Abstract
Despite standard drug therapy, sympathetic nerve activity (SNA) remains high in heart failure (HF) patients making the sympathetic nervous system a primary drug target in the treatment of HF. Studies in rabbits with pacing-induced HF have demonstrated that statins reduce resting SNA, in part, due to reductions in reactive oxygen species (ROS). Whether these findings can be extended to the clinical setting of human HF remains unclear. We first performed a study in seven statin-naïve HF patients (56 ± 2 yr; ejection fraction: 31 ± 4%) to determine if 1 mo of simvastatin (40 mg/day) reduces muscle SNA (MSNA). Next, to control for possible placebo effects and determine the effect of simvastatin on ROS, a double-blinded, placebo-controlled crossover design study was performed in six additional HF patients (51 ± 3 yr; ejection fraction: 22 ± 4%), and MSNA, ROS, and superoxide were measured. We tested the hypothesis that statin therapy decreases resting MSNA in HF patients and this would be associated with reductions in ROS. In study 1, simvastatin reduced resting MSNA (75 ± 5 baseline vs. 65 ± 5 statin bursts/100 heartbeats; P < 0.05). Likewise, in study 2, simvastatin also decreased resting MSNA (59 ± 5 placebo vs. 45 ± 6 statin bursts/100 heartbeats; P < 0.05). In addition, statin therapy significantly reduced total ROS and superoxide. As expected, cholesterol was reduced after simvastatin. Collectively, these findings indicate that short-term statin therapy concomitantly reduces resting MSNA and total ROS and superoxide in HF patients. Thus, in addition to lowering cholesterol, statins may also be beneficial in reducing sympathetic overactivity and oxidative stress in HF patients.
Keywords: reactive oxygen species, superoxide, norepinephrine, simvastatin
chronic sympathetic overactivity is well characterized in heart failure (HF) patients (2, 24). The elevated sympathetic activity further worsens the HF status being associated with poor prognosis. Indeed, a recent study (2) showed that increased muscle sympathetic nerve activity (MSNA) was a significant independent predictor of 1-yr cardiac mortality in HF patients. However, despite standard drug therapy, sympathetic nerve activity remains high in HF patients making the sympathetic nervous system a primary drug target in the treatment of HF (16, 24). As such, newer drug strategies are needed to reduce the excessive sympathetic overactivity and potentially improve the clinical prognosis for HF patients (17).
Multiple clinical trials have shown that statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, reduce cardiovascular events and improve survival in HF patients irrespective of cholesterol-lowering effects (19, 30, 38). Although more recent trials have challenged these findings (22, 40), it is interesting to note that previous trials used primarily lipophilic statins (e.g., simvastatin), whereas recent trials used a hydrophilic statin (i.e., rosuvastatin). This is an important distinction since the latter would not cross the blood brain barrier and thus obviate any potential central effects. Indeed, although the underlying mechanism(s) for the potential protective effect of statins in HF remain unclear, recent studies in rabbits with pacing-induced HF have demonstrated that statin therapy reduces central sympathetic outflow. This statin-induced decrease in sympathetic activity appeared to be due, in part, to a reduction in superoxide in the rostral ventrolateral medulla of the brainstem, the primary center for the central control of sympathetic nerve activity (9, 46). To our knowledge, no studies have examined the effect of statin therapy on oxidative stress in human HF. In addition, only two recent studies have investigated the effect of statin therapy on resting MSNA in HF patients. In the first study (14), although a reduction in MSNA was reported, all subjects were already prescribed statin treatment and upon entering the study were asked to discontinue taking their statin without any placebo control. Thus this may have introduced an experimental bias as the patients clearly knew when they were on or off the drug. In the second study (20), no statistically significant effect of statins on resting MSNA was reported even though a 16% reduction in MSNA was observed following statin treatment. Clearly, additional studies are needed to better understand the potential beneficial effect of statins to reduce MSNA in HF patients. Likewise, no attempt has been made in HF patients to understand the potential mechanisms by which statins may reduce MSNA. As noted above, studies (9) in rabbits with pacing-induced HF have demonstrated that statins reduce resting SNA, in part, due to reductions in reactive oxygen species (ROS). However, no studies have been performed to examine whether statins reduce ROS in patients with HF.
Thus the purpose of the present study was to rigorously test the influence of statin therapy on MSNA in HF patients. In addition, to begin to understand the potential underlying mechanisms, markers of total ROS and superoxide were measured. Protocols were performed in two separate groups of statin-naïve HF patients. First, we performed a proof of concept study to determine if 1 mo of statin therapy reduces MSNA, as originally reported in animals with experimental HF (9, 32). Next, a double blinded, placebo-controlled crossover design study was performed and MSNA, plasma norepinephrine, and total ROS and superoxide were measured. We tested the hypothesis that statin therapy decreases resting MSNA in HF patients and this would be associated with a reduction in ROS.
