Abstract
Injury caused by oxidative stress occurs in many clinical scenarios involving ischemia and reperfusion such as organ transplantation, hemorrhagic shock (HS), myocardial infarction and cerebral vascular accidents. Activation of the immune system as a result of disturbances in the redox state of cells appears to contribute to tissue and organ damage in these conditions. The link between oxidative stress and inflammatory pathways is poorly understood. Recently, Toll like receptors (TLRs) have been shown to mediate the inflammatory response seen in experimental ischemia and reperfusion (I/R). The TLR family of receptors involved in alerting the innate immune system of danger appears to be activated by damage associated molecular pattern molecules (DAMPs) that are released during condition of oxidative stress. In this review, we will examine the role of TLRs in various experimental models of oxidative stress such as HS and I/R. We will also report on potential DAMPs that may interact with TLRs in mediating injury. Finally, potential mechanisms by which reactive oxygen species from NADPH oxidase can signal the commencement of inflammatory pathways through TLRs will be explored.
Keywords: toll-like receptor (TLR), oxidative stress, inflammation, redox, ischemia-reperfusion, hemorrhagic shock, damage associated molecular pattern (DAMP)
Introduction
Disturbances in the reduction-oxidation (redox) equilibrium of tissues can lead to a proinflammatory state, classically seen in conditions such as ischemia reperfusion (I/R) or hemorrhagic shock (HS) induced injury. The mechanisms by which redox stress can activate an inflammatory response have not been fully elucidated. However, there is accumulating evidence that pattern recognition receptors of the innate immune system such as toll-like receptors (TLRs) may be involved in mediating this response. Multiple studies have shown the in vivo requirement of TLR signaling in mediating injury from oxidative stress in I/R or HS induced injury (Table 1).
Table 1.
In vivo studies on the role of TLRs in Ischemia / Reperfusion (I/R) Injury and Hemorrhagic Shock / Resuscitation (HS/R)
| Publication | Model | Findings |
|---|---|---|
| DeMaria J Trauma 1993 | Systemic HS/R | TLR4 deficient mice had lower levels of TNF-α and improved five day survival and survival time compared to TLR4 competent mice exposed to HS/R. |
| Pellicane J Surg Res 1994 | Systemic HS/R | TLR4 deficiency was associated with reduced TNF-α and faster return of lactate levels to baseline following HS/R. |
| Frink Mol Immunol 2007 | Trauma HS/R | Laparotomy and HS/R in TLR4 deficient mice showed attenuated levels of chemokines in plasma, lung tissue and Kupffer cell supernatants, and reduced lung neutrophil infiltration compared to TLR4 competent controls. |
| Prince J Am Coll Surg 2006 | Liver in HS/R | HS/R induced elevation in serum ALT, IL-6 and IL-10 were attenuated in TLR4 deficient mice, as were hepatic NF-κB activation and hepatic TNF-α, IL-10, and iNOS mRNA levels. |
| TLR2 deficient and CD14 deficient mice were not protected against HS/R induced liver injury or systemic inflammation. | ||
| Meng Am J Physiol Regul Integr Comp Physiol 2005 | Myocardium in HS | HS without resuscitation resulted in increased plasma and myocardial TNF-α and depressed myocardial contractility, which were both attenuated in TLR4 deficient mice. |
| Barsness Am J Physiol Regul Integr Comp Physiol 2004 | Lung in HS | Hemorrhage-induced lung injury was associated with lower levels of TNF-α, less neutrophil accumulation, and reduced protein permeability in TLR4 deficient mice. |
| Chen Shock 2008 | Lung HS/R | TLR4 deficiency was associated with reduced acute lung injury as evidenced by lower levels of IL-10, myeloperoxidase, and HO-1, and lower p38 MAPK activation, in response to HS/R. |
| Wu Hepatobiliary Pancreat Dis Int 2004 | Liver I/R | TLR4 deficient mice had lower systemic levels of AST and TNF-α, as well as lower hepatic TNF-α mRNA and myeloperoxidase after hepatic I/R compared to TLR4 competent mice. |
| Zhai J Immunol 2004 | Liver I/R | TLR4 deficient but not TLR2 deficient mice were protected against liver I/R injury as assessed by liver function, histology, and local cytokine / chemokine production. |
| Hepatocellular damage from I/R mediated by TLR4 required IRF3 but not MyD88 signaling. | ||
| Shen Am J Transplant 2005 | Liver I/R | TLR4 deficient mice had reduced I/R injury by liver function, histology, neutrophil infiltration, and TNF-α production compared to TLR2 deficient or wild type mice. |
| TLR4 deficiency was associated with increased HO-1 production, and tin protoporphyrin-mediated HO-1 inhibition removed protection against I/R induced hepatic damage in TLR4 deficiency. | ||
| Tsung J Exp Med 2005 | Liver I/R | TLR4 deficient mice had reduced damage in hepatic I/R, and neutralizing antibody to HMGB1 failed to protect TLR4 deficient mice from hepatic I/R injury but did protect TLR4 competent mice. In wild type mice, administration of recombinant HMGB1 worsened I/R injury. |
| Tsung J Immunol 2005 | Liver I/R | Chimeric wild type mice bearing TLR4 mutant hemopoietic cells and TLR4 mutant mice transplanted with their own bone marrow were protected against hepatic I/R injury, but TLR4 mutant mice transplanted with wild type bone marrow exhibited injury from I/R. Additionally, depletion of phagocytes reduced injury in wild type mice but did not afford additional protection to TLR4 mutant mice. |
| Tsung J Leukoc Biol 2007 | Liver I/R | Increasing DC numbers worsened I/R injury in wild type mice but not TLR4 deficient mice. |
| Tsung J Exp Med 2007 | Liver I/R | Hypoxia-induced HMGB1 release by hepatocytes was mediated by TLR4 dependent ROS production and downstream CaMK signaling. Antioxidant treatment or CaMK inhibition in vivo both reduced hepatic I/R injury in wild type mice but not TLR4 deficient mice. |
| Shishido Circulation 2003 | Cardiac I/R | TLR2 deficient mice had improved survival compared to wild type mice in response to myocardial infarction. They had reduced TFG-β1 and collagen type 1 mRNA, and reduced myocardial fibrosis in the non-infarct area accompanied by higher fractional shortening. Left ventricular dimensions at end diastole were smaller in TLR2 deficiency after injury. |
| Oyama Circulation 2004 | Cardiac I/R | TLR4 deficient mice had smaller infarcts, less neutrophil infiltration, fewer lipid peroxides, and less complement deposition in the myocardium following I/R compared to wild type mice. Systemic IL-12 and INF-γ levels were lower in TLR4 deficient mice after I/R. |
| Kaczorowski Transplantation 2007 | Cardiac I/R | A cardiac cold I/R model performed by transplantation of hearts between TLR4 deficient and wild type mice showed intermediate levels of serum IL-6 and MCP-1, and intragraft TNF- α, IL-6, IL-1β and ICAM-1 mRNA levels were found in TLR4 deficient to wild type or wild type to TLR4 deficient transplants, compared with low levels for TLR4 deficient to TLR4 deficient mice and high levels for wild type to wild type transplantation. |
| Kim BMC Physiol 2007 | Cardiac I/R | TLR4 deficiency was associated with reduced infarct size and inflammatory cytokines, but also reduced left ventricular developed pressure, and echocardiography showed no functional difference from wild type. |
| Hua J Immunol 2007 | Cardiac I/R | Protection against myocardial I/R in TLR4 deficient mice was abrogated by use of pharmacological inhibitors of PI3k. |
| Favre Arterioscler Thromb Vasc Biol 2007 | Cardiac I/R | TLR2 deficient mice had smaller infarct size, reduced reperfusion-associated production of ROS, and reduced leukocyte infiltration. |
| Chimeric studies showed a role for both parenchymal and myeloid cells in protection against I/R induced coronary endothelial dysfunction. | ||
| Shimamato Ann Thorac Surg 2006 | Lung I/R | TLR4 deficiency reduces vascular permeability, lung MPO, and leukocyte accumulation in broncho-alveolar lavage fluid, associated with reduced pro-inflammatory cytokine levels following lung I/R. |
| Leemans J Clin Invest 2005 | Renal I/R | TLR2 deficient mice had reduced levels of local cytokines and chemokines, leukocytes, and renal injury and dysfunction from I/R compared to wild type mice. |
| Chimeric studies showed that TLR2 expression on parenchymal cells was necessary for induction of inflammation from I/R. | ||
| Shigeoka J Immunol 2007 | Renal I/R | TLR2 deficient mice were better protected from ischemic renal injury than MyD88 deficient mice. There was no significant difference between TRIF deficient and wild type mice in response to ischemic injury. |
