Abstract
Background
Physician co-management, representing joint participation in the planning, decision-making, and delivery of care, is often cited in association with coordination of care. Yet little is known about how physicians manage tasks and how their management style impacts patient outcomes.
Objectives
To describe physician practice style using breast cancer as a model. We characterize correlates and predictors of physician practice style for 10 clinical tasks, and then test for associations between physician practice style and patient ratings of care.
Methods
We queried 347 breast cancer physicians identified by a population-based cohort of women with incident breast cancer regarding care using a clinical vignette about a hypothetical 65-year-old diabetic woman with incident breast cancer. To test the association between physician practice style and patient outcomes, we linked medical oncologists’ responses to patient ratings of care (physician n = 111; patient n = 411).
Results
After adjusting for physician and practice setting characteristics, physician practice style varied by physician specialty, practice setting, financial incentives, and barriers to referrals. Patients with medical oncologists who co-managed tasks had higher patient ratings of care.
Conclusion
Physician practice style for breast cancer is influenced by provider and practice setting characteristics, and it is an important predictor of patient ratings. We identify physician and practice setting factors associated with physician practice style and found associations between physician co-management and patient outcomes (e.g., patient ratings of care).
Keywords: Quality of care, physician practice style, physician co-management, patient ratings of care, breast cancer care, provider network restrictions
Recent work has described health care as fragmented, with an opaque structure and operating rules (Sofaer 2009). Patients with multiple chronic conditions may be at highest risk for this fragmented care. Yet little is known about how physicians caring for the same patient typically manage tasks. Without care coordination—efforts to integrate the highly technical, highly differentiated health system—patients may needlessly experience gaps in treatment or, at the other end of the spectrum, redundant or unnecessary care.
Many have touted patient-centered medical homes as a means of improving patients’ health care experiences (Colwill 2010). However, even the best medical homes may have little or no influence on what occurs when patients are referred into the “medical neighborhood,” for example, seeking care from specialist physicians (Pham 2010).
No single physician practice style has been identified as optimal across all aspects of care. However, for certain tasks associated with cancer diagnosis and treatment, co-management, where two or more physicians actively collaborate in caring for patients with regular communication and consultation, may be the best practice style to support continuity and coordination of care. While the prevalence of co-management between pediatricians and specialists has been studied (Forrest et al. 1999), the prevalence, predictors, and patient outcomes associated with physician co-management among specialists have not been reported.
Coordinated care has been strongly advocated as a means of improving care of multiple chronic conditions (Starfield et al. 2005; Stille et al. 2005; Smith, Allwright, and O'Dowd 2007), including cancer (Committee on Quality of Health Care in America, Institute of Medicine, Institute of Medicine 2001; Hewitt, Greenfield, and Stovall 2005; Schrag et al. 2006; Aiello Bowles et al. 2008) and survivorship care (Ganz and Hahn 2008; Hong et al. 2009). However, most studies report that coordinated care is not standard, that the current health care system is fragmented, and that poor communication among providers caring for the same patient is prevalent (Gandhi et al. 2000; Coleman et al. 2002, 2006; Earle and Neville 2004; Pham et al. 2007; O'Malley et al. 2009). While there is growing recognition of the importance of communication and collaboration among physicians in enhancing continuity of care, to date, most work has focused on describing collaboration between a primary care doctor and a patient (Carrier, Gourevitch, and Shah 2009), or between generalists and specialists (Starfield et al. 2005; Smith, Allwright, and O'Dowd 2007, 2008; Hong et al. 2009). A study of specialists’ practice style in the management of patients receiving treatments from multiple specialists could further inform the evolving study of physician communication and care coordination in health care delivery.
Breast cancer often involves treatment planning and delivery from at least three different specialists: medical oncologists, radiation oncologists, and surgeons. Depending on the tumor characteristics and other patient attributes, different specialists may be involved in treating patients. Little is known about if or when one of the specialists acts as lead in providing breast cancer care (e.g., surgeon for lumpectomy only, medical oncologist if prolonged adjuvant treatment is involved). Patients with multiple providers may be at risk for discontinuities in care (Coleman et al. 2002; Ayanian et al. 2005; Aiello Bowles et al. 2008; Sofaer 2009 ). For example, as patients transition from one specialist and treatment (e.g., surgical resection) to another (e.g., radiation and/or chemotherapy), if providers operate independently with no integrated plan of care, vulnerable patients, particularly those suffering adverse effects associated with treatment, may be adversely affected (Coleman et al. 2002). With all of these considerations, breast cancer is a model condition for the study of prevalence and predictors of physician practice style (Kahn et al. 2002), and the relationship between physician practice style and patient-level outcomes.
Here, we describe self-reported physician practice style of specialists associated with 10 clinically prevalent tasks in breast cancer care. We, then, test if physician practice style is associated with patient-level outcomes. We believe the study of physician practice style and its links to patient outcomes reveals pathways to improve the coordination of care for breast cancer and other conditions involving multiple specialty providers.
Objectives
In this study, we queried physicians delivering care to a population-based cohort of women with incident breast cancer about their usual practice style for 10 prevalent clinical tasks associated with the management of a hypothetical patient with incident breast cancer. Building on earlier work by Hadley et al. 1999; and Reschovsky, Hadley, and Landon 2006, we hypothesize that physician characteristics (e.g., medical specialty), practice setting characteristics (e.g., solo practice vs. medical group or HMO), physician reimbursement (fee for service vs. salary or capitated arrangements), and structural characteristics (e.g., restrictions to referrals) may be associated with variations in physician practice styles used to manage prevalent clinical tasks.
Based on our study of physician and structural characteristics, we developed several hypotheses. Given differences in medical training, we would expect less co-management and fewer referrals from medical oncologists compared with radiation oncologists and surgeons. With greater proximity to other specialists, we expect more frequent reports of co-management or referrals in medical groups and HMOs compared with solo practice settings. If providers face restrictions to referrals, and are limited in their ability to select physician consultants and clinicians, they may be more inclined to perform tasks themselves. In a separate set of analyses, we test for associations between physician practice styles and patient outcomes (e.g., patient ratings of care) for four clinical tasks. We believe co-management will be associated with higher patient ratings of care for these tasks. We believe this is the first study describing the prevalence of physician practice styles, identifying the predictors and correlates of physician co-management among cancer specialists and the relationship between physician practice style and patient outcomes.
Methods
Provider-Level Data Source
As part of the Los Angeles Women's (LAW) Health Study, using previously tested methods for identifying patients’ providers (Kahn et al. 2007), we asked a population-based cohort of women with incident breast cancer about their care and the providers who delivered that care. From the provider names and contact information, we initially identified 747 physicians, confirming names and addresses for 477 physicians (64 percent). We mailed the final, self-administered survey instrument to physicians between April and October of 2004, including 175 medical oncologists, 75 radiation oncologists, and 227 surgeons. The research team obtained 348 surveys from physicians associated with 298 unique office addresses, for a final response rate of 77 percent (63 percent for medical oncologists, 88 percent for radiation oncologists, and 75 percent for surgeons) (Tisnado et al. 2008, 2009). We excluded one physician who did not complete questions about specialty physician practice style. The sample for the provider-level analysis was 347 physicians. For the patient outcomes analyses, we limited our provider sample to patients associated with medical oncologists only (n for patients = 411, n for providers = 111).