METHODS
A total of twenty New York Heart Association (NYHA) class I-III HF patients with idiopathic dilated cardiomyopathy were initially recruited into this study. However, primarily due to medical problems leading to changes in medications during the study, some patients were not able to continue. Thus 13 patients successfully completed the protocols. Inclusion criteria included left ventricular ejection fraction <40% assessed via echocardiography before study enrollment. Exclusion criteria were use of statins, smoking, frequent cardiac arrhythmias that would interfere with MSNA signals, low blood pressure (BP; <100/60 mmHg), end-stage renal disease, chronic obstructive pulmonary disease, and limb neuropathy. The physical and clinical characteristics of the HF patients are provided in Table 1. All patient medications were stable for ≥2 mo before commencing the study and apart from the addition of simvastatin were unchanged for the duration of the study. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri-Columbia Health Sciences Institutional Review Board. After receiving a detailed verbal and written explanation of the experimental procedures and measurements, each subject provided written informed consent. All study visits were conducted at ∼8:00 AM after an overnight fast with the patients withholding medications on the morning of the experiments.
Table 1.
Patient characteristics
| Study 1 | Study 2 | |
|---|---|---|
| Male/female | 6/1 | 5/1 |
| Age, yr | 56 ± 2 | 51 ± 3 |
| Weight, kg | 90 ± 7 | 91 ± 7 |
| Height, cm | 173 ± 4 | 173 ± 3 |
| BMI, kg/m2 | 31 ± 3 | 30 ± 2 |
| EF, % | 31 ± 4 | 22 ± 4 |
| Medications | ||
| ACE inhibitor | 7 | 6 |
| β-Blocker | 7 | 6 |
| Diuretic | 3 | 6 |
| Digoxin | 2 | 3 |
| Warfarin | 1 | 1 |
Values are means ± SE. BMI, body mass index; EF, ejection fraction; ACE, angiotensin-converting enzyme.
We first performed a proof of concept study in which seven HF patients were studied before and after taking simvastatin (40 mg per day for 4 wk; study 1). This initial study was performed without placebo due to the difficulties of recruiting HF patients who met our inclusion-exclusion criteria and were willing to commit to 2 mo for a study, which is required if a placebo arm is included. However, given the positive results of our initial cohort, the next step needed was to comprise a placebo arm to eliminate concerns of a placebo effect from the initial study results. Thus, to control for a possible placebo effect and also determine the effect of simvastatin on oxidative stress, an additional six HF patients completed a double blind placebo-controlled crossover study (study 2).
Experimental Measurements
Patients were studied in the supine position in a quiet temperature-controlled room (23- 24°C). Heart rate (HR) was monitored using a lead II electrocardiogram (ECG; model Q710; Quinton Instrument, Bothell, WA). Arterial BP was measured by auscultation of the brachial artery of the right arm using an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA). Multiunit recordings of postganglionic MSNA were obtained by inserting unipolar tungsten microelectrodes percutaneously through the intact, unanaesthetized skin and positioned into muscle nerve fascicles of the peroneal nerve near the fibular head. The nerve signal was processed by a preamplifier and an amplifier (Dept. of Bioengineering, University of Iowa, Iowa City, IA), band pass filtered (bandwidth 700–2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. MSNA recordings were identified by their characteristic pulse-synchronous burst pattern and increased neural activity in response to an end-expiratory apnea or Valsalva maneuver, without any response to arousal stimuli or stroking of the skin. MSNA was quantified as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Blood samples were taken and sent to a commercial laboratory for analysis of lipids, liver enzymes, creatine phosphokinase (CPK), and norepinephrine (Boyce and Bynum Pathology Labs, Columbia, MO). Total cholesterol, triglycerides, high-density lipoproteins, and low-density lipoproteins (LDL) were measured using radioimmunoassay, while plasma norepinephrine was measured using high performance liquid chromatography.
Experimental Protocols
Study 1.
During each visit, a blood sample was taken for cholesterol, electrolytes, CPK, and liver enzymes. Patients were then instrumented for the measurement of HR, arterial BP, and respiration after which microneurography was performed to obtain MSNA. After a satisfactory MSNA recording was obtained, there was a minimum of 10 min of quiet rest before 20 min of continuous data collection.
Study 2.
Study 2 was performed to control for any placebo effect and also, attempt to provide further insight into the actions of statins by making additional measures including oxidative stress and plasma norepinephrine. A double-blinded placebo-controlled crossover design study was performed with the randomization and medication distribution controlled by the University of Missouri-Columbia Health Sciences pharmacy. For month 1, patients were supplied with 4 wk of blinded simvastatin (40 mg per day) or 4 wk of blinded placebo capsules and then the opposite treatment was provided for month 2. The placebo consisted of encapsulated lactulose, and the pharmacy also encapsulated the simvastatin so that the pills were identical and patients could not identify the statin. Capsules were dispensed per prescription, and the identity of capsules dispensed was determined by the randomization table. The website http://www.randomizer.org/ was used to generate a list of numbers 1–2 for the randomization process.