| Wu J Clin Invest 2007 | Renal I/R | TLR4 deficient and MyD88 deficient mice were protected against renal I/R injury as seen by less tubular dysfunction or damage, reduced proinflammatory cytokines / chemokines, and less neutrophil and macrophage accumulation. |
| Chimeric studies showed that TLR4 on parenchymal cells mediated kidney damage in I/R. | ||
| Pulskens PLoS ONE 2008 | Renal I/R | TLR4 deficiency was associated with lower levels of chemokines, fewer infiltrating granulocytes, less renal damage, and better preserved renal function. |
| Chimeric studies indicated that both epithelial and leukocyte TLR4 contribute to I/R injury. | ||
| MyD88 and TRIF deficient mice had similar inflammation and renal dysfunction as wild type mice. | ||
| Tang Proc Natl Acad Sci USA 2007 | Brain I/R | TLR2 and TLR4 deficiency were associated with reduced I/R induced brain damage and neurological deficit. |
| TLR2 and TLR4 expression were increased in wild type neurons in cortical I/R injury. | ||
| Ziegler Biochem Biophys Res Commun 2007 | Brain I/R | TLR2 deficient mice had reduced cerebral infarct size. |
| In wild type mice, TLR2 was the most significantly upregulated TLR compared to TLR4 and TLR9 in postischemic mouse brains, and TLR2-related genes were induced. | ||
| Lehnardt J Neuroimmunol 2007 | Brain I/R | TLR2 deficient mice had decreased injury after focal cerebral ischemia compared to wild type mice. |
| Wild type mice had upregulated expression of TLR2 mRNA after I/R, and TLR2 protein was found in lesion-associated microglia. | ||
| Cao Biochem Biophys Res Commun 2007 | Brain I/R | TLR4 deficient mice had reduced cerebral infarct size and neurological impairment after I/R, and lower TNF-α and IL-6 levels than TLR4 competent mice. |
| Caso Circulation 2007 | Brain I/R | TLR4 deficient mice had smaller infarct volumes and better outcomes in neurological and behavioral tests and their TLR4 competent counterparts. |
| Levels of IRF-1, iNOS, COX2, INF-β, malondialdehyde, and MMP-9 were also lower in TLR4 deficient mice after I/R. | ||
| Hua J Neuroimmunol 2007 | Brain I/R | TLR4 deficiency was associated with lower levels of NF-κB activity, Akt and GSK3β phosphorylation, cytokine expression and neuronal death / apoptosis in response to I/R. |
| Kilic Neurobiol Dis 2008 | Brain I/R | TLR4 deficiency was associated with reduced brain injury from I/R but increased densities of myeloperoxidase positive neutrophils and Iba1 positive microglial cells. Decreased levels of phosphorylated MAPKs ERK1/2, JNK, and p38, as well as decreased iNOS levels, were found in neurons from TLR4 deficient mice exposed to I/R. |
| Aprahamian Pediatr Crit Care Med 2008 | Gut I/R | TLR2 deficient mice had increased intestinal injury scores by histology, and elevated levels of INF-γ, IL-4, and IL-6 mRNA but not TNF-α mRNA in response to I/R compared to wild type mice. |
| Cavassani JEM 2008 | Gut I/R | TLR3 deficient mice had increased chemokine / cytokine levels and neutrophil infiltration following gut I/R, and in vivo TLR3 antibody in wild type mice attenuated tissue injury from I/R. |
| In TLR3 deficient mice, levels of TNF-α and chemokines returned to baseline quickly after the initial rise due to I/R, and TLR3 deficient mice were then protected from the lethal effects of sustained inflammation. | ||
| Khandoga Shock 2008 | Cremasteric muscle I/R | TLR 2 and TLR4 deficiency reduced I/R induced vascular leakage. |
| I/R induced leukocyte transmigration was attenuated in TLR2 deficiency, but I/R induced leukocyte adhesion was not affected by TLR2 or TLR4 deficiency. |
TLRs are a family of proteins which are mammalian homologues to the Drosophila Toll, a protein that functions in development and immunity [1]. TLRs are ubiquitously expressed pattern recognition receptors central to the inflammatory response in a broad array of species. In vertebrates, TLR expression was originally described in cells of the immune system, such as macrophages and neutrophils, but it is now becoming apparent that they are widely expressed throughout the body in cells as diverse as hepatocytes, vascular smooth muscle cells, and neurons. The TLR receptors have many structural similarities both extracellularly and intracellularly, but they differ from each other in ligand specificities and expression patterns, and have some variability in the signaling pathways they activate. Thirteen TLRs have been found in mammals, of which TLR3, TLR7, TLR8, and TLR9 reside in endosomal vesicles inside the cells, while TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are present on the cell surface.
In his “self, non-self model” for immune recognition, Charles Janeway Jr. [2] proposed nearly twenty years ago that the immune system was organized to recognize and respond to invading microbial pathogens through gene encoded pattern recognition receptors. The discovery that cell surface receptors such as CD14 [3] and TLRs [1, 4, 5] were essential components of innate immune defenses provided firm support for the role of pattern recognition receptors in microbial immunity. We now know that the interaction of TLRs with their microbial ligands, referred to as pathogen associated molecular pattern molecules (PAMPs), results in an activation of innate immune responses. One of the most studied is the recognition of the PAMP lipopolysaccharide (LPS) with the TLR4 surface complex. However, the “self, non-self” model for immune recognition could not account for the activation of innate immune responses in the setting of sterile insults such as those resulting from oxidative tissue stress or damage.
Polly Matzinger [6] in 1994 proposed that the immune system was not organized to just distinguish self from non-self but instead to recognize any threat dangerous to the host. In the “danger” model, she predicted that tissue injury would provide signals to the immune system that could activate or enhance the immune response. With time, data began to emerge showing that products of cells killed by necrosis were pro-inflammatory, followed by reports showing that endogenous molecules of host origins could trigger macrophage and dendritic cell activation. Since the initial observation that heat shock protein 60 could activate TLR4-dependent signaling, numerous cell constituents and matrix components have been shown to trigger immune cell activation through TLRs. The concept of cellular communication after damage via exogenous or endogenous molecules, or Damage Associated Molecular Patterns (DAMPs), allowed for reconciliation of the phenomena of immune activation by foreign invasion as well as sterile tissue damage during non-infectious inflammatory states [7-9]. While the term PAMP is restricted to patterns located on pathogens, these endogenous analogues, termed endogenous danger signals or “alarmins” [10], are equally effective at activating the immune system and are involved in both sterile and infectious inflammation. Hence, mobilization of DAMPs to activate TLR signaling may represent a link between oxidative stress and inflammation.
TLR activation has best been characterized in immune cells, where it proceeds to initiate an inflammatory response by way of intracellular adaptor molecules such as Myeloid differentiation factor 88 (MyD88) or Toll-receptor-associated activator of interferon (TRIF). The majority of TLRs utilize MyD88 to initiate intracellular signaling. The exceptions are TLR3, which utilizes TRIF exclusively, and TLR4, which interacts with both MyD88 and TRIF to initiate downstream signaling. TLR ligand binding stimulates MyD88 facilitation of phosphorylation of IL-1 receptor associated kinase (IRAK)-1 by IRAK4 [11-13]. Phosphorylated IRAK1 recruits and activates TNFR-associated factor 6 (TRAF6). TRAF6 can then activate protein kinase C (PKC), Extracellular Signal Regulated Kinase (ERK)-1/2, and Transforming Growth Factor(TGF)-β-activated kinase 1 (TAK1). TAK1 is a Mitogen Activated Protein Kinase (MAPK) kinase kinase able to phosphorylate p38 MAPK, c-Jun N-terminal kinase (JNK), and I-kappa kinase (IκK). Activation of IκK leads to the nuclear translocation of Nuclear Factor kappa-B (NF-κB) and subsequent transcription of genes associated with TLR activation. TLR signaling mediated via TRIF activates TRAF3, but can also interact with TRAF6. Activated TRAF3 can activate TANK-binding kinase 1 (TBK1), which stimulates Interferon Regulatory Factor 3 (IRF3) to activate Interferon-beta (INF-β) and thereby leads to activation of Signal Transduction and Activator of Transcription (STAT)-1 and transcription of genes associated with this pathway. Whether these adaptors and downstream signaling events operate in a similar manner in non-immune cells is not known.
