Abstract
Myocardial infarction (MI) results in adverse cardiac remodeling leading to heart failure and increased mortality. Experimental mouse models of MI are extensively used to identify mechanisms underlying adverse remodeling, but the extent of remodeling that occurs may be highly variable and can limit the utility to discover new disease pathways. The ability to predict the development of significant late post-MI remodeling would be invaluable in conducting such studies by increasing throughput and efficiency. This study aimed to identify potential thresholds of cardiac magnetic resonance imaging (MRI) parameters measured early after murine MI that would predict the development of significant adverse remodeling at 4 wk. MI was achieved by permanent coronary ligation and animals (n = 84) were followed up for 4 wk subsequently. MRI was used to assess left ventricular (LV) volumes, mass and ejection fraction, as well as infarct size (IS). Late gadolinium enhancement cine-MRI was performed at 2 days with standard cine-MRI at 30 days post-MI. Utilizing multiple logistic regression, we found that IS >36%, at 2 days post-MI, was the overall best single predictor of adverse remodeling at 30 days (sensitivity 80.7%, specificity 88.9%; C-statistic of 0.939 from receiver-operating curve analysis). LV end-systolic volume (LVESV) >32 μl was also an excellent predictor comparable to IS. The combination of IS >36% and/or LVESV >32 μl provided the highest predictive values for late adverse remodeling among multiple predictors. This study demonstrates that MRI-based estimation of IS and ESV during the acute phase of murine MI are good predictors of subsequent adverse remodeling that may aid experimental design.
Keywords: remodeling prediction, magnetic resonance imaging, infarct size, ejection fraction
myocardial infarction (MI) is commonly followed by adverse left ventricular (LV) remodeling involving significant changes in LV geometry and function (4). Such adverse remodeling contributes to impaired cardiac function and is a major cause of heart failure and increased mortality (25). Experimental models of MI, for example, as induced by permanent coronary ligation in gene-modified mice, are extensively utilized to elucidate pathophysiological and molecular mechanisms underlying cardiac remodeling that could be therapeutically targeted (5, 17, 18, 22, 23, 27). The extent of adverse remodeling that occurs after experimental MI in murine models, as manifest by an increase in LV end-diastolic and end-systolic volumes (LVEDV and LVESV, respectively) and a reduction in ejection fraction (EF), can be highly variable and can limit their utility to discover new disease pathways (23, 29). This is most likely related to variations in the severity and extent of myocardial injury that is induced by coronary ligation in mouse models (15, 23, 28, 29). The ability in such preclinical models to predict at an early stage whether a sufficient infarction has been induced to result in significant delayed remodeling would be invaluable in improving the design, throughput, and efficiency of such studies.
In clinical practice, the prediction of delayed cardiac remodeling is an important aim that aids decisions regarding early therapy (8, 10, 16). Many different methods of such prediction have been studied among which imaging modalities such as cardiac magnetic resonance imaging (MRI), which provide accurate and reproducible assessment of functional and volumetric parameters of the left ventricle in vivo, are considered especially promising (2, 6, 9, 13, 16). MRI has high accuracy in quantifying not only volumes and mass in remodeling murine heart but also infarct size (IS; Refs. 3, 7, 20, 21, 26) by the use of late gadolinium enhancement (LGE) in the acute phase (1, 3, 19). We therefore hypothesized that MRI-based parameters measured in the acute phase after experimental murine MI could predict the extent of delayed adverse remodeling. We found that the occurrence of significant adverse remodeling, as assessed by MRI studies performed 4 wk post-MI, could be predicted from the estimation of IS (by LGE) and LVESV in the acute phase after MI. Significant adverse remodeling was defined as an increase in LVEDV >84 μl after a statistical analysis.
METHODS
Animal model.
