Abstract
Acclimatization to hypoxia requires time to complete the adaptation mechanisms that influence oxygen (O2) transport and O2 utilization. Although decreasing hemoglobin (Hb) O2 affinity would favor the release of O2 to the tissues, increasing Hb O2 affinity would augment arterial O2 saturation during hypoxia. This study was designed to test the hypothesis that pharmacologically increasing the Hb O2 affinity will augment O2 transport during severe hypoxia (10 and 5% inspired O2) compared with normal Hb O2 affinity. RBC Hb O2 affinity was increased by infusion of 20 mg/kg of 5-hydroxymethyl-2-furfural (5HMF). Control animals received only the vehicle. The effects of increasing Hb O2 affinity were studied in the hamster window chamber model, in terms of systemic and microvascular hemodynamics and partial pressures of O2 (Po2). Pimonidazole binding to hypoxic areas of mice heart and brain was also studied. 5HMF decreased the Po2 at which the Hb is 50% saturated with O2 by 12.6 mmHg. During 10 and 5% O2 hypoxia, 5HMF increased arterial blood O2 saturation by 35 and 48% from the vehicle group, respectively. During 5% O2 hypoxia, blood pressure and heart rate were 58 and 30% higher for 5HMF compared with the vehicle. In addition, 5HMF preserved microvascular blood flow, whereas blood flow decreased to 40% of baseline in the vehicle group. Consequently, perivascular Po2 was three times higher in the 5HMF group compared with the control group at 5% O2 hypoxia. 5HMF also reduced heart and brain hypoxic areas in mice. Therefore, increased Hb O2 affinity resulted in hemodynamics and oxygenation benefits during severe hypoxia. This acute acclimatization process may have implications in survival during severe environmental hypoxia when logistic constraints prevent chronic acclimatization.
Keywords: microcirculation, oxygen release, partial pressure of oxygen at which hemoglobin is 50% saturated with oxygen, hemoglobin oxygen affinity, critical oxygen supply, high altitude
red blood cells (RBCs) contain hemoglobin (Hb), which reversibly binds oxygen (O2). The delivery of O2 to tissues is drastically affected by reductions in a fraction of inspired O2 (hypoxia), which decreases arterial Po2 and blood O2 saturation (So2) (20). Exposure and adaptation to hypoxia (and/or high altitude) decrease Hb O2 affinity, introducing a right shift to the blood O2 equilibrium curve (9). The decrease in Hb O2 affinity during hypoxia has been explained to favor O2 offload to tissues (9). When exposed to hypoxia, the magnitude of the decrease in Hb O2 affinity depends on the acid-base status (Bohr Effect and Haldane Effect), whereas, during acclimation to hypoxia, the change in Hb O2 affinity depends on the total concentration of organic phosphates in the RBC, mainly 2,3-diphosphoglycerate (2,3-DPG) and ATP (10).
Comparison between individuals acutely and chronically exposed to hypoxia showed that, when the partial pressure of O2 in air breathed is reduced by 60% (equivalent to altitudes >5,000 m), the arterial So2 decreased by more than 30% during acute exposure, whereas, in chronically exposed individuals, the arterial So2 decreased by only 20% (18). Therefore, accelerated adaptation to low inspired O2 produces severe hypoxemia and eventual death, whereas the lack of adaptation induces acute mountain sickness (AMS), high-altitude cerebral edema (HACE), high-altitude pulmonary edema (HAPE), or death within a few hours (22). Adaptation to hypoxia is the hallmark of survival at high altitude. Human and mammalian natives of high altitudes have adapted to the low inspired O2 by increasing their Hb O2 affinity compared with their relatives at sea level, thus enabling higher O2 uptake, increasing arterial So2 and O2 delivery at lower partial pressure of O2 in air breathed (32).
The Hb O2 affinity can be modified with Hb allosteric effectors. We have previously increased Hb O2 affinity during anemic conditions using 5-hydroxymethyl-2-furfural (5HMF) and found that a moderate increase in O2 affinity [a decrease in Po2 at which the Hb is 50% saturated with O2 (P50) of 6 mmHg] maintained hemodynamic conditions, O2 delivery, and increased tissue Po2 (6, 30). 5HMF is a low-molecular-weight five-carbon-ring aromatic aldehyde with limited toxicological response (21). 5HMF is active with high bioavailability, it can be administered orally, intraperiotoneally, or intravenously, and it has high red cell membrane permeability (2). 5HMF binds covalently with intracellular Hb to form a high-affinity Schiff-base Hb adduct in a symmetrical fashion with the NH2-terminal α Valine-1 of Hb, allosterically shifting the Hb O2 equilibrium curve at relatively low 5HMF concentrations (1).
This study investigates the protective effects of artificially increased Hb O2 affinity during acute hypoxia. We propose that 5HMF can be used for acute adaptation and acclimation of personnel exposed to high altitude, accelerating critical acclimation and preventing AMS, HACE, HAPE, or hypoxemic death. Hamsters fitted with the window chambers were treated with 5HMF (20 mg/kg), and the control group was given only the vehicle solution. We selected the hamster window chamber model because of its capacity to analyze the exchange of O2 in the microcirculation, accounting for the contributions of RBC and the processes associated with interactions between Hb and O2. 5HMF treatment was administered one hour before the onset of hypoxia (normobaric 10% inspired O2) followed by severe hypoxia (normobaric 5% inspired O2). Our experimental model allows us to measure systemic and microvascular hemodynamics and microvascular Po2 levels to calculate O2 transport. Validation of the benefit in O2 transport produced by 5HMF during acute hypoxia in the hamster model was confirmed in mice by immunohistochemistry staining for hypoxic zones in brain and heart sections using intraperitoneal pimonidazole. Pimonidazole acts as a probe specific for hypoxic cells, because, at Po2 <10 mmHg, it is reduced to a reactive intermediate that binds covalently to molecules containing a thiol group, including proteins, and can be detected by a specific monoclonal antibody. Based on our results, the beneficial effect of 5HMF will be most noticeable upon exposure to severe hypoxia where O2 uptake in the lungs is insufficient to maintain health. The use of 5HMF may reduce the need for chronic adaptation and improve performance at limited inspired O2, clearly, of practical importance for flight crews and military and emergency personnel. Moreover, the effects of left-shifted Hb O2 affinity during acute hypoxia is information useful in understanding the role of Hb O2 affinity and hypoxic hypoxia in O2 transport in the microcirculation.
MATERIALS AND METHODS
Hamster preparation.
Male Golden Syrian Hamsters (55–65 g; Charles River Laboratories, Boston, MA) were fitted with a dorsal window chamber. Animal handling and care followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere (7, 8). Briefly, the animal was prepared for chamber implantation with a 50 mg/kg ip injection of pentobarbital sodium anesthesia. After hair removal, sutures were used to lift the dorsal skin away from the animal, and one frame of the chamber was positioned on the animal's back. A chamber consisted of two identical titanium frames with a 15-mm circular window. With the aid of backlighting and a stereomicroscope, one side of the skin fold was removed following the outline of the window until only a thin layer of retractor muscle and the intact subcutaneous skin of the opposing side remained. The intact skin of the other side was exposed to the ambient environment. Animals were allowed 2 days for recovery. Finally, they were anesthetized again to implant arterial and venous catheters (PE-50) in the carotid artery and jugular vein. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Hamster inclusion criteria.
Hamsters were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) >340 beats/min, mean arterial blood pressure (MAP) >80 mmHg, systemic hematocrit (Hct) >45%, and arterial O2 partial pressure >50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under ×650 magnification did not reveal signs of edema or bleeding. Hamsters are a fossorial animal with a low arterial Po2 compared with other rodents; however, the intravascular O2 tension in the hamster window chamber model is similar to the window chamber model implanted on mice (5).
Test material.
Chemicals, including 5HMF, were purchased from Sigma Aldrich (St. Louis, MO). Stock solution at 14 mg/ml was prepared, before the experiment, in a degassed (O2 free) saline (0.9% NaCl) solution. Hypoxyprobe-1 Green Kits (pimonidazole and the correspondent monoclonal antibody conjugated to fluorescein) were purchased from Hypoxyprobe (Burlington, MA).
Systemic parameters.
MAP and HR were recorded continuously (MP 150; Biopac System, Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin; Hemocue, Stockholm, Sweden). Arterial blood was collected in heparinized glass capillaries (50 μl) and immediately analyzed for Po2, Pco2, base excess, and pH (Rapidlab 248; Bayer, Norwood, MA). Arterial Hb saturations were measured on the IL482 CO-Oximeter System (Instrumentation Laboratory, Lexington, MA).
Cardiac output.
Cardiac output (CO) was measured by a modified thermodilution technique (4). Animals instrumented for CO measurements were surgically prepared and recovered identically to animals studied for microvascular measurements. The complexity of the setup for the thermodilution prohibited positioning them on the microscope. Vascular resistance (VR) was calculated at relation between MAP and CO (VR = MAP/CO). O2 delivery (Do2) was calculated as the product of the total Hb by the O2-carrying capacity of saturated Hb (1.34 ml O2/gHb, where gHb is grams of Hb) by the arterial blood O2 saturation and CO (Do2 = [gHb × 1.34 × SA] × CO).
Blood O2 equilibrium curve.
O2 equilibrium curves for hamster blood were obtained by deoxygenation of O2-equilibrated samples in a Hemox buffer at 37°C, using a Hemox Analyzer (TCS Scientific, New Hope, PA). The Hemox buffer pH was adjusted to match the arterial blood pH of the animals using Tris and BisTris buffers. Tris and BisTris buffers were prepared by titrating the reagents with HCI before adjusting the pH of the solutions to keep Cl− concentration equal to the buffer at the pH values.
