Abstract
Earlier studies have demonstrated that aldose reductase (AR) plays a key role in mediating ischemia-reperfusion (I/R) injury. Our objective was to investigate if AR mediates I/R injury by influencing phosphorylation of glycogen synthase kinase-3β (p-GSK3β). To investigate this issue, we used three separate models to study the effects of stress injury on the heart. Hearts isolated from wild-type (WT), human expressing AR transgenic (ARTg), and AR knockout (ARKO) mice were perfused with/without GSK3β inhibitors (SB-216763 and LiCl) and subjected to I/R. Ad-human AR (Ad-hAR)-expressing HL-1 cardiac cells were exposed to hypoxia (0.5% O2) and reoxygenation (20.9% O2) conditions. I/R in a murine model of transient occlusion and reperfusion of the left anterior descending coronary artery (LAD) was used to study if p-GSK3β was affected through increased AR flux. Lactate dehydrogenase (LDH) release and left ventricular developed pressure (LVDP) were measured. LVDP was decreased in hearts from ARTg mice compared with WT and ARKO after I/R, whereas LDH release and apoptotic markers were increased (P < 0.05). p-GSK3β was decreased in ARTg hearts compared with WT and ARKO (P < 0.05). In ARKO, p-GSK3β and apoptotic markers were decreased compared with WT (P < 0.05). WT and ARTg hearts perfused with GSK3β inhibitors improved p-GSK3β expression and LVDP and exhibited decreased LDH release, apoptosis, and mitochondrial pore opening (P < 0.05). Ad-hAR-expressing HL-1 cardiac cells, exposed to hypoxia (0.5% O2) and reoxygenation (20.9% O2), had greater LDH release compared with control HL-1 cells (P < 0.05). p-GSK3β was decreased and correlated with increased apoptotic markers in Ad-hAR HL-1 cells (P < 0.05). Treatment with phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) inhibitor increased injury demonstrated by increased LDH release in ARTg, WT, and ARKO hearts and in Ad-hAR-expressing HL-1 cells. Cells treated with protein kinase C (PKC) α/β inhibitor displayed significant increases in p-Akt and p-GSK3β expression, and resulted in decreased LDH release. In summary, AR mediates changes in p-GSK3β, in part, via PKCα/β and Akt during I/R.
Keywords: aldose reductase, ischemia reperfusion, glycogen synthase kinase 3β, cardioprotection
while recanalization therapy using thrombolytic or surgical approaches have helped salvage ischemic myocardium, damage observed during the reperfusion phase attenuates the benefits of these interventions (2, 45). Therapeutic strategies that target both the ischemic and reperfusion components of myocardial injury are likely to afford protection against acute myocardial ischemia-reperfusion (I/R). In the quest for novel therapeutic strategies for acute I/R injury, we have focused on interventions that protect against myocardial I/R injury by modulating substrate metabolism. In this context, we and others have demonstrated that the aldose reductase (AR) pathway contributes to myocardial I/R injury and that the inhibition of AR protects hearts from I/R damage (1–2, 16–17, 19, 35–36, 40, 45).
AR, a member of the aldo-keto reductase family, is a monomeric NADPH-dependent enzyme and the first, rate-limiting step in the polyol pathway (11, 17, 21, 34). Glucose substrate flux via AR increases under ischemic conditions, (17, 41) even in the absence of diabetes, and negatively impacts the myocardium by increasing oxidative stress, impairing ATP production as a result of altered glucose metabolism, and impairing calcium homeostasis, conditions that favor the opening of the mitochondrial permeability transition pore (mPTP) (1, 6, 13–14, 20). Preserving mitochondrial function is essential for normal recovery after I/R (1, 4, 14, 24). Opening of the mPTP has been implicated in upregulation of apoptotic and necrotic cell death mechanisms due to loss of membrane potential and ATP depletion (1, 6, 13).
Studies have shown that use of cardioprotective agents increased the phosphorylation and inhibition of glycogen synthase kinase-3β (GSK3β), resulting in a delayed opening of the mPTP. GSK3β, a serine/threonine kinase, has been identified by several studies as a key signaling protein that mediates cardioprotection and reduces cell death (28–29). GSK3β phosphorylates numerous substrates, including transcription factors and metabolic proteins, and is involved in several cellular processes such as gene transcription, apoptosis, and cell division (22–23). A wide spectrum of signaling pathways have been associated with the phosphorylation and inhibition of GSK3β (5, 32). We have previously demonstrated that increased metabolic flux through AR mediates I/R injury, in part, via changes in protein kinase C (PKC) α/β-dependent signaling (18) and mPTP opening (1). Because phosphorylation of GSK3β is a key determinant of mPTP opening, we investigated if AR mediates I/R injury, in part, by adversely influencing GSK3β phosphorylation in murine hearts.
We used three separate models to study whether AR mediates I/R injury by influencing phosphorylation of GSK3β (p-GSK3β): HL-1 cardiomyocytes an in vitro I/R injury model, an ex vivo intact heart preparation subjected to I/R, and a transient occlusion and reperfusion of the left anterior descending coronary artery (LAD) in vivo model of I/R. Because mice AR activity is severalfold lower than that in rats and humans (16, 34), we employed transgenic mice overexpressing human aldose reductase (ARTg) as well as an aldose reductase knockout mouse model (ARKO) to determine whether altered flux via AR influenced GSK3β phosphorylation.
MATERIALS AND METHODS
Animals.
All animal experiments were approved by the Institutional Animal Care and Use Committees of Columbia University, New York University School of Medicine, and Baylor College of Medicine and conformed to the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Pub. No. 85–23, 1996). Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were used as control wild-type (WT) mice. Male mice weighing 25–30 g at age 12–14 wk were used in all experiments and maintained in a temperature-controlled room with alternating 12:12-h light-dark cycles. ARTg mice were obtained from Dr. Mitsuo Itakura (University of Tokushima), and a colony was established at our facility. Briefly, these transgenic mice were developed by injecting full-length human AR (hAR) cDNA with a mouse major histocompatibility antigen class I promoter (43). ARKO mice were generated as described recently (8). The hAR transgenic and ARKO mice have been backcrossed >10 generations to develop a C57BL/6J background.
Reagents.
The primary antibodies used were anti-phospho-GSK3β/total GSK3β, anti-phospho-protein kinase B (Akt; Thr308 and Ser473)/total Akt IgG (Cell Signaling), anti-cytochrome c IgG (BD Pharmingen), and anti-β-actin IgG (BD Biosciences Pharmingen). The secondary antibodies used were goat-anti-rabbit IgG-peroxidase antibody and rabbit-anti-mouse IgG-peroxidase antibody (Sigma). All primary antibodies were diluted 1:1,000 before use in Western blot studies. GSK3 inhibitors LiCl and SB-216763 and PKCα/β inhibitor Gö-6976 were purchased from Sigma. Phosphatidylinositol 3-kinase (PI3K)/Akt inhibitor LY-294002 was purchased from Calbiochem.
Isolated perfused heart preparation.
