Abstract
Doppler ultrasound measures of left ventricular (LV) active relaxation and diastolic suction are slowed with healthy aging. It is unclear to what extent these changes are related to alterations in intrinsic LV properties and/or cardiovascular loading conditions. Seventy carefully screened individuals (38 female, 32 male) aged 21–77 were recruited into four age groups (young: <35; early middle age: 35–49; late middle age: 50–64 and seniors: ≥65 yr). Pulmonary capillary wedge pressure (PCWP), stroke volume, LV end-diastolic volume, and Doppler measures of LV diastolic filling were collected at multiple loading conditions, including supine baseline, lower body negative pressure to reduce LV filling, and saline infusion to increase LV filling. LV mass, supine PCWP, and heart rate were not affected significantly by aging. Measures of LV relaxation, including isovolumic relaxation time and the time constant of isovolumic pressure decay increased progressively, whereas peak early mitral annular longitudinal velocity decreased with advancing age (P < 0.001). The propagation velocity of early mitral inflow, a noninvasive measure of LV suction, decreased with aging with the greatest reduction in seniors (P < 0.001). Age-related differences in LV relaxation and diastolic suction were not attenuated significantly when PCWP was increased in older subjects or reduced in the younger subjects. There is an early slowing of LV relaxation and diastolic suction beginning in early middle age, with the greatest reduction observed in seniors. Because age-related differences in LV dynamic diastolic filling parameters were not diminished significantly with significant changes in LV loading conditions, a decline in ventricular relaxation is likely responsible for the alterations in LV diastolic filling with senescence.
Keywords: aging, diastolic function, Doppler imaging, relaxation
heart failure hospitalization rates increase sharply with advancing age (15). Because many adults over the age of 65 years with heart failure have apparently preserved systolic function (ejection fraction >50%), diastolic dysfunction contributes substantially to the heart failure syndrome in adults ≥65 yr (seniors) (36, 42). Longitudinal measures of persistent or worsening left ventricular (LV) diastolic function are predictive of new-onset heart failure (26). Thus, there is an emerging need to comprehensively understand specific age-related changes in LV diastolic filling to distinguish pathological from healthy physiological variation.
With healthy aging, a slower peak early mitral annular longitudinal velocity (Em), a prolonged isovolumic relaxation time (IVRT), a slower early propagation velocity (VP), and a reduction in intraventricular pressure gradient (IVPG), which is consistent with slowed active ventricular relaxation and diastolic suction, have been reported previously (4, 19, 27, 34, 35). However, because Doppler parameters of diastolic filling are influenced by changes in LV loading conditions in healthy individuals (6, 7, 16, 35), it remains unclear to what extent age-related changes result from alterations in intrinsic LV properties and/or to cardiovascular loading conditions. Moreover, there is little information whether there is a concomitant reduction in the responsiveness of LV filling properties to alterations in LV filling pressure [pulmonary capillary wedge pressure (PCWP)] with advancing age. Such information is important to establish whether slowed relaxation and diastolic suction may compromise LV filling in seniors during conditions in which rapid changes in LV volume are required such as exercise or orthostatic stress.
Accordingly, the aims of this study were to determine: 1) at what stage of life are active relaxation and diastolic suction slowed with healthy aging and 2) whether the response of LV relaxation and diastolic suction properties to alterations in PCWP is reduced with healthy aging. To achieve these aims, Doppler measures of LV diastolic filling, LV end-diastolic volume (LVEDV), and hemodynamics were collected over a range of LV filling pressures in healthy individuals aged 21–77 yr at specifically defined stages of aging.
METHODS
Participant Characteristics
Subjects were recruited from the Dallas Heart Study, a population-based probability sample of ≈6,100 individuals in the Dallas-Fort Worth metroplex who have been carefully screened with a detailed history, clinical exam, and selected diagnostic tests as previously described (43). To enrich the total sample, subjects were additionally recruited from a random sample of ≈20,000 employees at Texas Health Resources, the third largest employer in the Dallas-Fort Worth metroplex and a diverse health care company. All subjects were nonsmokers, were not taking any cardiovascular medications, had a 24-h blood pressure <140/90 mmHg, body mass index ≤30 kg/m2, and had a normal electrocardiogram and exercise stress echocardiogram. Subjects were either sedentary or casual exercisers with all subjects exercising no more (and usually less than) than three times per week on a regular basis.
