Abstract
Mitofusins (Mfn-1 and Mfn-2) are transmembrane proteins that bind and hydrolyze guanosine 5′-triphosphate to bring about the merging of adjacent mitochondrial membranes. This event is necessary for mitochondrial fusion, a biological process that is critical for organelle function. The broad effects of mitochondrial fusion on cell bioenergetics have been extensively studied, whereas the local effects of mitofusin activity on the structure and integrity of the fusing mitochondrial membranes have received relatively little attention. From the study of fusogenic proteins, theoretical models, and simulations, it has been noted that the fusion of biological membranes is associated with local perturbations on the integrity of the membrane that present in the form of lipidic holes which open on the opposing bilayers. These lipidic holes represent obligate intermediates that make the fusion process thermodynamically more favorable and at the same time induce leakage to the fusing membranes. In this perspectives article we present the relevant evidence selected from a spectrum of membrane fusion/leakage models and attempt to couple this information with observations conducted with cardiac myocytes or mitochondria deficient in Mfn-1 and Mfn-2. More specifically, we argue in favor of a situation whereby mitochondrial fusion in cardiac myocytes is coupled with outer mitochondrial membrane destabilization that is opportunistically employed during the process of mitochondrial permeability transition. We hope that these insights will initiate research on this new hypothesis of mitochondrial permeability transition regulation, a poorly understood mitochondrial function with significant consequences on myocyte survival.
Keywords: membrane permeability, cell death, cardiac myocytes, hemifusion, dynamin-related proteins
this article is part of a collection on Mitochondria in Cardiovascular Physiology and Disease. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
A Common Membrane Fusion Principle for Multiple Biological Applications
Membrane fusion is a basic function that underlies numerous cellular processes. It is essential in viral entry, neurotransmitter exocytosis, endocytic cargo trafficking, autophagic degradation, endoplasmic reticulum (ER) morphogenesis, and mitochondrial dynamics. Fusion and its opposite process fission have evolved to maintain the biological membrane at a dynamic state that can better adapt to the changing needs of cells. Mutations in genes involved in membrane remodeling are linked to heritable neurodegenerative conditions such as optical nerve atrophy, spastic paraplegia, axonal neuropathy, and centronuclear myopathy (5, 92, 102, 145, 148, 149), illustrating the importance of membrane dynamics in human disease. The list of human pathologies associated with alterations in the activities of membrane remodeling proteins is likely to expand as we obtain more details on the workings of membrane dynamics. One topic in membrane dynamics that has remained relatively unexplored is to what extent fusion is capable of altering the barrier function of the membrane. Below, we discuss common principles in fusion and then present models that predict fusion-related membrane leakage.
Membrane fusion requires the merging of two lipid bilayers that, in biological systems, depends on specific protein catalysts (fusogens). The different steps necessary for a membrane fusion reaction have been delineated using prototypical fusogens such as the hemagglutinin (HA) protein of the influenza virus and the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complexes (40, 68). A concept that emerged from these investigations is that despite their essential roles in distinct biological processes, these unrelated fusogenic proteins induce the formation of intermediate fusion conformations in membranes that are strikingly similar (26, 115, 143). These data suggest that during membrane fusion, a universal pathway is in operation, regardless of the molecular identity of the protein catalyst that mediates the actual reaction (84, 139). A key membrane structure that appears to be common in many fusion reactions is the hemifusion stalk where the external leaflets of two adjoining bilayers become continuous (by forming a stalk), whereas the leaflets lining the distal sides of the bilayers remain intact and independent from each other (27). Given the widespread use of this mechanism in many fusion processes (28), it is reasonable to assume that fusion of mitochondrial membranes progresses through formation of hemifusion intermediates.
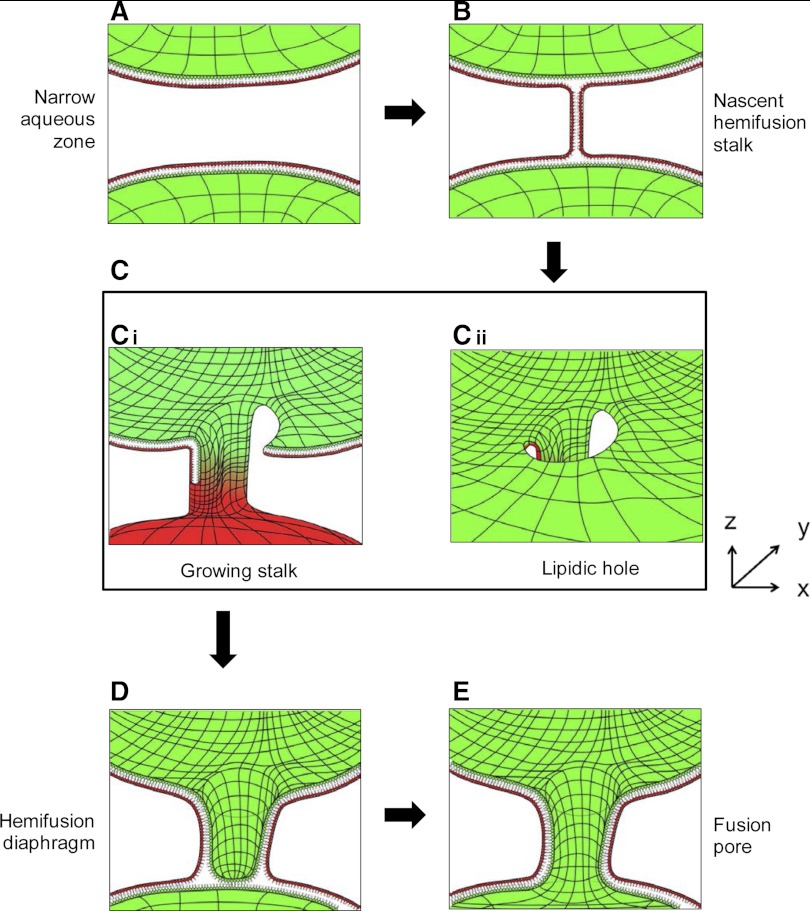
Membrane fusion is considered to be an energetically costly reaction, and among the many intermediate states that are formed during the fusion pathway, it appears that formation of the hemifusion intermediate is the most energetically favorable. In fact, given its relatively low state of free energy, it has been possible to observe the hemifusion stalk experimentally (144). Notably, however, the membranes are only fused halfway when hemifusion structures are formed. Therefore, additional rearrangements are required for the resolution of the hemifusion structure into a site of successful fusion (i.e., a fusion pore). Currently, two models of hemifusion resolution are available. The classical model posits that the neck-like hemifusion stalk is radially expanded into a hemifusion diaphragm, and, subsequently, a hole forms inside this bilayered septum leading to the opening of a fusion pore (71, 74). An alternative model suggests that the neck-like stalk does not expand radially into a diaphragm, but instead it assumes a filamentous configuration that elongates and encircles the area that will evolve into a fusion pore (Fig. 1). This crawling of the elongating stalk destabilizes the membranes and, importantly, elicits the opening of a hole in at least one of the two bilayers (Fig. 1). As the stalk grows in a circular motion, it circumscribes the newly formed hole, thereby creating a structure that is equivalent to the hemifusion diaphragm (95, 122). At this stage, a fusion pore opens on the bilayered septum that initiates the continuity between the two fusing compartments. Whether the classical or the alternative (Fig. 1) model will occur is likely to be influenced by the phospholipid and protein composition of the biological membranes. However, theoretical calculations support that the alternative model is generally more favorable (72). Consistent with these theoretical aspects, the formation of a lipidic hole near the stalk has been frequently observed in a number of membrane fusion simulations (83, 96, 103). Therefore, the formation of a lipidic hole during the stalk elongation step appears to be integral to the fusion process, and it is conceivable that this event can induce transient leakiness to the fusing membranes.
Fig. 1.
