Abstract
The antihypertensive influence of fish oil is controversial, and the mechanisms remain unclear. Because the inverse relation between fish oil and hypertension appears to be partially dependent on the degree of hypertension, we tested the hypothesis that fish oil would elicit more dramatic reductions in mean arterial pressure (MAP) and muscle sympathetic nerve activity (MSNA) in prehypertensive (PHT) compared with normotensive (NT) subjects. Resting MAP, MSNA, and heart rate (HR) were examined before and after 8 wk of fish oil (9 g/day; 1.6 g eicosapentaenoic acid and 1.1 g docosahexaenoic acid) or placebo (olive oil; 9 g/day) in 38 NT (19 fish oil; 19 placebo) and 29 PHT (15 fish oil; 14 placebo) volunteers. Fish oil did not alter resting MAP, MSNA, or HR in either NT (80 ± 1 to 80 ± 1 mmHg; 11 ± 2 to 10 ± 1 bursts/min; 71 ± 2 to 71 ± 2 beats/min) or PHT (88 ± 2 to 87 ± 1 mmHg; 11 ± 2 to 10 ± 2 bursts/min; 73 ± 2 to 73 ± 2 beats/min) subjects. When NT and PHT groups were consolidated, analysis of covariance confirmed that pretreatment resting MAP was not associated with changes in MSNA after fish oil. In contrast, pretreatment resting HR was correlated with changes in MSNA (r = 0.47; P = 0.007) and MAP (r = 0.42; P < 0.007) after fish oil but not placebo. In conclusion, fish oil did not alter sympathetic neural control in NT or PHT subjects. However, our findings suggest that fish oil is associated with modest sympathoinhibition in individuals with higher resting heart rates, a finding that is consistent with a recent meta-analysis examining the relations among fish oil, HR, and the risk of cardiovascular disease.
Keywords: muscle sympathetic nerve activity, autonomic activity, omega-3 fatty acids
omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil, have been suggested as a nonpharmacological treatment for cardiovascular disease. Specifically, fish oil has been reported to reduce resting heart rate and blood pressure (1, 11, 16–18). Tachycardia and hypertension are known risk factors for cardiovascular-related morbidity and mortality. The mechanisms by which fish oil lowers resting heart rate and blood pressure are unknown, but the autonomic nervous system has been suggested as an important contributor (6, 18, 19).
A previous study (20) in diabetic rats has shown that EPA supplementation decreases cardiac norepinephrine concentration. Others (6, 7, 18, 19) report an increase of heart rate variability after fish oil consumption. To date, only one study (15) has directly measured post-ganglionic sympathetic neural responses to omega-3 supplementation in humans using microneurographic techniques. Monahan et al. (15) studied normotensive adults and reported that 1 mo of fish oil treatment did not alter resting muscle sympathetic nerve activity (MSNA). Because fish oil treatment appears to lower blood pressure more effectively in hypertensive rather than normotensive individuals (11, 17), the primary purpose of the present study was to examine MSNA before and after 8 wk of fish oil in prehypertensive adults. We hypothesized that fish oil supplementation would reduce resting arterial blood pressure and MSNA in prehypertensive adults. Additionally, a recent meta-analysis reports that fish oil reduced resting heart rate in humans when values were greater than ∼70 beats per min (18). Therefore, our secondary hypothesis was that fish oil supplementation would elicit greater reductions in neural and cardiovascular activity in adults with higher resting heart rates.
METHODS
Subjects.
Sixty-seven subjects participated in a double-blind, randomly assigned, placebo based study. Subject characteristics are provided in Tables 1 and 2. Classification as normotensive included a resting systolic pressure <120 mmHg and diastolic pressure <80 mmHg, while classification as prehypertensive required a resting systolic pressure of 120–139 mmHg and/or a diastolic pressure of 80–89 mmHg. These classifications are consistent with the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (5). Participants signed an informed consent and refrained from caffeine, alcohol, and exercise for ≥12 h before testing. Exclusion criteria included smoking, diabetes, hypertension, autonomic dysfunction, and use of blood pressure medication. To participate, subjects confirmed they had not been taking any omega-3 fatty acid supplements for ≥2 mo before the start of the study. This study was approved by the Michigan Technological University Institutional Review Board.
Table 1.