Upon arrival to the laboratory, an intravenous catheter was placed in the antecubital vein for blood sampling. After a minimum of 10 min, blood samples were drawn for cholesterol, electrolytes, CPK, and liver enzymes as well as measures of ROS and superoxide, as described in detail below. Subsequently, patients were instrumented for the measurement of HR, arterial BP, and respiration after which microneurography was performed to obtain MSNA. Once a satisfactory MSNA recording was obtained, there was a minimum of 10 min of quiet rest, after which 20 min of continuous data were collected. A blood sample for plasma norepinephrine was then drawn. Measurements were performed during three visits: visit 1 (familiarization) and visits 2 and 3 (placebo or simvastatin).
At each visit, blood samples were obtained from the antecubital vein for the determination of total ROS and superoxide using the electron paramagnetic spin resonance (EPR) technique (34, 41). Samples contained 3.5 mM of deferoxamine methanesulfonate salt (Noxygen Science Transfer & Diagnostics, Elzach, Germany) and 9.08 mM of diethyldithiocarbamic acid sodium (Noxygen Science Transfer & Diagnostics). Initially, samples assigned for the measurements of total ROS and superoxide were incubated with Krebs-HEPES buffer solution and SOD (1,000 U/ml; Sigma Aldrich, St. Louis, MO), respectively, at 37°C for 15 min. Subsequently, both samples were incubated with methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (Noxygen Science Transfer & Diagnostics) spin probe at 37°C for 15 min. After complete mixing, 50 μl of each sample were loaded into a 1-cc syringe and flash frozen between buffer solutions to form a continuous frozen plug using liquid nitrogen. Samples were then stored at −80°C and shipped to the University of Nebraska Medical Center for analyses. Total ROS was measured directly using the methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine probe, while superoxide was calculated indirectly by subtracting SOD treated samples from total ROS.
Data analysis.
ECG and MSNA signals were sampled at 1,000 Hz and stored for off-line analysis (Chart v5.2 and Powerlab; ADInstruments, Bella Vista, NSW, Australia). HR, arterial BP, and MSNA were calculated as mean values over a 5-min steady-state period. MSNA was identified by two experienced microneurographers blinded to the treatment phase of the study. Sympathetic activity was quantified using standard measures; including burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats). The latter was used for our main comparison because of potential effects of statins on HR and the inherent cardiac synchronicity of MSNA (5, 6, 43).
Statistical analysis.
Adequate sample size was calculated for within group comparisons (e.g., placebo vs. simvastatin) for MSNA burst incidence by applying a desired power of 0.80 and an α-error of 5%. Using the effect size of 1.21 to 1.27, the minimum sample size was determined to be four subjects in each group. Statistical comparisons of physiological variables between baseline and poststatin therapy for study 1 as well as placebo and statin therapy in study 2 were conducted using paired Student's t-test. Statistical significance was set at P < 0.05, and analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL) for Windows. Results are presented as means ± SE.
RESULTS
Subject Characteristics
Table 1 presents general subject characteristics and existing medications for all HF patients. Patients in both studies were of similar age and had comparable body mass indexes with ejection fractions slightly lower in the study 2 cohort. At entry into the study, all patients were receiving standard treatment for HF primarily including angiotensin-converting enzyme inhibitors and β-blockers.
MSNA
Study 1.
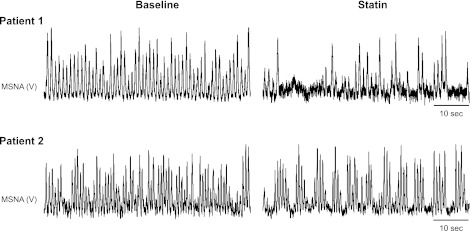
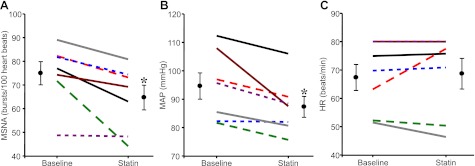
Treatment with simvastatin significantly reduced MSNA burst incidence. The decrease in MSNA was variable amongst patients (range of −1 to −28 bursts/100 heartbeats), as can be appreciated from the original records provided in Fig. 1. However, the individual data in Fig. 2 demonstrate the consistency of these results as MSNA was reduced in all patients. MSNA burst frequency was also lower after statin therapy (50 ± 4 baseline to 44 ± 5 statin bursts/min, P < 0.05).
Fig. 1.
Segments of original records showing resting muscle sympathetic nerve activity (MSNA) before (baseline) and after 1 mo of simvastatin (40 mg per day) in 2 heart failure (HF) patients. Patient 1 exhibited a very robust reduction in MSNA following simvastatin, whereas patient 2 exhibited a much more modest decrease in MSNA.
Fig. 2.
Individual and mean data showing changes in MSNA (bursts/100 heartbeats: A), mean arterial pressure (MAP; mmHg; B), and heart rate (HR; beats/min: C) in 7 HF patients before and after 1 mo of simvastatin (40 mg per day). Values are means ± SE. *P < 0.05 vs. baseline.