Patient-Level Data Source
We used patient self-report data from the LAW Study, a population-based, longitudinal, telephone health survey of women with breast cancer 50 years and older in Los Angeles County (Chen et al. 2008; Yoon et al. 2008a; Yoon et al. 2008b; Chen et al. 2009). Ninety-minute computer-assisted telephone interviews were conducted in English and Spanish. The sample for the survey was drawn from a census of incident breast cancer cases diagnosed March through November 2000 identified by Rapid Case Ascertainment (RCA) of the Los Angeles County Cancer Surveillance Program (LAC CSP) (Pearson et al. 2002). The LAC CSP staff screened pathology reports in all LAC hospitals at least monthly to identify incident cancers and enter relevant information (e.g., type of cancer, surgery, patient contact information) into a central database. Cancer researchers can then petition to use the information collected by RCA for research purposes. To minimize respondent burden, the LAC CSP did not release information pertaining to Asian American women 50–59 years of age and more than 75 years of age for this study because these women had already participated in another study.
A total of 2,745 patients were initially identified by RCA from 103 hospitals. Of these women, 1,269 completed the baseline telephone interview for a response rate of 64 percent. The baseline survey was conducted a mean of 223 days after diagnosis (median 185 days, interquartile range, 159–255). The response rate for the follow-up survey was 79 percent. A flow chart showing how the analytic sample was derived can be found in Yoon et al. (2008a). We restricted the patient-level sample to women who could be linked to their medical oncologist from whom we had provider survey data (n = 411).
Dependent Variables
Provider-Level Dependent Variable
The specifications for the provider-level dependent variable are derived from physicians’ descriptions of their typical management of 10 tasks associated with 3 clinical domains for a hypothetical patient (Table 2). The first domain represents initial consultation: that is, establishment of goals for cancer treatment and prognosis; assessment of patient preferences; and determination of the initial course of cancer treatment. The second domain represents physician decision-making for each patient, that is, decisions about type of breast surgery; possible use of radiation therapy; or possible use of chemotherapy. The third domain addresses treatment of symptoms and comorbidities: that is, prescribing opiates for pain; evaluation and treatment of cancer-related arm symptoms such as lymphedema; depressive symptoms; and management of non-cancer-related comorbidities (e.g., diabetes).
Table 2.
Physician Practice Style Items for 10 Clinical Tasks by Specialty
| Variable | All N = 347 (%) | Medical Oncologist N = 111 (%) | Radiation Oncologist N = 66 (%) | Surgeon N = 170 (%) |
|---|---|---|---|---|
| Domain I. Initial consultation | ||||
| a. Establish goals for cancer treatment‡ | ||||
| I manage | 49 | 76 | 24 | 36 |
| I co-manage | 48 | 24 | 74 | 59 |
| I refer | 2 | 0 | 0 | 4 |
| I do not handle | 1 | 0 | 2 | 1 |
| b. Assess patient preferences‡ | ||||
| I manage | 62 | 82 | 35 | 55 |
| I co-manage | 37 | 18 | 64 | 43 |
| I refer | 0 | 0 | 0 | 0 |
| I do not handle | 1 | 0 | 1 | 2 |
| c. Determine the initial course of cancer treatment ‡ | ||||
| I manage | 57 | 74 | 17 | 57 |
| I co-manage | 40 | 25 | 80 | 39 |
| I refer | 2 | 1 | 0 | 2 |
| I do not handle | 1 | 0 | 3 | 2 |
| Domain II. Physician decision-making | ||||
| d. Decide about type of breast surgery‡ | ||||
| I manage | 39 | 12 | 3 | 72 |
| I co-manage | 47 | 65 | 68 | 26 |
| I refer | 12 | 22 | 21 | 1 |
| I do not handle | 2 | 1 | 8 | 1 |
| e. Decide about possible use of radiation‡ | ||||
| I manage | 22 | 17 | 68 | 9 |
| I co-manage | 63 | 68 | 32 | 70 |
| I refer | 15 | 15 | 0 | 20 |
| I do not handle | 0 | 0 | 0 | 1 |
| f. Decide about possible use of chemotherapy‡ | ||||
| I manage | 37 | 93 | 3 | 4 |
| I co-manage | 39 | 6 | 47 | 63 |
| I refer | 23 | 1 | 47 | 32 |
| I do not handle | 1 | 0 | 3 | 1 |
| Domain III. Treatment of Symptoms and comorbidities | ||||
| g. Prescribe opiates for pain* | ||||
| I manage | 72 | 90 | 53 | 64 |
| I co-manage | 22 | 10 | 41 | 25 |
| I refer | 5 | 0 | 5 | 9 |
| I do not handle | 1 | 0 | 2 | 2 |
| h. Evaluate and treat cancer-related arm symptoms† | ||||
| I manage | 27 | 34 | 12 | 27 |
| I co-manage | 44 | 42 | 38 | 47 |
| I refer | 28 | 23 | 50 | 23 |
| I do not handle | 1 | 1 | 0 | 3 |
| i. Evaluate and treat depressive symptoms‡ | ||||
| I manage | 17 | 37 | 7 | 4 |
| I co-manage | 29 | 51 | 20 | 14 |
| I refer | 46 | 12 | 68 | 67 |
| I do not handle | 8 | 0 | 5 | 15 |
| j. Manage non-cancer-related comorbidities such as diabetes‡ | ||||
| I manage | 8 | 14 | 0 | 6 |
| I co-manage | 24 | 42 | 1 | 19 |
| I refer | 62 | 43 | 91 | 66 |
| I do not handle | 6 | 1 | 8 | 9 |
p < .05;
p < .01;
p < .001.
For each clinical task, physicians selected one of four response options to describe their practice style: I provide this care myself without much input from another clinician; I co-manage or decide jointly about this care with another clinician; I refer patients to another clinician for this aspect of care; I am not involved in this aspect of care. We categorize physician style as independent if physicians reported completing tasks without much input from another clinician, and as co-managing if the respondent indicated he or she would decide jointly about care with another clinician. Physicians’ responses did not suggest that specialists typically referred patients or did not handle tasks. Our goal here was not to characterize one practice style as more appropriate than another but to describe alternative styles physicians report for typically managing tasks.
Patient-Level Dependent Variable
The patient-level dependent variable is an overall score averaging responses associated with six items from the patient interview, adapted from CAHPS hospital survey (Marshall et al. 2001). Please see Appendix B for items. The interview asked: Did your provider explain enough about the risks and benefits of each diagnostic procedure and treatment? Did your provider take into account all of your medical problems when providing your care? Response options for each of the six items included Excellent, Very Good, Good, Fair or Poor, and they were scored from 100 (Excellent) to 0 (Poor).
