Abstract
The congestive heart failure (CHF) syndrome with soft tissue wasting, or cachexia, has its pathophysiologic origins rooted in neurohormonal activation. Mechanical cardiocirculatory assistance reveals the potential for reverse remodeling and recovery from CHF, which has been attributed to device-based hemodynamic unloading whereas the influence of hormonal withdrawal remains uncertain. This study addresses the signaling pathways induced by chronic aldosteronism in normal heart and skeletal muscle at organ, cellular/subcellular, and molecular levels, together with their potential for recovery (Recov) after its withdrawal. Eight-week-old male Sprague-Dawley rats were examined at 4 wk of aldosterone/salt treatment (ALDOST) and following 4-wk Recov. Compared with untreated, age-/sex-/strain-matched controls, ALDOST was accompanied by 1) a failure to gain weight, reduced muscle mass with atrophy, and a heterogeneity in cardiomyocyte size across the ventricles, including hypertrophy and atrophy at sites of microscopic scarring; 2) increased cardiomyocyte and mitochondrial free Ca2+, coupled to oxidative stress with increased H2O2 production and 8-isoprostane content, and increased opening potential of the mitochondrial permeability transition pore; 3) differentially expressed genes reflecting proinflammatory myocardial and catabolic muscle phenotypes; and 4) reversal to or toward recovery of these responses with 4-wk Recov. Aldosteronism in rats is accompanied by cachexia and leads to an adverse remodeling of the heart and skeletal muscle at organ, cellular/subcellular, and molecular levels. However, evidence presented herein implicates that these tissues retain their inherent potential for recovery after complete hormone withdrawal.
Keywords: intracellular calcium overloading, oxidative stress, mitochondria, cardiomyocyte necrosis
the clinical syndrome congestive heart failure (CHF) has reached epidemic proportions. Despite today's standard of care, the recurrence of its disabling symptoms and signs has now catapulted CHF into the number one admitting diagnosis to US hospitals. CHF represents a chronic stressor state in which neurohormonal activation features the endocrine properties of circulating effector hormones of the renin-angiotensin-aldosterone and adrenergic nervous systems acting on the heart and systemic tissues to evoke a progressive adverse remodeling. Mitochondria-based oxidative stress in myocardium contributes to an ongoing nonischemic necrosis of cardiomyocytes with release of troponins coupled to tissue repair and consequent microscopic myocardial scarring. Oxidative stress and a proinflammatory phenotype are inextricably linked to the progressive pathologic remodeling of the failing heart (19). A complex metabolic disorder, termed cardiac cachexia, involves systemic tissues, including muscle and adipose tissue wasting coupled with bone resorption.
The potential, however, does exist for recovery of the failing heart and from CHF with associated wasting (3, 25, 41, 44). In 1995, Kass, et al. (18) introduced the term “reverse remodeling.” After months of either mechanical circulatory assistance or resynchronization therapy, together with medical therapy, a reverse remodeling of the injured heart and wasted tissues at cellular and molecular levels has been reported (25, 41, 44). Important components of the molecular pathways involved in this reverse remodeling relate to intracellular calcium handling, redox signaling, and extracellular matrix turnover. In some patients, untoward removal of the assist device has been possible and has come to be referred to as a “bridge to recovery.”
Pathophysiologic mechanisms contributing to such recovery have largely been attributed to device-related hemodynamic unloading of the overburdened myocardium (14, 25, 41, 44). In 1963, Burch et al. (5) reported a prolonged period of bed rest, together with digitalis leaf and mercurial diuretic, led to the resolution of the CHF syndrome followed by a reduction in radiographic cardiomegaly. Taken together, these findings suggest neurohormonal withdrawal contributes to recovery and reverse remodeling. However, the gain in function and reverse remodeling of the heart and wasted tissues attributed to neurohormonal withdrawal remains to be elucidated. Toward this end, signaling pathways induced solely by neurohormonal activation that contribute to cardiac pathology and muscle degeneration first need to be identified before it can be ascertained whether they are reversible. It is difficult to resolve these issues in patients with CHF receiving cardiocirculatory support since the withdrawal of hemodynamic and hormonal burdens is essentially contemporaneous (20).
To begin dissecting these intrinsically linked variants we turned to rats receiving aldosterone/salt treatment (ALDOST), where plasma ALDOST levels are raised and sustained to those found in human CHF, while plasma renin activity and angiotensin II levels are each suppressed. A chronic immunostimulatory stressor state ensues accompanied by cachexia (12). Compared with untreated age-/sex-/strain-matched controls, this model allows us to address pathophysiologic responses that arise in normal myocardium and skeletal muscle due to chronic aldosteronism (7, 16). In working with intact tissue from the outset, we eliminate potential confounding variables, such as ongoing remodeling due to previous myocardial infarction or underlying cardiomyopathy. Furthermore, iterations in tissue integrity that could arise in response to proteins, released from necrotic cardiomyocytes, and recognized by the immune system as “danger signals,” are avoided. Morphologic evidence of nonischemic cardiac pathology first appears at 4-wk ALDOST and, therefore, was targeted as our starting point. Having identified tissue remodeling at organ, cellular/subcellular, and molecular levels in response to 4-wk ALDOST, we then studied their potential for recovery from these pathophysiologic sequelae attendant with a subsequent 4-wk period of neurohormonal withdrawal. Such reverse remodeling was critically examined to ascertain whether these tissues returned to or towards levels found in untreated controls.
METHODS
Animal Model
Eight-week-old male Sprague-Dawley rats were used throughout these experiments, approved by our Institution's Animal Care and Use Committee. Following uninephrectomy, an osmotic minipump containing ALDO was implanted subcutaneously to release ALDO (0.75 μg/h), while drinking water was fortified with 1% NaCl and 0.4% KCl to prevent hypokalemia (reviewed in Ref. 38).
