Abstract
Circadian rhythms are approximate 24-h oscillations in physiology and behavior. Circadian rhythm disruption has been associated with increased incidence of hypertension, coronary artery disease, dyslipidemia, and other cardiovascular pathologies in both humans and animal models. Mice lacking the core circadian clock gene, brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like protein (Bmal1), are behaviorally arrhythmic, die prematurely, and display a wide range of organ pathologies. However, data are lacking on the role of Bmal1 on the structural and functional integrity of cardiac muscle. In the present study, we demonstrate that Bmal1−/− mice develop dilated cardiomyopathy with age, characterized by thinning of the myocardial walls, dilation of the left ventricle, and decreased cardiac performance. Shortly after birth the Bmal1−/− mice exhibit a transient increase in myocardial weight, followed by regression and later onset of dilation and failure. Ex vivo working heart preparations revealed systolic ventricular dysfunction at the onset of dilation and failure, preceded by downregulation of both myosin heavy chain isoform mRNAs. We observed structural disorganization at the level of the sarcomere with a shift in titin isoform composition toward the stiffer N2B isoform. However, passive tension generation in single cardiomyocytes was not increased. Collectively, these findings suggest that the loss of the circadian clock gene, Bmal1, gives rise to the development of an age-associated dilated cardiomyopathy, which is associated with shifts in titin isoform composition, altered myosin heavy chain gene expression, and disruption of sarcomere structure.
Keywords: systolic dysfunction, mechanical stiffness, titin isoforms, myosin heavy chain
circadian rhythms are oscillations in animal physiology and behavior that cycle with a period of ∼24 h. Underlying circadian behavior is the function of the molecular clock. Molecular clocks are intrinsic to each mammalian cell and generate cell autonomous and self-sustaining rhythms, which are thought to prepare the organism for changes and/or stresses in the surrounding environment (26, 48, 54). Molecular clocks are composed of a series of interconnected transcriptional-translational positive and negative feedback loops, which generate rhythmicity in gene expression, protein abundance, physiological processes, and animal behavior (38, 67). Brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like protein (Bmal1) is one of the core molecular clock transcription factors and is a member of the basic helix loop helix-period ARNT single-minded (bHLH-PAS) family of transcription factors (7). Upon dimerization with circadian locomotor output cycles kaput (CLOCK), another member of the bHLH-PAS family, it binds to E-boxes to regulate transcription of members of the negative arm of the molecular clock, Period and Cryptochrome genes (21, 61), as well as other clock-controlled genes that regulate an array of cellular processes (3). Bmal1 is unique among members of the core molecular clock, since its loss in mice results in arrhythmic behavior as assessed by voluntary wheel running (7). In addition to disrupted circadian behavior, Bmal1−/− mice die young and are suggested to be a mouse model of accelerated aging (34), with additional pathologies including increased levels of liver and kidney function enzymes (55), defective glucose homeostasis (51), skeletal muscle weakness (2), increased sleep fragmentation (37), arthropathy (6), and infertility (1). In the cardiovascular system, Bmal1−/− mice lack the diurnal variation in heart rate and blood pressure and remain hypotensive throughout the circadian cycle (12). However, very little is known about the structure and function of the Bmal1−/− hearts and whether any changes occur in an age-associated manner.
A common pathology seen in aged humans and rodents is dilated cardiomyopathy (DCM) (11, 29, 56). DCM is a primary disease of the myocardium and one of the leading causes of congestive heart failure, causing significant morbidity and premature mortality (60). DCM is characterized by impaired myocardial contractility [as assessed by a reduction in ejection fraction, fractional shortening (FS), maximal left ventricular pressure, and rate of pressure development], dilation of the left ventricular chamber, and thinning of the ventricular walls (15, 40, 53). The reduction in myocardial contractility is often associated with changes in myosin heavy chain (MHC) isoform composition. Increased expression of MHC-β leads to a significant decrease in systolic function (58) and has been observed in canine DCM models and rat pressure overload models (20, 28). A recent canine study of tachycardia-induced DCM showed increased myocardial passive tension and the possible involvement of the striated muscle protein titin (65). Two titin isoforms, differing in stiffness properties, are coexpressed in the heart and compositional changes occur in response to different physiological demands imposed on the myocardium (18, 23). Titin plays an important role in myofilament repair and turnover (25), a process that could be disrupted in circadian dysfunction (5).
In this study, we evaluated cardiac performance starting at 4 through 36 wk of age using noninvasive echocardiography and we show the development of an age-associated DCM phenotype in the Bmal1−/− mice. Structural analysis using electron microscopy indicates a disruption of sarcomere architecture in the Bmal1−/− hearts. This is associated with downregulation of both MHC mRNA isoforms at ages before the development of DCM, but not MHC isoform protein levels. Biochemical analysis of whole heart extracts reveals a shift in titin isoforms, but we did not detect a change in passive stiffness at the level of the single cardiomyocyte. Systolic performance is decreased before the overt development of DCM in the Bmal1−/− mice, as shown by ex vivo functional measurements of isolated working hearts. Together, these findings suggest that loss of Bmal1 results in an age-associated pathology in the heart that shares some, but not all, characteristics with DCM.
MATERIALS AND METHODS
All animal procedures were conducted in compliance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committees at University of Kentucky and Wayne State University.
Animals.
The germline Bmal1−/− mice were previously backcrossed over 10 generations to the C57BL6 background (2). Both male and female mice are infertile (1), so the Bmal1−/− and wild-type mice for this study were littermates from heterozygote (Bmal1+/−) breeding. Genotypes were determined as previously described (7). Mice were housed four per cage and kept on a 14-h:10-h light-dark schedule with ad libitum access to food and water.
Echocardiograms.
Mice were placed on a heated platform set at 37°C and anesthetized with isoflurane gas, and transthoracic measurements were taken using a VisualSonics Vevo 660 with a RMV 707 30-MHz probe. M-mode images were acquired from the parasternal short-axis view at the papillary muscle level. Data analysis was performed with the use of the Vevo 660 analytic software.
Cardiomyocyte isolation.
Cardiomyocytes were isolated as previously described (45a, 68). The composition of all buffers is identical to those of O'Connell et al. (45a), except that trypsin was not included in the digestion buffer. Briefly, mice were euthanized by cervical dislocation, the heart was carefully excised, and the aorta was cannulated. The heart was hung in a Langendorff apparatus and perfused retrograde with aerated (95% O2-5% CO2, 37°C) perfusion buffer, followed by perfusion with digestion buffer until the heart was pale and swollen. Following digestion, the ventricles were minced, resuspended in digestion buffer, and triturated to create a cell suspension. An equal amount of Stop-1 solution was added and the cardiomyocytes were allowed to settle into a pellet. The pellet was resuspended in Stop-2 solution, and the isolation was considered successful when about 65% of the cells were rod shaped. Isolated cardiomyocytes were used for assessment of cell size or for passive tension measurements.
Cardiomyocyte size determination.
Cardiomyocytes in Stop-2 solution were treated with Hoechst dye (1:1,000) for 5 min. A hundred images were taken from each heart at ×100 magnification using a Nikon Eclipse E600 light microscope, averaging 275 cells per heart. Cardiomyocyte area was measured manually using National Institutes of Health (NIH) ImageJ software (10). Cardiomyocytes were binned according to their cell area in bins of 500 μm2, and histograms with the percentage of cardiomyocytes in each bin were generated using GraphPad Prism (43).
Histological sections.
At 36 wk of age, mice were euthanized by cervical dislocation; the heart was excised and placed in a dish containing warm phosphate-buffered saline until it stopped beating, placed in 4% paraformaldehyde (24 h at 4°C), and then in 70% ethanol. The fixed hearts were embedded in paraffin, and 5-μm sections were taken starting at the papillary muscle level. Sections were stained with Masson's Trichrome (Sigma-Aldrich, St. Louis, MO). Low-magnification images (×20) were taken using a Nikon Eclipse E600 light microscope. Left ventricular wall thickness and area were obtained manually using NIH ImageJ software (10).