All in vivo procedures were conducted in accordance with the Guidance on the Operation of the Animals (Scientific Procedures) Act, 1986 (UK Home Office) and approved by the Institutional Health Committee. Female C57Bl/6J mice (n = 84) weighing 18–24 g were subjected to MI by left coronary artery ligation surgery (11, 14). Mice were anesthetized with 2% isoflurane/98% oxygen and underwent endotracheal intubation and ventilation using a small animal ventilator (Hugo Sacks Elektronic). A lateral thoracotomy was made, the thorax was opened in the fourth intercostal space, and the pericardium was removed. The left coronary artery was ligated using 8/0 Ethilon suture at a level between 1 and 2 mm below the tip of the left atrium. Successful ligation was confirmed by regional blanching of the LV, extending to the apex. The chest wall was then repaired in layers, and the animals were weaned from the ventilator. Animals were recovered in a warmed chamber for ≥6 h. Perioperative analgesia with buprenorphine intramuscularly and flunixin subcutaneously was used.
Seven female C57Bl/6J mice weighing 18–24 g were used as control mice. No surgical operations were performed on those mice.
Animal MRI.
At 2 and 30 days after MI, cardiovascular MRI imaging was performed on a 7T horizontal MR scanner (Varian, Palo Alto, CA) with mice positioned in the prone position. The gradient coil had an inner diameter of 12 cm, 1,000 mT/m (100G/cm) gradient strength, and rise-time of 120 μs. A quadrature transmit/receive coil (RAPID Biomedical) with an internal diameter of 39 mm was used. Anesthesia was maintained with 1.5% isoflurane/98.5% oxygen, and body temperature was maintained at 37° using a warm air fan (SA Instruments, Stony Brook, NY). The ECG was monitored via two metallic needles placed subcutaneously into the front paws. A pressure-transducer for respiratory gating was placed on the abdomen. To synchronize data acquisition with the ECG and to compensate for respiratory motion, simultaneous ECG triggering and respiration gating (SA Instruments) were applied. LGE MRI was performed 20 min after intraperitoneal injection of a 30-μl bolus of 0.5 mmol/kg gadolinium-diethylenetri-amine-pentaacetic acid (Magnevist, Schering Healthcare; Ref. 19).
Cardiac MRI sequences.
LGE cine-MRI was used at day 2 post-MI to acquire gadolinium enhanced images as reported in our previous work (19). The same cine imaging technique (without LGE) was used at day 30 where no injection was needed. Cine-MRI allowed the acquisition of temporally resolved dynamic short-axis images of the heart. This gradient echo sequence maintains the steady-state during the entire scan. Spoiler gradients (1 ms, 100 mT/m) were applied after each data acquisition readout to dephase the transverse magnetization before the application of the next radio frequency excitation pulse. LGE cine-MRI was performed with single ECG gating and 20 min after gadolinium injection. Imaging parameters included the following: repetition time = RR-interval/number of frames (typically ∼ 9 to 10 ms); echo time = 1 ms; field of view = 25 × 25 mm; in-plane resolution = 0.195 × 0.195 mm; slice thickness = 1 mm; matrix size = 128 × 128; flip angle = 40°; 3 averages; 9 slices at 2 days, 11 slices at 30 days; 1 k-space line/frame; multiple frames were acquired per cardiac cycle to study the dynamic contraction of the heart; acquisition time was ∼8 min. Acquisitions were synchronized with the peak of the QRS complex. Systolic and diastolic frames were achieved and the following functional/volumetric parameters were estimated: EF, LVEDV, LVESV, stroke volume (SV), IS, and LV mass.
Infarct area analysis by MRI.
Infarct area analysis of MRI images at day 2 was performed using a semiautomated in-house-developed cardiac preclinical computer software program (19). This program allows delineation of the infarct region based on myocardial pixel intensity and thresholding, thus minimizing manual intervention. At 2 days post-MI, the myocardium did not demonstrate significant reduction in wall thickness compared with healthy animals. Hypertrophy was not observed either. Hence, the IS was calculated as follows:
| (1) |
where areainf is the LV infarcted area of the end diastolic frame as identified by LGE and areatot is the entire LV end-diastolic area. This method is valid since slice thickness is constant during MRI scanning, and hence actual areas may be summed to calculate infarct size.
At the 30-day time point, infarct size was calculated by applying a midline approach for quantification of thinned myocardium, which has been shown to be as reliable as the area or the length-based approach (23). The midline approach followed the following equation:
| 2 |
where midlineinf was achieved by estimating the length of the infarcted myocardium by manually drawing a line. Midlinetot represents the length of the midline drawing along the entire LV septum.
