Abstract
We reported previously that tempol attenuated the exercise pressor and muscle mechanoreceptor reflexes in rats whose femoral arteries were ligated, whereas tempol did not attenuate these reflexes in rats whose femoral arteries were freely perfused. Although the mechanism whereby tempol attenuated these reflexes in rats whose femoral artery was ligated was independent of its ability to scavenge reactive oxygen species, its nature remains unclear. An alternative explanation for the tempol-induced attenuation of these reflexes involves ATP-sensitive potassium channels (KATP) and calcium-activated potassium channels (BKCa), both of which are opened by tempol. We tested the likelihood of this explanation by measuring the effects of either glibenclamide (0.1 mg/kg), which blocks KATP channels, or iberiotoxin (20 or 40 μg/kg), which blocks BKCa channels, on the tempol-induced attenuation of the exercise pressor and muscle mechanoreceptor reflexes in decerebrated rats whose femoral arteries were ligated. We found that glibenclamide prevented the tempol-induced attenuation of both reflexes, whereas iberiotoxin did not. We also found that the amount of protein comprising the pore of the KATP channel in the dorsal root ganglia innervating hindlimbs whose femoral artery was ligated was significantly greater than that in the dorsal root ganglia innervating hindlimbs whose femoral arteries were freely perfused. In contrast, the amounts of protein comprising the BKCa channel in the dorsal root ganglia innervating the ligated and freely perfused hindlimbs were not different. We conclude that tempol attenuated both reflexes by opening KATP channels, an effect that hyperpolarized muscle afferents stimulated by static contraction or tendon stretch.
Keywords: static contraction, tendon stretch, thin fiber muscle afferents, peripheral artery disease, calcium-activated potassium channels, adenosine 5′-triphosphate
the exercise pressor reflex is evoked by both mechanical and metabolic stimuli arising in statically contracting muscle and results in increased arterial pressure, heart rate (HR), and ventilation (9, 23). The sensory arm of the reflex consists of thinly myelinated group III and unmyelinated group IV afferents (10, 23). Group III afferents primarily transmit information about mechanical stimuli arising in the exercising muscles, whereas the group IV afferents primarily transmit information about metabolic stimuli arising in these muscles (14, 18, 19).
The importance of the exercise pressor reflex in generating the cardiovascular adjustments to exercise has been controversial. Previously, the reflex was not thought to play a major role in the cardiovascular and ventilatory responses to low to moderate levels of exercise (11, 44) and was thought to be important only in situations where the exercising muscles were ischemic (27, 32). Although there is no doubt that the exercise pressor reflex plays an important role in generating the cardiovascular responses to exercise while the working muscles are ischemic, evidence has accumulated over the past 10–20 yr demonstrating conclusively that the reflex is also active at low levels of exercise when blood and oxygen supply to the working muscles match metabolic demand (1, 2, 3, 12, 15, 16, 25, 28, 43).
Recently, attention has shifted from the role played by the reflex in physiological conditions to the role played by the exercise pressor reflex in pathophysiological conditions, such as hypertension, heart failure, and peripheral artery disease (21, 36, 38, 42, 45, 46). Particular emphasis has been placed on the role played by oxidative stress in generating the exercise pressor reflex in animal models of these disease states. Recently, we (24) found that tempol, a widely used scavenger of superoxide radicals, attenuated the exaggerated exercise pressor reflex in a rodent model of peripheral artery disease in which one femoral artery of a rat was occluded 72 h before the start of the experiment. In contrast, we (24) found that tempol had no effect on the exercise pressor reflex in rats in which the femoral artery was freely perfused. We were surprised to find that the tempol-induced attenuation of the reflex in the rats with a ligated femoral artery could not be explained by its ability to scavenge superoxide radicals in contracting hindlimb muscles (24). In this regard, tempol has been shown to open both ATP-sensitive potassium channels (KATP; Ref. 7) and calcium-activated potassium channels (BKCa; Ref. 48), raising the possibility that this mechanism could be responsible for its attenuation of the exercise pressor reflex in rats with simulated peripheral artery disease. Specifically, we tested the hypotheses that tempol attenuated the exercise pressor reflex in decerebrated rats whose femoral artery was ligated for 72 h before the start of the experiment by opening either KATP or BKCa channels.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. Adult male rats (Sprague Dawley, n = 48, weighing between 345 and 500 g) were used in this study. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle. Rats were fed a standard diet and tap water ad libitum. Seventy-two hours before an experiment, the rats underwent surgery to ligate the left femoral artery according to the procedure described previously (42). Briefly, rats were anesthetized with a mixture of 4% isoflurane balanced with oxygen; the left femoral artery was isolated and then tightly ligated with 5–0 silk suture just distal to the inguinal ligament. The rats were allowed to recover 72 h before the experiments were started. Femoral artery occlusion has been reported to have no effect on normal cage activity (40). This technique has been shown to reduce blood flow capacity to ∼10–20% of normal while having little effect on resting blood flow (29, 49).
Surgical Preparation
On the day of the experiment, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The right jugular vein and common carotid artery were cannulated (PE-50) for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL; Statham). HR was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within normal range either by adjustment of ventilation or by intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp.
