Single-Neuron Dynamics in Human Focal Epilepsy.
Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Nat Neurosci 2011;14:635–641
Epileptic seizures are traditionally characterized as the ultimate expression of monolithic, hypersynchronous neuronal activity arising from unbalanced runaway excitation. Here we report the first examination of spike train patterns in large ensembles of single neurons during seizures in persons with epilepsy. Contrary to the traditional view, neuronal spiking activity during seizure initiation and spread was highly heterogeneous, not hypersynchronous, suggesting complex interactions among different neuronal groups even at the spatial scale of small cortical patches. In contrast to earlier stages, seizure termination is a nearly homogenous phenomenon followed by an almost complete cessation of spiking across recorded neuronal ensembles. Notably, even neurons outside the region of seizure onset showed significant changes in activity minutes before the seizure. These findings suggest a revision of current thinking about seizure mechanisms and point to the possibility of seizure prevention based on spiking activity in neocortical neurons.
Commentary
Countless articles in the field of epilepsy begin with a statement such as: “Epileptic seizures are caused by an imbalance of cerebral excitation and inhibition.” This seems to be a fundamental truth that is rarely questioned. The International League Against Epilepsy (ILAE) defines seizures as “excessive or synchronous neuronal activity”(1). Truccolo et al. report on single-neuron activity in humans before, during, and after seizures by utilizing microelectrode recordings. They find that neuronal firing during seizures is much more heterogeneous than we assumed and can probably not be explained based on a simple model of excitation and inhibition.
They implanted a 96-channel, tightly spaced microelectrode array in the neocortex of four patients (Neuroport, Blackrock Microsystems Inc., Salt Lake City, UT). The array was placed in an area that was likely to be resected in epilepsy surgery. It covered an area of 4 mm2 and recorded at a depth of 1 mm. They investigated single-neuron firing during eight seizures by routine intracranial EEG prior to epilepsy surgery. They distinguished between the interictal, preictal, ictal, and postictal periods. They found that neuronal firing rate increased in 45.5% of all recorded neurons and decreased in 9.9%. Firing patterns were heterogeneous and followed no uniform pattern.
What is the Beginning and the End?
At the onset of the seizure, neuronal firing did not uniformly increase as expected in a purely excitatory event. Heterogeneity of firing patterns increased at seizure onset, as reflected by a measure of variance, the Fano Factor (Figure 1). The authors suggest their findings argue “against homogeneous runaway excitation or widespread paroxysmal depolarization as the primary mechanism underlying seizure initiation.” However, firing patterns within patients seemed reproducible but neuronal recordings over prolonged periods of time are often too unstable to assess with certainty. The authors determine seizure onset as classically defined by intracranial electrocorticography via visual inspection (Figure 1) and show that in the preictal period, there is already a modulation in baseline firing as compared with the interictal state. Preictal firing rate changes are seen less frequently than ictal firing changes. Preictal firing could increase (11.8% of all neurons) or decrease (7.5% of neurons). These findings raise the question of whether there is a clear-cut seizure onset at all. Where does the preictal period end and the seizure start? Seizure onset may not be a single event in time but rather a continuum.
FIGURE.
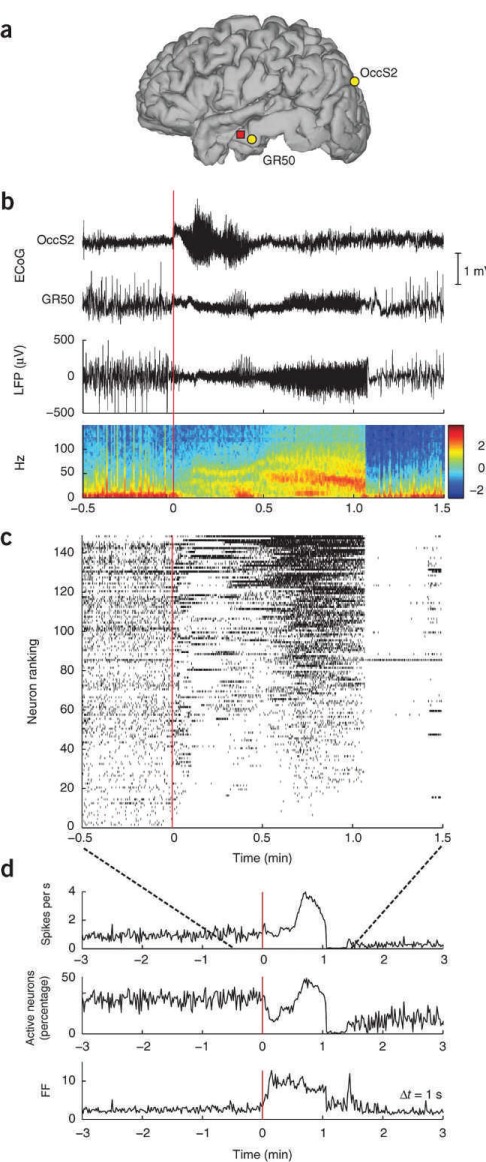
(a) Locations of the microelectrode array in participant A (red square), and subdural ECoG electrodes OccS2 and GR50 in occipital and middle temporal cortices, respectively. (b) ECoG traces recorded at the locations shown in Figure 1a during seizure 1. The ECoG-based onset area was identified to be under the occipital electrode OccS2. Seizure onset is at time 0. The local field potential (LFP) recorded from a single channel in the microelectrode array and the corresponding spectrogram (in dB) are shown below. (c) Neuronal spike raster plot including all recorded neurons (n = 149). Each hash mark represents the occurrence of an action potential. Neurons were ranked (vertical axis) in increasing order according of their mean spiking rate during the seizure. (This ranking number is unrelated to physical location.) Toward the end of the seizure, activity across the population became more homogeneous, until spiking was abruptly interrupted at seizure termination. With the exception of a few neurons, spiking in the recorded population remained suppressed for about 20 seconds. (d) The mean population rate, the percentage of active neurons, and the Fano factor (FF) of the spike counts across different neurons at a given time (determined in 1-second time bins). These were roughly stationary during the several minutes preceding the seizure onset. An increase in the FF, reflecting the heterogeneity in neuronal spiking, is observed around seizure onset and precedes an increase in the mean population rate.