Electron microscopy.
Mice were anesthetized with ketamine-xylazine, the chest cavity was opened, and the heart was exposed. Perfusion through the left ventricle was started first with phosphate-buffered saline (pH 7.4), followed by cold 2% paraformaldehyde-4% glutaraldehyde in 0.1 M sodium cocadylate buffer (pH 7.4) and 130 mM NaCl. The perfusion-fixed hearts were then taken to the electron microscopy core facility at the University of Kentucky for processing and sectioning (14). Images were obtained using a Philips Biotwin 12 transmission electron microscope.
Real-time PCR.
Total RNA was extracted from 4-wk-old wild-type and Bmal1−/− hearts using TRIzol (Invitrogen). Isolated RNA was quantified using the Nano Drop 2000 spectrophotometer (Thermo Scientific). First-strand cDNA synthesis was done using SuperScript III first-strand synthesis supermix kit (Invitrogen). Real-time PCR was performed using TaqMan probe-based chemistry (Applied Biosystems) and conducted in an ABI 7700 sequence detector. Probes and primers for Gapdh, Myh6, and Myh7 were purchased from Applied Biosystems. After normalization to Gapdh [change in cycle threshold (ΔCt)], gene expression was reported relative to the average expression for that gene in the wild-type group at 4 wk of age (ΔΔCt). Fold change relative to control was calculated as 2−ΔΔCt.
Sample preparation for MHC gels.
MHC protein expression was performed on ventricular tissue from 4-, 12-, and 36-wk-old wild-type and Bmal1−/− mice. Control tissue for MHC-α and MHC-β was from neonatal hearts. Frozen ventricular tissue was homogenized using nine volumes of homogenizing buffer/mg tissue, consisting of (in mM) 250 sucrose, 25 NaCl, and 20 Tris (pH 7.4), modified from Talmadge and Roy (57). The homogenate was spun at 20,000 g for 30 min at 4°C. The pellet, which was enriched in myofibrils, was resuspended in homogenizing buffer and protein concentration was determined using a Bradford assay. Sample buffer (3×), consisting of 1.15 M Tris (pH 6.8), 6% SDS, 75 mM DTT, 0.06% bromophenol blue, and 40% glycerol, was added to a final concentration of 1×. The samples were heated at 100°C for 2 min, and 0.8 μg/lane was immediately loaded on gels (57).
MHC electrophoresis and isoform determination.
Gel composition and preparation were identical to Talmadge and Roy (57). The upper and lower chamber buffer composition was the same as in Reiser and Kline (49). The gels were run at 70-V constant voltage for 38 h at 4°C [modified from (57)]. MHC isoforms were visualized with a silver stain plus kit (Bio-Rad, Philadelphia, PA) and scanned using an Epson Perfection V500 photo scanner. Total MHC and the percentage of MHC-α and MHC-β were determined using NIH ImageJ software (10).
Sample preparation for titin gels.
Frozen ventricular tissue was placed in sample buffer [described in Warren et al. (63), excluding bromophenol blue, buffer-to-tissue ratio 60:1 (vol/wt)]. Protein concentration of the homogenate was determined using the RC-DC protein assay (Bio-Rad). Samples were heated at 60°C for 10 min; glycerol and bromophenol blue were added to 30% (vol/vol) and 0.1% (vol/vol) final concentration, respectively. Following 10 min centrifugation at 13,000 g, the supernatant was aliquoted and loaded on gel or stored at −80°C.
Titin electrophoresis and isoform determination.
A Bio-Rad Protean II xi XL vertical electrophoresis unit was used. The gel size was 20 cm × 20 cm × 1.5 mm. The composition of the acrylamide plug, the agarose gel, and the upper and lower chamber buffers were the same as in Warren et al. (63). The gels were run at 4°C for a total of 12 h, starting at 80 V and increasing the voltage by 30 V every 100 min. After electrophoresis, the gels were stained with Sypro Ruby (Invitrogen, Eugene, OR) and scanned using a Typhoon scanner operating in fluorescence mode at 450 nm. Scanned images were analyzed using Image Quant software.
Ex vivo working heart preparation and functional measurements.
Cardiac function was measured in isolated working heart preparations of 18-wk-old male wild-type and Bmal1−/− mice. As described previously (16), the mice were heparinized and anesthetized before the heart was rapidly isolated. A modified 18-gauge, 6-mm-long, thin-walled needle was used as the aortic cannula. After retrograde perfusion was established, a modified 16-gauge needle was used to cannulate the pulmonary vein for antegrade perfusion through the left atrium. A 30-gauge needle was used to puncture the left ventricle from the apex to make a path for the insertion of a 1.2-Fr pressure-volume catheter (calibrated for pressure and volume at 37°C, model 898B, Scisense, London, Ontario, Canada).
Aortic pressure was measured using an MLT844 pressure transducer (Capto, Horten, Norway) that was placed at heart level and connected to the aortic cannula. A 0.5-ml air bubble was placed in the compliance chamber to mimic in vivo aortic compliance and a beveled polyethylene-50 tubing was used to cannulate the pulmonary artery to collect coronary effluent. In all experiments, the hearts were perfused with modified Krebs-Henseleit buffer aerated with 95% O2-5% CO2 at 37°C without recycling to exclude the effects of metabolic products and hormones. The buffer contents were as follows: (in mM) 118 NaCl, 4.7 KCl, 2.25 CaCl2, 2.25 MgSO4, 1.2 KH2PO4, 0.32 EGTA, 25 NaHCO3, 15 d-glucose, and 2 sodium pyruvate (pH 7.4, adjusted at 37°C). Heart rate was controlled at 480 beats/min by supraventricular pacing using an isolated stimulator (A365, World Precision Instrument) with two microplatinum electrodes attached to the right atrium. Cardiac output was measured by the actual aortic and pulmonary artery flows recorded in real time by calibrated drop counting using a pair of electrodes feeding to a PowerLab/16 SP digital data archiving system (AD Instruments). Baseline function of the isolated working hearts was measured at a preload of 10 mmHg and afterload of 55 mmHg for the maximum left ventricular pressure, the maximum rate of left ventricular pressure development (±dP/dt), and left ventricular volume. Left ventricular stroke volume (μl/mg heart tissue) was calculated from the sum of aortic flow and coronary effluents, normalized to heart rate.
Single cardiomyocyte mechanical measurements.
Passive tension was measured in single cardiomyocytes isolated from wild-type and Bmal1−/− hearts, using a method described by Warren et al. (62) with a few modifications. The composition of relaxing and pCa9 solutions was identical to Ferreira et al. (17). Cardiomyocytes were resuspended in relaxing solution and were chemically permeabilized in relaxing solution containing 0.5% (vol/vol) Triton X-100 (Thermo Scientific, Rockford, IL) for 6 min at room temperature. Cardiomyocytes were washed twice in relaxing solution and stored on ice for up to 12 h. Cardiomyocytes were attached between stainless steel insect pins extending from a motor (308B, Aurora Scientific, Ontario, Canada) and a force transducer (406, Aurora Scientific) using silicone adhesive. Once attached, the cardiomyocyte was lifted and placed in a well containing pCa9 solution at 15°C. This apparatus was placed on the stage of a Nikon TE2000-U-inverted microscope with the capacity for video capturing. Experiments were performed using SLControl software (8). Each cardiomyocyte was stretched to double its resting length at a rate of 0.1 l0/s, and then shortened to 50% l0 before being returned to its resting length. The SLControl software generated traces of passive tension and fiber length over the stretch time. A video of the stretch was captured for subsequent analysis. The SLControl data and the video file were transported to a MatLab program written by K. S. Campbell, which extracted the passive tension values from the SLControl data and plotted them against sarcomere length calculated from individual frames from the video file. We used 20–24 preparations from four mice from each age and genotype group. Experimental results from each group were binned according to their sarcomere lengths in bins of 0.05 μm, and a two-way ANOVA with a post hoc Bonferroni test was run to assess significance. Additionally, passive tension traces from each cardiomyocyte were analyzed with an exponential equation: passive tension = exp[k·(SL − SL0)] (19), where SL is the sarcomere length, k is the exponent of stiffness, and SL0 is the sarcomere length at zero load.