Study 2.
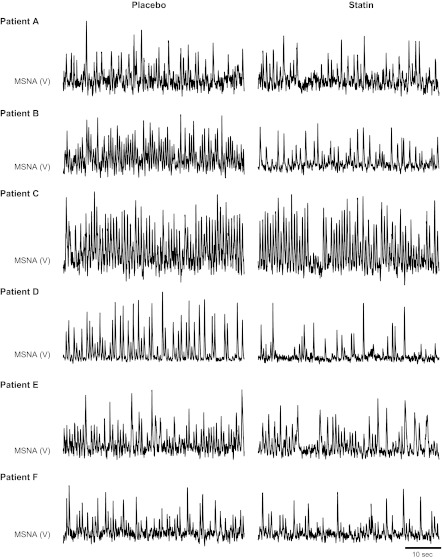
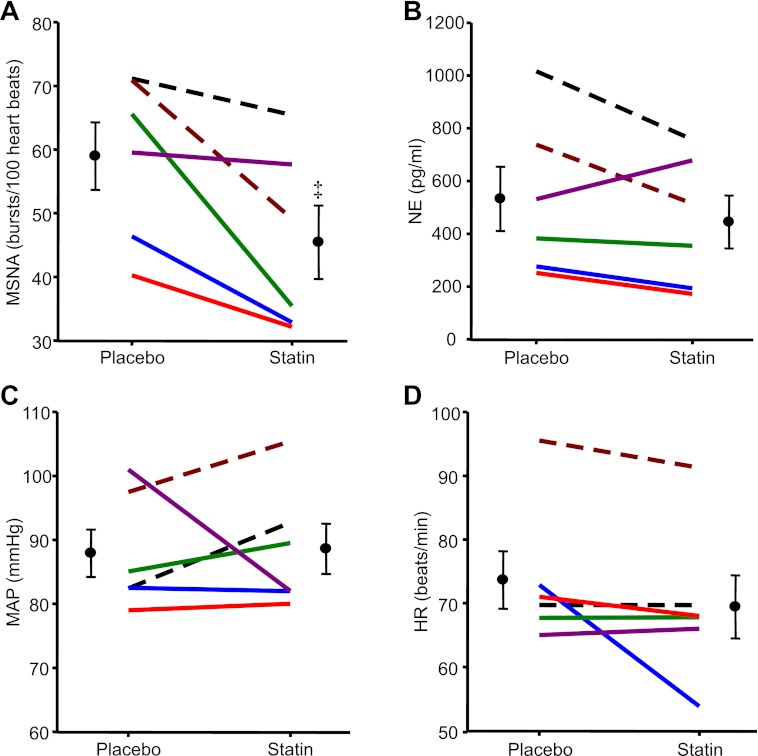
In accordance with study 1, HF patients also demonstrated significant reductions in MSNA burst incidence with simvastatin compared with placebo. Original records of MSNA for all six subjects are provided in Fig. 3. Again, although variable reductions were observed among patients (range of −2 to −30 bursts/100 heartbeats), there was a consistent reduction in MSNA (Fig. 4A). MSNA burst frequency was also lower after simvastatin compared with placebo (44 ± 6 placebo to 32 ± 5 statin bursts/min; P < 0.05). Although not statistically different from placebo, simvastatin reduced plasma norepinephrine concentrations in five of six HF patients (Fig. 4B).
Fig. 3.
Segments of original records showing resting MSNA during placebo and after 1 mo of simvastatin (40 mg per day) in all 6 HF patients who completed study 2.
Fig. 4.
Individual and mean data showing changes in MSNA (bursts/100 heartbeats; A), plasma norepinephrine (NE; pg/ml; B), MAP (mmHg; C), and HR (beats/min; D) in 6 HF patients after placebo and simvastatin (40 mg per day) therapy for 1 mo. Values are means ± SE. ‡P < 0.05 vs. placebo.
