Abstract
Diabetic cardiomyopathy is characterized, in part, by calcium handling imbalances associated with ventricular dysfunction. The cardiac Na+/Ca2+ exchanger 1 (NCX1) has been implicated as a compensatory mechanism in response to reduced contractility in the heart; however, its role in diabetic cardiomyopathy remains unknown. We aimed to fully characterize the Akitains2 murine model of type 1 diabetes through assessing cardiac function and NCX1 regulation. The CXCL12/CXCR4 chemokine axis is well described in its cardioprotective effects via progenitor cell recruitment postacute myocardial infarction; however, it also functions in regulating calcium dependent processes in the cardiac myocyte. We therefore investigated the potential impact of CXCR4 in diabetic cardiomyopathy. Cardiac performance in the Akitains2 mouse was monitored using echocardiography and in vivo pressure-volume analysis. The Akitains2 mouse is protected against ventricular systolic failure evident at both 5 and 12 mo of age. However, the preserved contractility was associated with a decreased sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a)/phospholamban ratio and increased NCX1 content. Direct myocardial injection of adenovirus encoding anti-sense NCX1 significantly decreased NCX1 expression and induced systolic failure in the Akitains2 mouse. CXCL12 and CXCR4 were both upregulated in the Akitains2 heart, along with an increase in IκB-α and NF-κB p65 phosphorylation. We demonstrated that CXCR4 activation upregulates NCX1 expression through a NF-κB-dependent signaling pathway in the cardiac myocyte. In conclusion, the Akitains2 type 1 diabetic model is protected against systolic failure due to increased NCX1 expression. In addition, our studies reveal a novel role of CXCR4 in the diabetic heart by regulating NCX1 expression via a NF-κB-dependent mechanism.
Keywords: systolic function, calcium cycling, action potential
diabetes mellitus is a leading risk factor in causing premature illness and death worldwide (10). Importantly, diabetes mellitus is a known independent risk factor for cardiovascular disease and heart failure and more than half of diabetic individuals will succumb to a cardiovascular event (11, 42). Diabetic cardiomyopathy is defined in part by diastolic and systolic impairment, myocardial remodeling, and inflammation absent from other independent risk factors such as hypertension and coronary artery disease (17, 35). Myocardium exposed to hyperglycemia not only alters the energetic efficiency of the cardiac myocyte, which relies heavily on fatty acid oxidation in the diabetic state, but also leads to significant alterations in activity and expression of Ca2+ transporters including cardiac sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), L-type Ca2+ channel (LTCC), and cardiac Na+/Ca2+-exchanger-1 (NCX1) (21, 31, 48).
The molecular mechanisms of diabetic cardiomyopathy are not well understood; however, impairment of Ca2+ homeostasis is a significant feature of type 1 and type 2 diabetic cardiomyopathies (7, 49). In normal excitation-contraction (EC) coupling, Ca2+ entry via LTCC triggers the release of approximately two-thirds of Ca2+ stored in sarcoplasmic reticulum (SR), thereby acutely raising cytosolic Ca2+, which activates the contractile apparatus (1, 3). During diastole, ∼95% of cytosolic Ca2+ in murine myocytes is resequestered into the SR by SERCA2a [under the control of phospholamban (PLB)], thereby lowering intracellular Ca2+ concentration ([Ca2+]i) and allowing myocyte relaxation (3). To maintain beat-to-beat Ca2+ balance, Ca2+ that has entered via LTCC is extruded by NCX1, with minor contributions from the plasmalemmal Ca2+-ATPase (3, 4). The exact role of NCX1 in disease and whether it participates as a compensatory or maladaptive mechanism remain controversial. In the initial stages of clinical and experimental heart failure, SERCA2a Ca2+ reuptake activity and expression are decreased leading to reduced SR Ca2+ load and increased diastolic [Ca2+]i (13, 15, 25, 38). Increased NCX1 expression and forward NCX current (Incx) is thought to contribute up to 50% of cytoplasmic calcium efflux to maintain diastolic [Ca2+]i levels as a compensatory mechanism to decreased SERCA2a activity (34, 43, 46). In addition, reverse Incx may contribute to Ca2+-induced Ca2+ release (CICR) by directly contributing to local Ca2+ for CICR and through refilling SR Ca2+ content (26). However, this may come at the cost of increased arrhythmogenesis at the cellular level (41, 45).
We utilized the Akitains2 mouse, a genetic model of type 1 diabetes, in which diabetes is evident by 4 wk of age with blood glucose levels consistently >600 mg/dl in males (47). Several groups (2, 6, 24, 28) have reported strikingly different results on the systolic and diastolic capacity of the Akitains2 hearts ranging from overt heart failure to minimal cardiac dysfunction. The conflicting data led us to initiate an in-depth cardiac physiological analysis of the Akitains2 mouse to accurately determine the exact nature of type 1 diabetic cardiac dysfunction and to identify potential mechanisms involved.
The CXCL12/CXCR4 chemokine pair has been identified as being cardioprotective in acute myocardial infarction. Augmenting the CXCL12/CXCR4 axis promotes endothelial progenitor cell recruitment, angiogenesis, and possibly cardiogenesis maintaining and preserving ventricular function postmyocardial infarction (5, 14). However, the CXCL12/CXCR4 chemokine axis may participate in additional mechanisms promoting cardiac myocyte survival and function. CXCR4 has direct signaling consequences in the cardiomyocyte (32) and may provide beneficial regulation of calcium homeostatic mechanisms. Due to the presence of Ca2+ handling imbalances, altered Ca2+ cycling proteins expression, and activity in diabetic cardiomyopathies, we sought to determine the role of the CXCL12/CXCR4 axis in this process.
MATERIALS AND METHODS
Animals, In Vivo Studies, and Virus Injection
Animals were handled as approved by the Mount Sinai Institutional Animal Care and Use Committees in accordance with the Principles of Laboratory Animal Care by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23, revised 1996).
Heterozygous male Akitains2 and wild-type controls were acquired from Jackson laboratories at 6–8 wk of age. Twenty- and fifty-two-week-old mice were anesthetized with intraperitoneal ketamine (100 μg/g) for echocardiographic analysis. Two-dimensional images and M-mode tracings were recorded on the short-axis at the level of the papillary muscle to determine percent fractional shortening and ventricular dimensions (GE Vivid 7 Vision). One day after echocardiography, in vivo hemodynamics were performed using a 1.2-Fr (2-electrode) pressure-volume (PV) conductance catheter Advantage System (Scisense, Canada). Mice were anesthetized with an intraperitoneal injection of urethane (1 mg/g), etomidate (10 μg/g), and morphine (1 μg/g) combination and intubated via a tracheotomy and mechanically ventilated at 7 μl/g tidal volume and 125 respirations/min. A central jugular venous cannula was placed for vascular access, and a thoracotomy was performed to expose the heart. The PV catheter was placed in the left ventricle via an apical stab approach as previously described (27). Hemodynamics data were obtained at baseline and after isoproterenol administration (20 pg·g−1·min−1 for 5 min; Sigma-Aldrich, St. Louis, MO). PV data were analyzed using IOX2 software (EMKA Technologies, Falls Church, VA).
For viral injection, wild-type and Akitains2 mice (20 wk old; n = 7) were anesthetized with 50 μl of ketamine/xylazine (50/5 μg/g) mix intraperitoneal injection, intubated, and ventilated. The chest was opened, and 30 μl of 1.5 × 109 particles/μl of either Ad.GFP or Ad.asNCX1 were directly injected into left ventricular free wall at five to six injection sites with 30-g needle-1-cc syringe. After 7 days postinjection (a window which constitutes the optimal time for Adeno gene expression), wall motion and wall thickening, percent fractional shortening, and ventricular dimensions were evaluated by echocardiography. One day after echocardiography in vivo hemodynamics were performed using a 1.2-Fr PV conductance catheter (Scisense) as described above.
Culture of Adult Rat Ventricular Myocytes
Adult rat ventricular myocytes (ARVM) were isolated from Sprague-Dawley rats. Hearts were excised and quickly cannulated in the ascending aorta. The hearts were initially perfused for 5 min with a low calcium Tyrode's buffer (containing in mM: 120.0 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, and 25.0 HEPES, pH 7.40 at 37°C) and followed by an enzyme solution containing collagenase (0.7 mg/ml; Worthington Type II, 258 μ/mg), and the hearts were perfused for another 10–15 min. The hearts were minced, filtered, and underwent a step-wise calcium challenge reaching a final calcium concentration of 1.2 mM. ARVMs were plated at a density of 2 × 104 cells/ml onto laminin-precoated coverslips (1 μg/cm2, Invitrogen) and cultured for 24 h in MEM (Sigma) containing 50 IU/ml penicillin/streptomyocin, 0.1% BSA, 10 mM 2,3-butanedione monoxime, and 1× insulin/transferrin/selenium.
Isolated Myocyte Calcium Transient Studies
Adult mouse cardiomyocytes from the Akitains2 mouse and wild-type controls were isolated as previously described (23). Freshly isolated mouse cardiomyocytes plated onto laminin-coated coverslips were loaded with fura 2-AM (Invitrogen) for 5 min at 27°C and subsequently washed. Cells included in the study were rod shaped with a clear striation pattern and were quiescent in the absence of electrical stimulation. Cells were placed in a chamber and paced at 1 Hz with a 4-ms duration. Sarcomeric shortening and whole cell calcium transients were determined on individual cardiomyocytes using a dual excitation spectrofluorometer and video-edge detection system (Ionoptix), as described previously (22). Three animals per group were used measuring ≥15 cardiomyocytes per animal to generate average sarcomeric shortening and whole cell calcium transients.
Immunoblotting
Isolated cardiomyocytes, cultured for 24 h, and whole left ventricular tissues, harvested from the Akitains2 and wild-type littermate controls, were lysed with RIPA buffer plus protease inhibitors (Roche) and protein concentration was determined using a standard Bradford assay (Bio-Rad). Cytoplasmic and nuclear fractions of adult cardiomyocytes were prepared using the NE-PER nuclear and cytoplasmic extraction reagents kit (Thermo Scientific) according to the manufacturer's instructions. Western blots were performed using antibodies against SERCA2a (1:5,000; custom-made in our laboratory); CXCR4 (1:2000; Abcam); CXCL12 (1:500, Cell Signaling); phospholamban (1:5,000; Barilla); Cav1.2 (1:500; Antibodies, Inc.); NCX1 (1:1,000; Invitrogen); β1-adrenergic receptor (β1-AR), β2-AR, and Gs (1:1,000; Santa Cruz Biotechnology); and IκBα and NF-κB (Cell Signaling Technology). Signal intensities were visualized by Chemiluminescence (Pierce). Films from at least three independent experiments were scanned, and densities of the immunoreactive bands were evaluated using NIH ImageJ software. Protein loadings were verified against GAPDH densities.