Experiments were performed using an isovolumic isolated heart preparation as published and modified for the use in mice hearts (1, 16). Mice were anesthetized using a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg). After deep anesthesia was achieved, hearts were rapidly excised, placed in iced saline, and retrogradely perfused at 37°C in a nonrecirculating mode through the aorta at a rate of 2.5 ml/min. Hearts were perfused with modified Krebs-Henseleit buffer containing (in mM) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 5 glucose, 0.4 palmitate, 0.4 BSA, and 70 mU/l insulin. The perfusate was equilibrated with a mixture of 95% O2-5% CO2, which maintained perfusate Po2 >600 mmHg. Left ventricular developed pressure (LVDP) was measured using a latex balloon in the left ventricle. LVDP and coronary perfusion pressure were monitored continuously on a four-channel Gould recorder.
Surgical procedures.
Surgical procedures relating to coronary artery ligation were carried out as previously described (16). Briefly, the LAD was ligated including the cannula for 30 min. After 30 min of ischemia, the LAD blood flow was restored. Heart samples from the affected ischemic area were further evaluated at 48 h of reperfusion. Appropriate sham-treated animals subjected to anesthesia and surgical procedure without occluding LAD were included.
I/R protocol.
Hearts from WT, ARTg, and ARKO hearts were perfused with Krebs-Henseleit buffer throughout the I/R protocol. After an equilibration period of 30 min, global ischemia was performed for 30 min followed by 60 min of reperfusion. Perfusate temperature was maintained at 37°C at all times during the protocol (i.e., during baseline, ischemia, and reperfusion).
To determine the link between AR and GSK3β after I/R injury, experiments were performed in the presence of the following GSK3β inhibitors: SB-216763 (3 μM) and LiCl (3 mM) as well as PI3K/Akt inhibitor (LY-294002, 10 μM). Hearts from WT and ARTg mice were perfused with modified Krebs-Henseleit buffer containing 3 μM SB-216763 or 3 mM LiCl. The doses of the inhibitors used in this study were based on publications in the literature (15, 31, 37). Inhibitors were present at the start of the equilibration period and continued throughout ischemia and reperfusion.
AR activity.
AR activity in the hearts of WT, hAR (ARTg), and AR null (ARKO) mice was evaluated by measuring NADPH consumption using d-glyceraldehyde as a substrate (17). One unit of AR activity is defined as nanomoles of NADPH consumed per milligram of heart tissue protein per minute.
Mitochondrial swelling as a measure of mPTP opening.
We employed the mitochondrial swelling as a measure of mPTP opening. For measurement of mPTP opening, mitochondria were suspended in freshly prepared swelling buffer (0.2 M sucrose, 10 mM Tris-MOPS, pH 7.4, 5 mM succinate, 1 mM phosphate, 2 μM rotenone, and 1.0 μM EGTA-Tris, pH 7.4) at 0.5 mg/ml, and swelling of mitochondria was monitored by decrease in absorbance at 540 nm in the presence of CaCl2 (5–100 μM). Extent of pore opening was expressed in terms of changes in absorbance at 540 nm/min in the presence and absence of Ca2+.
Culture and transfection of HL-1 cardiomyocytes.
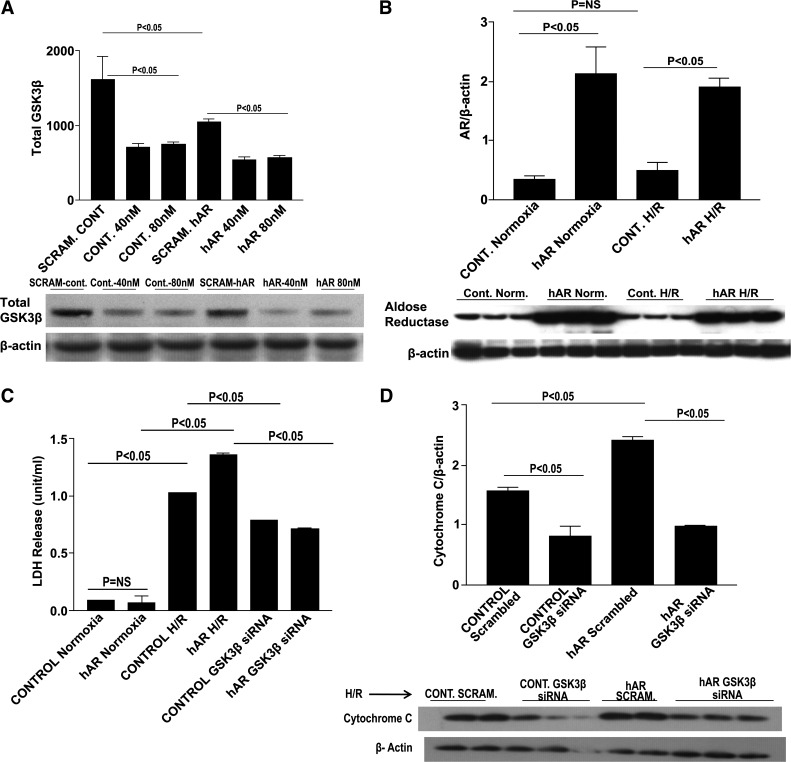
Immortalized HL-1 cardiomyocytes were a gift from Dr. William Claycomb (Louisiana State University, New Orleans, LA) (42). HL-1 cell line was chosen because they were derived from mice cardiac muscle cells (42). Cells were plated on gelatin/fibronectin-coated six-well plates at a density of 4 × 105 cells/well. HAR was overexpressed in HL-1 cardiomyocytes through viral-mediated transfection. Appropriate empty vector was transfected as a control. For small-interfering RNA (siRNA) transfection, HL-1 cells were cultured in six-well plates and transfected with control or 40 nM GSK3β siRNA using Lipofectamine RNAiMax reagent (Invitrogen) according to the manufacturer's instructions. HL-1 cardiomyocytes were subjected to hypoxic stress for 30 min (0.5% O2) using an In Vivo 400 hypoxic workstation maintained at 37°C, followed by 60 min of reoxygenation (20.9% O2). In specific experiments, cardiomyocytes were treated with either GSK3β siRNA and/or PI3K/Akt (10 μM) and PKCα/β inhibitors (1 μM).
Western blot analysis.
The tissue and cell protein concentration was determined using a DC Protein Assay kit (Bio-Rad). Equal amounts of protein were separated by SDS-PAGE (4–12% gradient gels), and proteins were transferred to a nitrocellulose membrane (Invitrogen). After blocking in 5% dry milk in TBST (20 mM Tris·HCl, pH 7.5, 250 mM NaCl, and 0.1% Tween 20), membranes were incubated overnight with target primary antibodies (1:1,000 dilution) according to the manufacturer's instructions. Membranes were incubated sequentially with secondary antibody for 1 h. Blots were visualized with an ECL Horseradish Peroxidase Western Blot Detection System (Cell Signaling), and quantitative analysis was performed using Image Quant TL software (Amersham).
LDH measurements.