Both oxidative stress and infective stress can share the same TLR signaling pathways [14-16]. Molecules found in oxidative stress, such as reactive oxygen species (ROS) and nitric oxide (NO), are found in response to microbial invasion during the neutrophil and macrophage respiratory burst. ROS and NO can be virucidal but can also contribute to an increase in influenza viral titer, possibly via NF-κB [17, 18]. There are important differences in the goals of oxidative stress compared to infective stress, in that oxidative stress is designed to lead to repair of tissue while infective stress should result in immunity. Differences in TLR signaling in response to DAMPs released by oxidative stress compared to PAMPs released by infective stress are beginning to emerge. HMGB1 activates both IKKα and IKKβ, but LPS increases activity of only IKKβ in cultured neutrophils and macrophages [19]. Ligand recognition by TLRs is accomplished with the aid of molecules such as MD2 and CD14, as seen during LPS recognition when MD2 heterodimerizes with TLR4 [20]. MD2 mediates TLR4 recognition of PAMPs, but CD14 mediates TLR4 recognition of DAMPs released by necrotic cells [21]. CD24 co-immunoprecipitates with DAMPs Hsp70, Hsp90, and HMGB1, which can reduce DAMP activation of TLR4, and a germline mutation of CD24 increased susceptibility to hepatocyte necrosis in an acetoaminophen-induced liver injury model [22, 23]. CD24 does not regulate the inflammatory response to LPS.
The inflammatory response to conditions of oxidative stress is designed to ward off invading pathogens and to initiate repair processes. When excessive, these responses lead to early organ damage and dysfunction due to an overexuberant inflammatory response, and can then render the victim susceptible to infection due to profound immune suppression and / or dysregulation. The consequences of inflammation from oxidative stress injuries, such as I/R and HS induced injury, can lead to death and disability (Table 1). Thus, understanding the pathways leading to the initial activation of inflammatory pathways in oxidative stress is essential to devising strategies to limit the detrimental consequences of the inflammatory response to injury. This review will focus on the role of TLRs in the response to ischemic stress.
Hemorrhagic Shock: global hypoperfusion
Hemorrhagic shock (HS) represents a global ischemic stress, resulting from acute blood loss in a number of clinically settings such as accidental or intentional injury, ruptured arterial aneurysms, and gastrointestinal hemorrhage. However, resuscitation after severe or persistent HS (HS/R) contributes to further end-organ dysfunction and damage as a result of a global ischemia and reperfusion phenomenon. Evidence that TLR signaling may be involved in the profound systemic inflammatory response after HS/R first appeared in 1993. DeMaria et al. [24] reported that endotoxin resistant mice due to a deficiency in TLR4 signaling (C3H/HeJ mice) exhibited lower circulating tumor necrosis factor (TNF) levels and improved survival compared to wild type mice when subjected to HS/R. At the time, it was not known that the C3H/HeJ mouse had a defect in the signaling domain of TLR4, and the connection between pattern recognition receptors and HS-induced inflammation could not be made. These observations were extended in 1994 with experiments showing that C3H/HeJ mice exhibited lower lactate levels following HS [25].
More recent work has further characterized the role of TLRs in the inflammatory response after HS/R. Our laboratory has shown that TLR4 deficient mice were protected from liver injury following HS/R with decreased circulating levels of plasma proinflammatory cytokines and decreased NF-κB activation [26]. Hepatic messenger RNA (mRNA) levels for TNF, IL-10, and inducible nitric oxide synthase (iNOS) were also lower in TLR4 deficient mice exposed to HS/R. Mice deficient in TLR2 or the TLR4 associated protein CD14 did not show similar protection from hepatic injury and systemic inflammation, indicating the specific role of TLR4 in mediating HS/R induced inflammation. In addition, myocardial and lung injury associated with global oxidative stress also appears to be TLR4-dependent. HS induced myocardial contractile depression and TNF-α expression in the heart was attenuated in the absence of functional TLR4 signaling [27]. In the lung, TLR4 signaling was required for HS induced lung injury as seen by attenuated lung TNF-α levels, protein permeability, and neutrophil accumulation in TLR4 deficient mice [28]. Lung NF-κB activation in response to HS was however independent of functional TLR4, but required TLR4 in endotoxemia induced lung injury, suggesting that the TLR4 dependent inflammatory response is different in HS compared to that induced by LPS. Interestingly, HS/R is known to sensitize rodents to the toxic effects of LPS [29]. There is an upregulation of TLR4 and TLR2 expression on alveolar macrophages in the lung [30-32], and the upregulation of TLR4 has been shown to be dependent on reactive oxygen species (ROS) formation [32]. TLR2 expression upregulation in the lung was found to be dependent on the TLR4 mediated recruitment and activation of neutrophils [33]. Liu et al. found that the DAMP High Mobility Group Box 1 protein (HMGB1) induced TLR4 dependent macrophage secretion of IL-23 and IL-17 in response to HS, which resulted in neutrophil recruitment from the bone marrow [34].
Other studies have also examined the changes in TLR expression in models of HS/R. TLR4 is expressed by both immune cells and parenchymal or non-immune cells [35-38]. Using studies involving bone marrow chimeric mice, it was established that the inflammatory response following HS involves TLR4 on both cells of bone marrow origin and cells not derived from bone marrow [38]. Levels of TLR2 and TLR4 mRNA were upregulated in the myocardium of mice exposed to HS/R or LPS compared to shams [39]. In rats exposed to HS/R, TLR2, TLR3, and TLR6 mRNA were upregulated in the lung but not the spleen [40]. HS/R induced lung injury was associated with increased expression of TLR4, p38 MAPK, and heme oxygenase (HO)-1 in wild type mice compared to TLR4 deficient mice [41]. In a model of murine hypoxia (exposure to 8% oxygen over 6 hours), TLR2 and TLR6 transcripts were induced in multiple organs, but this was abolished in hypoxia inducible factor (HIF)-1α mutant mice, indicating that hypoxia induces expression of at least some TLRs [42]. In vitro, binding sites for HIF-1α were found on putative TLR2 and TLR6 promoters, and the TLR2 and TLR6 promoters bound HIF-1α in chromatin immunoprecipitation assays. Additionally, stimulation of TLR9, like TLR4 may also be functioning to exacerbate the inflammatory response to HS. Not only did exposure to CpG-ODN (synthetic oligonucleotides containing TLR9 stimulating unmethylated CpG motifs) prior to HS significantly increase levels of TNF-α, interferon gamma (INF-γ), IL-6, and nitrite, TLR4 expression was also found to be increased in the liver [43].
TLR4 also appears to play a role in the immune response to models of trauma and HS/R. Although monocyte cell surface TLR4 expression in patients undergoing severe trauma was not found to be different from healthy controls [44], in experimental conditions of trauma simulated by laparotomy and HS/R, elevated levels of chemoattractants KC (murine equivalent of human interleukin (IL)-8) and monocyte chemoattractant protein (MCP)-1 were found in plasma, lung, and Kupffer cell supernatants in TLR4 competent mice but not in TLR4 deficient mice [45]. Additionally, there was an increase in lung neutrophil infiltration in TLR4 competent mice. Therefore in the setting of HS/R and trauma, TLR4 appears to have a functional role in increasing chemoattract expression and augmention of neutrophil recruitment, and the subsequent inflammatory response.
Warm Liver I/R
Warm I/R to the liver occurs in the setting of liver surgery or during vascular occlusion. There is considerable experimental evidence that I/R specifically to the liver induces TLR4 dependent inflammation and injury. Mice deficient in TLR4 signaling undergoing hepatic I/R injury had reduced liver damage as measured by serum aspartate transaminase (AST) levels, as well as decreased inflammation evidenced by lower levels of TNF-α and myeloperoxidase (MPO) [46]. Injury and inflammation as measured by serum alanine aminotransferase (ALT) levels, histology, and local induction of TNF-α, IL-6, and INF-inducible protein (IP)-10, was significantly lower in TLR4 deficient mice but not TLR2 deficient mice [47]. Other investigators found a similar protection from hepatic I/R injury in TLR4 deficiency but not TLR2 deficiency, as measured by ALT levels, histology, neutrophil infiltration, and TNF-α levels [48, 49].
TLR4 is expressed on both parenchymal cells or hepatocytes and cells of the immune system in the liver, such as Kupffer cells or dendritic cells. Using chimeric mice, our laboratory found that TLR4 mediated hepatic injury in I/R is principally dependent on cells of bone marrow origin [49]. Furthermore, depletion of phagocytic cells using gadolinium chloride reduced inflammation and injury in wild type mice but not TLR4 deficient animals. Conversely, injury was amplified when dendritic cell numbers in the liver were increased using plasmid GM-CSF pre-treatment [50]. Again, this was seen only in TLR4 competent mice.