Proposed pathway illustrating the relationship between fusion and permeabilization during the early stages of mitochondrial fusion. The underlying mechanism is derived from membrane fusion models where artificial amphiphiles were used to simulate the behavior of phospholipids (95). The mechanism is adapted here to describe the early events in fusion of the outer mitochondrial membranes (OMM). Only the OMMs of two adjacent mitochondria are shown, whereas the inner mitochondrial membranes (IMMs) are omitted for clarity. In these cross sections, the inner leaflet of the lipid bilayer (facing the intermembrane space) is shown in green, whereas the outer leaflet of the bilayer (facing the cytoplasm) is shown in red. Lines were added to the surface of the monolayers to provide perspective. The mitofusins (Mfn-1 or Mfn-2) are embedded in the OMM and likely participate throughout these series of events but were omitted here for clarity. A: close mitochondrial proximity. One mitochondrion (A, top) is brought into extreme proximity to the OMM of another mitochondrion (A, bottom). B: formation of a hemifusion stalk. At this stage, the phospholipids of the outer leaflet (red) traverse the very narrow sheath of water that separates the two bilayers to create a stalk that connects the outer leaflets (red), leaving the inner leaflets (green) intact. C: stalk growth and hole formation. During its establishment, the stalk acquires nearby phospholipids. This is accompanied by the thinning of the bilayer at the vicinity of the stalk, and this area eventually collapses into a hole. The stalk can now acquire phospholipids from both the outer and inner leaflets (Ci), which markedly potentiates its growth in the y-dimension. Thus the circular motion of the growing stalk (referred to as “crawling”) is energetically linked to the production of a hole. Cii: perspective of the newly formed hole while looking from the intermembrane space (IMS) and towards the cytoplasm. At this stage the IMS can become accessible to the external environment, i.e., the permeability barrier of the OMM is breached. D: encircling the hole and resealing. As the neck-like stalk continues to grow, its circular motion allows it to fully encircle the hole to form a structure resembling an empty cylinder (shown here in cross section). At this stage the membrane continuity has been reestablished and the permeability barrier is resealed. This new steady state of the fusing membranes can be envisioned as a hemifusion diaphragm. E: formation of the fusion pore. Shortly after closure into a hemifusion diaphragm, a second hole forms into the newly circumscribed bilayer. This event marks the onset of a fusion pore where the aqueous IMS of the two fusing mitochondria become contiguous. The early fusion pore can be expanded to the x and y plane to facilitate content exchange between IMSs and possibly accommodate the initiation of a fusion reaction between the underlying IMMs.
The phospholipid composition can be important in determining the fusogenic properties of membranes and could influence the formation of stalk/hole hemifusion intermediates. In human heart, phosphatidylcholine (PC) and phosphatidylethanolamine (PE), respectively, account for about 47 and 25% of total mitochondrial phospholipid content, whereas cardiolipin (CL) accounts for 12% (124). CL distribution is restricted to mitochondrial membranes; it is mainly present in the inner mitochondrial membrane (IMM), although a small but functionally important fraction is also located in the outer mitochondrial membrane (OMM) (47). Other less abundant mitochondrial phospholipids include phosphatidic acid (PA), phosphatidylserine (PS), and phosphatidylinositol (PI). The molecular shape of phospholipids can be cylindrical (e.g., PC, PI, and PS) or approximate that of a truncated cone (e.g., PE, CL, and PA), if there is a mismatch in the size of the polar head group relative to the hydrophobic domain (107). Conical phospholipids are subcategorized into cone-shaped and inverted cone-shaped species, depending on whether the diameter of the polar head is narrower or wider than the hydrophobic domain, respectively. These phospholipids and their distribution in bilayers are particularly important in determining the fusion properties of biological membranes (29). Thus it can be speculated that local enrichment of cone-shaped phospholipids such as PE and CL (and its derivative PA) produces curvature at regions where the stalk emerges from the otherwise flat monolayer. On the other hand, inverted cone-shaped phospholipids (e.g., lyso-PC and lyso-PE) may be found in regions delineating the hole (80). Hydrolytic/lipid-modifying enzymes and fence/scaffolding proteins that influence the availability and distribution of conical phospholipids are likely to act in concert with mechanoenzyme fusogens to locally alter the architecture of the membrane, thereby favoring the creation and resolution of these stalk-and-hole hemifusion intermediates.
Experimental Evidence for Leakiness of the Membrane During Fusion
The theoretical models described above suggest that the membrane fusion reaction is linked to transient loss of the permeability barrier (i.e., leakage). Although these conclusions were initially derived from experimental models simulating the behavior of protein-free phospholipid bilayers, similar observations have also been made in protein-regulated experimental systems that recapitulate more closely the fusion of biological membranes (41). In early studies that used HA to examine the fusion of the viral envelope to liposomes, it was found that the target membrane (liposome) could become permeabilized during the fusion process and allow the passage of dextrans with a molecular mass of up to 10 kDa (125). Subsequent experiments also detected leakage of encapsulated components (ranging from small water-soluble fluorophores to fluorescently-labeled dextrans) in membrane fusion reactions using HA (19, 51, 79). In another study, evidence for membrane permeabilization during HA-mediated cell fusion was obtained when the integrity of the membrane was monitored with conductance electrodes (45). This analysis detected increases in the permeability of the plasma membrane that coincided with the onset of fusion. This tight temporal correlation between fusion and permeabilization argues that the formation of leaky holes on membranes is inherent to the fusion reaction catalyzed by HA (45). Furthermore, the permeabilization in this system was found to be transient, in agreement with the theoretical predictions which suggest that the leaky holes are resealed as the fusion process progresses (see also Fig. 1D).
Leakage across membranes undergoing fusion has been found to occur in experimental systems employing proteins different from HA. In yeast, the homotypic fusion between vacuoles (a yeast lysosome) is primed when unpaired R-SNAREs expressed on one membrane interact with their cognate partner Q-SNARE on another membrane to form trans complexes (101, 140). SNARE pairing (an early event in vacuolar fusion) depends on the presence of proton (H+) gradient across membranes, generated by the vacuolar ATPase (133). Building the proton gradient requires that membranes remain sealed during early fusion, but it is unclear what happens to the proton gradient or the membrane itself during subsequent steps. What is known, however, is that the hemifusion intermediate is formed during this type of fusion (116). Interestingly, a number of studies examining intermediate or late fusion events reported the transient release of lumenal Ca2+ from vacuoles (90, 112) that occurs in the same time frame during which a hemifusion intermediate is formed. A number of studies have suggested that a proteinaceous channel is responsible for the observed Ca2+ efflux (14, 90, 112), but direct evidence is lacking. According to the working model presented here, the Ca2+ efflux observed during vacuolar fusion may be attributable, at least in part, to membrane destabilization at the site of hemifusion formation (Fig. 1). Furthermore, the finding that the Ca2+ flux vanishes as fusion progresses (90) is reminiscent of the situation where the leaky sites are resealed as the fusion pore expands.
More recently, experiments performed with purified vacuolar SNAREs reconstituted in proteoliposomes demonstrated that the minimum protein complement necessary for fusion is also sufficient to induce permeability in these vesicles (147), further corroborating the notion that fusion commonly involves the opening of the membrane barrier. More specifically, this study noted that concomitant with the fusion reaction (SNARE activation, membrane merging, and content exchange), the vacuole lumen became accessible to dithionite, a low molecular mass reducing agent. This study also distinguished between the different outcomes that fusion could have on proteoliposome integrity. On one hand, proteoliposomes undergoing fusion could become permeable for small molecules (e.g., dithionite, biotin) but prevent passage of larger ones, indicating the maintenance of overall membrane integrity. On the other hand, the localized increase in permeability could sometimes evolve into a more pervasive destabilization of the membrane that permitted the release of substantially larger molecules (i.e., dextrans of 10 or 70 kDa), a situation that is akin to lysis rather than transient permeability (147). In this regard, it had been previously found that enforced expression of the four vacuolar SNAREs not only could elevate membrane fusion rates but also promoted substantial lysis (128). One factor that regulates the progression from low-scale permeability to lysis appears to be the aberrant enrichment of SNAREs in the fusion microdomain. Furthermore, studies report different degrees of lysis in reconstituted fusion experiments (91, 142), and the induction of lysis is probably influenced by many other variables, including the presence of accessory proteins in the reaction, the phospholipid content and size of the fusing vesicles, the molecular ratio between proteins:phospholipids on the liposome, and the temperature of the fusion reaction. Regardless of whether the outcome is transient membrane permeability or lysis, all of these studies support the concept that a perturbation in membrane continuity is inherent to the fusion reaction per se.