Microvascular experimental setup.
The animal in the restraining tube with the protruding window chamber was fixed to the microscopic stage for transillumination with the intravital microscope (BX51WI; Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the tube environment before any measurement. Detailed mappings were made of the chamber vasculature so that the same vessels studied at baseline could be followed throughout the experiment. Observation of the fields was done systematically by displacing the microscopic field of view by a field width in 10–12 successive steps in the lateral direction (relative to the observer). Each step was viewed on the video monitor and was 240 μm long when referred to the tissue. Blood vessels were chosen by a distinctive anatomic landmark to easily and quickly reestablish the same fields and vessels at each observation time point. Six to eight arterioles and venules were selected in each preparation. The tissue image was projected onto a charge-coupled device (CCD) camera (COHU 4815) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a ×40 (LUMPFL-WIR, numerical aperture 0.8; Olympus) water immersion objective. The same sites of study were followed throughout the experiment so that comparisons could be made directly to baseline levels.
Functional capillary density.
Functional capillaries are defined as those capillaries with RBC transit of at least a single RBC within a period of 45 s. Functional capillary measurements include 10 successive microscopic fields with a total area of 0.46 mm2. Functional capillary density (FCD) is defined as the total length of RBC perfused capillaries divided by the area of the microscopic fields (cm−1).
Microhemodynamics.
A video image-shearing method was used to measure vessel diameter (D) (13). Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Arteriolar and venular centerline velocities were measured on-line by using the photodiode cross-correlation method (Photo Diode/Velocity Tracker Model 102B; Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity (19). Blood flow (Q) was calculated from the measured values as Q = π × V(D/2)2. This calculation assumes a parabolic velocity profile and has been found to be applicable to tubes of 15–80 μm internal diameters and for Hcts in the range of 6–60% (19). The same sites of study were followed throughout the experiment so that comparisons could be made directly to baseline levels.
Microvascular Po2 distribution.
High-resolution noninvasive microvascular Po2 measurements were made using phosphorescence quenching microscopy (PQM) (14). PQM is based on the relationship between the decay rate of excited palladium-mesotetra-(4-carboxyphenyl) porphyrin (Frontier Scientific Porphyrin Products, Logan, UT) bound to albumin and the O2 concentration according to the Stern-Volmer equation (29). The method was used previously in microcirculatory studies to determine Po2 levels in different tissue (14). Po2 measurements by PQM were obtained following these steps for both groups: 1) the probe was injected (iv injection of 15 mg/kg at a concentration of 10 mg/ml of the phosphorescence complex 10 min before O2 measurements); 2) the tissue was illuminated (pulsed of light at 420 nm wavelength) to excite the probe into its triplet state; 3) the emitted phosphorescence (680 nm wavelength) was collected and analyzed to yield the phosphorescence lifetime; and 4) the phosphorescence lifetime was converted into O2 concentration, Po2. The phosphorescence lifetimes are concentration independent, which permit extravascular fluid Po2 measurements, although the dye albumin complex that extravasates is very small. Perivascular Po2 was measured in regions in between functional capillaries.
Microvascular O2 saturations.
Intravascular Hb O2 saturations were calculated using the O2 equilibrium curves measured and the intravascular Po2 values measured with the PQM. The O2 equilibrium curves were measured at arterial blood pH as described above.
Do2 and extraction.
The microvascular methodology used in our studies allows a detailed analysis of O2 supply in the tissue. Calculations are made using Eqs. 1 and 2:
| (1) |
| (2) |
where, RBCHb is the total Hb [gHb/dlblood], γ is the O2 carrying capacity of saturated Hb [1.34 ml O2/gHb], SA is the arteriolar blood O2 saturation, A-V indicates the arteriolar/venular differences, and Q is the average microvascular blood flow (arterioles and venules). Intravascular Hb O2 saturations were calculated as described above.
Experimental groups.
Animals were randomly divided into the following experimental groups before the experiment: 1) 5HMF, treated with 20 mg/kg of 5HMF in sterile saline (0.9% NaCl) and administered in a single intravenous infusion of 100 μl; and 2) vehicle, treated with a single intravenous infusion of sterile saline (0.9% NaCl) solution in a volume of 100 μl. The dose of 20 mg/kg of 5HMF was selected based on previously published studies and various pilot experiments that appear to be sufficient to reduce P50 by 12 mmHg in hamsters and by 20 mmHg in mice. Additionally, higher doses of 5HMF have no extra effects on P50.
Acute hypoxia protocol.
The awake animals were placed in a restraining tube with a longitudinal slit from which the protruding window chamber provided a microscopic stage for observation. A plastic tent with an inlet valve connected to the gas tanks was placed in front of the restraining tube. The gas flow rate (0.2 l/min) into the tent was diffused by a cotton filter barrier so that the hamster was not subjected to a direct stream of gas flow. The hamsters were given 20 min to adjust to the experimental environment before baseline measurements were completed. Immediately after baseline measurements, the hamsters received a bolus intravenous injection of 5HMF or vehicle. After treatment (60 min), measurements for normoxia were completed. Palladium-mesotetra-(4-carboxyphenyl)porphyrin complex was administered 15 min before Po2 measurements. The initial decrease to 10% O2 hypoxia was induced by normobaric hypoxia (10% O2-balance N2). The hamsters were given 15 min to adjust to the change in the gas environment before measurements. Severe hypoxia to 5% O2 was induced by normobaric hypoxia (5% O2-balance N2), and 15 min were given to adjust to the gas change before measurements. At each time point, systemic parameters, microvascular hemodynamics, and Po2 measurements were performed. Blood P50 was measured at each time point. To prevent animal stress or discomfort, hypoxia was stopped if blood pressure dropped below 40 mmHg, and the animal was excluded from the study.
Brain and heart hypoxic areas in mice.
C57BL/6 mice (17–22 g; Charles River Laboratories) were used to study brain and heart hypoxic zones. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. To validate the results obtained in hamsters with 5HMF during hypoxia, we have expanded our study to include immunohistochemistry staining for pimonidazole bound to hypoxic zones in the brain and heart tissues of mice treated with 5HMF during hypoxia. Mice were restrained and exposed to a similar hypoxic protocol for the hamsters, as described above. Study mice received a bolus intraperitoneal injection of 5HMF or vehicle. After treatment (60 min), mice received an intraperitoneal injection of the hypoxic marker Hypoxyprobe-1 (40 mg/kg pimonidazole) and 5 mg/kg Hoechst 33342 (Invitrogen, Carlsbad, CA) diluted in PBS (total volume 100 μl) and were exposed to 10% O2 hypoxia for 30 min. Next, the mice received a second intraperitoneal injection of pimonidazole (40 mg/kg) and Hoechst 33342 (5 mg/kg) diluted in PBS (100 μl) and were exposed to severe hypoxia at 5% O2 for an additional 30 min. Mice blood P50 was measured at baseline, 60 min after 5HMF or vehicle treatment, and 15 min after exposing the animals to each hypoxic level from blood samples (20 μl) collected from the tail vein. Finally, mice were killed, and their brains and hearts were removed. In addition, a control group of mice, not exposed to hypoxia, was included in the study for comparison. Brain and heart were split sagittally and transverse, respectively. Tissues were fixed by immersion in formalin for 24 h at room temperature before transfer to 70% (vol/vol) ethanol. Last, tissues were cut into 100-μm-thick sections.
Pimonidazole immunohistochemistry.
Sections were cleaned and rehydrated according to standard procedures. Monoclonal antibody directed against pimonidazole (included in the Hypoxyprobe-1 green kit) was used for immunohistochemical staining of the brain and heart sections. Fluorescence microscopy was performed using an Olympus BX51WI equipped with a high-resolution digital CCD ORCA-285 (Hamamatsu, Hamamatsu City, Japan) illuminated with a mercury burner and the appropriate fluorescent cubes (XF100–2 and XF02–2; Omega Optical, Brattleboro, VT). Images for pimonidazole antibody-stained areas and Hoechst were prepared using Wasabi Imaging Software (Hamamatsu). The ratio of pixels stained for pimonidazole in each region to the total cellular area of the image was calculated. Ten images were analyzed, by sections, and the results were pooled to determine the mean and SD. To indicate the colocalization of pimonidazole and Hoechst in cells, images were superimposed.
Data analysis.
Results are presented as means ± SD. The Grubbs' method was used to assess closeness for all measured parameter values at baseline. Data within each group were analyzed using ANOVA for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunn's multiple-comparison test. Data comparison between groups was analyzed using two-way ANOVA (2-way ANOVA test). When appropriate, posttest analyses were performed with the Bonferroni posttests comparison. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. The same vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, San Diego, CA). Changes were considered statistically significant if P < 0.05.
RESULTS
Twenty-four hamsters entered the study; 14 were used for microvascular studies and 10 for CO measurements. Fourteen mice were used to study pimonidazole binding to brain and heart hypoxic tissue. All animals tolerated the entire hypoxia protocol. Animals were randomly assigned to the experimental groups, microvascular studies: 5HMF (n = 7; 66 ± 7 g) and vehicle (n = 7; 68 ± 5 g); CO studies: 5HMF (n = 5; 69 ± 6 g) and vehicle (n = 5; 70 ± 7 g); mice pimonidazole binding: 5HMF (n = 5; 18 ± 2 g), vehicle (n = 5; 19 ± 3 g), and control (not exposed to hypoxia, n = 4; 19 ± 2 g). All animals passed the Grubbs' test, ensuring that all the measured values at baseline were within a similar population (P < 0.05). Similarities between groups at baseline for hamsters and mice were statistically verified between groups (P > 0.30).