Statistical analysis.
Results from each group (Bmal1−/− vs. wild-type) at each time point are reported as means ± SE. Two-way ANOVA was performed to determine whether a significant interaction existed between factors for each dependent variable under consideration. If a significant interaction was detected, Bonferroni post hoc comparisons were performed to identify the source of significance, with P < 0.05.
RESULTS
Germline Bmal1−/− mice develop age-associated DCM.
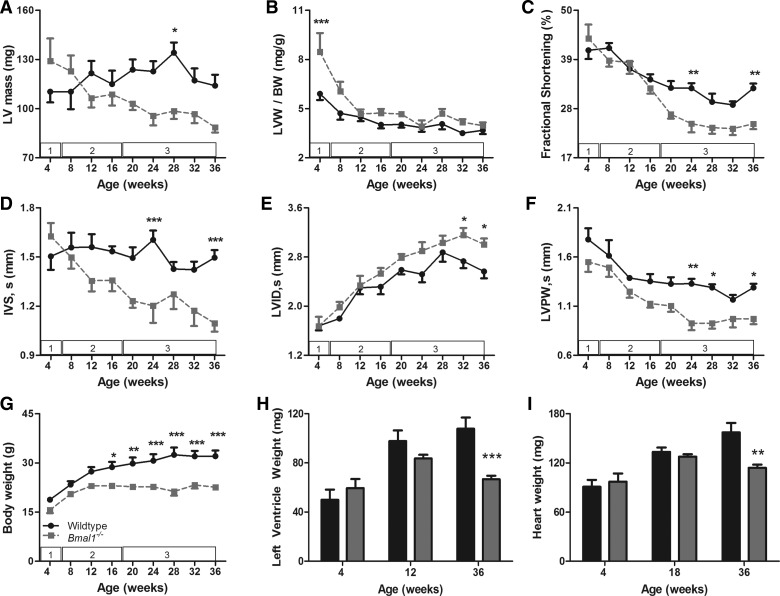
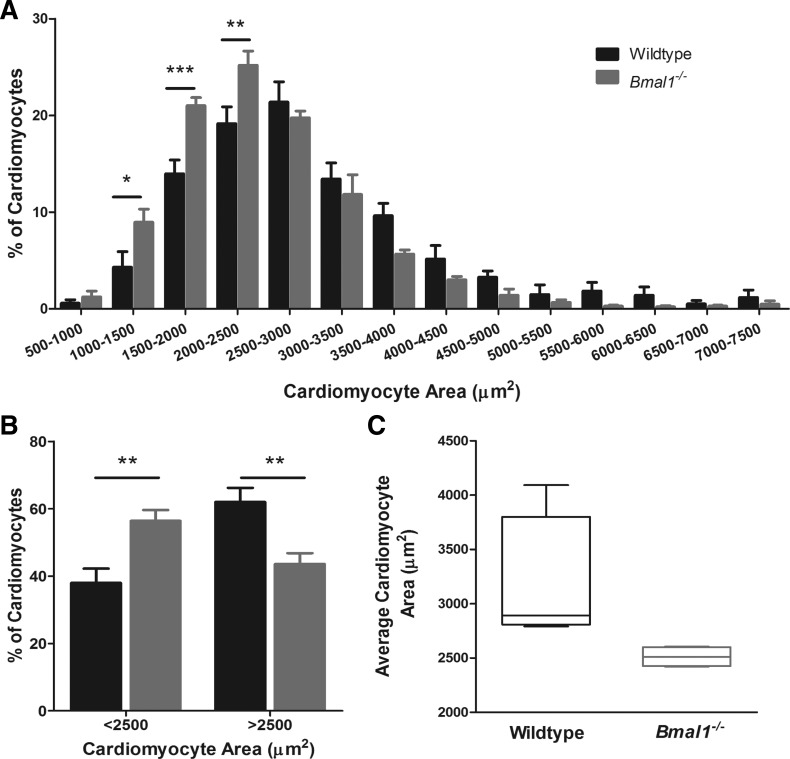
Echocardiogram data showed that at 4 wk of age, the germline Bmal1−/− mice had bigger hearts compared with wild-type littermates, as demonstrated by a 15% increase in the estimated left ventricular weight (LVW) (129 ± 13.79 vs. 110 ± 6.57 mg) (Fig. 1A) and a 43% increase in ratio of LVW to body weight (BW) (8.46 ± 1.14 vs. 5.9 ± 0.4 mg/g, P < 0.001) (Fig. 1B). We measured wet LVW and heart weight (HW) in a separate cohort of mice to complement echocardiographic estimates and to show that LVW was increased by 19% in the Bmal1−/− mice, but this increase was not statistically significant (59.47 ± 7.40 vs. 49.98 ± 8.31 mg) (Fig. 1H). However, because of the smaller body size in Bmal1−/− mice, wet LVW-to-BW ratio was significantly increased by 56% (4.74 ± 0.50 vs. 3.04 ± 0.64 mg/g, P < 0.05) as was the HW-to-BW ratio (6.26 ± 0.29 vs. 5.26 ± 0.37 mg/g) (data not shown). At this age, FS was preserved (43.65 ± 3.14 vs. 41.05 ± 1.96%) (Fig. 1C), the interventricular septum (IVS) was slightly, but not significantly, thicker (1.62 ± 0.08 vs. 1.54 ± 0.08 mm) (Fig. 1D), and no changes were detected in the left ventricular internal diameter (LVID) (1.67 ± 0.08 vs. 1.68 ± 0.07 mm) (Fig. 1E) or left ventricular posterior wall (LVPW) thickness (Fig. 1F). To evaluate whether the increase in LVW-to-BW ratio was associated with hypertrophy of cardiomyocytes, we isolated cardiomyocytes and measured cell area. We found that Bmal1−/− hearts have a greater percentage of smaller cardiomyocytes compared with wild-type hearts (Fig. 2A). The percentage of cardiomyocytes smaller than 2,500 μm2 is significantly higher in the Bmal1−/− hearts (56.41 ± 3.23 vs. 38 ± 4.22 μm2, P < 0.01), whereas the percentage of larger cardiomyocytes is significantly decreased (43.59 ± 3.23 vs. 62 ± 4.22 μm2, P < 0.01) (Fig. 2B). The average cardiomyocyte area is 19% smaller in the Bmal1−/− hearts (2,538 ± 470.88 vs. 3,152 ± 796 μm2) (Fig. 2C).
Fig. 1.
Age-associated dilated cardiomyopathy development in germline brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like protein-1 knockout (Bmal1−/−) mice. Graphs show longitudinal echocardiographic changes in left ventricular (LV) mass (A), LV weight (LVW) normalized to body weight (BW) (B), fractional shortening (C), systolic interventricular septum thickness (IVS; D) systolic LV internal diameter (LVID; E), systolic LV posterior wall thickness (LVPW; F), and BW (G). Data are means ± SE; n = 7. The numbers in the white boxes denote the stage of cardiac pathology observed in that age range. Summarized data for wet LVW (H) and wet heart weight (I) from wild-type (WT) and Bmal1−/− mice at each representative age are shown. Values are means ± SE; n = 4 (4 wk and 12 wk), 8 (18 wk), and 7 (36 wk). *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Bmal1−/− cardiomyocytes are smaller than WT cardiomyocytes at 4 wk of age. Average cardiomyocyte area from WT and Bmal1−/− mice at 4 wk of age is represented, showing that cardiomyocytes from Bmal1−/− hearts are smaller (C). Cardiomyocytes were binned according to their size in 500-μm2 bins, and the percentage of cardiomyocytes in each bin was calculated. There is a shift toward smaller cardiomyocytes in the Bmal1−/− mice (A). The percentage of cardiomyocytes smaller than 2,500 μm2 was increased in the Bmal1−/− hearts, whereas the percentage of larger cardiomyocytes (>2,500 μm2) was decreased (B). n = 880 cells from 4 mice (WT) and 1,330 cells from 4 mice (Bmal1−/−). *P < 0.05; **P < 0.01; ***P < 0.001.