All study procedures were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital, and all subjects signed an informed consent form.
Cardiovascular Assessment
LV function was assessed using previously described techniques (1, 35). Briefly, a 6-Fr balloon-tipped fluid-filled catheter (Edwards Lifesciences) was placed using fluoroscopic guidance through an antecubital vein in the pulmonary artery. The catheter was connected to a pressure transducer with the zero reference point set 5.0 cm below the sternal angle. Resting supine measures (baseline 1) were collected, and then LV filling was decreased using lower body negative pressure (LBNP) of −15 mmHg (LBNP15). Mean PCWP (as an index of mean LA pressure) and Doppler measurements were collected after 5 min of LBNP. After release of LBNP and confirmed return to hemodynamic baseline (baseline 2), defined by ≤10% difference in stroke volume from baseline 1 (coefficient of variation, 7.9%), LV filling was then increased through a rapid infusion of warm (37°C) isotonic saline solution at 200 ml/min to achieve total volume infusion of 10–15 ml/kg (NS15). Irrespective of age, we observed a 2- to 3-mmHg mean difference in PCWP between baseline 1 and baseline 2, which may influence Doppler measures. Accordingly, baseline 1 and 2 were treated as two separate loading conditions. LV end-diastolic transmural filling pressure (LVTMP) was calculated as PCWP − right atrial pressure (RAP) as previously described (17).
Cardiac output (Qc) was measured with the acetylene rebreathing method (41), which has been previously validated in this laboratory (23). Qc and stroke volume were indexed to body surface area (BSA) (QI and SVI, respectively). Systemic vascular resistance (SVR) was calculated by the following formula: SVR = MAP/Qc × 80, where MAP is mean arterial pressure and 80 is a conversion factor to dynes per second per centimeter to the fifth power.
Echocardiography Assessment
Echocardiographic images were digitally acquired using an iE33 (Philips) and ATL HDI5000 (Advanced Technology Laboratories, Bothell, WA) and were measured offline in an Xcelera cardiovascular image management system (Philips). LV volumes were determined using a modified Simpson's method as previously described (1). Because LV volumes by echocardiography have been reported to underestimate those by magnetic resonance imaging (MRI) (24), a correction factor was determined as the ratio of LVEDV by echocardiography at baseline 1 to that by cardiac MRI in each subject. This individual correction factor was used to correct LV volumes by echocardiography during loading and unloading conditions to eliminate errors due to foreshortening or suboptimal echo images.
Diastolic filling.
A 2-mm sample volume placed at the tips of the mitral valve leaflets was used to determine peak velocities of mitral inflow.
Tissue Doppler imaging.
In the apical four-chamber view, a 2-mm sample volume was placed at the septal and lateral side of the mitral annulus. Septal and lateral values were averaged to obtain a mean tissue Doppler imaging value (29, 35).
Vp of early mitral inflow.
A color M-mode image of LV inflow was obtained with the sampling area positioned to extend from midatrium to the apex, directly through the mitral valve orifice. The scale was reduced to produce a clear aliasing within the early portion of the mitral inflow. The slope of first aliasing velocity from the mitral plane to 4 cm into the ventricle was used to measure VP (18). With the use of a five-chamber apical view with a 4-mm sample volume, the interval between aortic valve closing and mitral valve opening (IVRT) was determined.
Time constant of isovolumic pressure decay.
The time constant of isovolumic pressure decay with zero asymptote assumption (τ) was calculated by the following formula (34, 37):
where P is systolic blood pressure and PCWP is mean PCWP. All parameters were collected at end-expiration, and at least three measurements were averaged.
Global systolic function.
Global systolic function was assessed by 1) peak systolic mitral annular velocity (Sm); 2) the ejection fraction; and 3) the end-systolic blood pressure/end-systolic volume index (ESBP/ESVI) relationship, where ESBP was estimated as brachial systolic pressure × 0.9 (10).