Oxidative Stress Measurements
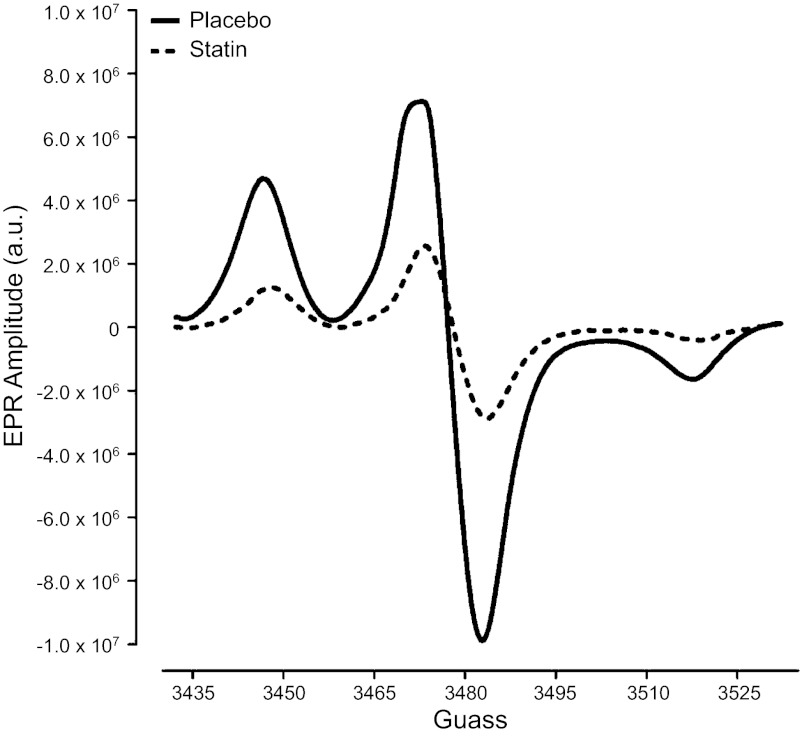
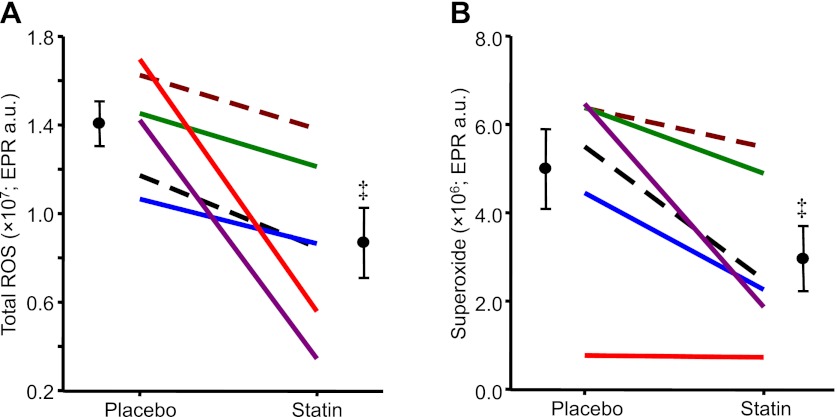
Original total ROS EPR spectra for one HF patient during placebo and statin therapy are provided in Fig. 5. Compared with placebo treatment, simvastatin reduced total ROS and superoxide in all six patients (Fig. 6). Similar to MSNA responses, these reductions in oxidative stress following statin therapy were also variable among the patients (total ROS: range of −2.0 × 106 to −11.4 × 106 EPR arbitrary units and superoxide: range of −0.4 × 105 to −4.6 × 106 EPR arbitrary units). Although there were relationships between the magnitude of the reduction in total ROS and MSNA burst incidence (r2 = 0.41; P = 0.17) as well as superoxide and MSNA burst incidence (r2 = 0.27; P = 0.29), these were not significant.
Fig. 5.
Original electron paramagnetic spin resonance (EPR) spectra showing total ROS for a HF patient during placebo and statin therapy (simvastatin; 40 mg per day); a.u., arbitrary units.
Fig. 6.
Individual and mean data showing changes in total reactive oxygen species (total reactive oxygen species (ROS; EPR a.u.: A) and superoxide (EPR a.u.: B) in 6 HF patients after placebo and simvastatin (40 mg per day) therapy for 1 mo. Values are means ± SE. ‡P < 0.05 vs. placebo.
Cardiovascular Parameters
Simvastatin treatment did not affect resting HR (Figs. 2C and 4D) or systolic BP (Table 2). Although diastolic BP and mean BP were reduced following simvastatin in study 1 (Fig. 2B and Table 2), no differences in BP were observed between simvastatin and placebo in study 2 (Fig. 4C and Table 2).
Table 2.
Effect of simvastatin therapy on cardiovascular and lipid parameters
|
Study 1 |
Study 2 |
|||
|---|---|---|---|---|
| Baseline | Statin | Placebo | Statin | |
| Systolic BP, mmHg | 126 ± 4 | 119 ± 4 | 119 ± 5 | 118 ± 2 |
| Diastolic BP, mmHg | 79 ± 5 | 72 ± 4* | 72 ± 4 | 74 ± 5 |
| Cholesterol, mg/dl | 213 ± 13 | 156 ± 11* | 187 ± 13 | 136 ± 11‡ |
| Triglycerides, mg/dl | 281 ± 112 | 306 ± 155 | 178 ± 57 | 119 ± 33 |
| LDL, mg/dl | 125 ± 5 | 73 ± 9* | 112 ± 6 | 71 ± 8‡ |
| HDL, mg/dl | 40 ± 3 | 39 ± 5 | 39 ± 4 | 41 ± 5 |
Values are means ± SE BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
P < 0.05 vs. baseline;
P < 0.05 vs. placebo.
Lipid Parameters
As expected, fasting blood cholesterol and LDL levels were significantly reduced following simvastatin therapy, whereas triglyceride and high-density lipoprotein levels were unchanged. There was no relationship between the reduction in low-density lipoproteins and the decrease in MSNA burst incidence following simvastatin (r2 = 0.04; P = 0.52).