We cannulated (PE-10) the right femoral artery in a retrograde direction and advanced the tip to the bifurcation of the abdominal aorta. This allowed us to inject drugs into the arterial supply of the left hindlimb. A snare was placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. When tightened, the snare helped to keep the injectate within the circulation of the left hindlimb.
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brainstem edema. The left common carotid artery was tied off, and a precollicular decerebration was performed. The plane of section was 1 mm anterior to the superior colliculi. All neural tissue rostral to the section was removed. To minimize cerebral hemorrhage, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with cotton. In our experiments, rats were decerebrated instead of anesthetized because the preponderance of the evidence indicates that anesthesia prevents the exercise pressor reflex in this species (37).
A laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1–L5) was performed. The rat was then secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, we formed a pool that was filled with warm (37°C) mineral oil. The dura was cut and reflected allowing visual identification of the spinal roots. The left L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. The calcaneal bone of a left hindlimb was severed, and the triceps surae muscles were isolated. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air. After decerebration, we waited a minimum of 90 min before beginning any experimental protocol.
Experimental Protocols
The peripheral cut ends of the L4 and L5 ventral roots were placed on shielded stimulating electrodes. The left calcaneal tendon was attached to a force transducer (model FT 10, Grass), which in turn was attached to a rack-and-pinion. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, ∼2 times motor threshold) the L4 and L5 ventral roots. A muscle mechanoreceptor reflex was evoked by stretching the triceps surae muscles by manually turning the rack-and-pinion that was attached to the calcaneal tendon (39). Baseline tension was set between 80 and 100 g. Both muscle contraction and tendon stretch lasted for 60 s. The order of presentation of the two stimuli was varied randomly.
Protocol 1.
In six ligated rats, we injected tempol (10 mg in 0.2 ml) retrogradely into the right femoral artery catheter following the initial evaluation of the exercise pressor and muscle mechanoreceptor reflexes. In these rats, we tightened the snare placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation before injection of drugs to partially trap the injectate in the circulation of the left limb. The snare was maintained for 5 min, after which it was released and the hindlimb was reperfused for 15 min. The dose of tempol used in our experiments was identical to that used by McCord et al. (24), Koba et al. (21), and about twice that used by Wang et al. (46). Tempol injection decreased arterial pressure below baseline levels for ∼10–15 min. Consequently, we waited for arterial pressure to return to near baseline levels before evoking the exercise pressor and muscle mechanoreceptor reflexes.
Protocol 2.
In eight ligated rats, we blocked KATP channels by injecting glibenclamide (0.1 mg/kg in 0.2 ml), retrogradely into the right femoral artery catheter. We evaluated the exercise pressor and muscle mechanoreceptor reflexes before and after intra-arterial injection of glibenclamide. Next, we injected tempol (10 mg) as described in protocol 1 and again evaluated the exercise pressor and muscle mechanoreceptor reflexes. Our purpose was to determine if glibenclamide prevented the attenuation of the exercise pressor and muscle mechanoreceptor reflexes by tempol in the ligated rats.
Protocol 3.
In seven ligated rats, we blocked calcium-sensitive BKCa channels by injecting iberiotoxin (20 or 40 μg/kg in 0.2 to 0.4 ml), retrogradely into right femoral artery catheter (26). The dose of iberiotoxin used in our experiments was based on that used by other investigators in which they injected this antagonist intravenously in a dose of 100–150 μg/kg (4, 47). We evaluated the exercise pressor and muscle mechanoreceptor reflexes before and after iberiotoxin. Next, we injected tempol (10 mg) as described in protocol 1. Our purpose was to determine if iberiotoxin prevented the attenuation of the exercise pressor and muscle mechanoreceptor reflexes by tempol in the ligated rats. At the conclusion of the experiment, the rat was humanely killed with overdose of pentobarbital followed by an injection of saturated KCl solution.