Statistical Analysis
Baseline 1 was used for group comparisons of supine resting measures. A one-way ANOVA was used to test the effect of age on group subject characteristics, resting hemodynamics, and Doppler measures. A repeated-measures two-factor ANOVA (age, condition) was used to determine the effect of aging on Doppler parameters during LV loading and unloading. To determine whether there is a reduction in the responsiveness of LV relaxation and diastolic suction properties to alterations in PCWP with advancing age, an ANOVA was conducted on the delta change (%) in LVEDV index and Doppler filling parameters from baseline 1 to LBNP −15 mmHg (LBNP15), and from baseline 2 to saline infusion of 10–15 ml/kg (NS15). Tukey adjustments were used to control for multiple comparisons. Nonparametric data were analyzed via Kruskal-Wallis ANOVA on ranks. P < 0.05 was considered statistically significant. All statistical analysis was performed using SigmaStat (Systat Software) and SAS version 9.1 (SAS Institute). Data are presented as means ± SD in Tables 1 and 2 and means ± SE in Figs. 1–3.
Table 1.
Participant characteristics
| Young | Early Middle Age | Late Middle Age | Seniors | ANOVA P Value | |
|---|---|---|---|---|---|
| Subjects (n, % female) | 14 (43) | 19 (68) | 23 (52) | 14 (50) | |
| Age, yr | 28 ± 4 | 42 ± 5* | 57 ± 4* | 70 ± 3* | <0.001 |
| Weight, kg | 69 ± 9 | 68 ± 14 | 77 ± 14 | 73 ± 10 | 0.105 |
| BSA, m2 | 1.80 ± 0.14 | 1.77 ± 0.22 | 1.90 ± 0.21 | 1.85 ± 0.17 | 0.163 |
Values presented are means ± SD. BSA, body surface area.
P < 0.05 vs. young group.
Table 2.
Left ventricular hemodynamics and volumes
| Young | Early Middle Age | Late Middle Age | Seniors | ANOVA P Value | |
|---|---|---|---|---|---|
| QI, l/m2 | 3.3 ± 0.8* | 2.6 ± 0.5* | 2.6 ± 0.4* | 2.6 ± 0.3* | <0.001 |
| Heart rate, beats/min | 73 ± 14 | 68 ± 9 | 64 ± 8 | 71 ± 10 | 0.058 |
| SVI, ml/m2 | 47 ± 9 | 38 ± 6* | 41 ± 7 | 38 ± 9* | 0.008 |
| SBP, mmHg | 113 ± 11 | 110 ± 3 | 113 ± 4 | 134 ± 4* | <0.001 |
| DBP, mmHg | 64 ± 7 | 71 ± 12 | 72 ± 7 | 78 ± 9* | 0.003 |
| MAP, mmHg | 81 ± 7 | 84 ± 11 | 86 ± 8 | 96 ± 11* | <0.001 |
| SVR, dynes · s−1 · cm5 | 1,131 ± 291 | 1,541 ± 400* | 1,438 ± 237* | 1,617 ± 263* | <0.001 |
| EDVI, ml/m2 | 66 ± 7 | 62 ± 10 | 60 ± 9 | 50 ± 7* | <0.001 |
| ESVI, ml/m2 | 24 ± 4 | 21 ± 7 | 17 ± 5* | 14 ± 3* | <0.001 |
| Ejection fraction, % | 64 ± 5 | 67 ± 7 | 72 ± 6* | 73 ± 4* | <0.001 |
| ESBP/ESVI, mmHg · ml−1 · m−2 | 4 ± 1 | 5 ± 2 | 7 ± 2* | 9 ± 2* | <0.001 |
| Sm, cm/s | 10 ± 2 | 9 ± 1* | 8 ± 1# | 8 ± 1# | 0.034 |
| LVMI, g/m2 | 54 ± 7 | 54 ± 13 | 50 ± 8 | 48 ± 7 | 0.228 |
| PCWP, mmHg | 12.6 ± 1.3 | 11.9 ± 2.6 | 11.9 ± 2.4 | 11.3 ± 1.2 | 0.472 |
| RAP, mmHg | 9.1 ± 1.6 | 8.6 ± 1.9 | 8.8 ± 1.8 | 7.8 ± 1.6 | 0.216 |
| LVTMP, mmHg | 3.4 ± 0.8 | 3.4 ± 1.2 | 3.1 ± 1.3 | 3.5 ± 1.5 | 0.802 |
Values presented are means ± SD. QI, cardiac index; SVI, stroke volume index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SVR, systemic vascular resistance; EDVI, end-diastolic volume index; ESVI, end-systolic volume index; ESBP, end-systolic blood pressure; Sm, peak systolic mitral annular velocity; LVMI, left ventricular mass index; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; LVTMP, left ventricular transmural pressure. Left ventricular mass, left ventricular end-systolic volume index, and ejection fraction were determined using magnetic resonance imaging as previously described (1).