This increase in LVW-to-BW seen in the 4-wk-old Bmal1−/− mice began to regress by 8 wk of age and declined to wild-type levels by 12 wk of age as assessed by echocardiography (4.67 ± 0.28 vs. 4.48 ± 0.25 mg/g) (Fig. 1B) and wet weight measurements (3.38 ± 0.18 vs. 3.56 ± 0.05 mg/g) (data not shown). Weeks 8–16 were characterized by a regression of LVW-to-BW ratio and myocardial wall thickness, whereas FS continued to be preserved (Fig. 1C). The IVS and LVPW started to get thinner in the Bmal1−/− mice compared with wild-type mice, but these differences did not reach statistical significance by 16 wk of age (IVS, 1.36 ± 0.07 vs. 1.53 ± 0.03 mm; and LVPW, 1.13 ± 0.04 vs. 1.35 ± 0.07 mm) (Fig. 1, D and F). Additionally, the LVID was similar in wild-type and Bmal1−/− hearts (Fig. 1E).
Weeks 20–36 were characterized by a significant decline in FS, indicating systolic dysfunction in the Bmal1−/− mice. By 36 wk of age, FS was 24.3% smaller in the Bmal1−/− mice compared with wild-type mice (24.6 ± 1.16 vs. 32.5 ± 1.06%, P < 0.01) (Fig. 1C). During this time period, the IVS and the LVPW continued to thin and by 36 wk of age were on average 26.67 and 24.8% smaller in the Bmal1−/− mice (IVS, 1.10 ± 0.05 vs. 1.50 ± 0.05 mm, P < 0.001; and LVID, 0.97 ± 0.05 vs. 1.29 ± 0.04 mm, P < 0.05) (Fig. 1, D and F). The LVID got progressively larger in the Bmal1−/− mice during this time, becoming 17.12% larger in the Bmal1−/− mice compared with wild-type by 36 wk of age (3.01 ± 0.10 vs. 2.57 ± 0.11 mm, P < 0.05) (Fig. 1E). LVW declined during this time and by 36 wk of age was 22.33% smaller in the Bmal1−/− mice (88.54 ± 3.16 vs. 114.0 ± 6.65 mg) (Fig. 1A). This was during a period of time in which body weight continued to decline (Fig. 1G), thus LVW-to-BW ratio remained unchanged as estimated by echocardiography (3.96 ± 0.20 vs. 3.68 ± 0.24 mg/g) (Fig. 1B) and wet weight measurements (3.46 ± 0.28 vs. 3.48 ± 0.28 mg/g) (data not shown). It is also worth noting that body weight (19.90 ± 1.40 vs. 31.11 ± 1.40 g, P < 0.001), wet LVW (66.67 ± 2.91 vs. 107.74 ± 9.13 mg, P < 0.001), and wet HW (113.86 ± 3.92 vs. 157.06 ± 11.62 mg, P < 0.01) were significantly smaller in the Bmal1−/− mice at 36 wk of age (Fig. 1, G–I).
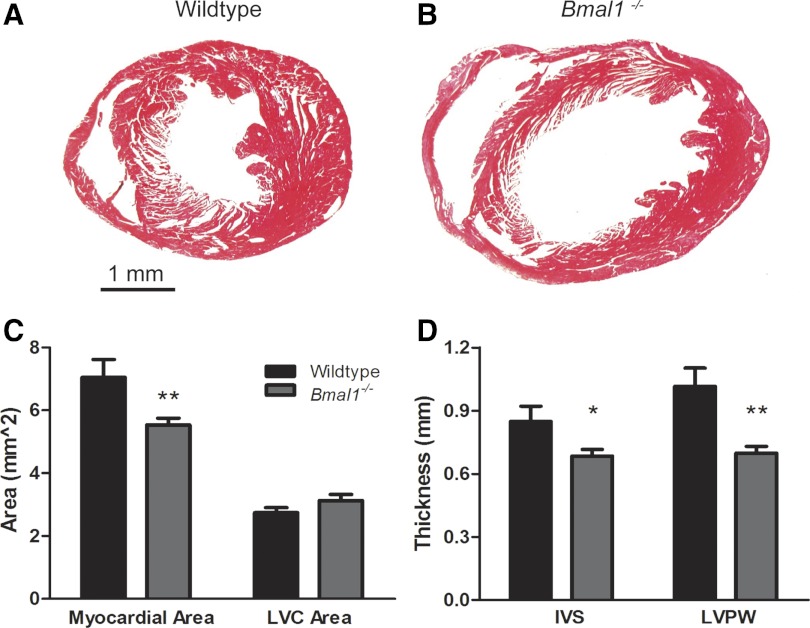
At 36 wk of age, we evaluated histological cross sections of the heart at the level of the papillary muscles, and this analysis supported the echocardiography data. Representative images are shown in Fig. 3, A and B. We found that the area occupied by myocardium was decreased by 21% in the Bmal1−/− mice (5.54 ± 0.21 vs. 7.05 ± 0.57 mm2, P < 0.05) (Fig. 3C). IVS thickness was decreased by 20% (0.68 ± 0.03 vs. 0.85 ± 0.07 mm, P < 0.05), and LVPW thickness was decreased by 31% (0.70 ± 0.03 vs. 1.02 ± 0.09 mm, P < 0.01) in the Bmal1−/− mice compared with wild-type controls (Fig. 3D). The left ventricular cavity area was slightly increased (3.13 ± 0.20 vs. 2.75 ± 0.16 mm2), but this difference did not reach statistical significance (Fig. 3C).
Fig. 3.
Thinning of the myocardial walls and dilation of the ventricular cavity in 36-wk-old Bmal1−/− mice. Representative Masson's trichrome-stained myocardial sections at the level of the papillary muscles, show enlargement of the ventricular cavity and wall thinning in the 36-wk-old Bmal1−/− myocardium (B) compared with an age- and sex-matched WT control myocardium (A). Graphs of combined data show a decrease in the area of myocardium (C) and the thickness of both the IVS and LVPW (D). LVC, LV cavity. n = 5 WT and 10 Bmal1−/− mice. *P < 0.05; **P < 0.01.
Bmal1−/− myocardium exhibits disorganized sarcomeres.
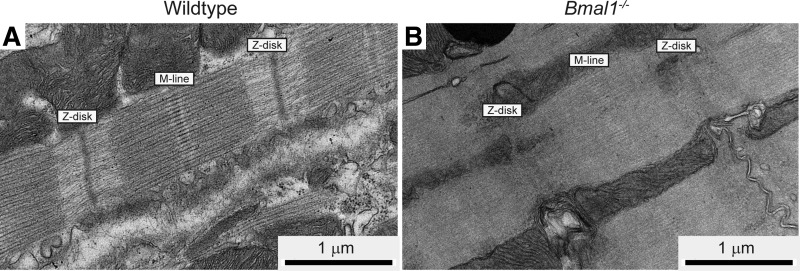
To assess whether the cardiac functional changes were associated with disruptions in sarcomere architecture, we obtained electron micrographs from wild-type and Bmal1−/− myocardial sections at 14 wk of age. An evaluation of electron micrographs found that some regions had normal sarcomere architecture in the Bmal1−/− myocardium, whereas other areas exhibited sarcomere disorganization, with diffuse M lines, A bands, and Z disks. We found regions where the distinction between A and I bands was diminished in the Bmal1−/− myocardium, and this was not seen in any of the images from the wild-type hearts (Fig. 4, A and B).