Cardiac injury due to I/R stress was assessed by measuring LDH release in the perfusates that were collected during 60 min of reperfusion. In HL-1 cardiomyocytes, injury due to hypoxia/reoxygenation (H/R) stress was measured in supernatants that were collected after H/R. Lactate dehydrogenase (LDH) was measured using the commercially available kits (Pointe Scientific) as published earlier (1, 37).
Statistical analysis.
All data are presented as means ± SE. Statistical significance of differences between various groups of heart was determined by ANOVA. Post hoc comparisons were performed with Tukey or Dunnett procedures using GraphPad software as indicated. Statistical significance was ascribed to the data when P < 0.05.
RESULTS
GSK3β phosphorylation expression in WT, ARTg, and ARKO mice.
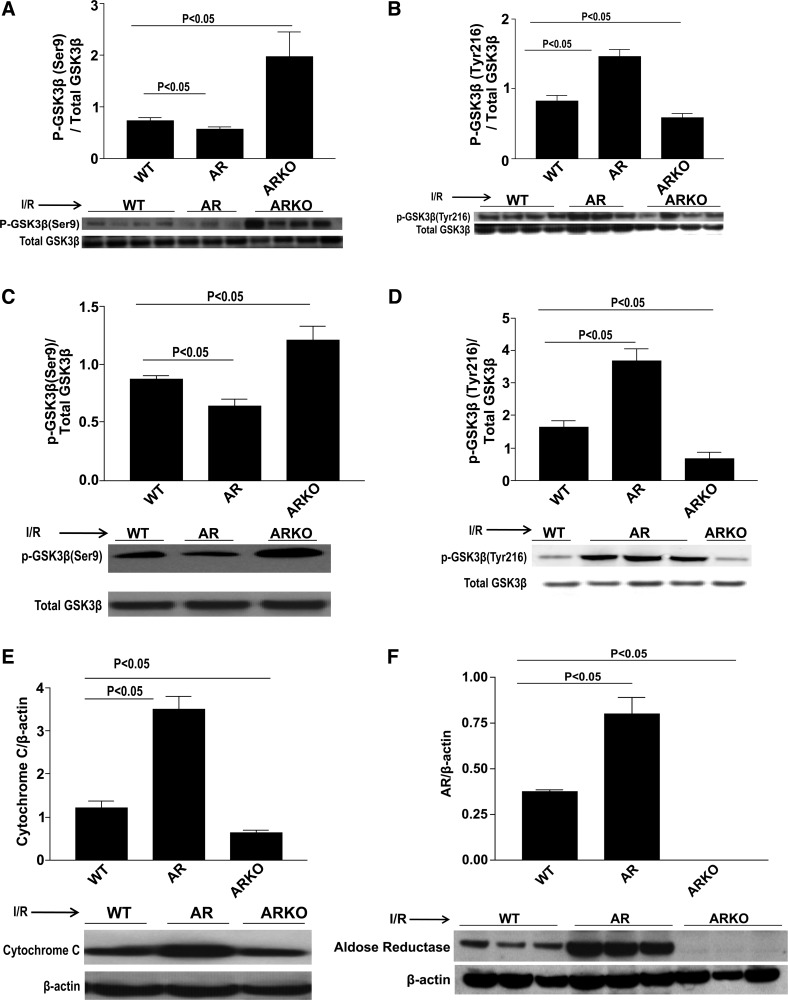
We used the isolated Langendorff perfusion system and intact transient occlusion and reperfusion of the LAD to establish the influence, if any, of AR-mediated ischemic injury on the phosphorylation and inhibition of GSK3β. Previous studies have shown that the phosphorylation and inhibition of GSK3β is cardioprotective (28–29). We first examined GSK3β phosphorylation at both the Ser9 and Tyr216 sites on GSK-3β after a 30-min baseline period only. Results show that there are no differences in GSK3β phosphorylation in WT, ARTg, and ARKO hearts after baseline (data not shown). We then determined whether GSK3β phosphorylation was influenced after I/R. Induction of I/R resulted in decreased inhibition of GSK3β demonstrated by decreased levels of p-GSK3β phosphorylated at Ser9 and increased levels of p-GSK3β phosphorylated at Tyr216 in ARTg mice hearts compared with WT mice (P < 0.05, Fig. 1, A and B). Phosphorylation of GSK3β on the Ser9 site was also significantly increased in ARKO hearts compared with WT (Fig. 1A). Similarly, 30 min of LAD occlusion followed by 48 h of reperfusion resulted in a decreased p-GSK3β expression on Ser9 and increased p-GSK3β on Tyr216 (Fig. 1, C and D). Furthermore, ARTg hearts displayed a more than threefold increase in apoptosis compared with WT and ARKO hearts as measured by cytochrome c expression in total lysates (P < 0.05, Fig. 1E). ARTg mice displayed a more than twofold increase in AR expression compared with WT mice, whereas ARKO hearts had negligent AR expression (Fig. 1F). Additionally, AR activity assay revealed that AR activity in ARTg mice hearts was greater, 16 nmol of NADPH consumed·min−1·mg of protein−1, than when compared with WT hearts, 1.63 nmol of NADPH consumed·min−1·mg of protein−1. ARKO hearts had no detectable units of AR activity.
Fig. 1.
Glycogen synthase kinase-3β (GSK3β) phosphorylation expression in wild-type (WT), aldose reductase transgenic (ARTg), and aldose reductase knockout (ARKO) mice. Western blot analysis for phospho (p)-GSK3β-Ser9 [isolated heart (A) and LAD ligation (C)], p-GSK3β-Tyr216 [isolated heart (B) and LAD ligation (D)], cytochrome c (E), and aldose reductase (AR) expression (F) in untreated WT, ARTg, and ARKO mice hearts subjected to ischemia-reperfusion (I/R). ARTg hearts showed decreased p-GSK3β inhibition compared with WT hearts. ARKO hearts had significant increases in p-GSK3β (Ser9) (P < 0.05). ARTg hearts displayed a more than threefold increase in apoptosis compared with WT and ARKO hearts as was measured by cytochrome c expression (P < 0.05). ARKO hearts displayed significant decreases in apoptotic levels compared with WT (P < 0.05). ARTg hearts displayed a twofold increase in AR expression compared with WT (n = 4–16 mice/group).
Pharmacological inhibition of GSK3β protects mice hearts upon I/R.