Hemodynamic and limb vascular responses to 8 wk of fish oil or placebo in normotensive humans
| Fish Oil (n = 19) |
Placebo (n = 19) |
|||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | Time | Time × Drug Interaction |
| Sex (male/female) | 9/10 | – | 9/10 | – | – | – |
| Age, yr | 24 ± 2 | – | 24 ± 2 | – | – | – |
| Height, cm | 170 ± 2 | – | 170 ± 2 | – | – | – |
| Body mass, kg | 68 ± 3 | 69 ± 3 | 70 ± 2 | 71 ± 2 | 0.17 | 0.92 |
| BMI, kg/m2 | 24 ± 1 | 24 ± 1 | 24 ± 1 | 24 ± 1 | 0.85 | 0.53 |
| SAP, mmHg | 110 ± 1 | 107 ± 1 | 107 ± 2 | 107 ± 2 | 0.19 | 0.17 |
| DAP, mmHg | 66 ± 1 | 66 ± 1 | 65 ± 1 | 66 ± 1 | 0.28 | 0.69 |
| MAP, mmHg | 80 ± 1 | 80 ± 1 | 79 ± 1 | 80 ± 1 | 0.78 | 0.42 |
| HR, beats/min | 71 ± 2 | 71 ± 2 | 74 ± 2 | 76 ± 2 | 0.46 | 0.43 |
| RRI, ms | 931 ± 59 | 985 ± 35 | 971 ± 29 | 910 ± 23 | 0.90 | 0.05 |
| RRISD, ms | 66 ± 4 | 77 ± 7* | 81 ± 7 | 69 ± 6 | 0.89 | 0.02 |
| FBF, ml·100 ml tissue−1·min−1 | 2.5 ± 0 | 2.8 ± 0 | 2.2 ± 0 | 2.5 ± 0 | 0.05 | 0.81 |
| FVC, 100 * ml·100 ml tissue−1· min−1·mmHg−1 | 3.1 ± 0 | 3.5 ± 0 | 2.8 ± 0 | 3.0 ± 0 | 0.06 | 0.59 |
| CBF, ml·100 ml tissue−1·min−1 | 2.0 ± 0 | 2.2 ± 0 | 2.2 ± 0 | 2.3 ± 0 | 0.14 | 0.70 |
| CVC, 100 * ml·100 ml tissue−1·min−1·mmHg−1 | 2.4 ± 0 | 2.8 ± 0 | 2.8 ± 0 | 2.9 ± 0 | 0.17 | 0.47 |
Values are means ± SE.
BMI, body mass index; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; HR, heart rate; FBF, forearm blood; FVC, forearm vascular conductance; CBF, calf blood flow; CVC, calf vascular conductance.
Forearm vascular measurements: n = 18 fish oil and n = 18 placebo; calf vascular measurements: n = 16 fish oil and n = 16 placebo.
Significantly different from corresponding Pre value (P < 0.05, post hoc analysis).
Table 2.
Hemodynamic and limb vascular responses to 8 wk of fish oil or placebo in prehypertensive humans
| Fish Oil (n = 15) |
Placebo (n = 14) |
|||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | Time | Time × Drug Interaction |
| Sex (male/female) | 15/0 | – | 13/1 | – | – | – |
| Age, yr | 23 ± 1 | – | 25 ± 3 | – | – | – |
| Height, cm | 179 ± 2 | – | 179 ± 2 | – | – | – |
| Body mass, kg | 88 ± 4 | 89 ± 3 | 87 ± 2 | 87 ± 2 | 0.71 | 0.71 |
| BMI, kg/m2 | 28 ± 1 | 28 ± 1 | 27 ± 1 | 27 ± 1 | 0.47 | 0.69 |
| SAP, mmHg | 127 ± 1 | 125 ± 2 | 126 ± 2 | 123 ± 2 | 0.04 | 0.59 |
| DAP, mmHg | 68 ± 2 | 68 ± 2 | 74 ± 2 | 74 ± 2 | 0.52 | 0.93 |
| MAP, mmHg | 88 ± 2 | 87 ± 1 | 92 ± 1 | 90 ± 2 | 0.22 | 0.79 |
| HR, beats/min | 73 ± 2 | 73 ± 2 | 76 ± 3 | 74 ± 3 | 0.26 | 0.53 |
| RRI, ms | 909 ± 34 | 941 ± 29 | 928 ± 34 | 941 ± 38 | 0.21 | 0.59 |
| RRISD, ms | 60 ± 5 | 75 ± 8* | 79 ± 12 | 80 ± 12 | 0.08 | 0.04 |
| FBF, ml·100 ml tissue−1·min−1 | 3.9 ± 0 | 3.5 ± 0 | 3.2 ± 0 | 3.4 ± 1 | 0.80 | 0.37 |
| FVC, 100 * ml·100 ml tissue−1·min−1·mmHg−1 | 4.5 ± 0 | 4.1 ± 0 | 3.5 ± 0 | 3.7 ± 1 | 0.75 | 0.49 |
| CBF, ml·100 ml tissue−1·min−1 | 2.8 ± 0 | 3.0 ± 0 | 2.4 ± 0 | 2.5 ± 0 | 0.48 | 0.82 |
| CVC, 100 * ml·100 ml tissue−1·min−1·mmHg−1 | 3.3 ± 0 | 3.5 ± 0 | 2.6 ± 0 | 2.7 ± 0 | 0.46 | 0.76 |
Values are means ± SE. Forearm vascular measurements: n = 14 fish oil and n = 13 placebo; calf vascular measurements: n = 14 fish oil and n = 11 placebo.
Significantly different from corresponding Pre value (P < 0.05, post hoc analysis).
Experimental protocol.