Fig. 4.
Bmal1−/− myocardium exhibits disorganized sarcomeres. Representative electron micrographs from WT (A) and Bmal1−/− (B) hearts are shown. Bmal1−/− sarcomeres have less defined A bands and I bands and diffuse M lines and Z disks compared with sarcomeres from WT hearts.
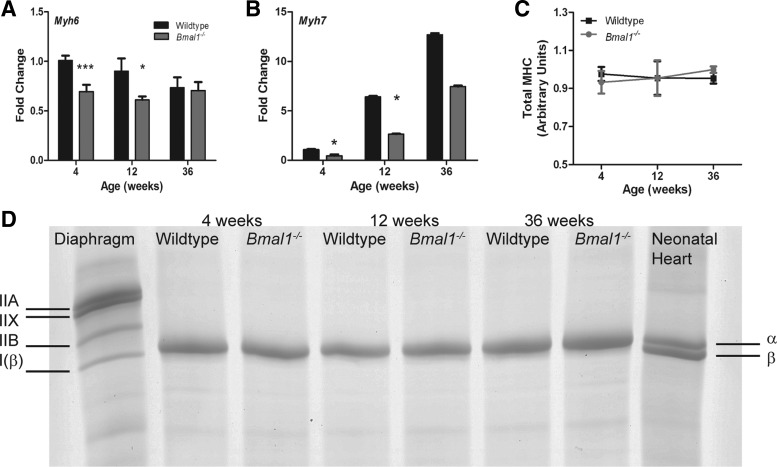
mRNA, but not protein, levels of MHC isoforms were downregulated in the Bmal1−/− myocardium at 4 and 12 wk of age.
We measured mRNA expression of MHC-α and MHC-β in the Bmal1−/− and wild-type hearts at all three ages (Fig. 5, A and B). MHC-α mRNA levels were significantly decreased by 31 and 32% at 4 and 12 wk, respectively, and were unchanged at 36 wk (Fig. 5A). MHC-β mRNA expression was significantly lower by 57 and 59% at 4 and 12 wk, respectively, but was not statistically different at 36 wk (Fig. 5B). MHC-β mRNA increased with age in both the wild-type and Bmal1−/− groups. However, we found no difference in MHC isoform composition or total MHC content at the protein level between wild-type and Bmal1−/− hearts (Fig. 5, C and D).
Fig. 5.
Myosin heavy chain (MHC) RNA is downregulated at 4 and 12 wk of age, whereas protein content and isoform composition is not different in the Bmal1−/− myocardium. Real-time PCR data show downregulation of both MHC-α (A) and MHC-β (B) in Bmal1−/− hearts at 4 and 12 wk of age, but not at 36 wk. Representative SDS-PAGE for MHC isoform separation show that the left ventricles of WT and Bmal1−/− mice contain only the MHC-α isoform (D). Total MHC is expressed relative to the neonatal heart sample to normalize for gel to gel differences (C). For the RT-PCR experiment, n = 7 (4 wk), 4 (12 wk), and 6 (36 wk). *P < 0.05; ***P < 0.001. For the MHC gels, n = 4 mice for each age and genotype.
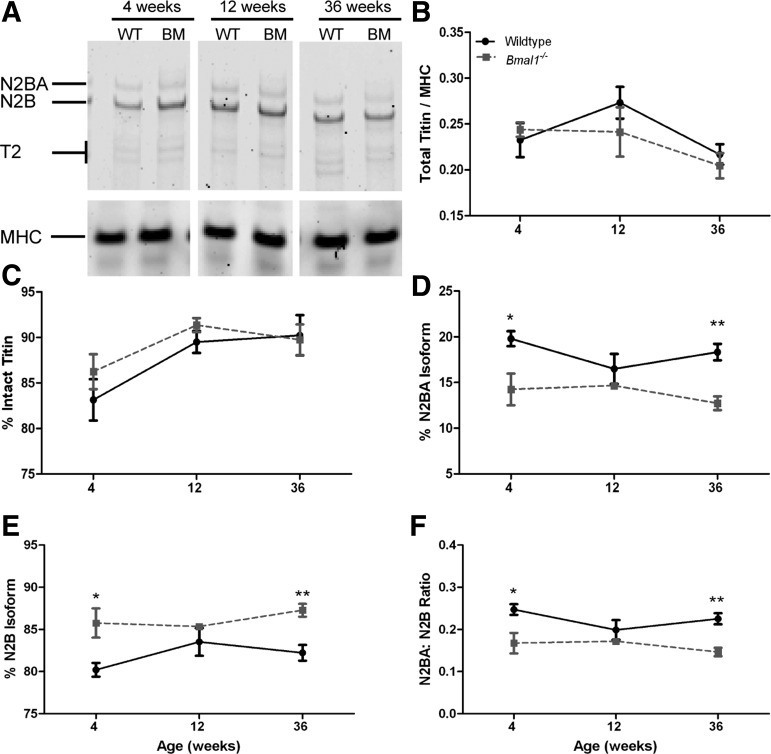
Bmal1−/− mice show increase of N2B and decrease of N2BA titin isoforms.
Total titin, reflecting the sum of the N2BA, N2B, and T2 bands, and normalized to MHC and was not different between wild-type and Bmal1−/− hearts at any age (Fig. 6B). Additionally, the percentage of intact titin, calculated as the sum of N2BA and N2B bands over the sum of N2BA, N2B, and T2, was not affected by genotype and ranged between 83 and 90% in the wild-type hearts and 86 and 91% in the Bmal1−/− hearts (Fig. 6C). The T2 band is a degradative product of titin and is a measure of both in vivo degradation as well as degradation that could have occurred during processing of the samples. The levels of total titin normalized to MHC were similar in Bmal1−/− and wild-type hearts during stage 1, increased LVW-to-BW ratio (0.24 ± 0.07 vs. 0.23 ± 0.019); stage 2, regression (0.24 ± 0.027 vs. 0.27 ± 0.017); and stage 3, dilation and failure (0.20 ± 0.014 vs. 0.22 ± 0.011), suggesting that the cardiac pathology is not a result of changes in total titin protein levels.
Fig. 6.
Bmal1−/− mice (BM) show increase of N2B and decrease of N2BA titin isoforms. Representative SDS-vertical agarose gel electrophoresis for titin protein is shown (A). Total titin-to-MHC ratio (B) and the percentage of intact titin (C) were not different between WT and Bmal1−/− mice at any age. The percentage of the compliant N2BA isoform is decreased in the Bmal1−/− mice compared with age-matched controls (D), and this is associated with an increase in the percentage of the stiffer N2B isoform (E). These changes result in a decrease in the N2BA-to-N2B ratio in the 4- and 36-wk-old Bmal1−/− mice compared with WT controls (F). n = 4. *P < 0.05; **P < 0.01.
However, we found that the percentage of the compliant N2BA isoform, calculated as N2BA/(N2BA + N2B), was lower in the Bmal1−/− left ventricles, especially at 4 wk (14.24 ± 1.73 vs. 19.79 ± 0.82%, P < 0.05) and 36 wk of age (12.73 ± 0.76 vs. 18.32 ± 0.90%, P < 0.01) (Fig. 6D). This was at the expense of the stiffer N2B titin isoform, which was in turn increased in Bmal1−/− hearts compared with age-matched wild-type controls (4 wk, 85.76 ± 1.7 vs. 80.21 ± 0.82%, P < 0.05; 12 wk, 85.34 ± 0.29 vs. 83.52 ± 1.64%; and 36 wk, 87.27 ± 0.76 vs. 82.22 ± 0.94%, P < 0.01) (Fig. 6E). The N2BA-to-N2B ratio, a well-accepted measure of myocardial stiffness, was decreased in the Bmal1−/− hearts (Fig. 6F) during stage 1 when LVW to BW is increased (0.17 ± 0.02 vs. 0.25 ± 0.01, P < 0.05) and stage 3 when the heart is dilated and failing (0.15 ± 0.01 vs. 0.23 ± 0.01, P < 0.01) but not during stage 2 (0.17 ± 0.004 vs. 0.20 ± 0.02) when the wild-type and Bmal1−/− hearts were functionally similar.