P < 0.05 and
P = 0.051–0.055 vs. young group.
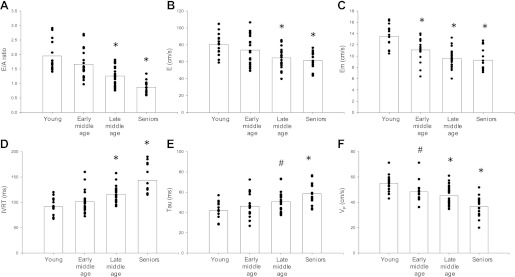
Fig. 1.
The effect of healthy aging on the E/A ratio (A), E (B), peak early mitral annular longitudinal velocity (Em, C), isovolumic relaxation time (IVRT, D), the time constant of isovolumic pressure decay with zero asymptote assumption (τ, E), and propagation velocity (VP, F). Boxes represent group means. *P < 0.05 and #P < 0.070–0.076 vs. young group.
Fig. 2.
The effect of aging on left ventricular (LV) volumes: stroke volume index (SVI, A) was smaller in the seniors compared with the youngest group during baseline, whereas end-diastolic volume index (EDVI) was considerably smaller in the seniors during unloading and loading compared with all other age groups irrespective of whether pulmonary capillary wedge pressure (PCWP) or LV end-diastolic transmural filling pressure is used (B and C). Note the scale change used for B and C. LBNP15, lower body negative pressure of −15 mmHg; NS15, isotonic saline solution at 200 ml/min to achieve total volume infusion of 10–15 ml/kg. *P < 0.05 vs. young group at the same loading condition. Values are means ± SE.
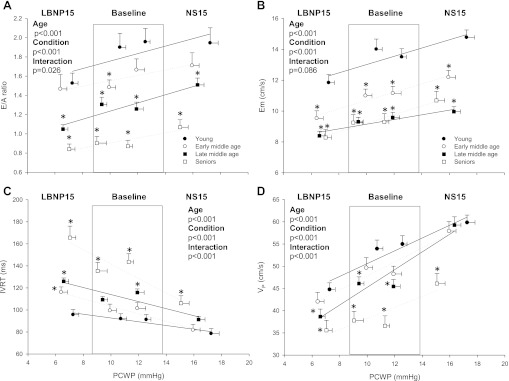
Fig. 3.
The effect of aging and changes in PCWP on Doppler measures of LV diastolic filling. A: the E/A ratio varied in relation to PCWP (loading condition main effect, P < 0.001) but remained lower in the late middle age and seniors compared with the young group during all conditions (age main effect, P < 0.001). B: Em was altered in response to changes in PCWP (loading condition main effect, P < 0.001). Across all loading conditions, Em was faster in the young group compared with all other groups. Em of the two older groups did not reach baseline levels of the younger groups even when PCWP was increased (age main effect, P < 0.001). C: IVRT was slower in the seniors vs. all other groups irrespective of loading conditions. D: while age-associated differences in baseline VP were attenuated in the middle age groups during loading, the seniors group had an attenuated VP response, and consequently remained lower compared with the other groups across all LV filling pressures (age × loading condition interaction effect, P < 0.001). *P < 0.05 vs. young group at the same loading condition. Values are means ± SE.
RESULTS
Participant Characteristics and Baseline Global LV Hemodynamics
There were no significant age-related changes in total body mass or BSA (Table 1). As a result of a larger SVI and an increased heart rate, QI was greater in the young group compared with the other groups (Table 2). Blood pressures were increased in seniors compared with the younger groups (ANOVA age effect, P < 0.001). Whereas indexed EDVI and ESVI were smaller, ejection fraction increased with aging and was greatest in seniors (ANOVA age effect, P < 0.001) (Table 1). Because of a smaller ESVI and a greater ESBP, ESBP/ESVI (mmHg·ml−1·m−2) was greatest in the seniors (ANOVA age effect, P < 0.001); however, Sm, a measure of longitudinal shortening during systole, declined with age (P < 0.001). Likewise, SVR increased with aging (ANOVA age effect, P < 0.001). LV mass index, PCWP, RAP, and LVTMP did not differ significantly among age groups (Table 2).