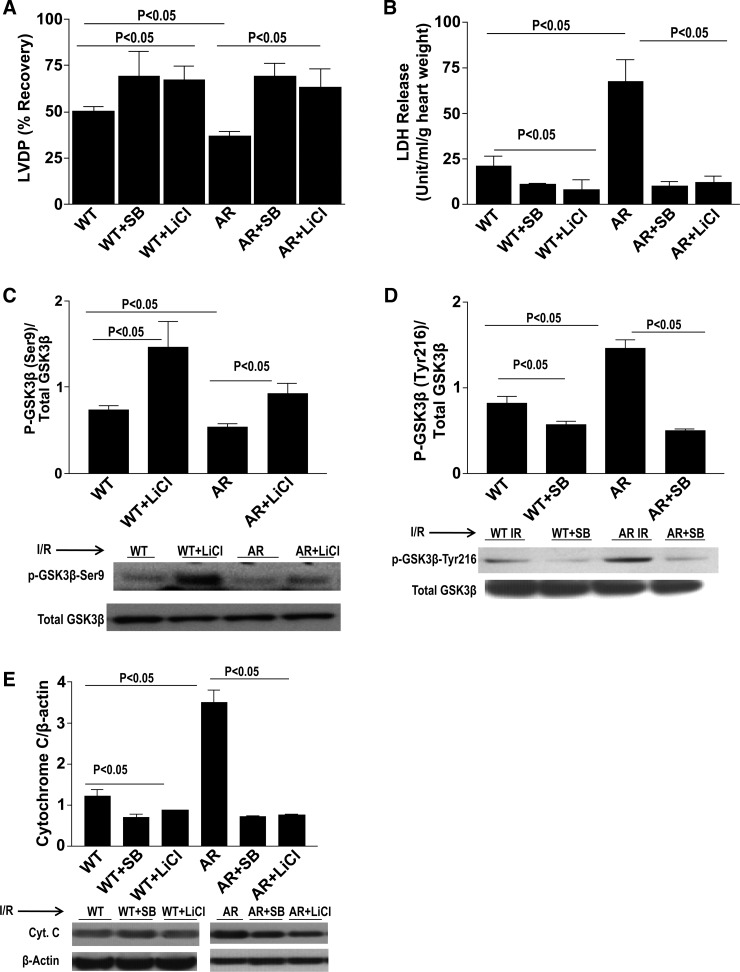
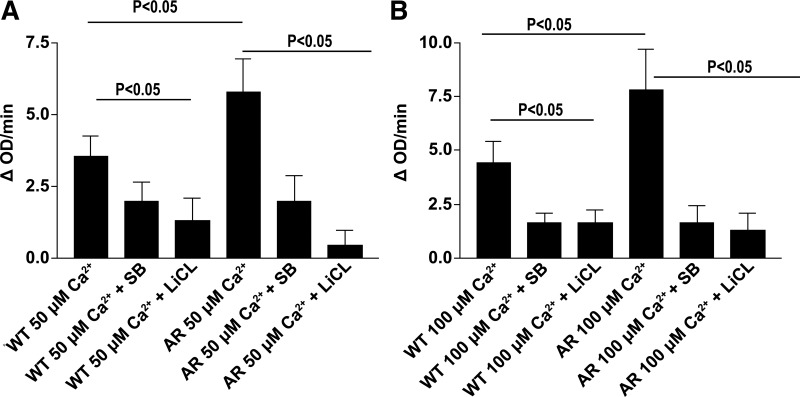
Because ARTg hearts displayed decreased inhibition of GSK3β, we next sought to further test the role of GSK3β in mediating I/R injury. WT and ARTg hearts were treated with GSK3β inhibitors SB-216763 and LiCl and were perfused under normoxic conditions for 30 min, followed by 30 min of ischemic conditions and 60 min of reperfusion conditions. LiCl targets the inhibitory phosphorylation site of GSK3β-Ser9 and works to increase phosphorylation, whereas SB-216763 targets the stimulatory phosphorylation site GSK3β-Tyr216 and works to decrease phosphorylation. Treatment with SB-216763 and LiCl protected WT and ARTg mice hearts significantly as shown in Fig. 2, A and B. Improvement of LVDP (P < 0.05, Fig. 2A) was observed in WT and ARTg hearts treated with both SB-216763 and LiCl. Similarly, Fig. 2B shows decreased release of LDH (P < 0.05, Fig. 2B) in WT and ARTg hearts upon treatment with both inhibitors. Treatment with LiCl and SB-216763 significantly inhibited GSK3β in both WT and ARTg hearts (P < 0.05, Fig. 2, C and D). WT and ARTg hearts perfused with both GSK3β inhibitors displayed markedly reduced apoptotic levels as was demonstrated by reduced cytochrome c (P < 0.05, Fig. 2E) expression. Pharmacological inhibition of GSK3β abolished mPTP pore opening in mitochondria from WT and ARTg hearts, as shown by the mitochondrial swelling changes in response to increasing amounts of added calcium (Fig. 3).
Fig. 2.
Pharmacological inhibition of GSK3β protects mice hearts upon I/R. Determination of myocardial ischemic injury and function with and without GSK3β inhibitor treatment as shown by left ventricular developed pressure (LVDP) recovery (A) and lactate dehydrogenase (LDH) release (B). Western blot analysis for p-GSK3β-Ser9 (C), p-GSK3β-Tyr216 (D), and cytochrome c (E) in WT and ARTg hearts perfused with and without GSK3β inhibitors SB-216763 and LiCl. Treatment with SB-216763 and LiCl protected WT and ARTg mice hearts, significantly increased p-GSK3β inhibition (P < 0.05), and decreased apoptosis (n = 4–16 mice/group).
Fig. 3.
Mitochondrial permeability in WT and ARTg hearts treated with and without GSK3β inhibitors SB-216763 and LiCl and subjected to I/R. Mitochondrial permeability transition pore opening (mPTP) was determined by monitoring swelling of mitochondria by measuring light scattering at 540 nm in the presence and absence of 50 μM (A) and 100 μM (B) Ca2+. Pore opening was reduced in WT and ARTg hearts treated with SB-216763 and LiCl in response to increasing calcium concentrations (P < 0.05) (n = 4–16/group).
