Abstract
After an infection, cytotoxic T lymphocyte precursors proliferate and become effector cells by recognizing foreign peptides in the groove of major histocompatibility complex (MHC) class I molecules expressed by antigen-presenting cells (APCs)1. Professional APCs specialized for T-cell activation acquire viral antigen either by becoming infected themselves (direct presentation) or by phagocytosis of infected cells, followed by transfer of antigen to the cytosol, processing and MHC class I loading in a process referred to as cross-presentation2. An alternative way, referred to as ‘cross-dressing’, by which an uninfected APC could present antigen was postulated to be by the transfer of preformed peptide–MHC complexes from the surface of an infected cell to the APC without the need of further processing3. Here we show that this mechanism exists and boosts the antiviral response of mouse memory CD8+ T cells. A number of publications have demonstrated sharing of peptide-loaded MHC molecules in vitro4–7. Our in vitro experiments demonstrate that cross-dressing APCs do not acquire peptide–MHC complexes in the form of exosomes released by donor cells. Rather, the APCs and donor cells have to contact each other for the transfer to occur. After a viral infection, we could isolate cross-dressed APCs able to present viral antigen in vitro. Furthermore, using the diphtheria toxin system to selectively eliminate APCs that could only acquire viral peptide–MHC complexes by cross-dressing, we show that such presentation can promote the expansion of resting memory T cells. Notably, naive T cells were excluded from taking part in the response. Cross-dressing is a mechanism of antigen presentation used by dendritic cells that may have a significant role in activating previously primed CD8+ T cells.
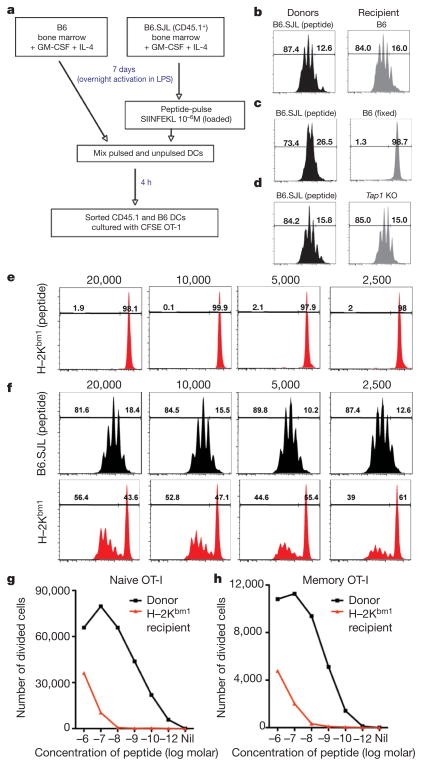
We investigated the capacity of dendritic cells to use peptide–MHC I complexes acquired from other cells to drive CD8+ T-cell activation and expansion. Bone-marrow-derived dendritic cells (BMDCs) from B6.SJL-PtprcaPep3/BoyJ (B6.SJL) mice pulsed with SIINFEKL peptide (donor) were mixed with BMDCs from B6.GFP mice (recipient). After a 4-h incubation period the dendritic cell populations were separated using the markers CD45.1 and green fluorescent protein (GFP) to distinguish donor and recipient populations (Supplementary Fig. 1). Sorted dendritic cells were cultured with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled OT-I TCR transgenic cells, which express a TCR recognizing SIINFEKL in the context of H–2Kb (Fig. 1a). Although only the CD45.1+ donor dendritic cells were pulsed with peptide, both dendritic cell populations could drive OT-I division (Fig. 1b). Fixation of the recipient dendritic cells before mixing with peptide-loaded donor dendritic cells abolished their ability to drive OT-I division (Fig. 1c). Antigen transfer could also occur when recipient dendritic cells were deficient in transporter associated with antigen processing (TAP), indicating that it is not conventional cross-presentation (Fig. 1d).