One group of rats received ALDOST for 4 wk when cardiac pathology first appears (36). A separate group received ALDOST for 4 wk that was then discontinued and rats followed for 4 wk of recovery (Recov). Unoperated, untreated age-/sex-/strain-matched rats served as controls. Each experimental group consisted of six rats. In preliminary studies, we did not find any difference in our experimental variables in control rats between 12 and 16 wk of age and therefore did not include a 16-wk-old control group.
Cardiac Pathology
Myocyte area measurement.
The cross-sectional area of myocytes was measured from hematoxylin and eosin-stained sections. The outline of 50–60 myocytes was traced in each section. An image system software (Universal Imaging, Media, PA) was used to determine myocyte cross-sectional area.
Fibrosis.
The extent of myocardial fibrosis was assessed by collagen-specific picrosirius red staining in 6-μm coronal sections of the ventricles using a computer image system as previously reported (36).
Isolation of Cardiomyocytes and Mitochondria
Ventricular myocytes were isolated from excised hearts as previously reported (11). Mitochondria were isolated by differential centrifugation from heart tissue homogenates. Only the subsarcolemmal (SSM) population was harvested by centrifugation at 10,000 g, and the purity of the SSM preparation and integrity of their membranes were assessed according to our routine protocol as previously reported (11, 16).
Cardiomyocyte Cytosolic Free and Mitochondrial Free Calcium Concentrations
Cytosolic free ([Ca2+]i) and mitochondrial free ([Ca2+]m) calcium concentrations were measured ratiometrically using the Ca2+-specific fluorophore fura-2 (Invitrogen, Eugene OR) as reported previously (16).
Mitochondrial H2O2 Production
We monitored H2O2 released by these organelles in response to succinate stimulation as we previously reported (16).
Skeletal Muscle 8-Isoprostane
8-Isoprostane levels were measured in gastrocnemius muscle using a competitive enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) as previously reported (16).
Mitochondrial Mitochondrial Permeability Transition Pore Opening
Mitochondrial permeability transition pore (mPTP) opening was determined by CaCl2-induced swelling of isolated cardiac mitochondria (n = 6 samples for each group) as previously reported (16).
Transcriptome: Myocardium and Skeletal Muscle
Ventricular tissue and skeletal muscle were each homogenized and RNA extracted using a tri-reagent as previously described (23). Transcriptome data was acquired using the rat 230 2.0 expression array from Affymetrix, which covers expression of 31,042 probe sets (hereto referred as genes). Verification of RNA integrity and analysis of gene expression on the arrays have been described previously (12). Normalized data were filtered to eliminate genes that were not expressed in at least one sample and analyzed by ANOVA to identify genes that revealed significant differential expression between the experimental groups. Hierarchical clustering yielded a gene tree organization of these differentially expressed genes and visualized in a heat map. Within the gene tree, “branches” showing expression patterns of targeted candidates were selected, as previously described (42). The pattern identified herein related to genes affected by 4-wk ALDOST and reversed towards normal after the 4-wk recovery phase, which were then subjected to further interrogation using Ingenuity Pathways (www.ingenuity.com) as previously described (42). Expression of selected genes were graphed using scaled and normalized numbers as “relative expression” units.
Statistical Analysis
Group data are presented as means ± SE and analyzed by one-way ANOVA in SPSS software (ver. 18.0; SPSS, Chicago, IL) with P < 0.05 as significant. Multiple-group comparisons between groups were made by Scheffé's F-test.
RESULTS
Organ Remodeling and Recovery
Body, skeletal muscle, and heart.
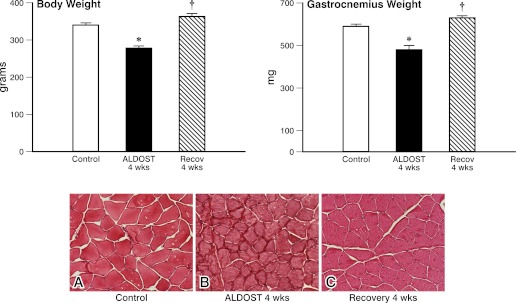
As seen in Fig. 1, there was an 18% difference between body weight in 12-wk-old male Sprague-Dawley rats receiving 4-wk ALDOST compared with untreated age-/sex-/strain-matched controls. Gastrocnemius weight also differed from controls by 18%. Microscopic evidence of muscle fiber atrophy was seen without fibrosis. After 4-wk Recov body and muscle weight each returned to control levels and muscle fiber atrophy resolved.
Fig. 1.
Reverse remodeling at the organ level. Body weight, gastrocnemius weight, and morphology in hematoxylin-eosin stained tissue for age-/sex-/strain-matched controls (A), 4-wk aldosterone/salt treatment (ALDOST; B), and 4-wk recovery (Recov; C) from ALDOST. *P < 0.05 vs. controls, †P < 0.05 vs. ALDOST; magnification = ×400.
Mean arterial pressure was elevated (P < 0.05) at 4-wk ALDOST compared with controls (160.4 ± 4.9 vs. 103.7 ± 2.8 mmHg) and returned toward control levels after 4-wk Recov (116.4 ± 1.9 mmHg). Total heart weight was increased at 4-wk ALDOST but was not statistically different compared with controls (1.08 ± 0.02 vs. 0.94 ± 0.02 g) and was reduced toward control levels after 4-wk Recov (1.04 ± 0.02 g).
Cardiac pathology.