Independent Variables
Provider-Level Independent Variables
Physician independent variables included physician demographic characteristics (age, gender); specialty type (medical oncologist, radiation oncologist, surgeon); and physician practice characteristics (working full- or part-time; physician volume, defined as physician-reported number of new cancer patients in the last month).
Physician compensation methods and financial incentives
Building on examples from the literature (Hadley et al. 1999; Reschovsky, Hadley, and Landon 2006), we asked physicians about the percentage of patients covered by payment sources: Medicare, Medicaid, or, private health insurance (for each payment source, we asked if it was fee-for-service or managed care) or if the patient was uninsured. We also asked about payment mechanisms (e.g., salary, salary with bonuses, fee-for-service reimbursement, and capitation or pre-paid reimbursement). From physician report of proportion of payment sources and mechanisms, we derived measures of salary or fee-for-service reimbursement, dichotomized as low or high (< vs. ≥50 percent). Capitation or prepaid reimbursement was dichotomized as any or none. In addition, we queried physicians if they ever had any financial incentives to increase practices or services versus none (e.g., we asked medical oncologists if they received financial incentives for the use of parenteral chemotherapy or growth factor injections in the office) (Tisnado et al. 2008).
Practice setting characteristics
We asked physicians to describe their practice. Practice setting was categorized as solo practice (reference group); county government or medical school or university; staff/group model HMO; or medical group. We also asked physicians about the number of full-time physicians in their practice: 1, 2–5, 6–15, 16–24, 25–49, or 50 or more. Because of small numbers of observations in some of the practice size responses, we indicated large practice size as 50 or more full-time physicians.
Physicians were asked whether they experienced barriers to arranging high-quality referrals for their patients, including the following: provider network restrictions imposed by a health plan, medical group, or IPA; lack of established professional relationships with high-quality providers; or because Medicaid is not accepted by high-quality providers. We also queried physicians about tumor board involvement, deriving a measure indicating weekly or monthly tumor board attendance versus less frequent attendance (Scher et al. 2011).
Patient-Level Independent Variables
In the analyses testing for associations between physician practice style and patient-level outcomes, patient-level controls include age, race/ethnicity, marital status, tumor stage (stage 0, 1 or 2 as reference vs. stage 3 or stage 4) and number of comorbid conditions.
Analytic Methods
Provider-Level Analytic Methods
In the analyses of specialty physician practice style, we fit population-average panel-data models using generalized estimation equations (Liang and Zeger 1993) to estimate how often physicians reported co-managing tasks, adjusting for multiple questions associated with each provider. We ranked the four response options reflecting varying degrees of physician involvement in managing each task: independent or provision of care without much input from others; co-managing or joint decision-making with another physician; referring to another physician for care; or no involvement in care. To test whether our results were dependent on response option order, we conducted sensitivity analyses switching co-management and referral. Results were consistent, indicating that our results were robust across ordering schemes.
Tests for correlations showed salary was highly correlated with measures of practice setting (physician practices in an HMO or big practice variables; 0.47 and 0.46, respectively), so the variable was excluded from the multivariate analyses. Sensitivity analyses tested a priori hypothesized interactions between physician characteristics; practice setting and practice size; and between physician compensation methods, financial incentives, and practice settings (Conrad and Christianson 2004). No statistically significant terms were found.
Patient-Level Analytic Methods
In the linked patient-provider-level analyses, we used multilevel mixed-effect linear models to predict patient ratings of care, while controlling for multiple patients clustering within medical oncologists. We conducted analyses with and without providers who reported not managing tasks; the results were the same. To simplify presentation, we present the results excluding those who reported not managing tasks.
Results
Provider-Level Descriptive Results
Among the 347 physician respondents, 32 percent were medical oncologists, 19 percent were radiation oncologists, and 49 percent were surgeons (Table 1). The mean age of physicians was 52 years (SD: 9.3 years); 82 percent were male, and two-thirds were Caucasian. Physicians reported 20 new cancer patients during the last month, on average. Thirteen percent of physicians reported working part-time (30 hours or less per week).
Table 1.
Provider Self-Report of Physician and Office Characteristics
| Variable | All N = 347 | Medical Oncologist N = 111 | Radiation Oncologist N = 66 | Surgeon N = 170 |
|---|---|---|---|---|
| Physician demographics | ||||
| Mean physician age in years and SD (range) | 52 SD 9.3 (34–79) | 53 SD 8.6 (35–79) | 49 SD 9.9 (34–78) | 53 SD 9.2 (35–77) |
| Physician gender | ||||
| Male | 82% | 76% | 79% | 87% |
| Physician race/ethnicity | ||||
| Non-Hispanic White | 66% | 64% | 65% | 69% |
| Non-Hispanic Black | 3% | 3% | 3% | 8% |
| Hispanic | 5% | 3% | 3% | 2% |
| Asian | 20% | 23% | 24% | 16% |
| Other | 6% | 7% | 5% | 5% |
| Physician volume | ||||
| Mean number of new cancer patients during last month and SD (range) | 20 SD 21 (0–180) | 28 SD 29 (2–180) | 31 SD 16 (10–100) | 10 SD 8 (0–55) |
| Physician working full-time or part-time in patient care | ||||
| Physicians working ≤30 hours in direct patient care | 13% | 15% | 13% | 12% |
| Practice type‡ | ||||
| Solo | 30% | 24% | 8% | 42% |
| County or medical school or university | 8% | 12% | 9% | 5% |
| HMO | 18% | 16% | 13% | 21% |
| Medical group | 45% | 50% | 70% | 32% |
| Practice size* | ||||
| Large practice (50 or more full-time physicians in main practice) | 17% | 15% | 4% | 23% |
| Financial incentives and payment | ||||
| Physician reported any financial incentives to expand clinical practices or services to patients† | 21% | 39% | 9% | 11% |
| Physician reported predominantly payment on a salary basis | 46% | 53% | 57% | 36% |
| Physician reported predominantly fee-for-service payment* | 32% | 25% | 22% | 40% |
| Physician reported receiving any capitated payment | 16% | 20% | 15% | 14% |
| Barriers to referrals | ||||
| Provider reported barriers to referral of high-quality providers because of: | ||||
| Provider network restrictions | 21% | 25% | 23% | 17% |
| Lack of established relationship with high-quality provider | 8% | 9% | 8% | 7% |
| Medi-Cal was not accepted by high-quality provider | 38% | 47% | 39% | 31% |
| Tumor board participation | ||||
| Weekly or monthly tumor board participation | 84% | 88% | 99% | 75% |
p < .05;
p < .01;
p < .001.
Provider-Level Bivariate Results by Domain of Care and Provider Specialty
Across the domains, radiation oncologists reported co-managing more (4.6 of 10 tasks), followed by surgeons (4.0 tasks) and medical oncologists (3.5 tasks) (Figure 1). Medical oncologists reported independently managing 5 of 10 tasks.