All subjects were tested before (pre) and after (post) 8 wk of fish oil or placebo supplementation. Specifically, subjects reported to the laboratory for 3 consecutive days before each testing session (i.e., pre vs. posttesting). During each of the three days, multiple seated resting arterial blood pressure and heart rates were measured (described below). On the final day of pre and posttesting sessions, a 10-min supine resting arterial pressure, heart rate and MSNA were recorded. All pre and posttesting sessions were performed at the same time of day to avoid the potential confounder of diurnal fluctuation. Following the pretesting session, participants were randomly assigned to one of two groups in which they consumed either 9 g of fish oil pills (1.6 g EPA, 1.1 g DHA) or 9 g of placebo pills (olive oil) per day for 8 consecutive weeks. Subjects were asked to maintain their current diet and exercise routines. A pill diary and weekly reminders were issued to the subjects to track compliance.
Measurements.
Seated resting arterial blood pressure and heart rate were determined with an IntelliSense automated sphygmomanometer (model HEM-907XL; Omron Healthcare, Bannockburn, IL). Three seated resting measurements were taken after a 5 min quiet rest period over 3 consecutive days before and after treatment (3 measurements with 1-min intervals each day). Thus the reported pre- and posttreatment seated resting arterial blood pressures and heart rates (Table 1) each represent the mean of 9 readings taken over 3 consecutive days. Supine heart rates were measured using a three-lead electrocardiogram, and supine beat-to-beat arterial pressure was recorded using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). Heart rate variability was assessed using time domain analysis of the electrocardiogram to determine the R-R interval standard deviation.
Multifiber recordings of MSNA were recorded using the microneurography technique. A tungsten microelectrode was inserted into the common peroneal nerve located in the popliteal region of the knee or at the base of the fibular head of the lower leg. A reference electrode was inserted subcutaneously 2–3 cm away from the recording electrode. Both electrodes were connected to a preamplifier and amplifier. The signal was amplified 80,000 times, band-pass filtered (700–2000 Hz), and integrated (time constant, 0.1 s) to obtain a mean voltage display of nerve activity. MSNA was determined by observing spontaneous multifiber bursts of activity and confirmed by having the subject perform end-expiratory apnea with a resultant increase in MSNA. The signal was distinguished from skin sympathetic activity by performing auditory stimulation with no subsequent neural reaction.
Data analysis.
Data were recorded using WinDaq/Pro data acquisition software (DATAQ Instruments, Akron, OH) and imported into WinCPRS (Absolute Aliens, Turku, Finland) software package for analysis. R-waves from the electrocardiogram were detected and marked in the time series. Integrated bursts of muscle sympathetic nerve recordings were detected as a 3:1 burst-noise ratio within a search window of 0.5 s based on an average expected burst peak latency of 1.3 s following the previous R-wave. Sympathetic bursts of activity were expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Forearm and calf blood flows were recorded via venous occlusion plethysmography (D.E. Hokanson, Bellevue, WA). Forearm and calf vascular conductances were calculated as the respective limb blood flow divided by mean arterial pressure from the automated sphygmomanometer.
