Abstract
Background
Analyses from double-blind randomized trials have reported lower mortality among participants who were more adherent to placebo compared with those who were less adherent. We explored this phenomenon by analyzing data from the placebo arm of the Heart and Estrogen/Progestin Replacement Study (HERS), a randomized, double-blind, placebo-controlled trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women.
Aims
Our primary aim was to measure and explain the association between adherence to placebo and total mortality among the placebo-allocated participants in the HERS trial. Secondary aims included assessment of the association between placebo adherence and cause-specific morbidity and mortality.
Methods
Participants with "higher placebo adherence" were defined as having taken at least 75% of their placebo study medication during each individual’s participation in the study, while those with “lower placebo adherence” took <75%. The primary outcome was in-study all-cause mortality.
Results
More adherent participants had significantly lower total mortality compared to less-adherent participants (HR = 0.52, 95% Confidence Interval: 0.29–0.93). Adjusting for available confounders did not change the magnitude or significance of the estimates. Analyses revealed that the association of higher adherence and mortality might be explained, in part, by time-dependent confounding.
Conclusions
Analyses of the HERS trial data support a strong association between adherence to placebo study medication and mortality. While probably not due to simple confounding by healthy lifestyle factors, the underlying mechanism for the association remains unclear. Further analyses of this association are necessary to explain this observation.
Keywords: Double-blind clinical trials, Placebo, Adherence
Introduction
Double-blind clinical trials provide a unique opportunity to measure the health effects of adherence itself by studying only the placebo-allocated participants. In post-hoc analyses of several clinical trials, participants with higher adherence to placebo had substantially reduced mortality compared to those with lower adherence 1–7. Possible explanations for this relationship include publication bias, adherence as a proxy for a healthy lifestyle, and time-dependent confounding (i.e., a serious underlying disease process causing reduced compliance as well as death). This report is part of a series of detailed analyses examining the association of placebo adherence with mortality 8,9.
Methods
Study Description
We used data from the Heart and Estrogen/Progestin Replacement Study (HERS), a randomized, double-blind, placebo-controlled trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women 10. Eligible participants were postmenopausal women younger than 80 years with an intact uterus and established coronary heart disease. Participants were randomized to either 1 tablet daily containing both 0.625 mg conjugated equine estrogens and 2.5 mg medroxyprogesterone acetate, or an identical-appearing placebo. Follow-up visits occurred every 4 months for 4–5 years. The trial showed no difference in the risk of all-cause mortality nor coronary heart disease between the two treatment arms and significantly more thromboembolic events in the active-treatment group 11. Data for these analyses were obtained from the HERS Coordinating Center and were analyzed in collaboration with the original study investigators 10.
Analytic Methods
Our primary aim was to measure and elucidate the association between adherence to placebo and total mortality among the placebo-allocated women in the HERS trial. Secondary aims included assessment of the association between placebo adherence and cause-specific mortality, incident cardiovascular disease events, and cancer. Additional details regarding the methods for these analyses have been published previously 8.
Participants with "higher placebo adherence" were defined as having taken at least 75% of their placebo study medication over the entire course of each individual’s participation in the study. We conducted a sensitivity analysis on this definition by recalculating the association between placebo adherence and total morality while varying the definition of adherence from 50% to 95%. For the primary analyses, the total mean adherence was calculated as the total number of pills taken (as determined by the difference between number of pills dispensed and number of pills returned over the course of the study) divided by the total number of pills that should have been taken if adherence was 100% (as determined by total number of days assigned to study medication). We also calculated adherence as a cumulative mean adherence variable at each visit (i.e., cumulative adherence up to the study visit just prior to the current visit), and also as a simple time-dependent adherence variable (i.e., the adherence for the time between the most recent visit and visit prior to that one only; all other adherence measurements were ignored for this calculation). In addition, total mean adherence was modeled as a continuous variable in one set of analyses. No missing data were imputed.
Some participants had adherence measurements that, for some visits, exceeded 100%. The adherence determinations at these visits were adjusted as follows: those measurements between 100% and 125% were capped at 100%; those measurements that exceeded 125% were set to missing (i.e., we assumed that these values were data-entry errors).