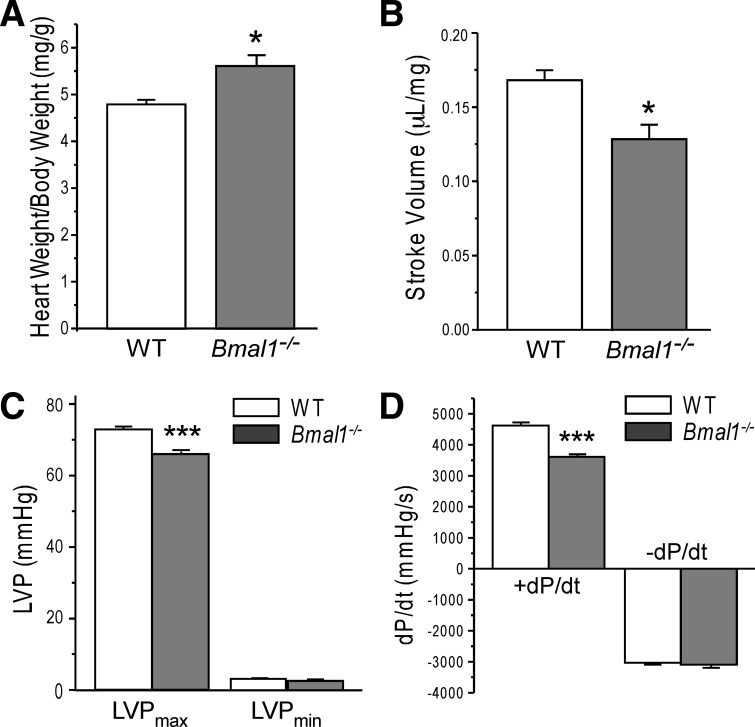
Bmal1−/− hearts exhibit compromised systolic function in ex vivo preparations.
To complement the echocardiography data, we measured cardiac function in isolated working hearts from wild-type and Bmal1−/− mice at 18 wk of age, the beginning of stage 3. As seen in Fig. 7, hearts from Bmal1−/− mice exhibited significant depression in left ventricular systolic function as demonstrated by a 9.7% decrease in maximal left ventricular pressure (Fig. 7C), a 22.3% decrease in the rate of contraction (dP/dt) (Fig. 7D), and a subsequent 24.7% decrease in stroke volume (Fig. 7B). At this time point, the heart was larger as shown by the increase in HW-to-BW ratio (Fig. 7A), whereas the rate of relaxation (−dP/dt) was not affected (Fig. 7D).
Fig. 7.
Decreased systolic heart function in Bmal1−/− mice. Wet heart weight (in mg) normalized to BW (in g) was significantly higher in Bmal1−/− mice compared with the WT group (A). The functional measurements of ex vivo working heart at preload of 10 mmHg and afterload of 55 mmHg with heart rate paced at 480 beats/min detected that Bmal1−/− hearts had deceased stroke volume (B), lower maximum left ventricular pressure (LVPmax; C), and slower systolic velocity (+dP/dt; D) compared with the WT group. No changes were found in diastolic velocity (−dP/dt; D) or end-diastolic left ventricular pressure (LVPmin; C). Values are means ± SE; n = 4 WT and 5 Bmal1−/− mice. *P < 0.05 and ***P < 0.001 compared with WT in Student's t-test.
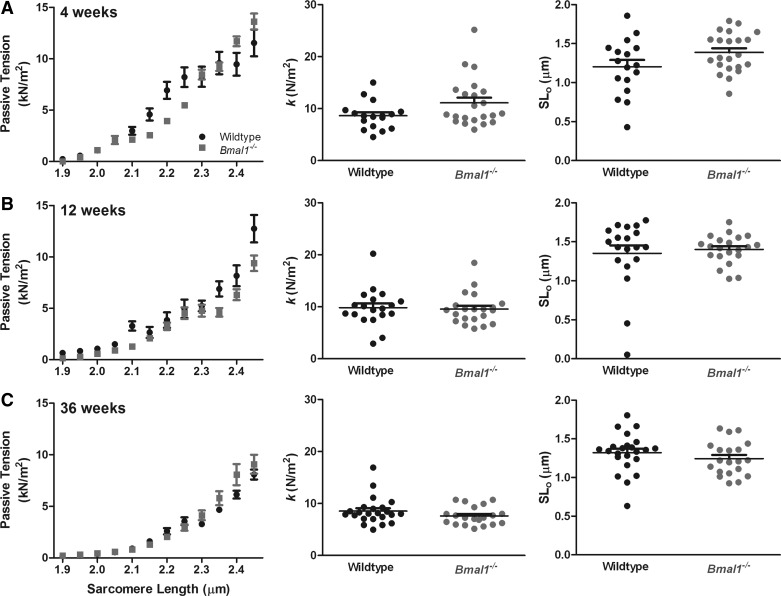
Passive tension is not different in Bmal1−/− cardiomyocytes compared with wild-type controls.
We found no difference in passive tension between Bmal1−/− and wild-type cardiomyocytes at any age (at sarcomere length of 2.45 μm, 4 wk: 13.63 ± 0.75 vs. 11.54 ± 1.28 kN/m2; 12 wk: 10.16 ± 0.72 vs. 12.54 ± 1.25 kN/m2; 36 wk: 8.78 ± 0.65 vs. 8.78 ± 0.32 kN/m2) (Fig. 8). Additionally, the exponential values, k and SL0 were not different between Bmal1−/− and wild-type cardiomyocytes at any age (Fig. 8).
Fig. 8.
Passive tension is not different in Bmal1−/− cardiomyocytes compared with WT controls. Passive tension measurements in single cardiomyocytes from WT and Bmal1−/− mice at 4 wk (A), 12 wk (B), and 36 wk (C) are shown. Individual data points are binned according to their sarcomere length in bins of 0.05 μm to generate graphs (left). Additionally, each curve was fit to an exponential equation: passive tension = exp[k*(SL − SL0)], where SL is the sarcomere length, k is the exponent of stiffness, and SL0 is the sarcomere length at zero load. k and SL0 are plotted on the scatter plots (middle and right). Data are means ± SE; n = 20–24 cardiomyocytes from 4 mice/group.
DISCUSSION
Data presented in this study demonstrate that germline Bmal1−/− mice develop age-associated DCM associated with myofilament disorganization that is preceded by changes in titin isoform composition and downregulation of MHC transcript levels. Whereas there is a rich body of evidence linking circadian rhythm dysfunction to the development of cardiovascular pathologies (27, 32, 33, 42), data are lacking on the role of Bmal1 and/or circadian rhythms on the structure and function of the heart with age.
We followed a cohort of Bmal1−/− and wild-type mice with echocardiograms starting at 4 wk of age until they reached 36 wk of age. We chose 36 wk as our end point because it has been previously reported that Bmal1−/− mice have an average lifespan of 37 ± 12 wk (34). We identified three distinct stages in the progression of the cardiac pathology in the Bmal1−/− mice, with stage 1 at 4 wk, characterized by larger hearts in the Bmal1−/− mice, whereas FS was not affected. We referred to this first stage as a transitory increase in LVW-to-BW ratio, because it was not sustained past 4 wk of age. This indicates that the germline Bmal1−/− mice are different from wild-type mice beginning shortly after birth and have bigger hearts for their body weight.