Western Blot Analysis
In 27 rats, we ligated one femoral artery as described above. Seventy-two hours afterwards, the rats were anesthetized with a mixture of 4% isoflurane balanced with oxygen and the L4 and L5 dorsal root ganglia were removed from the ligated and freely perfused sides. All ganglia were stored at −80°C. We combined the L4 and L5 dorsal root ganglia innervating hindlimbs whose femoral arteries were freely perfused into nine samples, each of which contained material from three rats. Likewise, we combined the L4 and L5 dorsal root ganglia innervating hindlimbs whose femoral arteries were ligated into nine samples, each of which contained material from three rats. This allowed us to compare protein expression for KCa1.1, Kir6.1, and Kir6.2 in the dorsal root ganglia between freely perfused and ligated hindlimbs. The dorsal root ganglia were homogenized in 20 μl [(3-cholamidopropyl)-dimethylammonio]-l-propanesulfonate (CHAPS) homogenization buffer (40 mM HEPES pH 7.4, 120 mM NaCl, 1 mM EDTA, 10 mM Na pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, 1.5 mM Na3VO4, and 0.3% CHAPS) containing 1 mM DTT, and EDTA-free protease inhibitor and PhosSTOP phosphatase inhibitor cocktail tablets (Roche Diagnostics). Samples were incubated on ice for 10 min and centrifuged (3,000 rpm, 1 min), and supernatants were collected. The pellet was resuspended in CHAPS buffer, and the process was repeated. Supernatants were combined, and protein concentration was determined using the Microplate DC protein assay (Bio-Rad, Hercules, CA). Protein (25 μg) was analyzed by SDS-PAGE using precast 8–16% Tris·HCl Criterion gels (Bio-Rad) and transferred onto polyvinylidene difluoride membranes (GE Health Care, Piscataway, NJ) in a buffer containing 10 mM CHAPS pH 11.0 and 10% MeOH. Membranes were blocked for 1 h in buffer (5% nonfat milk, 20 mM Tris base, 140 mM NaCl, and 0.1% Tween 20); incubated overnight with anti-KCa1.1, anti- Kir6.1, or anti-Kir6.2 polyclonal antibodies (1:200; Alomone Labs, Jerusalem, Israel); and visualized with a goat anti-rabbit horseradish peroxidase (1:10,000; Cell Signaling, Danvers, MA). Membrane bound proteins were detected using Amersham's ECL Prime enhanced chemiluminescence (GE Health Care) and imaged with a FLuoroChem M imager (Cell Biosciences, Santa Clara, CA). The protein bands were then quantified using Alphaview software (Cell Biosciences). The membrane was also stripped and reprobed with a β-tubulin antibody (1:1,000; Cell Signaling). Bands were expressed as a ratio to tubulin.
Data Analysis
Arterial blood pressure, HR, and tension developed by the triceps surae muscles were recorded with a Spike 2 data acquisition system (CED, Cambridge) and stored on a computer hard drive (Dell). Mean arterial pressure is expressed in millimeters of mercury and HR in beats per minute. The tension-time index was calculated by integrating the area between the tension trace and the baseline level (spike 2) and is expressed in kilogram per seconds.
All values are expressed as means ± SE. Statistical analyses of arterial pressure, HR, and tension development were performed with either a one-way repeated-measures ANOVA or two-way repeated-measures ANOVA. Post hoc tests were performed with the Tukey's test between individual means. Bonferroni post hoc tests were used to determine significant differences between time course means. Statistical analyses of Western blots were performed with an unpaired t-test. The criterion for statistical significance was set at P < 0.05.
RESULTS
Protocol 1: Effect of Tempol on the Exercise Pressor and Muscle Mechanoreceptor Reflexes
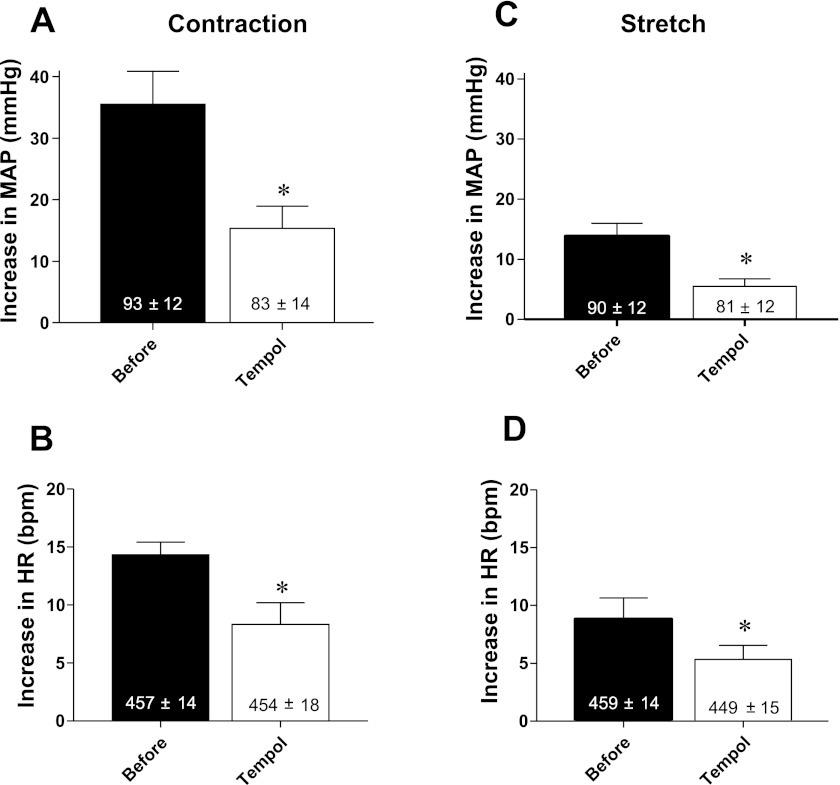
We first confirmed our previous findings that tempol attenuated both the exercise pressor and the muscle mechanoreceptor reflexes in six rats whose femoral artery had been ligated for 72 h before the start of the experiment (P <0.05; Figs. 1 and 2). The tension-time indexes evoking the two reflexes did not differ from each other before and after tempol for either static contraction (P = 0.70) or tendon stretch (P = 0.66; Table. 1).