The mechanisms and ligands leading to TLR4 activation in the setting of liver I/R are poorly understood. It is likely that numerous endogenous molecules can activate TLR4 signaling during I/R. The majority of the experimental work in this area has used models of partial hepatic occlusion to avoid intestinal congestion and the release of microbial products such as LPS into the liver. Furthermore, germ-free mice exhibit a similar inflammatory response to that seen in germ-bearing mice, suggesting that LPS is not required [51]. However, the importance of LPS has not been firmly established. We have shown that neutralizing antibody to the nuclear protein HMGB1 mimics the TLR4 deficient state [49]. In addition, administration of HMGB1 worsened hepatic injury during I/R only in TLR4 competent mice. Thus HMGB1 may be one endogenous activator in liver I/R. Zhai et al. provided further evidence of an activator of TLR4 endogenous to the liver using an isolated perfusion liver system [52]. These investigators rigorously excluded a role for LPS and found no role for HMGB1.
The TLR4 dependent inflammatory response to hepatic I/R appears to be independent of the MyD88 pathway based on studies on MyD88 knockout mice [48]. In these studies, IRF3 knockout mice were markedly protected, implicating the activation of TRIF-dependent signaling. TLR4 activation of the TRIF / IRF3 pathway leads to STAT1 phosphorylation and subsequent inflammatory gene expression such as expression of IP-10. HO-1 was found to downregulate phosphorylated STAT1, which may be one of the mechanisms of cytoprotection in liver I/R by HO-1 [53]. HO-1 overexpression reduced liver damage in vivo, and in vitro suppressed release of IP-10. TLR4 signaling may downregulate HO-1 expression in liver I/R as TLR4 mutant mice had higher levels of HO-1 expression than wild type mice [48]. TLR4 protein levels were decreased on induction of HO-1 in vivo, suggesting that HO-1 and TLR4 both downregulate one another's expression in liver I/R. Further work has implicated the role of INF-β and the type I INF receptor [54]. It has also been shown that the transcription factor Interferon Regulatory Factor-1 (IRF-1) is central to the hepatic I/R injury / inflammatory response [55]. As type I interferons are strong inducers of IRF-1, this supports a picture where TLR4 dependent production of type I interferon drives the inflammatory response in warm liver I/R.
Warm Cardiac I/R
The most clinically relevant model of warm cardiac I/R is that of myocardial infarction, one of the major causes of death across industrialized nations. In this model of I/R injury, both TLR2 and TLR4 deficient mice have smaller infarcts compared to their wild type counterparts [56-58]. TLR2 deficient mice had a smaller infarct size, reduced reperfusion-associated production of ROS, and reduced leukocyte infiltration [59]. TLR4 deficient mice similarly had a smaller infarct size, fewer lipid peroxides, and less complement deposition in the myocardium, as well as lower systemic levels of IL-12 and INF-γ as a result of cardiac I/R compared to TLR4 competent mice [57]. A specific TLR4 antagonist, eritoran, reduced cardiac infarct size, JNK phosphorylation, NF-κB activity, and cytokine expression in wild type mice undergoing cardiac I/R [60]. A recent review by Chao [61] covers how TLR4, TLR2, and MyD88 deficiency are all associated with reduced myocardial inflammation and infarct size, as well as reduced ventricular remodeling after cardiac I/R. A reduction in collagen type 1 mRNA and reduced myocardial fibrosis in the non-infarct area accompanied by higher fractional shortening was found in TLR2 deficient mice subjected to warm I/R, and hearts from TLR2 deficient mice had higher left ventricular end diastolic pressures ex vivo [56, 62]. TLR4 deficient mice, in contrast, had reduced left ventricular end diastolic pressure compared to wild type controls in warm cardiac I/R [63].
Cardiac myocytes express many of the TLRs, and ligand activation of TLR2, TLR4, and TLR5 results in activation of NF-κB and expression of IL-6, KC, MIP-2, and intercellular adhesion molecule (ICAM)-1 in vitro [64]. Cardiac I/R in vivo was associated with reduced JNK, NF-κB and AP-1 activation, as well as lower levels of IL-1β, monocyte chemotactic factor (MCF)-1 and IL-6 in the reperfused myocardium of TLR4 deficient mice [58]. TLR4 expression in cardiac myocytes can be enhanced by IL-1β or LPS, and this appears to be mediated by ROS as the effect is abolished by the oxygen radical scavenger PDTC [65]. LPS pretreatment of hearts subjected to I/R injury was associated with reduced infarct size, reduced cardiac myocyte apoptosis, and lower caspase-3 activity, as well as increased levels of Hsp-27, phosphorylated protein kinase B (Akt), and phosphorylated glycogen synthase kinase (GSK)-3β [66]. Cardioprotection by LPS pretreatment was abolished in mice deficient in Akt or by using inhibitors of phosphoinositide 3 kinase (PI3k). It is possible that pre-stimulation of TLR4 activates inhibitory pathways that reduce TLR4 mediated inflammatory signaling.
Warm Lung I/R
Hypoxia appears to elicit a TLR4 dependent inflammatory response in the lung. In a model of lung I/R injury using 1 h of ischemia followed by 3 h of reperfusion, TRL4 deficient mice demonstrated reduced vascular permeability, lung myeloperoxidase (MPO) activity, and leukocyte accumulation in broncho-alveolar lavage (BAL) fluid [67]. Phosphorylation of JNK, and activation of NF-κB and AP-1 were reduced in the absence of TLR4 signaling, as were the presence of pro-inflammatory cytokines (KC, TNF-α, MIP, and MCP) in the BAL. Using a model of acid-induced lung injury and various knock-out strains, it was found that the TLR4-TRIF-TRAF6 pathway determined the severity of lung injury, and deletion of a component of the NADPH oxidase also improved the severity of lung injury [68].
Hyperoxia, on the other hand, is associated with reduced severity of lung injury in the presence of functional TLR4. Inducing hyperoxia for 72 h followed by normoxia resulted in the survival of 55% of wild type mice (n=18) at five days but none of the TLR4 deficient mice survived [69]. Mice lacking functional TLR4 signaling had significantly greater oxidative DNA damage, inflammation, and lung permeability in response to hyperoxia. TLR4 was required for the upregulation of anti-apoptotic protein Bcl-2 and to phosphorylate Akt. It is also possible that TLR4 activation in the lung in response to hyperoxia results in the upregulation of HO-1, as exogenous transfer of the HO-1 gene reduced five day mortality in TLR deficient mice to 50% and attenuated hyperoxia-induced lung injury.
Warm Renal I/R
Both TLR2 and TLR4 appear to play a role in I/R induced renal inflammation and injury. TLR2 and TLR4 mRNA and protein were upregulated up to fivefold five days following sterile renal I/R [70]. Expression of MIP-2, KC, TNF-α, INF-γ, and MHC class I and class II molecules were concurrently increased. The expression of TLR2 and TLR4 by renal tubular cells was increased in response to renal I/R along with a rise in the DAMP Hsp 70 [71]. TLR2 deficient mice had reduced levels of pro-inflammatory cytokines KC, MCP-1, IL-1β, and IL-6, reduced leukocyte infiltrate, and better preserved renal function as assessed by plasma urea and creatinine, and apoptotic tubular cells in renal I/R, compared to TLR2 competent mice [72]. This was also seen by using TLR2-antisense treatment compared to nonsense oligonucleotide treatment in wild type mice exposed to renal I/R. Chimeric mice demonstrated that TLR2 on parenchymal cells is principally responsible for the TLR2 mediated inflammatory response to I/R, as TLR2 deficient mice given wild type leukocytes had lower levels of creatinine, urea, infiltrating granulocytes, and apoptotic tubular cells following renal I/R than wild type mice given TLR2 deficient leukocytes. Interestingly, TLR2 signaling deficient mice were more protected from renal I/R injury than MyD88 or double MyD88 and TRIF knockout mice, while TRIF deficient or wild type mice were the least protected [73].