Figure 1. Transfer of peptide-loaded class I molecules between dendritic cells in vitro.
a, Scheme of the experiment. DC, dendritic cell; GM-CSF, granulocyte macrophage colony-stimulating factor. b–d, In-vitro-generated bone-marrow-derived B6.SJL dendritic cells were peptide pulsed and cultured with unloaded bone-marrow-derived B6.GFP dendritic cells (b), fixed B6.GFP dendritic cells (c) or Tap1−/− dendritic cells (d). Dendritic cells were separated by cell sorting and 2 ×104 cells were cultured with CFSE-labelled OT-I cells for 60 h. Representative flow cytometry profiles are depicted. e, H–2Kbm1 dendritic cells were pulsed with peptide before culture with CFSE-labelled OT-I cells. f, Peptide-pulsed B6.SJL dendritic cells were cultured with unloaded H–2Kbm1 dendritic cells. Dendritic cells were then separated and cultured with CFSE-labelled OT-I cells. Representative flow cytometry profiles are shown. The numbers above the plots indicate the number of dendritic cells cultured per well. Bone-marrow-derived B6.GFP dendritic cells were pulsed with varying concentrations of SIINFEKL peptide and cultured with unloaded H–2Kbm1 dendritic cells. g, h, Dendritic cells were then separated and 4 ×104 cells were cultured with CFSE-labelled naive (g) or memory (h) OT-I cells for 60 h. The absolute numbers of divided cells are shown.
It was unclear whether the recipient dendritic cells were merely acquiring free peptide from the culture or if they were ‘stealing’ an intact MHC I–peptide complex. Thus, we repeated the above experiment only this time B6.SJL peptide-loaded dendritic cells were mixed with dendritic cells from H–2Kbm1 mice (Supplementary Fig. 2). H–2Kbm1 mice bear a mutation in the Kb molecule and although SIINFEKL can bind it is not recognized by OT-I T cells (Fig. 1e). Nonetheless, culturing H–2Kbm1 dendritic cells with peptide-loaded B6.SJL donor dendritic cells for a short period before purification resulted in OT-I T cell division (Fig. 1f). Once again, fixation of H–2Kbm1 dendritic cells before mixing with peptide-loaded donor dendritic cells abolished their ability to drive T-cell division, indicating that it was not simply contaminating donor dendritic cells that initiated OT-I expansion (Supplementary Fig. 3). Therefore it seems that H–2Kbm1 dendritic cells drive OT-I division due to their ability to acquire peptide-loaded class I molecules from other dendritic cells. Titrating the amount of peptide used to load donor dendritic cells showed that the recipient ‘cross-dressed’ dendritic cells were about three orders of magnitude less efficient than the donor dendritic cells in driving naive and memory T-cell proliferation (Fig. 1g, h and Supplementary Fig. 4).
Exosomes are membrane vesicles of endocytic origin that are secreted by a range of cell types, including dendritic cells. Fusion of exosomes with a target cell membrane could facilitate the exchange of membrane proteins between two cell types8,9. We explored if exosomes served as the means by which dendritic cells acquired loaded class I molecules in culture. Exosomes isolated from the supernatants of SIINFEKL peptide-loaded BMDCs were attached to beads and their phenotype assessed by flow cytometry. Exosomes stained positive for CD9, a member of the tetraspanin protein family routinely found on the surface of dendritic-cell-derived exosomes and MHC class II molecules. An antibody that recognizes SIINFEKL in the groove of H–2Kb revealed that they also express peptide-loaded MHC class I molecules (Supplementary Fig. 5). Exosomes recovered from the supernatant of B6 dendritic cells pulsed with SIINFEKL peptide were added to cultures containing CFSE-labelled OT-I cells and either B6 or H–2Kbm1 dendritic cells. Exosomes alone as well as exosomes in the presence of B6 dendritic cells resulted in OT-I division (Fig. 2a). Interestingly, the addition of H–2Kbm1 dendritic cells to the exosome-T-cell culture resulted in the ablation of OT-I division. We speculate that this is due to the H–2Kbm1 dendritic cells engulfing exosomes and degrading the contents or reloading the peptide onto their own, non-presenting class I molecule. To explore this possibility further, we isolated exosomes from the supernatant of peptide-pulsed H–2Kbm1 dendritic cells. Exosomes from H–2Kbm1 dendritic cells alone failed to drive OT-I division (Fig. 2a). However, feeding these exosomes to B6 dendritic cells resulted in OT-I T cell division, indicating that dendritic cells can use exosomes as a source of antigen but do this by reloading the peptide onto their endogenous MHC class I molecules. Hence, exosomes are not the means by which intact MHC class I–peptide complexes are picked up by dendritic cells, but may serve as an effective means to transfer peptide antigen between cells.