The efflux of Ca2+ during membrane fusion has also been detected in other experimental systems. Assays designed to study the homotypic fusion of small vesicles during tubular ER formation have typically detected Ca2+ efflux from these vesicles (135). This phenomenon was so robust that the release of Ca2+ was used as a readout of the effectiveness of the fusion reaction (135). Furthermore, the Ca2+ flux correlated with the addition of GTP that stimulated fusion in this in vitro system, indicating that GTP-hydrolyzing enzymes drive vesicle fusion and concomitantly trigger Ca2+ release.
In agreement with the GTP requirement, a key mediator of ER membrane fusion is the GTP-hydrolyzing protein atlastin (64, 106). Atlastins form a conserved family of proteins within the broader class of dynamin-related proteins (DRPs) (114). The atlastin polypeptide traverses the ER membrane twice and presents both its NH2- and COOH-termini to the cytoplasm. The GTPase activity is mapped to the globular domain that is proximal to the NH2-terminus. When bound to GTP, this globular domain is capable of forming homotypic interactions and mediates the engagement of an adjacent atlastin molecule on the opposing ER membrane (18, 23). Following GTP hydrolysis and inorganic phosphate release, the nascent atlastin dimer undergoes conformational changes, distorting the underlying bilayers, and it relaxes only after the membranes have undergone fusion (63). Additionally, the amphipathic COOH-terminus of atlastins is suggested to interact with the proximal leaflet of the bilayer and this could potentiate the formation of the hemifusion intermediate during the membrane fusion process (94). Because atlastin-mediated membrane fusion has been successfully recapitulated in vitro using the Drosophila isoform following reconstitution in proteoliposomes (18, 106), it would be interesting to test whether these dynamin-like GTPase proteins are able to induce transient loss in the permeability of fusing compartments using experimental systems of minimal complexity.
Membrane Permeability and Mitochondrial Transition
Early experiments performed with isolated mitochondria observed the ability of these organelles to undergo abrupt increases in membrane permeability through a process that was triggered by exogenously added Ca2+ (65). Furthermore, this increase in permeability was found to be reversible, and membranes could reseal if Ca2+ was diluted with other divalent cations or chelated (4, 65). During this mitochondrial transition, various solutes (sucrose, glucose, K+, H+, Mg2+, and Ca2+) could be observed to enter or exit the mitochondrial matrix with no selectivity for charge, whereas the passage of molecules with a size >1.5 kDa was restricted or very slow (55). Together, these studies have led to the concept that the mitochondrial permeability transition (MPT) is a regulated process that is mediated by a proteinaceous channel with Ca2+ binding sites (56). A consensus of pharmacological, biochemical and genetic evidence has established that the Ca2+ sensitivity necessary for the MPT is conferred by the matrix chaperone cyclophilin D (Cyp-D) (9, 13, 21, 35, 53, 97, 123). On the other hand, the evidence with regard to the identity of a proteinaceous pore that spans the dual mitochondrial membranes and mediates the passage of solutes has been inconclusive. Affinity purification with Cyp-D and reconstitution experiments in liposomes indicated that the adenine nucleotide translocator and voltage-dependent anion channel (VDAC) are important elements for MPT (17, 22, 36, 87, 118), but mouse genetic studies demonstrated that the loss of adenine nucleotide translocator and VDAC had very little or no impact on the MPT process (10, 75, 77).
To date, it has been difficult to demonstrate that a certain channel or a transporter is essential for MPT, raising the possibility that nonconventional mechanisms may operate to permeabilize mitochondria. Along these lines, it has been proposed that mitochondrial transition may be triggered nonspecifically by various misfolded proteins that form aggregates inside the mitochondrial membranes (57). The identification of a single proteinaceous channel has been further complicated by the fact that mitochondria are surrounded by two sets of biological membranes. The importance of the OMM in MPT has been discounted on the basis that the initial stimulus for MPT occurs primarily in the matrix and the IMM (16). Furthermore, the OMM is viewed as a low permeability barrier to the organelle because it is enriched in VDAC, presumably making it readily passable to ions and metabolites (33). However, altering the barrier properties of the OMM by genetically removing VDAC has no effect on the MPT process (10). Although the OMM is thought to be only secondarily implicated in MPT, data suggest that the status of the OMM can significantly alter the MPT process. For example, a comparison of the MPT in mitochondria and mitoplasts (mitochondria that are stripped from their outer membrane) showed that mitoplasts were more susceptible to undergo MPT than mitochondria bearing the native double-membrane arrangement (127). Based on these considerations, it can be suggested that, although the OMM is not the primary site for MPT initiation, it can modulate the MPT process and that proteins with the potential to alter the stability or structure of the OMM may play a role in potentiating the transition rather than functioning in a classical proteinaceous pore structure.
In summary, although the MPT presents as an acute event, it probably occurs as a multistep process with early (i.e., Ca2+ uptake/Cyp-D activation), intermediate (i.e., breach of the IMM permeability barrier), and late (i.e., loss of OMM continuity) steps. These sequential steps do not have to be spatially linked into a single channel that spans the two membranes and connects the matrix with the cytosol, but they do have to occur in the same time frame to allow a complete MPT. Therefore, proteins with the ability to remodel or rearrange membranes may be particularly important in overcoming the mitochondrial permeability barriers and promoting this transition.
Mitofusins and Membrane Fusion
The mitochondrial boundaries are defined by two membrane systems that undergo constant turnover through regulated merging and splitting. The OMM can be remodeled because of the membrane-fusing activities of mitofusins (Mfn-1 and Mfn-2) and the membrane-splitting activity of Drp-1. Mitofusins pass the lipid bilayer twice where they presumably catalyze the unification of adjacent OMMs, whereas Drp-1, which lacks a bilayer-spanning segment, forms oligomeric spirals and severs the OMM at neck-like junctions (88, 117, 121). In inducing membrane merger, the mitofusins require the binding and hydrolysis of GTP (66, 89). It can be speculated that the role of mitofusins during fusion is to transmit the energy released from hydrolyzed GTP to the lipid bilayers and to trigger their rearrangement toward fusion.
Although clues about the mechanism of mitofusin action may be obtained from studies of related membrane remodeling proteins, such as the previously discussed ER-fusogens atlastins, a detailed mechanism of how mitofusins operate to fuse OMMs remains elusive, and studies of mitofusins reconstituted in proteoliposomes are currently lacking. Nevertheless, information is available on the crystal structure of a truncated Mfn-1 isoform (76). This work demonstrates that Mfn-1 isoforms that lack the NH2-terminal GTPase but contain the middle and COOH-terminal regions can undergo dimerization via their COOH-terminal coiled-coil domains. When ectopically expressed in cells, these truncated isoforms are capable of tethering mitochondria at a distance estimated to be 10–15 nm (76). However, membrane fusion is not induced, presumably because the tether distance is too long to allow the formation of a hemifusion stalk, which is favored when the interbilayer distance is about 1–3 nm. It can therefore be suggested that additional domain interactions conferred by the full-length mitofusin might facilitate closer apposition between lipid bilayers that are required for the formation of hemifusion intermediates. In this mode, the binding and hydrolysis of GTP by mitofusin complexes is likely to be important, similar to the mechanism described for atlastin.