The distribution of the following baseline characteristics were examined by adherence: age, race, education, marital status, systolic blood pressure, diabetes medication use, body mass index, smoking status, LDL and HDL cholesterol, exercise status, alcohol use and perceived health status. Differences in baseline variables were tested for statistical significance with t-tests for continuous variables and chi-squared tests for categorical variables.
The primary outcome was total in-study mortality. Secondary outcomes included coronary heart disease mortality (fatal myocardial infarction, sudden death within 1 hour of onset of symptoms, unobserved death that occurred out of the hospital in the absence of other known cause, and death due to coronary revascularization or congestive heart failure), all cardiovascular disease mortality (coronary heart disease, and stroke mortality), and non-cardiovascular disease mortality. We also examined the incidence of fatal or non-fatal coronary heart disease and cardiovascular disease events and incident cancer to capture additional outcomes that may be related to healthy lifestyle behaviors.
The primary analytic approach was survival analysis 12. The association between placebo adherence and each outcome was analyzed by constructing separate Kaplan-Meier curves for higher-adherent and lower-adherent participants and testing the significance of the difference between the curves by log-rank tests. Multivariable analyses were conducted with Cox proportional hazards models 12. Baseline values of covariates were used for adjustment in the multivariable analyses, as in the prior analyses of other datasets for this study 8.
An important potential bias is time-dependent confounding or "effect-cause artifact": i.e., that some ultimately fatal condition with a prodrome caused the participant's death and also resulted in reducing the participant's adherence in the 4-to-8 month period prior to the fatal event. In order to examine this possibility, we repeated the analyses for total mortality after deleting each participant's last adherence measurement and last two measurements (these procedures remove the effect of the adherence measurements in the months just prior to a participant's death). We also repeated the proportional-hazards models, introducing a lagged and a twice-lagged adherence variable, which also eliminates the influence of the last and last two adherence measurements, respectively; these procedures were conducted on both the cumulative-adherence variable and the time-dependent adherence variable analyses. All analyses were performed with SAS v. 9.213 and STATA v. 10.014
Results
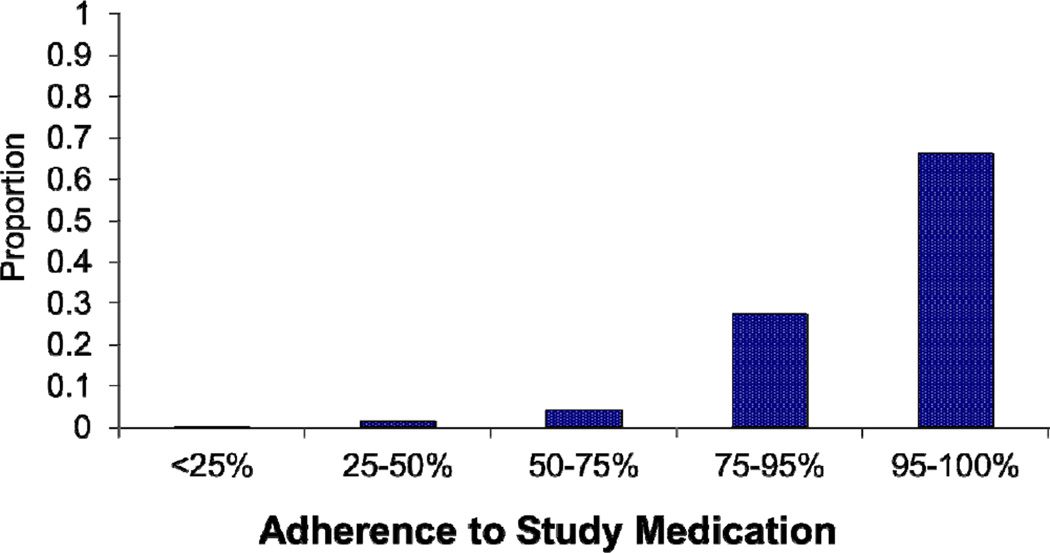
In the HERS study, 1375 participants were randomized to receive a placebo. Among the placebo-allocated participants, 85 (6.2%) took less than 75% of their prescribed study medication (Figure 1). Lower adherence was associated with African-American and Hispanic race/ethnicity as well as higher LDL-cholesterol. (Table 1). Overall, there were 120 in-study deaths (Table 2) in the placebo group, with 68 (57%) attributable to cardiovascular causes and, of those, the majority (84%) were due to coronary heart disease. Additionally, there were 184 coronary heart disease events and 99 cases of incident cancer.