Quantitative Real-Time PCR
RNA was extracted from isolated ARVMs using TRIzol reagent (Invitrogen). Total RNA (250 ng) was used for cDNA synthesis using SuperScript reverse transcriptase (BioRad) following manufacturer's protocol. CXCL12 and CXCR4 expression levels were determined using SYBR Green PCR Master Mix (Applied Biosystems). One microliter from cDNA synthesis was used with a final primer concentration of 0.4 μM in each 25-μl PCR reaction. Real-time PCR conditions included a 95°C for a 10-min denaturation, followed by 40 amplification cycles: 15 s at 95°C (denaturation) and 60 s at 59°C (annealing and extension). Postamplification dissociation curves were performed to verify the presence of a single amplification product in the absence of genomic DNA contamination or contribution from primer dimers. Fold changes in gene expression were determined using the ΔΔCt method with normalization to endogenous controls. Primers sequences include the following: CXCL12 forward: 5′-CTTCATCCCCATTCTCCTCA-3′; CXCL12 reverse: 5′-GACTCTGCTCTGGTGGAAGG-3′ (NM_021704); CXCR4 forward: 5′-CGTCGTGCACAAGTGGATCT-3′; CXCR4 reverse: 5′-GTTCAGGCAACAGTGGAAGAAG-3′ (NM_022205); 28S rRNA forward: 5′-CTCG CTGGCCCTTGAAAATCC-3′; and 28s rRNA reverse: 5′-CCCAGCCCTTAGAGCCAATCCTTA-3′ (NR_046246).
Histology and Fluorescent Immunostaining
Heart tissue.
Whole ventricular tissue was frozen in optimum cutting temperature using liquid nitrogen (TissueTek). Frozen sections (10 μm) were mounted and subsequently stained with hematoxylin-eosin using routine procedures. For CXCL12 immunostaining, sections were incubated at 37°C for 30 min. Slides were then fixed in 100% cold acetone for 10 min at −20°C; nonspecific binding sites were blocked using 10% goat serum (DAKO) for 30 min at 37°C in a humid chamber. Ventricular sections were coincubated with anti-CXCL12 (1:100; Santa Cruz Biotechnology) and anti-α sarcomeric actin (1:100; Abcam) in blocking buffer overnight at 4°C in a humid chamber. CXCL12 and sarcomeric actin were visualized with goat anti-rabbit-FITC and goat anti-mouse-Cy3, respectively. Sections were counterstained with DAPI to visualize cell nuclei and mounted with a coverslip (Vector Labs) for confocal microscopy.
Isolated myocytes.
Adult rat cardiomyocytes, isolated as described above, were fixed with 4% formaldehyde and stained with an anti-NF-κB p65 monoclonal antibody (Cell Signaling; 1:400). Primary antibodies were detected with an anti-mouse Alexa Fluor 555 IgG secondary antibodies (Invitrogen). Images were collected using a Olympus IX71 fluorescence microscope. Cells were counterstained with DAPI to visualize cell nuclei and mounted with a coverslip (Vector Labs). Nonspecific staining for NF-κB was assessed by omission of the primary antibody and examination of the cells in the presence of the secondary antibody alone.
Assessment of [Ca2+] in SR and Na+-Ca2+ Exchange Activity
All chemicals were purchased from the Sigma-Aldrich unless otherwise indicated. Normal Tyrode (NT) solution was composed of the following (in mM): 140 NaCl, 1.2 CaCl2 4 KCl, 1 MgCl2, 10 glucose, and 5 HEPES, pH to 7.4 with NaOH. The 0 Na+ 0 Ca2+ NT had the same concentrations of the aforementioned chemicals, with the exception of no added CaCl2, the addition of 10 EGTA, and 140 LiCl substituted for NaCl; the solution was brought up to a pH of 7.4 with LiOH.
Isolated mouse ventricular myocytes were loaded with 10 μM fluo-3 AM (Biotium, Hayward, CA), a single-wavelength cytosolic calcium dye, for 30 min at room temperature (20–22°C) and allowed to deesterify for at least 30 min. Cells were then placed in a laminin-coated experimental chamber and left to settle for 5 min so that the cells could adhere. Changes in [Ca2+]i were recorded using a laser-scanning confocal microscope (Zeiss 5 Exciter) operating in line-scan mode, with excitation at 488 nm and emission >505 nm. Cells were perfused with NT and electrically stimulated at 0.5 Hz for 2 min to establish a steady-state SR Ca2+ load. Stimulation was halted, a line-scan recording was initiated, and NT containing 20 mM caffeine was rapidly perfused onto the cells after ∼10 s. After again being perfused with NT and electrically stimulated for ≥2 min to reload the SR, a second application of 20 mM caffeine was performed. In this case, after stimulation was ceased, cells were perfused with 0 Na+ 0 Ca2+ solutions for 30 s, and then 0 Na+ 0 Ca2+ solution containing 20 mM caffeine was applied.
These measurements served multiple purposes. The peak increase in fluorescence upon application of 20 mM caffeine in 0 Na+ 0 Ca2+ solutions was used to estimate the total Ca2+ content of the SR {see below for a more detailed explanation of the calculation of [Ca2+] in SR ([Ca2+]SR)}. The difference in the rates of Ca2+ decay in the presence vs. the absence of extracellular Na+ was used as an index of NCX activity in each cell. Analysis of these data was performed using routines written in the MATLAB programming environment that performed the following steps: 1) average the line-scan image over the cell area; 2) convert from fluorescence to intracellular [Ca2+]; 3) determine the maximum intracellular [Ca2+] and convert to the equivalent [Ca2+]SR; and 4) fit the decaying phase of the Ca2+ signal to a decaying exponential curve.
Analysis and Calculation of [Ca2+]SR
The data analysis performed using MATLAB incorporates fluorescence data taken from images recorded via confocal microscopy with well-established mathematical equations to calculate [Ca2+]SR accurately. Initially, ΔF/F0 is determined by subtracting baseline fluorescence from the peak of the caffeine-induced calcium transient. Total cellular Ca2+ bound to fluo-3 AM ([Ca2+]i) can be determined from fluorescence and established buffering properties using the following formula (in nM):
Total cellular Ca2+ calculation incorporates [Ca2+]i with additional properties of binding and dissociation of other buffering properties in the cell (in μM):
Total [Ca2+]SR can be calculated by dividing the total cellular calcium by the percentage of cellular volume occupied by the SR (in μM):
Finally, with the use of known buffering properties of [Ca2+]SR, [Ca2+]SRfree can be determined (in μM):
where [Ca2+]i is total cellular Ca2+ bound to fluo-3 AM (nM); [Ca2+]SR is SR Ca2+ concentration (μM); [Ca2+]SR_free is unbound [Ca2+]SR (μM); [Ca2+]tot is total cellular [Ca2+] (μM); Bmax_fluo3 is binding capacity of fluo-3 AM to Ca2+ (150 μM/l cytosol); Bmax_i is binding capacity for Ca2+ binding within the cell (150 μM/l cytosol); Bmax_SR is binding capacity for Ca2+ binding within the SR (3.25 mM/l SR); Cacyt_rest is cytosolic [Ca2+] at rest (100 nM); F/F0 is relative fluorescence (arbitrary units); Kd_fluo3 is dissociation constant between fluo-3 AM and Ca2+ (1.1 μM); Kd_i is dissociation constant of Ca2+ binding within cell (1.1 μM); Kd_SR is dissociation constant of Ca2+ binding within the SR (0.63 mM/l); and SRratio is %cellular volume attributed to SR (0.05).
Action Potential Recording and Analysis
An additional group of myocytes were loaded with 5 μM Di-8-ANEPPS (Biotium, Hayward, Ca), a membrane-bound voltage indicator dye, for 20 min at 20–22°C. While membrane potential voltage cannot be quantified, the changes in fluorescence observed relative to resting fluorescence directly correlate to changes in membrane voltage relative to resting membrane potential, thus enabling an accurate measurement of action potential duration (APD). Cells were placed in a laminin-coated chamber and left to settle and adhere for 5 min before initiating gravity-driven perfusion with NT. This solution additionally contained 20 μM blebbistatin, an inhibitor of myosin's ATPase activity, to prevent motion artifacts. Fluorescence was excited with an He-Ne laser at 543 nm, and fluorescence >560 nm was recorded. Line scan images reflecting changes in transmembrane potential were obtained during steady-state pacing at 1 Hz. Data were analyzed using a MATLAB routine that averages the change in fluorescence of the series of APs recorded in a single cell, normalizes all cells to a uniform amplitude, and calculates the time it takes for a cell to repolarize to a predetermined percentage of the amplitude.
Statistics
Numeric data are presented as means ± SD. One-way ANOVA and Student's t-test were utilized with P values <0.05 considered statistically significant.