O2 equilibrium curves.
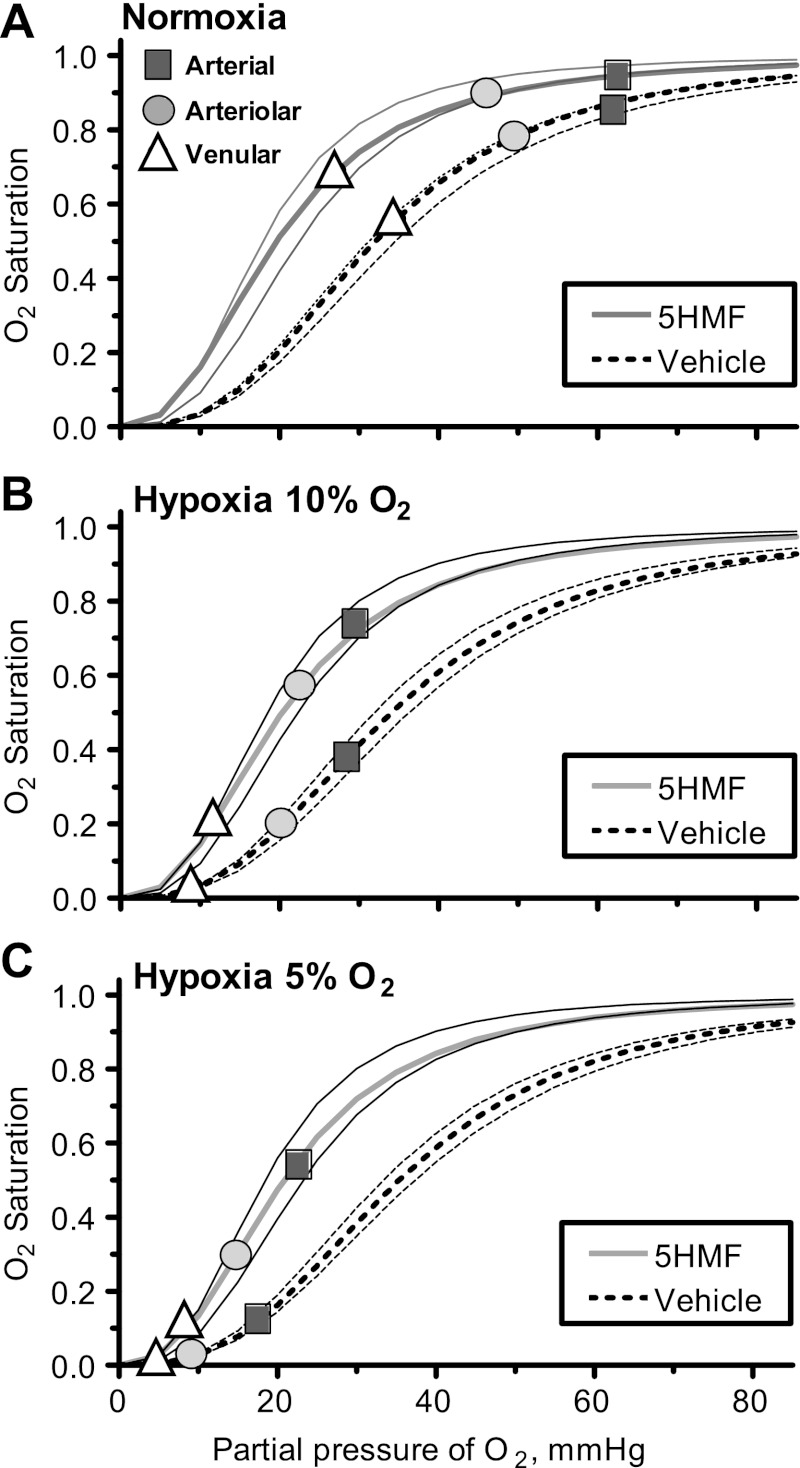
Blood O2 equilibrium curves are presented in Fig. 1 for normoxia, hypoxia 10% O2, and hypoxia 5% of O2. Treatment with 5HMF decreased blood P50 from 32.2 ± 1.2 mmHg at baseline to 19.6 ± 1.8 mmHg within 60 min. The decrease in blood P50 produced by 5HMF was maintained through the entire protocol, and the P50 of 5HMF animals was 20.3 ± 1.9 mmHg during the 10% hypoxia and 20.8 ± 2.4 mmHg during the 5% hypoxia. The P50 of the animals treated with the vehicle was not different from baseline at normoxia, 32.6 ± 1.4 mmHg; however, during 10 and 5% O2 hypoxia, their P50 increased to 34.1 ± 2.2 and to 35.4 ± 2.0 mmHg, respectively.
Fig. 1.
Changes in blood O2 equilibrium curves during normoxia, 10% O2 hypoxia, and 5% O2 hypoxia. Each panel includes arterial (square), arteriolar (circle), and venular (triangle) O2 saturation for each group. BL, baseline; Nor, normoxia (A); Hyp10, 10% O2 hypoxia (B); Hyp5, 5% O2 hypoxia (C). Severe hypoxia was induced by normobaric hypoxia with 10% O2 balanced N2, and, after characterization, the animals were exposed to extreme hypoxia using 5% O2 balanced N2. Reduction of the fraction of inspired O2 limits the amount of O2 bound to the Hb in pulmonary circulation; therefore, increasing hemoglobin (Hb) O2 affinity augments the O2 transported by the blood to the tissues. 5-Hydroxymethyl-2-furfural (5HMF) increased the blood O2 saturation at lower Po2.
Blood chemistry and gas parameters.
Blood gas parameters are presented in Table 1. 5HMF did not produce any significant change in blood gas parameters during normoxia. The multiple samples for blood gases and O2 affinity determination produced a small and not significant decrease in Hct and Hb in both groups. O2 hypoxia (10%) decreased arterial Po2 in both groups compared with baseline. Arterial Pco2 also decreased compared with baseline during 10% O2 hypoxia. The arterial CO2 tension decreased due to hyperventilation, which should produce an increase in blood pH. However, arterial pH decreased in both groups during hypoxia. Thus the acid-base status changed toward a mixed respiratory alkalosis and metabolic acidosis, the latter dominating and decreasing blood pH. O2 hypoxia (5%) further decreased arterial Po2 in both groups, although 5HMF had higher arterial Po2 compared with the vehicle. During 5% O2 hypoxia, the arterial blood pH further decreased, triggering a reflex response to metabolic acidosis that decreased arterial Pco2, producing a more profound respiratory alkalosis, as indicated by the blood biochemical analysis.
Table 1.
Blood gas parameters
| Fraction of Inspired O2 |
|||||
|---|---|---|---|---|---|
| Baseline | Norm (21% O2) | Hyp10 (10% O2) | Hyp5 (5% O2) | ||
| Hct, % | 49.1 ± 1.2 | 5HMF | 48.8 ± 0.7 | 48.4 ± 0.8 | 47.9 ± 0.7 |
| Vehicle | 49.4 ± 0.9 | 48.8 ± 0.7 | 48.4 ± 0.6 | ||
| Hb, g/dl | 14.7 ± 0.8 | 5HMF | 14.6 ± 1.0 | 14.4 ± 0.8 | 14.1 ± 0.6 |
| Vehicle | 14.9 ± 0.9 | 14.6 ± 0.8 | 14.2 ± 0.7 | ||
| Po2, mmHg | 56.8 ± 4.6 | 5HMF | 63.1 ± 7.2 | 30.2 ± 4.7†‡ | 24.6 ± 4.4†‡§ |
| Vehicle | 62.3 ± 5.9 | 28.8 ± 4.8†‡ | 18.7 ± 3.5†‡§ | ||
| Pco2, mmHg | 50.1 ± 5.4 | 5HMF | 48.7 ± 4.9 | 33.6 ± 5.1†‡ | 27.6 ± 3.6†‡§ |
| Vehicle | 49.5 ± 5.3 | 32.5 ± 4.2†‡ | 24.5 ± 4.1†‡§ | ||
| pH | 7.331 ± 0.028 | 5HMF | 7.335 ± 0.029 | 7.328 ± 0.026 | 7.316 ± 0.017†‡§ |
| Vehicle | 7.337 ± 0.024 | 7.319 ± 0.020 | 7.295 ± 0.016†‡§ | ||
| BE, mmol/l | 2.6 ± 1.8 | 5HMF | 1.5 ± 1.9 | −2.4 ± 2.1†‡ | −5.6 ± 1.8†‡§ |
| Vehicle | 2.4 ± 1.3 | −2.6 ± 1.8†‡ | −7.7 ± 1.5†‡§ | ||
Values are means ± SD. Baseline included all the animals in the study. No significant differences were detected at baseline between groups, before treatment. Norm, normoxia; Hyp10, 10% O2 hypoxia; and Hyp5, 5% O2 hypoxia; Hct, systemic hematocrit; Hb, hemoglobin; Po2, arterial partial O2 pressure; Pco2, arterial partial pressure of CO2; BE, base excess; 5HMF, 5-hydroxymethyl-2-furfural. P < 0.05 compared with baseline (
), compared with normoxia (
), and compared with 10% O2 hypoxia (
).