Figure 1.
Distribution of total in-study adherence levels among placebo-allocated participants in the HERS study
Table 1.
Characteristics of placebo-allocated participants, overall and by adherence level
| Baseline Characteristic |
Overall N=1375 |
Higher Adherence1 N=1290 (93.8%) |
Lower Adherence2 N=85 (6.2%) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age in years, mean (SD) | 66.8 (6.7) | 66.8 (6.7) | 66.6 (6.7) | 0.80 |
| Race3, N (%) | <0.01 | |||
| White | 1232(89.6) | 1169 (90.6) | 63 (74.1) | |
| African-American | 102 (7.4) | 84 (6.5) | 18 (21.2) | |
| Asian | 5 (0.4) | 5 (0.4) | 0 (0) | |
| Hispanic | 28 (2.0) | 24 (1.9) | 4 (4.7) | |
| Other | 8 (0.7) | 8 (0.8) | 0 (0) | |
| Married, N (%) | 791 (57.5) | 740 (57.4) | 51 (60.0) | 0.63 |
| Education, N (%) | 0.41 | |||
| Less than high school | 279 (20.3) | 257 (19.9) | 22 (25.9) | |
| High school graduate | 547 (39.8) | 513 (39.8) | 34 (40.0) | |
| Some college | 347 (25.2) | 331 (25.7) | 16 (18.8) | |
| College graduate | 202 (14.7) | 189 (14.7) | 13 (15.3) | |
| Clinical Characteristics | ||||
| Systolic Blood Pressure, mmHg, mean (SD) | 135.1 (19.3) | 135.0 (19.1) | 137.6 (22.7) | 0.22 |
| Diabetes Medications, N (%) | 244 (17.8) | 227 (17.6) | 17 (20.0) | 0.57 |
| Body mass index, N (%) | 0.30 | |||
| ≤25 | 390 (28.4) | 360 (27.9) | 30 (35.3) | |
| 26 – 29 | 519 (37.8) | 488 (37.8) | 31 (36.5) | |
| ≥ 30 | 466 (33.9) | 442 (34.3) | 24 (28.2) | |
| Current smoker, N (%) | 0.31 | |||
| Never | 537 (39.1) | 502 (38.9) | 35 (41.2) | |
| Current | 181 (13.6) | 166 (12.9) | 15 (17.7) | |
| Former | 657 (47.8) | 622 (48.2) | 35 (41.1) | |
| Laboratory Parameters | ||||
| LDL-cholesterol (mg/dl), mean (SD) | 144.9 (37.3) | 144.4 (36.7) | 152.7 (44.9) | 0.05 |
| HDL-cholesterol (mg/dl), mean (SD) | 50.4 (13.2) | 50.5 (13.2) | 49.8 (13.3) | 0.63 |
| Health Characteristics/Status | ||||
| Exercise Status (> 3 times per week), N (%) | 527 (38) | 498 (38.6) | 29 (34.1) | 0.41 |
| Alcohol use (average drinks per day), N (%) | ||||
| None | 817 (59.4) | 762 (59.1) | 55 (64.7) | 0.43 |
| 1–2 | 492 (35.8) | 467 (36.2) | 25 (29.4) | |
| >2 | 66 (4.8) | 61 (4.7) | 5 (5.9) | |
| Perceived Health Status4, N (%) | ||||
| Excellent | 54 (3.9) | 53 (4.1) | 1 (1.2) | 0.44 |
| Very Good | 336 (24.5) | 316 (24.5) | 20 (23.5) | |
| Good | 656 (47.7) | 617 (47.9) | 39 (45.9) | |
| Fair | 298 (21.7) | 275 (21.3) | 23 (27.1) | |
| Poor | 30 (2.2) | 28 (2.1) | 2 (2.4) |
“Higher Adherence” defined as >= 75% total mean in-study placebo medication adherence.
“Lower Adherence” defined as < 75% total mean in-study placebo medication adherence.
Asian/Hispanic/Other categories of race were combined for the Chi-square test.
