- 17. Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcohol Clin Exp Res 25: 269– 276, 2001 [PubMed] [Google Scholar]
- 18. Cudd TA, Chen WJ, West JR. Fetal and maternal sheep hypothalamus pituitary adrenal axis responses to chronic binge ethanol exposure during the third trimester equivalent. Alcohol Clin Exp Res 25: 1065– 1071, 2001 [PubMed] [Google Scholar]
- 19. Cudd TA, Chen WJ, West JR. Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcohol Clin Exp Res 26: 53– 58, 2002 [PubMed] [Google Scholar]
- 20. Dow-Edwards DL, Trachtman H, Riley EP, Freed LA, Milhorat TH. Arginine vasopressin and body fluid homeostasis in the fetal alcohol exposed rat. Alcohol 6: 193– 198, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol 25: 413– 416, 1990 [PubMed] [Google Scholar]
- 22. Fortina P, Surrey S. Digital mRNA profiling. Nat Biotechnol 26: 293– 294, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 322: 95– 99, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: the Atherosclerosis Risk in Communities Study. Hypertension 37: 1242– 1250, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Gabriel K, Hofmann C, Glavas M, Weinberg J. The hormonal effects of alcohol use on the mother and fetus. Alcohol Health Res World 22: 170– 177, 1998 [PMC free article] [PubMed] [Google Scholar]
- 26. Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26: 317– 325, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 230: 394– 406, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res 94: 1408– 1417, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Gu JW, Elam J, Sartin A, Li W, Roach R, Adair TH. Moderate levels of ethanol induce expression of vascular endothelial growth factor and stimulate angiogenesis. Am J Physiol Regul Integr Comp Physiol 281: R365– R372, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Gundogan F, Elwood G, Longato L, Tong M, Feijoo A, Carlson RI, Wands JR, de la Monte SM. Impaired placentation in fetal alcohol syndrome. Placenta 29: 148– 157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341: 549– 555, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Halmesmaki E, Autti I, Granstrom ML, Stenman UH, Ylikorkala O. Estradiol, estriol, progesterone, prolactin, and human chorionic gonadotropin in pregnant women with alcohol abuse. J Clin Endocrinol Metab 64: 153– 156, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Horiguchi T, Suzuki K, Comas-Urrutia AC, Mueller-Heubach E, Boyer-Milic AM, Baratz RA, Morishima HO, James LS, Adamsons K. Effect of ethanol upon uterine activity and fetal acid-base state of the rhesus monkey. Am J Obstet Gynecol 109: 910– 917, 1971 [DOI] [PubMed] [Google Scholar]
- 34. Husain K. Vascular endothelial oxidative stress in alcohol-induced hypertension. Cell Mol Biol (Noisy-le-grand) 53: 70– 77, 2007 [PubMed] [Google Scholar]
- 35. Ireland MA, Vandongen R, Davidson L, Beilin LJ, Rouse IL. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin Sci (Lond) 66: 643– 648, 1984 [DOI] [PubMed] [Google Scholar]
- 36. Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol 11: 195– 208, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension 55: 1005– 1011, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones PJ, Leichter J, Lee M. Placental blood flow in rats fed alcohol before and during gestation. Life Sci 29: 1153– 1159, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol 182: 682– 688, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Kay HH, Tsoi S, Grindle K, Magness RR. Markers of oxidative stress in placental villi exposed to ethanol. J Soc Gynecol Investig 13: 118– 121, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Kelly JJ, Mangos G, Williamson PM, Whitworth JA. Cortisol and hypertension. Clin Exp Pharmacol Physiol Suppl 25: S51– S56, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Kelly SJ, Mahoney JC, West JR. Changes in brain microvasculature resulting from early postnatal alcohol exposure. Alcohol 7: 43– 47, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Kim CK, Giberson PK, Yu W, Zoeller RT, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal responses to chronic cold stress in rats. Alcohol Clin Exp Res 23: 301– 310, 1999 [PubMed] [Google Scholar]
- 44. Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol 25: 447– 458, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: The Endocrinology of Pregnancy Bazer. Totowa, NJ: Humana, 1998, p. 507– 539 [Google Scholar]
- 46. Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17β-estradiol. Am J Physiol Heart Circ Physiol 275: H731– H743, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol Heart Circ Physiol 272: H1730– H1740, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol 280: H1692– H1698, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Maier SE, Miller JA, West JR. Prenatal binge-like alcohol exposure in the rat results in region-specific deficits in brain growth. Neurotoxicol Teratol 21: 285– 291, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health 25: 168– 174, 2001 [PMC free article] [PubMed] [Google Scholar]