Statistical analysis.
Statistical analysis was done using SPSS 18.0 (SPSS Chicago, IL). Data are presented as means ± SE. Both normotensive and prehypertensive were compared using a 1-within (time: pre vs. post), 1-between (drug: fish oil vs. placebo) repeated-measures ANOVA. We also combined the normotensive and prehypertensive data and utilized a 1-within (time: pre vs. post), 1-between (drug: fish oil vs. placebo) repeated-measures analysis of covariance in which pretreatment resting heart rate and blood pressure were included as covariables. Time × drug interactions were probed with Bonferroni correction post hoc analyses, while time × covariable interactions were probed with Pearson correlation. We also performed an analysis with age and body mass index as covariables; this additional analysis did not alter our findings. Significance was determined as P < 0.05.
RESULTS
Responses to fish oil in normotensive humans.
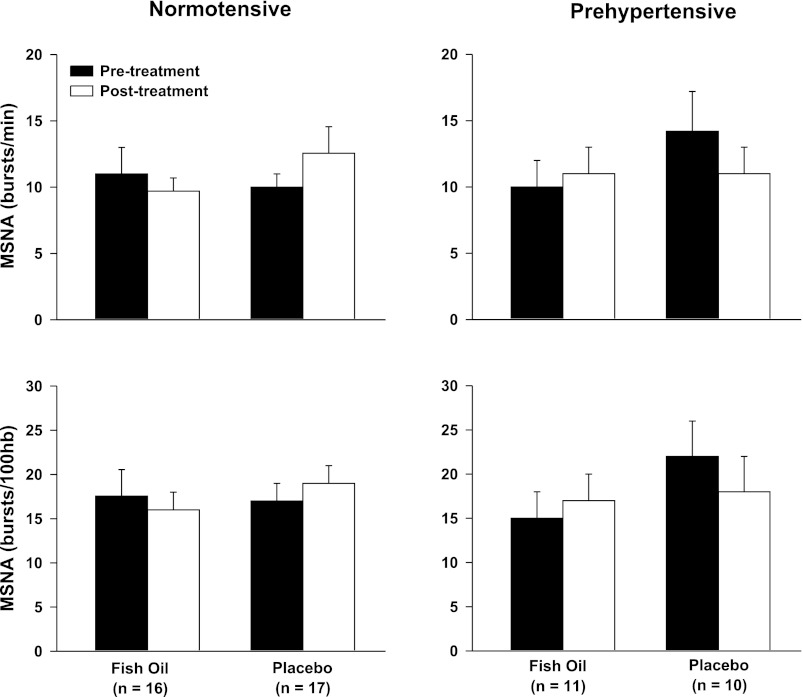
Table 1 depicts resting hemodynamic and limb vascular responses to fish oil or placebo supplementation in normotensive humans. Fish oil did not significantly alter resting heart rate, blood pressure, limb blood flow, or limb vascular conductance. Figure 1 demonstrates that fish oil did not alter resting MSNA in normotensive humans.
Fig. 1.
Resting muscle sympathetic nerve activity (MSNA) before and after 8 wk of fish oil and placebo supplementation in both normotensive (n = 33) and prehypertensive humans (n = 21). There were no significant differences between treatments or groups.
Responses to fish oil in prehypertensive humans.
Table 2 depicts resting hemodynamic and limb vascular responses to fish oil or placebo supplementation in prehypertensive humans. Fish oil did not significantly alter resting heart rate, blood pressure, limb blood flow, or limb vascular conductance. Fish oil did not alter resting MSNA in prehypertensive humans (Fig. 1).
Covariate analysis: influence of resting blood pressure.