RESULTS
Echocardiography Indicates Normal Systolic Function in the Akitains2 Mouse
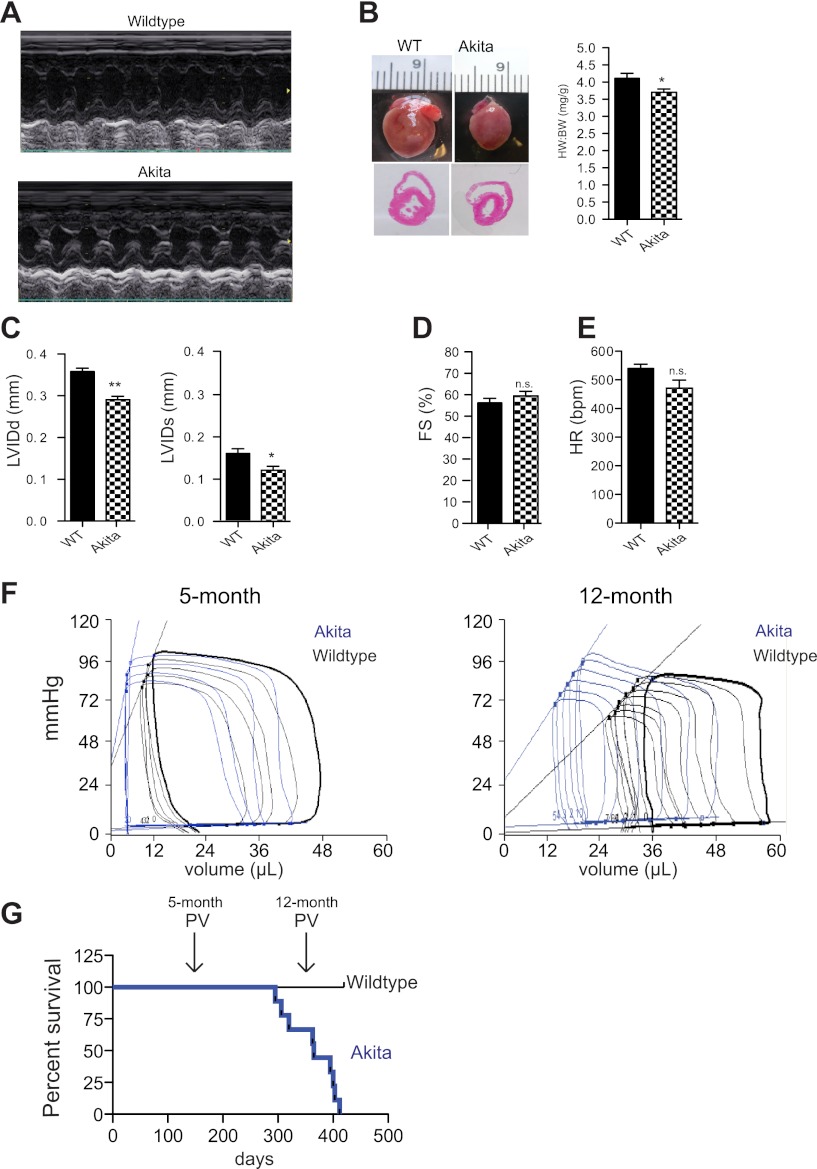
We first utilized noninvasive transthoracic echocardiography in the Akitains2 and age-matched wild-type controls at 20 wk of age. M-mode analysis determined that the Akitains2 hearts had smaller ventricular dimensions including decreased left ventricular inner diameter at diastole (3.58 ± 0.18 vs. 2.90 ± 0.24 mm; P < 0.001) and systole (1.58 ± 0.24 vs. 1.18 ± 0.24 mm; P < 0.05; Fig. 1, A–C). Importantly, percent fractional shortening between wild-type and AkitaIns2 was indistinguishable (56.3 ± 5.3 vs. 59.2 ± 5.9%) (Fig. 1D). Heart rates were not significantly different between the two groups (Fig. 1E), and gross cardiac examination indicated modest cardiac atrophy with a diminished heart weight:body weight ratio in the Akitains2 mouse (Fig. 1B).
Fig. 1.
Echocardiographic analysis and gross examination of the Akitains2 heart at 20 weeks of age compared with wild-type (WT) controls. A: representative M-mode images of WT and Akitains2 mice. B: gross examination of the hearts of WT and Akitains2 shows decreased cardiac size as well as significantly decreased heart weight/body weight ratio. C: ventricular indexes: left ventricular inner diameter diastole and systole (LVIDd/s) are smaller in the Akitains2 heart. D: cardiac function was not significantly different between the Akitains2 and WT controls as determined by percent fractional shortening (%FS). E: heart rates were not significantly different (n = 5 WT; n = 7 Akita). F: pressure-volume analysis of Akitains2 mice and WT controls. Representative baseline pressure-volume loops of the Akitains2 mouse (blue) compared with WT (black) at 5 and 12 mo of age (n = 4–5 Wild-type ; n = 4–7 Akita) are shown. G: percent survival rates of Akitains2 mice; the Akitains2 cumulative survival drastically decreases at 52 wk of age. *P < 0.05; **P < 0.01; ns, nonspecific.
In Vivo Hemodynamics Reveals Diastolic Dysfunction with Enhanced Inotropy in the Akitains2 Mouse
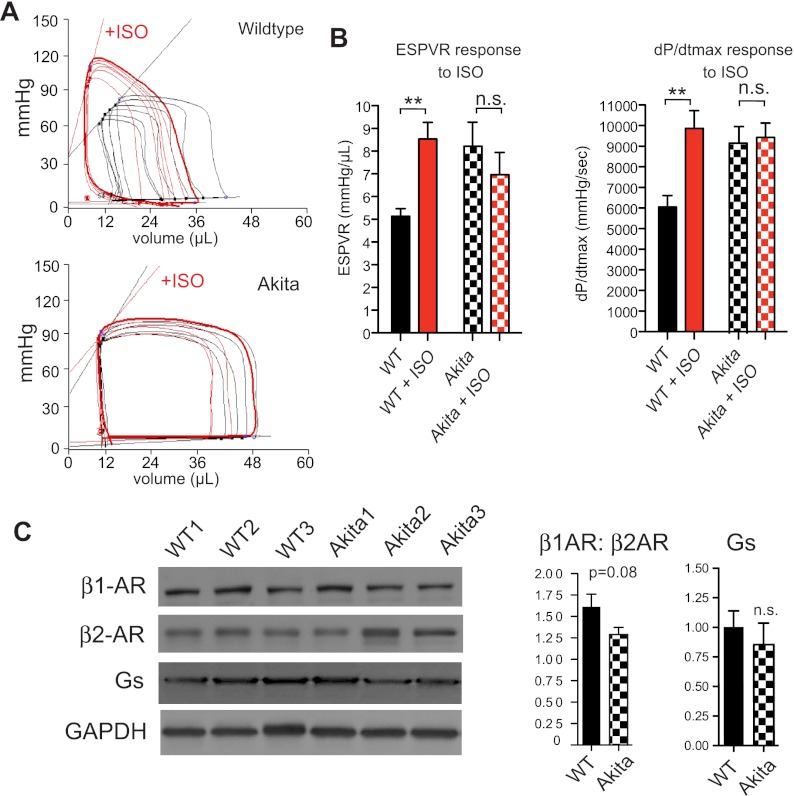
To further examine Akitains2 cardiac function, we conducted left ventricular PV analysis. PV analysis of the Akitains2 at 20-wk (5-mo) revealed abnormalities in diastolic measurements including increased relaxation time and tau (Table 1). Upon inferior vena cava occlusion to determine load-independent indexes of contraction and relaxation, an increased end-diastolic PV relationship was observed (Table 1). Lusitropic defects are classical indicators of cardiac dysfunction accompanying type 1 diabetes. In addition to the diastolic abnormalities observed in the Akitains2, the systolic function was enhanced with increased dP/dtmax (Table 1). The end-systolic PV relationship (ESPVR) was also significantly increased indicating an enhanced inotropic state of the Akitains2 (Fig. 1F; Table 1). When we administered the nonspecific β-AR agonist isoproterenol wild-type mice responded immediately with increased dP/dtmax and ESPVR. However, Akitains2 mice lacked an inotropic response to isoproterenol infusion with minimal changes in dP/dtmax and ESPVR (Fig. 2, A and B). Additionally, we show subtle alterations in the total expression of the β1- and β2-AR in the Akitains2 heart (Fig. 2C). A modest switching of β1- to β2-AR was observed with a slight reduction in the β1:β2-AR ratio, a sensitive marker of chronic β-AR stimulation. Furthermore, we investigated Gαs content, the main G-protein effector of β-adrenergic receptors; we did not detect a significant change in total Gαs between Akitains2 and wild-type controls (Fig. 2C). These data suggest saturation, desensitization, and/or uncoupling of the β-AR signaling system in the Akitains2.
Table 1.
Left ventricular invasive hemodynamics by pressure-volume conductance catheters in WT and Akita mice at 5 mo and 12 mo of age
| 5 mo |
12 mo |
|||
|---|---|---|---|---|
| Hemodynamics | WT (n = 5) | Akita (n = 7) | WT (n = 4) | Akita (n = 4) |
| Pes, mmHg | 87.1 ± 5.9 | 94.2 ± 6.8 | 91.2 ± 13.1 | 100.3 ± 8.1 |
| Ped, mmHg | 5.1 ± 1.1 | 4.8 ± 1.3 | 7.6 ± 3.5 | 6.0 ± 3.0 |
| dPmax, mmHg/s | 6,091 ± 617 | 9,111 ± 978‡ | 4,474 ± 152 | 5,180 ± 392* |
| dPmin, mmHg/s | −4,777 ± 998 | −5,008 ± 1,001 | −3,560 ± 185 | −4,238 ± 401 |
| RT, ms | 16.0 ± 1.2 | 22.3 ± 2.1† | 55.3 ± 7.1 | 51.0 ± 2.5 |
| Tau, ms | 4.21 ± 0.42 | 6.11 ± 0.22* | 7.55 ± 1.5 | 6.34 ± 0.58 |
| EDV, μl | 40.9 ± 3.1 | 38.2 ± 5.3 | 54.3 ± 1.2 | 43.2 ± 1.8‡ |
| ESV, μl | 9.7 ± 2.6 | 6.7 ± 5.1 | 34.0 ± 9.1 | 19.3 ± 2.3* |
| SV, μl | 29.9 ± 3.8 | 32.2 ± 4.1 | 29.6 ± 2.5 | 31.7 ± 2.9 |
| CO, μl/min | 15,299 ± 1,840 | 14,445 ± 2,001 | 14,825 ± 2,277 | 10,248 ± 325* |
| EF, % | 75.7 ± 5.1 | 78.8 ± 10.1 | 54.3 ± 5.6 | 63.9 ± 3.0 |
| SW, mmHg/μl | 1958 ± 258 | 2133 ± 122 | 2309 ± 343 | 2513 ± 534 |
| HR, beats/min | 578 ± 31 | 554 ± 42 | 467 ± 37 | 385 ± 34* |
| Load-independent indexes | ||||
| ESPVR | 5.05 ± 0.33 | 8.12 ± 1.87* | 3.49 ± 0.18 | 3.81 ± 0.70 |
| EDPVR | 0.11 ± 0.01 | 0.27 ± 0.09* | 0.14 ± 0.01 | 0.18 ± 0.04 |
| PRSW | 89.6 ± 10.4 | 98.2 ± 16.7 | 71.68 ± 3.48 | 77.5 ± 11.34 |
Values are means ± SD. WT, wild type; Pes and Ped, end-systolic and -diastolic pressure; dPmax and dPmin, maximum and minimum changes in pressure; RT, relaxation time; EDV and ESV, end-diastolic and end-systolic volume; SV, stroke volume; CO, cardiac output; EF, ejection fraction; SW, stroke work; HR, heart rate; ESPVR and EDPVR, end-systolic and -diastolic pressure-volume relationship; PRSW, preload recruitable stroke work.
P < 0.05;
P < 0.01;
P < 0.001.
Fig. 2.