Figure 1.
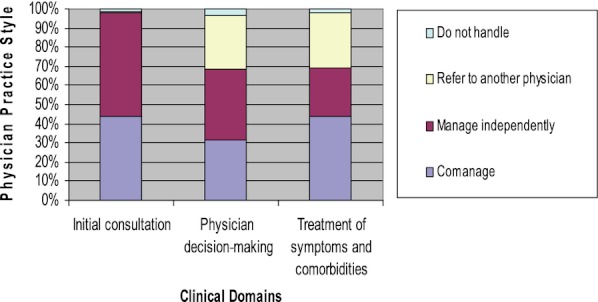
Prevalence of Physician Co-Management by Domain
For the initial consultation domain (Table 2), all physicians reported either independently managing or co-managing tasks. Medical oncologists frequently reported independently managing these tasks, for example, without much input from other physicians, while radiation oncologists frequently reported co-managing these tasks.
In the decision-making domain, the vast majority of physicians reported independently managing decisions about treatments within their own specialty, but they reported co-managing patients or referring patients for decisions about treatments delivered by other specialists.
In the treatment of symptoms and comorbidities domain, physician reports of practice style varied. Most physicians (72 percent) reported independently prescribing opiates for pain. Medical oncologists varied in reports of how they treated arm symptoms associated with lymphedema: 42 percent reported co-managing care, 34 percent reported independently managing care, and 23 percent reported referring the patient to another clinician for treatment. Approximately half the surgeons and radiation oncologists reported referring patients with lymphedema to another clinician for treatment. For the treatment of depressive symptoms, half of medical oncologists (46 percent) said that they referred patients to another clinician for treatment, and 37 percent said that they would independently manage care. In contrast, two-thirds of surgeons and radiation oncologists said that they referred patients for treatment of depressive symptoms.
For treatment of non-cancer-related comorbidities (e.g., diabetes), medical oncologists were equally likely to report co-managing care (42 percent) or referring (43 percent) patients to other clinicians for treatment. In contrast, most surgeons (66 percent) and almost all (91 percent) radiation oncologists reported referring patients for treatment of non-cancer-related comorbidities. Eight percent of radiation oncologists and 9 percent of surgeons reported that they would not perform this task at all.
Provider-Level Multivariate Results
Physician Characteristics
As shown in Table 3, older physicians were less likely to co-manage or refer patients (Coefficient: −0.003, 95% CI: −0.006, −0.0004, p < .05). Consistent with differences in specialist training and expertise, physician specialty was a significant predictor of physician practice style. Compared with medical oncologists (reference group), radiation oncologists and surgeons reported co-managing or referring significantly more tasks across all three domains (Coefficient: 0.493, 95% CI: 0.416, 0.570, p < .001 for radiation oncologists; and coefficient for surgeons: 0.307, 95% CI: 0.239, 0.376, p < .001).
Table 3.
Multivariate Analysis of Specialty Physician Practice Style Associated with 10 Clinical Tasks 1
| Variable | Specialty Physician Practice Style Associated with 10 Clinical Tasks Coefficient (95% Confidence Interval) |
|---|---|
| Physician demographics | |
| Age | −0.003* (−0.006, −0.0004) |
| Male | −0.125 (−0.054, 0.079) |
| Physician specialty | |
| Medical oncologist | Reference |
| Radiation oncologist | 0.493‡ (0.416, 0.570) |
| Surgeon | 0.307‡ (0.239, 0.376) |
| Physician volume | |
| Mean number of new cancer patients during last month | −0.000 (−0.002, 0.001) |
| Full-time or part-time | |
| Part-time: physicians working ≤30 hours in direct patient care | 0.046 (−0.028, 0.120) |
| Practice type | |
| Solo | Reference |
| County or medical school or university | 0.004 (−0.099, 0.108) |
| HMO | −0.150† (−0.251, −0.049) |
| Medical group | 0.047 (−0.251, 0.112) |
| Practice size | |
| Large practice (50 or more full-time physicians in main practice) | −0.067 (−0.152, 0.017) |
| Financial incentives and payment | |
| Physician reported any financial incentives to expand clinical practices or services to patients† | −0.080* (−0.150, −0.010) |
| Physician reported predominantly fee-for-service payment | −0.022 (−0.083, 0.040) |
| Physician reported receiving any capitated payment | 0.072 (−0.003, 0.147) |
| Barriers to referrals | |
| Provider reported barriers to referral of high-quality providers because of: | |
| Provider network restrictions | −0.200‡ (−0.266, −0.135) |
| Medi-Cal was not accepted by high-quality provider | 0.035 (−0.022, 0.092) |
| Tumor board participation | |
| Weekly or monthly tumor board participation | −0.056 (−0.127, 0.015) |
Note. Statistically significant coefficients appear in bold.
Coefficients were derived from population-averaged panel-data models using generalized estimating equations.
p < 0.05;
p < .01;
p < .001.
Practice Setting Characteristics
Physicians in HMOs report co-managing or referring more tasks compared with physicians in solo practice (Coefficent: −0.150, 95% CI: −0.251, −0.049, p < .01). Physicians who reported having (any) financial incentives were less likely to report co-managing or referring patients (Coefficient: −0.080, 95% CI: −0.150, −0.010, p < .05). Physicians who reported that provider network referrals imposed by a health plan, medical group, or IPA are barriers to high-quality referrals reported less co-management or referral of patients to other physicians (Coefficient: −0.200, 95% CI: −0.266, −0.135, p < .001).
Patient-Level Descriptive Results
The patients include women ranging from ages 50 to 99. Forty-one percent were age 50–59, 17 percent were age 60–64, 26 percent were age 65–74, and 16 percent were 75 years or older (Table 4). The sample is mostly Non-Hispanic White (74 percent), with 8 percent Non-Hispanic Black women, and 13 percent Hispanic women. Eight percent of women were English-speaking Hispanics (e.g., completed the survey in English), and 5 percent were Spanish-speaking Hispanic women. Five percent of the sample is categorized as “Other” race/ethnicity. Most of the women (94 percent) had breast cancer tumors with the following stages: 0, 1, 2, or no stage information. Eight percent could be described as late-stage, 6 percent with Stage 3, and 2 percent with Stage 4 tumors. Many women in our sample were married (83 percent), 20 percent identified themselves as divorced or separated, and 7 percent reported that they were never married. The average number of comorbid conditions was 2 (SD: 1.6, range: 0–8), and patient ratings of care averaged around 80 points (SD: 23.4 points, range: 0–100 points).
Table 4.