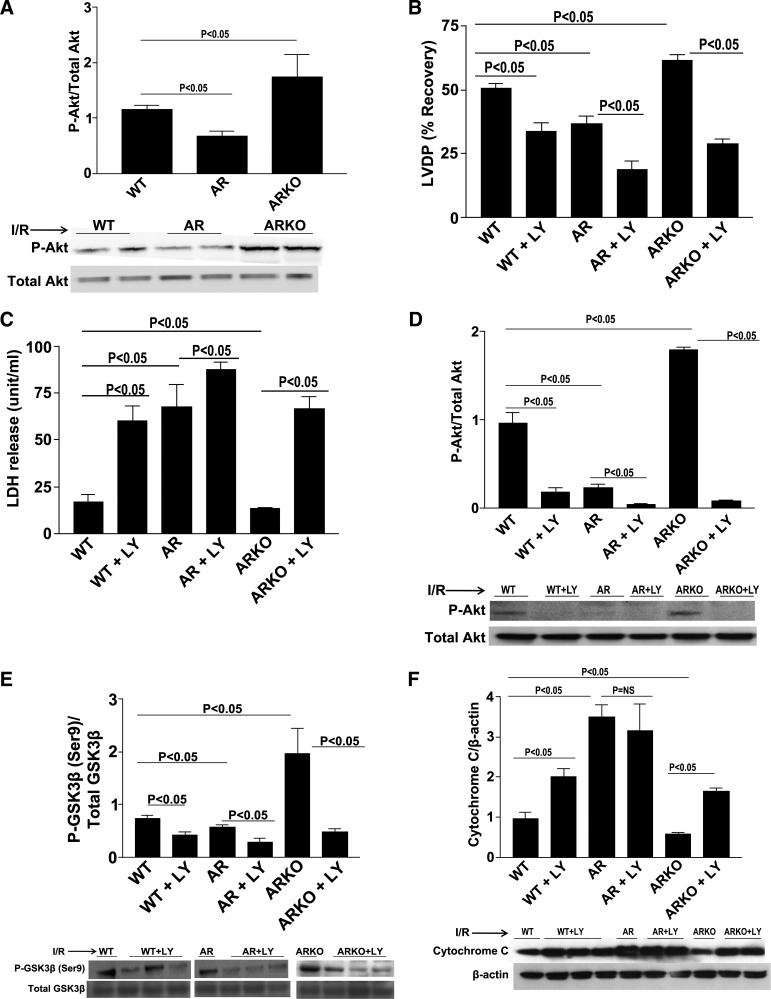
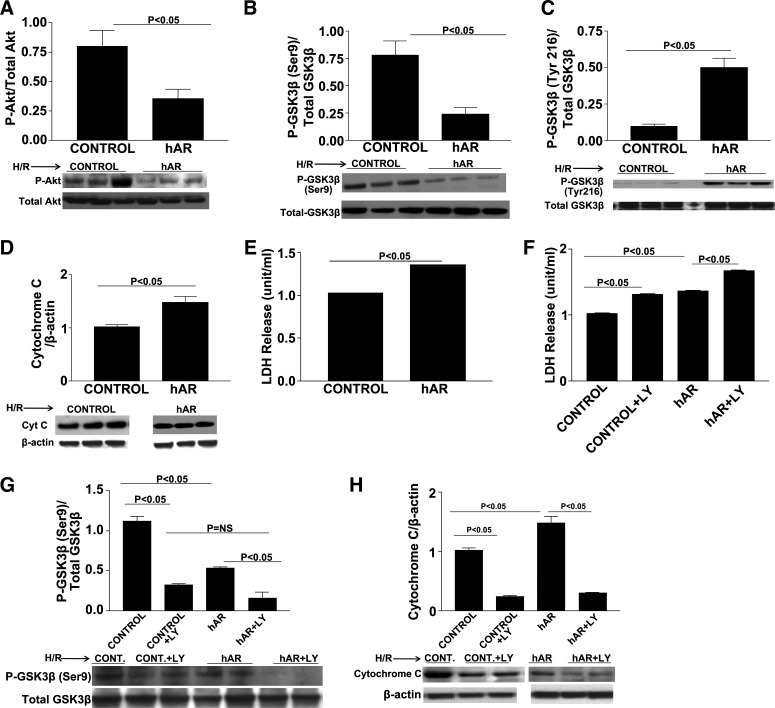
PI3K/Akt is a well-established upstream kinase that has been shown to phosphorylate and inhibit GSK3β at the serine residue. We investigated Akt phosphorylation expression patterns in WT, ARTg, and ARKO hearts after I/R. Transient LAD occlusion for 30 min followed by 48 h of reperfusion resulted in significant decreases in p-Akt expression in ARTg hearts when compared with WT hearts (Fig. 4A). p-Akt expression in ARKO hearts was significantly lower compared with WT hearts (Fig. 4A). To determine whether Akt is a key player by which AR mediates I/R injury, we used the PI3K/Akt inhibitor LY-294002 during I/R. Functional recovery was impaired in WT, ARTg, and ARKO hearts in the presence of LY-294002 as was demonstrated by significant decreases in LVDP (P < 0.05, Fig. 4B). As shown in Fig. 4C, LDH release was increased significantly in WT, ARTg, and ARKO hearts subjected to I/R stress in the presence of LY-294002. Marked reductions in p-Akt levels were observed in WT, ARTg, and ARKO hearts treated with LY-294002 (P < 0.05, Fig. 4D). Consequentially, p-GSK3β levels were decreased in all three groups after I/R when in the presence of the PI3K/Akt inhibitor (P < 0.05, Fig. 4E). Inhibition of the PI3K/Akt pathway resulted in significant increases in apoptotic levels as was measured by cytochrome c expression (P < 0.05, Fig. 4F). These data indicate that reduction in GSK3β phosphorylation is in part due to reduced PI3K/Akt phosphorylation.
Fig. 4.
p-Protein kinase B (Akt). Western blot analysis for p-Akt in LAD ligation of WT, ARTg, and ARKO hearts (A), determination of myocardial ischemic injury and function, as shown by LVDP (B) and LDH release (C) in WT, ARTg, and ARKO perfused with and without phosphatidylinositol 3-kinase (PI3K)/AKT inhibitor LY-294002. Western blot analysis for p-Akt (D), p-GSK3β-Ser9 (E), and cytochrome c (F) in WT, ARTg, and ARKO hearts perfused with and without PI3K/Akt inhibitor LY-294002. Treatment with LY-294002 decreased p-Akt and p-GSK3β levels in all 3 groups (P < 0.05). Cytochrome c levels were increased in WT and ARKO hearts (P < 0.05), but not ARTg upon perfusion with LY-294002 (n = 4–16 mice/group).
Akt and GSK3β phosphorylation expression in hAR-overexpressing HL-1 cells.
We employed the murine cardiomyocyte HL-1 cell line to identify where the signaling mechanisms are occurring in the myocardium. We opted to use the HL-1 cardiomyocyte cell line due to the problems we encountered in our previous attempts to isolate adult cardiomyocytes, including cell death within a very short period of time, which made transfection studies extremely difficult, and failed attachment of the cells to culture plates. We used siRNA to inhibit GSK3β and to determine whether GSK3β knock down would decrease cellular injury and apoptosis. Different concentrations of GSK3β siRNA were used to determine the least amount necessary for efficient GSK3β knock down. Approximately 60% GSK3β knock down was achieved in hAR-overexpressing HL-1 cardiomyocytes with a 40 nM siRNA concentration (Fig. 5A). Cells were transiently transfected with hAR virus (Fig. 5B). LDH measured in the supernatants after GSK3β siRNA transfection and H/R stress was markedly decreased in both control and hAR-expressing cells (Fig. 5C). As was expected, GSK3β siRNA knock down in control and hAR-expressing cells significantly reduced cytochrome c levels (P < 0.05, Fig. 5D). HL-1 cells expressing hAR and empty control vectors were exposed to H/R stress to determine p-Akt and p-GSK3β patterns. Similar to what was found in ARTg hearts during I/R, the expression of p-Akt was reduced significantly in Ad-hAR-expressing cells during H/R stress (P < 0.05, Fig. 6A). Decreased expression of p-Akt corresponded with decreased phosphorylation and inhibition of GSK3β. We analyzed the expression of p-GSK3β at both the Ser9 and Tyr216 residues in hAR-overexpressing cells and found that phosphorylation of GSK3β was decreased significantly on the Ser9 site and increased on the Tyr216 site (P < 0.05, Fig. 6, B and C) after H/R. As was observed in ex vivo heart I/R injury, decreased phosphorylation and inhibition of GSK3β correlated with increased cell death after H/R as was measured by the increased expression of cytochrome c in hAR-overexpressing cells (Fig. 6D). Additionally, cardiac injury as measured by LDH release in supernatants from hAR-overexpressing cells after H/R was increased significantly (Fig. 6E). HL-1 cells overexpressing hAR and control cells were treated with the PI3K/Akt signaling inhibitor LY-294002 during H/R to establish if Akt phosphorylation is a key event that mediates injury. As shown in Fig. 6F, both control and hAR-overexpressing cells treated with the Akt signaling inhibitor displayed marked increases in LDH release after H/R, which was parallel to experiments in the ex vivo I/R heart preparations. In addition to the increased LDH release, LY-294002 treatment significantly reduced p-GSK3β expression (P < 0.05, Fig. 6G) and resulted in increased apoptotic levels as shown by increased cytochrome c levels (P < 0.05, Fig. 6H).