In vivo hemodynamic response to isoproterenol infusion (A). Akitains2 displays little response to isoproterenol (ISO) as indicated by the lack of change in end-systolic PV relationship (ESPVR) and dp/dtmax post-ISO infusion (B). Representative Western blots analysis with histograms showing relative expression of the β1-adrenergic receptor (β1-AR), β2-AR and Gs (normalized to GAPDH) in the Akitains2 mouse and WT to determine the integrity of the β-AR signaling system (C). Data shown are means ± SD from 3 independent experiments. **P < 0.01.
The Akitains2 cumulative survival drastically decreases at 52 wk (12-mo) of age (Fig. 1G). We performed PV analysis at this time to determine if the Akitains2 cardiac function had decompensated to systolic failure. Surprisingly, the Akitains2 systolic function was maintained compared with age-matched controls (Fig. 1F), excluding the possibility that increased mortality is due to systolic failure. These data indicate for the first time, to our knowledge, that the Akitains2 heart is protected against cardiac systolic failure within the setting of chronic, severe diabetes.
Isolated Cardiomyocytes from the Akitains2 Have Diastolic Dysfunction with Preserved Contractility
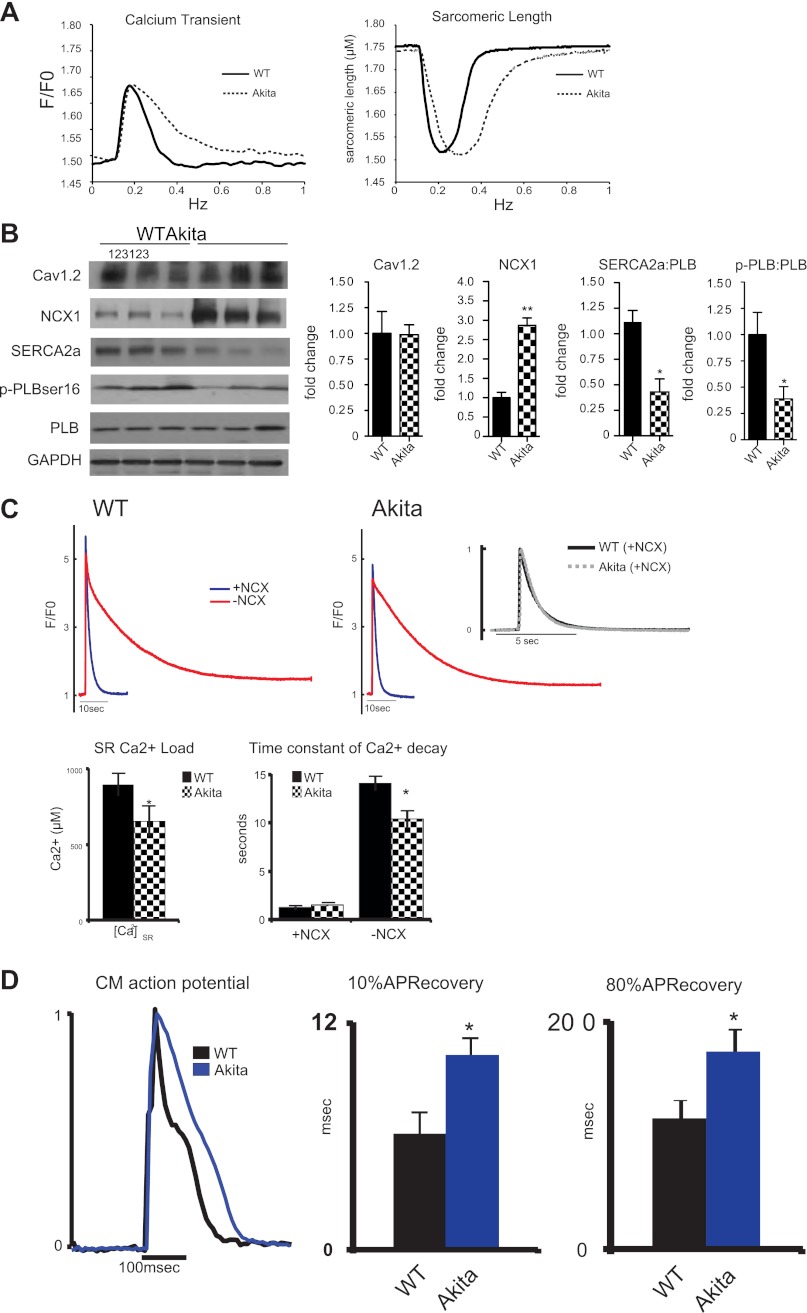
We isolated cardiomyocytes from 20-wk-old Akitains2 mice and their wild-type controls. Upon field stimulation, Ca2+ transient analysis indicated increased Ca2+ reuptake time and prolonged myocyte relaxation (Fig. 3A). There was a significant increase in tau and time to 10, 50, and 90% relaxation (Table 2). These results are in concordance with diastolic dysfunction observed in the Akitains2 in vivo. Importantly, we observed no alterations in peak Ca2+ or peak sarcomeric shortening. Similar peak [Ca2+]i transient amplitudes but decreased tau of [Ca2+]i transient decline suggest reduced SR Ca2+ uptake activity in Akitains2 myocytes.
Fig. 3.
Cardiomyocyte mechanics and expression of calcium regulatory proteins. Isolated cardiac myocyte studies with representative whole cell calcium transients and sarcomeric shortening (A). Akitains2 myocytes (dashed) display diastolic dysfunction with increased sarcomeric relaxation and calcium reuptake compared with wild-type (solid); however, peak sarcomeric shortening and peak calcium transient are unchanged between the WT and Akitains2. B: representative Western blot analyses of Cav1.2, Na+/Ca2+ exchanger 1 (NCX1), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), and phospholamban (PLB) in the Akitains2 mouse, with quantitative densitometry. pPLB:PLB and SERCA2a:PLB ratios were decreased to <50% of WT levels. Data shown are means ± SD; n = 5 animals/group. *P < 0.05; **P < 0.01. C: caffeine-induced sarcoplasmic reticulum (SR) Ca2+ content in Akitains2 compared with WT. SR Ca2+ and transient decay rates (NCX forward mode function) were both determined by rapid exposure to 20 mM caffeine in 0 Ca2+ and 0 Na+ Tyrode buffer, effectively blocking NCX (−NCX, red tracing) and 0 Ca2+ and 140 mM Na+ to monitor decay rates with functional NCX (+NCX, blue tracing). Time constant of decay (inset) did not show a significant change between the two groups when NCX was active. D: action potential kinetics in cardiomyocytes (CM) from Akitains2 and WT measured using di-8-ANEPPS; 10% and 80% APD recovery in the Akitains2 was significantly longer than WT controls (*P < 0.05) (n = 4 animals/group; >15 cells/animal).
Table 2.
Calcium transients and contractility parameters of cardiomyocytes isolated from control or Akita mice
| Calcium Transient |
Sarcomeric Shortening |
|||
|---|---|---|---|---|
| Control | AkitaIns2 | Control | AkitaIns2 | |
| Baseline | 1.74 ± 0.05 | 1.73 ± 0.02 | 1.54 ± 0.06 | 1.57 ± 0.05 |
| Peak height | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.13 ± 0.01 |
| Time to peak, ms | ||||
| 10% | 36.0 ± 1.6 | 41.5 ± 3.0* | 16.68 ± 0.02 | 18.05 ± 0.06* |
| 50% | 63.9 ± 1.3 | 73.7 ± 4.1* | 35.06 ± 0.04 | 37.17 ± 0.07* |
| 90% | 96.1 ± 5.0 | 110.7 ± 6.4* | 58.41 ± 0.18 | 60.61 ± 0.11 |
| Time to baseline, ms | ||||
| 10% | 145.2 ± 5.1 | 178.8 ± 9.5* | 113.5 ± 3.6 | 126.7 ± 6.7* |
| 50% | 178.9 ± 3.8 | 225.7 ± 6.0* | 188.1 ± 0.5 | 233.9 ± 2.8* |
| 90% | 244.1 ± 20.4 | 357.8 ± 29.4* | 378.5 ± 23.3 | 508.5 ± 39.6* |
Values are means ± SD.
P < 0.05.
Analysis of Ca2+ Handling Proteins Expression Profile in the Akitains2 Heart
Alterations in key Ca2+ transporters influence ventricular function in diabetes. We examined how LTCC, NCX1, SERCA2a, and PLB expressions were affected in the Akitains2 heart. LTCC (Cav1.2) expression was unaffected between the two groups; however, SERCA2a expression levels were significantly decreased in the Akitains2 myocardium (Fig. 3B). PLB expression was unchanged but serine-16 phosphorylation level of PLB (Fig. 3B) was significantly decreased, leading to a decreased SERCA2a:PLB ratio and further inhibition of SERCA2a, consistent with diastolic dysfunction of the Akitains2 cardiomyocytes seen in vitro and in vivo. Notably, NCX1 expression was significantly increased in the Akitains2 mouse (Fig. 3B). This led us to hypothesize that increased expression of NCX1 was a potential mechanism to compensate for the decreased activity of SERCA2a in the Akitains2 heart and maintain ventricular contractility.
Akitains2 Cardiomyocytes Have Reduced SR Ca2+ Content and Prolonged Action Potential Duration
To measure NCX1 activity in cells and determine the effects of decreased SERCA2a expression on SR Ca2+ content, we rapidly applied 20 mM caffeine to quiescent ventricular myocytes in the absence or presence of extracellular Na+ (Fig. 3C). From these measurements we determined that Akitains2 myocytes have a 27% reduction in SR Ca2+ load compared with controls (655 ± 74 vs. 899 ± 77 μM; P < 0.05; Fig. 3C), likely resulting from decreased SERCA2a activity. Surprisingly, however, we observed no change in NCX1 activity, as assessed by the decay rate of caffeine-induced Ca2+ transients in the presence of extracellular Na+ (Fig. 3C), suggesting increased NCX1 expression in Akitains2 hearts does not result in increased NCX1 function in myocytes.
Therefore, we investigated how the Akitains2 has preserved systolic function in the presence of depressed SERCA2a function and decreased SR Ca2+ load. One potential mechanism is that during each cardiac cycle NCX1 spends more time operating in reverse (Ca2+ entry) mode, as previously observed in failing human myocytes (9). To evaluate this possibility, we optically measured action potentials in isolated myocytes. APD at 80% was significantly prolonged 51% (115 ± 16 vs. 174 ± 19 ms; P < 0.05) in the Akitains2 cardiomyocyte compared with wild type (Fig. 3D). Importantly, the early part of the APD at 10 %, when NCX1 operates in reverse mode, was prolonged in the Akitains2 cardiomyocyte by 66% (6.2 ± 1.1 vs. 10.3 ± 1.6 ms; P < 0.05). Increased Ca2+ entry via NCX1 during the early phase of the AP may support calcium homeostasis and contractility in Akitains2 cardiomyocytes.