Characteristics of Women from the Los Angeles Women's Health Study Associated with 111 Medical Oncologists (n = 411)
| Patient-Level Variables | |
|---|---|
| Age | |
| Age 50–59 | 41% |
| Age 60–64 | 17% |
| Age 65–74 | 26% |
| Age 75–99 | 16% |
| Race/ethnicity | |
| Non-Hispanic White | 74% |
| Non-Hispanic Black | 8% |
| Hispanic, English-speaking | 8% |
| Hispanic, Spanish-speaking | 5% |
| Other race/ethnicity | 5% |
| Breast cancer tumor stage | |
| Stage 0, 1, or 2 or no stage information | 92% |
| Stage 3 | 6% |
| Stage 4 | 2% |
| Marital status | |
| Married | 83% |
| Never married | 7% |
| Divorced or separated | 20% |
| Number of comorbid conditions | 2 |
| Mean number and SD (range) | SD 1.6 (0–8) |
| Patient ratings of care | 80 points |
| Mean score and SD (range) | SD 23.4 (0–100) |
Patient-Level Results: Multi-Level Multivariate Analysis of Patient Ratings of Care
In multivariate analyses of patients' ratings of care provided by their medical oncologists, controlling for patient-level, physician-level, and practice setting characteristics, we found associations between medical oncologist practice style and patient ratings of care (Table 5). We found similar results in bivariate analyses shown in Appendix C. For the decision-making domain, patients whose medical oncologists reported referring patients for decision-making about surgery and/or possible use of radiation, rather than co-managing care, had lower ratings of care (Coefficient: −8.3, 95% CI: −15.1, −1.4, p < .05 for surgery and −10.8, 95% CI: −18.1, −3.6, p < .05 for radiation).
Table 5.
Multi-Level Multivariate Analyses of Patient Ratings of Care (Provider n = 111, Patient n = 411)1
| Patient Ratings of Care | ||||
|---|---|---|---|---|
| Clinical Task Associated with Breast Cancer Care | Decision-Making About Type of Breast Cancer Surgery Coefficient (95% Confidence Interval) | Decision-Making About Possible Use of Radiation Coefficient (95% Confidence Interval) | Evaluation and Treatment of Depressive Symptoms Coefficient (95% Confidence Interval) | Evaluation and Treatment of Arm Symptoms, e.g., Lymphedema Coefficient (95% Confidence Interval) |
| Provider-level variables | ||||
| Physician demographics | ||||
| Age | −0.3 (−1.2, 0.7) | −0.2 (−1.1, 0.7) | −0.2 (−1.1, 0.8) | −0.2 (−1.2, 0.7) |
| Male | 0.1 (−6.3, 6.5) | −1.7 (−8.2, 4.8) | −2.7 (−9.3, 3.9) | −2.7 (−9.2, 3.8) |
| Physician volume | ||||
| Mean number of new cancer patients during last month (range) | −0.1 (−6.6, 6.5) | 0.8 (−5.8,7.3) | 0.3 (−6.3, 6.9) | 0.8 (−5.9, 7.4) |
| Full-time or part-time | ||||
| Part-time: physicians working ≤30 hours in direct patient care | 0.5 (−9.1, 10.1) | 0.8 (−8.8, 10.4) | 1.2 (−8.5, 10.9) | 0.4 (−9.2, 9.9) |
| Practice type | ||||
| Solo | Reference | Reference | Reference | Reference |
| County or medical school or university | 7.0 (−3.5, 17.4) | 6.4 (−3.9, 16.7) | 2.1 (−8.6, 12.8) | 2.3 (−8.2, 12.8) |
| HMO | 6.2 (−5.8, 12.7) | 5.0 (−6.7, 16.6) | −1.0 (−13.2, 11.1) | 1.3 (−10.4, 13.0) |
| Medical group | 5.8 (−2.4, 13.9) | 6.0 (−2.0, 13.9) | 2.7 (−5.6, 11.0) | 3.4 (−4.8, 11.5) |
| Practice size | ||||
| Large practice (50 or more full-time physicians in main practice) | 3.4 (−5.9, 12.7) | 2.8 (−6.3, 12.0) | 0.4 (−8.8, 9.7) | 1.1 (−8.0, 10.3) |
| Financial incentives and payment | ||||
| Physician reported any financial incentives to expand clinical practices or services to patients | −3.9 (−10.8, 3.0) | −4.3 (−11.1, 2.4) | −2.8 (−9.5, 3.9) | −0.8 (−7.6, 5.9) |
| Physician reported predominantly fee-for-service payment | 4.0 (−2.9, 10.4) | 3.0 (−3.4, 9.3) | 3.6 (−2.8, 10.0) | 2.9 (−3.9, 9.7) |
| Physician reported receiving any capitated payment | 1.3 (−6.4, 9.1) | 0.9 (−6.7, 8.5) | −0.4 (−7.4, 5.4) | −1.4 (−9.1, 6.2) |
| Barriers to referrals | ||||
| Provider reported barriers to referral of high-quality providers because of: | ||||
| Provider network restrictions | −1.1 (−7.6, 5.3) | −1.9 (−8.2, 4.5) | −1.0 (−7.4, 5.4) | 0.6 (−5.9, 7.1) |
| Medi-Cal was not accepted by high-quality provider | −1.8 (−11.5, 7.9) | −2.4 (−8.2, 3.5) | −2.3 (−8.3, 3.6) | −0.3 (−6.2, 5.5) |
| Tumor board participation | ||||
| Weekly or monthly tumor board participation | −1.8 (−11.4, 7.9) | −2.4 (−12.0, 7.3) | −1.0 (−10.9, 8.8) | −1.8 (−11.8, 8.1) |
| Physician practice style | ||||
| Physician manages task without input from other clinicians | 4.9 (−5.9, 15.6) | 4.5 (−4.4, 13.4) | −4.5 (−10.6, 1.7) | −7.8* (−14.4, −1.1) |
| Physician co-manages task | Reference | Reference | Reference | Reference |
| Physician refers task to another clinician | −8.4* (−15.2, −1.6) | −10.9* (−18.1, −3.6) | −13.1† (−21.5, −4.6) | −4.0 (−10.3, 2.4) |
| Patient-level variables | ||||
| Age | ||||
| Age 50–59 | Reference | Reference | Reference | Reference |
| Age 60–64 | 4.5 (−5.0, 14.0) | 4.2 (−5.1, 13.6) | 3.0 (−6.5, 12.4) | 4.4 (−5.0, 13.9) |
| Age 65–74 | 5.9 (−8.7, 20.5) | 4.8 (−9.6, 19.3) | 5.1 (−6.5, 19.6) | 5.9 (−8.5, 20.2) |
| Age 75–99 | 4.1 (−19.1, 27.3) | 2.7 (−20.2, 25.6) | 2.0 (−21.0, 25.0) | 5.2 (−17.6, 28.0) |
| Patient race/ethnicity | ||||
| Non-Hispanic White/English-speaking Hispanic | Reference | Reference | Reference | Reference |
| Non-Hispanic Black | −4.8 (−13.4, 3.9) | −5.0 (−13.5, 3.6) | −7.1 (−15.7, 1.6) | −4.5 (−13.3, 4.2) |
| Spanish-speaking Hispanic | −1.1 (−11.7, 9.6) | −1.5 (−12.3, 9.2) | −1.1 (−11.7, 9.5) | −0.1 (−10.7, 10.6) |
| Stage of breast cancer tumor | ||||
| Stage 0, 1, or 2 | Reference | Reference | Reference | Reference |
| Stage 3 | 4.0 (−5.9, 13.9) | 3.2 (−6.6, 13.0) | 3.5 (−6.3, 13.3) | 3.5 (−6.3, 13.2) |
| Stage 4 | −7.5 (−24.9, 10.0) | −7.4 (−24.7, 10.0) | −6.0 (−23.4, 11.4) | −7.2 (−24.4, 10.1) |
| Marital status | ||||
| Married | Reference | Reference | Reference | Reference |
| Never married | 6.5 (−3.1, 16.2) | 6.4 (−3.3, 16.0) | 7.6 (−2.0, 17.2) | 7.0 (−2.6, 16.5) |
| Divorced or separated | −0.3 (−6.2, 5.6) | −0.1 (−6.0, 5.7) | −0.2 (−6.1, 5.7) | −0.9 (−6.8, 4.9) |
| Number of comorbid conditions | −0.5 (−2.1, 1.1) | −0.5 (−2.0, 1.1) | −0.3 (−1.8, 1.3) | −0.3 (−1.9, 1.3) |
Note.