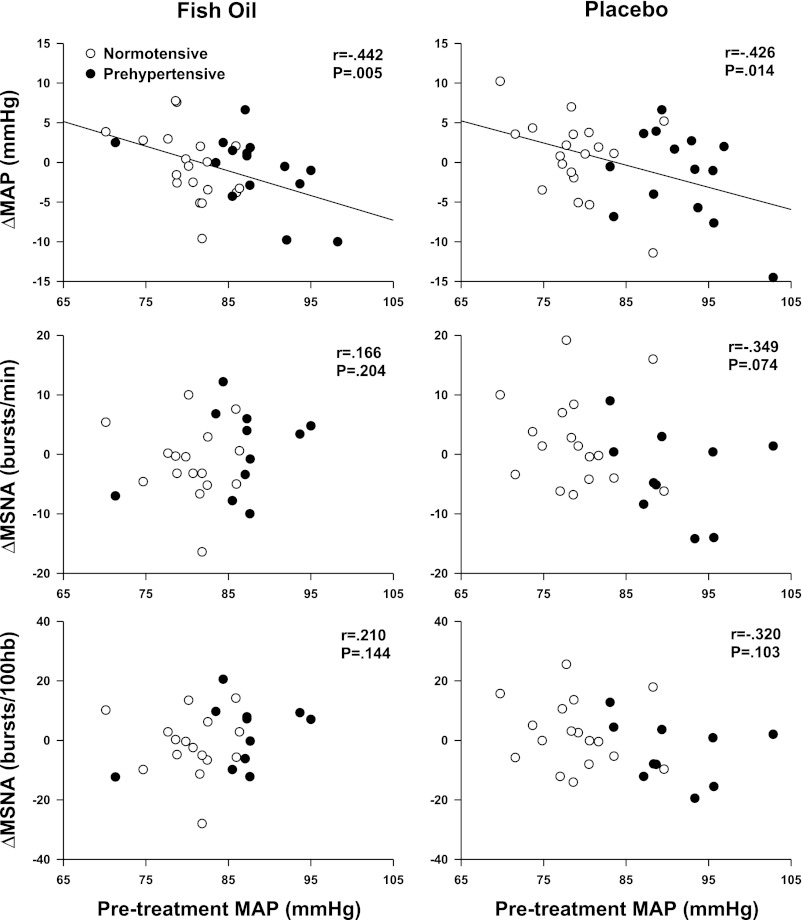
A significant interaction was observed between pretreatment MAP (covariable) and changes in systolic arterial pressure (time × covariable, P < 0.01), diastolic arterial pressure (time × covariable, P < 0.01), and MAP (time × covariable, P < 0.001). However, Fig. 2 demonstrates that significant relations between pretreatment MAP and changes in arterial blood pressure were observed after both fish oil and placebo. There were no significant interactions between pretreatment MAP and changes in HR, MSNA, or limb blood flow.
Fig. 2.
Relations between pretreatment resting mean arterial pressure (MAP) and changes in MSNA and MAP. Elevated pretreatment resting MAP was associated with greater reductions in MAP after fish oil, but this relationship was also observed with placebo (i.e., olive oil).
Covariate analysis: influence of resting heart rate.
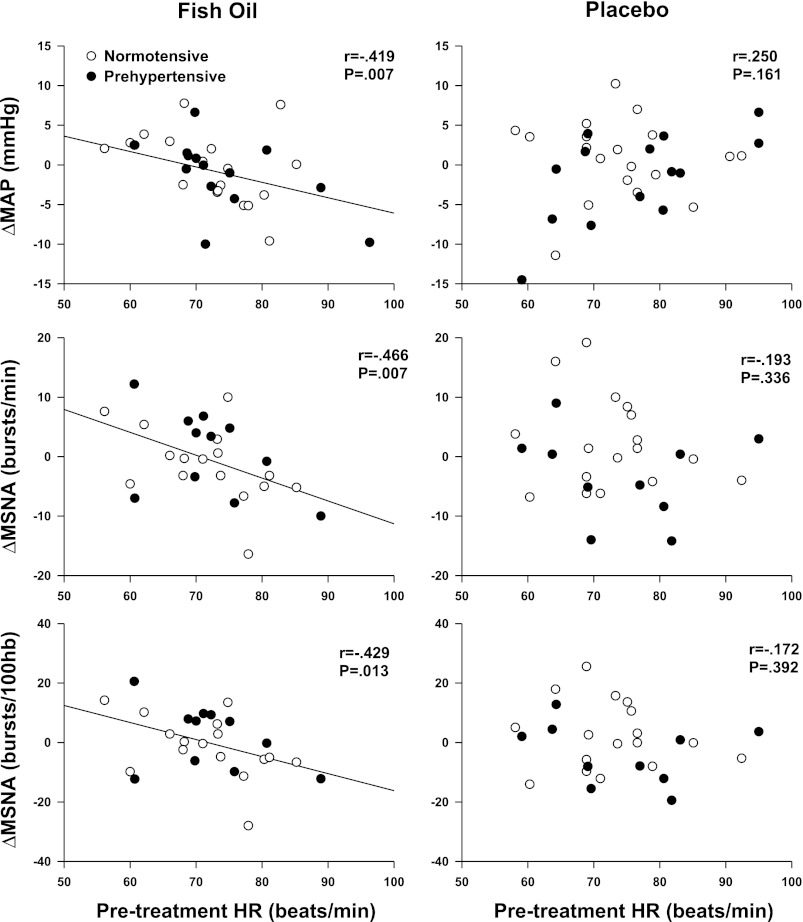
A significant interaction was observed between pretreatment HR (covariable) and changes in MSNA burst frequency (time × covariable, P = 0.02) and burst incidence (time × covariable, P = 0.03). Figure 3 demonstrates an inverse relationship between resting HR and changes in MAP and MSNA after fish oil but not placebo. There were no significant interactions between pretreatment HR and changes in HR or limb blood flow.