NCX1 Knockdown Using Adenovirus Encoding Antisense-NCX1 Induced Systolic Failure in the Akitains2 Mouse
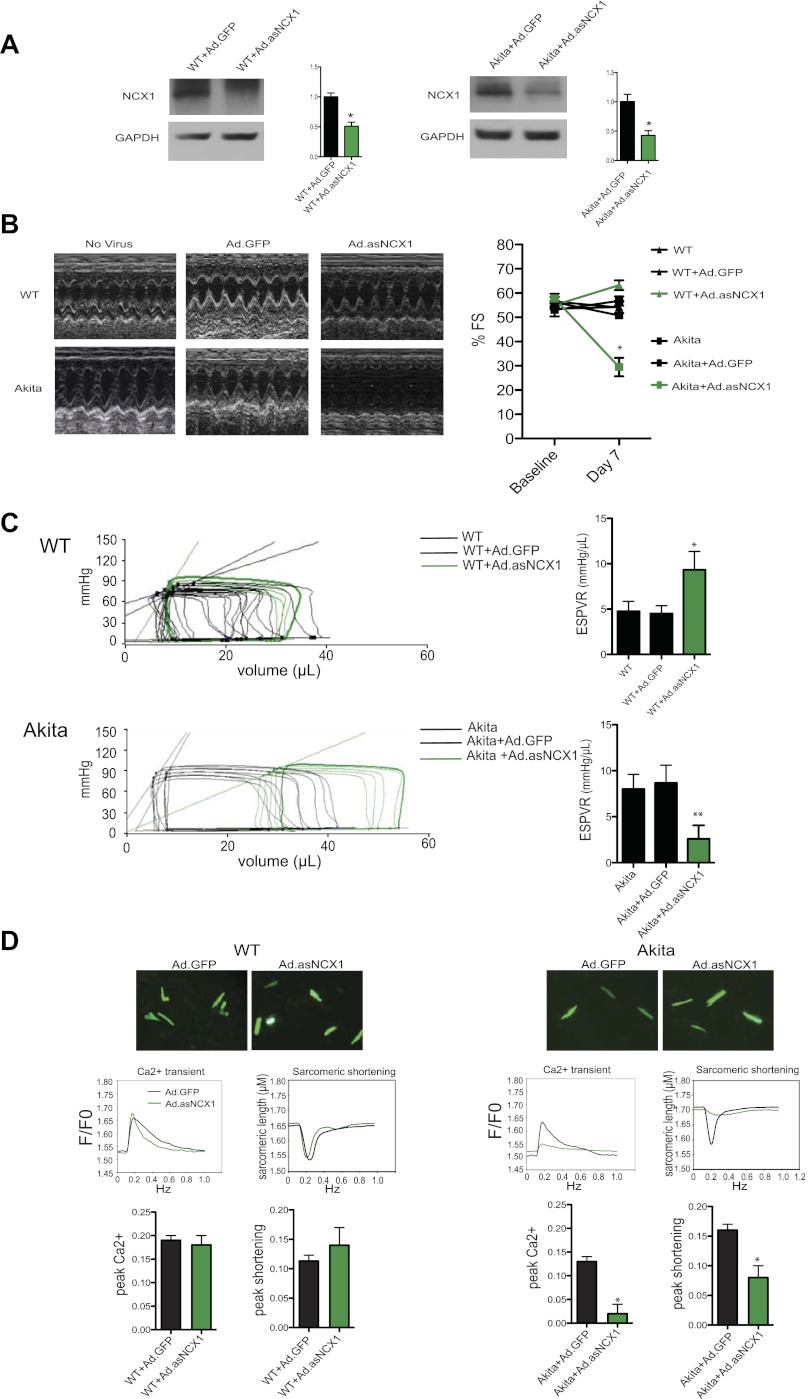
We tested the functional significance of NCX1 in the Akitains2 heart through direct myocardial injection of adenoviral vectors engineered to encode antisense-NCX1 (Ad.asNCX1). In wild-type and Akitains2 mice, Ad.asNCX1 achieved ∼50% reduction in NCX1 content compared with Ad.GFP controls 7 days postinfection (Fig. 4A). In wild-type mice treated with Ad.asNCX1, there were mild elevations in contractile parameters indicated by echocardiography and PV analyses; however, peak calcium or sarcomeric shortening were unchanged in isolated wild-type GFP+ cardiomyocytes treated with Ad.asNCX1. Strikingly, in Akitains2 mice receiving gene transfer of Ad.asNCX1, echocardiography revealed a significant decrease in ventricular function with decreased percent fractional shortening compared with Akita + Ad.GFP controls (33.8 ± 4.9 vs. 58.2 ± 4.4%, n = 7; P < 0.001; Fig. 4B; Table 3). In addition, PV analysis indicated depressed myocardial contractility with reductions in ESPVR (3.07 ± 1.82 vs. 8.53 ± 2.44, n = 7; P < 0.001) with Akita + Ad.asNCX1 (Fig. 4C, Table 4). Additionally, isolated GFP+ cardiomyocytes from the Akitains2 mouse treated with Ad.asNCX1 displayed a significant reduction in peak calcium and myocyte shortening compared with Ad.GFP controls (Fig. 4D). These in vivo results, coupled with the biochemical and electrophysiological data, determine NCX1 is functioning as a compensatory mechanism to maintain ventricular function in the Akitains2 mouse.
Fig. 4.
Adenovirus knockdown of NCX1 leads to systolic failure in the Akitains2. Direct myocardial injection of adenovirus encoding anti-sense NCX1 (Ad.asNCX1) knocked down NCX1 protein expression ∼50% compared with Ad.GFP controls (A). Seven days postinjection echocardiography was performed on WT and Akitains2. Representative M-mode images and fractional shortening (%FS; B) and in vivo hemodynamic occlusion studies and ESPVR analysis (C) of WT and Akitains2 treated with vehicle only, Ad.GFP, or Ad.asNCX1. *P < 0.05. D: calcium transients and sarcomeric shortening parameters of GFP+ ventricular myocytes from WT and Akitains2 mice infected with Ad.GFP or Ad.asNCX1 for 7 days. Histograms comparing means (±SD) peak Ca2+ and peak shortening determined in GFP+ myocytes are shown. *P < 0.05; **P < 0.01.
Table 3.
Echocardiographic analyses
| WT + Ad.GFP | WT + Ad.asNCX1 | |
|---|---|---|
| IVSd, mm | 1.31 ± 0.19 | 1.13 ± 0.12 |
| LVIDd, mm | 3.09 ± 0.53 | 2.96 ± 0.31 |
| LVPWd, mm | 1.29 ± 0.19 | 1.33 ± 0.19 |
| IVSs, mm | 1.96 ± 0.40 | 1.94 ± 0.15 |
| LVIDs, mm | 1.41 ± 0.03 | 0.99 ± 0.30 |
| LVPWs, mm | 2.00 ± 0.28 | 2.19 ± 0.06* |
| %FS | 54.8 ± 2.4 | 67 ± 7.4† |
| HR, beats/min | 535 ± 25 | 559 ± 18 |
| Akita + Ad.GFP | Akita + Ad.asNCX1 | |
| IVSd ,mm | 0.86 ± 0.09 | 0.77 ± 0.07 |
| LVIDd, mm | 2.78 ± 0.15 | 3.48 ± 0.29† |
| LVPWd, mm | 0.97 ± 0.09 | 0.90 ± 0.04 |
| IVSs, mm | 1.50 ± 0.09 | 1.22 ± 0.14* |
| LVIDs, mm | 1.16 ± 0.07 | 2.31 ± 0.32‡ |
| LVPWs, mm | 1.75 ± 0.03 | 1.35 ± 0.12‡ |
| %FS | 58.2 ± 4.4 | 33.8 ± 4.9‡ |
| HR, beats/min | 528 ± 30 | 541 ± 32 |
Values are means ± SD. Seven days postinjection echocardiography was performed. WT or Akita mice received gene transfer of Ad.GFP or Ad.asNCX1. IVSd, intraventricular septum in diastole; LVIDd and LVIDs, left ventricular internal diameter at diastole and systole ; LVPWd and LVPWs, left ventricle posterior wall diastole and systole.
P < 0.05;
P < 0.01;
P < 0.001.
Table 4.
Left ventricular invasive hemodynamics 7 days post-Ad.GFP or Ad.asNCX1 gene transfer in 20 wk WT or Akita mice
| WT + Ad.GFP | WT + Ad.asNCX1 | Akita + Ad.GFP | Akita + Ad.asNCX1 | |
|---|---|---|---|---|
| Pes, mmHg | 88.6 ± 3.4 | 99.9 ± 5.1* | 102.0 ± 8.4 | 101.5 ± 9.7 |
| Ped, mmHg | 3.6 ± 0.5 | 5.2 ± 1.6 | 6.0 ± 3.1 | 4.8 ± 2.6 |
| dPmax, mmHg/s | 5,870 ± 400 | 6,864 ± 378 | 7,133 ± 188 | 7,331 ± 954 |
| dPmin, mmHg/s | −3,664 ± 181 | −4,932 ± 624 | −4,864 ± 391 | −5,605 ± 757 |
| Tau, ms | 4.96 ± 0.2 | 4.38 ± 0.50* | 6.00 ± 0.2 | 5.25 ± 0.75 |
| EDV, ul | 41.6 ± 8.6 | 42.8 ± 8.2 | 44.7 ± 8.6 | 54.8 ± 9.78 |
| ESV, ul | 10.0 ± 2.4 | 9.6 ± 4.0 | 10.9 ± 2.4 | 22.1 ± 6.0† |
| SV, ul | 25.0 ± 4.0 | 32.6 ± 5.2 | 38.8 ± 5.2 | 32.6 ± 8.5 |
| CO, ul/min | 11,951 ± 2,357 | 15,784 ± 1,613 | 16,788 ± 2,800 | 16,317 ± 1,613 |
| EF, % | 72.6 ± 8.2 | 79.8 ± 9.7 | 79.6 ± 3.2 | 59.8 ± 2.1‡ |
| SW, mmHg/μl | 1,533 ± 347 | 2614 ± 480 | 3,313 ± 470 | 2,722 ± 256* |
| ESPVR | 4.78 ± 0.54 | 9.03 ± 2.47* | 8.53 ± 2.44 | 3.07 ± 1.82‡ |
| EDPVR | 0.10 ± 0.01 | 0.13 ± 0.07 | 0.23 ± 0.03 | 0.30 ± 0.08‡ |
| PRSW | 80.1 ± 9.53 | 121 ± 27.22* | 116.3 ± 11.1 | 93 ± 17.45 |
| HR, beats/min | 508 ± 26 | 538 ± 35 | 519 ± 26 | 489 ± 35 |
Values are means ± SD.