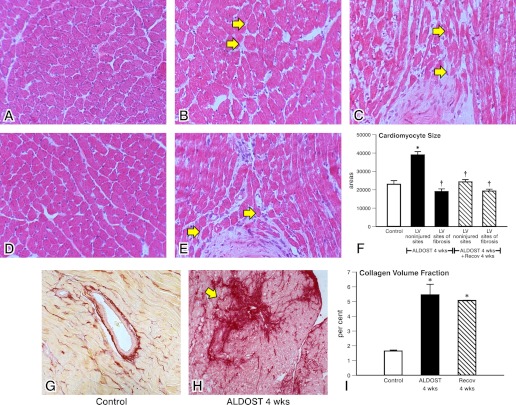
At 4-wk ALDOST, cardiac myocyte cross-sectional area at noninjured sites of myocardium distant to microscopic scarring was increased (P < 0.05) in keeping with hypertrophy and returned largely toward control levels following 4-wk Recov (see Fig. 2). At sites of scarring, where cardiomyocytes were enveloped by fibrillar fibrous tissue, myocyte size was attenuated in keeping with atrophy. Together with the loss of necrotic cardiomyocytes, this may have offset any rise in heart weight attributed solely to hypertrophy.
Fig. 2.
Reverse remodeling at the organ level. Light microscopic images of hematoxylin-eosin-stained myocardium (A-E, top and middle) at 4-wk ALDOST. Morphometric determination of cardiomyocyte size (F) for control tissue, noninjured left ventricle (LV) and at site of fibrosis at 4-wk ALDOST and after 4-wk Recov. *P < 0.05 vs. controls; †P < 0.05 vs. ALDOST. Arrows in B identify hypertrophied while arrows in C indicate atrophic muscle fibers seen at 4-wk ALDOST. E, middle: atrophic fibers surrounded by fibrillar collagen are again found at 4-wk Recov. F: regression in cardiomyocyte hypertrophy was seen after 4-wk Recov while atrophied fibers were unchanged during Recov. Control tissue (G) and a microscopic scar are seen at 4-wk ALDOST (H). Collagen volume fraction in picrosirius red-stained myocardium, where scarring accounts for the severalfold rise in collagen volume fraction seen at 4-wk ALDOST (I) and that does not regress after 4-wk Recov. Magnification = ×400.
Compared to controls, microscopic scars were seen scattered throughout the left and right ventricles with a more than twofold rise in collagen volume fraction (CVF) found in ventricular myocardium at 4-wk ALDOST (see Fig. 2). However, scarring with attendant myocyte atrophy was not altered during 4-wk Recov. Likewise, the rise in collagen volume fraction that occurred during 4-wk ALDOST remained unchanged after the Recov period and compatible with no further accumulation of fibrous tissue.
Cellular/Subcellular Remodeling and Recovery
Cardiomyocyte cytosolic free [Ca2+]i.
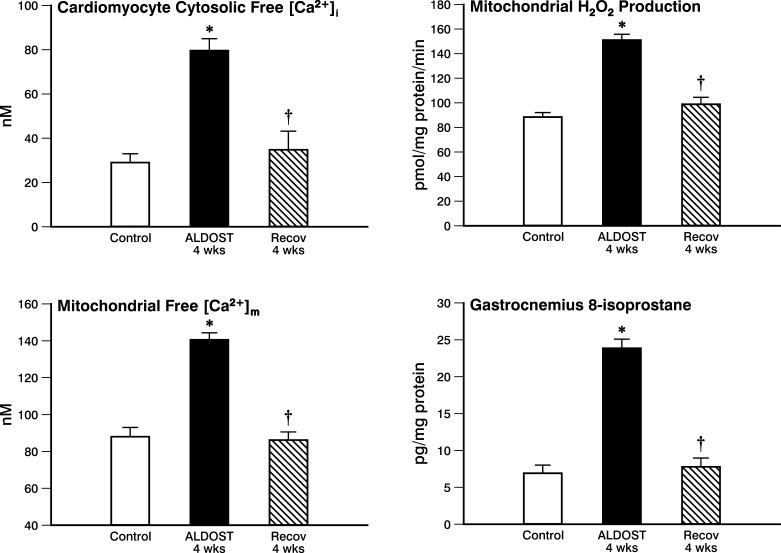
As seen in Fig. 3 and compared with controls, cardiomyocyte free [Ca2+]i was significantly increased at 4-wk ALDOST. This excessive intracellular Ca2+ accumulation, which accompanied secondary hyperparathyroidism associated with chronic aldosteronism (7), returned to control levels after 4-wk Recov without ALDOST.
Fig. 3.
Reverse remodeling at cellular/subcellular levels. Cardiomyocyte cytosolic free Ca2+ ([Ca2+]i; top left) and mitochondrial free Ca2+ ([Ca2+ ]m; bottom left) are each increased at 4-wk ALDOST but recover to control levels after 4-wk Recov. Cardiac mitochondrial Ca2+ overloading is accompanied by evidence of oxidative stress with increased H2O2 production by these organelles, which returns to controls levels with 4-wk Recov. Evidence of lipid peroxidative and 8-isoprostane content is seen in the gastrocnemius muscle at 4-wk ALDOST and then resolves with 4-wk Recov. *P < 0.05 vs. controls; †P < 0.05 vs. ALDOST.
Mitochondrial free [Ca2+]m.
In SSM mitochondria harvested from rat hearts at 4-wk ALDOST, mitochondrial free [Ca2+]m was significantly increased compared with controls (see Fig. 3) and returned to control levels following 4-wk Recov.
Mitochondrial H2O2 production.
At 4-wk ALDOST, H2O2 production by SSM was significantly increased compared with these organelles harvested from hearts of untreated controls (see Fig. 3). This evidence of mitochondria-based oxidative stress generation resolved after 4-wk Recov.
mPTP opening.