DISCUSSION
The major novel finding of the present study is that short-term statin therapy concomitantly reduced resting MSNA and ROS in HF patients. Thus, in addition to lowering cholesterol, statins may also be beneficial in reducing sympathetic overactivity and oxidative stress in HF patients.
In the current study, 40 mg per day of simvastatin resulted in a consistent decrease in MSNA in all patients leading to an overall reduction of ∼18%. These findings are in agreement with previous animal studies using a pacing-induced HF model and demonstrating that simvastatin reduced renal sympathetic nerve activity (9). Furthermore, our findings extend the recent results of Gomes et al. (14) in which discontinuation of existing statin therapy in HF patients caused an increase in resting MSNA and when statins were added back, MSNA was again lower. Indeed, our double blind, placebo-controlled crossover design study eliminates the possibility of experimental bias potentially contributing to the changes in MSNA observed in eliminating and restarting statin therapy. In addition, although Horwich et al. (20) recently reported no statistically significant effect of 10 mg per day of atorvastatin on resting MSNA in HF patients; a 16.2% reduction of MSNA was observed in the treatment group compared with a 2.5% decrease in the placebo group. As noted by the authors, the observed lack of statistical significance may have been because the study was powered to detect a large reduction in MSNA (≥13 bursts/min), meaning that changes in MSNA of a smaller magnitude may have been missed. These findings also raise the possibility of dose-dependent effects of statins on MSNA. In this regard, studies including varying dosages and types of statins are needed to better understand their potential effects on the sympathetic nervous system.
Findings of 16% (20) and 18% (current study) reductions in MSNA with statin therapy are quite promising given the strong association between resting MSNA and mortality in HF patients (2, 3). This is particularly important considering that these effects were seen when statins were added to existing standard medical therapy including angiotensin-converting enzyme inhibitors and β-blockers, which may also reduce MSNA (4, 15, 29). Recent findings (28) comparing MSNA in NYHA class IV HF patients reported a 24% lower resting MSNA in the group that survived after an 18 month follow-up. These data lend insight into the potential clinical significance of an 18% MSNA reduction in HF patients following statin therapy. Also, even though clonidine (α2- and I1-receptor agonist) and moxonidine (selective I1 receptor agonist) have both been shown to reduce MSNA in HF patients, both pharmacological agents have undesirable side effects, which can negate any potential beneficial effects of these drugs on sympathetic overactivity (7, 39). Thus other treatment strategies aimed at reducing the sympathetic overactivity in HF patients, such as statins, are needed. Indeed, larger scale prospective, placebo-controlled, randomized clinical trials are warranted to validate the initial positive findings in these smaller cohort studies.
In the present study, we have demonstrated for the first time that in statin-naïve HF patients the reduction in MSNA following statin treatment was accompanied by a decrease in total ROS and superoxide. Thus an additional beneficial effect of statin therapy in HF may be to reduce the known elevations in oxidative stress associated with this condition (35). This becomes quite significant given the well-known deleterious consequences of increased oxidative stress including elevations in central sympathetic outflow (27).
Numerous studies performed in HF animals have shown that oxidative stress is increased in key brain areas involved in the central regulation of sympathetic outflow, such as the rostral ventrolateral medulla (RVLM; Refs. 9, 10). The increase in ROS in HF appears to be driven by an upregulation of angiotensin II type 1 (AT1) receptors and NADPH oxidase pathways (10). One way in which these elevations in ROS production lead to increases in sympathetic outflow is via scavenging of nitric oxide in the RVLM (21). However, increases in ROS have also been proposed to directly activate or sensitize sympathetic neurons via alterations in membrane ion channel function (31, 44). Nevertheless, statin treatment has been shown to normalize sympathetic overactivity in association with downregulation of AT1 receptors and NADPH oxidase expression in the RVLM leading to reductions in local superoxide production (9). Interestingly, in the present study, the magnitude of the reduction in both total ROS and superoxide in the HF patients appeared to be related to the decreases in MSNA following statin therapy. Although the relationships did not reach statistical significance, these findings are in general agreement with animal studies indicating that greater resting sympathetic outflow in HF is reduced following statin therapy possibly by a reduction in central ROS. These novel findings provide the first insight into the potential mechanism by which statin therapy lowers sympathetic outflow in human HF. Additional studies in this area are warranted.
There has been a growing interest in the potential pleiotropic effect of statin therapy to reduce sympathetic outflow in disease states. Two recent studies (36, 37) in patients with chronic kidney disease and primary hypertension have demonstrated a reduction in MSNA following statin treatment. Although the underlying mechanisms were not specifically addressed in these studies, it is of interest that both of these conditions are also associated with elevations in oxidative stress (26, 33). Furthermore, salt-sensitive hypertensive rats exhibited greater central sympathoexcitation along with higher central oxidative stress due to the activation of NADPH oxidase (8). These data further support the role of central ROS in mediating sympathetic overactivity in disease and thereby, providing a potential target by which statin therapy reduces sympathetic outflow in pathological conditions.