TLR4 deficient mice, like MyD88 deficient mice, are also protected from renal I/R injury as demonstrated by reduced tubular dysfunction or damage, reduced pro-inflammatory cytokines, and less leukocyte accumulation in TLR4 or MyD88 deficient mice compared to their wild type controls undergoing renal I/R [74]. Similar to the findings of the TLR2 deficient chimeric studies in renal I/R, chimeric studies using TLR4 deficient mice showed that presence of TLR4 on parenchymal cells was the principal determinant of end organ dysfunction in renal I/R rather than TLR4 on immune cells. However, another study indicated that both epithelial and leukocyte TLR4 may contribute to I/R injury, and these investigators found that MyD88 and TRIF deficient mice had similar inflammatory profiles to renal I/R as wild type mice but TLR4 deficient mice had lower levels of chemokines, infiltrating granulocytes, and less renal damage [37].
Warm Cerebral I/R
Neuronal tissue is highly sensitive to hypoxia. Ischemia usually manifests as a “stroke” where neurons in the affected region undergo cell death. Using a middle cerebral artery occlusion / reperfusion model, Tang et al. demonstrated that stroke-induced brain damage and neurological deficits measured 3 days following injury were significantly less in TLR2 or TLR4 deficient mice [75]. The ischemic cortex showed increased levels of TLR2, TLR4, Hsp-70, and phosphorylated JNK. Neurons in the ischemic cortex exhibited robust TLR2 and TLR4 immunoreactivity. In vitro, astrocytes responded to the TLR2 and TLR4 ligands peptidoglycan and LPS, respectively, with NF-κB and JNK activation, but neurons did not respond to these PAMPs. Endogenous ligands such as Hsp-70 or hyaluronic acid (HA) may be acting as DAMPs in cerebral I/R to cause neuronal cell apoptosis via TLR2 or TLR4 activation. The presence of HA degradation products has been found in association with acute stroke in post-mortem samples of brain tissue and in the serum of patients [76]. HA was found intracellularly in the nuclei of peri-infarct neurons, as well as deposited in blood vessels. Phagocytic cells showed increased HA synthase and hyaluronidase expression in the stroke and peri-infarct areas of the brain.
Ziegler et al. [77] used a cerebral I/R model to show that the infarct size was much smaller in TLR2 deficient mice than in wild type mice. Wild type mice had upregulation of TLR2, TLR4, and TLR9 in the ipsilateral brain hemisphere. TLR2 protein expression was predominantly on phagocytic microglial cells, but was also seen in neurons, astrocytes, and endothelial cells. Focal cerebral ischemia in TLR2 deficient mice was also associated with decreased injury compared to wild type mice, and upregulation of TLR2 in wild type mice following injury was found mainly in lesion-associated microglia [78]. Hyperoxic resuscitation following fetal asphyxia in newborn sheep was associated with an upregulation of IL-1β, 1L-12p40, TLR2, and TLR4 mRNA in the cortex/subcortex after resuscitation with 100% oxygen compared to 21% oxygen [79]. Therefore TLR2 and TLR4 appear to be upregulated in the brain in response to a variety of oxidative stressors.
TLR4 deficient mice had improved functional outcome as well as a reduced cerebral infarct size following cerebral I/R, and lower TNF-α and IL-6 levels than TLR4 competent mice [80]. This was also seen in a permanent occlusion of the middle cerebral artery model where two different strains of TLR4 deficient mice had lower infarct volumes and better outcomes in neurological and behavioral tests [81]. The TL4 deficient mice were found to express lower levels of stroke-induced IRF-1, INF-β, iNOS, cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-9, and lipid peroxidation marker malondialdehyde (MDA). In vivo use of a specific iNOS inhibitor or a COX-2 inhibitor administered after ischemic insult showed a partial protective effect in TLR4 competent mice with recovery in neurological deficit and a reduction in infarct volume, indicating that TLR4 mediated iNOS and COX-2 contribute to inflammatory injury in the brain. In another study, the cysteine donor N-acetylcysteine (NAC) reduced LPS sensitized ischemic injury in neonatal rats when NAC was given prior to injury and/or immediately post-injury, but not when NAC treatment was delayed by even 2 h post injury [82]. Protection by NAC was associated with reduced nitrotyrosine formation, increased levels of antioxidants glutathione and thioredoxin, and inhibition of caspase activation. TLR4 deficient mice undergoing cerebral I/R had lower levels of NF-kB activity, reduced Akt and GSK-3β phosphorylation, and less cell apoptosis in response to I/R [83]. Decreased levels of phosphorylated MAPKs as well as decreased iNOS levels were found in neurons from TLR4 deficient mice exposed to cerebral I/R [84].
Warm Intestinal I/R
The intestine is commonly subjected to ischemic insults following acute vascular occlusions due to thrombus or embolus. TLR2 and TLR4 appear to have a protective effect in I/R injury of the gut. TLR2 deficient mice have exacerbated intestinal injury in response to intestinal I/R [85]. TLR2 deficient mice had elevated levels of intestinal INF-γ, IL-4, and IL-6 mRNA in response to I/R compared to wild type mice, and increased intestinal injury on histology. LPS, a TLR4 agonist, reduced gut I/R induced intestinal permeability, lipid peroxidation and glutathione depletion [86]. A reduction in the commensal microflora, which can stimulate TLR4 via LPS, was associated with increased villi apoptosis, decreased TLR4 expression, and exacerbated damage to the gut by I/R, as well as increased NF-κB and AP-1 activity, Hsp-70 protein expression, and decreased Bcl-w and TNF-α mRNA expression of the intestinal mucosa.
TLR3 deficient mice also had increased levels of cytokines following gut I/R, and had increased neutrophil infiltration in the gut, but levels of pro-inflammatory cytokines returned to baseline more quickly in TLR3 deficient mice and these mice were ultimately protected from the lethal effects of ongoing inflammation compared to wild type mice [87]. Neutralizing antibody to TLR3 reduced gut injury from I/R and decreased sepsis-induced mortality. TLR3 recognizes double stranded RNA but also RNA from necrotic cells. RNA from necrotic neutrophils may have been acting as endogenous DAMPs in wild type mice in gut I/R, as macrophages from TLR3 deficient mice responded normally to other TLR ligands but not to RNA from necrotic neutrophils.
Cold I/R injury
Cold I/R injury is classically seen in the setting of transplantation and can result in early graft injury and dysfunction, and may also contribute to a greater incidence of rejection [88]. Although significant overlap in the pathophysiology of cold and warm I/R likely exists, there are clear differences in the manifestation of these two insults. For example, although cold I/R results in a decreased rate of anoxic injury, more severe endothelial cell disruption occurs during reperfusion than in warm I/R [89]. Cold I/R in an orthotopic syngeneic rat liver transplantation model resulted in increased NF-κB and AP-1 activity, as well as upregulation of multiple components of the LPS signaling pathway, including mRNA for LPS binding protein, CD14, and TLR2 [90]. Increased expression of TLR2 and TLR4 on monocytes of liver transplant patients was associated with acute rejection, and steroid pulse therapy considerably reduced this expression [91]. Shen et al. [92] provided proof that the early organ injury and inflammatory response to prolonged cold I/R in the liver is strongly dependent on TLR4 by carrying out syngeneic liver transplants using TLR4 knockout mice as donors. The inflammatory response induced by cold I/R in the heart also involves TLR4 [93]. By using combinations of wild type and TLR4 mutant mice as donors and recipients, we showed that TLR4 on both recipient and donor cells were part of the response leading to increases in serum TNF, IL-6, MCP-1, IL-1β, and troponin I levels, as well as intragraft TNF, IL-1β, IL-6, EGR-1, ICAM-1, and iNOS mRNA levels. Syngenic heart transplants in MyD88 or TRIF deficient mice had significantly lower levels of intragraft TNF-α, IL-6, and ICAM1, and grafts revealed HMGB1 translocation out of the nucleus of cardiac myocytes (Transplantation, in press Kaczorowski). Biopsies performed during human lung transplantation showed that mRNA levels for several TLRs and cytokines correlated with the intubation time of donors [94]. Following reperfusion, mRNA levels of the heat shock protein (Hsp)-70, a known DAMP, were elevated. Taken together, the experimental and clinical findings implicate TLR4 as central to the initial inflammatory response induced not only by warm I/R but also by cold I/R.