Figure 2. MHC I–peptide transfer does not require exosomes but does require cell contact between donor and recipient cells.
a, Exosomes recovered from the supernatants of peptide-pulsed B6 or H–2Kbm1 dendritic cells were incubated alone (exosomes alone) or with B6 dendritic cells or H–2Kbm1 dendritic cells and cultured with CFSE-labelled OT-I cells for 60 h. Representative flow cytometry profiles are shown. b, Dendritic cells labelled with either CMFDA (cytoplasmic dye (green)) or Dil (membrane dye (red)) were cultured together. A representative frame is shown. c, d, B6.SJL peptide-pulsed dendritic cells were cultured with unloaded H–2Kbm1 dendritic cells mixed together (c; −transwell) or separated by a transwell (d; +transwell). Dendritic cells were then separated by cell sorting and cultured with CFSE-labelled OT-I cells for 60 h. Representative flow cytometry profiles are shown. Numbers above the plots indicate the number of dendritic cells cultured per well.
Dendritic cells may acquire preformed peptide–MHC complexes from neighbouring cells using a mechanism termed trogocytosis10–13. Consistent with a previous study12, we visualized membrane transfer during live imaging of cultures containing dendritic cells labelled with either a membrane (red) or a cytoplasmic dye (green) (Fig. 2b and Supplementary Movie 1). To investigate whether trogocytosis was the means by which dendritic cells acquired peptide-loaded MHC class I molecules, we used a transwell system with peptide-loaded B6 dendritic cells in one chamber and unloaded H–2Kbm1 dendritic cells in the other to see if this separation would alter the ability of the H–2Kbm1 dendritic cells to drive OT-I division. Physical separation of donor and recipient dendritic cells prevented MHC I–peptide acquisition by the H–2Kbm1 dendritic cells (Fig. 2c, d). Hence, dendritic cells acquire MHC class I–peptide complexes capable of driving CD8+ T-cell activation via a mechanism that requires cell contact.
We next wanted to determine if dendritic cells could act as cross-dressing APCs in vivo by acquiring class I–peptide complexes derived during a viral infection. We generated bone marrow chimaeras where we injected B6.GFP bone marrow into lethally irradiated F1[BALB/c × C57BL/6] mice. In this chimaera, bone-marrow-derived cells are of B6 origin and can be readily identified and sorted based on GFP expression, whereas the parenchyma cells are of F1 origin and express both B6 and BALB/c MHC molecules. F1[BALB/c × C57BL/6] mice receiving B6.GFP bone marrow were infected with lymphocytic choriomeningitis virus (LCMV) and on day 2 and 3 after infection CD11c+GFP+ cells were sorted from the spleen and cultured with a T-cell hybridoma specific for the BALB/c H–2Ld restricted LCMV epitope NP(118–226). As negative and positive controls, respectively, CD11c+ cells were sorted from B6 mice receiving B6.GFP bone marrow and from BALB/c mice. CD11c+GFP+ dendritic cells isolated from LCMV-infected F1[BALB/c ×C57BL/6] mice receiving B6.GFP bone marrow could drive IL-2 production to a level 6-fold above background levels generated when CD11c+ cells were recovered from LCMV-infected B6 mice receiving B6.GFP bone marrow (where there is no available source of H–2Ld) (Fig. 3 and Supplementary Fig. 6a). This ability to present to the hybridoma was limited to CD11c+cells, as isolated CD11c−GFP+cells from the same animals were unable to stimulate the Ld restricted hybridoma (Supplementary Fig. 6b, c). Furthermore, dendritic cell subsets sorted from LCMV-infected F1[BALB/c × C57BL/6] mice receiving B6.GFP bone marrow revealed that CD8α− dendritic cells were superior to their CD8α+ counterparts at antigen presentation via cross-dressing (Supplementary Fig. 7), which is consistent with previous in vitro studies7.
Figure 3. Dendritic cells can acquire MHC I–peptide in vivo after viral infection.