MRI is subject to the problem of out-of-plane motion due to twisting of the heart but this is minimal at end diastole. Intraobserver variability for IS was attained from the measurement differences between the first and second segmentation of observer A. Interobserver variability was estimated by the difference between data sets segmented by two observers A and B. Variability was assessed on 10 animals at 2 and 30 days, respectively.
Calculation of SV, EF, and LV mass.
Parameters such as SV, EF, and LV mass were obtained from cine-MR images at 2 and 30 days (21).
| 3 |
expressed in microliters.
| 4 |
expressed in percentages.
| 5 |
expressed in grams (12). The specific gravity (γ) of the myocardium is 1.055 g/cm2.
The aforementioned cardiac preclinical computer software automatically calculated all the parameters after detecting epicardial and endocardial LV borders for end diastolic and end systolic frames.
Statistical analysis.
Based on an analysis of LVEDV and EF at the 30-day time point, the animals subjected to MI could be divided into two groups, one that showed significant adverse remodeling as indicated by increased LVEDV [high remodeling (HR)] and a group that showed little or no remodeling [low remodeling (LR); see results]. Significant late LV remodeling was defined as an increase in LVEDV >84 μl that corresponded to an increase of 68% to the average LVEDV value of noninfarcted animals (control). An independent t-test was used to compare the HR and LR group data. P < 0.05 was considered significant. The relation between LVEDV at 30 days and variables at 2 days was initially established by applying a receiver operating curves (ROC) analysis with IBM SPSS software.
Subsequently a multiple logistic regression analysis was used to assess predictors of delayed severe remodeling from the early stage data set, i.e., parameters that could distinguish between the HR and LR groups. Analyses were performed on IBM SPSS. Sensitivity was calculated as the number of animals predicted to be in the HR group from the 2-day data set divided by the number that were actually found to be in the HR group (here called eHR) after the application of the 2-day cutoff condition. Specificity was calculated as the number predicted to be in the LR group from the 2-day data set divided by the number actually found to be in the LR group (here called eLR) after the application of the 2-day cutoff condition. Positive (+LH) and negative (−LH) likelihood ratios were calculated as:
| 6 |
| 7 |
RESULTS
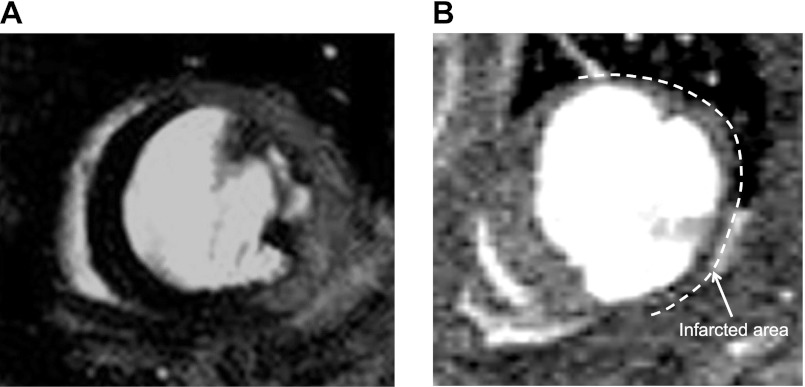
Adverse remodeling post-MI markedly modifies the geometry of the ventricle and functional and volumetric parameters. Figure 1 shows representative MR images of an infarcted heart at 2 and 30 days at slice-matched positions. Figure 1A shows an LGE short axis image where the infarcted region is characterized by signal enhancement. Infarcted, viable myocardium and blood are well differentiated as reported in our previous article (19). At 30 days, dilatation of the LV and thinning of the infarcted region are shown (Fig. 1B).
Fig. 1.
Representative magnetic resonance (MR) images of the same mouse at 2 and 30 days post infarction. A: late gadolinium enhancement (LGE) image of infarcted myocardium at 2 days post-myocardial infarction (MI). Enhanced myocardium, corresponding to the infarcted area, viable myocardium and blood can be readily distinguished. B: corresponding slice at the 30 days time point (without LGE). Markedly thinned infarct (arrow) and dilated left ventricular (LV) are evident. Image contrast has been adjusted to better delineate the infarct.