Mitofusin Roles in MPT and Cardiomyocyte Viability
The mitofusin genes have been highly conserved during evolution, indicating that the encoded proteins are essential for the overall mitochondrial function and energetics of the cell (24). Nevertheless, as discussed previously, any membrane fusion reaction has the potential to compromise the integrity of the membrane at least transiently, or even permanently, if the fusion catalyst is overactive or deregulated. Cardiac myocytes are highly enriched in mitochondrial mass, and the heart is one of the principal tissues where both Mfn-1 and Mfn-2 are expressed at very high levels. Because of a high content in mitochondria, it can be argued that the viability of cardiac myocytes is extremely labile to death-inducing pathways that operate through these organelles, such as the MPT that is a very potent inducer of myocyte death (8, 52). Along these lines, we initiated studies to explore the roles of mitofusins in cardiac myocytes using gene targeting approaches in mice and found that abrogation of Mfn-1 or Mfn-2 could alter the MPT process and, surprisingly, improve the survival of myocytes (108, 109). These data suggest that, through their ability to promote membrane fusion, mitofusins can transiently increase the permeability of the OMM. We reason that although this event alone is unlikely to be deleterious for the cell under nonstress conditions, if it occurs concomitantly with loss of the permeability barrier at the IMM, it can be employed in the MPT process and ultimately become detrimental for the viability of the myocyte.
The first evidence in support of the above hypothesis came from studies with mice that lack Mfn-2. Being broadly interested in the role of mitochondrial fusion in cardiac mitochondria, we examined the phenotype of mice that lacked Mfn-2 specifically in cardiac myocytes (108). The deficient mice were viable and their heart function at baseline was very similar to that of wild-type littermates. Nevertheless, the cardiac myocytes were hypertrophic and exhibited a contractility deficit when stimulated with isoproterenol (108). Although the respiratory capacity of isolated Mfn-2-deficient mitochondria was normal, the structure of the organelle was perturbed and enlarged mitochondria became prevalent when Mfn-2 was absent.
Unexpectedly, multiple lines of evidence showed that the Mfn-2-deficient cardiomyocytes and mitochondria exhibited significant latency in MPT formation and improved tolerance against stressful stimuli (108). First, when isolated cardiac mitochondria were exposed to increasingly higher doses of Ca2+, the time required for MPT was significantly extended if Mfn-2 was absent. Second, the magnitude of mitochondrial swelling downstream of Ca2+ exposure (where swelling indicates MPT) was significantly attenuated in Mfn-2-deficient mitochondria. Third, the rate of shrinkage after exposure to polyethylene glycol was delayed in Mfn-2-depleted mitochondria. Fourth, when isolated adult cardiomyocytes were subjected to local photochemical stress, the activation of MPT was delayed in cells that lacked Mfn-2. Even when these myocytes were exposed to the reactive oxygen species (ROS)-generating agent H2O2, the propensity of their mitochondria to depolarize was significantly attenuated. Taken together, these observations led us to hypothesize that Mfn-2 in wild-type myocytes facilitates the MPT.
To examine whether the contribution to the MPT process was specific to Mfn-2 or whether a delayed MPT was a general consequence of deficiency in either mitofusin, we constructed and analyzed mice that lacked Mfn-1 in cardiac myocytes. As was the case with the Mfn-2 knockout mice, the heart function of Mfn-1 knockout mice did not differ significantly from that of wild-type mice. Furthermore the respiratory capacity and membrane potential of Mfn-1-deficient mitochondria was similar to wild-type controls. Notably, however, Mfn-1-deficient mitochondria were significantly smaller than wild-type mitochondria (109). Thus the deletion of Mfn-1 resulted in the opposite result on mitochondrial size from that observed when Mfn-2 was deleted. Despite this strikingly different effect on mitochondrial structure, there were considerable similarities in the MPT behavior between Mfn-1- and Mfn-2-deficient myocytes. More specifically, when isolated Mfn-1-deficient adult cardiomyocytes were challenged with H2O2, the depolarization of their mitochondria was significantly retarded (109). Similarly, isolated mitochondria that lacked Mfn-1 exhibited a reduction in their MPT downstream of ROS exposure.
We interpret the protection against MPT observed after Mfn-1 or Mfn-2 ablation to be the direct consequence of a reduction in membrane fusion rates or in the frequency of hemifusion events. As discussed above, the fusion pathway contains intermediate steps that require the opening of the membranes and the nucleation of lipidic holes. Although these OMM holes can probably open and resolve many times during the lifetime of fusing mitochondria, they alone are unlikely to have a major impact on organelle physiology in the absence of stressors. Indeed, full permeabilization of mitochondria requires overcoming two membrane barriers, and a transient perturbation in the OMM alone would not be expected to trigger MPT. However, by overloading mitochondria with Ca2+ or ROS, the IMM is more likely to become permeabilized, and this may coincide with the transient opening of holes on the OMM undergoing fusogenic transitions. Once these two separate events occur simultaneously, the ion balance across the IMM becomes rapidly dysregulated and MPT is triggered. Therefore, the detrimental effects of mitofusins in permeability are unmasked when mitochondria are under primary stress.
These new observations on the relationship between MPT and mitofusins, coupled with the established role of MPT in promoting cell death, prompted us to assess the viability of cardiomyocytes with or without mitofusins. To test the connection between cell death and mitofusins, we treated freshly isolated adult myocytes with H2O2, an agent commonly used to induce cell death via MPT activation (2, 3). Consistently, we found that the course of myocyte death was significantly delayed when Mfn-1 or Mfn-2 was genetically ablated (108, 109). Furthermore, by using the Mfn-2-deficient strain, we demonstrated that single myocytes exposed to conditions of hypoxia/reoxygenation had reduced rates of cell death when Mfn-2 was absent. In addition, Mfn-2-deficient, Langendorff-perfused hearts had significantly better recovery of function after global ischemia and also the extent of tissue damage caused by in situ myocardial ischemia-reperfusion injury was reduced in these hearts (108). These results are in agreement with our working hypothesis that endogenous mitofusin levels undermine the viability of myocytes in conditions of stress by potentiating the MPT process.
We view the above as evidence of a reciprocal relationship between membrane fusion and permeabilization. However, we acknowledge that the absence of Mfn-1 or Mfn-2 from mitochondria and myocytes could be protective against MPT through mechanisms that are not related to membrane fusion. For example, a recent study found that isolated mouse brain mitochondria bearing mutated human Mfn-2 had undergone opening of the mitochondrial ATP-sensitive K+ channel (mKATP) (50). Opening of mKATP channels is reported to counteract Ca2+ loading in cardiac mitochondria and could potentially prevent MPT (59). mKATP channel activity was not tested in our studies with Mfn-2-deficient mitochondria (108), and conversely the MPT activity was not tested in the studies on mKATP (50); therefore, it is difficult to directly compare the two studies. A number of other important differences also apply. For example, our studies used ablation of endogenous Mfn-2, whereas Guillet et al. (50) used transgene-mediated upregulation of mutant Mfn-2 (Tg-R94Q). Additionally, the respiratory function of Mfn-2-deficient mitochondria was not severely impaired (108), whereas respiration in Tg-R94Q mitochondria did exhibit defects that were attributed to dysfunction in complexes II and V (50). Another function that could potentially alter the MPT response is the reported ability of Mfn-2 to align mitochondria with sarcoplasmic reticulum (SR) and ER membranes (38, 46). According to these findings, it can be suggested that tight coupling between the two compartments in wild-type myocytes could expose mitochondria to steep gradients of Ca2+ during pathological conditions and trigger MPT. In Mfn-2-deficient myocytes, the gap between the SR and the OMM could be sufficiently large to spare mitochondria from a hazardous rise in Ca2+ concentration. However, it remains to be addressed experimentally whether the OMM is functionally disconnected from the SR in Mfn-2-deficient cardiac myocytes and to what extent this protects mitochondria from Ca2+ overload under stress conditions. Thus it is recognized that perturbing Mfn-2 may evoke collateral mechanisms with the potential to impact the MPT activity, but this does not rule out a more direct effect of Mfn-2 in the integrity of the fusing membrane.