Fig. 5.
GSK3β small-interfering RNA (siRNA) knock down in HL-1 cells. Western blot analysis for total GSK3β dose-dependent siRNA knockdown (A) and AR expression (B). Determination of cardiac injury as determined by LDH release in control and human AR (hAR) siRNA knock down of GSK3β (C). D: Western blot analysis of cytochrome c expression in control and hAR-overexpressing cells transfected with GSK3β siRNA. No change in LDH release was observed in control and hAR-overexpressing cells exposed to 30 min of normoxia alone. Thirty minutes of hypoxia followed by 60 min of reoxygenation increased LDH release (P < 0.05), whereas inhibiting GSK3β with siRNA significantly reduced LDH release and injury (P < 0.05). hAR-transfected cells, as expected, had greater AR expression than control cells. There was no difference in AR expression between HL-1 control cells subjected to normoxic and H/R conditions. GSK3β siRNA knock down significantly reduced GSK3β expression in both control and hAR-overexpressing cells. Inhibition of GSK3β significantly reduced apoptotic levels in both control and hAR-overexpressing cells. NS, not significant. Error bars not visible. Control normoxia: SE ± 0.003; control hAR: SE ± 0.006; control GSK3β siRNA: ±0.005.
Fig. 6.
HL-1 cardiomyocyte studies. Western blot analysis for p-Akt (A), p-GSK3β-Ser9 (B), p-GSK3β-Tyr216 (C), and cytochrome c (D) in control and hAR-overexpressing HL-1 cells subjected to H/R alone. E: determination of cardiomyocyte H/R injury as shown by LDH release in control and hAR-overexpressing HL-1 cell supernatants. F: LDH release in control and hAR-overexpressing HL-1 cells treated with and without PI3K/Akt inhibitor LY-294002. Western blot analysis for p-GSK3β-Ser9 (G) and cytochrome c (H) in control and hAR-overexpressing HL-1 cells treated with and without PI3K/Akt inhibitor LY-294002. Cells were incubated with LY-294002 (10 μM) or its vehicle control dimethyl sulfoxide (DMSO) for 1 h, followed by 30 min of hypoxia and 1 h reoxygenation (H/R). Treatment with LY-294002 decreased p-Akt levels, decreased inhibition of GSK3β, and increased apoptosis in both control and hAR-overexpressing cells. LDH release in supernatants in both control and hAR-overexpressing cells was increased with PI3K/Akt inhibitor LY-294002 treatment. Error bars not visible. Control: SE ± 0.006; and hAR: SE ± 0.011.
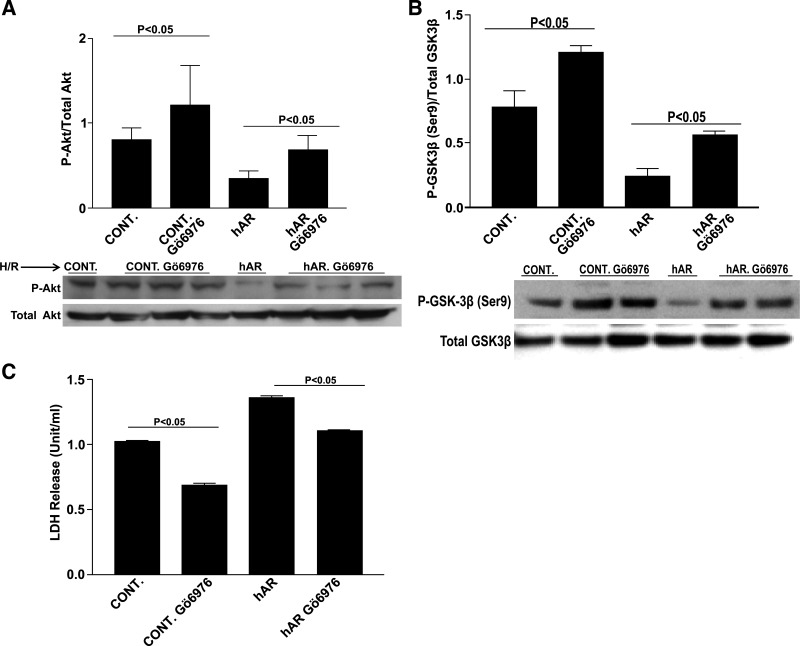
To further elaborate and clarify the role of PKC in AR-mediated I/R injury, we have previously demonstrated that increased metabolic flux through AR mediates I/R injury, in part, via changes in PKCα/β-dependent signaling. Flux via the polyol pathway increases the cytosolic ratio of NADH/NAD+, which results in increased levels of diacylglycerol and, subsequently, increased PKC activation (18). We have also shown earlier that flux via AR leads to activation of PKCα/β and that inhibition of PKCα/β with Gö-6976 attenuates I/R injury (18). In addition, the PI3K/Akt pathway has been shown to be a downstream target of PKCα/β (9). In the current study, to investigate if AR-driven changes in PKCα/β influence GSK3β phosphorylation, we treated HL-1 cells with PKCα/β inhibitor Gö-6976 and subjected them to H/R stress. PKC inhibition resulted in increased p-Akt (P < 0.05, Fig. 7A) and p-GSK3β (P < 0.05, Fig. 7B) in Ad-hAR-expressing HL-1 cells. Furthermore, treatment of HL-1 cells with Gö-6976 resulted in a marked reduction of injury as shown by decreased LDH release (P < 0.05, Fig. 7C). These data establish that AR modulates p-GSK3β, in part, via PKCα/β in cardiac cells.
Fig. 7.
Protein kinase C (PKC) α/β inhibition. Western blot analysis for p-Akt (A) and p-GSK3β (B). C: determination of cardiomyocyte injury as shown by LDH release. Treatment with Gö-6976 resulted in decreased cellular injury and an increase in p-GSK3β. Cells were incubated with Gö-6976 (1 μM) or its vehicle control DMSO for 1 h, followed by 30 min of hypoxia and 1 h reoxygenation (H/R).