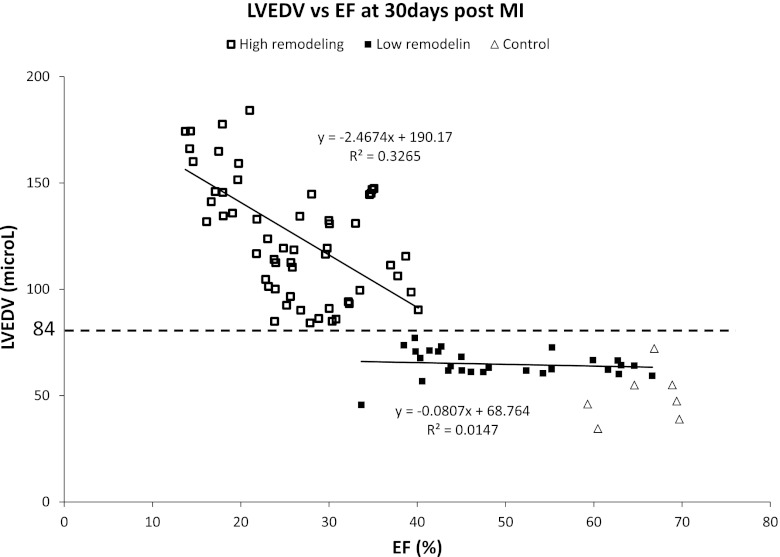
The range of delayed remodeling observed after experimental MI in our cohort of 84 mice was analyzed by quantifying LVEDV and EF at 30 days post-MI. When the data for LVEDV vs. EF were plotted, two groups could be distinguished, which displayed different gradients (slopes). Although a complex fit of the entire data set could be attempted, as a simple approach to distinguish between the groups, we fit the data using a linear relationships (Fig. 2). One group showed relatively modest reductions in EF down to ∼40% (i.e., LR). The HR group showed, instead, substantial reductions in EF to ∼15% and LVEDV increased by an average of 154% (i.e., HR). A similar separation could also be defined on the basis of an LVESV against EF or IS plot (data not shown). Based on this analysis, we defined significant adverse remodeling as an increase in LVEDV >84 μl that corresponded to an increase of 68% to the average LVEDV value of controls animals. LVEDV value of 84 μl at 30 days post-MI was used as the cutoff between HR and LR groups (Fig. 2).
Fig. 2.
LV end-diastolic volume (LVEDV) vs. ejection fraction (EF) at 30 days post-MI. Animals can de divided into two groups, one with high remodeling (□; high remodeling) and the other with low remodeling (■; low remodeling group). LVEDV cutoff value of 84 μl separated the two groups (dotted line). Values from control animals are also reported (△).
An LVEDV >84 μl cutoff at 30 days divided the group of 84 MI mice into a HR group comprising 57 animals (68%) and a LR group comprising 27 animals (22%). Table 1 reports the mean values (±SD) for various contractile parameters and for IS in these two groups, both at 30-day and at the 2-day time point. When comparing these results to that of the control group, also reported in Table 1, the LR group is significantly different from the latter only in few of the parameters. In addition, it is evident that the HR group had a larger IS at 2 days as well as a lower EF and higher LVEDV and LVESV. Inter- and intraobserver mean difference for measurement of IS were −0.9 ± 1.2 and 0.8 ± 1.0% at 2 days post-MI. At 30 days, post-MI inter- and intraobserver mean differences were 1.3 ± 4.6 and −1.9 ± 5.1% respectively.
Table 1.