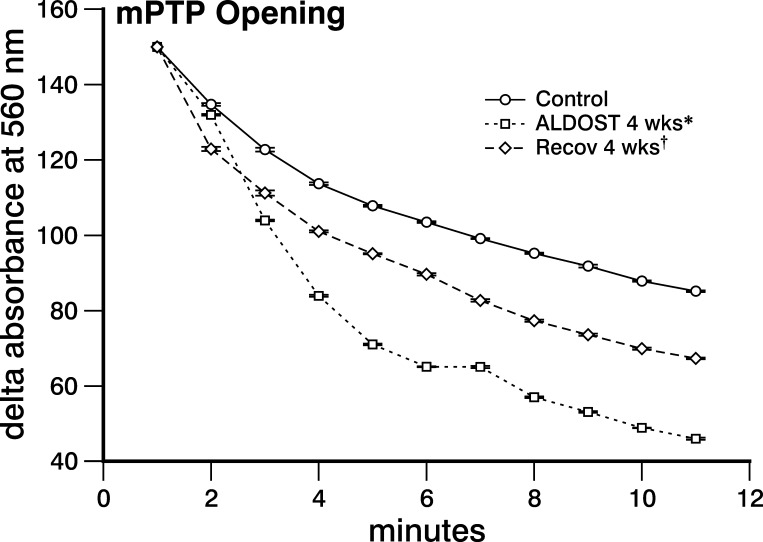
The decline in changes in absorbance at 560 nm due to mPTP opening induced by a 200 μM CaCl2 challenge in mitochondria harvested at 4-wk ALDOST was significantly greater (P < 0.05) than that seen in controls (see Fig. 4). This response implicates an increased propensity for mPTP opening and a greater susceptibility of cardiomyocytes to necrosis during chronic ALDOST. This propensity was significantly attenuated by 4-wk Recov (P < 0.05).
Fig. 4.
Reverse remodeling at subcellular level. Mitochondrial permeability transition pore (mPTP) opening was measured as a change in absorbance at 560 nm induced by 200 μM CaCl2 and found to be significantly increased compared with controls at 4-wk ALDOST and is then attenuated with 4-wk Recov. *P < 0.05, controls vs. ALDOST 4-wk; †P < 0.05, ALDOST 4-wk vs. Recov 4-wk.
Skeletal muscle 8-isoprostane.
At 4-wk ALDOST, 8-isoprostane levels in gastrocnemius were markedly increased compared with controls (see Fig. 3). This evidence of oxidative stress and lipid peroxidation was abrogated at 4-wk Recov.
Molecular Remodeling and Recovery
Heart.
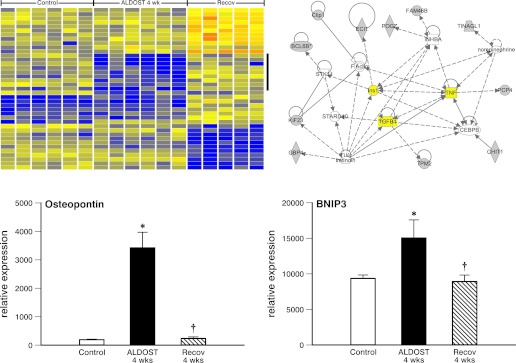
We analyzed five to six independent samples on the MOE230.2 array from each of the three groups. We first filtered out probe sets for which there were no signs of expression in any of the samples and then used ANOVA with a strict statistical criteria (Benjamini-Hochberg multiple test correction and P < 0.01) to reduce the false positive rate of our data set to 1%. The analysis produced a list of 1,312 genes with significant differential expression among the three groups, which were then subjected to hierarchical clustering to define the specific patterns of group differences within these genes. Figure 5, top left, shows a portion of the heat map produced by this hierarchical clustering. To the right of the heat map and marked by a vertical bar are nine genes that form a single cluster within the data and clearly follow a pattern of downregulation (blue color) in ALDOST 4-wk heart samples compared with those from controls and 4-wk Recov groups. We examined the heat map of all the data to find clusters of genes that were either upregulated or downregulated at 4-wk ALDOST hearts compared with each of the two other groups (i.e., genes affected by ALDOST, but normalized after Recov). This process produced a list of 57 differentially expressed genes. To evaluate how these genes were connected to each other we conducted pathway analysis using the ingenuity pathways system. One of the three pathways produced is shown in Fig. 5 (top right). The pathway shown in Fig. 5 centers around TNF-α, transforming growth factor (TGF-β1), and insulin-related signaling pathways, suggesting that these proinflammatory and fibrogenic cytokines/growth factors are central to regulating the abnormalities in gene expression induced by 4-wk ALDOST but are normalized during Recov. We selected specific genes, osteopontin and BNIP3, among these 57 differentially expressed genes to show their relative expression levels (lower panels of Fig. 5). Osteopontin, a matricellular protein involved in tissue repair and extracellular matrix remodeling, revealed low level expression in control myocardium but increased markedly at 4-wk ALDOST when cardiomyocyte necrosis was taking place and before returning to baseline levels at 4-wk Recov. BNIP3 is a protein that localizes to mitochondrial membrane during stressor states (e.g., hypoxia) and promotes autophagy to rid cardiomyocytes of “debris” arising from dysfunctional and injured organelles. Its expression was increased during 4-wk ALDOST when mPTP opening and mitochondrial degeneration were ongoing and downregulated to control levels following 4-wk Recov.
Fig. 5.
Reverse remodeling at molecular level for heart. A heat map of differentially expressed (P < 0.01) genes seen for 6 controls, 6 samples at 4-wk ALDOST, and 5 samples after 4-wk Recov (top left) is shown. Vertical bar identifies 9 genes forming a cluster which followed an expression pattern of decreased expression (blue color) at 4-wk ALDOST compared with controls and 4-wk Recov. The ingenuity pathway system was used to identify interconnecting pathways, one of which includes TNF-α, transforming growth factor (TGF)-β1, and insulin signaling (top right). Osteopontin and BNIP3 gene expression are shown at bottom left and right (see text).