P < 0.05;
P < 0.01;
P < 0.001.
CXCL12/CXCR4 Axis Is Upregulated in the Akitains2 Myocardium with No Presence of Inflammatory Infiltrate
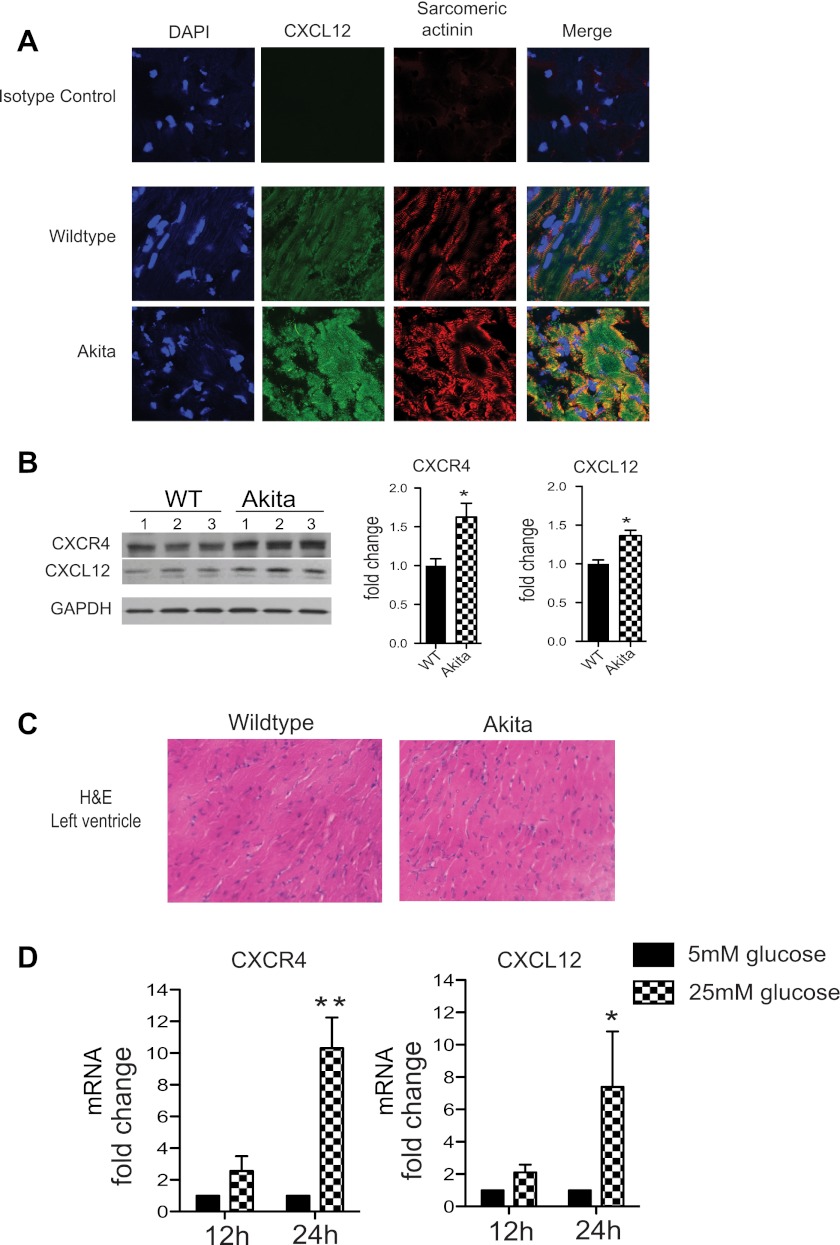
Several studies (30) have demonstrated a role for CXCL12 in type 1 diabetes. CXCR4/CXCL12 signaling pathway has been shown to protect NOD mice from autoimmune diabetes via inhibition of T-cell-mediated inflammation and pancreatic islet cell death. How CXCL12/CXCR4 signaling network functions in other organ systems in the diabetic state is unknown. CXCL12 is a critical paracrine signal during acute cardiac ischemic events for circulating progenitor cells to initiate the myocardial repair process. However, CXCL12 may also act in an autocrine fashion on the cardiomyocyte regulating Ca2+ homeostasis and promoting cell survival. In the setting of the diabetic heart with calcium perturbations, the CXCL12/CXCR4 axis could represent a novel protective mechanism in maintaining ventricular function and calcium homeostasis. In the myocardium of the Akitains2 mouse, we observed a significant increase in CXCL12 (Fig. 5, A and B) and CXCR4 expression (Fig. 5B). Importantly, the Akitains2 heart has been reported to have low levels of myocardial inflammation and we also show no significant inflammatory infiltration (Fig. 5C). This suggests that the upregulated CXCL12/CXCR4 expression is not involved in chemotactic, inflammatory mechanisms but potentially is enhanced due to hyperglycemia and calcium-handling disturbances in the cardiomyocyte. We tested if hyperglycemia in vitro induces CXCL12/CXCR4 expression in cardiomyocytes. Isolated ARVMs were cultured in a high glucose (25 mM) media for 24 h, and CXCL12 and CXCR4 expression was determined by quantitative PCR. We demonstrate both CXCL12 and CXCR4 expression was upregulated seven and ninefold, respectively (Fig. 5D). The upregulation of CXCL12/CXCR4 in the Akitains2 myocardium in the absence of acute ischemia details an additional, potentially cardioprotective, mechanism of the CXCL12/CXCR4 axis within diabetic cardiomyopathy.
Fig. 5.
CXCL12/CXCR4 expression in the Akitains2 mouse. Cryosections of left ventricular tissue were immunostained for CXCL12 (green), sarcomeric actinin (red), and DAPI (blue). CXCL12 expression is enhanced on the sarcomeric Z-lines of the cardiac myocyte in the Akitains2 myocardium (A). Representative Western blots analysis with quantitative densitometry indicate increased protein expression of CXCL12 and CXCR4 in the Akitains2 heart (B). Data shown are means ± SD; *P < 0.05. Hematoxylin and eosin staining of the left ventricle in Akitains2 and WT control show no significant inflammatory infiltrate in the myocardium (C). D: glucose induces expression of CXCL12/CXCR4 in isolated adult rat cardiomyocytes treated with either normal culture media containing 5.5 mM glucose or media supplemented with 25 mM glucose final concentration for 12 and 24 h. Total RNA was isolated and quantitative PCR of CXCL12 and CXCR4 mRNA were determined (n = 3 independent experiments; *P < 0.05; **P < 0.01). rRNA 28S was used as a control.
CXCR4 Regulates the Expression of NCX1 via NF-κB
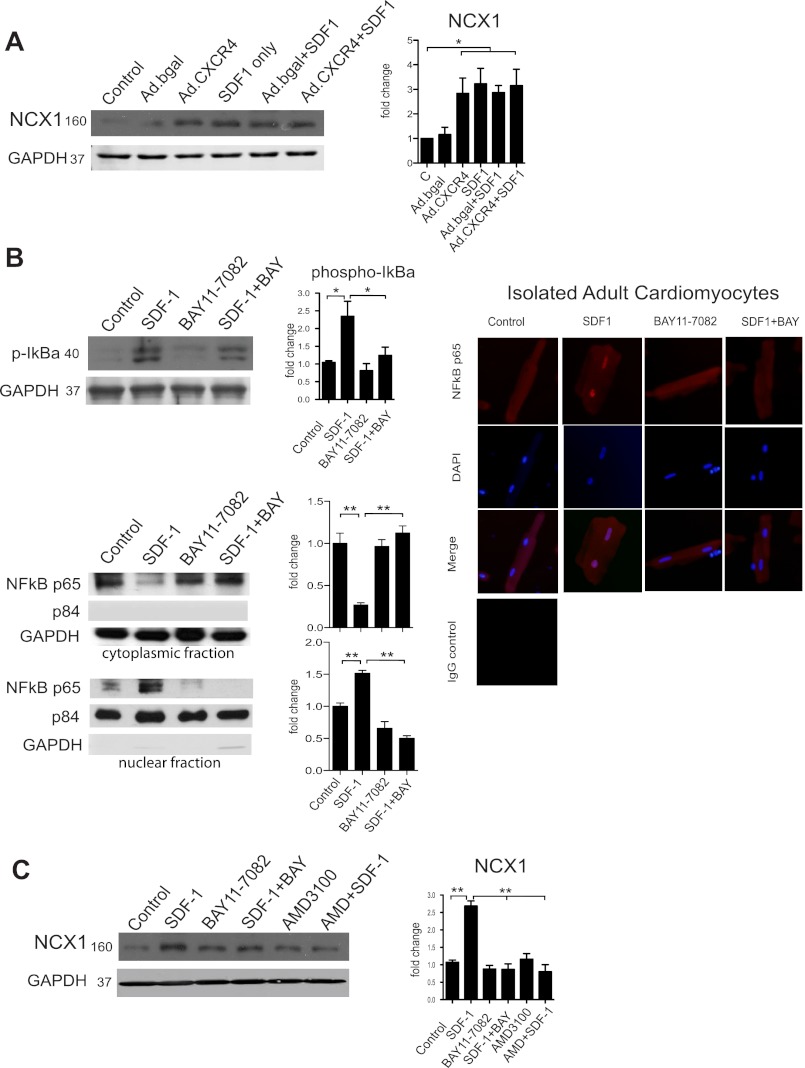
Our data reveal the increased NCX1 expression in Akitains2 cardiomyocytes may potentially be regulated by a CXCR4-dependent mechanism. To test this hypothesis, we treated isolated ARVMs with CXCL12 (SDF-1; 100 ng/ml) for 24 h. CXCL12 exposure generated a significant increase in NCX1 expression in the ARVM (Fig. 6A). Additionally, adenoviral overexpression of CXCR4 (Ad.CXCR4) in ARVM resulted in a significant increase in NCX1 expression compared with control adenovirus encoding β-gal (Ad.β-gal; Fig. 6A). These data demonstrate CXCR4 as a novel regulator of NCX1 expression.
Fig. 6.