Another aspect to consider is that Mfn-2 and Mfn-1 deficiency is protective against MPT even in isolated mitochondria preparations (108, 109), where the fusion between mitochondria is unfavorable. One possibility could be that the presence per se of mitofusins in the membrane microenvironment alters the local distribution of conical phospholipids, which deforms the membrane and makes it more prone for permeabilization. Alternatively, it is possible that membranes of isolated mitochondria still assume fusogenic conformations through a potential interaction between the closely apposed inner and outer membrane. In this regard, sites of extreme proximity between the inner and the outer membranes have been described, and some have speculated that these sites may serve as microdomains for MPT activation while they sometimes envision them to have nonbilayer structures (i.e., hemifusion) (34, 48, 104). Currently, there is no evidence to implicate mitofusins in the formation of these OMM-IMM contact sites, but the yeast homolog of mitofusins (Fzo-1) is reported to perform such a task (44, 54). A mitofusin-mediated cross talk between OMM and IMM in the formation of contact sites may be supported by studies reporting the physical interaction between Mfn-2 or Mfn-1 with optic atrophy-1 (Opa-1), which resides in the IMM (31, 49).
Finally, it should be noted that haploinsufficiency of Opa-1 in mice has been recently reported to be protective against MPT in cardiac myocytes (113). Similar to the examples of cardiomyocyte-specific ablation of Mfn-1 and Mfn-2, Opa-1+/− mice had normal heart function. Furthermore, Opa-1+/− cardiac myocytes contained a subset of mitochondria that were enlarged, and the Opa-1 heterozygous hearts underwent more pronounced hypertrophy in response to pressure overload (113). However, most interestingly from the standpoint of the current discussion, Opa-1 haploinsufficiency was protective against MPT both in cardiac myocytes and in isolated mitochondria (113). These results provide additional evidence in support of a relationship between mitochondrial membrane fusion and MPT function. Furthermore, they raise the possibility that Opa-1 and mitofusins operate in concert to regulate the permeability of mitochondria in physiological processes (e.g., the channeling of adenine nucleotides) (113). Finally, Opa-1 and mitofusins may functionally interact at sites of hemifusion between the OMM and the IMM, which can explain the ability of isolated wild-type mitochondria to undergo MPT in vitro in the absence of net mitochondrial fusion.
The Role of Mitofusins in Death Is Defined by the Internal Structure and the Bioenergetic Demands of the Cells
To extend our studies on the relationship between mitofusins and MPT, we tested the effects of Mfn-2 deficiency in cellular contexts different from the adult cardiac myocyte. One system involved the neonatal rat cardiac myocytes (NRCMs). In contrast to freshly isolated adult myocytes, the NRCMs are derived from short-term tissue culture, and although they contain considerable respiratory reserve, this is usually dormant since they primarily rely on glucose oxidation for their ATP supply (58, 120). Furthermore, NRCMs exhibit little or no contractility and contain a relatively small number of highly motile mitochondria that often enter or depart from an integrated mitochondrial network that surrounds the nucleus (60). Contrary to our observations with freshly isolated adult cardiac myocytes, experimentation with NRCMs revealed that depletion of Mfn-2 from these cells made them more susceptible to membrane depolarization downstream of H2O2, indicative of an accelerated MPT response. This also correlated with elevated levels of cell death markers in the Mfn-2 knocked-down cells (108). The effects of Mfn-2 knockdown on MPT were also examined in endothelial cells, another cell type derived from tissue culture that exhibits little dependence on oxidative phosphorylation (37). In these cells, the mitochondria are motile and assume tubular shapes and fuse to form even more elongated mitochondrial structures (15). In human umbilical vein endothelial cells, the knockdown of Mfn-2 was associated with accelerated MPT and reduced viability (82). Finally, we examined the consequences of Mfn-2 ablation on the MPT in elicited peritoneal macrophages from mice. Macrophage activation involves enhanced mitochondrial biogenesis, increased substrate oxidation, and ROS production (134, 137). When elicited macrophages deficient for Mfn-2 were exposed to H2O2, the MPT was delayed and death markers were decreased (108), consistent with results in adult myocytes. In sum, a contributing effect of Mfn-2 in MPT was detectable in some (adult cardiac myocytes, elicited macrophages) but not in other cell types (NRCMs, human umbilical vein endothelial cells) thereby suggesting that the cellular context critically influences the impact of mitofusin activity on MPT.
Before our work which focused on the relationship between mitofusins and MPT, several studies had examined the roles of these proteins in cell survival and death pathways. Most, but not all, of these studies have concluded that mitofusins have a protective rather than a deleterious effect on mitochondrial function and cell survival. For example, in HeLa cells, the elevated expression of Mfn-1 or Mfn-2 promoted mitochondrial fusion and delayed the cell death response induced by etoposide (129). Furthermore, the expression of an overactive isoform of Mfn-2 in COS-7 cells increased fusion and attenuated the MPT process (99). A similar activation of Mfn-2 in primary neurons was also shown to be protective against H2O2-induced cell death (67). Conclusions in agreement with a cytoprotective role of mitofusins were also drawn from loss-of-function analyses. For example, the genetic deletion of Mfn-1 in mouse embryonic fibroblasts (MEFs) diminished the ability of mitochondria to undergo fusion and sensitized these cells to the death-inducing stimuli such as actinomycin-D and UV irradiation (131); however, Mfn-1-deficient MEFs were not sensitive to other stresses such as H2O2 or staurosporine (43). More recently, we have shown that MEFs deficient in Mfn-2 are sensitized to cell death through mechanisms that involve ER stress (100).
With regard to the cardiac myocyte lineage, the first study examining the role of Mfn-2 in cell survival identified a pro-death role of this protein (126). Specifically, this study showed that Mfn-2 promotes death downstream of H2O2 exposure in NRCMs or H9C2 cardiomyocytes and the death-inducing signal was more effectively relayed after enhanced Mfn-2 expression (126). In contrast, others showed that the knockdown of Mfn-2 in NRCMs made mitochondria more susceptible to C2-ceramide and promoted the MPT (110). More recently, it was reported that the transfection of HL-1 cardiac myocytes with plasmids encoding Mfn-1 or Mfn-2 significantly delayed the MPT process and protected cells from death induced by in vitro simulated ischemia-reperfusion in vitro (105). Collectively, these studies show that although mitofusins are centrally involved in cell life and death decisions, their outcome can differ significantly depending on the cell type examined.
Why do mitofusins have different effects on MPT or survival in different cell types? It is currently difficult to predict how the cellular context may alter the impact of mitofusins on MPT and ultimately improve or compromise cell viability. In a simple scenario, it can be argued that cells with a very high mitochondrial content (e.g., adult cardiac myocytes) become extremely labile to even minor mitochondrial insults. Thus, a locally restricted and apparently benign effect of mitofusins in transiently breaking the OMM barrier (Fig. 1) can be amplified severalfold in adult cardiac myocytes in conditions of preexisting mitochondrial stress and accelerate the MPT. By contrast, in cells that have withdrawn from oxidative metabolism and maintain a mitochondrial content that is much lower than that of the contractile myocyte (e.g., cells found in nonstriated tissues, cell lines and neonatal myocytes maintained in culture), even if mitofusins facilitate the MPT locally during fusion, this effect would lack the amplification component that is found in cardiac myocytes and would not be sufficient to trigger cell-wide mitochondrial dysfunction and death. In these cell types, the cytoprotective activity of mitofusins may become more apparent. By promoting fusion between mitochondria, mitofusins help maintain a self-renewing population of mitochondria that actively excludes dysfunctional parts by mitophagy and maintains an error-free genetic pool (25, 98, 132). This role of mitofusins in mitochondrial quality control can partly explain why mitofusins exhibit a cytoprotective role in some of the studies described above. In addition, it should be noted that the beneficial effects on mitochondrial fusion or the hazardous membrane-opening activity of the mitofusins are not necessarily mutually exclusive events. Cell-specific characteristics, such as the cell's mitochondrial content, lipidic composition in the membrane, Bax levels or the status of the ER, could influence which arm of mitofusin activity becomes more dominant in determining the fate of the cell during stress.