Baseline Doppler Echocardiography
The peak of the E wave declined 20% from the young group until late middle age, and then plateaued (ANOVA age effect, P < 0.001) (Fig. 1B). The E/A ratio declined in a linear trend from 2.0 in the young group to below 1.0 (0.9 ± 0.2) in the seniors (ANOVA age effect, P < 0.001) (Fig. 1A). Em slowed (≈1 cm/decade) while IVRT increased 57% from the young group (91 ± 17 ms) to the seniors (144 ± 28 ms), resulting in an overall aging effect for both measures (P < 0.001) (Fig. 1, C and D). The relationship between advancing age and slowed relaxation was supported by a progressive slowing (≈3.5 ms/decade) in τ, increasing 40% from the youngest to oldest group (42 ± 9 to 59 ± 11 ms) (ANOVA age effect, P < 0.001) (Fig. 1E). There was an early decline in VP from 55 ± 7 cm/s in the young group to 46 ± 6 cm/s in the early middle age group. VP then plateaued during late middle age, and then declined further in the seniors. Overall, there was a 35% decline in VP from the youngest to oldest group (Fig. 1F).
Influence of LV PCWP
The relative changes in PCWP from baseline 1 to LBNP15 and baseline 2 to NS15 were not significantly different between age groups (P = 0.076 and 0.233). The relative change (%) in SVI to LBNP was smallest in the seniors and largest in the young group (P = 0.052), but was similar between groups in response to saline infusion (P = 0.287). As a result, SVI remained larger in the young group compared with the other age groups at any given PCWP (Fig. 2A). The change in EDVI during unloading or loading conditions was not age dependent (P = 0.899 and 0.906, respectively). As a result, EDVI remained smaller in the oldest group during these conditions compared with the younger age groups (Fig. 2B), an effect that was preserved relative to LVTMP (Fig. 2C). As expected, the E/A ratio varied directly and predictably in response to changes in PCWP, decreasing with LV unloading and increasing with loading in similar proportions in all groups (Fig. 3A). Thus, while seniors achieved an E/A ratio over one with LV loading, this value remained lower compared with the younger subjects during all conditions. Likewise, Em was very preload dependent, with the relative change in response to loading and unloading being unaffected by age (P = 0.339 and 0.847, respectively). Nevertheless, the Em of the late middle age and seniors groups failed to reach baseline levels of those in the early middle age and young groups despite PCWP being increased ≈75–80% (Fig. 3B). IVRT decreased in seniors from 135 ± 27 to 106 ± 26 ms (P < 0.001) with LV loading, suggesting earlier mitral value opening. However, the change in IVRT in response to an increased volume load was not significantly influenced by aging (P = 0.537); thus, IVRT remained slower in seniors compared with the two youngest groups (P = 0.004 compared with the young group and P = 0.007 compared with the early middle age group) (Fig. 3C). Although the relative decrease in VP during LV unloading was less prominent in seniors, VP still remained slower compared with all other groups (Fig. 3D). The increase in VP in response to increased LV load increased with aging (P = 0.018); however, VP still remained slower in seniors compared with all other groups (p≤0.001).
DISCUSSION
The key finding of this study is a progressive slowing of LV relaxation beginning in early middle age, with a significant concomitant reduction in ventricular diastolic suction in those ≥65 yr. Because these differences among age groups occur despite a similar LV filling pressure, heart rate, and mass, and remained during large changes in cardiac loading conditions, we interpret these findings to suggest that intrinsic ventricular properties are primarily responsible for the age-related changes in diastolic filling with advancing age. A marked reduction of relaxation and diastolic suction in healthy seniors is particularly relevant given the emergence of diastolic dysfunction as an important determinant of heart failure in seniors (36, 42).
Although there is a plethora of studies investigating the effect of healthy aging on Doppler measures of LV function, very few studies have collected Doppler measures over a wide range of LV filling pressures in healthy subjects varying in age (35). Given that Doppler measures are load-dependent in healthy individuals (11, 16), it is therefore very difficult to determine from previous findings whether age-related alterations in LV diastolic filling are related to changes in intrinsic ventricular properties and/or differences in LV loading conditions, in particular left atrial and ventricular pressures. To address this limitation, we collected multiple measures of LV dynamic diastolic filling over a wide range of PCWP (2.8–20.3 mmHg) in a large cohort of healthy individuals over a broad age range. This current study demonstrated that neither an increase in PCWP in the older subjects nor a reduction in PCWP in the younger subjects was sufficient to change fundamental age-related differences in LV diastolic filling parameters. Therefore, a deterioration in baseline levels of LV relaxation and diastolic suction resulting from changes in intrinsic ventricular function seems to be the most prominent effect of aging on dynamic LV diastolic filling.