Skeletal muscle.
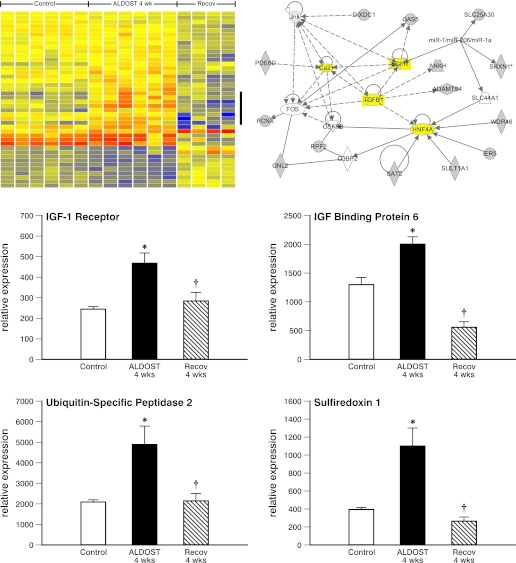
Gene expression analysis of skeletal muscle tissue from these experimental groups (controls, 4-wk ALDOST, and 4-wk natural recovery) was also conducted using the identical expression arrays and statistical analysis used for heart tissue. The ANOVA (P < 0.01 and Benjamini-Hochberg multiple test correction) produced a list of 654 genes with significant expression differences among the groups. Figure 6, top left, shows a part of the heat map generated by this hierarchical clustering. To the right of the heat map, a vertical bar marks seven genes that are part of a single cluster within the data set and were clearly upregulated in ALDOST 4-wk samples compared with both control and Recov samples. We examined the heat map of all 654 genes from the ANOVA to identify clusters of genes that were either upregulated or downregulated in ALDOST 4-wk samples compared with controls or recovery groups (i.e., genes affected by ALDOST but normalized after Recov). This process produced a list of 71 differentially expressed genes. Ingenuity analysis of these genes produced three networks; one is shown in Fig. 6, top right. The pathway shown in Fig. 6 is centered around Ca2+, TGF-β1, epidermal growth factor (EGF) receptor, and hepatocyte nuclear factor (HNF)4-α, suggesting their significance in regulating the abnormalities in gene expression induced by 4-wk ALDOST but normalized during 4-wk Recov.
Fig. 6.
Reverse remodeling at molecular level for skeletal muscle. Differentially expressed (P < 0.01) genes are presented as heat map with upregulated genes in red and downregulated genes in blue (top left). Vertical bar identifies 7 genes as a cluster with increased expression compared with 6 controls, 6 ALDOST 4-wk, and 4 samples after 4-wk Recov. Ingenuity pathway analysis (top right) reveals a pathway (in yellow) centered around Ca2+, TGF-β1, epidermal growth factor (EGF) receptor, and hepatocyte nuclear factor (HNF)-4a. Middle right and left: upregulation of IGF-1 receptor and IGF-binding protein-6 at 4-wk ALDOST and return to baseline with Recov, respectively. Bottom right and left: upregulation of ubiquitin-specific peptidase 2 and sulfiredoxin 1 at 4-wk ALDOST and that were normalized after Recov, respectively. See text.
The appearance of skeletal muscle atrophy reflects a dysequilibrium between anabolic and catabolic signaling pathways and a shift in homeostatic balance between protein synthesis and degradation. Not unexpectedly, the muscle wasting that accompanies the cachexia of 4 wk ALDOST was associated with a downregulation of insulin growth factor (IGF)-1, a growth-promoting anabolic cytokine (data not shown). Of interest, the expression of the IGF-1 receptor and of IGF-binding protein-6, which prolongs the half-life of IGF, was each upregulated (see Fig. 6, bottom) among the 71 differentially expressed genes found in atrophic muscle at 4 wk ALDOST. Furthermore, both IGF-1 receptor and IGF-binding protein-6 gene expression returned to control levels at 4-wk Recov. The ubiquitin-proteasome system (UPS) is involved in the regulation of muscle proteolysis during catabolic states. As seen in Fig. 6, bottom left, ubiquitin-specific peptidase (USP)2 was upregulated in atrophic muscle at 4-wk ALDOST and returned to baseline following 4-wk Recov when muscle atrophy had resolved. Intracellular Ca2+ overloading and the generation of reactive oxygen species contribute to UPS regulation. Sulfiredoxin, a small redox protein of the peroxiredoxin family of endogenous antioxidants, was also upregulated at 4-wk ALDOST before returning to control levels when skeletal muscle Ca2+ overloading and oxidative stress abated with 4-wk Recov.
DISCUSSION
In this study, we gained insights pertinent to the molecular mechanisms involving pathophysiologic remodeling of rat myocardium and skeletal muscle attributed to chronic aldosteronism. We further addressed the potential for reverse remodeling and natural recovery of these tissues with the withdrawal of ALDOST, which would simulate hormonal deactivation in patients with CHF after a period of mechanical circulatory assist.
Reverse Remodeling at the Organ Level
Body and skeletal muscle.
Over the course of 4-wk ALDOST, young adult 8-wk-old male Sprague-Dawley rats failed to maintain the gain in body weight corresponding to their untreated 12-wk-old male counterparts serving as controls. The failure to gain body weight was comparable to the failure to gain muscle mass and was accompanied by morphologic evidence of significant muscle fiber atrophy. A marked resorption of bone and reduction in tibial and femoral mineral density and flexor strength accompany the secondary hyperparathyroidism seen with ALDOST (8). Thus the failure to gain body weight can be attributed to a failure of its major determinants, muscle mass and bone mineral density.