CXCR4 regulates NCX1 expression via a NF-κB dependent mechanism. Representative western blots are shown with quantitative densitometry. Adenoviral overexpression of CXCR4 (Ad.CXCR4) in isolated adult rat cardiomyocytes cultured for 24 h produced a significant increase in NCX1 expression compared with Ad.βgal (A). Isolated myocytes were also treated with CXCL12 (SDF1; 100 ng/ml, 24 h) inducing significant expression of NCX1 (A). B: Western blot analysis of CXCL12/SDF1 and BAY11–7082 (5 μM) treatment on IκB-α phosphorylation and NF-κB p65 translocation. Additionally, immunofluorescence staining of NF-κB p65 is shown in similarly treated cardiomyocytes indicating increased p65 nuclear localization with CXCL12/SDF-1. C: isolated myocytes were treated with the IκB-α inhibitor, BAY11–7082 (5 μM) along with SDF1 or AMD3100, a CXCR4 inhibitor, for 24 h. Myocytes treated with BAY11–7082 or AMD3100 prevented CXCL12 induced NCX1 expression indicating a NF-κB-dependent mechanism. Protein loading and purity of fractions were verified by GAPDH (cytoplasmic) and p84 (nuclear). *P < 0.05; **P < 0.01
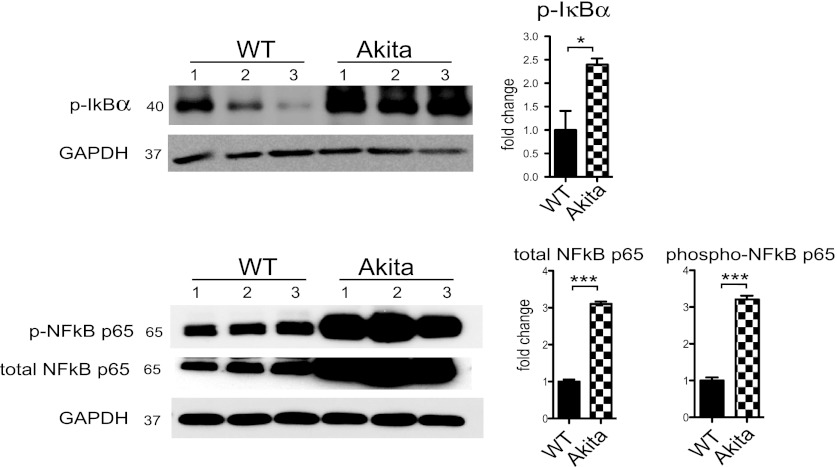
NF-κB is a transcriptional mediator involved in the regulation of stress-induced genes related to cardiomyocyte hypertrophy and cell survival (12). Importantly, Sirabela et al. (40) reported NF-κB is involved in transcriptional regulation of NCX1 in cortical neurons. We sought to determine if CXCR4 could activate NF-κB in cardiomyocytes and if this pathway was involved in regulating NCX1 expression. Indeed, we show CXCR4 activation by CXCL12/SDF-1 induces phosphorylation of IκB-α (an endogenous inhibitor of NF-κB) in cardiomyocytes (Fig. 6B). Phosphorylation and subsequent ubiquitination and degradation of IκB-α are essential for NF-κB activation and nuclear translocation. Furthermore, cardiac myocytes treated with CXCL12 and separated into cytoplasmic and nuclear fractions indicated significant NF-κB p65 nuclear translocation (Fig. 6B). Additionally, immunostaining revealed CXCL12 enhanced NF-κB p65 localization within the cardiomyocyte nuclei (Fig. 6B). Cardiomyocytes were treated with the IκB-α inhibitor Bay11–7082, which selectively inhibits NF-κB activation through inhibition of IkB-α phosphorylation. When IκB-α phosphorylation is inhibited, CXCL12 failed to increase NCX1 expression (Fig. 6C). Likewise, treatment with the CXCR4 antagonist AMD3100 prevented CXCL12-induced NCX1 upregulation. These results suggest NCX1 expression is regulated by CXCR4 activation of NF-κB. Finally, in the Akitains2 myocardium there is significantly increased IκB-α and NF-κB p65 total and phosphorylation levels (Fig. 7). Therefore, elevated CXCL12/CXCR4 and the increased NF-κB may be a potential mechanism for the increased NCX1 expression and systolic preservation in the Akitains2 heart.
Fig. 7.
Western blot analysis of IκB-α and NF-κB p65 in the Akitains2 whole ventricular tissue. Akitains2 heart showed ∼3-fold elevation of phospho-IκB-α and NF-κB p65 as well as total NF-κB p65. GAPDH was used as loading control. Data shown are means ± SD from at 3 independent experiments; *P < 0.05; ***P < 0.001.
DISCUSSION
Diabetic cardiomyopathy is an important clinical entity predisposing individuals to ventricular dysfunction and arrhythmogenesis. Calcium cycling is disturbed in the diabetic heart and understanding the involvement of calcium cycling in the cardiomyocyte will give insight into the pathogenesis of diabetic cardiomyopathy. This study identifies an important compensatory function of NCX1 in the diabetic heart mediated by a CXCR4-NF-κB pathway.
The Akitains2 mouse is a genetic model of type 1 diabetes presenting with consistent blood glucose levels >600 mg/dl (47). However, in the 5-mo Akitains2 mice, there is only modest evidence of myocardial dysfunction. Echocardiography revealed no difference in fractional shortening between Akitains2 and wild-type littermate controls. However, hemodynamic measurements using a PV conductance catheter indicated elevated diastolic parameters, including relaxation time tau, and end-diastolic pressure volume relationship, correlating with diastolic dysfunction. Interestingly, we did observe enhanced systolic function in the Akitains2 mouse with increased dP/dtmax, and ESPVR; however, there was no correlative increase in peak calcium transient or sarcomeric shortening of isolated Akitains2 myocytes. Of note, the Akitains2 survival significantly diminishes at 12 mo, ∼50%. Echocardiography and PV analysis at 12 mo indicated no systolic impairment, thus the decrease in the 12-mo survival is not due to a worsening cardiac systolic function or failure. Grossly, there was mild cardiac atrophy; however, evidence of fibrosis or inflammation that could also lead to decreased survival at 12 mo was not detected. In agreement with our findings, Basu et al. (2) have also reported that Akitains2 mouse exhibits diastolic dysfunction with preserved systolic function at 3 and 6 mo of age. Maintained ventricular function with such prolonged exposure to severe diabetes is surprising as other models of type 1 and type 2 diabetes generally present with significant systolic deterioration (33).
Maintenance of cardiac systolic function during diabetes includes not only adaptive alterations in metabolic glucose and free-fatty acid metabolism but also initiates adaptive mechanisms involving calcium cycling proteins (8, 48). Type 1 and type 2 diabetes both decrease myocardial SERCA2a content and activity leading to diastolic and systolic dysfunction shown in animal models and in human hearts (7, 19, 30). In the streptozotocin (STZ)-induced type 1 diabetes model, mice progress into systolic failure within weeks to months postdiabetes onset (7, 49), although other studies showed preserved function (8). In both the STZ and the Akitains2 models, there are reductions in SERCA2a expression; however, a key difference between the STZ model and the Akitains2 is the expression of NCX1, which is decreased in the former (7) but significantly elevated in the latter. In Akitains2 myocytes, reduced SR Ca2+ content was primarily a result of decreased SR Ca2+ uptake due to decreased SERCA2 expression and/or increased SERCA inhibition by unphosphorylated PLB. Despite decreased SR Ca2+ content, [Ca2+]i transient amplitudes were unchanged. Three possibilities to account for this result include 1) increased Ca2+ entry via reverse-mode NCX, 2) an increase in the gain of CICR, and 3) an increase in intracellular [Na+], which would alter the electrochemical potentials that dictate the direction of NCX1 activity. We did not address the second or third possibilities, but the prolonged action potentials observed in the Akitains2 myocytes support increased reverse-mode NCX1 activity. Slower phase 1 repolarization in the Akitains2 myocytes allows more time for Ca2+ entry via NCX, and this Ca2+ influx may both contribute to CICR and help maintain SR load, as seen in developing mouse myocytes (19). The prolonged action potential likely results from decreased Ito potassium channel currents (3) and can be interpreted as a potential adaptive mechanism regulating NCX1 activity and preventing contractile deterioration. It is also interesting to note that in the absence of both NCX1 and SERCA2a function the time constant of Ca2+ decay was faster in Akitains2 myocytes. This observation suggests that the activities of mitochondrial Ca2+ uniporter and sarcolemmal Ca2+-ATPase are also increased, perhaps as an additional compensatory response to decreased SERCA2a function in Akitains2 myocytes. However, the elevated NCX1 activity and expression providing systolic support to the Akitains2, along with the prolonged action potential duration, may be at the cost of generating an arrhythmogenic substrate in the cardiac myocyte (41). The possibility of sudden cardiac death as a contributing factor to the decreased 12-mo survival in the Akitains2 mouse is currently being investigated.
The observation that NCX1 expression was increased in Akitains2 hearts without a detectable change in NCX1 function suggests increased NCX1 expression might represent an important compensatory mechanism, required simply to maintain normal NCX1 function. To evaluate this possibility, we utilized a direct myocardial injection of anti-sense NCX1. NCX1 reduction in the Akitains2 mouse heart exhibited a marked impairment in systolic function, in stark contrast to wild-type control, with significantly depressed percent fractional shortening, ejection fraction, and ESPVR. Importantly, cardiac-specific NCX1 knockout in otherwise healthy, nondiabetic mice display normal Ca2+ dynamics and have little to no contractile depression (18). This indicates that in the Akitains2 mouse elevated NCX1 is compensating and stabilizing cardiac contractility in the setting of reduced SR Ca2+ and SERCA2a expression. This observation is relevant since Hasenfuss et al. (16) have also shown the compensatory mechanism of increased NCX1 in the setting of reduced SERCA2a expression in failing human cardiomyocytes. However, the increased NCX1 expression in the Akitains2 mouse did not correlate with increased NCX1 forward mode function as there was no alteration in caffeine-induced Ca2+ decay rates, but the prolonged APD suggests increased NCX1 reverse mode activity. Compared with failing human myocytes, NCX1 operating in the reverse mode contributes to maintenance of systolic function (9). This indicates the Akitains2 mouse is upregulating NCX1 transcription and translation to maintain basal NCX1 activity and myocyte contractility. Thereby, reducing NCX1 expression in the setting of reduced SERCA2a is deleterious, as is observed in the STZ model (49), and NCX1 upregulation indicates a compensatory mechanism of the cardiomyocyte. Importantly, even though manipulating the expression of NCX1 in vivo by direct myocardial gene transfer of antisense NCX1 has significant impact on cardiac performance and isolated cardiac myocyte contractility, this does not address the question of which cell types are affected by the transgene. It is well known that local injection of adenoviral transgenes would certainly transduce myocytes, fibroblasts, smooth muscle cells, as well as endothelial cells. As such, the impact of antisense NCX1 on cardiac function in vivo is more likely a global effect on the myocardium by NCX1 regardless of which cell types are infected. However, in isolated GFP positive myocytes we showed that decreased NCX1 expression led to reduced contractility and peak Ca2+ transients, thus indicating the acute decompensation of cardiac function in the Akita heart is primarily due to cardiomyocyte contractile dysfunction. Although fibroblasts may also be infected with antisense NCX1, their contribution to myocardial fibrosis is not expected, as we did not observe such phenomenon in the Akita heart. In addition, other groups reported that they found no evidence of fibrosis in the Akita heart as demonstrated by Trichrome staining or PCR of profibrotic genes (2).