Fig. 3.
Relations between pretreatment resting heart rate (HR) and changes in MSNA and MAP. Elevated pretreatment resting HR was associated with greater reductions in MAP and MSNA after fish oil; this relationship was not observed in the placebo group.
DISCUSSION
In contrast to our primary hypothesis, fish oil did not alter resting arterial blood pressure or MSNA in prehypertensive humans. Moreover, when normotensive and prehypertensive subjects were pooled, the modest inverse relations between pretreatment resting arterial blood pressure and the change in blood pressure after treatment were observed in both fish oil and placebo groups. Therefore, our data suggest that 8 wk of fish oil supplementation does not significantly alter neurovascular control in prehypertensive adults. In contrast, fish oil appeared to influence neural cardiovascular control in adults with higher resting heart rates. Specifically, there was a modest inverse relationship between pretreatment resting heart rate and the changes in both MAP and MSNA after fish oil but not placebo. These relations between pretreatment resting heart rate and MSNA responses to fish oil may provide new mechanistic insights into a recent meta-analysis suggesting that cardiovascular benefits of fish oil are greater in adults with higher resting heart rates (18).
The influence of fish oil on autonomic activity in humans remains equivocal. The vast majority of studies on this topic focus on heart rate variability (HRV) analyses. In a recent review, Christensen (6) examined all double-blind, placebo-based interventional studies and reported that <50% of the studies revealed results that directly supported a beneficial effect of omega-3 fatty acids on HRV. The potential beneficial effect of omega-3 fatty acids on HRV increased to 75% when subanalyses were considered, but 25% of the interventional trials demonstrated no benefit of omega-3 fatty acids on HRV. The inconsistencies among HRV trials are likely due to a variety of factors, including subject heterogeneity, limited sample sizes, and the variable methods for assessing HRV. Moreover, while HRV offers a cost-effective, noninvasive approach to assessing cardiac autonomic activity, there is controversy surrounding its use to estimate sympathetic neural outflow (9, 12–14, 21, 23). Therefore, we utilized microneurography to assess MSNA, which is strongly correlated to cardiac norepinephrine spillover during resting conditions (27). To date, only one prior study (15) has examined the influence of omega-3 fatty acids on MSNA. Monahan et al. (15) reported that 1 mo of fish oil supplementation did not alter resting MSNA in normotensive humans, but it did augment MSNA responsiveness to certain sympathoexcitatory maneuvers. The inverse relationship between fish oil and blood pressure appears to be stronger in individuals with higher blood pressure (11, 17), yet the effect of fish oil on MSNA in subjects with elevated blood pressure remains unclear.
Our motivation to study prehypertensive adults was severalfold. First, prehypertension is increasingly being recognized as an important predictor of future development of hypertension (5, 24, 25), and it is currently estimated that ∼70 million Americans are prehypertensive. Second, prehypertension represents an “early stage” at-risk group in which modest lifestyle and nutritional changes are recommended. Third, prehypertensive adults are not typically taking blood pressure medications yet, thus eliminating an important potential confounder in examining the relation between fish oil and neurovascular control. Finally, while the effects of omega-3 fatty acids on blood pressure have been extensively studied in normotensive and hypertensive adults, there is paucity of information on prehypertensive adults. Contrary to our initial hypothesis, our data demonstrate that 8 wk of fish oil supplementation did not significantly alter resting blood pressure and MSNA in prehypertensive adults.