Excellent/Very Good and Fair/Poor perceived health status categories were combined for the Chi-square test.
Table 2.
Number and Percent of Events and Unadjusted Hazard Ratios for Association Between Total Mean Adherence and Several Outcomes
| Outcome | Higher-Adherent Participants (n=1290) N (%) |
Lower-Adherent Participants (n=85) N (%) |
HR | 95% CI |
|---|---|---|---|---|
| Total Mortality | 107 (8) | 13 (15) | 0.52 | (0.29, 0.93) |
| Cardiovascular Disease Mortality |
62 (5) | 6 (7) | 0.66 | (0.28, 1.52) |
| Non- Cardiovascular Disease Mortality |
45 (3) | 7 (8) | 0.40 | (0.18, 0.90) |
| Coronary Heart Disease Mortality |
51 (4) | 6 (7) | 0.54 | (0.23, 1.26) |
| Incident Coronary Heart Disease Events |
173 (13) | 11 (13) | 0.95 | (0.52, 1.76) |
| Incident Cancer | 80 (6) | 11 (13) | 0.42 | (0.26, 0.79) |
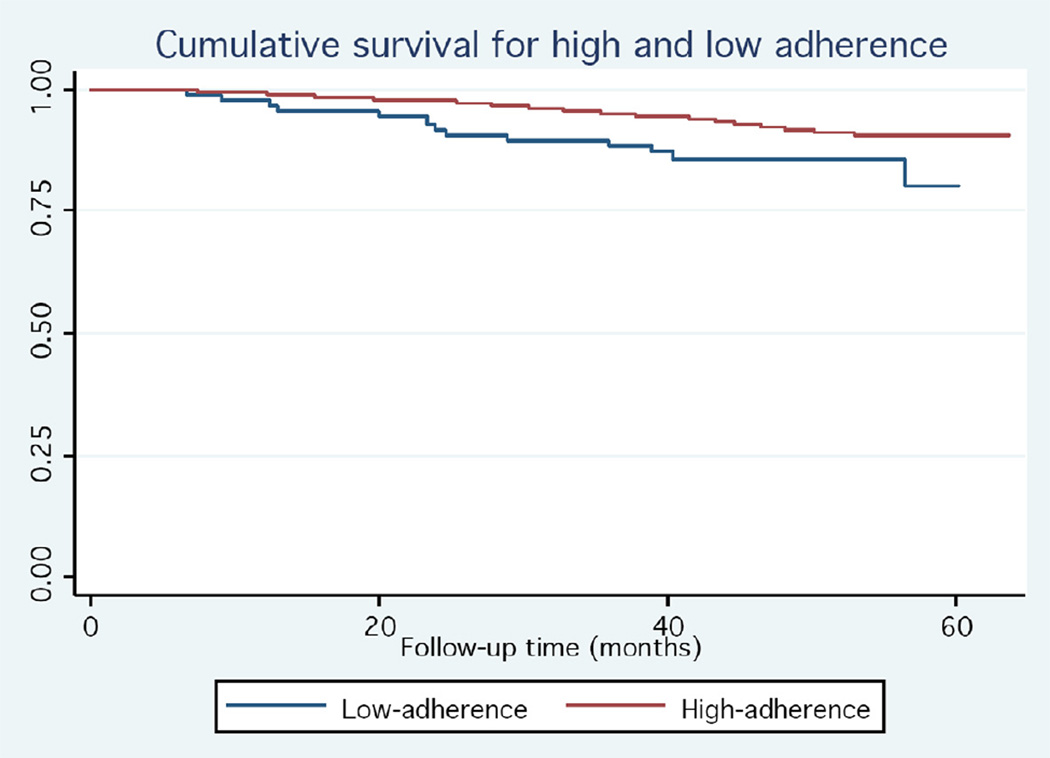
Participants with ≥75% adherence had a significantly lower total mortality compared to less-adherent participants (HR = 0.52, 95% Confidence Interval (CI): 0.29 to 0.93; Table 2 and Figure 2). For cause-specific mortality, the association was found to be statistically significant for non- cardiovascular disease mortality, but was attenuated and non-significant for cardiovascular disease or coronary heart disease - related deaths. Higher-adherent participants were also significantly less likely to be newly diagnosed with cancer during the study period (Table 2).