We hypothesize that two apparently opposite roles can be ascribed to mitofusins in cells under stress, i.e., a protective role related to the long term beneficial effects of mitochondrial fusion vs. an acute and potentially deleterious effect related to the brief opening of the membrane during a fusion reaction. Given this dichotomy, it may be difficult to discern between the two functions even in contexts that favor one versus the other. For example, even when performing mitofusin loss-of-function experiments in mitochondria-rich cell types such as adult cardiac myocytes, the window available for detecting stress resistance might be quite narrow relative to the long-term protective effects. In this regard, to avoid the multiple effects evoked by long-term gene deletion, it would be useful to explore the outcome of acute deletion or overexpression of mitofusins in cardiac myocytes. Such temporally oriented experiments could shed additional light into the workings of mitofusins and more clearly dissect their contributions to cell death during stress.
Interplay Between Bax and Mitochondrial Fission/Fusion Processes
The activation of programmed cell death is frequently preceded by cytochrome-c efflux from mitochondria, and this event is under the control of the Bcl-2 family of proteins (1, 20, 73). Among the different Bcl-2 family factors, the key effectors of cytochrome-c release are Bax and Bak (136, 146). Bax is soluble or peripherally associated with intracellular membranes (OMM, the nuclear envelope, and the ER), and in the presence of death stimuli, Bax undergoes changes in conformation and inserts itself into the OMM and forms oligomers (6, 42, 62, 141). Although this cascade of events is followed by release of cytochrome c and other proteins of the intermembrane space, the mechanism by which active Bax breaches the barrier of the OMM is not fully resolved. Reconstitution experiments in liposomes indicate that Bax oligomers form intramembrane protein complexes with discrete channeling properties (7, 119). In this regard, Bax channels were identified in the context of apoptosis (often termed MAC, for mitochondrial apoptosis-induced channel) and suggested to provide hydrophilic conduits with diameters 5 to 6 nm that are wide enough to accommodate cytochrome c (39, 85, 111). Nevertheless, other experiments indicate that activated Bax is able to permeate phospholipid bilayers in a more general fashion and the properties of this permeabilization cannot be explained by the presence of proteinaceous channels (11). Consistent with this notion, it was found that Bax oligomers could make mitochondrial membranes and liposomes passable to extremely large macromolecules (i.e., dextrans of 2,000 kDa), an observation that can be explained by the induction of large lipidic pores by activated Bax (78). The aforementioned study and others have also indicated that the ability of Bax to provoke membrane permeabilization is critically influenced by the lipidic composition and local architecture of the membrane (12, 78, 81, 130).
More relevant to the topic of mitochondrial dynamics, it has been recently demonstrated that the structural rearrangement induced by the mitochondrial division factor Drp-1 makes membranes susceptible to Bax insertion and oligomerization (93). Specifically, it was found that Bax oligomerization inside liposome membranes was enhanced by the presence of Drp-1, a protein that is generally thought to form spiral collars and sever mitochondrial membranes. Paradoxically, Drp-1 was found to induce clustering rather than separation of artificial vesicles in vitro (93). In this context, Drp-1 allowed the mixing of lipids between the tethered vesicles but not the exchange of their aqueous contents. These data suggest that the vesicles were joined through a stalled hemifusion configuration. This study also went on to show that it is the rearrangement of the membranes in the hemifusion mode that is the essential element that triggers Bax oligomerization and that the role of Drp-1 in this setting is to facilitate formation of this membrane intermediate rather than to directly interact with Bax (93).
This key insight that hemifusion intermediates provide a permissive substrate for Bax oligomerization within membranes suggests that proteins with similar abilities in rearranging membranes toward hemifusion could also favor Bax activation. In this regard, endogenous Mfn-2 is noted to cluster with activated Bax in discrete focal regions of the mitochondrial membrane during programmed cell death (69). Interestingly, a functional relationship between Mfn-2 and Bax (and Bak) is also noted in healthy, viable cells (70). In this context, monomeric Bax assists Mfn-2 in forming fusion-competent complexes at focal regions of the OMM. Consistently, in vitro mitochondria-fusion assays show that monomeric Bax augments the fusogenic activity of Mfn-2 (61). The precise relationship between endogenous Bax and Mfn-2 is currently unclear, although immunoprecipitation experiments support binding of Bax (through its helix 5) to Mfn-2 (32). However, the clustering of Mfn-2 and Bax at focal points during mitochondrial fusion may not necessitate direct interactions between the two molecules as discussed below.
Considering the model developed for Drp-1 and fission (86, 93), we suggest the following scenario for Mfn-1 and Mfn-2 and fusion (Fig. 2): mitofusins induce the formation of a hemifusion intermediate between OMMs, which attracts monomeric Bax to focal points of membrane fusion. The presence of Bax in the area reduces the threshold for hole formation (e.g., through Bax-phospholipid interactions that perturb the lipidic landscape). Lowering the threshold for hole formation positively influences the fusion process, thereby explaining the ability of Bax to augment fusion in the presence of mitofusins. However, facilitating the formation of the hole may turn out to be favorable for MPT under conditions of stress. Additionally, if there is a high density of Bax in the region (e.g., Bax is excessively recruited to the OMM after apoptotic stress), Bax oligomers may induce uncontrolled expansion of the hole and this would facilitate the efflux of cytochrome c.
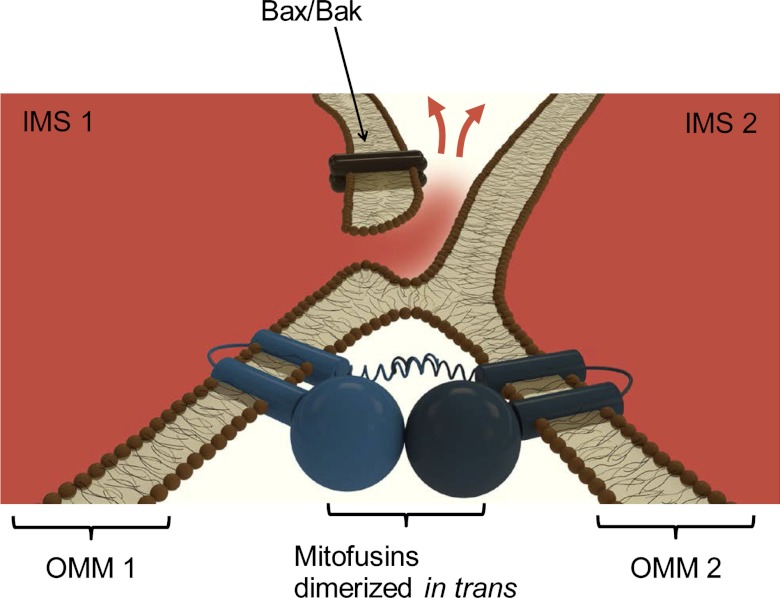
Fig. 2.
Hypothetical model for the relationship between mitofusins and Bax activation. The implications of membrane hemifusion in Bax oligomerization were explored using dynamin-related protein-1, and the working model developed therein (86, 93) is adapted here for mitofusins (Mfn-1 and Mfn-2). Only the OMMs of two mitochondria fusing horizontally are shown, and the IMMs are omitted. The intermembrane space is colored red, and the cytoplasm, white. Fusogenic interactions between membranes could be facilitated by the conversion of cardiolipin into phosphatidic acid following phospholipase D activation (30). In this cross section, mitofusins are shown to dimerize and create a membrane hemifusion intermediate. The large globular GTPase domain, the two cylindrical intermembrane segments, and the spiral coiled-coil domain at the COOH-terminus are shown schematically. Bax is recruited to the OMM and selectively localizes to regions of hemifusion intermediates. Following accumulation, Bax undergoes multimerization and the Bax oligomer stabilizes a preexisting hole or potentiates its expansion. The hole can be large enough to allow the efflux of IMS-resident proteins (red arrows) that trigger apoptotic cascades in the cytoplasm (e.g., cytochrome-c release).