Functional/volumetric parameters
| 2-Day MI |
30-Day MI |
||||
|---|---|---|---|---|---|
| HR | LR | HR | LR | Healthy Mice | |
| EF, % | 34.8 ± 6.9 | 47.0 ± 9.1 | 25.7 ± 7.2 | 48.4 ± 10.1 | 65.6 ± 4.3 |
| P < 0.0001 | P < 0.0001 | ||||
| IS, μl | 41.6 ± 5.9 | 23.5 ± 10.2 | 41.6 ± 6.5 | 16.7 ± 8.7 | NA |
| P < 0.0001 | P < 0.0001 | ||||
| LVEDV, μl | 66.4 ± 15.0 | 49.8 ± 10.3 | 126.8 ± 31.3 | 64.8 ± 6.4 | 49.9 ± 12.4 |
| P = 0.009 | P < 0.0001 | ||||
| LVESV, μl | 43.4 ± 11.0 | 26.6 ± 8.8 | 95.6 ± 30.5 | 33.4 ± 7.4 | 17 ± 4.1 |
| P < 0.0001 | P < 0.0001 | ||||
| SV, μl | 23.1 ± 7.0 | 23.2 ± 9.2 | 31.3 ± 8.2 | 31.4 ± 6.2 | 28.8 ± 7.0 |
| P = 0.027 | P = 0.2230 | ||||
| Mass, mg | 86.7 ± 13.3 | 78.3 ± 11.2 | 112.4 ± 18.4 | 88.9 ± 10.9 | 75.4 ± 10.8 |
| P = 0.1882 | P < 0.0001 | ||||
| Heart rate, beats/min | 410 ± 40 | 400 ± 50 | 415 ± 30 | ||
Values are means ± SD, and P values related to positive and negative groups are reported. Myocardial infarction (MI) functional and volumetric parameter for high remodeling (HR) and low remodeling (LR) groups at 2 and 30 days. EF, ejection fraction; IS, infarct size; LVEDV and LVESV, left ventricular end-systolic and end-diastolic volume; SV, stroke volume; NA, not applicable.
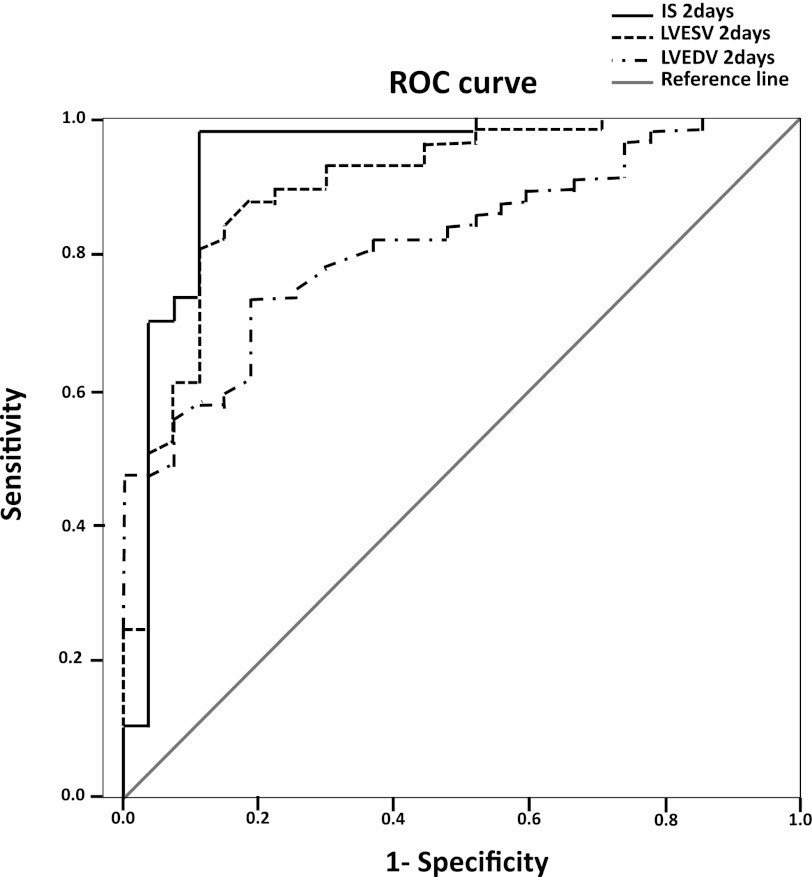
We then assessed the relationship between 2-day values of IS, LVEDV, and LVESV and the 30-day value of LVEDV >84 μl by ROC analysis (Fig. 3). EF was found to be a nonsignificant predictor (data not shown).This analysis showed that IS, LVESV, and LVEDV were significant independent predictors of 30 days LVEDV. IS appeared as the best early stage predictor with a C-statistic (area under the curve) of 0.939. The C-statistic for LVESV and LVEDV was 0.899 and 0.820, respectively. SV and EF reported nonsignificant values.