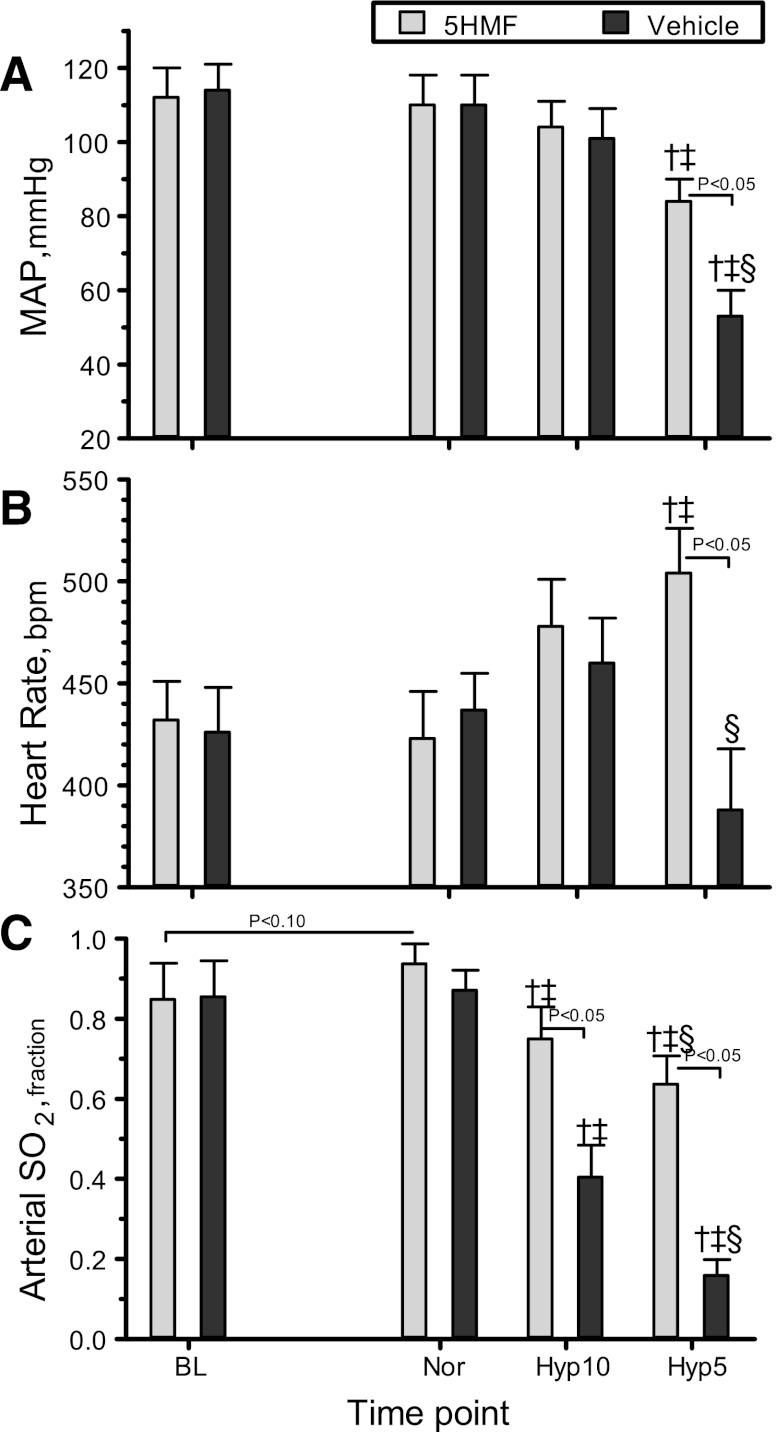
Blood pressure and HR.
MAP and HR are presented in Fig. 2, A and B. Treatment with 5HMF did not affect MAP or HR compared with baseline. No difference in MAP or HR was measured between groups at normoxia. MAP and HR were not changed significantly during 10% O2 hypoxia in either group. O2 hypoxia (5%) decreased MAP significantly in both groups compared with baseline and normoxia. During 5% O2 hypoxia, the vehicle-treated animals showed a significant decrease in MAP compared with 10% O2 hypoxia and with the 5HMF-treated animals. At 5% O2 hypoxia, the 5MHF-treated animals significantly increased their HR compared with baseline, normoxia, and the vehicle-treated animals. The vehicle-treated animals had lower HR compared with 10% O2 hypoxia.
Fig. 2.
Changes in mean arterial pressure (MAP, A), heart rate (B), and arterial O2 saturation (arterial So2, C) during the acute hypoxia protocol. †P < 0.05 relative to baseline. ‡P < 0.05 compared with normoxia. §P < 0.05 compared with 10% O2 hypoxia.
Arterial O2 saturation.
Arterial So2 are presented in Fig. 2C. The 5HMF group showed an increase in arterial So2 (P < 0.10) compared with baseline and the vehicle group during normoxia. O2 hypoxia (10%) decreased arterial So2 in both groups compared with baseline and normoxia, although the 5HMS group had significantly higher arterial So2 compared with the vehicle group. Similarly, at 5% O2 hypoxia, arterial So2 in both groups decreased compared with baseline, normoxia, and 10% hypoxia. During 5% O2 hypoxia, the 5HMS group had significantly higher arterial So2 compared with the vehicle group.
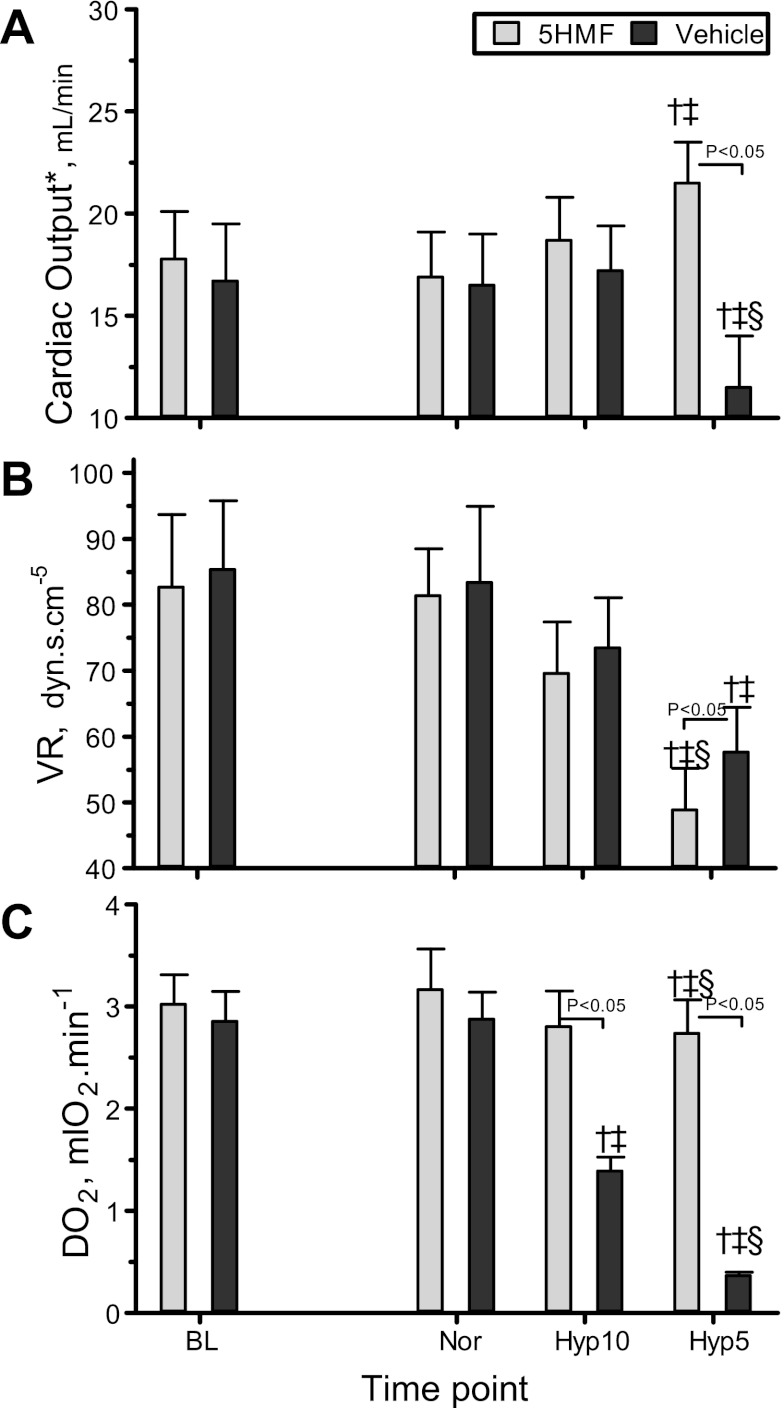
CO, VR, and systemic Do2.
CO, VR, and Do2 are presented in Fig. 3, A–C. CO, VR, and Do2 at normoxia did not change after treatment with 5HMF compared with baseline. In addition, 5HMF- and vehicle-treated animals were not different from each other at normoxia. 5HMF and vehicle groups were not different from baseline or normoxia at 10% O2 hypoxia. CO was not different between 5HMF- and vehicle-treated animals at 10% O2 hypoxia; however, at 5% O2 hypoxia, CO was significantly higher in the 5HMF group compared with the vehicle group. At 5% O2 hypoxia, the 5HMF-treated animals increased CO compared with baseline and normoxia, whereas the vehicle-treated animals decreased their CO compared with baseline, normoxia, and 10% O2 hypoxia. The changes in MAP and CO induced changes in VR in both groups, especially at 5% O2 hypoxia. The 5HMF group systematically decreased in VR as the hypoxia increased; thus, at 5% O2 hypoxia, VR was lower compared with baseline, normoxia, and 10% O2 hypoxia, whereas, in the vehicle group, VR decreased only significantly compared with baseline and normoxia. The increase in CO and the preservation of arterial O2 saturation in the 5HMF group preserved the calculated Do2 as hypoxia increased, even at 5% O2 hypoxia. In the vehicle group, Do2 decreased proportionally to the hypoxia.