DISCUSSION
Previous studies demonstrated the important role of AR in mediating myocardial I/R injury (1, 16–19, 34–36, 40). Our goal in this study was to investigate if AR-mediated I/R injury is linked to changes in phosphorylation of GSK3β and whether these changes would correlate with the impaired ability of the myocardium to recover after an ischemic insult. Consistent with earlier results, we show that transgenic mice expressing hAR have increased I/R injury (1, 18). Hearts from ARKO mice demonstrated a significant increase in LVDP recovery compared with WT mice. Our earlier studies showed that inhibition of AR in WT mice reduced injury and also improved function. We have previously shown that, after treatment with Zopolrestat, an aldose reductase inhibitor, WT mice had significant decreases in the opening of the mPTP. This can be explained in part because flux via AR increases after ischemia (34). Here, we show that inhibition of AR (ARKO) significantly protects the myocardium as a result of increased phosphorylation of GSK3β and a reduction in apoptosis. We demonstrate that AR mediates I/R injury, in part, via decreased phosphorylation of GSK3β (Ser9) thereby impairing mitochondrial function, as well as functional recovery of the heart. In these studies, impaired mitochondrial function in ARTg hearts after I/R resulted in both necrotic and apoptotic cell death mechanisms as demonstrated by increased mPTP opening and increases in cytochrome c expression. Phosphorylation of GSK3β was decreased significantly in ARTg mouse hearts as well as in hAR-overexpressing HL-1 cells. Further experiments indicated that, upon pharmacological inhibition of GSK3β, significantly higher levels of p-GSK3β were observed in ARTg hearts subjected to I/R and hAR-overexpressing cells subjected to H/R. The increases in levels of p-GSK3β correlated with decreased apoptosis in GSK3β-inhibited ARTg hearts. Our efforts to further elucidate the signaling mechanisms that contribute to the decreased phosphorylation of GSK3β highlight signaling via Akt, which functions upstream of GSK3β as a key player in mediating I/R injury. Inhibition of the PI3K/Akt pathway resulted in significant cardiac injury and impairment of cardiac recovery function. Treatment with PI3K/Akt inhibitor decreased p-Akt and p-GSK3β levels.
Two mammalian isoforms of GSK3 have been identified [GSK3α and GSK3β (29)]. The GSK3β isoform regulates numerous cell processes, including apoptosis and proliferation, and has been shown to enhance cardioprotection in several studies (3, 10, 12). The activity of GSK3β is regulated by phosphorylation (22–23, 29). The phosphorylation and inhibition of GSK3β is a major component of its cardioprotective role. Several studies have shown that the inhibition of GSK3β reduces apoptosis and confers cardioprotection depending on the degree and duration of inhibition (29, 32). The use of cardioprotective agents in a cardiomyocyte model has been shown to phosphorylate and inhibit GSK3β and further protects by inhibiting mPTP opening (23). Cardiomyocytes from mice with a constitutively active GSK3β were not protected upon treatment with cardioprotective agents. Other studies have shown a reduction in infarct size and an improvement in postischemic cardiac function with the use of GSK3β inhibitors (23, 27, 29, 39). Consistent with these data of cardioprotection achieved by inhibiting the activity of GSK3β, our data show significantly enhanced phosphorylated GSK3β upon treatment with GSK3β inhibitors after I/R injury, which correlated with enhanced cardioprotection and a reduction in apoptosis in ARTg mice hearts. Analogous to heart ex vivo I/R experiments, siRNA knock down of GSK3β in hAR-expressing cells was protected against H/R stress.
Several kinases upstream converge and inhibit GSK3β. GSK3β has emerged as the integration point of many of these pathways and plays a central role in transferring protective signals downstream to target(s) (23, 26). One of these upstream regulators includes Akt/PKB, a Ser/Thr kinase that has been shown to phosphorylate and inhibit GSK3β on the serine residue (5). Fujio et al. (7) have shown that Akt functions to promote cardiomyocyte survival and protects against I/R in the mouse hearts. Our results show that the metabolic flux through AR mediates I/R injury, in part, via decreased Akt activation. Furthermore, in hearts from WT and ARKO mice perfused with PI3K/Akt signaling inhibitors, we observed marked increases in LDH and impaired functional recovery during reperfusion. The PI3K/Akt inhibitor resulted in marked decrease in the levels of phosphorylated GSK3β that consequentially led to increased cell death and apoptosis. These data lead us to believe that Akt and GSK3β are key kinases that participate in the signaling mechanisms that mediate functional and cardiac recovery after I/R injury as a result of increased flux via AR. Decreased phosphorylation of these kinases resulted in the impairment of cardiac recovery that contributed to the vulnerability of the myocardium to recover after an ischemic insult.
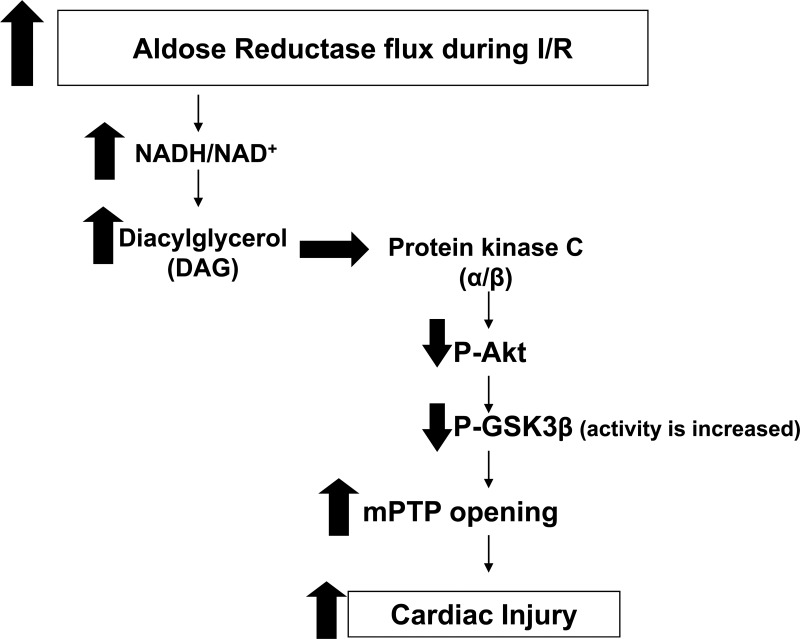
Studies by us (18) and others (33, 38) have demonstrated that increased flux via AR leads to activation of PKC (α/β) in euglycemic and hyperglycemic conditions. Furthermore, previous reports in the literature have alluded to the selective inhibition of PI3K/Akt as a result of PKC activation in endothelial cells (30). The mechanism of PI3K/Akt inhibition has been attributed to activation of the PKCβ isoform in particular, due to hyperglycemia. Hence, we investigated the role of PKC activation and GSK3β phosphorylation. Naruse et al. (30) have demonstrated that inhibition of PKCβ resulted in improved glomerular endothelial cell function and improved insulin action. Furthermore, translocation of PKCβII from the cytosol to membrane has been shown to contribute to I/R injury, and either genetic or pharmacological inhibition of PKCβII protects ischemic hearts (25). Additionally, activation of PKCα has been shown to contribute to cardiac hypertrophy, and inhibition of this PKC isoform led to attenuation of inflammation, cardiomyocyte growth, and cardiac hypertrophy (44). Here, we demonstrate reductions in LDH release upon treatment with PKCα/β inhibitor Gö-6976. Furthermore, we demonstrate that Akt and GSK3β phophorylation was increased in both control and Ad-hAR HL-1 cells treated with Gö-6976. Taken together, our data demonstrate that increases in AR flux lead to PKCα/β activation followed by decreases in Akt and GSK3β phosphorylation and is linked to increases in I/R injury (Fig. 8).