Studies examining the influence of statin therapy on cardiovascular variables such as HR and BP have provided equivocal results. Hypertensive animals treated with statins exhibited a reduction in HR (18). In agreement, simvastatin administered to hypertensive patients decreased resting HR, while improving cardiac baroreflex sensitivity compared with a placebo treated group (25). However, other studies have demonstrated no effect of statin treatment on HR in hypertensive as well as hypercholestrolemic patients (1, 37). Likewise, in the present study, we did not observe any changes in resting HR following simvastatin in HF patients. These findings are not surprising given the equivocal results in pacing-induced HF animals treated with simvastatin (9, 11). In addition, a plausible explanation for the lack of an effect in HF is that all patients were taking β-blockers as part of their standard treatment. Indeed, imaging studies in patients with dilated cardiomyopathy have suggested that statins decrease HR via a reduction in cardiac sympathetic nerve activity (42). However, an effect of statins on parasympathetic nerve activity cannot be discounted (45). In regards to BP changes following statin therapy, there is also equivocal evidence with studies demonstrating reductions or no changes in BP (1, 12, 13, 37). Likewise, we observed a reduction in mean BP in study 1 but not in study 2. The reason for these variable responses is unclear. Overall, discrepancies in findings regarding statin treatment need to be carefully evaluated as studies have used diverse patient groups, as well as different statins at varying dosages and durations making comparisons between studies difficult.
The influence of lowering cholesterol on MSNA remains unclear. Although we observed a significant reduction in LDL demonstrating the effectiveness of simvastatin as well as patient compliance with the treatment regimen, no relationship was observed between the decreases in LDL and MSNA following statin therapy. Likewise, animal studies (32) have indicated that the reduction in central sympathetic outflow in HF with statins appears independent of cholesterol lowering in that cholesterol levels were not significantly changed with simvastatin treatment. Despite the relatively consistent reduction of resting MSNA following statin therapy in the present study, a significant decrease in plasma norepinephrine was not observed. However, this appeared to be due to one patient who exhibited a slight increase in norepinephrine with statins. Interestingly, this patient had the smallest change in MSNA following statin therapy (Fig. 4). Nevertheless, it should be noted that plasma norepinephrine measures are dependent on rates of removal of the neurotransmitter from plasma and not just sympathetic outflow and norepinephrine release, whereas MSNA constitutes a direct measure of central sympathetic outflow. This may explain why statin treatment reduces MSNA but does not have a consistent effect on plasma norepinephrine (14, 18, 23).
In summary, the present study is the first to demonstrate that short-term statin therapy lowers resting MSNA along with systemic oxidative stress in HF patients. Collectively, these findings highlight that aside from lowering cholesterol, statin therapy may provide additional benefits towards lowering the risks associated with cardiovascular disease including reductions in sympathetic activity and oxidative stress.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-038690-22 and by University of Missouri Research Board Grant No. 3301.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H.D., J.P.F., L.C.V., A.K., A.C., and P.J.F. performed experiments; S.H.D., J.P.F., M.C.Z., and P.J.F. analyzed data; S.H.D., J.P.F., L.C.V., M.C.Z., I.H.Z., and P.J.F. interpreted results of experiments; S.H.D. and M.C.Z. prepared figures; S.H.D. drafted manuscript; S.H.D., J.P.F., L.C.V., A.K., A.C., M.C.Z., I.H.Z., and P.J.F. edited and revised manuscript; S.H.D., J.P.F., L.C.V., A.K., A.C., M.C.Z., I.H.Z., and P.J.F. approved final version of manuscript; I.H.Z. and P.J.F. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Kevin Gallagher, Dr. Chelif Junor, Dr. Azamuddin Khaja, Dr. Abrar Ahmed, and Charla Jay for assistance with patient recruiting and screening. We also thank Jocelyn Jones for technical assistance with the EPR measures.