Slowed LV Active Relaxation With Healthy Aging
This current study showed that Em, IVRT, and τ were slowed in a linear fashion with advancing age, even in the absence of manifest comorbid conditions such as hypertension, coronary artery disease, or obesity. Likewise, in 1,333 subjects aged from 10–89 (mean age 55 yr) with no known heart disease, Em declined progressively with advancing age (33), whereas IVRT is prolonged in older vs. younger adults (22, 44). Because these measures represent early active relaxation, these findings are consistent with the hypothesis of slowed ventricular active relaxation with aging.
This current study demonstrated that these measures were not restored to youthful levels even when PCWP was significantly increased in those over the age of 50 yr. Thus, these age-related changes in LV active relaxation are unlikely to be related to loading conditions, but rather intrinsic LV relaxation properties. Diastolic relaxation is heavily dependent on Ca2+ reuptake by sarcoplasmic reticulum (5). Biochemical studies in rodents have demonstrated a reduction in intracellular Ca2+ clearance with aging (30, 40). The etiology for the decline in intracellular Ca2+sequestration is not fully understood but is most likely to be multifactorial, including decreased levels of sarcoplasmic reticulum Ca2+-ATPase (SERCA) mRNA and protein (9, 38), as well an overexpression and/or reduction in phosphorylation of phospholamban (25, 28). The relative importance of Ca2+-regulating proteins such as SERCA on slowed LV relaxation with aging is demonstrated by more youthful levels of τ and −dP/dt in aged rodent hearts with adenoviral transfer of the SERCA gene (38).
Diastolic suction results from the development of restoring forces created in part by contraction below the equilibrium volume (the volume when transmural pressure = 0 mmHg) and the complex three-dimensional twisting deformation of the myocardium during systole (3, 31, 32). This stored energy is released during the subsequent diastolic period and contributes to the generation of an IVPG, a regional pressure difference within the ventricle (14) that acts as a suction force drawing blood from the base to the apex. Recent studies show that IVPGs can be estimated noninvasively using color M-mode Doppler (20), and the magnitude of IVPGs is reduced in seniors compared with younger healthy adults over a wide range of LV filling pressures (34), which is consistent with the current VP results. While it is acknowledged that VP is not a direct measure of IVPGs, these findings are not surprising given that VP is influenced by several physiological factors that would also impact IVPGs (global systolic function, rapid active relaxation, LV minimal diastolic pressure, and ventricular geometry) (2, 8, 39).
Whereas these current findings underscore the effect that LV relaxation and diastolic suction may exert on Doppler measures with aging, the influence of alterations in LV compliance with age requires mention. We have recently reported in this same cohort that there is LV atrophy and stiffening from early middle age onward, with the greatest effects being observed in seniors (17). We speculate that ventricular stiffening with aging results from alterations in myocardial tissue, including an increased volume fraction, cross-linking of collagen (advanced glycation end-products), and the accumulation of interstitial fibrosis, which have been discussed in detail elsewhere (17). However, given that the ventricle is likely to be relatively compliant during early mitral inflow, it is unlikely that overall ventricular stiffness exerts a meaningful effect during this period of diastole. It is more likely that the impact of ventricular stiffness is exerted later in the diastolic filling period, when LV filling volumes and pressure are increased.
Although the clinical relevance of a reduction of ventricular diastolic suction and prolonged relaxation with senescence has not been fully elucidated, it could compromise LV filling during conditions in which diastolic filling duration is shortened significantly, such as during exercise tachycardia. Mathematical models and experimental animal models have shown that slowed active relaxation and less vigorous diastolic suction during exercise tachycardia are associated with a significant increase in left atrial filling pressure (12, 13, 21), which, if excessive, may result in breathlessness and limit exercise performance. However, to date, no studies have simultaneously assessed LV filling pressures and diastolic filling during exercise in a large cohort of subjects of various ages; therefore, this hypothesis remains purely speculative.