Finally, the role of nonoligomerized Bax and Bak in mitochondrial fusion was recently evaluated in the context of MPT and necrotic cell death (138). This study confirmed that Bax/Bak double-knockout (DKO) cells contain fragmented mitochondria and demonstrated that DKO cells were resistant to MPT and necrotic cell death. Mfn-2-deficient cells, tested in the same study (138), also exhibited resistance against MPT, corroborating the observation that Mfn-2 promotes this process (108). Furthermore, the monomeric Bax is demonstrated to be responsible for the observed sensitivity to MPT. This may be in agreement with the notion that localization of Bax to the membrane lowers the threshold for forming hemifusion-related holes, and if there is sufficient stress overload in the matrix/IMM, this hole can be employed in the precipitous exchange of ions during MPT.
Concluding Remarks
Fusion of mitochondrial membranes is a complex and energetically costly reaction, but it has been retained through evolution and enables the organelles to better accommodate the metabolic demands of the cell. Uncovering the details of this process has begun only recently with the identification of its key mediators, such as the OMM-resident proteins Mfn-1 and Mfn-2. On the other hand, the structural conformations that occur to the mitochondrial membranes as they undergo rearrangement and fusion are far from being understood. Knowledge obtained from simulations and a growing body of experimental evidence in systems other than mitochondria indicate that membranes undergoing fusion are prone to permeabilization. Maintaining permeability barriers and ion balance across membranes is of utmost importance for mitochondria, and loss of this function, through a process termed MPT, can be catastrophic for the organelle and the cell.
In this article we have discussed the potential relationship between mitochondrial membrane fusion and MPT. This concept was developed to explain the unexpected phenotypes that were observed with cardiomyocyte-specific Mfn-1 and Mfn-2 knockout mice (108, 109). A prominent feature of Mfn-1- and Mfn-2-deficient cardiomyocytes is that they both exhibit a latent activation of MPT after stress overload. We have suggested that the membrane fusing activity of these proteins creates holes on the OMM and therefore facilitates the stress-induced MPT process. These insights may help improve our understanding on how the MPT occurs, and future experiments should test the relationship between mitofusins and permeabilization more rigorously. For example, reconstitution of recombinant mitofusins into proteoliposomes will allow a closer examination of their operation during vesicle fusion and could provide a clear and quantitative assessment of whether and how they impart membrane permeabilization. Furthermore, at the cellular and organismal level, it would be interesting to evaluate whether short-term abrogation of mitofusins in differentiated cardiomyocytes affords protection from MPT-inducing stressors such as ROS overload in myocytes and ischemia-reperfusion injury in hearts.
In addition to their participation in MPT, we have also highlighted a potential role of mitofusins in cooperating with Bax to activate the permeabilization of the OMM, another mitochondrial checkpoint with significant impact on cell survival. Current evidence suggests that Drp-1, a protein with considerable similarities but also differences with mitofusins, is important in Bax oligomerization and membrane permeabilization. It would therefore be interesting to investigate whether mitofusins also participate in the process of Bax activation similar to Drp-1. Again, experimental systems of minimal complexity, such as proteoliposomes bearing mitofusins, can help identify functional interactions between mitofusins and Bax and delineate a potential permeabilization effect.
GRANTS
This work was supported by National Institute of Health Grants HL-102874, AG-34972, AG-15052, and HL-68758 (to K. Walsh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.N.P., M.M.P., and K.W. conception and design of research; K.N.P. drafted manuscript; K.N.P. and K.W. edited and revised manuscript; M.M.P. prepared figures; K.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Ryan Walsh for a graphical illustration.
REFERENCES
- 1. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Akao M, O'Rourke B, Kusuoka H, Teshima Y, Jones SP, Marban E. Differential actions of cardioprotective agents on the mitochondrial death pathway. Circ Res 92: 195–202, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Akao M, O'Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res 92: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Al-Nasser I, Crompton M. The reversible Ca2+-induced permeabilization of rat liver mitochondria. Biochem J 239: 19–29, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J 24: 2096–2103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J 345: 271–278, 2000 [PMC free article] [PubMed] [Google Scholar]
- 8. Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci USA 96: 5492–5497, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem 277: 49360–49365, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 280: 18558–18561, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Bayer MJ, Reese C, Buhler S, Peters C, Mayer A. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol 162: 211–222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bereiter-Hahn J, Voth M, Mai S, Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol J 3: 765–780, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273: 2077–2099, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between hexokinase, mitochondrial porin and adenylate translocator in brain: regulation of hexokinase, oxidative phosphorylation and permeability transition pore. Biochem Soc Trans 25: 151–157, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Bian X, Klemm RW, Liu TY, Zhang M, Sun S, Sui X, Liu X, Rapoport TA, Hu J. Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes. Proc Natl Acad Sci USA 108: 3976–3981, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonnafous P, Stegmann T. Membrane perturbation and fusion pore formation in influenza hemagglutinin-mediated membrane fusion. A new model for fusion. J Biol Chem 275: 6160–6166, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17: 37–49, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264: 7826–7830, 1989 [PubMed] [Google Scholar]
- 22. Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 35: 8483–8488, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Byrnes LJ, Sondermann H. Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A. Proc Natl Acad Sci USA 108: 2216–2221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol 140: 1369–1382, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell 123: 375–382, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol 15: 675–683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J Cell Biol 175: 201–207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol 8: 1255–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ 18: 235–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels 4: 169–202, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999 [PMC free article] [PubMed] [Google Scholar]
- 35. Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255: 357–360, 1988 [PMC free article] [PubMed] [Google Scholar]
- 36. Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 258: 729–735, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007 [DOI] [PubMed] [Google Scholar]
- 38. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell 16: 2424–2432, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem 70: 777–810, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Engel A, Walter P. Membrane lysis during biological membrane fusion: collateral damage by misregulated fusion machines. J Cell Biol 183: 181–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Fritz S, Rapaport D, Klanner E, Neupert W, Westermann B. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J Cell Biol 152: 683–692, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frolov VA, Dunina-Barkovskaya AY, Samsonov AV, Zimmerberg J. Membrane permeability changes at early stages of influenza hemagglutinin-mediated fusion. Biophys J 85: 1725–1733, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garcia-Perez C, Schneider TG, Hajnoczky G, Csordas G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am J Physiol Heart Circ Physiol 301: H1907–H1915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19: 2133–2139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis 12: 841–855, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Guillery O, Malka F, Landes T, Guillou E, Blackstone C, Lombes A, Belenguer P, Arnoult D, Rojo M. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biol Cell 100: 315–325, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Guillet V, Gueguen N, Cartoni R, Chevrollier A, Desquiret V, Angebault C, Amati-Bonneau P, Procaccio V, Bonneau D, Martinou JC, Reynier P. Bioenergetic defect associated with mKATP channel opening in a mouse model carrying a mitofusin 2 mutation. FASEB J 25: 1618–1627, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Gunther-Ausborn S, Praetor A, Stegmann T. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J Biol Chem 270: 29279–29285, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, Griffith J, Mann M, Reggiori F, Neupert W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J 30: 4356–4370, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467, 1979 [DOI] [PubMed] [Google Scholar]
- 56. Haworth RA, Hunter DR. Allosteric inhibition of the Ca2+-activated hydrophilic channel of the mitochondrial inner membrane by nucleotides. J Membr Biol 54: 231–236, 1980 [DOI] [PubMed] [Google Scholar]
- 57. He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J 424: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol 519: 347–360, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hom J, Sheu SS. Morphological dynamics of mitochondria—a special emphasis on cardiac muscle cells. J Mol Cell Cardiol 46: 811–820, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell 41: 150–160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–13834, 1997 [DOI] [PubMed] [Google Scholar]