Figure 2.
Kaplan Meier curves of total mortality for higher-adherent (blue line) and lower-adherent (red line) placebo-allocated participants in the HERS study.
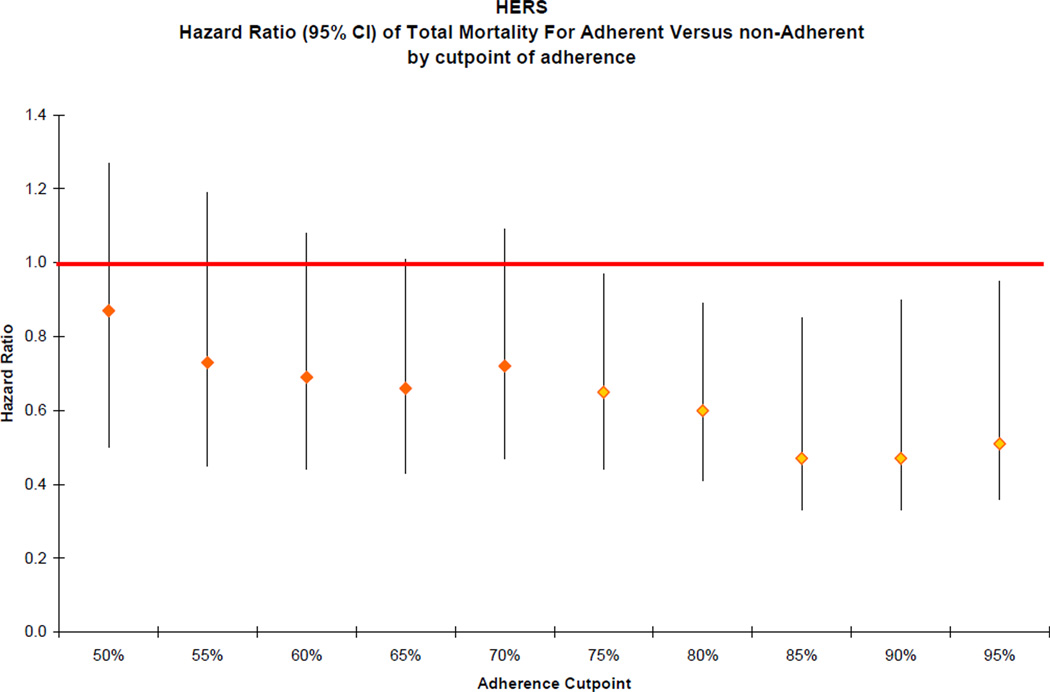
The association between placebo adherence and total mortality persisted when adherence was treated as a continuous measurement though it was not statistically significant (HR = 0.91, 95% CI: 0.81–1.03 for every 10% increase in adherence). The association was stronger when adherence was used as a time-varying covariate (HR = 0.16, 95% CI: 0.11 to 0.25, for participants with ≥75% adherence versus those who were less adherent). When adherence was calculated as a mean cumulative adherence variable, the association remained significant (HR = 0.27, 95% CI: 0.15 to 0.48). The cutpoints for defining increased adherence for which the association with mortality was strongest were found to be at 85% and 90% adherence (HR = 0.47, 95% CI: 0.33 to 0.67 for both; Figure 3).
Figure 3.
Hazard ratios (95% CI) of total mortality for more-adherent versus less-adherent participants by cutpoints of adherence in the HERS study
Adjustment for potential confounders did not result in a meaningful change in the association for any outcome, though the association for total mortality attenuated slightly and was no longer significant (Table 3). Adjustment for modifiable and non-modifiable cardiovascular disease risk factors, as well as psychosocial variables, had little effect on the hazard ratios (Table 3).
Table 3.