Fig. 8.
Schematic diagram. Increased flux via AR after I/R resulted in generation of diacylglycerol (DAG) and activation of PKCα and -β isoforms, leading to decreased phosphorylation of Akt and GSK3β kinases. Decreased GSK3β ultimately decreased cardioprotection via increased apoptotic mechanisms and mitochondrial permeability opening.
In summary, our data demonstrate decreased levels of p-GSK3β in ARTg mice hearts compared with WT hearts. Inhibition of GSK3β increased levels of p-GSK3β and was associated with decreased injury, improved functional recovery, and decreased apoptosis after I/R in ARTg hearts. ARKO mice had increased levels of p-GSK3β, improved functional recovery, and decreased apoptosis after I/R compared with WT and ARTg hearts. Furthermore, we show that AR modulates changes in levels of p-GSK3β via the Akt pathway. Taken together, our data demonstrate that AR mediates I/R injury, in part, via modulation of GSK3β phosphorylation.
GRANTS
This work was supported by National Institutes of Health Grants AG-026467, HL-61783, HL-60901, and HL-102022, the Harry B. & Aileen B. Gordon Foundation (K. H. Gabbay), and the Jacob & Louise Gabbay Foundation (K. H. Gabbay).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A., R.A., L.S., Z.Z., R. Rosario, and H.Z. performed experiments; M.A., R.A., L.S., Z.Z., and H.Z. analyzed data; M.A., S.V., H.Z., and R. Ramasamy interpreted results of experiments; M.A. prepared figures; S.V., K.M.B., K.H.G., and R. Ramasamy edited and revised manuscript; R. Ramasamy conception and design of research; R. Ramasamy drafted manuscript; R. Ramasamy approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Latoya Woods for assistance in manuscript preparation.
REFERENCES
- 1. Ananthakrishnan R, Kaneko M, Hwang YC, Quadri N, Gomez T, Li Q, Caspersen C, Ramasamy R. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol 296: H333–H341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest 76: 1713–1719, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng H, Woodgett J, Maamari M, Force T. Targeting GSK-3 family members in the heart: a very sharp double-edged sword. J Mol Cell Cardiol 51: 607–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Connern CP, Halestrap AP. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem J 284: 381–385, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Dorn GW., 2nd Mechanisms of non-apoptotic programmed cell death in diabetes and heart failure. Cell Cycle 9: 3442–3448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabbay KH, Bohren KM, Morello R, Bertin T, Liu J, Vogel P. Ascorbate synthesis pathway: dual role of ascorbate in bone homeostasis. J Biol Chem 285: 19510–19520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319–1331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 117: 2761–2768, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Grimshaw CE, Bohren KM, Lai CJ, Gabbay KH. Human aldose reductase: rate constants for a mechanism including interconversion of ternary complexes by recombinant wild-type enzyme. Biochemistry 34: 14356–14365, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Gross ER, Hsu AK, Gross GJ. GSK3beta inhibition and K(ATP) channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol 102: 341–349, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Halestrap AP. Mitochondria and reperfusion injury of the heart–a holey death but not beyond salvation. J Bioenerg Biomembr 41: 113–121, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta 1787: 1402–1415, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Hasselbaink DM, Glatz JF, Luiken JJ, Roemen TH, Van der Vusse GJ. Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem J 371: 753–760, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J 18: 1192–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hwang YC, Sato S, Tsai JY, Yan S, Bakr S, Zhang H, Oates PJ, Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischemia. FASEB J 16: 243–245, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hwang YC, Shaw S, Kaneko M, Redd H, Marrero MB, Ramasamy R. Aldose reductase pathway mediates JAK-STAT signaling: a novel axis in myocardial ischemic injury. FASEB J 19: 795–797, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Iwata K, Matsuno K, Nishinaka T, Persson C, Yabe-Nishimura C. Aldose reductase inhibitors improve myocardial reperfusion injury in mice by a dual mechanism. J Pharmacol Sci 102: 37–46, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res 45: 360–369, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Jez JM, Penning TM. The aldo-keto reductase (AKR) superfamily: an update. Chem Biol Interact 130–132: 499–525, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 104: 1240–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol Heart Circ Physiol 276: H496–H502, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, Homma S, Liu R, Zou YS, Leitges M, Yan SD, Ramasamy R, Schmidt AM, Yan SF. PKCbeta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol 294: H1862–H1870, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal 20: 206–218, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Murphy E. Inhibit GSK-3beta or there's heartbreak dead ahead. J Clin Invest 113: 1526–1528, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res 103: 226–228, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy E, Steenbergen C. Inhibition of GSK-3beta as a target for cardioprotection: the importance of timing, location, duration and degree of inhibition. Expert Opin Ther Targets 9: 447–456, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55: 691–698, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res 103: 307–314, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273: 19929–19932, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes 54: 818–829, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res 106: 1449–1458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 46: 292–300, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Ramasamy R, Trueblood N, Schaefer S. Metabolic effects of aldose reductase inhibition during low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol 275: H195–H203, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Shang L, Ananthakrishnan R, Li Q, Quadri N, Abdillahi M, Zhu Z, Qu W, Rosario R, Toure F, Yan SF, Schmidt AM, Ramasamy R. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One 5: e10092, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem 278: 30634–30641, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 90: 377–379, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Tracey WR, Magee WP, Ellery CA, MacAndrew JT, Smith AH, Knight DR, Oates PJ. Aldose reductase inhibition alone or combined with an adenosine A(3) agonist reduces ischemic myocardial injury. Am J Physiol Heart Circ Physiol 279: H1447–H1452, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho YS, Maulik N, Barski OA, Conklin DJ, Bhatnagar A. Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J Biol Chem 285: 26135–26148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol 286: H823–H829, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Yamaoka T, Nishimura C, Yamashita K, Itakura M, Yamada T, Fujimoto J, Kokai Y. Acute onset of diabetic pathological changes in transgenic mice with human aldose reductase cDNA. Diabetologia 38: 255–261, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Yan L, Huang H, Tang QZ, Zhu LH, Wang L, Liu C, Bian ZY, Li H. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem 109: 1158–1171, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]