Fig. 3.
Receiver operating curves (ROC) analysis of infarct size (IS), LV end-systolic volume (LVESV), and LVEDV at 2 days as predictors of high remodeling (LVEDV >84 μl) at 4 wk.
To generate and assess cutoff values from measurements at the 2-day time point that could be used to predict HR, we took the mean value of parameters in the HR group at 2 days plus 1 SD (for EF) or −1 SD (for other parameters) and then tested the sensitivity, specificity, and likelihood values for predicting LVEDV >84 μl at 30 days (Table 2). Such multiple logistic regression analysis showed that 2-day cutoff values of an IS >36% or an LVESV >32 μl both provided excellent prediction. The IS cutoff provided the overall best combination of sensitivity, specificity, and likelihood values. The combination of IS and LVESV (IS >36% and/or LVESV >32 μl) was also an excellent predictor of adverse remodeling. EF resulted in a better prediction than LVEDV.
Table 2.
Multiple logistic regression analysis
| Cutoff Predictors at 2 Days Post-MI | eHR | HR-eHR | eLR | LR-eLR | Sensitivity, % | Specificity, % | +LH | −LH |
|---|---|---|---|---|---|---|---|---|
| EF <41% | 48 | 9 | 19 | 8 | 84.2 | 70.4 | 2.8 | 4.5 |
| IS >36% | 46 | 11 | 24 | 3 | 80.7 | 88.9 | 7.3 | 4.6 |
| LVEDV >51 μl | 49 | 8 | 12 | 15 | 86.0 | 44.4 | 1.5 | 3.2 |
| LVESV >32 μl | 51 | 6 | 21 | 6 | 89.5 | 77.8 | 4.0 | 7.4 |
| IS >36% and LVEDV >51 μl | 39 | 18 | 26 | 1 | 68.4 | 96.3 | 18.5 | 3.0 |
| IS >36% and LVESV >32 μl | 42 | 15 | 26 | 1 | 73.7 | 96.3 | 19.9 | 3.7 |
| LVEDV >51 μl and LVESV >32 μl | 47 | 10 | 21 | 6 | 82.5 | 77.8 | 3.7 | 4.4 |
| IS >36% or LVEDV >51 μl | 56 | 1 | 10 | 17 | 98.2 | 37.0 | 1.6 | 21.1 |
| IS >36% or LVESV >32 μl | 55 | 2 | 19 | 8 | 96.5 | 70.4 | 3.3 | 20.1 |
| LVEDV >51 or LVEDV >32 μl | 51 | 6 | 12 | 15 | 89.5 | 44.4 | 1.6 | 4.2 |
Predictive patterns are reported for single and multiple variables. Sensitivity, specificity, and likelihood values are calculated from a multiple logistic regression analysis. The best predictors are shown in italics. HR group at 2 days corresponds to 57 animals. eHR group represents animals that satisfy the 2-day cutoff condition. HR-eHR group includes animals that belong to the HR group at 2 days but do not satisfy the 2-day cutoff condition. LR group at 2 days corresponds to 27 animals. eLR group includes animals that belong to the LR group but do not satisfy the 2-day cutoff. LR-eLR group includes animals that satisfy the 2-day cutoff condition. +LH and −LH, positive and negative likelihood ratios.
DISCUSSION
Cardiac remodeling after experimentally induced MI in the mouse is recognized to be variable, related to the difficulty in achieving equivalent infarcts across a group of animals, and also dependent on the skill and training of the mouse surgeon. The ability to predict delayed remodeling from measurement of early stage functional/volumetric parameters would be extremely valuable for instance in the study of MI in animals with different phenotype. Without the “baseline” values and cutoff conditions reported in this work, the understanding of an improvement or worsening in adverse remodeling of, for example, transgenic mice model, would be very challenging and probably resulting in misleading outcomes.