The authors propose that the changes in preictal firing may ultimately lead to a viable seizure prediction algorithm. After decades of searching for a reliable seizure prediction algorithm, this may be another venue to pursue. Rightly, the authors caution that their data are too premature to be conclusive. More single-unit recordings of seizures are necessary to test whether this is a workable algorithm.
We may have an easier time with determining the end of a seizure. The authors' second major observation is the “abrupt and widespread suppression of neuronal action potentials at seizure end.” This is a very striking and consistent finding (Figure 1). There is neuronal silencing for 20 to 30 seconds after seizure termination.
Seizure termination is thought to be a result of depolarization block owing to changing ion concentrations in the extracellular space (2). However, amplitude of action potentials did not decrease towards the end of the seizure as expected if depolarization block had occurred. The authors argue that depolarization block is not the “primary local factor responsible for the observed marked suppression [of neuronal] spiking”; thus introducing another shift in conventional thinking about the generation and termination of seizures.
Which Cells Do What?
A consistent finding in the study is that neurons that are active interictally have increased firing rates during the ictal and preictal period. Neurons were divided into principal neurons and interneurons in one patient. Both types of cells showed modulation of neuronal firing during the ictal and preictal periods, but in the preictal period, interneurons seemed to be more involved.
In models of seizures, interneurons and pyramidal cells seem to play very distinct roles (3). As it is difficult to record a sufficient number of interneurons and principal cells, the role of these distinct cells needs further investigation.
At the termination of seizure, both interneurons and principal cells were silenced, and the authors hypothesize that subcortical structures may initiate seizure termination. This hypothesis is certainly not easy to confirm in humans.
The Sampling Error in Human Neurophysiology
Truccolo et al. investigated single neurons in four patients, and in only one patient the recordings were performed in what is traditionally called the seizure-onset zone. This patient had a reduction of seizures but was not seizure free after seizure-onset zone resection. This raises the question of whether recordings performed in the seizure-onset zone would show different results and would be more homogeneous.
Single units were only recorded over a small cortical area, so conclusions about greater seizure dynamics may be premature. Recording from a small cortical patch does not account for the spatial sampling error, which is inherent in human electrophysiology. It was also demonstrated that neuronal recordings can be quite diverse depending on the cortical depth of the microelectrode (4). This could mean that findings in layer III, which reflects the depth of the microelectrode array, could be different than in deeper layers of the cortex such as layer IV or V. Another problem with single-unit recordings is certainly the problem of sheer numbers. Of the estimated many billion neurons of the human brain, recording from 241 cells represents a minuscule amount. Any conclusions could be over- or underestimating the effect.
Single-Neuron Recordings
Single-neuron recordings are technically difficult to perform. Artifact contamination and the limited number of neurons that show any firing pose significant hurdles. In addition, neuronal firing in humans is not consistent over time, so comparisons even within patients in recordings days apart are difficult to interpret. Newer technology on a submillimeter scale may allow for further insights into seizure dynamics, such as spiral waves during feline seizures, but may take time to implement in humans (5).
Truccolo et al. certainly made a significant contribution to understanding human single-neuron dynamics during seizures. Their investigation during spikes showed similarly interesting results and also speaks to the heterogenicity of epileptiform activity (6). Certainly, further investigation of single units in humans will help us to further investigate the question “What is a seizure?” with the ultimate goal to find more effective treatments.
Footnotes
Editor's Note: Authors have a Conflict of Interest disclosure which is posted under the Supplemental Materials (192KB, docx) link.
References
- 1.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Bragin A, Penttonen M, Buzsaki G. Termination of epileptic afterdischarge in the hippocampus. J Neurosci. 1997;17:2567–2579. doi: 10.1523/JNEUROSCI.17-07-02567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol. 2006;95:3948–3954. doi: 10.1152/jn.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: Current source density and multiunit analysis in vivo. Epilepsia. 2004;45(suppl):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- 5.Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller CJ, Truccolo W, Gale JT, Eskandar E, Thesen T, Carlson C, Devinsky O, Kuzniecky R, Doyle WK, Madsen JR, Schomer DL, Mehta AD, Brown EN, Hochberg LR, Ulbert I, Halgren E, Cash SS. Heterogeneous neuronal firing patterns during interictal epileptiform discharges in the human cortex. Brain. 2010;133(pt 6):1668–1681. doi: 10.1093/brain/awq112. [DOI] [PMC free article] [PubMed] [Google Scholar]