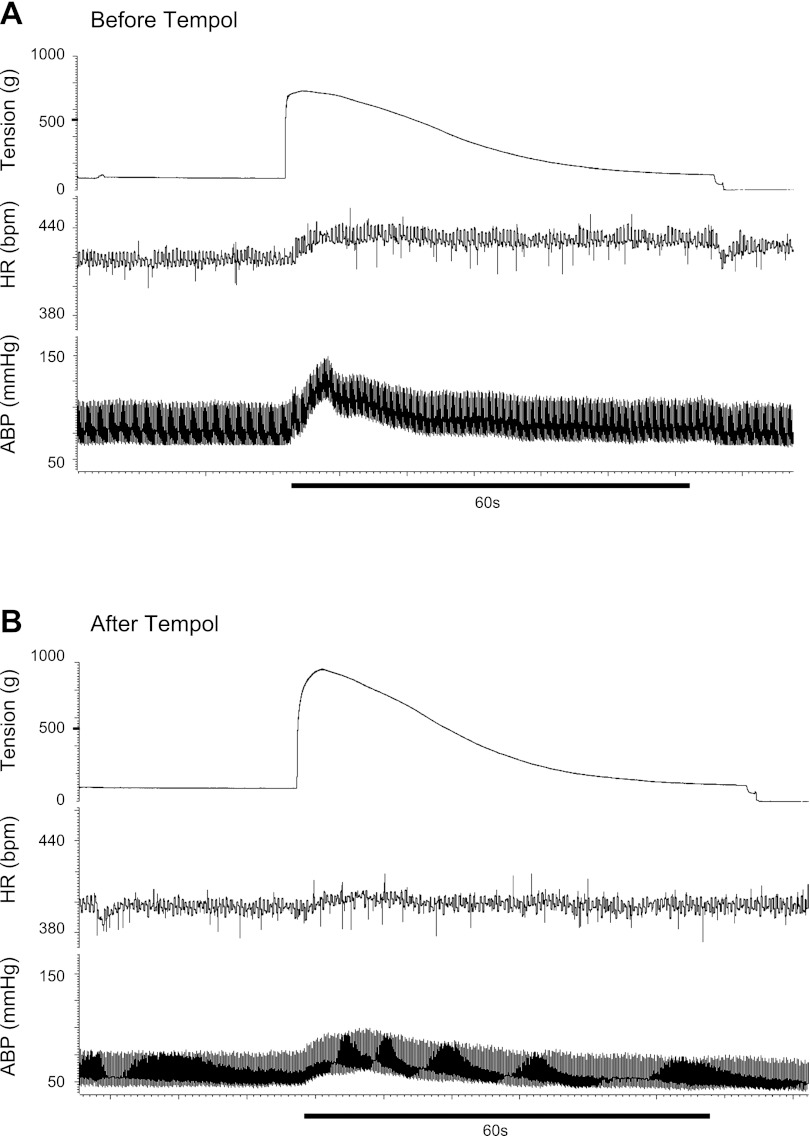
Fig. 1.
Pressor and cardioaccelerator responses to static contraction before (A) and after (B) retrograde injection of tempol (10 mg) into the right femoral artery of a rat whose left femoral artery was ligated 72 h before the start of the experiment. Note that the contraction-induced increases in arterial blood pressure (ABP) and heart rate (HR) were attenuated by tempol.
Fig. 2.
Pressor (A) and cardioaccelerator (B) responses to static contraction before (filled bars) and after (open bars) retrograde injection of tempol (10 mg) into the right femoral artery of rats whose left femoral arteries were ligated. Pressor (C) and cardioaccelerator (D) responses to tendon stretch before (filled bars) and after (open bars) intra-arterial injection of 10 mg of tempol in rats whose femoral arteries were ligated. Values inside bars represent baseline means ± SE. *P < 0.05, significant differences between means before and after tempol.
Table 1.
Tension time indexes
| TTI, kg/s |
||||
|---|---|---|---|---|
| n | Before | After | ||
| Tempol | Before | Tempol | ||
| Static contraction | 6 | 17.1 ± 2.4 | 16.7 ± 2.2 | |
| Tendon stretch | 6 | 26.5 ± 2.0 | 28.4 ± 2.4 | |
| Glibenclamide + tempol | Before | Glibenclamide | Tempol | |
| Static contraction | 8 | 14.5 ± 1.4 | 18.8 ± 1.9* | 16.3 ± 2.1 |
| Tendon stretch | 8 | 24.5 ± 2.2 | 25.6 ± 2.3 | 25.9 ± 2.7 |
| Iberiotoxin + tempol | Before | Iberiotoxin | Tempol | |
| Static contraction | 7 | 11.9 ± 1.5 | 13.2 ± 1.5 | 13.0 ± 1.6 |
| Tendon stretch | 7 | 21.4 ± 3.0 | 22.4 ± 3.2 | 22.3 ± 3.0 |
Values are means ± SE. Tension time indexes (TTI) for static contraction and tendon stretch before and after the injection of tempol (10 mg), glibenclamide (0.1 mg/kg), and iberiotoxin (20–40 μg/kg). *P < 0.05, significant difference between Before and Glibenclamide conditions.