Role of DAMPs as activators of TLR signaling in I/R
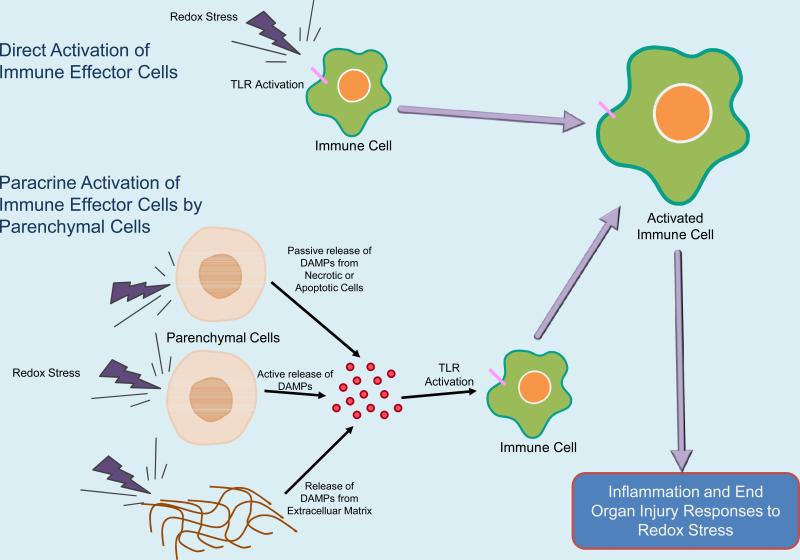
Proteins, lipids, and nucleic acids have all been shown to act as DAMPs that can activate TLR signaling when presented in the appropriate context [31, 52, 74, 95-98]. DAMPs appear to be made available to the immune system by one of three mechanisms which include, 1) cell necrosis where cell constituents are liberated, 2) regulated cell secretion where intracellular constituents are mobilized and secreted, or 3) the liberation of matrix components by enzymes (e.g. proteases). TLR activation in sterile injury can therefore proceed via direct activation of immune cells or by paracrine stimulation via release of DAMPs from parenchymal cells as illustrated in Figure 1.
Figure 1.
Proposed Models of TLR Activation by DAMPs in Ischemia/Reperfusion Injury
Evidence has evolved for a family of putative danger signals that may activate TLR signaling or may be released as a consequence of TLR signaling. Heat shock proteins (Hsp) are one of the first potential DAMPs shown to activate TLR4-dependent signaling after oxidative stress. Circulating Hsp70 levels were found to be increased following cardiac I/R in patients undergoing coronary artery bypass grafting and in those suffering an acute myocardial infarction, as well as following hepatic I/R in patients undergoing liver resection [99-101]. Hsp70 has also been shown to mediate inflammatory responses in a variety of I/R models such as renal and intestinal I/R [71, 86]. Hsp70 may function to activate TLR4 signaling as stimulation of TLR4 competent macrophages with Hsp70 results in the release of TNF-α, but not in TLR4 deficient macrophages [99]. Hsp72 can also activate TLR2 and TLR4 signaling in hepatocytes in vitro to release macrophage inflammatory protein (MIP)-2 but not TNF-α or IL-6 [98]. The calcium regulating protein S100 is an example of DAMP that can be released as a consequence of TLR4 signaling [102]. Cardiac tissue exposed to LPS upregulated S100A8 and S100A9 in mice, and S100A8 and S100A9 were shown to coimmunoprecipitate with the receptor for advanced glycation end products (RAGE). S100A8 and S100A9 overexpression led to a RAGE-dependent decrease in calcium influx and a decreased cardiac ejection fraction in live mice. Overexpression of human S100B has been shown to increase infarct size and worsen neurological deficits in cerebral ischemia [103].
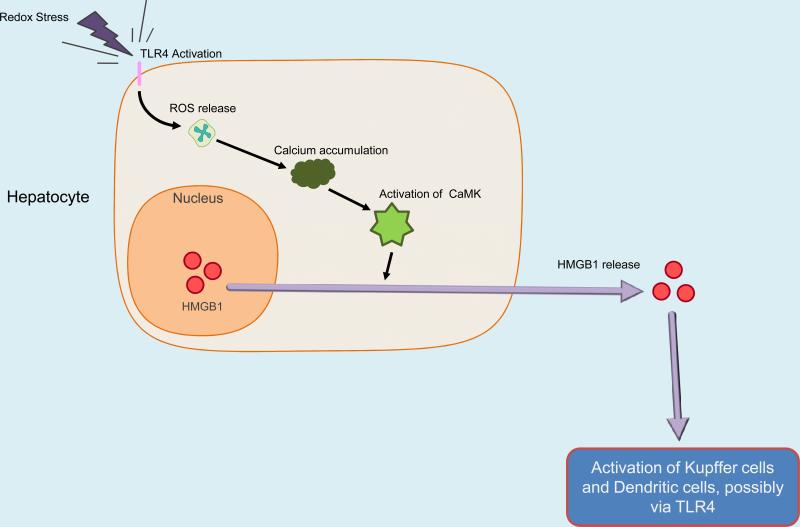
Another molecule, High mobility group box -1 (HMGB1), represents a DAMP that is both released as a consequence of TLR4 activation and may in turn stimulate TLR4 activation in conditions of oxidative stress. HMGB1, an abundant nuclear protein known to bind to the minor groove of DNA [104], has received particular attention as fulfilling the functions of a DAMP by being involved in many important infectious and non-infectious inflammatory conditions. Its role in inflammation was identified when it was shown to act as a late mediator in sepsis [105]. That HMGB1 could also act as a DAMP came from studies showing that necrotic cells release HMGB1 which then can activate inflammatory and tissue repair processes [106]. Furthermore, HMGB1 also contributes to the initiation of inflammatory signaling in models of I/R and HS/R (Table 2). HMGB1 serum levels were found to be increased in HS/R and treatment of animals with neutralizing antibody to HMGB1 resulted in improved survival [107]. Neutralizing antibody to HMGB1 also reduced systemic inflammation in response to HS/R, as demonstrated by reduced levels of systemic cytokines IL-6 and IL-10 and reduced gut permeability and bacterial translocation. Work from our laboratory demonstrated that HMGB1 blockade using neutralizing antibodies reduced inflammation and injury in models of liver I/R and trauma [49, 108]. This protection only occurred in TLR4 competent mice, not TLR4 deficient mice, suggesting that HMGB1 functions through TLR4 activation. The ability of HMGB1 to activate TLR4 was further supported by experiments showing that recombinant HMGB1 worsens I/R induced hepatic injury only in TLR4 competent mice [49]. Most recently, in a cold I/R model of syngenic cardiac transplantation, neutralizing antibody to HMBG1 has been found to reduce systemic IL-6 levels compared to controls (In press, Kaczorowski). The source of HMGB1 that mediates inflammatory responses after oxidative stress is uncertain. We have observed an increase in total as well as cytoplasmic levels of HMGB1 in the parenchymal cells in warm liver [49] and cold cardiac (In press, Kaczorowski) I/R, suggesting that parenchymal cells may be a source of DAMPs such as HMGB1 in ischemia. Although release through necrosis may be one source of HMGB1, there is evidence that hypoxic cells mobilize and release HMGB1. Our in vitro observations indicate that hypoxia stimulates the release of HMGB1 by hepatocytes through a process that requires TLR4, ROS production, and the activation of Calcium /Calmodulin-dependent protein kinases (CaMKs), a family of proteins involved in a wide range of calcium-linked signaling events [109]. The production of ROS by hypoxic hepatocytes was found to be TLR4 dependent, and in vivo studies confirmed the role of TLR4, ROS, and CaMKs in HMGB1 release in hepatic I/R (Figure 2). It is likely that the HMGB1 is dependent on its interaction with other molecules such as DNA [110], cytokines [111], or LPS [112]. Furthermore, post-translational modifications may be required for both its regulated release and activity [113]. Recent data indicates that redox modification of HMGB1 may be a determinant of its immune regulatory properties. Oxidation of critical thiols switches the activity of HMGB1 from proinflammatory to inducing tolerance [114].
Table 2.
Studies on the DAMP HMGB1 in Hemorrhagic Shock / Resuscitation (HS/R) and Ischemia / Reperfusion (I/R) in vivo models
| Publication | Model | Findings |
|---|---|---|
| Yang Mol Med 2006 | HS / R | Serum HMGB1 levels were higher in mice undergoing HS/R. Neutralizing antibody to HMBG1 improved survival, and was associated with lower levels of systemic IL-6 and IL-10, reduced gut permeability, and reduced bacterial translocation. |
| Tsung J Exp Med 2005 | Liver I/R | TLR4 deficient mice had reduced damage in hepatic I/R, and neutralizing antibody to HMGB1 failed to protect TLR4 deficient mice from hepatic I/R injury but did protect TLR4 competent mice. In wild type mice, administration of recombinant HMGB1 worsened I/R injury. |
| Tsung J Leuko Biol 2007 | Liver I/R | HMGB1 increased TLR4 expression in hepatic DC in vitro, and in vivo increased hepatic DC worsened I/R injury in wild type but not in TLR4 deficient mice. |
| Tsung J Exp Med 2007 | Liver I/R | TLR4 dependent ROS production and downstream CaMK signaling mediated hypoxia-induced HMGB1 release by hepatocytes. CaMK inhibition reduced injury in liver I/R, and was associated with HMGB1 accumulation in the cytoplasm of hepatocytes. |
Figure 2.