The skeletal muscle atrophy can be attributed to a Ca2+-activated, oxidative stress-induced ubiquitin proteasome-mediated pathway (vide infra) that accompanies the secondary hyperparathyroidism of aldosteronism with bone resorption (7, 8, 31, 35). The skeletal muscle myopathy seen in human primary or secondary hyperparathyroidism resolves after parathyroidectomy (32). Increments in body and gastrocnemius weights comparable to controls and resolution of gastrocnemius atrophy were attained after 4-wk Recov. The capacity of muscle to recover highlights its plasticity and the relevance of absolute neurohormonal withdrawal. Sukhanov et al. (35) have emphasized the role of neurohormonal factors in the development of muscle wasting, including angiotensin-induced ubiquitin proteasome-mediated proteolysis in the presence of oxidative stress. The recovery of atrophic skeletal muscle highlights the putative role of autophagy in minimizing accumulation of protein aggregates and abnormal mitochondria (26).
Myocardium.
During ALDOST, a heterogeneity in cardiomyocyte cross-sectional area was anticipated ranging from the nonpressure-overloaded RV to the hypertrophied hemodynamically overburdened LV. Cardiomyocyte hypertrophy would cause heart weight to rise significantly; however, this did not occur because of other factors. First, nonischemic cardiomyocyte necrosis with consequent replacement fibrosis, a major component to the pathologic remodeling of the explanted, failing human heart, occurred (2). Second, myocytes at sites of scarring are enveloped by fibrillar collagen, which reduces their contractile work and hence cardiomyocyte cross-sectional area declined and they became atrophic. A similar fall in cardiomyocyte size has been seen with the scar tissue that ensnares cardiomyocytes of the endocardium following isoproterenol-induced injury (15). Additional lines of evidence that suggest workload (vis-à-vis a circulating substance) regulates cardiomyocyte size is demonstrated by the atrophy of both the subpulmonic and systemic ventricles that accompanies banding of the thoracic segment of the inferior vena cava (24). Atrophy secondary to mechanical unloading also accompanies heterotopic transplantation of the heart where, like skeletal muscle, it is associated with an activation of the ubiquitin-proteasome system (29). Finally, apoptosis also contributes to cardiomyocyte loss and prevents the rise in heart weight (21). Apoptotic cardiomyocytes are rapidly phagocytized by macrophages, and their death is neither accompanied by inflammatory cell nor fibroblast responses with consequent tissue repair. Unlike the regression in cardiomyocyte hypertrophy that accompanied the decline in arterial pressure with ALDOST withdrawal, we did not find scar tissue to regress or fibrillar collagen-ensnared atrophic cardiomyocytes to return to control size after 4-wk Recov from ALDOST. Cardiomyocyte hypertrophy and myocardial fibrosis present at the time of left ventricular assist device (LVAD) implantation predict the degree of improvement in ventricular function after device removal and whether recovery is sustainable (4).
Reverse Remodeling at Cellular and Subcellular Levels
Cardiomyocytes and mitochondria.
Cellular/subcellular remodeling were also taken into consideration (10, 13). In cardiomyocytes harvested at 4-wk ALDOST, we found intracellular Ca2+ overloading, which included both cytosolic free [Ca2+]i and SSM mitochondrial free [Ca2+]m domains. The latter was coupled to the induction of oxidative stress in cardiac tissue (17, 34) and these organelles, as evidenced by their significantly increased production of H2O2, and opening potential for their mPTP. This pathophysiologic sequence to nonischemic cardiomyocyte necrosis is embodied by a mitochondriocentric signal-transducer-effector pathway occurring in SSM and interventions targeting specific pathway components have proven cardioprotective (6, 34).
Skeletal muscle.
The Ca2+ concentration of skeletal muscle is increased during ALDOST and is accompanied by oxidative stress and lipid peroxidation with increased 8-isoprostane levels and the atrophy of gastrocnemius muscle. We did not find any evidence of muscle scarring. A recovery of atrophic muscle was seen with the 4-wk withdrawal of ALDOST.
Reverse Remodeling at the Molecular Level
Myocardium.
Cardiomyocytes and mitochondria exhibited a considerable plasticity, including their pathologic remodeling during ALDOST and subsequent recovery upon its withdrawal. This cellular/subcellular remodeling was accompanied by the differential expression of 57 genes. During 4-wk ALDOST, altered profile included genes contributing to inflammatory cell-myofibroblast responses involved in tissue repair following cardiomyocyte necrosis (36). Microarray analysis revealed the reexpression of osteopontin, a known regulator of myofibroblast-based fibrillar collagen production that is integral to wound healing and regulated by TGF-β1 signaling and oxidative stress (27). In osteopontin knockout mice, cardiac remodeling is attenuated in response to ALDOST (33). Upregulated expression of BNIP3, a key regulator of mitochondrial function and turnover via autophagy, was also seen in compromised cardiomyocytes in which mPTP opening potential was significantly increased (30). Using the ingenuity pathway system, we examined the gene pathways involved and found a proinflammatory/fibrogenic phenotype that included TGF-β1, TNF-α, and insulin signaling as has also been reported by Brooks et al. (3) in spontaneously hypertensive rats.
These findings in the rat heart at 4-wk ALDOST are similar to LV tissue obtained at the time of human LVAD implantation, which reveals a proinflammatory/proapoptotic phenotype with increased TNF-α expression and Ca2+ dyshomeostasis with a reduced ratio of sarcoplasmic reticulum Ca2+ ATPase: Na+/Ca2+ exchanger (1, 28, 37). The transcriptome of myocardial tissue harvested at the time of LVAD explantation in patients who had recovered from their CHF revealed this phenotype to have been largely resolved while mRNAs related to transcription factors, DNA repair, Ca2+ handling, cytoskeletal proteins, metabolism, and IGF-1 were significantly upregulated (25, 41, 44).