Protocol 2: Effect of Glibenclamide on the Tempol-Induced Attenuation of the Exercise Pressor and Muscle Mechanoreceptor Reflexes
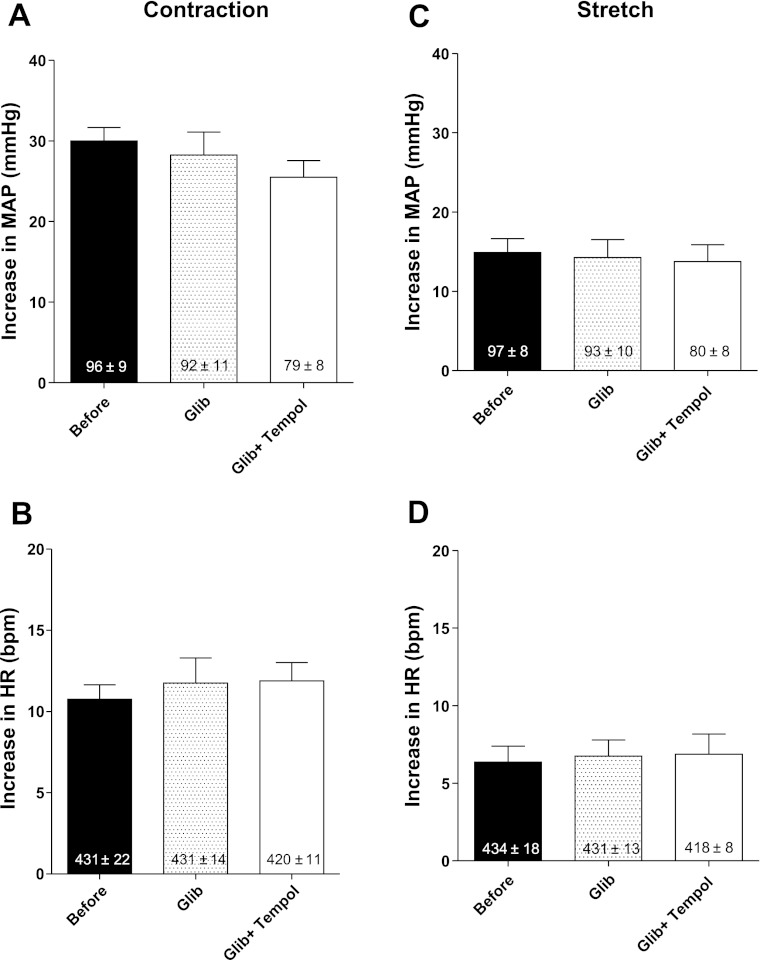
In eight rats whose left femoral artery had been ligated 72 h before the start of the experiment, the mean pressor and cardioaccelerator responses to static contraction were not attenuated by retrograde injection of glibenclamide (0.1 mg/kg) into the right femoral artery (Fig. 3, A and B). Subsequent injection of tempol, in contrast to the findings reported in protocol 1, failed to attenuate the reflex. For example, the pressor and cardioaccelerator responses (26 ± 2 mmHg and 12 ± 1 beats/min) to static contraction after tempol and in the presence of glibenclamide were not significantly different from the responses (28 ± 3 mmHg and 12 ± 2 beats/min; both P > 0.05) to static contraction before tempol (Fig. 3, A and B). Likewise, the pressor and cardioaccelerator responses to tendon stretch after tempol in the presence of glibenclamide (0.1 mg/kg) were not significantly different from those to stretch before tempol (Fig. 3, C and D).
Fig. 3.
Pressor (A) and cardioaccelerator (B) responses to static contraction before, immediately after retrograde injection of glibenclamide (0.1 mg/kg) into the right femoral artery (Glib) and after subsequent retrograde injection of tempol (10 mg; Glib + Tempol) in rats whose left femoral arteries were ligated. Pressor (C) and cardioaccelerator (D) responses to tendon stretch, before, immediately after retrograde injection of glibenclamide (0.1 mg/kg), and after subsequent retrograde injection of tempol (10 mg) in rats whose femoral arteries were ligated. Values inside bars represent baseline means ± SE.