TLR4 dependent ROS activate CaMK to release HMGB1 from hepatocytes, leading to Hepatic Ischemia/Reperfusion Injury
Interactions between TLR4 and NADPH oxidase
As mentioned previously, DAMPs may function in activating inflammatory cascades by either activating TLR signaling or by being released as a consequence of TLR signaling. In our studies of the regulation of HMGB1 release by hepatocytes, TLR4 activation under oxidative stress induces ROS production which can function as signaling molecules contributing to CaMK activation and HMBG1 release (Figure 2) [109]. Thus, ROS function not only in their classical role of causing injury by disruption of cell membranes, DNA strand breaks, or enzyme inactivation as they do when released at high concentrations in the neutrophil “respiratory burst”, but as precise signaling molecules. The various types of ROS and their functions are beyond the scope of this review, but are reviewed elegantly by other authors [115-119]. As signaling molecules, ROS can oxidize cysteine residues to form sulfenic acid moieties, which are unstable but are able to form disulfide bridges with one another or with the abundant intracellular protein glutathione (GSH) in S-glutathiolyation, thereby resulting in a change in structural configuration and activity [120]. Akin to protein phosphorylation in cell signaling, this is a reversible process, with molecular reductants such as thioredoxin allowing reversal back to the original configuration of the protein. Singh et al. [121] found that although ROS produced by B Cell receptor activation continued to accumulate over a five minute time course, signal transmission was much briefer. ROS transmitted signal by oxidation of the protein tyrosine phosphatase (PTP) SHP-1, but maximal PTP inhibition occurred within thirty seconds and had returned to baseline within one minute. Additionally, SHP-1 inhibition was localized, with no effect on the cytoplasmic pool of SHP-1. Interestingly, the antioxidant peroxiredoxin 4 has been found close to the BCR. This implies that ROS can function locally and be inactivated locally by antioxidants.
The mechanism by which TLR4 mediates the production of ROS during states of redox stress has not been fully elucidated but could involve the membrane associated enzyme complex NADPH oxidase [122]. There is accumulating evidence associating TLR activation with NADPH oxidase function. Sasada et al. [123] in 1983 reported that activation of mouse peritoneal macrophages by LPS resulted in an increase in functional activity of NADPH oxidase. The NADPH oxidase inhibitor apocynin and deletion of Nox, a subunit of NADPH oxidase, protected mice from LPS-induced lethality as well as decreasing the expression level of inflammatory cytokines in vivo [124]. In dendritic cells, the NADPH subunits p47phox and Nox were increased when dendritic cells were stimulated with a variety of PAMPs known to activate various TLRs [125]. However, dendritic cell maturation did not occur when immune stimuli such as CD40 ligand was used. Interestingly, administration of DPI, a NADPH oxidase inhibitor, reduced the upregulation of TLR2, TLR4, and TLR9 in a model of alcohol-induced fatty liver injury [126]. These studies provide evidence that TLR and NADPH oxidase activities may be linked in various inflammatory conditions. Interestingly, recent findings suggest that there may be a direct interaction between TLR4 and NADPH oxidase in mediating LPS-induced production of ROS. Using a yeast two hybrid and GST pull-down assay model, Park et al. [127] demonstrated that the carboxy-terminal region of Nox4, a subunit of NADPH oxidase, interacted directly with the TIR domain of TLR4 after LPS stimulation.
There is also evidence suggesting interplay between ROS derived from NADPH can modulate the TLR4 canonical pathway initiated by MyD88 and calcium-related signaling events. As was mentioned earlier, MyD88 facilitates phosphorylaton of IRAK1 by IRAK4. In a T cell line, recruitment of IRAK1 to the type I IL-1 receptor was regulated by intracellular oxidant balance [128]. Neutrophil activation by LPS is aborted by antioxidants at a step as early as IRAK4 activation [129]. Interestingly, the NADPH oxidase subunit p47phox has also been found to negatively regulate MyD88 or TRIF mediated TLR4 signaling through an interaction with TRAF4 and TRAF6 / TRIF [130]. ROS can also contribute to calcium mobilization and CaMK activation. Exposure of macrophages (THP-1 cells) to hydrogen peroxide resulted in peroxide-induced mobilization of annexin VI from lipid rafts to the cytosol and a subsequent increase in cytosolic calcium and phosphorylation of CaMK II [131].
Conclusion
TLR activation has been shown to be important in conditions of oxidative stress such as HS/R and I/R injury. Understanding the mechanisms by which inflammatory cascades are activated as a result of oxidative stress have important clinical implications as these conditions, as seen during myocardial infarction, stroke, and trauma, are major causes of morbidity and mortality. Systematic review of the current literature on TLRs in various models of HS/R and I/R demonstrates how the activation of TLRs is important in the initiation and propagation of inflammation and end organ injury. During ischemic insults, release of DAMPs have been shown to contribute to inflammation through processes that may involve activation of TLRs. Alternatively, DAMPs may also be released as a consequence of TLR4 activation, thereby forming a paracrine loop of amplification of inflammation and leading to end organ injury. During states of oxidative stress, ROS may participate in signaling events downstream of TLRs, with some evidence that TLR4 activation may lead to ROS signaling via direct interaction between TLR4 and NADPH oxidase. In summary, the relationship of oxidation to inflammation is complex, ranging from fine-tuned signaling by ROS during TLR4 activation leading to the active mobilization of DAMPs, to cellular injury from redox stress leading to release of DAMPs triggering TLR4-mediated inflammation and organ injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, Liu MY, Van HC, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 7.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Zheng W, Rock KL. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2000;97:14590–5. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 11.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci U S A. 2002;99:5567–72. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki N, Suzuki S, Yeh WC. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–6. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 14.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–12. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 15.Lee KM, Yeo M, Choue JS, Jin JH, Park SJ, Cheong JY, et al. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter. 2004;9:632–42. doi: 10.1111/j.1083-4389.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- 16.Deva R, Shankaranarayanan P, Ciccoli R, Nigam S. Candida albicans induces selectively transcriptional activation of cyclooxygenase-2 in HeLa cells: pivotal roles of Toll-like receptors, p38 mitogen-activated protein kinase, and NF-kappa B. J Immunol. 2003;171:3047–55. doi: 10.4049/jimmunol.171.6.3047. [DOI] [PubMed] [Google Scholar]
- 17.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr. 1997;127:962S–5S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 18.Zaki MH, Akuta T, Akaike T. Nitric oxide-induced nitrative stress involved in microbial pathogenesis. J Pharmacol Sci. 2005;98:117–29. doi: 10.1254/jphs.crj05004x. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 20.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–17. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2009 doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Chen GY, Zheng P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–61. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMaria EJ, Pellicane JV, Lee RB. Hemorrhagic shock in endotoxin-resistant mice: improved survival unrelated to deficient production of tumor necrosis factor. J Trauma. 1993;35:720–4. [PubMed] [Google Scholar]
- 25.Pellicane JV, Gore DC, DeMaria EJ. Decreased lactate in endotoxin-resistant mice undergoing hemorrhage is independent of tumor necrosis factor availability. J Surg Res. 1994;56:361–6. doi: 10.1006/jsre.1994.1056. [DOI] [PubMed] [Google Scholar]
- 26.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, et al. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 2006;202:407–17. doi: 10.1016/j.jamcollsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Ao L, Song Y, Raeburn CD, Fullerton DA, Harken AH. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-alpha receptor. Am J Physiol Regul Integr Comp Physiol. 2005;288:R600–R606. doi: 10.1152/ajpregu.00182.2004. [DOI] [PubMed] [Google Scholar]
- 28.Barsness KA, Arcaroli J, Harken AH, Abraham E, Banerjee A, Reznikov L, et al. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–R599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 29.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–7. [PubMed] [Google Scholar]
- 30.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–21. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 31.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 32.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, et al. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–61. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L738–L746. doi: 10.1152/ajplung.00280.2005. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y, et al. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol. 2009;182:572–80. doi: 10.4049/jimmunol.182.1.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noulin N, Quesniaux VF, Schnyder-Candrian S, Schnyder B, Maillet I, Robert T, et al. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J Immunol. 2005;175:6861–9. doi: 10.4049/jimmunol.175.10.6861. [DOI] [PubMed] [Google Scholar]
- 36.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/ reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–8. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 37.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS ONE. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, et al. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–8. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 39.Hang T, Jiang SS, Gong JB, Lu TF, Song Y, Zhuge HH. [Expression of Toll-like receptor 2/4 mRNA in myocardium in mice with hemorrhagic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2006;18:554–7. [PubMed] [Google Scholar]
- 40.Chen H, Koustova E, Shults C, Sailhamer EA, Alam HB. Differential effect of resuscitation on Toll-like receptors in a model of hemorrhagic shock without a septic challenge. Resuscitation. 2007;74:526–37. doi: 10.1016/j.resuscitation.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Wang Y, Zhang Z, Wang C, Peng M. TLR4 REGULATE HEME OXYGENASE-1 EXPRESSION AFTER HEMORRHAGIC SHOCK INDUCED ACUTE LUNG INJURY IN MICE: REQUIREMENT OF p38MAPK ACTIVATION. Shock. 2008 doi: 10.1097/SHK.0b013e318188f7e1. [DOI] [PubMed] [Google Scholar]