Skeletal muscle.
Muscle also demonstrated a plasticity and ability to recover from ALDOST. Seventy-one genes were differentially expressed in the gastrocnemius muscle among controls, 4-wk ALDOST, and 4-wk Recov. The dysequilibrium between anabolic and catabolic signaling included the downregulation of IGF-1 gene which, however, was accompanied by the upregulation of the IGF-1 receptor and IGF-binding protein-6 genes during ALDOST. Whether these responses facilitate the resolution of atrophy seen with Recov remains unknown.
The upregulation of ubiquitin-specific peptidase-2 expression was coincident with the proteolysis that accompanied muscle atrophy and the appearance of oxidative stress but that was reversible upon hormonal withdrawal and coincident with restitution in muscle mass. In a similar fashion, the loss of muscle mass that accompanies space flight and zero gravity is associated with upregulation of myostatin mRNA and protein, a negative regulator of muscle growth, and a decrease in IGF-2 mRNA, each of which are normalized with restoration of normal gravity (22).
Beyond the interplay that exists between IGF and UPS in muscle wasting, a complex crosstalk exists between various growth factors and cytokines that are regulated by cations and hormones, including Ca2+, Mg2+, angiotensin II, and aldosterone. Our ingenuity pathway analysis draws attention to the interrelationships that include Ca2+, oxidative stress, TGF-β1, the EGF receptor, and HNF-4a, which occurred during ALDOST and recovery. By inhibiting the Mg2+ transporter (TRPM6) of the distal convoluted tubule and intestine, where EGF and HNF are operative and magnesiotropic, aldosteronism enhances urinary and fecal Mg2+ and Ca2+ excretion, which leads to ionized hypomagnesemia and hypocalcemia (7, 43). Furthermore, aldosterone enhances the expression and phosphorylation of the Na+/H+ exchanger via activation of the EGF receptor and reactive oxygen species formation (9). The role of Mg2+ deficiency in the appearance of muscle wasting and progressive nature of heart failure needs to be addressed (39, 40).
We readily acknowledge several limitations to our study. We did not compare the reverse remodeling seen with hormonal withdrawal to that with hemodynamic unloading, such as would accompany banding the inferior vena cava or heterotopic transplantation, and therefore cannot comment on the relative importance of each. Given the importance of the SSM population of cardiac mitochondria to the induction of cardiomyocyte necrosis and remodeling, it is essential to monitor their proteome to identify signaling pathways involved, which could be targeted as cardioprotective strategies. Thirdly, we allowed the carryover effects of ALDOST to resolve gradually over 4-wk Recov; we did not promptly correct the hypocalcemia, hypomagnesemia, and hypozincemia using cation supplementation.
In summary, chronic ALDOST is accompanied by a pathologic remodeling of the previously intact myocardium and skeletal muscle, which takes place at the organ, cellular/subcellular, and molecular levels. Hence, neurohormonal activation can lead to tissue wasting, with cardiomyocyte necrosis and skeletal muscle atrophy, thereby contributing to the progressive and systemic nature of CHF. A reverse remodeling and recovery of these organelles and the transcriptome of these wasted tissues are plausible.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grants R01-HL73043 and R01 HL90867 (K. T. Weber). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.C., R.A.A., I.C.G., S.K.B., and K.T.W. conception and design of research; Y.C., W.Z., T.Z., M.U.K., K.D.G., R.A.A., and S.K.B. performed experiments; Y.C., R.A.A., I.C.G., S.K.B., and K.T.W. analyzed data; Y.C., R.A.A., I.C.G., S.K.B., and K.T.W. interpreted results of experiments; S.K.B. and K.T.W. drafted manuscript; K.T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for K. D. Green: Cardiovascular Medicine Division, Vanderbilt University School of Medicine, Nashville, TN.
REFERENCES
- 1. Bartling B, Milting H, Schumann H, Darmer D, Arusoglu L, Koerner MM, El-Banayosy A, Koerfer R, Holtz J, Zerkowski HR. Myocardial gene expression of regulators of myocyte apoptosis and myocyte calcium homeostasis during hemodynamic unloading by ventricular assist devices in patients with end-stage heart failure. Circulation 100: II216–II223, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89: 151–163, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Brooks WW, Shen S, Conrad CH, Goldstein RH, Deng LL, Bing OH. Transcriptional changes associated with recovery from heart failure in the SHR. J Mol Cell Cardiol 49: 390–401, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bruckner BA, Razeghi P, Stetson S, Thompson L, Lafuente J, Entman M, Loebe M, Noon G, Taegtmeyer H, Frazier OH, Youker K. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J Heart Lung Transplant 23: 36–42, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Burch GE, Walsh JJ, Black WC. Value of prolonged bed rest in management of cardiomegaly. JAMA 183: 81–87, 1963 [DOI] [PubMed] [Google Scholar]
- 6. Cheema Y, Sherrod JN, Shahbaz AU, Zhao W, Zhao T, Ahokas RA, Sun Y, Bhattacharya SK, Gerling IC, Weber KT. Mitochondriocentric pathway to cardiomyocyte necrosis in aldosteronism: cardioprotective responses to carvedilol and nebivolol. J Cardiovasc Pharmacol 58: 80–86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, Smith RA, Gerling IC, Weber KT. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation 111: 871–878, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, Smith RA, Gerling IC, Weber KT. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol 287: H2023–H2026, 2004 [DOI] [PubMed] [Google Scholar]
- 9. De Giusti VC, Nolly MB, Yeves AM, Caldiz CI, Villa-Abrille MC, Chiappe de Cingolani GE, Ennis IL, Cingolani HE, Aiello EA. Aldosterone stimulates the cardiac Na+/H+ exchanger via transactivation of the epidermal growth factor receptor. Hypertension 58: 912–919, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Dhalla NS, Saini-Chohan HK, Rodriguez-Leyva D, Elimban V, Dent MR, Tappia PS. Subcellular remodelling may induce cardiac dysfunction in congestive heart failure. Cardiovasc Res 81: 429–438, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Gandhi MS, Deshmukh PA, Kamalov G, Zhao T, Zhao W, Whaley JT, Tichy JR, Bhattacharya SK, Ahokas RA, Sun Y, Gerling IC, Weber KT. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol 52: 245–252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerling IC, Sun Y, Ahokas RA, Wodi LA, Bhattacharya SK, Warrington KJ, Postlethwaite AE, Weber KT. Aldosteronism: an immunostimulatory state precedes the proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol 285: H813–H821, 2003 [DOI] [PubMed] [Google Scholar]