Protocol 3: Effect of Iberiotoxin on the Tempol-Induced Attenuation of the Exercise Pressor and Muscle Mechanoreceptor Reflexes
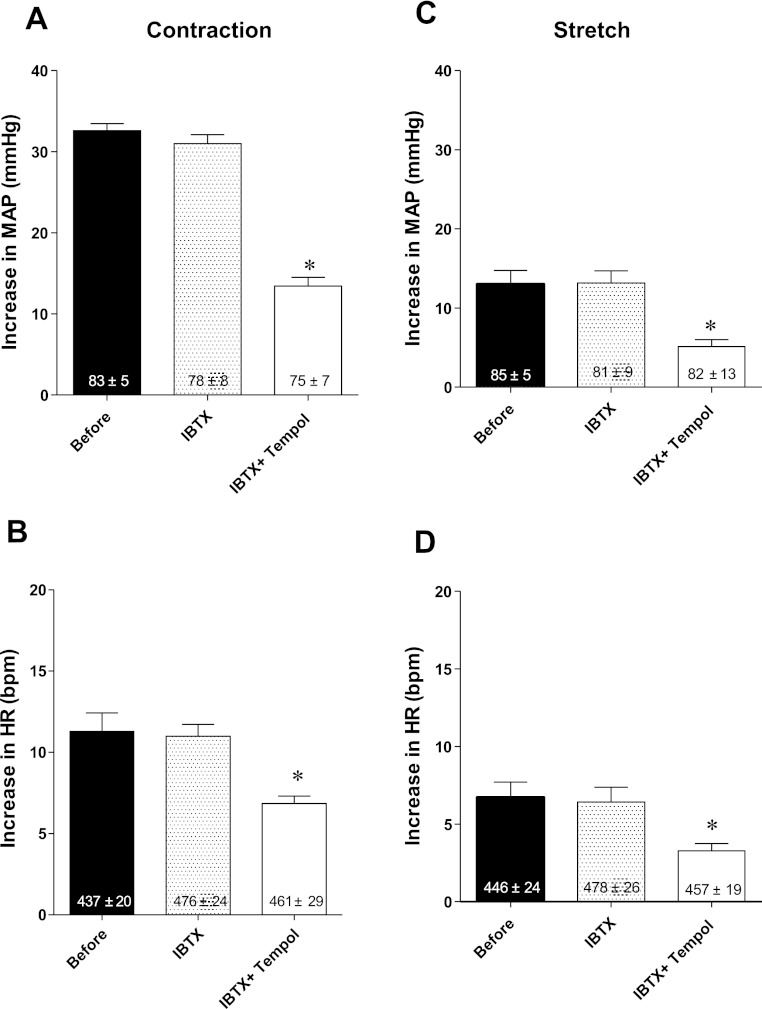
We determined the effects of iberiotoxin, injected retrogradely into the right femoral artery, on the exercise pressor and muscle mechanoreceptor reflexes before and after tempol in seven “ligated” rats. In five of the rats, we injected iberiotoxin in a dose of 20 μg/kg, and in the remaining two, we injected iberiotoxin in a dose of 40 μg/kg. Neither dose of iberiotoxin, injected before tempol, had any effect on the two reflexes (Fig. 4). Subsequent injection of tempol (10 mg), however, still significantly decreased both reflexes in each of the seven rats tested (P < 0. 05; Fig. 4).
Fig. 4.
Pressor (A) and cardioaccelerator (B) responses to static contraction before, immediately after retrograde injection of iberiotoxin (IBTX; 20–40 μg/kg) into the right femoral artery, and after subsequent retrograde injection of tempol (10 mg; IBTX + Tempol) in rats whose left femoral arteries were ligated. Pressor (C) and cardioaccelerator (D) responses to tendon stretch before, immediately after retrograde injection of IBTX (20–40 μg/kg) into the right femoral artery and after subsequent retrograde injection of tempol (10 mg; IBTX + Tempol) in rats whose left femoral arteries were ligated. Values inside bars represent baseline means ± SE. *P < 0.05, significant difference between means for IBTX + Tempol and either before or after IBTX.
Western Blots
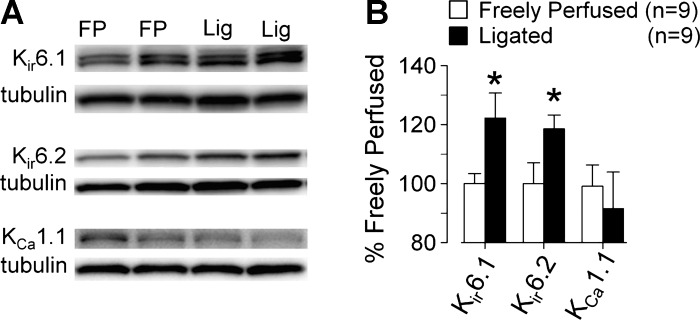
We found that the amounts of the Kir6.1 and Kir6.2 protein in the L4 and L5 dorsal root ganglia innervating the hindlimbs whose femoral arteries were ligated for 72 h were significantly but modestly greater (P < 0.05) than the amounts of Kir6.1 and Kir6.2 protein in the dorsal root ganglia innervating the hindlimbs whose femoral arteries were freely perfused (Fig. 5). In contrast, the amount of KCa1.1 protein in the L4 and L5 dorsal root ganglia innervating the hindlimbs whose femoral arteries were ligated was not significantly different than the amount of KCa1.1 protein in the L4 and L5 dorsal root ganglia innervating the hindlimbs whose femoral arteries were freely perfused. In addition, the amount of KCa1.1 protein in the dorsal root ganglia regardless of whether the femoral arteries were ligated or not appeared to be much less than the amounts of Kir6.1 and Kir6.2 protein (Fig. 5).
Fig. 5.
Individual Western blots (A) and summary data (B) showing that ligation of the left femoral artery 72 h before removing the left L4 and L5 dorsal root ganglia increased the pore-forming proteins (Kir6.1 and Kir6.2) of the KATP channel, but had no effect on the proteins comprising the BKCa channel (KCa1.1). *Significant difference between dorsal root ganglia taken from rats whose left femoral arteries were freely perfused (FP) and dorsal root ganglia taken from rats whose left femoral arteries were ligated (Lig).