B6 mice receiving B6.GFP bone marrow (B6.GFP→B6), F1(BALB/c ×B6) mice receiving B6.GFP bone marrow (B6.GFP→F1), and BALB/c mice were infected with LCMV and on day 2 after infection CD11c+cells were sorted from the spleen and cultured with an Ld–NP(118–226)-specific hybridoma. The amount of IL-2 produced was determined by ELISA. Error bars represent the mean + s.e.m. pooled from eight independent experiments.
To investigate whether cross-dressing functions to drive a CD8+ T-cell response in vivo, we generated another set of bone marrow chimaeras. BALB/c.CD11cDTR bone marrow was used to reconstitute lethally irradiated F1[BALB/c × C57BL/6] mice, creating a setting where dendritic cells are of BALB/c origin, express only H–2d class I molecules and are removable with diphtheria toxin treatment, whereas the parenchyma is of F1 origin and expresses both H–2d and H–2b MHC molecules. After reconstitution we adoptively transferred equal numbers of naive OT-I.CD45.1 and memory OT-I.GFP+ T cells into these mice. The mice were infected with recombinant vesicular stomatitis virus that expresses ovalbumin (VSV-OVA) and either treated with diphtheria toxin to ablate CD11c+ BALB/c-derived cells or left untreated. On day 7 after infection we determined if the H–2Kb-restricted OT-I T cells expanded in an environment where bone-marrow-derived APCs are of BALB/c origin.
Memory OT-I T cells expanded markedly in the intact, virus-infected F1[BALB/c × C57BL/6] mice receiving BALB/c.CD11cDTR bone marrow, with the OT-I cells accumulating to ~8.5% of the total CD8+ T-cell population in the spleen (Fig. 4a, b). Importantly, this expansion was not the result of direct antigen presentation by infected F1 parenchyma cells or due to direct or classical cross-presentation by radio-resistant F1 APCs, because when we ablated the BALB/c bone-marrow-derived CD11c+cells using diphtheria toxin treatment, OT-I expansion was greatly attenuated. It is unlikely that this effect is due to the acquisition of the diphtheria toxin receptor (DTR) either by the T cells themselves or by non-professional APCs capable of stimulating memory T cells, because in vitro co-cultures of peptide-loaded CD11cDTR dendritic cells with DTR− dendritic cells, or with naive or memory OT-I T cells, did not render the non-transgenic cells susceptible to diphtheria toxin (Supplementary Fig. 8).
Figure 4. Cross-dressed dendritic cells in VSV-infected mice stimulate memory CD8+ T cells.
B6 mice or F1(BALB/c × B6) mice receiving BALB/c.CD11cDTR bone marrow (BALB/c.CD11cDTR→F1) were seeded with naive OT-I.CD45.1 and memory OT-I.GFP T cells before infection with VSV-OVA. BALB/c.CD11cDTR→F1 chimaeras were treated with diphtheria toxin (+) or were left untreated (−). On day 7 after infection, spleens were recovered and the proportion of OT-I cells of the total CD8+ T-cell population was determined by flow cytometry. a, Representative flow cytometry profiles of the spleen gated on CD8+ T cells. b, c, The absolute numbers of memory OT-I.GFP (b) and naive OT-I.CD45.1 (c) T cells in the spleen on day 7 after infection are shown. Data are pooled from two independent experiments with 4–5 mice per group. Shown is the mean + s.e.m. d, Naive (CD45.1) or memory (GFP+) OT-I T cells were adoptively transferred into mice before infection with VSV-OVA. Representative pictures of the spleen on day 2 after infection showing staining for B220 (purple), CD11c (blue), CD45.1 (naive OT-I; yellow) and GFP (memory OT-I; green) are shown.