One recent longitudinal study in a large cohort of community-dwelling late middle age subjects and seniors reported that persistent or worsening LV diastolic filling over the 4-yr period resulted in an increased risk of new-onset heart failure during a subsequent 6-yr follow up (26). Not surprisingly, being 65 yr or older was predictive of the development of LV diastolic dysfunction. Therefore, the findings of this current study may provide greater understanding of specific age-related changes in sedentary but healthy aging adults with normal LV filling pressures. Such information may be useful for advancing the current knowledge of what may constitute “age-appropriate” changes in LV diastolic filling in seniors.
Methodological Limitations
There are several limitations to this current study. First, the number of subjects was relatively small in each age group, primarily as a result of the invasive experimentation nature of this study. We chose to investigate age-related differences derived from predetermined age groups based on important physiological aging milestones. This approach increases statistical power to detect group differences but comes at the expense of age resolution. Therefore, the current results do not imply that there is a sudden and dramatic change in Doppler measures as a person advances from age 64 to age 65, but rather emphasize that, as men and women advance through the stages of life, there are real and measureable alterations in Doppler measures of LV diastolic filling. Second, whereas the collection of LV end-diastolic pressure would allow for the direct determination of the transmitral pressure gradient, given that PCWP could be used as a surrogate for left atrial pressure, the advantage of a left heart catheter for the measurement of LV pressures did not outweigh the risk for these experiments in our healthy volunteers. Finally, although not presented within this current manuscript, more novel and sensitive echocardiography techniques (twist and untwisting) may provide more detailed analysis of age-related changes in LV diastolic filling associated with healthy aging, particularly during perturbations in LV filling conditions (i.e., exercise, tilt table, LBNP).
In summary, this current study demonstrated a slowing of LV relaxation that begins in early middle age with sedentary, but otherwise healthy, aging. Diastolic suction seems to be relatively well preserved until 65 yr, but there is a clear deterioration thereafter. These age-related changes occur despite a similar LV mass, PCWP, and heart rate, further demonstrating the reduction in intrinsic properties of relaxation and diastolic suction with senescence.
GRANTS
This study was supported by National Institute on Aging Grant AG-17479-02.
DISCLOSURES
There are no author conflicts to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: G.C.C.-R., J.L.H., P.S.B., S.S., N.F., M.D.P., K.B., and B.D.L. performed experiments; G.C.C.-R. analyzed data; G.C.C.-R., J.L.H., P.S.B., S.S., N.F., and B.D.L. interpreted results of experiments; G.C.C.-R. prepared figures; G.C.C.-R. drafted manuscript; G.C.C.-R. and B.D.L. edited and revised manuscript; J.L.H., P.S.B., and B.D.L. conception and design of research; B.D.L. approved final version of manuscript.
REFERENCES
- 1. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Barbier P, Grimaldi A, Alimento M, Berna G, Guazzi MD. Echocardiographic determinants of mitral early flow propagation velocity. Am J Cardiol 90: 613–619, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res 87: 235–240, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Levy D, Anderson KM, Wolf PA, Plehn JF, Evans JC, Comai K, Fuller DL, Sutton MS. Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study). Am J Cardiol 70: 508–515, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Bers D. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res 87: 275–281, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huet B, Thomas JD, Grayburn PA, Levine BD. Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging 4: 482–489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 50: 1570–1577, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Buakhamsri A, Popovic ZB, Lin J, Lim P, Greenberg NL, Borowski AG, Tang WH, Klein AL, Lever HM, Desai MY, Thomas JD. Impact of left ventricular volume/mass ratio on diastolic function. Eur Heart J 30: 1213–1221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cain BS, Meldrum DR, Joo KS, Wang JF, Meng X, Cleveland JC, Jr, Banerjee A, Harken AH. Human SERCA2a levels correlate inversely with age in senescent human myocardium. J Am Coll Cardiol 32: 458–467, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38: 2028–2034, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Cheng C, Freeman G, Santamore W, Constantinescu M, Little W. Effect of loading conditions, contractile state, and heart rate on early diastolic left ventricular filling in conscious dogs. Circ Res 66: 814–823, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Cheng C, Igarashi Y, Little W. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res 70: 9–19, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Cheng CP, Noda T, Nozawa T, Little WC. Effect of heart failure on the mechanism of exercise-induced augmentation of mitral valve flow. Circ Res 72: 795–806, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Courtois M, Kovacs S, Ludbrook P. Transmitral pressure-flow velocity relation. Importance of regional pressure gradients in the left ventricle during diastole. Circulation 78: 661–671, 1988 [DOI] [PubMed] [Google Scholar]