We defined the changes in the heart between 8 and 16 wk of age as stage 2, and this was characterized by regression of LVW-to-BW ratio with preservation of FS. During stage 3 (20–36 wk), there was significant thinning of the ventricular walls and enlargement of the LVID. These morphological changes were associated with a progressive decline in FS to around 55% of the initial value and a significant depression in cardiac systolic function as shown by decreased stroke volume, maximum left ventricular pressure, and dP/dt in the Bmal1−/− hearts at the beginning of stage 3. These findings indicate that loss of Bmal1 contributes to the development of features consistent with DCM. The Bmal1−/− mice have been considered a model of accelerated aging (34), and as such the pathologies observed here could be the result of accelerated systemic aging.
One feature we found early in the Bmal1−/− mouse is an enlarged heart seen with echocardiography and LVW-to-BW analysis. However, upon analysis of cardiomyocyte size we found that the Bmal1−/− hearts at 4 wk of age had larger percentages of smaller cardiomyocytes and average cardiomyocyte size was reduced by 19%. This suggests that there were more cells, possibly hyperplasia of cardiomyocytes or other cell types to support the larger heart size. Since cardiomyocyte hyperplasia occurs mainly during embryonic development and up to 1 wk postnatally (46), our data suggest a possible role of Bmal1 in regulating the timing of cardiac development and growth. The link between loss of Bmal1 in the heart and heart size in our studies is consistent with the findings by Durgan et al. (13), in which cardiac specific loss of Bmal1 was associated with increased biventricular weight-to-BW ratio and increased expression of the hypertrophic marker, mcip1. However, the hypertrophy we observed in the germline Bmal1−/− mouse occurs much earlier in life, at 4 wk of age, than that observed in the cardiac specific Bmal1−/− mouse, and the heart hypertrophy we detect is transient. Whether the increased size in the Durgan study is due to hyperplasia and whether the hypertrophy persists are not known. Thus there is still much to learn about the interaction among Bmal1, cardiomyocyte number, and size in the heart.
Much of the data in this study comes from longitudinal echocardiography studies, and, like others, we found that heart rate in the Bmal1−/− mice was significantly lower compared with wild-type mice at all ages (means ± SD across all time points, 406 ± 49 vs. 482 ± 41 beats/min). To minimize any potential problems with interpretation, we attempted to match heart rate for echocardiography through an adjustment of isoflurane concentration; however, Bmal1−/− mice were more sensitive to isoflurane and displayed a quicker drop in heart rate. Previous studies have shown that cardiac parameters measured through M-mode echocardiography were stable over a 20-min time span and a range of heart rates from 450–550 beats/min (50), whereas others have found increased LVW and LVID, decreased FS, and no effect on IVS and LVPW thickness when heart rate decreased by 120 beats/min (64). In the current study, we see a 76 beats/min decrease in heart rate in the Bmal1−/− mice, whereas our wild-type values for heart rate and FS are consistent with previously published work (22, 64). The findings that IVS and LVPW thickness and LVW are decreased in our Bmal1−/− mice cannot be explained by the decrease in heart rate and suggest that the changes seen in the Bmal1−/− hearts are due to the pathology they develop and not an artifact of their decreased heart rate.
One characteristic of DCM is impairment in force generation. The observed decline in the rate of contraction in the Bmal1−/− hearts ex vivo suggested the involvement of MHC, since a shift to the slower MHC-β isoform is both correlated with decreased dP/dt (41) and commonly associated with DCM (20). It has been shown that a shift toward MHC-β occurs in canine models of DCM and a rat model of pressure overload (20, 28). Additionally, a forced expression of MHC-α protects against cardiomyopathy in the rabbit left ventricle (31). We found that levels of MHC-β mRNA were not increased in the Bmal1−/− hearts at any of the ages in which significant functional changes were detected with ex vivo analysis or echocardiography. This indicates that the pathology observed in the Bmal1−/− hearts is not associated with MHC isoform changes and does not directly mimic models of DCM. What we did find is that expression of MHC-α and MHC-β mRNAs were downregulated in the Bmal1−/− hearts at 4 and 12 wk. This is consistent with previous reports from skeletal muscle of clock-compromised mice, showing downregulation of the mRNAs for many sarcomeric and structural genes (39). We were surprised to observe decreases in both MHC isoform mRNA levels with no differences in protein levels at the 4 and 12 wk ages. This suggests that in the early ages, loss of Bmal1 leads to altered MHC gene expression but posttranscriptional, potentially protein turnover, mechanisms are in place to maintain normal MHC protein levels. If MHC protein turnover is altered, it is possible that the MHC protein present in the Bmal1−/− hearts may be modified and thus exhibit altered function which could contribute to the observed decline in force development and the rate of contraction in the Bmal1−/− hearts. Additionally, a decrease in calcium release; mutations in myosin light chain, actin, and troponin T; and altered phosophorylation status of troponin I are common findings in DCM and affect cross-bridge cycle dynamics, actin-myosin ATPase activity, and excitation-contraction coupling leading to decreased force generation (30, 52, 66). Much remains to be done to understand the relationship between Bmal1 and cardiomyocyte specific gene expression and maintenance of mechanical function in the heart.
Titin plays a critical role in maintaining sarcomere structure. Our total titin/MHC values were similar to previously published work (9, 44, 65) and were not different between wild-type and Bmal1−/− mice at any age, suggesting that changes in total titin are not mediators of the structural or functional cardiac pathology observed in the Bmal1−/− mice. In the myocardium, two titin isoforms are expressed, the larger and more compliant N2BA and the smaller, stiffer N2B (23). The N2BA-to-N2B ratio, a measure of cardiac compliance, in our wild-type mice was consistent with previously reported values for mouse myocardium (45, 47). The N2BA-to-N2B ratio significantly decreased in the Bmal1−/− mice, specifically at 4 wk of age when mice had increased LVW-to-BW ratio and at 36 wk of age when mice showed most symptoms of DCM. These data are consistent with the canine study by Wu and colleagues (65), in which rapid-pacing induced DCM resulted in a decrease in the N2BA-to-N2B ratio and a concomitant increase in titin based passive tension. These data suggest that loss of Bmal1 shifts titin isoform composition toward the stiffer isoform and this shift potentially mediates the pathologies seen in the hearts of the Bmal1-deficient mice.
Titin-based passive force constitutes the majority of the total passive tension in cardiac muscle, and previous studies have shown a correlation between changes in titin isoform composition and myocardial stiffness (24). Although we found a decrease in the N2BA-to-N2B ratio, we did not find the expected increase in passive tension. Our reported passive tension measurements are somewhat lower than what has been reported in mice at the same sarcomere length (9), possibly reflecting the lower N2BA-to-N2B ratio reported in this study. However, it is worth noting that others have found similar N2BA-to-N2B ratios to ours, but those studies did not assess passive tension (45, 47). These data suggest the presence of compensatory mechanisms to offset the isoform induced increase in passive tension. Previous studies have shown that phosphorylation of titin decreases passive tension (19, 35, 36). Thus it is possible that in addition to increasing the N2BA-to-N2B ratio, loss of Bmal1 increases basal levels of titin phosphorylation, resulting in a decrease in passive tension even in light of the isoform changes observed. Another potential explanation for the discrepancy between our titin isoform data and the passive tension data might be due to sampling. For our titin isoform determination, we generated a homogenate from a portion of the ventricle taken from the apex of the heart, whereas for the mechanical measurements, we digested the entire heart and performed experiments on 5–7 viable cells selected following digestion. Titin isoform expression varies with the region of heart (4). It could be that by using the apex of the heart, we got an equal representation of all three myocardial layers (epi, mid, and endo), and maybe we have lost this equal representation of the three layers when using only 5–7 cells per mouse for the mechanical studies. Another possibility could be the selective loss of stiffer cells (lower N2BA-to-N2B ratio) during the cardiomyocyte isolation process for the mechanical measurements. With the isolation protocol, roughly 30% of cardiomyocytes are lost during the isolation (45a).