Coefficients were derived from population-averaged panel-data models using generalized estimating equations.
Statistically significant coefficients appear in bold.
p < .05;
p < .01;
p < .001.
In the domain for treatment of symptoms and comorbid conditions, patients whose medical oncologists reported referring patients with depressive symptoms had lower ratings of care (Coefficient: −13.2, 95% CI: −21.7, −4.8, p < .01), compared with medical oncologists who reported co-managing care. Patients whose medical oncologists reported caring for arm symptoms themselves without other clinicians’ involvement had lower ratings of care (Coefficient: −7.8, 95% CI: −14.4, −1.1, p < .05) compared with medical oncologists co-managing arm symptoms with another clinician.
Discussion
We surveyed 1,269 patients and 347 physicians identified as providers filling key roles by a population-based cohort of women with incident breast cancer in Los Angeles County. We described specialty physician practice styles in performing 10 tasks, and then identified predictors and correlates of physician practice style in breast cancer care. In unadjusted and adjusted analyses, we found variations exist in report of specialty physician practice style. As hypothesized, physician specialty (radiation oncology and surgical specialty versus medical oncology), practice setting (HMO versus solo practice), and structural characteristics (e.g., physician report of having provider network restrictions imposed by health plans, medical groups or IPAs) were associated with physician practice style.
Among specialty differences, we observed that medical oncologists report independently managing more tasks (e.g., without input from other clinicians). Medical oncologists, who are typically boarded in internal medicine, may be more comfortable managing tasks associated with comorbid conditions compared with radiation oncologists and surgeons. Medical oncologists may certainly provide effective care without input from other clinicians, but more specialized physicians may be less able to provide all needed care. This is particularly important since evaluations of comorbidities (e.g., diabetes) are often limited in the setting of initial cancer management and survivorship (Rosenblatt et al. 1998; Earle et al. 2003; Earle and Neville 2004; Snyder et al. 2009).
As for practice setting differences, compared with physicians in solo practice, physicians in HMOs appear to co-manage or refer more tasks. This is consistent with physicians in integrated health settings having greater access to other clinicians (e.g., physical therapists for lymphedema, mental health providers for depression, and other internists for diabetes care). Another possible explanation is that physicians in these settings receive some sort of institutional support for co-management or referral compared with physicians in solo practice.
We did not find a difference in practice styles for physicians in medical groups. Perhaps similar financial structures or incentives cause physicians in medical groups to act more similarly to physicians in solo practice than expected. Physicians who reported either financial incentives to expand clinical practices, or services or barriers to referrals because of provider network restrictions, were more likely to report handling tasks themselves (vs. co-managing or referring tasks to other physicians).
The association between financial incentives and physician practice style has been documented in the literature (Grumbach et al. 1998; Hadley et al. 1999), although our findings provide additional insights. Physicians responsive to incentives may perform tasks on their own because they consciously or unconsciously believe they will financially benefit from doing more, and work with other clinicians less. Others have identified provider network restrictions as barriers to coordination of care among generalists and specialists (Grumbach et al. 1998; O'Malley et al. 2009). These restrictions on referrals are imposed by health plans, medical groups, or IPAs to contain costs and encourage collaboration among in-network providers. However, our findings suggest that if a plan's provider network differs from a physician's referral base, physicians may manage more tasks on their own, without input from other clinicians. Monitoring the impact of restrictions on specialty physician practice style may help avoid unintended clinical consequences.
In addition to our analysis of specialty physician practice style, we conducted an analysis exploring the potential relationship between medical oncologists’ practice style and patient-level outcomes (e.g., patient ratings of care). As we noted earlier, we do not see co-management as the ideal style for all 10 tasks studied here, so we limited our analysis to tasks where co-management could plausibly be argued as most appropriate (e.g., decision-making about type of breast surgery and/or possible use of radiation; management of depression; and management of lymphedema). For these four tasks, patients consistently assigned higher ratings to physicians who co-managed these tasks rather than utilizing other practice styles, demonstrating a clinically and statistically significant link between use of the physician practice style of co-management and patient-reported quality of care ratings.
As we noted earlier, the nature and causes of fragmentation of the health care are increasingly being examined (Sofaer 2009). Patterns of redundant specialty care have been demonstrated as patients with chronic disease frequently saw multiple generalists and specialists in different settings (Starfield et al. 2005; Kahn et al. 2007; Pham et al. 2007). Here, we describe how physicians interact in managing patients with complex diseases. Future studies should focus on physicians’ goals and expectations in patient co-management (Chen and Yee 2009; Forrest 2009).
Two barriers to physician co-management include financial and workforce issues. Pham et al. (2009) assert that the current health care delivery system places the burden of coordination of care on the primary care provider, without financial support of efforts needed to successfully coordinate care. From our work here, we believe that future studies on specialty physician reimbursement should also include incentives for time spent communicating and collaborating with other physicians. As the oncologist shortage emerges (Erikson et al. 2007), specialty physicians may be less willing or able to take time needed to co-manage care.
Limitations
The study is an observational cross-sectional analysis of data collected in Los Angeles County in 2004. We used clinical vignettes to identify differences in physicians’ approaches to a standardized patient (Dresselhaus et al. 2004). The use of clinical vignettes may be associated with physicians’ selection of socially desirable responses (Landon et al. 2001). However, we offered multiple responses options to physicians consistently avoiding a “correct” response, to lessen the possibility of social desirability bias. In addition, we did not validate physician report of any financial incentives to expand clinical practices or services to patients. Physicians may have under-reported financial incentives because they gave socially desirable (e.g., altruistic) responses. Nevertheless, we found no evidence of systematic bias in our responses, and physicians did respond affirmatively to questions about the presence of financial incentives that may impact their practice.