Fig. 3.
Changes in cardiac output (A), vascular resistance (VR, B), and systemic O2 delivery (Do2, C) during the acute hypoxia protocol. †P < 0.05 relative to baseline. ‡P < 0.05 compared with normoxia. §P < 0.05 compared with 10% O2 hypoxia.
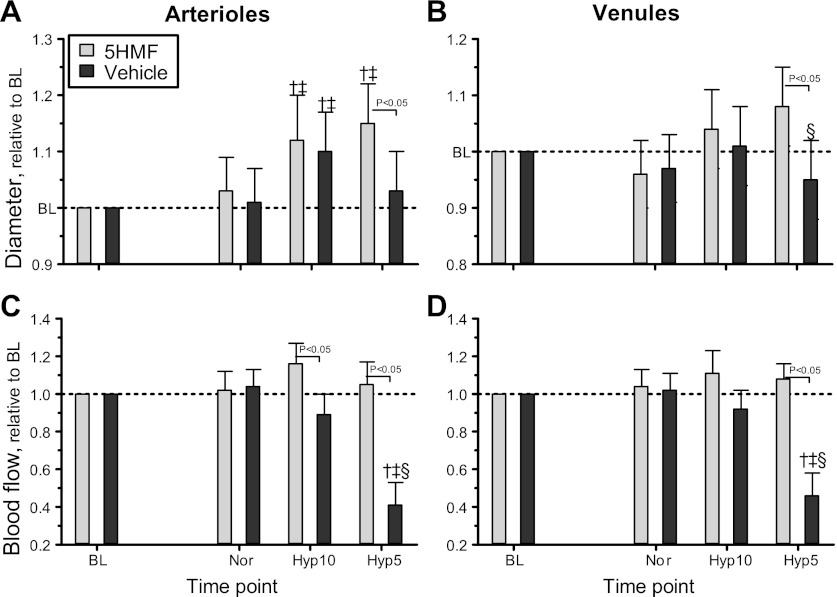
Microhemodynamics.
The following microvascular changes were monitored: diameter, RBC velocity, and blood flow in arterioles (range 42–74 μm) and venules (range 41–78 μm) were measured at each time point. Microvascular diameter and blood flow relative to baseline are presented in Fig. 4 for arterioles (Fig. 4A) and venules (Fig. 4B), and absolute values are presented in the legend. Arteriolar and venular diameters were not affected by 5HMF treatment. O2 hypoxia (10%) produced an increase in arteriolar and venular diameters in both groups compared with baseline and normoxia. O2 hypoxia (5%) produced arteriolar and venular vasodilation only in the 5HMF group compared with baseline and normoxia, and the arteriolar and venular diameters of the vehicle group were not different from baseline or normoxia. The 5HMF group showed significant arteriolar and venular vasodilation compared with the vehicle group. Arteriolar and venular RBC velocities decreased at 5% O2 hypoxia in both groups compared with baseline, although the decrease in arteriolar and venular RBC velocity was significantly more drastic in the vehicle group compared with the 5HMF group. Arteriolar and venular blood flows relative to baseline are presented in Fig. 4, C (arterioles) and D (venules), and absolute values are presented in the legend. Arteriolar and venular blood flows were not affected by 5HMF treatment during normoxia. O2 hypoxia (10%) increased arteriolar blood flow in the 5HMF group compared with the vehicle group. No changes in venular blood flow compared with baseline, normoxia, or between groups were measured at 10% O2 hypoxia. Analogous to the changes in microvascular diameters, the 5HMF group preserved higher arteriolar and venular blood flow compared with the vehicle group during the 5% O2 hypoxia. The blood flows in the vehicle group during the 5% O2 hypoxia were also significantly decreased in arterioles and venules compared with baseline, normoxia, and 10% O2 hypoxia.
Fig. 4.
Relative changes to baseline in arteriolar and venular hemodynamics during the acute hypoxia protocol. Broken line represents baseline level. A: arteriolar diameter. B: venular diameter. C: arteriolar blood flow. D: venular blood flows. Diameters at baseline in each animal group were as follows: 5HMF [arterioles (A): 64.5 ± 7.7 μm, n = 42; venules (V): 69.5 ± 8.9 μm, n = 49] and vehicle (A: 62.4 ± 6.8 μm, n = 44; V: 67.8 ± 9.2 μm, n = 47). n, No. of vessels studied. Red blood cell (RBC) velocities at baseline in each animal group were as follows: 5HMF (A: 4.6 ± 0.6 mm/s; V: 1.7 ± 0.5 mm/s) and vehicle (A: 4.5 ± 0.8 mm/s; V: 1.8 ± 0.6 mm/s). Blood flows at baseline in each animal group were as follows: 5HMF (A: 14.4 ± 4.3 nl/s; V: 6.7 ± 2.3 nl/s) and vehicle (A: 14.6 ± 4.2 nl/s; V: 6.9 ± 2.5 nl/s). †P < 0.05 relative to baseline. ‡P < 0.05 compared with normoxia. §P < 0.05 compared with 10% O2 hypoxia.
Functional capillary density.
FCD at baseline was 114 ± 7 cm−1 for 5HMF and 109 ± 9 cm−1 for the vehicle. 5HMF treatment did not affect FCD (5HMF: 108 ± 10 cm−1; vehicle: 104 ± 9 cm−1). During 10% O2 hypoxia, the FCD was not different from baseline or normoxia (5HMF: 104 ± 8 cm−1; vehicle: 97 ± 10 cm−1) in both groups. O2 hypoxia (5%) produced a significant decrease in FCD (5HMF: 78 ± 6 cm−1; vehicle: 42 ± 10 cm−1) compared with baseline and normoxia in both groups. As a consequence of the hemodynamic changes produced by 5% O2 hypoxia in the vehicle group, the vehicle group had significantly lower FCD compared with the 5HMF group.
Microvascular O2 distribution.
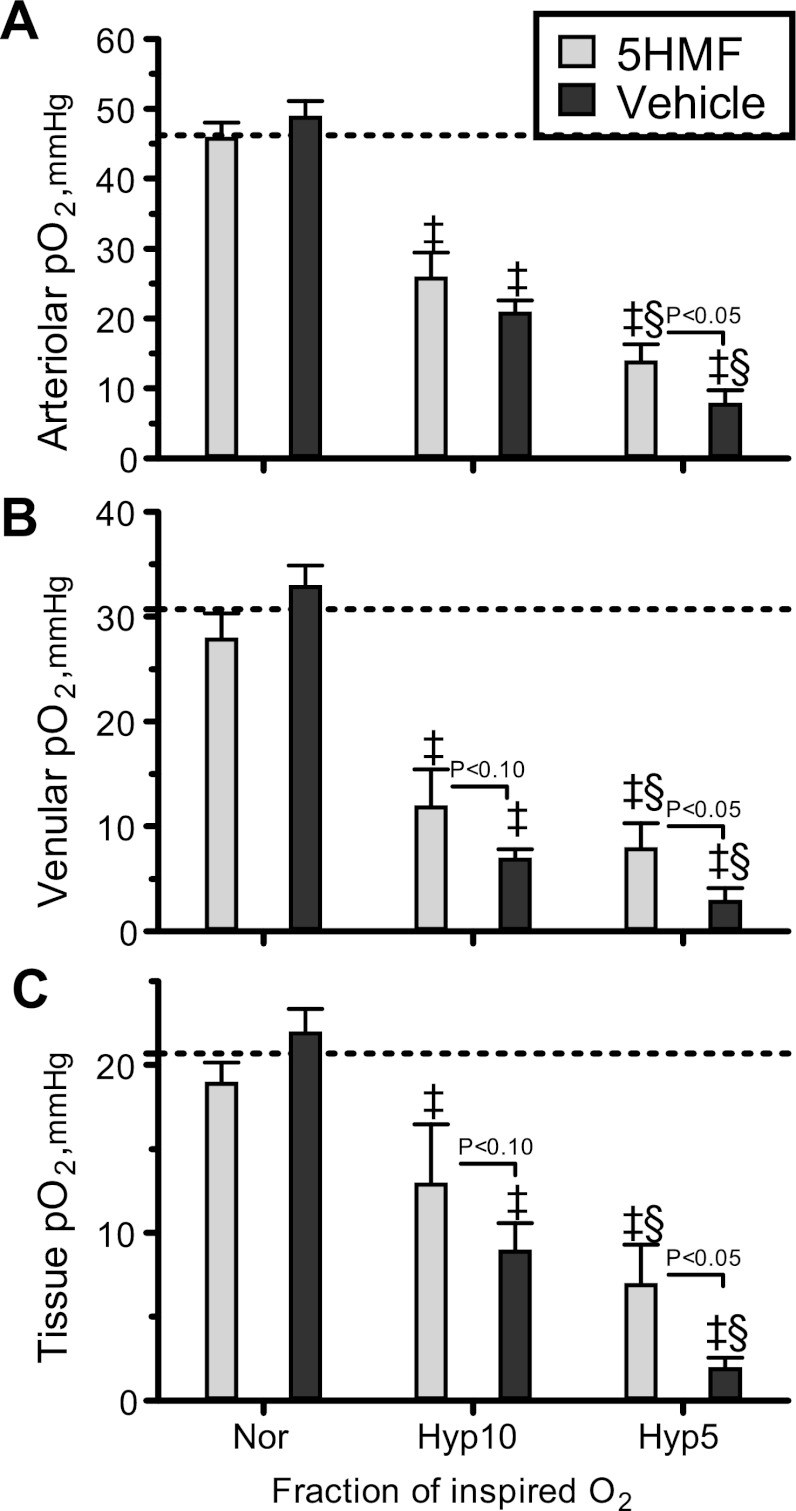
Intravascular and perivascular (tissue) O2 tensions are presented in Fig. 5. During normoxia, 5HMF treatment did not affect arteriolar, venular, or perivascular Po2 compared with the vehicle group. Additionally, 5HMF treatment at normoxia appears not to have a significant effect compared with previous measurements in O2 tension for the specie and the window model preparation. According to previous studies during normoxia, the perivascular Po2 for the hamster window model is 20.9 ± 2.9 mmHg, and intravascular Po2 in arterioles and venules were 46.2 ± 4.8 and 27.0 ± 3.6 mmHg, respectively (3). O2 hypoxia (10%) decreased arteriolar, venular, and tissue Po2 in both groups. The 5HMF group had significantly higher venular and tissue Po2 compared with the vehicle group (P < 0.10). O2 hypoxia (5%) further decreased arteriolar, venular, and tissue Po2 in both groups compared with normoxia and 10% O2 hypoxia, although the vehicle group was significantly reduced compared with the 5HMF group. Perivascular Po2 decreased with the hypoxic level, although 5HMF partially prevented the drastic decrease measured in the vehicle group. Arteriolar O2 saturation in the 5HMF group was 89.1 ± 5.2% during normoxia, 65.0 ± 4.6% during 10% O2 hypoxia, and 26.6 ± 3.5% during 5% O2 hypoxia, whereas the arteriolar O2 saturation in the vehicle group was 74.1 ± 5.0% during normoxia, 32.2 ± 4.0% during 10% O2 hypoxia, and 6.4 ± 1.6% during 5% O2 hypoxia. Therefore, 5HMF increased two- and fourfold in arteriolar So2 relative to vehicle-treated animals at 10 and 5% O2 hypoxia.