- 13. González A, Ravassa S, Beaumont J, López B, Díez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol 58: 1833–1843, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Ibrahim M, Terracciano CM, Yacoub MH. Bridge to recovery: what remains to be discovered? Cardiol Clin 29: 531–547, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Jalil JE, Janicki JS, Pick R, Abrahams C, Weber KT. Fibrosis-induced reduction of endomyocardium in the rat after isoproterenol treatment. Circ Res 65: 258–264, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Kamalov G, Ahokas RA, Zhao W, Johnson PL, Shahbaz AU, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Temporal responses to intrinsically coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria during aldosteronism. Am J Physiol Heart Circ Physiol 298: H385–H394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamalov G, Deshmukh PA, Baburyan NY, Gandhi MS, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol 53: 414–423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kass DA, Baughman KL, Pak PH, Cho PW, Levin HR, Gardner TJ, Halperin HR, Tsitlik JE, Acker MA. Reverse remodeling from cardiomyoplasty in human heart failure. External constraint versus active assist. Circulation 91: 2314–2318, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal 13: 1033–1049, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AH. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J 30: 805–812, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Kramer F, Sandner P, Klein M, Krahn T. Plasma concentrations of matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-1 and osteopontin reflect severity of heart failure in DOCA-salt hypertensive rat. Biomarkers 13: 270–281, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezzat S, Gonzalez-Cadavid NF. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J Endocrinol 167: 417–428, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Lenchik NI, Desiderio DM, Gerling IC. Two-dimensional gel electrophoresis characterization of the mouse leukocyte proteome, using a tri-reagent for protein extraction. Proteomics 5: 2202–2209, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lisy O, Redfield MM, Jovanovic S, Jougasaki M, Jovanovic A, Leskinen H, Terzic A, Burnett JC., Jr Mechanical unloading versus neurohumoral stimulation on myocardial structure and endocrine function in vivo. Circulation 102: 338–343, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Margulies KB. Reversal mechanisms of left ventricular remodeling: lessons from left ventricular assist device experiments. J Card Fail 8: S500–505, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Masiero E, Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6: 307–309, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Okamoto H. Osteopontin and cardiovascular system. Mol Cell Biochem 300: 1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Razeghi P, Mukhopadhyay M, Myers TJ, Williams JN, Moravec CS, Frazier OH, Taegtmeyer H. Myocardial tumor necrosis factor-alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 72: 2044–2050, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, Taegtmeyer H. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation 108: 2536–2541, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res 91: 226–231, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Rossi E, Perazzoli F, Negro A, Sani C, Davoli S, Dotti C, Casoli MC, Regolisti G. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension. Am J Hypertens 11: 8–13, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Ruff RL, Weissmann J. Endocrine myopathies. Neurol Clin 6: 575–592, 1988 [PubMed] [Google Scholar]
- 33. Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens 17: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Shahbaz AU, Kamalov G, Zhao W, Zhao T, Johnson PL, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, Weber KT. Mitochondria-targeted cardioprotection in aldosteronism. J Cardiovasc Pharmacol 57: 37–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342: 143–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol 161: 1773–1781, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torre-Amione G, Stetson SJ, Youker KA, Durand JB, Radovancevic B, Delgado RM, Frazier OH, Entman ML, Noon GP. Decreased expression of tumor necrosis factor-alpha in failing human myocardium after mechanical circulatory support: a potential mechanism for cardiac recovery. Circulation 100: 1189–1193, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Weber KT. From inflammation to fibrosis: a stiff stretch of highway. Hypertension 43: 716–719, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Weglicki WB, Chmielinska JJ, Kramer JH, Mak IT. Cardiovascular and intestinal responses to oxidative and nitrosative stress during prolonged magnesium deficiency. Am J Med Sci 342: 125–128, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Weglicki WB, Mak IT, Stafford RE, Dickens BF, Cassidy MM, Phillips TM. Neurogenic peptides and the cardiomyopathy of magnesium-deficiency: effects of substance P-receptor inhibition. Mol Cell Biochem 130: 103–109, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Wohlschlaeger J, Schmitz KJ, Schmid C, Schmid KW, Keul P, Takeda A, Weis S, Levkau B, Baba HA. Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc Res 68: 376–386, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Lenchik NI, Gerling IC. Approaches to reduce false positives and false negatives in the analysis of microarray data: applications in type 1 diabetes research. BMC Genomics 9, Suppl 2: S12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yogi A, Callera GE, O'Connor SE, He Y, Correa JW, Tostes RC, Mazur A, Touyz RM. Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J Hypertens 29: 1400–1410, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Zweier JL, Chen CA, Talukder MA. Cardiac resynchronization therapy and reverse molecular remodeling: importance of mitochondrial redox signaling. Circ Res 109: 716–719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]