In addition to the randomized, double-blinded, placebo-based approach, a strength of the present study was the inclusion of both normotensive and prehypertensive adults. This allowed us to pool data to obtain a statistically robust number of subjects with a wide range of both resting blood pressures and heart rates. The covariate analysis of resting MAP confirmed that the neurovascular responses to fish oil were indeed not dependent on resting blood pressure. Specifically, the modest inverse relationship between pretreatment resting MAP and changes in MAP after fish oil (i.e., greater reductions of MAP in individuals with higher pretreatment MAP) was also observed after placebo. The similar MAP responses after both fish oil and placebo likely represents a regression toward the mean, but they may also represent a true placebo effect on blood pressure. Olive oil is the most frequently utilized placebo in randomized, double-blinded fish oil studies because it does not contain DHA or EPA found in fish oils. However, it is important to acknowledge that olive oil has been suggested to have cardioprotective effects independent of DHA and EPA (8). Nevertheless, our findings clearly indicate that the DHA and EPA supplementation through fish oil did not significantly alter neural and cardiovascular control in normotensive and prehypertensive humans.
In contrast to resting MAP, there was a significant association between pretreatment resting heart rate and both arterial blood pressure and MSNA that was specific to fish oil. Mozaffarian et al. (18) recently reported that fish oils appear to elicit modest reductions of resting heart rate, particularly in adults with higher pretreatment baseline levels. Our data demonstrate more dramatic reductions of resting MSNA after fish oil in adults with higher pretreatment resting heart rate. We acknowledge that the noted reductions of resting MSNA did not result in end-organ responsiveness with changes in either heart rate or limb vascular conductance. Moreover, the observed associations were modest, and the physiological significance of these relations remains unclear. It is important to note that in addition to baseline tachycardia, Mozaffarian et al. (18) also noted that longer treatment durations were also associated with reductions in heart rate after fish oil. Thus it is possible that the 8 wk of supplementation used in the present study was enough to influence the relationship between pretreatment resting heart rate and changes in MSNA, but not long enough to elicit end-organ responsiveness.
Heart rate is modulated by both the sympathetic and parasympathetic divisions of the autonomic nervous system, and our MSNA findings focus only on the sympathetic branch. Nevertheless, resting MSNA is strongly correlated to cardiac norepinephrine spillover (27); thus it is reasonable to speculate that the observed reductions of resting MSNA reflect modest reductions in cardiac norepinephrine in humans with higher pretreatment heart rates. This would be consistent with Nishimura et al. (20) who reported EPA supplementation reduced cardiac norephinephrine concentrations in diabetic rats. Future studies examining the influence of fish oil on sympathetic neural control in humans might include measurements of cardiac norepinephrine spillover (10) and may want to include supplementation durations longer than 8 wk.
Although fish oil did not appear to reduce resting heart rate, we observed some alteration in heart rate variability after fish oil. Specifically, R-R interval standard deviation increased after fish oil in both the normotensive and prehypertensive groups. Coupled with the lack of overall change in resting MSNA (Fig. 1), one could argue our data support a prevailing concept that fish oil influences parasympathetic control more than sympathetic control (6). However, our lack of change in overall resting heart rate suggests that if this is the case, the influence on parasympathetic control may be very limited. Moreover, some recent studies (2–4, 26) in animals suggest that omega-3 fatty acids might not reduce resting heart rate through changes in cardiac autonomic control but instead reduce heart rate via a reduced intrinsic pacemaker rate.
We acknowledge several limitations. First, our study was restricted to an 8-wk supplementation period. Longer duration (i.e., chronic) fish oil supplementation may be necessary to observe a more robust sympathoinhibitory influence of fish oil. Second, the present study did not measure the amount of omega-3 fatty acid incorporated into plasma or red blood cells. It is possible that variable incorporation rates of omega-3 fatty acid could have influenced the treatment response in our study. Third, the present study was limited to younger individuals. It is possible that the effects of fish oil on resting MSNA may be more dramatic in older subjects, who have much higher levels of MSNA than younger subjects (22). Moreover, tachycardia is more prevalent in older subjects. Future work might examine older subjects and other populations with higher resting MSNA (and higher resting heart rates).