Fig. 5.
Intravascular and extravascular (tissue) partial pressure of O2 during the acute hypoxia protocol. ‡P < 0.05 compared with normoxia. §P < 0.05 compared with 10% O2 hypoxia. Broken lines represent baseline Po2 values [A: 46.2 mmHg (A); V: 30.7 mmHg (B); tissue: 20.9 mmHg (C)] (3).
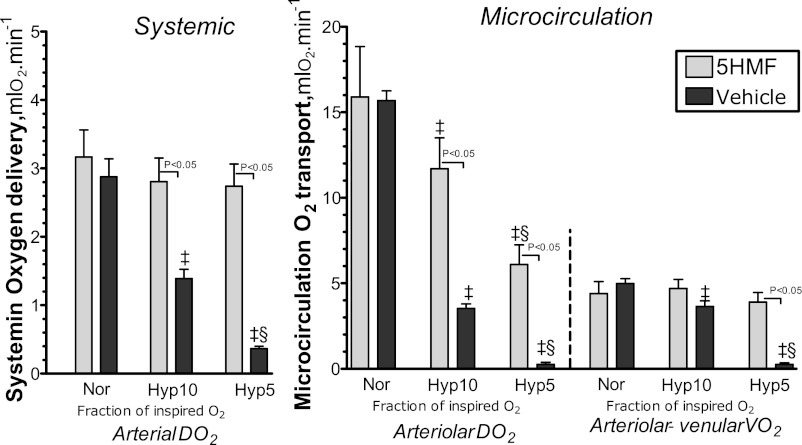
Calculated O2 transport.
Figure 6 shows the analysis of arterial and arteriolar O2 transport, and microvascular O2 extraction. Arterial O2 supply decreased with the degree of hypoxia in both groups. The vehicle group had significantly lower arterial O2 supply compared with the 5HMF group at 10 and 5% O2 hypoxia. Arteriolar O2 supply also decreased with the degree of hypoxia. The vehicle groups had lower arteriolar O2 supply compared with the 5HMF group during hypoxia. Microvascular (arteriolar-venular) extraction reflects the O2 consumed by the tissue, and it remained stable at 10% O2 hypoxia compared with normoxia, but at 5% O2 hypoxia the vehicle group had significantly lower microvascular extraction compared with normoxia, 10% O2 hypoxia, and the 5HMF group. Therefore, at 5% O2 hypoxia, the tissue of the vehicle group was not able to extract sufficient O2 from the O2 transported by the blood.
Fig. 6.
Arterial Do2 and extraction during the acute hypoxia protocol. O2 transport is not directly measurable; however, it can be calculated using the measured parameters. The delivery and extraction were calculated by averaging arterioles and venules of each animal. ‡P < 0.05 compared with normoxia. §P < 0.05 compared with 10% O2 hypoxia.
Pimonidazole binding to mice brain and heart hypoxic tissues.
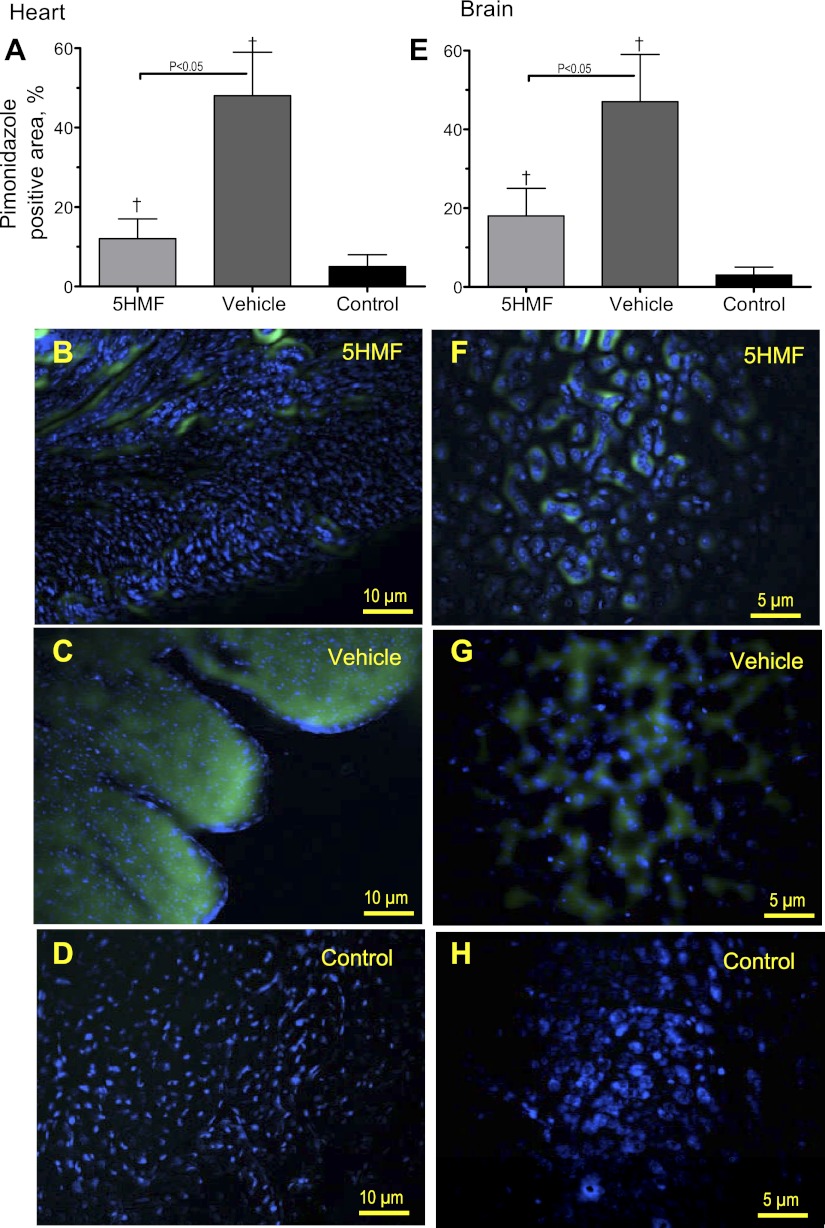
To expand and validate the results obtained using the hamster window model, C57BL/6 mice were treated with 5HMF or vehicle and exposed to an identical hypoxic protocol. Treatment with 20 mg/kg of 5HMF decreased mice blood P50 from 41.5 ± 2.0 mmHg at baseline to 23.8 ± 2.0 mmHg within 60 min, and the change in P50 was maintained through the entire protocol (10% O2 hypoxia: 22.5 ± 1.7 mmHg and 5% O2 hypoxia: 22.0 ± 1.5 mmHg). The P50 of the animals treated with the vehicle was not different from baseline (41.0 ± 2.3 mmHg) at normoxia; however, it decreased at 10 and 5% O2 hypoxia to 39.7 ± 1.9 and 39.2 ± 2.3 mmHg, respectively. Pimonidazole was injected to detect hypoxic areas in the brain and heart tissues. The degree of hypoxia was assessed from binary masked chromogenic images from different areas of the heart and brain. Pimonidazole staining was viewed covering large areas of the heart and brain in mice exposed to hypoxia compared with the control group (Fig. 7, A and E). A groupwise comparison revealed highly significant differences; the vehicle mice demonstrated an approximately fourfold and double higher degree of pimonidazole reactivity than the 5HMF mice in the heart and brain, respectively. Superimposed images (Fig. 7, B-D and F-H) indicate clear colocalization of pimonidazole (green) and Hoechst (blue). Very few areas seemed to be hypoxic in control mice that were not exposed to hypoxia (Fig. 7, D and H). In 5HMF mice (Fig. 7, B and F), some cells stained positive for hypoxia; these cells were scattered and nonuniformly distributed. In the vehicle mice (Fig. 7, B and F), the number of positive cells was visibly higher, and cells were often grouped in multicellular foci. Moreover, in 5HMF mice, the intensely labeled cells often were surrounded by areas of low-intensity intercellular and intracellular staining.