- 51. Maier SE, West JR. Regional differences in cell loss associated with binge-like alcohol exposure during the first two trimesters equivalent in the rat. Alcohol 23: 49– 57, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol 11: 147– 156, 1994 [DOI] [PubMed] [Google Scholar]
- 53. May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 15: 176– 192, 2009 [DOI] [PubMed] [Google Scholar]
- 54. McGivern RF, Handa RJ, Raum WJ. Ethanol exposure during the last week of gestation in the rat: inhibition of the prenatal testosterone surge in males without long-term alterations in sex behavior. Neurotoxicol Teratol 20: 483– 490, 1998 [DOI] [PubMed] [Google Scholar]
- 55. McGivern RF, Roselli CE, Handa RJ. Perinatal aromatase activity in male and female rats: effect of prenatal alcohol exposure. Alcohol Clin Exp Res 12: 769– 772, 1988 [DOI] [PubMed] [Google Scholar]
- 56. Mena MA, Zorzano A, Herrera E. Acute effects of ethanol on brain, plasma and adrenal monoamine concentrations in virgin and pregnant rats and their fetuses. Neurochem Int 9: 371– 378, 1986 [DOI] [PubMed] [Google Scholar]
- 57. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23: 947– 954, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ogata M, Mendelson JH, Mello NK, Majchrowicz E. Adrenal function and alcoholism. II. Catecholamines. Psychosom Med 33: 159– 180, 1971 [DOI] [PubMed] [Google Scholar]
- 59. Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000– 1006, 1992 [PubMed] [Google Scholar]
- 60. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185– 194, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Persson MG, Cederqvist B, Wiklund CU, Gustafsson LE. Ethanol causes decrements in airway excretion of endogenous nitric oxide in humans. Eur J Pharmacol 270: 273– 278, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Persson MG, Gustafsson LE. Ethanol can inhibit nitric oxide production. Eur J Pharmacol 224: 99– 100, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Phillips DE, Cummings JD, Wall KA. Prenatal alcohol exposure decreases the number of nitric oxide synthase positive neurons in rat superior colliculus and periaqueductal gray. Alcohol 22: 75– 84, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Pinardi G, Brieva C, Vinet R, Penna M. Effects of chronic ethanol consumption on alpha-adrenergic-induced contractions in rat thoracic aorta. Gen Pharmacol 23: 245– 248, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Portoles M, Sanchis R, Guerri C. Thyroid hormone levels in rats exposed to alcohol during development. Horm Metab Res 20: 267– 270, 1988 [DOI] [PubMed] [Google Scholar]
- 66. Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. Am J Physiol Heart Circ Physiol 289: H1084– H1090, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Ramadoss J, Jobe SO, Magness RR. Alcohol and maternal uterine vascular adaptations during pregnancy-part I: effects of chronic in vitro binge-like alcohol on uterine endothelial nitric oxide system and function. Alcohol Clin Exp Res 35: 1686– 1693, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramadoss J, Liao WX, Chen DB, Magness RR. High-throughput caveolar proteomic signature profile for maternal binge alcohol consumption. Alcohol 44: 691– 697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ramadoss J, Magness RR. 2-D DIGE uterine endothelial proteomic profile for maternal chronic binge-like alcohol exposure. J Proteomics 74: 2986– 2994, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramadoss J, Magness RR. Multiplexed digital quantification of binge-like alcohol-mediated alterations in maternal uterine angiogenic mRNA transcriptome. Physiol Genomics 44: 622– 628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Randall CL, Saulnier JL. Effect of ethanol on prostacyclin, thromboxane, and prostaglandin E production in human umbilical veins. Alcohol Clin Exp Res 19: 741– 746, 1995 [DOI] [PubMed] [Google Scholar]
- 73. Reynolds JD, Penning DH, Dexter F, Atkins B, Hrdy J, Poduska D, Brien JF. Ethanol increases uterine blood flow and fetal arterial blood oxygen tension in the near-term pregnant ewe. Alcohol 13: 251– 256, 1996 [DOI] [PubMed] [Google Scholar]
- 74. Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 230: 357– 365, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol Heart Circ Physiol 232: H231– H235, 1977 [DOI] [PubMed] [Google Scholar]