Remarkably, naive OT-I T-cell numbers in the same animals were not affected by the presence or absence of BALB/c APCs (Fig. 4a, c). The naive T cells may be activated by recognizing antigen expressed either on residual, radio-resistant F1 dendritic cells or on infected parenchymal cells. But it is clear that the naive CD8+ T cells are not able to respond to cross-dressed antigen presentation in this in vivo model. This was not due to the naive T cells being out-competed by memory OT-I T cells because even when we transferred the naive T cells alone they failed to respond to this form of presentation (Supplementary Fig. 9). Although memory and naive OT-I T cells seem to reside in similar locations within the spleen in this experimental set-up (Fig. 4d and Supplementary Fig. 10), memory T cells may be more responsive to cross-dressed antigen presentation due to more frequent and productive interactions with the dendritic cell subset actively engaged in trogocytosis. In addition, the differential response of naive and memory T cells may reflect a disparity in their epitope density requirements for activation in vivo, considering that cross-dressed antigen presentation is likely to be extremely low density (see Supplementary Fig. 4).
Cross-dressing serves as an alternative mode of antigen presentation to memory T cells during viral infection. It eliminates the need for antigen processing by the presenting dendritic cell and allows the prompt presentation of peptide epitopes that very accurately reflect those expressed on infected cells.
METHODS SUMMARY
Bone-marrow-derived dendritic cells were generated and matured as described14. Dendritic cells loaded with OVA peptide (donors) were mixed at a 1:1 ratio with unloaded dendritic cells (recipient) for 4 h at 37 °C. Dendritic cells were separated by cell sorting and cultured with 2 × 104 CFSE-labelled naive OT-I.CD45.1 T cells. Cultures were analysed for proliferation after 60 h.
Naive OT-I cells were purified from pooled spleen and lymph node prepared from OT-I mice by depletion of non-CD8+ cells using a MACs CD8 enrichment kit (Miltenyi Biotec) following the manufacturer’s instructions.
Mice were infected intranasally with 5 × 104 plaque-forming units (p.f.u.) of recombinant vesicular stomatitis virus that expresses GFP and a truncated form of OVA15. The lymphocytic choriomeningitis virus (LCMV) Armstrong 53b was grown and titred as described previously16. Mice were infected intraperitoneally with 2 × 105 p.f.u. of LCMV Armstrong 53b.
Exosomes were isolated following the procedure described in detail previously17.
To visualize membrane transfer, dendritic cells were labelled with the lipophilic probe l’dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil; Molecular probes) or the thiol-reactive chloromethyl probe 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen). A mixture of cells labelled with Dil or CMFDA were introduced into an ibiTreat, 35-mm μ-Dish (Ibidi GmbH) and images were collected every 4 min for each of the illumination conditions.
The T-cell hybridoma specific for the Ld restricted NP(118–226) LCMV immunodominant epitope was prepared by fusing T cells from LCMV-primed BALB/c mice with BWZ.36/CD8 (ref. 18). For analysis of antigen presentation, 1 × 105 dendritic cells were cultured with 1 × 105 hybridoma cells in a U-bottom 96-well plate. Secretion of IL-2 was measured by ELISA.
Supplementary Material
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and National Institutes of Health. L.M.W is supported by an Overseas Biomedical Fellowship from the National Health and Medical Research Council of Australia.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions L.M.W. and M.J.B. devised experiments and wrote the paper. L.M.W. performed the work.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nature Rev Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 3.Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- 4.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 5.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Tumor-specific CD4+ T cells are activated by “cross-dressed” dendritic cells presenting peptide-MHC class II complexes acquired from cell-based cancer vaccines. J Immunol. 2006;176:1447–1455. doi: 10.4049/jimmunol.176.3.1447. [DOI] [PubMed] [Google Scholar]
- 6.Qu C, Nguyen VA, Merad M, Randolph GJ. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol. 2009;182:3650–3659. doi: 10.4049/jimmunol.0801532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth LA, et al. The relative efficiency of acquisition of MHC:peptide complexes and cross-presentation depends on dendritic cell type. J Immunol. 2008;181:3212–3220. doi: 10.4049/jimmunol.181.5.3212. [DOI] [PubMed] [Google Scholar]
- 8.Théry C, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nature Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 9.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 10.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nature Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 11.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 12.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 13.Matheoud D, et al. Cross-presentation by dendritic cells from live cells induces protective immune responses in vivo. Blood. 2010;115:4412–4420. doi: 10.1182/blood-2009-11-255935. [DOI] [PubMed] [Google Scholar]
- 14.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrancois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J Exp Med. 2008;205:1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3(Unit 3.22) doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson S, Shastri N. LacZinducible, antigen/MHC-specific T cellhybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.