Fig. 7.
Brain and heart pimonidazole binding to hypoxic areas in mice. C57BL/6 mice treated with 5HMF or vehicle were exposed to an identical hypoxic protocol as hamster. Pimonidazole preferentially binds to hypoxic cells, so detection of pimonidazole adducts using monoclonal antibodies can serve as a method for measuring tissue hypoxia. Pimonidazole stained areas in the heart (A–D) and brain (E–H) sections after hypoxia. Superimposed images [hearts (B–D) and brains (F–H)] indicate clear colocalization of pimonidazole (green) and Hoechst (blue). Control animals received pimonidazole but were not exposed to hypoxia. †P < 0.05 compared with control.
DISCUSSION
The principal finding of the study is that 5HMF increased Hb O2 affinity, prevented hemodynamic disturbance produced by severe hypoxia, and partially maintained microvascular oxygenation compared with animals with normal Hb O2 affinity. The decrease in the fraction of inspired O2 decreased arterial So2 and blood O2 content. In animals with normal Hb O2 affinity, hypoxia produced systemic and microvascular hemodynamic changes that further limited tissue oxygenation. 5HMF increased Hb O2 affinity and arterial So2 and O2 content and partially prevented the hemodynamic disturbances induced by severe hypoxia by preserving MAP, CO, and microvascular blood flow. The increase in Hb O2 affinity during hypoxia partially preserved systemic O2 delivery (Fig. 3C). These results suggest that the hemodynamic adjustments to maintain blood flow and O2 supply during hypoxia are limited by arterial and arteriolar So2. Increased Hb O2 affinity during normoxia had minor effects on perivascular Po2, although it maintained tissue O2 extraction by generating the appropriate Po2 gradients to extract sufficient O2. Thus, 5HMF treatment increased Hb O2 affinity, provided an advantage for blood O2 loading, and increased arterial So2 during severe hypoxia, preserving blood pressure and HR, compared with vehicle-treated animals, and supported microvascular blood flow and oxygenation. These changes consequently increased microvascular O2 delivery and maintained O2 extraction and tissue Po2 compared with animals with normal Hb O2 affinity during severe hypoxia. Additionally, these results appeared not to be species specific, since staining for hypoxic tissues in heart and brain of mice suggests that the increased Hb O2 affinity after 5HMF treatment reduced hypoxic areas in mice in a similar hypoxia protocol.
5HMF acute adaptation to extreme hypoxic environments is very significant since chronic physiological adaptation requires time, as several systems (i.e., cardiovascular, pulmonary, endocrine) gradually adjust. Our results suggest that 5HMF may prevent tissue anoxia during severe hypoxia and reduce the incidence of AMS, HACE, and HAPE. 5HMF has also been reported to prevent sickling and hemorheological complications induced by hypoxia in sickle mice (1). 5HMF protective effects in sickle cell disease and during severe hypoxia are associated with an increase in Hb O2 affinity and the maintenance of central hemodynamics. While cardiac function is limited by myocardial O2 delivery (12), the overall cardiac dysfunction observed in animals with normal Hb O2 affinity during severe hypoxia restricted the maximum hydraulic cardiac power (MAP × HR) to 50% of the hydraulic cardiac power in the 5HMF-treated animals. The preservation of cardiac function induced by 5HMF increased CO and systemic O2 delivery to vital organs, including the heart and brain. Moreover, 5HMF effects in O2 delivery to vital organs are derived from the increase in Hb O2 affinity, as confirmed by pimonidazole hypoxic staining in heart and brain tissues in mice. Although no neurological function was measured in this study, during 5% O2 hypoxia, 5HMF animals were alert, whereas control animals were lethargic. These results suggest that 5HMF can improve survivability at 5% O2 (38 mmHg) without a hypoxic adaptation process at an O2 partial pressure lower than the highest place on earth (Mount Everest, 43 mmHg, 5.6% O2).
A decrease of 50% in the fraction of inspired O2 is mostly compensated for through vasodilation and increased blood flow, although, as the inspired O2 reaches a critical limit, the changes in vascular tone and resistance become ineffective to maintain O2 delivery. 5HMF-increased Hb O2 affinity allowed the physiological response to hypoxia to be extended to 5% inspired O2, where vasodilation and the increase in blood flow favored oxygenation. At 5% O2 hypoxia, VR in hamsters treated with 5HMF decreased to 58% of baseline, whereas the resistance in vehicle-treated hamsters was 70% of baseline. Increasing blood O2 content during hypoxia by increased Hb O2 affinity also has positive systemic hemodynamic effects. Hamsters treated with 5HMF had an 87% higher CO compared with vehicle-treated hamsters. Similar advantage was previously described in studies by Turek et al. (27, 28) as enhanced on O2 pulmonary uptake. Their results showed that an increase in Hb O2 affinity provides an advantage for tissue O2 delivery under severe hypoxemia, which increased CO and redistributed blood flow to vital organs (28). Although FCD decreased in both groups with the reduction of inspired O2, the increased Hb O2 affinity preserved FCD above 50%, suggesting redistribution of blood flow for 5HMF-treated animals. The arteriolar vasoconstriction in the vehicle-treated hamsters during severe hypoxia, with a consequential effect on perfusion, can be attributed to the autoregulatory process that attempts to maintain O2 supply to vital organs while compromising peripheral tissues (17). 5HMF treatment mediated a rapid adaptive process that maintained microvascular function and partially preserved oxygenation during severe hypoxia.
The hamster chamber model includes muscle and connective tissue with stable O2 consumption; therefore, changes of O2 supply determine tissue oxidative state. Comparing our published intravascular and perivascular Po2 for the hamster chamber model (3), 5HMF did not affect intravascular Po2 and So2 values for arterioles and venules; however, perivascular (tissue) Po2 appeared to decrease in 5HMF-treated animals (P < 0.10), whereas, during 10 and 5% hypoxia, the 5HMF group arteriolar So2 was two- and fourfold in the arteriolar So2 of the vehicle group, respectively. At 10% O2 hypoxia, venule So2, which reflects O2 extraction, increased fourfold for the 5HMF compared with the control group, and, at 5% O2 hypoxia, the difference in venular So2 was sixfold. Although these changes in So2 appear dramatic, they reflect the range in the Hb O2 equilibrium curve where each group of animals manages their O2 exchange (Fig. 2). During 10 and 5% O2 hypoxia, the 5HMF group maneuvers O2 within the steepest section of the Hb O2 equilibrium curve, whereas the vehicle group operates at a lower section of their Hb O2 equilibrium curve. In the present study, hypoxia increased the P50 from 32.6 to 34.1 and to 35.4 mmHg at 10 and 5% O2 in the hamsters. This appears to result from an augmented metabolic acidosis, whereas the P50 in mice decreased from 41.0 to 39.7 and 39.2 at 10 and 5% O2, suggesting a respiratory alkalosis. Hypoxia may induce different ventilation changes in both species; however, 5HMF's protective effect during hypoxia appears to be specific to increasing Hb O2 affinity and is independent of the animal specie.
Experimental evidence in isolated canine muscle and humans supports that the rate at which O2 diffuses from the RBC to the tissue determines maximal O2 uptake (11). The O2 diffusion can be divided in the O2 conductance within the tissue and the release from the RBC Hb to the tissue (11). For a given O2 transported by the blood (flow × O2 content), the O2 extracted by tissue is determined by the Po2 gradient between the blood and the tissue. Therefore, as the O2 conductance is held constant, Hb O2 affinity regulates the rate at which the O2 is released. Therefore, changes in Hb O2 affinity affect the radial Po2 gradient between blood vessels and perivascular tissues required to satisfy the flux of O2, leaving the blood vessel to maintain the O2 consumed by perivascular tissues (26). Previous results obtained by Stein and Ellsworth (24) suggest that an increase in Hb O2 affinity did not affect the RBC supply rate, a measurement of convective O2 transport, between animals with high O2 affinity and controls during hypoxia. Their first study concludes that the amount of O2 lost across the capillary network, which reflects the O2 extraction during hypoxia (10% inspired O2), was not affected by Hb O2 affinity (24). Their results are consistent with the present results, where the high-affinity and control groups at normoxia and moderate hypoxia had similar arteriolar-venular O2 extraction (Fig. 5) even though arteriolar supply was higher for the group with increased Hb O2 affinity (24). Stein and Ellsworth (24) also found that blood pressure and arteriolar blood flow were higher in animals with increased Hb O2 affinity during hypoxia. In a subsequent paper, Stein and Ellsworth (23) found a beneficial effect of an increase in O2 affinity during anemia (40% reduction in systemic Hct) and hypoxia (10% inspired O2). The practicality and limited toxicity of the acute treatment with 5HMF (20 mg/kg) provides significant benefits compared with sodium cyanate (0.2 mg/kg 5 times/2 wk) used by Stein and Ellsworth, which creates similar changes in Hb O2.