- 15. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the US, 1979 to 2004. J Am Coll Cardiol 52: 428–434, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, Zuckerman J, Thomas JD. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol 36: 1664–1669, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Fujimoto N, Hastings J, Bhella P, Shibata S, Gandhi N, Carrick-Ranson G, Palmer D, Levine B. Effect of aging on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol 590: 1871–1880, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia MJ, Ares MA, Asher C, Rodriguez L, Vandervoort P, Thomas JD. An index of early left ventricular filling that combined with pulsed Doppler peak E velocity may estimate capillary wedge pressure. J Am Coll Cardiol 29: 448–454, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Gardin JM, Arnold AM, Bild DE, Smith VE, Lima JA, Klopfenstein HS, Kitzman DW. Left ventricular diastolic filling in the elderly: the cardiovascular health study. Am J Cardiol 82: 345–351, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg NL, Vandervoort PM, Firstenberg MS, Garcia MJ, Thomas JD. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am J Physiol Heart Circ Physiol 280: H2507–H2515, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol 288: H1203–H1208, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hees PS, Fleg JL, Dong SJ, Shapiro EP. MRI and echocardiographic assessment of the diastolic dysfunction of normal aging: altered LV pressure decline or load? Am J Physiol Heart Circ Physiol 286: H782–H788, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol 103: 867–874, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Jenkins C, Bricknell K, Chan J, Hanekom L, Marwick TH. Comparison of two- and three-dimensional echocardiography with sequential magnetic resonance imaging for evaluating left ventricular volume and ejection fraction over time in patients with healed myocardial infarction. Am J Cardiol 99: 300–306, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Jiang MT, Moffat MP, Narayanan N. Age-related alterations in the phosphorylation of sarcoplasmic reticulum and myofibrillar proteins and diminished contractile response to isoproterenol in intact rat ventricle. Circ Res 72: 102–111, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. J Am Med Assoc 306: 856–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitzman DW, Sheikh KH, Beere PA, Philips JL, Higginbotham MB. Age-related alterations of Doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol 18: 1243–1250, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Lim CC, Liao R, Varma N, Apstein CS. Impaired lusitropy-frequency in the aging mouse: role of Ca(2+)-handling proteins and effects of isoproterenol. Am J Physiol Heart Circ Physiol 277: H2083–H2090, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107–133, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Narayanan N. Comparison of ATP-dependent calcium transport and calcium-activated ATPase activities of cardiac sarcoplasmic reticulum and sarcolemma from rats of various ages. Mech Ageing Dev 38: 127–143, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Nikolic S, Feneley M, Pajaro O, Rankin J, Yellin E. Origin of regional pressure gradients in the left ventricle during early diastole. Am J Physiol Heart Circ Physiol 268: H550–H557, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Nikolic S, Yellin E, Tamura K, Vetter H, Tamura T, Meisner J, Frater R. Passive properties of canine left ventricle: diastolic stiffness and restoring forces. Circ Res 62: 1210–1222, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, Yamagishi H, Toda I, Yoshiyama M, Yoshikawa J, Yoshida K. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging 2: 41–46, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol 290: H1454–H1459, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. J Am Med Assoc 289: 194–202, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Scalia GM, Greenberg NL, McCarthy PM, Thomas JD, Vandervoort PM. Noninvasive assessment of the ventricular relaxation time constant (tau) in humans by Doppler echocardiography. Circulation 95: 151–155, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation 101: 790–796, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Takatsuji H, Mikami T, Urasawa K, Teranishi J, Onozuka H, Takagi C, Makita Y, Matsuo H, Kusuoka H, Kitabatake A. A new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol 27: 365–371, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Tate C, Taffet G, Hudson E, Blaylock S, McBride R, Michael L. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained rats. Am J Physiol Heart Circ Physiol 258: H431–H435, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Envir Med 48: 203–209, 1977 [PubMed] [Google Scholar]
- 42. Vasan R, Larson M, Benjamin E, Evans J, Reiss C, DL Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol 33: 1948–1955, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93: 1473–1480, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Wierzbowska-Drabik K, Krzeminska-Pakula M, Chrzanowski L, Plewka M, Waszyrowski T, Drozdz J, Kurpesa M, Trzos E, Kasprzak JD. Age-dependency of classic and new parameters of diastolic function. Echocardiography 25: 149–155, 2008 [DOI] [PubMed] [Google Scholar]