REFERENCES
- 1. Antonicelli R, Onorato G, Pagelli P, Pierazzoli L, Paciaroni E. Simvastatin in the treatment of hypercholesterolemia in elderly patients. Clin Ther 12: 165–171, 1990 [PubMed] [Google Scholar]
- 2. Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrao CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135: 302–307, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Benedict CR, Shelton B, Johnstone DE, Francis G, Greenberg B, Konstam M, Probstfield JL, Yusuf S. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation 94: 690–697, 1996 [DOI] [PubMed] [Google Scholar]
- 4. De Matos LD, Gardenghi G, Rondon MU, Soufen HN, Tirone AP, Barretto AC, Brum PC, Middlekauff HR, Negrao CE. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail 10: 496–502, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 6. Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972 [DOI] [PubMed] [Google Scholar]
- 7. Floras JS. The “unsympathetic” nervous system of heart failure. Circulation 105: 1753–1755, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 50: 360–367, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation 112: 1763–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Wang W, Zucker IH. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J Pharmacol Exp Ther 326: 278–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glorioso N, Troffa C, Filigheddu F, Dettori F, Soro A, Parpaglia PP, Collatina S, Pahor M. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension 34: 1281–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med 168: 721–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomes ME, Lenders JW, Bellersen L, Verheugt FW, Smits P, Tack CJ. Sympathoinhibitory effect of statins in chronic heart failure. Clin Auton Res 20: 73–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Pozzi M, Morganti A, Carugo S, Mancia G. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation 96: 1173–1179, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Grassi G, Seravalle G, Dell'Oro R, Facchini A, Ilardo V, Mancia G. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J Hypertens 22: 1747–1753, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Head GA, Burke SL. I1 imidazoline receptors in cardiovascular regulation: the place of rilmenidine. Am J Hypertens 13: 89S–98S, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Herring N, Lee CW, Sunderland N, Wright K, Paterson DJ. Pravastatin normalises peripheral cardiac sympathetic hyperactivity in the spontaneously hypertensive rat. J Mol Cell Cardiol 50: 99–106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol 43: 642–648, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Horwich TB, Middlekauff HR, Maclellan WR, Fonarow GC. Statins do not significantly affect muscle sympathetic nerve activity in humans with nonischemic heart failure: a double-blind placebo-controlled trial. J Card Fail 17: 879–886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kishi T, Hirooka Y, Mukai Y, Shimokawa H, Takeshita A. Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens 21: 379–386, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 357: 2248–2261, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Krum H, Ashton E, Reid C, Kalff V, Rogers J, Amarena J, Singh B, Tonkin A. Double-blind, randomized, placebo-controlled study of high-dose HMG CoA reductase inhibitor therapy on ventricular remodeling, pro-inflammatory cytokines and neurohormonal parameters in patients with chronic systolic heart failure. J Card Fail 13: 1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Lewandowski J, Sinski M, Bidiuk J, Abramczyk P, Dobosiewicz A, Ciarka A, Gaciong Z. Simvastatin reduces sympathetic activity in men with hypertension and hypercholesterolemia. Hypertens Res 33: 1038–1043, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Massy ZA, Stenvinkel P, Drueke TB. The role of oxidative stress in chronic kidney disease. Semin Dial 22: 405–408, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Molavi B, Mehta JL. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr Opin Cardiol 19: 488–493, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Munhoz RT, Negrao CE, Barretto AC, Ochiai ME, Cardoso JN, Morgado PC, Del Carlo CH, Ramires JA. Microneurography and venous occlusion plethysmography in heart failure: correlation with prognosis. Arq Bras Cardiol 92: 46–53, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Newton GE, Parker JD. Acute effects of beta 1-selective and nonselective beta-adrenergic receptor blockade on cardiac sympathetic activity in congestive heart failure. Circulation 94: 353–358, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation 108: 839–843, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation 107: 2493–2498, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaugh EG, Roat JW, Gao L, Yang RF, Manickam DS, Yin JX, Schultz HD, Bronich TK, Batrakova EV, Kabanov AV, Zucker IH, Zimmerman MC. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 31: 5218–5226, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sawyer DB. Oxidative stress in heart failure: what are we missing? Am J Med Sci 342: 120–124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siddiqi L, Joles JA, Oey PL, Blankestijn PJ. Atorvastatin reduces sympathetic activity in patients with chronic kidney disease. J Hypertens 29: 2176–2180, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Sinski M, Lewandowski J, Ciarka A, Bidiuk J, Abramczyk P, Dobosiewicz A, Gaciong Z. Atorvastatin reduces sympathetic activity and increases baroreceptor reflex sensitivity in patients with hypercholesterolaemia and systemic arterial hypertension. Kardiol Pol 67: 613–620, 2009 [PubMed] [Google Scholar]
- 38. Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol 47: 332–337, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Swedberg K, Bristow MR, Cohn JN, Dargie H, Straub M, Wiltse C, Wright TJ. Effects of sustained-release moxonidine, an imidazoline agonist, on plasma norepinephrine in patients with chronic heart failure. Circulation 105: 1797–1803, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1231–1239, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Tong J, Zimmerman MC, Li S, Yi X, Luxenhofer R, Jordan R, Kabanov AV. Neuronal uptake and intracellular superoxide scavenging of a fullerene (C60)-poly(2-oxazoline)s nanoformulation. Biomaterials 32: 3654–3665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsutamoto T, Sakai H, Ibe K, Yamaji M, Kawahara C, Nakae I, Fujii M, Yamamoto T, Horie M. Effect of atorvastatin vs. rosuvastatin on cardiac sympathetic nerve activity in non-diabetic patients with dilated cardiomyopathy. Circ J 75: 2160–2166, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 44. Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Zoccali C, Seravalle G, Grassi G. Sympathoinhibitory effects of statins in chronic kidney disease: are they clinically relevant? J Hypertens 29: 2064–2067, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Zucker IH, Gao L. Statins and sympathetic nerve activity in heart failure. J Card Fail 12: 759, 2006 [DOI] [PubMed] [Google Scholar]