Conclusions
Within a few months of receiving a breast cancer diagnosis, many patients receive treatments from multiple providers. In these circumstances, where co-management could be expected to make a significant difference in patients’ experiences, our study found variation in physician practice style. To further understand the clinical relevance of this variation, we analyzed the relationship between physician practice style and patient outcomes. Our analyses found a positive relationship between physician co-management and patient ratings of care. This type of research provides much needed evidence about the relationship between physician practice style and patient outcomes, which is critical, as efforts to improve quality of health suggest that better outcomes are associated with better coordination of care.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was funded by the California Breast Cancer Research program grant 7PB-0126S and the National Cancer Institute and the Agency for Health Care Research and Quality grant 1-R01-CA81338-01A1. Dr. Tisnado received support from the University of California, Los Angeles, Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under NIH/NIA Grant P30-AG021684, and the content does not necessarily represent the official views of the NIA or the NIH. Dr. Rose received support from the UCLA Cancer Education and Career Development Program, National Cancer Institute grant R25 CA087949. We thank the two anonymous referees for suggestions that significantly strengthened the analyses. We acknowledge Diane Fitzpatrick and Ann Zisser for their data collection efforts (under supervision); Fang Ashlee Hu for programming and data management, and Judith Magee for administrative support. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosures: None.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Description of Samples for the Los Angeles Women's Health Study Patient and Provider Surveys.
Appendix SA3: Patient Ratings of Care Measure.
Appendix SA4: Multi-level, Bivariate Results Testing Associations between Physician Practice Style and Patient Ratings of Care.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aiello Bowles EJ, Tuzzio L, Wiese CJ, Kirlin B, Greene SM, Clauser SB, Wagner EH. Understanding High-Quality Cancer Care: A Summary of Expert Perspectives. Cancer. 2008;112(4):934–42. doi: 10.1002/cncr.23250. [DOI] [PubMed] [Google Scholar]
- Ayanian JZ, Zaslavsky AM, Guadagnoli E, Fuchs CS, Yost KJ, Creech CM, Cress RD, O'Connor LC, West DW, Wright WE. Patients’ Perceptions of Quality of Care for Colorectal Cancer by Race, Ethnicity, and Language. Journal of Clinical Oncology. 2005;23(27):6576–86. doi: 10.1200/JCO.2005.06.102. Epub August 22, 2005. [DOI] [PubMed] [Google Scholar]
- Carrier E, Gourevitch MN, Shah NR. Medical Homes: Challenges in Translating Theory into Practice. Medical Care. 2009;47(7):714–22. doi: 10.1097/MLR.0b013e3181a469b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AH, Yee HF., Jr Improving the Primary Care-Specialty Care Interface: Getting from Here to There. Archives of Internal Medicine. 2009;169(11):1024–6. doi: 10.1001/archinternmed.2009.140. [DOI] [PubMed] [Google Scholar]
- Chen J, Tao ML, Tisnado DM, Malin JL, Adams JA, Ganz PA, Kahn KL. Impact of Physician-Patient Discussions on Patient Satisfaction. Medical Care. 2008;46(11):1157–62. doi: 10.1097/MLR.0b013e31817924bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Malin J, Ganz PA, Ko C, Trisnado D, Tao ML, Timmer M, Adams JL, Kahn KL. Variation in Physician-Patient Discussion of Breast Reconstruction. Journal of General Internal Medicine. 2009;24(1):99–104. doi: 10.1007/s11606-008-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and Testing of a Measure Designed to Assess the Quality of Care Transitions. International Journal of Integrated Care. 2002;2:e02. doi: 10.5334/ijic.60. Epub June 1, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman EA, Parry C, Chalmers S, Min SJ. The Care Transitions Intervention: Results of a Randomized Controlled Trial. Archives of Internal Medicine. 2006;166(17):1822–8. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- Colwill JM. Narrative Matters. A Case of ‘Medical Homelessness’. Health Affairs (Millwood) 2010;29(5):1067–70. doi: 10.1377/hlthaff.2009.0224. [DOI] [PubMed] [Google Scholar]
- Committee on Quality of Health Care in America, Institute of Medicine, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Conrad DA, Christianson JB. Penetrating the “Black Box”: Financial Incentives for Enhancing the Quality of Physician Services. Medical Care Research and Review. 2004;61(3 suppl):37S–68S. doi: 10.1177/1077558704266770. [DOI] [PubMed] [Google Scholar]
- Dresselhaus TR, Peabody JW, Luck J, Bertenthal D. An Evaluation of Vignettes for Predicting Variation in the Quality of Preventive Care. Journal of General Internal Medicine. 2004;19(10):1013–8. doi: 10.1007/s11606-004-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle CC, Neville NA. Under Use of Necessary Care among Cancer Survivors. Cancer. 2004;101(8):1712–9. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of Non-breast Cancer Health Maintenance among Elderly Breast Cancer Survivors. Journal of Clinical Oncology. 2003;21(8):1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future Supply and Demand for Oncologists: Challenges to Assuring Access to Oncology Services. Journal of Oncology Practice. 2007;3(2):79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest CB. A Typology of Specialists’ Clinical Roles. Archives of Internal Medicine. 2009;169(11):1062–8. doi: 10.1001/archinternmed.2009.114. [DOI] [PubMed] [Google Scholar]
- Forrest CB, Glade GB, Baker AE, Bocian AB, Kang M, Starfield B. The Pediatric Primary-Specialty Care Interface: How Pediatricians Refer Children and Adolescents to Specialty Care. Archives of Pediatrics and Adolescent Medicine. 1999;153(7):705–14. doi: 10.1001/archpedi.153.7.705. [DOI] [PubMed] [Google Scholar]
- Gandhi TK, Sittig DF, Franklin M, Sussman AJ, Fairchild DG, Bates DW. Communication Breakdown in the Outpatient Referral Process. Journal of General Internal Medicine. 2000;15(9):626–31. doi: 10.1046/j.1525-1497.2000.91119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Hahn EE. Implementing a Survivorship Care Plan for Patients with Breast Cancer. Journal of Clinical Oncology. 2008;26(5):759–67. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- Grumbach K, Osmond D, Vranzian, Jaffe D, Bindman AB. Primary Care Physicians’ Experiences of Financial Incentives in Managed-Care Systems. New England Journal of Medicine. 1998;339:1516–21. doi: 10.1056/NEJM199811193392106. [DOI] [PubMed] [Google Scholar]