- 76. Russell M, Cooper ML, Frone MR, Welte JW. Alcohol drinking patterns and blood pressure. Am J Public Health 81: 452– 457, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev 87: 407– 414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saunders JB. Alcohol: an important cause of hypertension. Br Med J (Clin Res Ed) 294: 1045– 1046, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Signore C, Aros S, Morrow JD, Troendle J, Conley MR, Flanigan EY, Cassorla F, Mills JL. Markers of oxidative stress and systemic vasoconstriction in pregnant women drinking > or =48 g of alcohol per day. Alcohol Clin Exp Res 32: 1893– 1898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Siler-Khodr TM, Yang Y, Grayson MH, Henderson GI, Lee M, Schenker S. Effect of ethanol on thromboxane and prostacyclin production in the human placenta. Alcohol 21: 169– 180, 2000 [DOI] [PubMed] [Google Scholar]
- 81. Smith R. Parturition. N Engl J Med 356: 271– 283, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA 290: 2996– 2999, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Solonskii AV, Logvinov SV, Kutepova NA. Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol. Neurosci Behav Physiol 38: 373– 376, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Spiers A, Padmanabhan N. A guide to wire myography. Methods Mol Med 108: 91– 104, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Stevens RG, Cohen RD, Terry MB, Lasley BL, Siiteri P, Cohn BA. Alcohol consumption and serum hormone levels during pregnancy. Alcohol 36: 47– 53, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Surgeon General. Surgeon General's advisory on alcohol and pregnancy. FDA Drug Bull 11: 9– 10, 1981 [PubMed] [Google Scholar]
- 87. Tan W, Bailey AP, Shparago M, Busby B, Covington J, Johnson JW, Young E, Gu JW. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol Ther 6: 1211– 1217, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Toda N, Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol 45: 347– 355, 2010 [DOI] [PubMed] [Google Scholar]
- 89. Tufan AC, Satiroglu-Tufan NL. The effect of ethanol exposure on extraembryonic vascular development in the chick area vasculosa. Cells Tissues Organs 175: 84– 97, 2003 [DOI] [PubMed] [Google Scholar]
- 90. van Leer EM, Seidell JC, Kromhout D. Differences in the association between alcohol consumption and blood pressure by age, gender, and smoking. Epidemiology 5: 576– 582, 1994 [DOI] [PubMed] [Google Scholar]
- 91. Venkov CD, Myers PR, Tanner MA, Su M, Vaughan DE. Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost 81: 638– 642, 1999 [PubMed] [Google Scholar]
- 92. Ward IL, Bennett AL, Ward OB, Hendricks SE, French JA. Androgen threshold to activate copulation differs in male rats prenatally exposed to alcohol, stress, or both factors. Horm Behav 36: 129– 140, 1999 [DOI] [PubMed] [Google Scholar]
- 93. Ward OB, Ward IL, Denning JH, French JA, Hendricks SE. Postparturitional testosterone surge in male offspring of rats stressed and/or fed ethanol during late pregnancy. Horm Behav 41: 229– 235, 2002 [DOI] [PubMed] [Google Scholar]
- 94. Weinberg J, Bezio S. Alcohol-induced changes in pituitary-adrenal activity during pregnancy. Alcohol Clin Exp Res 11: 274– 280, 1987 [DOI] [PubMed] [Google Scholar]
- 95. Weiner CP, Thompson LP, Liu KZ, Herrig JE. Endothelium-derived relaxing factor and indomethacin-sensitive contracting factor alter arterial contractile responses to thromboxane during pregnancy. Am J Obstet Gynecol 166: 1171– 1178, 1992 [DOI] [PubMed] [Google Scholar]
- 96. West JR, Goodlett CR, Kelly SJ. Alcohol and brain development. NIDA Res Monogr 78: 45– 60, 1987 [PubMed] [Google Scholar]
- 97. Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry 10: 961– 971, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab 287: E318– E326, 2004 [DOI] [PubMed] [Google Scholar]
- 99. Witteman JC, Willett WC, Stampfer MJ, Colditz GA, Kok FJ, Sacks FM, Speizer FE, Rosner B, Hennekens CH. Relation of moderate alcohol consumption and risk of systemic hypertension in women. Am J Cardiol 65: 633– 637, 1990 [DOI] [PubMed] [Google Scholar]
- 100. Wood CE. Control of parturition in ruminants. J Reprod Fertil Suppl 54: 115– 126, 1999 [PubMed] [Google Scholar]
- 101. Ylikorkala O, Halmesmaki E, Viinikka L. Urinary prostacyclin and thromboxane metabolites in drinking pregnant women and in their infants: relations to the fetal alcohol effects. Obstet Gynecol 71: 61– 66, 1988 [PubMed] [Google Scholar]
- 102. Ylikorkala O, Stenman UH, Halmesmaki E. Testosterone, androstenedione, dehydroepiandrosterone sulfate, and sex-hormone-binding globulin in pregnant alcohol abusers. Obstet Gynecol 71: 731– 735, 1988 [PubMed] [Google Scholar]
- 103. Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 230: 376– 388, 2005 [DOI] [PubMed] [Google Scholar]