DISCUSSION
Our interest in the actions of tempol on the exercise pressor reflex stemmed from our previous findings that this supposed antioxidant had no effect on the reflex in healthy rats whose femoral arteries were freely perfused but attenuated the reflex in rats whose femoral arteries were ligated (24). We further found that tiron, an antioxidant that does not open KATP channels (48) (47), did not attenuate the exercise pressor reflex in either group of rats (24). These findings prompted us to measure the interstitial concentrations of 8-isoprostaglandin F2α, an index of oxidative stress (17), before and during static contraction of the triceps surae muscles in rats with ligated femoral arteries as well as in rats with freely perfused femoral arteries. Although the exercise pressor reflex in rats with ligated femoral arteries was significantly greater than it was in rats with freely perfused femoral arteries, the interstitial concentrations of 8-isoprostaglandin F2α, increased equally (24). When considered together, these findings suggested that tempol attenuated the exaggerated exercise pressor reflex in rats with a ligated femoral artery by some action other than scavenging superoxide radicals. The most likely explanation for the effect of tempol in our experiments was its well-documented ability to open KATP (7) and BKCa channels (48).
To test this possible explanation, we first replicated our previous finding (24) that tempol attenuated the exercise pressor reflex in rats whose femoral artery was ligated for 72 h. We then proceeded to show that this attenuation was prevented by glibenclamide-induced blockade of KATP channels but was not prevented by iberiotoxin-induced blockade of BKCa channels. Last, we showed that Kir6.1 and Kir6.2 protein levels in the L4 and L5 dorsal root ganglia innervating the hindlimbs of rats whose femoral arteries were ligated for 72 h were ∼20% greater than those in the L4 and L5 dorsal root ganglia innervating the hindlimbs of rats whose femoral arteries were freely perfused.
The significance of the last finding is that the Kir6.1 and Kir6.2 proteins comprise the pore of the KATP channel (13), which when blocked by glibenclamide in our experiments prevented the tempol-induced attenuation of the exercise pressor reflex in rats whose femoral arteries were ligated. The ligation-induced increase in the pore-forming proteins of the KATP channel in dorsal root ganglia cells allows us to speculate as to why tempol attenuated the reflex in “ligated rats” but did not attenuate the reflex in the “freely perfused rats.” Specifically, we speculate that femoral arterial ligation increased the number of KATP channels in the group III and IV afferents innervating the contracting hindlimb muscles. This increase in KATP channels exceeded some threshold, which when opened by tempol hyperpolarized the afferents, rendering them less susceptible to stimulation by static contraction. In contrast, in the “freely perfused” rats, the number of opened channels was below the threshold needed to decrease the sensitivity of the afferents to contraction.
In contrast to the levels of the proteins comprising the KATP channel, we found that femoral artery ligation had no effect on the levels of the protein comprising the BKCa channel in the L4 and L5 dorsal root ganglia. This finding is consistent with our finding that iberiotoxin, a specific antagonist to BKCa channels (26), had no effect on the exercise pressor reflex in the “ligated rats.” BKCa channels, therefore, appear to play little or no role in the tempol-induced attenuation of the exercise pressor reflex in rats whose femoral arteries were ligated 72 h before the start of the experiment.
Previous studies have indicated that agents that open KATP and BKCa channels hyperpolarize excitable membranes, an effect that will inhibit sensory neurons (34) and will relax vascular smooth muscle (30, 48). Tempol, which opens both KATP and BKCa channels (7, 48), applied topically to the renal sympathetic nerve inhibited its spontaneous discharge in anesthetized rats (35). Likewise, cromokalim, which opens KATP channels, relaxed vascular smooth muscle (33). Opening either or both of these potassium channels can function to decrease arterial blood pressure in intact preparations and, as a result, has led to investigations of tempol as an antihypertensive agent (7).
Both KATP and BKCa channels are believed to play important roles in the generation of neuropathic pain. Specifically, nerve injury has been found to decrease KATP and BKCa channels in dorsal root ganglion cells, effects that in turn are believed to be at least in part responsible for the increased excitability of nociceptors and, in turn, result in either allodynia or hyperalgesia (6, 8). The possibility that a decrease in KATP and BKCa channels in group III and IV hindlimb muscle afferents is responsible for the exaggerated exercise pressor reflex seen in rats with a ligated femoral artery (42) seems to us to be unlikely for two reasons. First, femoral artery ligation for 72 h in our experiments did not significantly decrease the amount BKCa protein in dorsal root ganglion cells. Second, ligation increased the amount of protein comprising part of the KATP channel. The failure of ligation to decrease the two potassium channels is not too surprising because the procedure has been shown to provide adequate blood flow to the hindlimb of cage-restrained rats, making the likelihood of tissue injury low (40, 50).