- Hadley J, Mitchell JM, Sulmasy DP, Bloche MG. Perceived Financial Incentives, HMO Market Penetration, and Physicians’ Practice Styles and Satisfaction. Health Services Research. 1999;34(1 Pt 2):307–21. [PMC free article] [PubMed] [Google Scholar]
- Hewitt M, Greenfield S, Stovall E. Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- Hong S, Nekhlyudov L, Didwania A, Olopade O, Ganschow P. Cancer Survivorship Care: Exploring the Role of the General Internist. Journal of General Internal Medicine. 2009;24(suppl 2):S495–500. doi: 10.1007/s11606-009-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn KL, Malin JL, Adams J, Ganz PA. Developing a Reliable, Valid, and Feasible Plan for Quality-of-Care Measurement for Cancer: How Should We Measure? Medical Care. 2002;40:III-73–85. doi: 10.1097/00005650-200206001-00011. [DOI] [PubMed] [Google Scholar]
- Kahn KL, MacLean CH, Liu H, Rubenstein LZ, Wong AL, Harker JO, Chen WP, Fitzpatrick DM, Bulpitt KJ, Traina SB, Mittman BS, Hahn BH, Paulus HE. The Complexity of Care for Patients with Rheumatoid Arthritis: Metrics for Better Understanding Chronic Disease Care. Medical Care. 2007;45(1):55–65. doi: 10.1097/01.mlr.0000237425.58554.66. [DOI] [PubMed] [Google Scholar]
- Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, Organizational, and Market Level Influences on Physicians’ Practice Patterns: Results of a National Survey of Primary Care Physicians. Medical Care. 2001;39(8):889–905. doi: 10.1097/00005650-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Regression Analysis for Correlated Data. Annual Review of Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Morales LS, Elliott M, Spritzer K, Hays RD. Confirmatory Factor Analysis of the Consumer Assessment of Health Plans Study (CAHPS) 1. 0 Core Survey. Psychological Assessment. 2001;13(2):216–29. doi: 10.1037//1040-3590.13.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley AS, Tynan A, Cohen GR, Kemper N, Davis MM. Coordination of Care by Primary Care Practices: Strategies, Lessons and Implications. Research Briefs. 2009;April(12):1–16. [PubMed] [Google Scholar]
- Pearson ML, Ganz PA, McGuigan K, Malin JR, Adams J, Kahn KL. The Case Identification Challenge in Measuring Quality of Cancer Care. Journal of Clinical Oncology. 2002;20(21):4353–60. doi: 10.1200/JCO.2002.05.527. [DOI] [PubMed] [Google Scholar]
- Pham HH. Good Neighbors: How Will the Patient-Centered Medical Home Relate to the Rest of the Health-Care Delivery System? Journal of General Internal Medicine. 2010;25(6):630–4. doi: 10.1007/s11606-009-1208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care Patterns in Medicare and Their Implications for Pay for Performance. New England Journal of Medicine. 2007;356(11):1130–9. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- Pham HH, O'Malley AS, Bach PB, Saiontz-Martinez C, Schrag D. Primary Care Physicians’ Links to Other Physicians through Medicare Patients: The Scope of Care Coordination. Annals of Internal Medicine. 2009;150(4):236–42. doi: 10.7326/0003-4819-150-4-200902170-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschovsky JD, Hadley J, Landon BE. Effects of Compensation Methods and Physician Group Structure on Physicians’ Perceived Incentives to Alter Services to Patients. Health Services Research. 2006;41(4 Pt 1):1200–20. doi: 10.1111/j.1475-6773.2006.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt RA, Hart LG, Baldwin LM, Chan L, Schneeweiss R. The Generalist Role of Specialty Physicians: Is There a Hidden System of Primary Care? Journal of the American Medical Association. 1998;279(17):1364–70. doi: 10.1001/jama.279.17.1364. [DOI] [PubMed] [Google Scholar]
- Scher KS, Tisnado DM, Rose DE, Adams JL, Ko CY, Malin JL, Ganz PA, Kahn KL. Physician and Practice Characteristics Influencing Tumor Board Attendance: Results from the Provider Survey of the Los Angeles Women's Health Study. Journal of Oncology Practice. 2011;7(2):103–10. doi: 10.1200/JOP.2010.000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag D, Xu F, Hanger M, Elkin E, Bickell NA, Bach PB. Fragmentation of Care for Frequently Hospitalized Urban Residents. Medical Care. 2006;44(6):560–7. doi: 10.1097/01.mlr.0000215811.68308.ae. [DOI] [PubMed] [Google Scholar]
- Smith SM, Allwright S, O'Dowd T. Effectiveness of Shared Care across the Interface between Primary and Specialty Care in Chronic Disease Management. Cochrane Database of Systematic Reviews. 2007;July 18(3):CD004910. doi: 10.1002/14651858.CD004910.pub2. [DOI] [PubMed] [Google Scholar]
- Smith SM, Allwright S, O'Dowd T. Does Sharing Care Across the Primary-Specialty Interface Improve Outcomes in Chronic Disease? A Systematic Review. American Journal of Managed Care. 2008;14(4):213–24. [PubMed] [Google Scholar]
- Snyder CF, Frick KD, Kantsiper ME, Peairs KS, Herbert RJ, Blackford AL, Wolff AC, Earle CC. Prevention, Screening, and Surveillance Care for Breast Cancer Survivors Compared with Controls: Changes from 1998 to 2002. Journal of Clinical Oncology. 2009;27:1054–61. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofaer S. Navigating Poorly Charted Territory: Patient Dilemmas in Health Care ‘nonsystems’. Medical Care Research. 2009;66(1 Suppl):75S–93S. doi: 10.1177/1077558708327945. [DOI] [PubMed] [Google Scholar]
- Starfield B, Lemke KW, Herbert R, Pavlovich WD, Anderson G. Comorbidity and the Use of Primary Care and Specialist Care in the Elderly. Annals of Family Medicine. 2005;3(3):215–22. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating Care Across Diseases, Settings, and Clinicians: A Key Role for the Generalist in Practice. Annals of Internal Medicine. 2005;142(8):700–8. doi: 10.7326/0003-4819-142-8-200504190-00038. [DOI] [PubMed] [Google Scholar]
- Tisnado DM, Rose-Ash DE, Malin JL, Adams JL, Ganz PA, Kahn KL. Financial Incentives for Quality in Breast Cancer Care. American Journal of Managed Care. 2008;14(7):457–66. [PMC free article] [PubMed] [Google Scholar]
- Tisnado DM, Malin JL, Tao ML, Ganz P, Rose-Ash D, Hu AF, Adams J, Kahn KL. The Structural Landscape of the Health Care System for Breast Cancer Care: Results from the Los Angeles Women's Health Study. Breast Journal. 2009;15(1):17–25. doi: 10.1111/j.1524-4741.2008.00666.x. Epub 2008 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Malin JL, Tisnado DM, Tao ML, Adams JL, Timmer MJ, Ganz PA, Kahn KL. Symptom Management after Breast Cancer Treatment: Is It Influenced by Patient Characteristics? Breast Cancer Research and Treatment. 2008a;108(1):69–77. doi: 10.1007/s10549-007-9580-1. [DOI] [PubMed] [Google Scholar]
- Yoon J, Malin JL, Tao ML, Tisnado DM, Adams JL, Timmer MJ, Ganz PA, Kahn KL. Symptoms after Breast Cancer Treatment: Are They Influenced by Patient Characteristics? Breast Cancer Research and Treatment. 2008;108(2):153–65. doi: 10.1007/s10549-007-9599-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