In this work, we demonstrate that measurements of IS and LVESV quantified by LGE MRI in the acute phase after MI provide excellent prediction of significant late cardiac remodeling. This is consistent with the knowledge from clinical human studies that IS is a good predictor of delayed remodeling (6, 13), but measurement of IS in the mouse heart is of course much more challenging than in the human heart. The data are also consistent with prior studies (26) in other animals models showing a good correlation between IS and other functional/volumetric parameters.
In the longitudinal study reported herein, it was interesting to note that the animals studied 30 days after MI separated into two broad groups, where one of the groups showed significant LV dilatation. This group shows also lower EF and larger IS. This suggests that myocardial injury below a threshold level may induce only relatively minor reduction in global contractile function (down to an EF of ∼40%) and that this is not accompanied by substantial LV dilatation. A similar separation of groups could also be obtained with the use of IS instead of EF. Based on analysis of data at the 2-day time point in the group that showed significant remodeling vs. the one that did not, we identified potential predictors of remodeling.
Cutoff points at 2 days post-MI were derived from values of the HR group at early stage. Such cutoffs were used in a multiple logistic regression analysis to identify the best parameters to predict remodeling at 4 wk. An IS >36% as quantified by LGE MRI provided the best single predictor of positive remodeling. Such a choice was justified by high sensitivity, specificity, and likelihood values. Likelihood values give a measure of how well sensitivity and specificity work providing essential information in the selection of the best overall predictor. LVESV >32 μl was also a good predictor working almost as well as IS. Although single parameter predictors are easier to use than the more sophisticated combinations of multiple predictors, the combination of IS and/or LVESV achieved excellent predictive accuracy and might also be useful in studies where even higher accuracy of remodeling forecasting is required. The properties of the early IS measurement by LGE MRI (2 days post-MI) are also revealed in the excellent correlation between this measurement and the area of scar measured at 30 days. None of the other functional/volumetric parameters correlated this well. Note that a multiple logistic regression analysis is essential in providing specific cutoff values while the ROC curve is only able to indicate which parameter can be consider to be a good predictor.
There are some limitations to the current study. We only studied female C57Bl6 mice and so the precise thresholds might be different in male mice, different ages, or other strains. However, C57Bl6 is the most commonly studied strain in such studies. The use of intraperitoneal as opposed to gadolinium administration to estimate IS could introduce some variation although it achieves simplicity. We assumed that IS would be very similar or equivalent to area at risk since we used a permanent coronary ligation model. The occurrence of arrhythmia after infarction could complicate functional measurements, although this was not found to be a significant problem in the current study.
From a practical point of view, the MRI scans could be undertaken in ∼10 min using a single cine-FLASH MRI acquisition for each animal at each time point (2 and 30 days). For the 2-day scan, gadolinium was injected 20 min before LGE acquisition. Therefore, a single MRI scan at the 2-day time point could easily be used to categorize the animals into those that had a high likelihood of developing significant LV remodeling. This can be extremely advantageous in the study of mouse remodeling after MI. Echocardiography is a commonly used method in mouse MI studies and allows serial noninvasive imaging. Conventional mouse echocardiography systems are limited by the fact that the imaging is only two-dimensional so that volume quantification may be subject to error in the asymmetrically remodeling heart after MI. Although three-dimensional echocardiography could overcome this limitation, the major advantage of MRI for the purpose of predicting remodeling is the ability to estimate IS by LGE. Additional MRI techniques such as myocardial tagging or DENSE could also be implemented to obtain information on regional myocardial function. The use of MRI does not obviate the utility of invasive approaches to assessment of contractile function such as pressure-volume analysis, which indeed provide more information on contractile function, but these are terminal procedures and therefore unsuitable for serial studies.
In conclusion, the assessment of early IS and LVESV with the MRI cine-FLASH LGE technique provides a powerful way of predicting which mice are likely to develop significant late remodeling (ventricular dilatation). This method provides accurate data to establish precise cutoff conditions, such as an IS >36% or LVESV >32 μl, that can be used to significantly increase the efficiency of experiments designed to study delayed remodeling in mouse models.
GRANTS
This work was supported by the British Heart Foundation and a Fondation Leducq Transatlantic Network of Excellence Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.P. conception and design of research; A.P., X.D., and A.S. performed experiments; A.P. analyzed data; A.P., R.B., and A.M.S. interpreted results of experiments; A.P. prepared figures; A.P. drafted manuscript; A.P., R.B., and A.M.S. edited and revised manuscript; A.P., R.B., and A.M.S. approved final version of manuscript.