In the dose used, glibenclamide, given alone, had no effect on the exercise pressor and muscle mechanoreceptor reflexes in our experiments. Our findings suggest that KATP channels on group III and IV muscle afferents do not play a role in evoking these reflexes in rats with ligated femoral arteries. Although we cannot exclude the possibility that a higher dose of glibenclamide might have increased their magnitudes, the dose used in our experiments was sufficient to prevent the attenuation of the two reflexes by tempol, a potent KATP channel opener (48). KATP channels on vascular smooth muscle, in contrast to those on group III and IV afferents, appear to have an important function during exercise. Specifically, KATP channels on vascular smooth muscle are responsible, at least in part, for sympatholysis in both rats (41) and humans (20). We note with interest that KATP channels on vascular smooth muscle can be opened by metabolic by-products of contraction, such as adenosine (22) and prostaglandins (5), of which only the latter are capable of stimulating group III and IV afferents (31). Why these metabolic by-products appear to function on KATP channels on vascular smooth muscle cells but do not appear to function on KATP channels on thin fiber muscle afferents is unclear. Perhaps the effect of opening these channels on the group III and IV afferents is overwhelmed by the effect of prostaglandins opening endoperoxide receptors. Alternatively, vascular smooth muscle cells may have a greater number of KATP channels than do the endings of thin fiber muscle afferents. Last, there might be a difference between KATP channels on vascular smooth muscle cells and those on sensory nerve endings in their sensitivities to the various metabolic by-products of contraction.
In summary, we have shown that tempol attenuates the exercise pressor reflex in rats with ligated femoral arteries by opening KATP channels. This mechanism appears to be far more likely than does the scavenging of superoxide radicals as an explanation as to why tempol attenuates the reflex in with simulated peripheral artery disease (24, 46). Perhaps the most important message to be taken from our findings is that effects of tempol should not be attributed to its antioxidant action unless the other potent “side effects” of this compound are excluded. The most important of these “side effects” appear to be the fact that tempol opens ATP-sensitive and calcium-sensitive potassium channels, effects that can often be confused with an antioxidant action.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-096570.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.Y. and M.P.K. conception and design of research; K.Y. and S.D.S. performed experiments; K.Y. and A.J.S. analyzed data; K.Y. and S.D.S. interpreted results of experiments; K.Y., A.J.S., and S.D.S. prepared figures; K.Y. and M.P.K. drafted manuscript; K.Y., A.J.S., S.D.S., and M.P.K. edited and revised manuscript; K.Y., A.J.S., S.D.S., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hirotsugu Tsuchimochi for advice as well as Joyce Kim and Shane Miller for technical assistance.
REFERENCES
- 1. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg T, Koteng O. Signalling pathways in bradykinin- and nitric oxide-induced hypotension in the normotensive rat; role of K+-channels. Br J Pharmacol 121: 1113–1120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouchard JF, Dumont E, Lamontagne D. Evidence that prostaglandins I2, E2, and D2 may activate ATP sensitive potassium channels in the isolated rat heart. Cardiovasc Res 28: 901–905, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Chen SR, Cai YQ, Pan HL. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J Neurochem 110: 352–362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 293: H3246–H3253, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chi XX, Jiang X, Nicol GD. ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons. Brain Res 1145: 28–40, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol 208: 261–278, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol 59: 313–337, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anesthesia in man. J Physiol 420: 281–293, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev 90: 799–829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 587: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, FitzGerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study. III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic Biol Med 38: 711–718, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol 561: 273–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J Physiol 587: 5227–5237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol 472: 1–9, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCord JL, Tsuchimochi H, Yamauchi K, Leal AK, Kaufman MP. Tempol attenuates the exercise pressor reflex independently of neutralizing reactive oxygen species in femoral arterial ligated rats. J Appl Physiol 111: 971–979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 27. O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Pickar JG, Hill JM, Kaufman MP. Dynamic exercise stimulates group III muscle afferents. J Neurophysiol 71: 753–760, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Quast U, Guillon JM, Cavero I. Cellular pharmacology of potassium channel openers in vascular smooth muscle. Cardiovasc Res 28: 805–810, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Rowell L, O'Leary D. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 270: H1649–H1654, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Sarantopoulos C, McCallum B, Sapunar D, Kwok WM, Hogan Q. ATP-sensitive potassium channels in rat primary afferent neurons: the effect of neuropathic injury and gabapentin. Neurosci Lett 343: 185–189, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 41: 266–273, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 40. Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest 99: 2602–2609, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Handbook of Physiology Section. Exercise: Regulation and Integration of Multiple Systems. edited by Rowell LB, Shepherd JT. Bethesda, MD: Am Physiol Soc; 1996, sect 12, p. 333–380 [Google Scholar]
- 45. Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107: 450–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu H, Bian X, Watts SW, Hlavacova A. Activation of vascular BK channel by tempol in DOCA-salt hypertensive rats. Hypertension 46: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Jackson WF, Fink GD, Galligan JJ. Activation of potassium channels by tempol in arterial smooth muscle cells from normotensive and deoxycorticosterone acetate-salt hypertensive rats. Hypertension 48: 1080–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Yang HT, Terjung RL. Angiotensin-converting enzyme inhibition increases collateral-dependent muscle blood flow. J Appl Physiol 75: 452–457, 1993 [DOI] [PubMed] [Google Scholar]