In summary, fish oil did not alter arterial blood pressure or MSNA in normotensive or prehypertensive adults. However, fish oil does appear to elicit modest sympathoinhibitory and hypotensive responses in individuals with higher pretreatment heart rates. This inverse relation between pretreatment resting heart rate and MSNA responsiveness to fish oil may provide new mechanistic insight into a recent meta-analysis reporting that cardiovascular benefits of fish oil are greater in adults with higher resting heart rates (18). Collectively, evidence is accumulating to suggest that while the supplementation of fish oil is widely recommended for any adult for a variety of potential health benefits (cardiovascular health included), it may be particularly important for individuals with elevated resting heart rates.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant HL-088689.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.C. and M.J.J. conception and design of research; J.R.C., C.E.S., and H.Y. performed experiments; J.R.C., C.E.S., and H.Y. analyzed data; J.R.C., C.E.S., H.Y., and M.J.J. interpreted results of experiments; J.R.C. drafted manuscript; J.R.C., C.E.S., H.Y., and M.J.J. edited and revised manuscript; J.R.C., C.E.S., H.Y., and M.J.J. approved final version of manuscript; H.Y. prepared Figs..
ACKNOWLEDGMENTS
We thank John Durocher, Thomas Drummer, and Sarah Stream for assistance in data collection and analysis. We also thank all the subjects for participation.
REFERENCES
- 1. Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with “fish oil” reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med 153: 1429– 1438, 1993 [PubMed] [Google Scholar]
- 2. Billman GE. Effect of dietary omega-3 polyunsaturated fatty acids on heart rate and heart rate variability in animals susceptible or resistant to ventricular fibrillation. Front Physiol 3: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billman GE, Harris WS. Effect of dietary omega-3 fatty acids on the heart rate and the heart rate variability responses to myocardial ischemia or submaximal exercise. Am J Physiol Heart Circ Physiol 300: H2288– H2299, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, Carnes CA, Janssen PM. Effects of dietary omega-3 fatty acids on ventricular function in dogs with healed myocardial infarctions: in vivo and in vitro studies. Am J Physiol Heart Circ Physiol 298: H1219– H1228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560– 2572, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Christensen JH. Omega-3 polyunsaturated fatty acids and heart rate variability. Front Physiol 2: 84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen JH, Gustenhoff P, Korup E, Aaroe J, Toft E, Moller J, Rasmussen K, Dyerberg J, Schmidt EB. Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomised controlled trial. BMJ 312: 677– 678, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Covas MI, Konstantinidou V, Fito M. Olive oil and cardiovascular health. J Cardiovasc Pharmacol 54: 477– 482, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 96: 3224– 3232, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol Endocrinol Metab 247: E21– E28, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 20: 1493– 1499, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Houle MS, Billman GE. Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity. Am J Physiol Heart Circ Physiol 276: H215– H223, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation 90: 234– 240, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482– 492, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Monahan KD, Wilson TE, Ray CA. Omega-3 fatty acid supplementation augments sympathetic nerve activity responses to physiological stressors in humans. Hypertension 44: 732– 738, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation 102: 1264– 1269, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 88: 523– 533, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation 112: 1945– 1952, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation 117: 1130– 1137, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Nishimura M, Nanbu A, Komori T, Ohtsuka K, Takahashi H, Yoshimura M. Eicosapentaenoic acid stimulates nitric oxide production and decreases cardiac noradrenaline in diabetic rats. Clin Exp Pharmacol Physiol 27: 618– 624, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Parati G, Mancia G, Di Rienzo M, Castiglioni P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 101: 676– 678; discussion 681–672, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621– 637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor JA, Studinger P. Counterpoint: cardiovascular variability is not an index of autonomic control of the circulation. J Appl Physiol 101: 678– 681; discussion 681, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345: 1291– 1297, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet 358: 1682– 1686, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Verkerk AO, den Ruijter HM, Bourier J, Boukens BJ, Brouwer IA, Wilders R, Coronel R. Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm 6: 1485– 1492, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453: 45– 58, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]