Adjusted Hazard Ratios for Association Between Total Mean Adherence and Several Outcomes (N=1375)
| Outcome | Non-modifiable Risk Factors1 |
Modifiable Risk Factors2 |
Full Model3 |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Total Mortality | 0.55 (0.31, 0.98) | 0.61 (0.34, 1.10) | 0.64 (0.36, 1.16) |
| Cardiovascular Disease Mortality |
0.71 (0.31, 1.65) | 0.81 (0.35, 1.88) | 0.88 (0.38, 2.08) |
| Non- Cardiovascular Disease Mortality |
0.41 (0.18, 0.91) | 0.45 (0.20, 1.01) | 0.44 (0.20, 1.01) |
| Coronary Heart Disease Mortality |
0.60 (0.25, 1.40) | 0.69 (0.29, 1.61) | 0.76 (0.32, 1.81) |
| Incident Coronary Heart Disease Events |
1.03 (0.56, 1.91) | 1.13 (0.61, 2.09) | 1.25 (0.67, 2.32) |
| Incident Cancer | 0.43 (0.23, 0.81) | 0.41 (0.22, 0.77) | 0.41 (0.21, 0.78) |
Model 1- Age, race
Model 2- Smoking status, LDL-cholesterol, HDL-cholesterol, exercise status, BMI category, mean systolic BP, diabetes (diagnosis or taking insulin)
Model 3- All of the above-listed variables and education, marital status, perceived health status
Several analyses were conducted to examine the possibility that the association between placebo adherence and mortality was the result of a fatal illness that caused both the participant's death and reduced adherence. First, we estimated the association after eliminating the last and the last two adherence measurements; this procedure resulted in substantial attenuation in the association as well as a profound reduction in the precision of the estimate (HR = 0.81, 95% CI: 0.30 to 2.20 and HR = 1.23, 95% CI: 0.30 to 4.99). Next, we lagged the adherence variable (i.e., did not include the ultimate and penultimate observations) in the survival models with cumulative adherence by one and by two measurements (HR = 0.65, 95% CI: 0.24 to 1.77 and HR = 1.09, 95% CI: 0.27 to 4.43, respectively). Finally, when adherence was entered as a single time-dependent variable, the association attenuated and was no longer statistically significant after lagging the adherence predictor (HR = 0.63, 95% CI: 0.28 to 1.45 for one lag and HR = 1.04, 95% CI: 0.33 to 3.30 for two lags).
Tests of violation of the proportionality assumption in the primary Cox model were not significant.
Discussion
Participants in the HERS trial who were randomly assigned to placebo and demonstrated higher adherence to placebo medication had a 48% lower total mortality rate compared to those with lower adherence to placebo. Placebo, by definition, has no specific biologic activity, therefore, it may be counterintuitive that better adherence to placebo would be associated with improved health outcomes. However, those who adhere to any medication may be different from those who do not in ways that affect their survival. Despite adjustment for the numerous available co-variables, however, these factors only slightly attenuated the association. This was not surprising given the lack of association of adherence with most of these baseline characteristics (Table 1). In addition, there may be unmeasured confounding that we were not able to capture in the secondary analysis of these data.
One possible explanation for this relationship is that placebo adherence is merely a marker for adherence to other life-prolonging medications, but older data suggest this is unlikely. The association between placebo adherence and survival was first described by Canner et al. using data from the Coronary Drug Project, a large, double-blind secondary prevention trial of cholesterol-lowering medications, conducted between 1966 and 1975 6. It is unlikely that the survival advantage of the more-adherent participants in the placebo arm of this study could be explained by greater adherence with other life-prolonging medications, since few such drugs existed at the time (e.g., statins, angiotensin-converting enzyme inhibitors, and calcium channel blockers were developed only after the study was completed). While beta blockers and thiazide diuretics were available, adjustment for blood pressure did not attenuate the association in this study 6.
Another possibility is that some patients developed fatal illnesses with a prodrome (such as cancer or progressive congestive heart failure), which could be responsible for both the participant's death and a reduction in their adherence to study medication (because of the participant’s decline in health prior to their death). Using our lag-adherence methodology to investigate this possibility, we found that dropping the last one-to-two adherence measurements (or lagging the adherence variable by one-to-two measurements) resulted in substantial attenuation of the HR with both the cumulative mean adherence and time-dependent adherence variables. This suggests that some of the observed effect of adherence on mortality in the HERS data could be a consequence of this "effect-cause" artifact. Taken together, the results suggest that the apparently protective effect and the relatively tight CIs of the unlagged analyses are substantially driven by what happens in the last two reporting periods before death. Nevertheless, the wider confidence intervals of the time-dependent analysis indicate that the influence of this potential bias remains uncertain.