REFERENCES
- 1. Bohl S, Lygate CA, Barnes H, Medway DJ, Stork LA, Schulz-Menger J, Neubauer S, Schneider JE. Advanced methods for quantification of infarct size in mice using three-dimensional high-field late gadolinium enhancement MRI. Am J Physiol Heart Circ Physiol 296: H1200–H1208, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Celic V, Dekleva M, Majstorovic A, Radivojevic N, Kostic N, Caparevic Z. Myocardial performance index: prediction and monitoring of remodeling and functioning of the left ventricle after first myocardial infarction. Med Pregl 63: 652–655, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Chapon C, Herlihy AH, Bhakoo KK. Assessment of myocardial infarction in mice by late gadolinium enhancement MR imaging using an inversion recovery pulse sequence at 9.4T. J Cardiovasc Magn Reson 10: 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35: 569–582, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res 45: 330–338, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Ichikawa Y, Sakuma H, Suzawa N, Kitagawa K, Makino K, Hirano T, Takeda K. Late gadolinium-enhanced magnetic resonance imaging in acute and chronic myocardial infarction. Improved prediction of regional myocardial contraction in the chronic state by measuring thickness of nonenhanced. J Am Coll Cardiol 45: 901–909, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94: 3318–3326, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Kramer CM, Rogers WJ, Theobald TM, Power TP, Geskin G, Reichek N. Dissociation between changes in intramyocardial function and left ventricular volumes in the eight weeks after first anterior myocardial infarction. J Am Coll Cardiol 30: 1625–1632, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 113: 2733–2743, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Lavine SJ. Prediction of heart failure post myocardial infarction: comparison of ejection fraction, transmitral filling parameters, and the index of myocardial performance. Echocardiography 20: 691–701, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Manning WJ, Wei JY, Katz SE, Litwin SE, Douglas PS. In vivo assessment of LV mass in mice using high-frequency cardiac ultrasound: necropsy validation. Am J Physiol Heart Circ Physiol 266: H1672–H1675, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Masci PG, Dymarkowski S, Rademakers FE, Bogaert J. Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology 250: 50–60, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol Heart Circ Physiol 269: H2147–H2154, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Orn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol 99: 1109–1114, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Pegg TJ, Selvanayagam JB, Jennifer J, Francis JM, Karamitsos TD, Dall'Armellina E, Smith KL, Taggart DP, Neubauer S. Prediction of global left ventricular functional recovery in patients with heart failure undergoing surgical revascularisation, based on late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 12: 56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol Heart Circ Physiol 260: H1406–H1414, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979 [DOI] [PubMed] [Google Scholar]
- 19. Protti A, Sirker A, Shah AM, Botnar R. Late gadolinium enhancement of acute myocardial infarction in mice at 7T: cine-FLASH vs. inversion recovery. J Cardiovasc Magn Reson 32: 878–886, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 98: 1006–1014, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Schneider JE, Wiesmann F, Lygate CA, Neubauer S. How to perform an accurate assessment of cardiac function in mice using high-resolution magnetic resonance imaging. J Cardiovasc Magn Reson 8: 693–701, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101: 2981–2988, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102: 2104–2111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102: 2104–2111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Touboul P, Andre-Fouet X, Leizorovicz A, Itti R, Lopez M, Sayegh Y, Milon H, Kirkorian G. Risk stratification after myocardial infarction. A reappraisal in the era of thrombolysi.s The Groupe d'Etude du Pronostic de l'Infarctus du Myocarde (GREPI). Eur Heart J 18: 99–107, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Watzinger N, Lund GK, Higgins CB, Wendland MF, Weinmann HJ, Saeed M. The potential of contrast-enhanced magnetic resonance imaging for predicting left ventricular remodeling. J Magn Reson Imaging 16: 633–640, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100: 15883–15888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol 87: 547–555, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Yang Z, Berr SS, Gilson WD, Toufektsian MC, French BA. Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast-enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation 109: 1161–1167, 2004 [DOI] [PubMed] [Google Scholar]