- 63. Hu J, Prinz WA, Rapoport TA. Weaving the web of ER tubules. Cell 147: 1226–1231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138: 549–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251: 5069–5077, 1976 [PubMed] [Google Scholar]
- 66. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117: 6535–6546, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem 282: 23788–23798, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell 112: 519–533, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159: 931–938, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion. I. Hemifusion mechanism. Biophys J 87: 3277–3290, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion: II. Mechanism of a stalk-hole complex. Biophys J 90: 915–926, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136, 1997 [DOI] [PubMed] [Google Scholar]
- 74. Knecht V, Marrink SJ. Molecular dynamics simulations of lipid vesicle fusion in atomic detail. Biophys J 92: 4254–4261, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1−/− mitochondria. Biochim Biophys Acta 1757: 590–595, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Lee KK. Architecture of a nascent viral fusion pore. EMBO J 29: 1299–1311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lucken-Ardjomande S, Martinou JC. Newcomers in the process of mitochondrial permeabilization. J Cell Sci 118: 473–483, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Lucken-Ardjomande S, Montessuit S, Martinou JC. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ 15: 929–937, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Lugus JJ, Ngoh GA, Bachschmid MM, Walsh K. Mitofusins are required for angiogenic function and modulate different signaling pathways in cultured endothelial cells. J Mol Cell Cardiol 51: 885–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marrink SJ, Mark AE. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J Am Chem Soc 125: 11144–11145, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol 9: 543–556, 2008 [DOI] [PubMed] [Google Scholar]
- 85. Martinez-Caballero S, Dejean LM, Kinnally MS, Oh KJ, Mannella CA, Kinnally KW. Assembly of the mitochondrial apoptosis-induced channel, MAC. J Biol Chem 284: 12235–12245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21: 92–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 187: 1261–1271, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol 18: 20–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science 305: 1747–1752, 2004 [DOI] [PubMed] [Google Scholar]
- 90. Merz AJ, Wickner WT. Trans-SNARE interactions elicit Ca2+ efflux from the yeast vacuole lumen. J Cell Biol 164: 195–206, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J 27: 2031–2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Montenegro G, Rebelo AP, Connell J, Allison R, Babalini C, D'Aloia M, Montieri P, Schule R, Ishiura H, Price J, Strickland A, Gonzalez MA, Baumbach-Reardon L, Deconinck T, Huang J, Bernardi G, Vance JM, Rogers MT, Tsuji S, De Jonghe P, Pericak-Vance MA, Schols L, Orlacchio A, Reid E, Zuchner S. Mutations in the ER-shaping protein reticulon 2 cause the axon-degenerative disorder hereditary spastic paraplegia type 12. J Clin Invest 122: 538–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142: 889–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moss TJ, Andreazza C, Verma A, Daga A, McNew JA. Membrane fusion by the GTPase atlastin requires a conserved C-terminal cytoplasmic tail and dimerization through the middle domain. Proc Natl Acad Sci USA 108: 11133–11138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Muller M, Katsov K, Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys J 85: 1611–1623, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Muller M, Katsov K, Schick M. New mechanism of membrane fusion. J Chem Phys 116: 2342–2345, 2002 [Google Scholar]
- 97. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem 280: 25060–25070, 2005 [DOI] [PubMed] [Google Scholar]
- 100. Ngoh GA, Papanicolaou KN, Walsh K. Loss of Mitofusin 2 Promotes Endoplasmic Reticulum Stress. J Biol Chem. 2012. April 17 [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387: 199–202, 1997 [DOI] [PubMed] [Google Scholar]
- 102. Nicot AS, Toussaint A, Tosch V, Kretz C, Wallgren-Pettersson C, Iwarsson E, Kingston H, Garnier JM, Biancalana V, Oldfors A, Mandel JL, Laporte J. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 39: 1134–1139, 2007 [DOI] [PubMed] [Google Scholar]
- 103. Noguchi H, Takasu M. Fusion pathways of vesicles: a Brownian dynamics simulation. J Chem Phys 115: 9547–9551, 2001 [Google Scholar]
- 104. Ohlendieck K, Riesinger I, Adams V, Krause J, Brdiczka D. Enrichment and biochemical characterization of boundary membrane contact sites from rat-liver mitochondria. Biochim Biophys Acta 860: 672–689, 1986 [DOI] [PubMed] [Google Scholar]
- 105. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 106. Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460: 978–983, 2009 [DOI] [PubMed] [Google Scholar]
- 107. Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol 192: 7–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31: 1309–1328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Papanicolaou KN, Ngoh GA, Dabkowski ER, O'Connell KA, Ribeiro RF, Jr, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol 302: H167–H179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res 77: 387–97, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol 155: 725–731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396: 575–580, 1998 [DOI] [PubMed] [Google Scholar]
- 113. Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, Huynh LH, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, Joubert F. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94: 408–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5: 133–147, 2004 [DOI] [PubMed] [Google Scholar]
- 115. Reese C, Heise F, Mayer A. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature 436: 410–414, 2005 [DOI] [PubMed] [Google Scholar]
- 116. Reese C, Mayer A. Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J Cell Biol 171: 981–990, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rojo M, Legros F, Chateau D, Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674, 2002 [DOI] [PubMed] [Google Scholar]
- 118. Ruck A, Dolder M, Wallimann T, Brdiczka D. Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett 426: 97–101, 1998 [DOI] [PubMed] [Google Scholar]
- 119. Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol 2: 553–555, 2000 [DOI] [PubMed] [Google Scholar]
- 120. Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem Biol Interact 191: 288–295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874, 2001 [DOI] [PubMed] [Google Scholar]
- 122. Schick M. Membrane fusion: the emergence of a new paradigm. J Stat Phys 142: 1317–1323, 2011 [Google Scholar]
- 123. Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102: 12005–12010, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schlame M, Kelley RI, Feigenbaum A, Towbin JA, Heerdt PM, Schieble T, Wanders RJ, DiMauro S, Blanck TJ. Phospholipid abnormalities in children with Barth syndrome. J Am Coll Cardiol 42: 1994–1999, 2003 [DOI] [PubMed] [Google Scholar]
- 125. Shangguan T, Alford D, Bentz J. Influenza-virus-liposome lipid mixing is leaky and largely insensitive to the material properties of the target membrane. Biochemistry 35: 4956–4965, 1996 [DOI] [PubMed] [Google Scholar]
- 126. Shen T, Zheng M, Cao C, Chen C, Tang J, Zhang W, Cheng H, Chen KH, Xiao RP. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem 282: 23354–23361, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Sileikyte J, Petronilli V, Zulian A, Dabbeni-Sala F, Tognon G, Nikolov P, Bernardi P, Ricchelli F. Regulation of the inner membrane mitochondrial permeability transition by the outer membrane translocator protein (peripheral benzodiazepine receptor). J Biol Chem 286: 1046–1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci USA 104: 13551–13558, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279: 52726–52734, 2004 [DOI] [PubMed] [Google Scholar]
- 130. Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem 279: 30081–30091, 2004 [DOI] [PubMed] [Google Scholar]
- 131. Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28: 1589–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ungermann C, Wickner W, Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc Natl Acad Sci USA 96: 11194–11199, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab 4: 13–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124: 573–586, 2006 [DOI] [PubMed] [Google Scholar]
- 136. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472: 476–480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, O'Rourke B, 2nd, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 109: 6566–6571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol 43: 189–219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol 26: 115–136, 2010 [DOI] [PubMed] [Google Scholar]
- 141. Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139: 1281–1292, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xu H, Zick M, Wickner WT, Jun Y. A lipid-anchored SNARE supports membrane fusion. Proc Natl Acad Sci USA 108: 17325–17330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol 12: 417–422, 2005 [DOI] [PubMed] [Google Scholar]
- 144. Yang L, Huang HW. Observation of a membrane fusion intermediate structure. Science 297: 1877–1879, 2002 [DOI] [PubMed] [Google Scholar]
- 145. Zhao X, Alvarado D, Rainier S, Lemons R, Hedera P, Weber CH, Tukel T, Apak M, Heiman-Patterson T, Ming L, Bui M, Fink JK. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat Genet 29: 326–331, 2001 [DOI] [PubMed] [Google Scholar]
- 146. Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15: 1481–1486, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zucchi PC, Zick M. Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis. Mol Biol Cell 22: 4635–4646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet 36: 449–451, 2004 [DOI] [PubMed] [Google Scholar]
- 149. Zuchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA, Speer MC, Stenger JE, Walizada G, Zhu D, Pericak-Vance MA, Nicholson G, Timmerman V, Vance JM. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet 37: 289–294, 2005 [DOI] [PubMed] [Google Scholar]