Most prior published studies are consistent with the presence and strength of the bivariate and multivariate associations identified in HERS, but few had specifically examined the possibility of an effect-cause bias. In a prior analysis of the two Studies Of Left Ventricular Dysfunction trials, we did not find strong evidence for the presence of this bias though the results were not entirely uniform and were compatible with a small element of this effect 8.
The HERS data had several strengths for these analyses, including a large sample of women, a substantial event rate, good follow-up and high data quality. However, the HERS data had limited information about many important lifestyle covariates such as daily exercise, self-perceived well-being, and psycho-social measures. Finally, all adherence measures were based on pill counts. Although they may be susceptible to manipulation by study participants, the pill counts were necessary for comparison to other studies of this association between adherence and mortality. The cutpoint of 75% is consistent with the definition used in previous studies3,4,9,15 and in-between those used in others1,16. The proportion of lower-adherent study participants is small (6%) yet similar to that in previous studies using this definition3,4,9,15.
In conclusion, adherence to placebo medication was associated with a lower risk of total in-study mortality and other outcomes in this secondary analysis of the HERS data and these associations were not due to confounding by measured healthy lifestyle factors. The association of higher adherence with reduced mortality was markedly attenuated when the effect of time periods proximal to death were removed, suggesting that some of the observed association could be due to an external factor (such as a fatal illness), which caused both mortality and the lower adherence. However, this finding is highly imprecise and whether this phenomenon is generally true requires analysis of additional, larger datasets. These analyses do not fully explain the association of placebo adherence and reduced mortality, which should be examined in analyses of other studies. It is likely that prospective studies designed to answer this question specifically will be required to more definitively understand this phenomenon.
Acknowledgements
Supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI), no. R01 HL081195. The Heart and Estrogen/Progestin Replacement Study (HERS) was conducted and supported by Wyeth-Ayerst Research. The manuscript does not necessarily reflect the opinions or views of the HERS investigators or Wyeth-Ayerst Research.
Funding: National Heart, Lung, Blood Institute (R01 HL081195).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None declared.
Authorship: All authors had access to the data and a role in writing the manuscript.
References
- 1.Irvine J, Baker B, Smith J, et al. Poor adherence to placebo or amiodarone therapy predicts mortality: results from the CAMIAT study. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial. Psychosom Med. 1999;61:566–575. doi: 10.1097/00006842-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher EJ, Viscoli CM, Horwitz RI. The relationship of treatment adherence to the risk of death after myocardial infarction in women. JAMA. 1993;270:742–744. [PubMed] [Google Scholar]
- 4.Horwitz RI, Viscoli CM, Berkman L, et al. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336:542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 5.Walker AS, Ford D, Mulenga V, et al. Adherence to both cotrimoxazole and placebo is associated with improved survival among HIV-infected Zambian children. AIDS Behav. 2009;13:33–41. doi: 10.1007/s10461-008-9382-4. [DOI] [PubMed] [Google Scholar]
- 6.Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303:1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 7.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 8.Avins AL, Pressman A, Ackerson L, Rudd P, Neuhaus J, Vittinghoff E. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. J Gen Intern Med. 2010;25:1275–1281. doi: 10.1007/s11606-010-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pressman A, Avins AL, Neuhaus J, Ackerson L, Rudd P. Adherence to placebo and mortality in the Beta Blocker Evaluation of Survival Trial (BEST) Contemp Clin Trials. 2012 doi: 10.1016/j.cct.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998;19:314–335. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 11.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 12.Kleinbaum DGKM. Survival analysis. 2nd ed. ed. New York: Springer; 2005. [Google Scholar]
- 13.SAS statistical software. Edition 9.2. 2008. [Google Scholar]
- 14.STATA. Edition 10.0. College Station, TX: Stata Corporation; 2008. [Google Scholar]
- 15.Avins AL, Pressman A, Ackerson L, Rudd P, Neuhaus J, Vittinghoff E. Placebo adherence and its association with morbidity and mortality in the studies of left ventricular dysfunction. Journal of general internal medicine. 2010;25:1275–1281. doi: 10.1007/s11606-010-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazajkowski SMCM, Smith AW, editors. Adherence and placebo effect. New York: Springer Publishing Company, Inc.; 1990. [Google Scholar]