- 42.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luyer MD, Buurman WA, Hadfoune M, Wolfs T, van't Veer C, Jacobs JA, et al. Exposure to bacterial DNA before hemorrhagic shock strongly aggravates systemic inflammation and gut barrier loss via an IFN-gamma-dependent route. Ann Surg. 2007;245:795–802. doi: 10.1097/01.sla.0000251513.59983.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lendemans S, Kreuzfelder E, Rani M, Bayeeh E, Schade FU, Flohe SB, et al. Toll-like receptor 2 and 4 expression after severe injury is not involved in the dysregulation of the innate immune system. J Trauma. 2007;63:740–6. doi: 10.1097/01.ta.0000240451.42238.d1. [DOI] [PubMed] [Google Scholar]
- 45.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, et al. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007;44:2625–30. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250–3. [PubMed] [Google Scholar]
- 47.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–9. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 48.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 49.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol. 2007;81:119–28. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 51.Yang R, Gallo DJ, Baust JJ, Watkins SK, Delude RL, Fink MP. Effect of hemorrhagic shock on gut barrier function and expression of stress-related genes in normal and gnotobiotic mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1263–R1274. doi: 10.1152/ajpregu.00278.2002. [DOI] [PubMed] [Google Scholar]
- 52.Zhai Y, Qiao B, Shen XD, Gao F, Busuttil RW, Cheng G, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–22. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation. 2007;83:1628–34. doi: 10.1097/01.tp.0000266917.39958.47. [DOI] [PubMed] [Google Scholar]
- 54.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 55.Tsung A, Stang MT, Ikeda A, Critchlow ND, Izuishi K, Nakao A, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1261–G1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 56.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, et al. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–10. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 57.Oyama J, Blais C, Jr., Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–9. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 58.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, et al. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128:170–9. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 59.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, et al. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–71. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 60.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 61.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol. 2009;296:H1–12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, et al. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 63.Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, et al. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 2007;7:5. doi: 10.1186/1472-6793-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72:384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 65.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, et al. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res. 2008;78:546–53. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 67.Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg. 2006;82:2017–23. doi: 10.1016/j.athoracsur.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 68.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van LG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, et al. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834–8. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 70.Wolfs TG, Buurman WA, van SA, de VB, Daemen MA, Hiemstra PS, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–93. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 71.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–7. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 72.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol. 2007;178:6252–8. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 74.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–59. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, et al. Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain. 2006;129:2158–76. doi: 10.1093/brain/awl139. [DOI] [PubMed] [Google Scholar]
- 77.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, et al. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun. 2007;359:574–9. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 78.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Markus T, Hansson S, Amer-Wahlin I, Hellstrom-Westas L, Saugstad OD, Ley D. Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatr Res. 2007;62:71–7. doi: 10.1203/PDR.0b013e31811ead6e. [DOI] [PubMed] [Google Scholar]
- 80.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–14. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 81.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Svedin P, Nie C, Lapatto R, Zhu C, Gustavsson M, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol. 2007;61:263–71. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 83.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–11. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA. Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med. 2008;9:105–9. doi: 10.1097/01.PCC.0000288717.44702.C0. [DOI] [PubMed] [Google Scholar]
- 86.Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563–70. doi: 10.1097/SHK.0b013e31816a3458. [DOI] [PubMed] [Google Scholar]
- 87.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–21. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneeberger H, Schleibner S, Illner WD, Messmer K, Land W. The impact of free radical-mediated reperfusion injury on acute and chronic rejection events following cadaveric renal transplantation. Clin Transpl. 1993:219–32. [PubMed] [Google Scholar]
- 89.de GH, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–4. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Tsoulfas G, Takahashi Y, Ganster RW, Yagnik G, Guo Z, Fung JJ, et al. Activation of the lipopolysaccharide signaling pathway in hepatic transplantation preservation injury. Transplantation. 2002;74:7–13. doi: 10.1097/00007890-200207150-00003. [DOI] [PubMed] [Google Scholar]
- 91.Deng JF, Geng L, Qian YG, Li H, Wang Y, Xie HY, et al. The role of toll-like receptors 2 and 4 in acute allograft rejection after liver transplantation. Transplant Proc. 2007;39:3222–4. doi: 10.1016/j.transproceed.2007.02.102. [DOI] [PubMed] [Google Scholar]
- 92.Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–43. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 93.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–87. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 94.Andrade CF, Kaneda H, Der S, Tsang M, Lodyga M, Chimisso Dos SC, et al. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J Heart Lung Transplant. 2006;25:1317–23. doi: 10.1016/j.healun.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 95.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–4. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 96.de GR, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect. 2006;8:1859–65. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 97.Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, et al. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol. 2008;19:2321–30. doi: 10.1681/ASN.2007111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galloway E, Shin T, Huber N, Eismann T, Kuboki S, Schuster R, et al. Activation of hepatocytes by extracellular heat shock protein 72. Am J Physiol Cell Physiol. 2008;295:C514–C520. doi: 10.1152/ajpcell.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–90. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 100.Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte Toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8:810–5. doi: 10.1016/j.ejheart.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Kimura F, Itoh H, Ambiru S, Shimizu H, Togawa A, Yoshidome H, et al. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg. 2004;187:777–84. doi: 10.1016/j.amjsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 102.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–46. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 103.Mori T, Tan J, Arendash GW, Koyama N, Nojima Y, Town T. Overexpression of human S100B exacerbates brain damage and periinfarct gliosis after permanent focal ischemia. Stroke. 2008;39:2114–21. doi: 10.1161/STROKEAHA.107.503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Einck L, Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985;156:295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- 105.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 106.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 107.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–14. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, et al. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 109.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 111.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–7. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 112.El MR, El GM, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007;282:16336–44. doi: 10.1074/jbc.M608467200. [DOI] [PubMed] [Google Scholar]
- 114.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–53. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 116.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 117.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–64. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 120.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem. 2000;283:214–21. doi: 10.1006/abio.2000.4623. [DOI] [PubMed] [Google Scholar]
- 121.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–93. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 122.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 123.Sasada M, Pabst MJ, Johnston RB., Jr. Activation of mouse peritoneal macrophages by lipopolysaccharide alters the kinetic parameters of the superoxide-producing NADPH oxidase. J Biol Chem. 1983;258:9631–5. [PubMed] [Google Scholar]
- 124.Kim JH, Na HJ, Kim CK, Kim JY, Ha KS, Lee H, et al. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic Biol Med. 2008;45:885–96. doi: 10.1016/j.freeradbiomed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 125.Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, Riboldi E, et al. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol. 2004;173:5749–56. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- 126.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 127.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 128.Bol GF, Jurrmann N, Brigelius-Flohe R. Recruitment of the interleukin-1 receptor (IL-1RI)-associated kinase IRAK to the IL-1RI is redox regulated. Biol Chem. 2003;384:609–17. doi: 10.1515/BC.2003.068. [DOI] [PubMed] [Google Scholar]
- 129.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–9. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 130.Takeshita F, Ishii KJ, Kobiyama K, Kojima Y, Coban C, Sasaki S, et al. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur J Immunol. 2005;35:2477–85. doi: 10.1002/eji.200526151. [DOI] [PubMed] [Google Scholar]
- 131.Cuschieri J, Bulger E, Garcia I, Maier RV. Oxidative-induced calcium mobilization is dependent on annexin VI release from lipid rafts. Surgery. 2005;138:158–64. doi: 10.1016/j.surg.2005.03.018. [DOI] [PubMed] [Google Scholar]