The present study was carried out to determine the systemic and microvascular hemodynamic changes induced by acute modifications in Hb O2 affinity during severe and extreme hypoxia. The principal hemodynamic change identified in the study was the preservation of central and peripheral hemodynamics in the animals with higher Hb O2 affinity by partially preserving O2 delivery. A reduced fraction of inspired O2 limits the amount of O2 that can be bound to the Hb in pulmonary circulation; therefore, by simply increasing Hb O2 affinity, the O2 transported to the tissues is increased. VR was not affected by the increase in Hb O2 affinity during normoxia. O2 hypoxia (10%) decreased VR equally, independently of Hb O2 affinity, whereas, at 5% O2 hypoxia, the group with increased Hb O2 affinity showed lower VR compared with unmodified Hb O2 affinity. The VR in the vehicle group decreased to 67% of baseline during 5% O2 hypoxia, whereas in the animals with increased Hb O2 affinity the VR decreased to 60% of baseline. Previous studies had shown similar effects in VR by increasing P50 at any given level of arterial O2 content, consistent with the concept that O2 transport is regulated in specific vascular beds (16).
Limitation of the study.
Hamsters are fossorial animals, which explains their low arterial Po2 at normoxia, since their respiratory exchange is adapted to their burrow environment (25). Other fossorial rodents withstand conditions of extreme hypoxia; however, hamsters are sensitive to hypoxia and hypercapnia (25, 31). Hamsters and humans have different ventilation regulation during hypoxia; hamsters increase their ventilation during hypoxia primarily by increasing frequency with minor changes in tidal volume, which results in minimal activation of pulmonary stretch receptors (31). Additionally, hamster's vasodilatation during hypoxia might be limited for humans, since hypoxic hypocapnia in humans produces central inhibition of the peripheral chemoreflex and induces peripheral vasoconstriction (15). Small animals have a high HR; this high HR reduces the fraction of time spent in diastole, thus shortening the time for subendocardial perfusion. Therefore, hamster and mice critical fraction of inspired O2 level may be higher than other larger mammals with slower HR. Hamster's high baseline HR limits the increase in CO during hypoxia; therefore, their limited CO response to hypoxia appears to produce metabolic acidosis. Therefore, the early phase of human acclimatization to high altitude is different from the hamster, since it includes respiratory alkalosis producing a small increase in Hb O2 affinity, which is later offset by a decrease by 2,3-DPG (33). The benefits of 5HMF in humans may be limited by hypocapnia, which decreases P50. The results of the current study are limited to hypoxic hypoxia; they do not account for the changes in barometric pressure and air dryness that occur at high altitude. The extent of hypoxia used in our study was selected to determine the effects of increased Hb O2 affinity in the early acclimatization process to hypoxia, before 2,3-DPG and erythropoietin affect O2 transport.
In conclusion, this study indicates that 5HMF increases tolerance to normobaric severe hypoxia without an adaptation process. The potent effects on Hb O2 affinity, low molecular weight, high bioavailability, and low toxicity of 5HMF, make it an attractive candidate drug for further detailed studies as a treatment for acute adaptation to extreme hypoxic environments. 5HMF protective mechanisms appear closely associated to the increase of Hb O2 affinity, extending physiological hemodynamic responses and changing microvascular O2 transport. This suggests that O2 content rather than Po2 is the critical determinant of O2 supply during hypoxia. Further studies may be necessary to better understand how the protective role of 5-HMF in response to severe hypoxia affects O2 transport from the blood to the mitochondria.
GRANTS
This work was partially supported by Bioengineering Research Partnership Grant R24-HL-64395, Program project P01-HL-071064, and Grants R01-HL-52684, R01-HL-62354, and R01-HL-62318 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: O.Y. and P.C. performed experiments; O.Y. and P.C. prepared figures; O.Y. and P.C. drafted manuscript; P.C. conception and design of research; P.C. analyzed data; P.C. interpreted results of experiments; P.C. edited and revised manuscript; P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
REFERENCES
- 1. Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol 128: 552–561, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Antal MJ, Jr, Mok WS, Richards GN. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose an sucrose. Carbohydr Res 199: 91–109, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Cabrales P. Low oxygen-affinity hemoglobin solution increases oxygenation of partially ischemic tissue during acute anemia. J Am Coll Surg 210: 271–279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabrales P, Acero C, Intaglietta M, Tsai AG. Measurement of the cardiac output in small animals by thermodilution. Microvasc Res 66: 77–82, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Cabrales P, Tsai AG, Frangos JA, Intaglietta M. Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic Biol Med 39: 1229–1237, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cabrales P, Tsai AG, Intaglietta M. Modulation of perfusion and oxygenation by red blood cell oxygen affinity during acute anemia. Am J Respir Cell Mol Biol 38: 354–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol Heart Circ Physiol 246: H508–H517, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med 177: 125–134, 1980 [DOI] [PubMed] [Google Scholar]
- 9. Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, Berger EM. Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest 62: 593–600, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hepple RT. Skeletal muscle: microcirculatory adaptation to metabolic demand. Med Sci Sports Exerc 32: 117–123, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Hogan MC, Bebout DE, Wagner PD. Effect of increased Hb-O2 affinity on V̇o2max at constant O2 delivery in dog muscle in situ. J Appl Physiol 70: 2656–2662, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Holloway CJ, Montgomery HE, Murray AJ, Cochlin LE, Codreanu I, Hopwood N, Johnson AW, Rider OJ, Levett DZ, Tyler DJ, Francis JM, Neubauer S, Grocott MP, Clarke K. Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 25: 792–796, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res 5: 309–312, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Kerger H, Groth G, Kalenka A, Vajkoczy P, Tsai AG, Intaglietta M. pO2 measurements by phosphorescence quenching: characteristics and applications of an automated system. Microvasc Res 65: 32–38, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Koehler RC, McDonald BW, Krasney JA. Influence of CO2 on cardiovascular response to hypoxia in conscious dogs. Am J Physiol Heart Circ Physiol 239: H545–H558, 1980 [DOI] [PubMed] [Google Scholar]
- 16. Koehler RC, Traystman RJ, Jones MD., Jr Influence of reduced oxyhemoglobin affinity on cerebrovascular response to hypoxic hypoxia. Am J Physiol Heart Circ Physiol 251: H756–H763, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Ledingham JM. Autoregulation in hypertension: a review. J Hypertens Suppl 7: S97–S105, 1989 [PubMed] [Google Scholar]
- 18. Lenfant C, Sullivan K. Adaptation to high altitude. N Engl J Med 284: 1298–1309, 1971 [DOI] [PubMed] [Google Scholar]
- 19. Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res 15: 93–101, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Mairbaurl H, Oelz O, Bartsch P. Interactions between Hb, Mg, DPG, ATP, and Cl determine the change in Hb-O2 affinity at high altitude. J Appl Physiol 74: 40–48, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Rasmussen A, Hessov I, Bojsen-Moller M. General and local toxicity of 5-hydroxymethyl-2-furfural in rabbits. Acta Pharmacol Toxicol (Copenh) 50: 81–84, 1982 [DOI] [PubMed] [Google Scholar]
- 22. Roach RC, Hackett PH. Frontiers of hypoxia research: acute mountain sickness. J Exp Biol 204: 3161–3170, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Stein JC, Ellsworth ML. Capillary oxygen transport during severe hypoxia: role of hemoglobin oxygen affinity. J Appl Physiol 75: 1601–1607, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Stein JC, Ellsworth ML. Microvascular oxygen transport: impact of a left-shifted dissociation curve. Am J Physiol Heart Circ Physiol 262: H517–H522, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Tomasco IH, Del Rio R, Iturriaga R, Bozinovic F. Comparative respiratory strategies of subterranean and fossorial octodontid rodents to cope with hypoxic and hypercapnic atmospheres. J Comp Physiol B 180: 877–884, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev 83: 933–963, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Turek Z, Kreuzer F, Ringnalda BE. Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch 376: 7–13, 1978 [DOI] [PubMed] [Google Scholar]
- 28. Turek Z, Kreuzer F, Turek-Maischeider M, Ringnalda BE. Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch 376: 201–207, 1978 [DOI] [PubMed] [Google Scholar]
- 29. Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem 262: 5476–5482, 1987 [PubMed] [Google Scholar]
- 30. Villela NR, Cabrales P, Tsai AG, Intaglietta M. Microcirculatory effects of changing blood hemoglobin oxygen affinity during hemorrhagic shock resuscitation in an experimental model. Shock 31: 645–652, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Walker BR, Adams EM, Voelkel NF. Ventilatory responses of hamsters and rats to hypoxia and hypercapnia. J Appl Physiol 59: 1955–1960, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Winslow RM, Monge CC, Statham NJ, Gibson CG, Charache S, Whittembury J, Moran O, Berger RL. Variability of oxygen affinity of blood: human subjects native to high altitude. J Appl Physiol 51: 1411–1416, 1981 [DOI] [PubMed] [Google Scholar]
- 33. Winslow RM, Samaja M, West JB. Red cell function at extreme altitude on Mount Everest. J Appl Physiol 56: 109–116, 1984 [DOI] [PubMed] [Google Scholar]