In conclusion, this is the first study to show that Bmal1-deficient mice develop a cardiac pathology that starts with a transitory increase in myocardial mass and progresses to dilation and failure until their time of death at ∼36 wk of age. The systolic dysfunction observed is associated with early downregulation of both MHC isoform transcripts, but not with protein, implicating both transcriptional and post-transcriptional changes in myosin gene expression. Bmal1−/− hearts show loss of the normal sarcomere architecture that is associated with a shift in titin isoform composition toward the stiffer isoform. However, titin-based passive tension is not affected, suggesting the presence of other compensatory mechanisms, such as phosphorylation and/or altered intermediate filament changes. Collectively, our results suggest that Bmal1 and potentially the molecular circadian clock play an important role in maintaining the structural and functional integrity of cardiac muscle through regulation of titin and MHC.
GRANTS
This work was supported by NIH Grants R01-AR-055246 and RC1ES018636 (to K. A. Esser) and R01-AR-048816 and R01-HL-098945 grants (to J. P. Jin) and by American Heart Association Predoctoral Fellowship 10PRE3900047 (to M. Lefta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.L. and K.A.E. conception and design of research; M.L. and H.-Z.F. performed experiments; M.L., K.S.C., H.-Z.F., and J.-P.J. analyzed data; M.L. and K.A.E. interpreted results of experiments; M.L. prepared figures; M.L. drafted manuscript; M.L., K.S.C., J.-P.J., and K.A.E. edited and revised manuscript; M.L., K.S.C., H.-Z.F., J.-P.J., and K.A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank C. E. Kiper for help with echocardiography; C. Long for help with histology; M. G. Engle for help with electron microscopy; M. Mitov for help with titin gels; M. Miyazaki for help with myosin gels; and J. J. McCarthy, G. Wolff, and E. A. Schroder for technical advice and support.
REFERENCES
- 1. Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms 23: 26–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA 107: 19090–19095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aschoff J. Human circadian rhythms in activity, body temperature and other functions. Life Sci Space Res 5: 159–173, 1967 [PubMed] [Google Scholar]
- 4. Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res 87: 235–240, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res 77: 667–675, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41: 122–132, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell KS, Moss RL. SLControl: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol 285: H2857–H2864, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86: 59–67, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Collins TJ. ImageJ for microscopy. Biotechniques 43: 25–30, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Coughlin SS, Tefft MC, Rice JC, Gerone JL, Baughman KL. Epidemiology of idiopathic dilated cardiomyopathy in the elderly: pooled results from two case-control studies. Am J Epidemiol 143: 881–888, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104: 3450–3455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int 28: 187–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenberg BR. Adaptability of ultrastructure in the mammalian muscle. J Exp Biol 115: 55–68, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Feldman MD, Alderman JD, Aroesty JM, Royal HD, Ferguson JJ, Owen RM, Grossman W, McKay RG. Depression of systolic and diastolic myocardial reserve during atrial pacing tachycardia in patients with dilated cardiomyopathy. J Clin Invest 82: 1661–1669, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM, Jin JP. Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis. J Physiol 586: 3537–3550, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferreira LF, Moylan JS, Stasko S, Smith JD, Campbell KS, Reid MB. Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J Appl Physiol 112: 1538–1545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med 84: 446–468, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Fukuda N, Wu Y, Nair P, Granzier HL. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol 125: 257–271, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuller GA, Bicer S, Hamlin RL, Yamaguchi M, Reiser PJ. Increased myosin heavy chain-beta with atrial expression of ventricular light chain-2 in canine cardiomyopathy. J Card Fail 13: 680–686, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Gentry-Smetana S, Redford D, Moore D, Larson DF. Direct effects of volatile anesthetics on cardiac function. Perfusion 23: 43–47, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail Rev 10: 211–223, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68: 1027–1044, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Curr Opin Cell Biol 11: 18–25, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Grundschober C, Delaunay F, Puhlhofer A, Triqueneaux G, Laudet V, Bartfai T, Nef P. Circadian regulation of diverse gene products revealed by mRNA expression profiling of synchronized fibroblasts. J Biol Chem 276: 46751–46758, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health 47: 89–95, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Haddad F, Qin AX, Bodell PW, Zhang LY, Guo H, Giger JM, Baldwin KM. Regulation of antisense RNA expression during cardiac MHC gene switching in response to pressure overload. Am J Physiol Heart Circ Physiol 290: H2351–H2361, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Harding P, Yang XP, Yang J, Shesely E, He Q, LaPointe MC. Gene expression profiling of dilated cardiomyopathy in older male EP4 knockout mice. Am J Physiol Heart Circ Physiol 298: H623–H632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasenfuss G, Mulieri LA, Leavitt BJ, Allen PD, Haeberle JR, Alpert NR. Alteration of contractile function and excitation-contraction coupling in dilated cardiomyopathy. Circ Res 70: 1225–1232, 1992 [DOI] [PubMed] [Google Scholar]
- 31. James J, Martin L, Krenz M, Quatman C, Jones F, Klevitsky R, Gulick J, Robbins J. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation 111: 2339–2346, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knutsson A. Health disorders of shift workers. Occup Med (Lond) 53: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Koller M. Health risks related to shift work. An example of time-contingent effects of long-term stress. Int Arch Occup Environ Health 53: 59–75, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kruger M, Kotter S, Grutzner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104: 87–94, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil 27: 435–444, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 28: 395–409, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lefta M, Wolff G, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. Curr Top Dev Biol 96: 231–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DA, Seidman CE, Seidman JG. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest 104: 1235–1244, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meyer M, Trost SU, Bluhm WF, Knot HJ, Swanson E, Dillmann WH. Impaired sarcoplasmic reticulum function leads to contractile dysfunction and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H2046–H2052, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Mosendane T, Raal FJ. Shift work and its effects on the cardiovascular system. Cardiovasc J Afr 19: 210–215, 2008 [PMC free article] [PubMed] [Google Scholar]
- 43. Motulsky H. Fitting Models to Biological Data Using Linear and Non-Linear Regression. A Practical Guide to Curve Fitting. Oxford, UK: Oxford University Press, 2004 [Google Scholar]
- 44. Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 110: 155–162, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 24: 175–189, 2003 [DOI] [PubMed] [Google Scholar]
- 45a. O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Oparil S, Bishop SP, Clubb FJ., Jr Myocardial cell hypertrophy or hyperplasia. Hypertension 6: III38–III43, 1984 [DOI] [PubMed] [Google Scholar]
- 47. Opitz CA, Linke WA. Plasticity of cardiac titin/connectin in heart development. J Muscle Res Cell Motil 26: 333–342, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Reiser PJ, Kline WO. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol Heart Circ Physiol 274: H1048–H1053, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J., Jr Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol 282: H2134–H2140, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2: e377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schonberger J, Seidman CE. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am J Hum Genet 69: 249–260, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104: 557–567, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Sun Y, Yang Z, Niu Z, Wang W, Peng J, Li Q, Ma MY, Zhao Y. The mortality of MOP3 deficient mice with a systemic functional failure. J Biomed Sci 13: 845–851, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D'Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 120: 1585–1597, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol 278: H412–H419, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Towbin JA, Bowles NE. The failing heart. Nature 415: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mech Dev 121: 1301–1312, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis 24: 1695–1702, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Wu J, Bu L, Gong H, Jiang G, Li L, Ma H, Zhou N, Lin L, Chen Z, Ye Y, Niu Y, Sun A, Ge J, Zou Y. Effects of heart rate and anesthetic timing on high-resolution echocardiographic assessment under isoflurane anesthesia in mice. J Ultrasound Med 29: 1771–1778, 2010 [DOI] [PubMed] [Google Scholar]
- 65. Wu Y, Bell SP, Trombitas K, Witt CC, Labeit S, LeWinter MM, Granzier H. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation 106: 1384–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Zakhary DR, Moravec CS, Stewart RW, Bond M. Protein kinase A (PKA)-dependent troponin-I phosphorylation and PKA regulatory subunits are decreased in human dilated cardiomyopathy. Circulation 99: 505–510, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol 11: 764–776, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000 [DOI] [PubMed] [Google Scholar]