Furthermore, increased NCX1 has been associated with reduced β-adrenergic responses in the cardiac myocyte. Cardiac myocytes moderately overexpressing NCX1 have shown reduced β-AR responsiveness to isoproterenol through the enhancement of β2-AR/Gi coupling thereby inhibiting β-AR inotropic capacity (37). β2-AR/Gi signaling elicits cardioprotective pathways in response to excessive cathecholamine stimulation (29). NCX1 may augment pathways promoting cardiac myocyte survival. We also observed intravenous administration of isoproterenol elicited a blunted inotropic response in the Akitains2, further establishing a functional relationship between elevated NCX1 and the β-AR signaling system.
We subsequently aimed to identify mechanisms regulating NCX1 expression in the cardiomyocyte. Interestingly, the chemokine receptor CXCR4 is known to regulate β-AR signaling in the cardiac myocyte inhibiting β-AR-induced L-type calcium channel activity, as well as physically associating with β2-AR (20). In addition, CXCR4 has been the subject of many studies evaluating the beneficial effects of CXCR4-dependent recruitment of progenitor cells in acute myocardial ischemia (5). However, CXCR4 signaling affecting calcium handling, survival, or metabolic responses within the cardiomyocyte is not well delineated. CXCR4 is known to induce cellular migration in part by activating NF-κB. Recently, NF-κB was shown to transcriptionally regulate NCX1 expression in cortical neurons exposed to anoxia-glucose deprivation injury (40). In the cardiomyocyte, we demonstrated CXCL12 activates NF-κB through IκB-α phosphorylation and NF-κB p65 isoform nuclear translocation. IκB-α is an associated inhibitory protein that upon phosphorylation by IKK is degraded allowing NF-κB nuclear translocation. We determined in cardiomyocyte NCX1 is also regulated by NF-κB through a CXCR4-dependent mechanism. The Akitains2 heart has significantly elevated IκB-α and NF-κB p65 total and phosphorylation levels. Therefore, the increased CXCL12/CXCR4 expression in vivo is potentially a source for IκB-α phosphorylation and p65 nuclear translocation resulting in the upregulation of NCX1 expression in the Akitains2 heart, promoting cardiac systolic function. The functional role of NF-κB within the cardiac myocyte has remained difficult to define as it participates in hypertrophy, myocyte survival, apoptosis, and myocardial inflammatory responses. The adaptive and maladaptive implications of NF-κB signaling have been well documented, and NF-κB downstream targets are considered to be dependent on the contextual myocardial microenvironment. Several studies have shown activation of NF-κB postmyocardial infarction is a cardioprotective mechanism leading to enhanced myocyte survival and a reduction in infarct size. Specifically, NF-κB p50 null mice have been shown to have worsening cardiac function postmyocardial infarction with increased apoptosis (44). Also, p65 has been described as antiapoptotic through repression of Bnip3 (39). Additionally, patients diagnosed with NYHA III/VI heart failure, having the p50 ATTG1/1 polymorphism with diminished myocardial p50 expression, have worse cardiac performance and decreased survival (36). Here we show the prolonged activation of NF-κB irrespective of acute ischemia in the Akita myocardium is not cardiotoxic and does not lead to increased apoptosis, inflammation, or ventricular dysfunction. This study shows long-term NF-κB activation in diabetic cardiomyopathy is cardioprotective and may be cardioprotective through its influence on NCX1 and promotion of calcium homeostasis.
In summary, our results define an adaptive role for NCX1 in promoting contractile function in diabetic cardiomyopathy. They also reveal a novel mechanism for CXCR4 in the Akitains2 diabetic heart by regulating NCX1 expression via a NF-κB. The identification of CXCR4 involvement in NCX1 regulation further establishes CXCR4 as a central mediator of calcium homeostasis in the cardiac myocyte. The CXCR4/NF-κB/NCX1 module represents a potential therapeutic target in diabetic cardiomyopathy.
GRANTS
Support was provided in part by the National Heart, Lung, and Blood Institute Ruth L. Kirschstein National Research Service Award 1F30HL096344 (to T. J. LaRocca), Mount Sinai School of Medicine Medical Scientist Training Program GM007280 (to T. J. LaRocca), and Juvenile Diabetes Research Foundation (to D. Lebeche).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.J.L. and D.L. conception and design of research; T.J.L., F.F., J.C., D.B., S.Z., and L.T.M. performed experiments; T.J.L., F.F., E.A.S., and D.L. analyzed data; T.J.L., J.Y.C., E.A.S., and D.L. interpreted results of experiments; T.J.L. and F.F. prepared figures; T.J.L. and D.L. drafted manuscript; T.J.L., A.D.S., J.Y.C., E.A.S., R.J.H., and D.L. edited and revised manuscript; D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We are thankful to Kathryn Vreeland for providing technical assistance.
REFERENCES
- 1. Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 74: 555–564, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol 297: H2096–H2108, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bers DM, Despa S, Bossuyt J. Regulation of Ca2+ and Na+ in normal and failing cardiac myocytes. Ann NY Acad Sci 1080: 165–177, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Brunner S, Winogradow J, Huber BC, Zaruba MM, Fischer R, David R, Assmann G, Herbach N, Wanke R, Mueller-Hoecker J, Franz WM. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. FASEB J 23: 351–361, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes 57: 2924–2932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283: H1398–H1408, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol 32: 985–996, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Dipla K, Mattiello JA, Margulies KB, Jeevanandam V, Houser SR. The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ Res 84: 435–444, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Federation ID.International Diabetes Foundation. Diabetes Atlas. Homepage Diabetes Atlas. (Online). http://www. diabetesatlas.org/ [11 Oct 2010]
- 11. Fox CS, Coady S, Sorlie PD, D′Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115: 1544–1550, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 61: 70–76, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103: 1300–1308, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hasenfuss G, Mulieri LA, Leavitt BJ, Allen PD, Holubarsch C, Just H, Alpert NR. Contractile protein function in failing and nonfailing human myocardium. Basic Res Cardiol 87, Suppl 1: 107–116, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99: 641–648, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 107: 539–557, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Ishikawa T, Kajiwara H, Kurihara S. Alterations in contractile properties and Ca2+ handling in streptozotocin-induced diabetic rat myocardium. Am J Physiol Heart Circ Physiol 277: H2185–H2194, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Larocca TJ, Schwarzkopf M, Altman P, Zhang S, Gupta A, Gomes I, Alvin Z, Champion HC, Haddad G, Hajjar RJ, Devi LA, Schecter AD, Tarzami ST. β2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol 56: 548–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med 5: 715–724, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lebeche D, Kaprielian R, del Monte F, Tomaselli G, Gwathmey JK, Schwartz A, Hajjar RJ. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation 110: 3435–3443, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Liao R, Jain M. Isolation, culture, and functional analysis of adult mouse cardiomyocytes. Methods Mol Med 139: 251–262, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Lu Z, Jiang YP, Xu XH, Ballou LM, Cohen IS, Lin RZ. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes 56: 2780–2789, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Mercadier JJ, Lompre AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C, Allen PD, Komajda M, Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest 85: 305–309, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nuss HB, Houser SR. Sodium-calcium exchange-mediated contractions in feline ventricular myocytes. Am J Physiol Heart Circ Physiol 263: H1161–H1169, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park HJ, Zhang Y, Du C, Welzig CM, Madias C, Aronovitz MJ, Georgescu SP, Naggar I, Wang B, Kim YB, Blaustein RO, Karas RH, Liao R, Mathews CE, Galper JB. Role of SREBP-1 in the development of parasympathetic dysfunction in the hearts of type 1 diabetic Akita mice. Circ Res 105: 287–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med 32: 1041–1048, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Penpargkul S, Fein F, Sonnenblick EH, Scheuer J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13: 303–309, 1981 [DOI] [PubMed] [Google Scholar]
- 31. Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98: 596–605, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothetis DE, Hajjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol 41: 834–844, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radovits T, Korkmaz S, Loganathan S, Barnucz E, Bomicke T, Arif R, Karck M, Szabo G. Comparative investigation of the left ventricular pressure-volume relationship in rat models of type 1 and type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 297: H125–H133, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Reuter H, Pott C, Goldhaber JI, Henderson SA, Philipson KD, Schwinger RH. Na+-Ca2+ exchange in the regulation of cardiac excitation-contraction coupling. Cardiovasc Res 67: 198–207, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602, 1972 [DOI] [PubMed] [Google Scholar]
- 36. Santos DG, Resende MF, Mill JG, Mansur AJ, Krieger JE, Pereira AC. Nuclear factor (NF) kappaB polymorphism is associated with heart function in patients with heart failure. BMC Med Genet 11: 89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. Loss of beta-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J Mol Cell Cardiol 36: 43–48, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 30: 1929–1937, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Shaw J, Yurkova N, Zhang T, Gang H, Aguilar F, Weidman D, Scramstad C, Weisman H, Kirshenbaum LA. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc Natl Acad Sci USA 105: 20734–20739, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sirabella R, Secondo A, Pannaccione A, Scorziello A, Valsecchi V, Adornetto A, Bilo L, Di Renzo G, Annunziato L. Anoxia-induced NF-kappaB-dependent upregulation of NCX1 contributes to Ca2+ refilling into endoplasmic reticulum in cortical neurons. Stroke 40: 922–929, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Sobie EA, Cannell MB, Bridge JH. Allosteric activation of Na+-Ca2+ exchange by L-type Ca2+ current augments the trigger flux for SR Ca2+ release in ventricular myocytes. Biophys J 94: L54–L56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spector KS. Diabetic cardiomyopathy. Clin Cardiol 21: 885–887, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res 95: 807–813, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res 104: 699–706, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH. Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol 588: 4249–4260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents, and action potential duration. Am J Physiol Heart Circ Physiol 297: H590–H601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation 105: 1861–1870, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Zhong Y, Ahmed S, Grupp IL, Matlib MA. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 281: H1137–H1147, 2001 [DOI] [PubMed] [Google Scholar]