Abstract
A practical synthesis of resveratrol 3-O-β-D-glucuronide, suitable for preparation of large quantities, was developed using selective deacetylation of resveratrol triacetate with ammonium acetate. A simplified procedure for large scale preparation of resveratrol is also reported.
Keywords: resveratrol, glucuronide, preparation
INTRODUCTION
Resveratrol 3-O-β-D-glucuronide is one of the key metabolites of resveratrol,[1] a natural compound with a variety of biological activity.[1,2] For purposes of biological studies we were faced with a task of preparation of multigram quantities (also potentially scalable to tens of grams) of resveratrol 3-O-β-D-glucuronide. Several methods have been reported for preparation of this compound.[1,3,4] However, we found them inconvenient for our purposes due to use of relatively expensive starting materials and reagents, or relying on low yielding steps and difficult separations. We thus have ventured to develop a method for its preparation using cheap and available starting materials and reagents.
RESULTS AND DISCUSSION
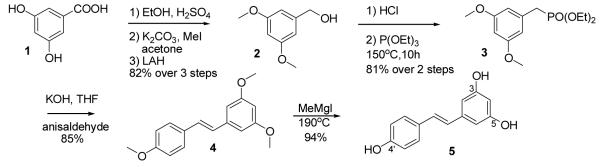
For preparation of resveratrol we selected the reported method using olefination of anisaldehyde with benzylic phosphonate,[5] opting to simplify the conditions. Starting from cheap and readily available 3,5-dihydroxybenzoic acid we prepared diethyl (3,5-dimethoxyphenyl) methylphosphonate using a modification of the reported method. [5,6] While preparation of methyl (3,5-dimethoxyphenyl)benzoate by a direct trimethylation of 3,5-dihydroxybenzoic acid with iodomethane and potassium carbonate in acetone has been reported,[6] we found it more convenient to use the two step sequence of esterification and methylation (Scheme 1), since in our hands the trimethylation reaction suffered from slow conversion, with the reaction still not complete after the reflux for 3 days. LAH reduction of the resulting ester led to 3,5-dimethoxybenzylic alcohol. We found that it was possible to use concentrated aqueous HCl to convert it to the corresponding chloride. The chloride was converted to the phosphonate as described, by Arbuzov reaction.
Scheme 1.
Preparation of resveratrol
Several sets of conditions have been reported for performing the olefination step. However, they were relatively inconvenient for large scale preparations. After attempting several variations of conditions we have found that the use of solid potassium hydroxide in THF as a base was effective for promoting the reaction (Scheme 1).
Demethylation of the obtained trimethylresveratrol (4) has been performed using several methods. Boron tribromide, most commonly employed for this purpose,[7] is rather expensive, an inconvenient in handling. Of other demethylation methods, use of pyridinium hydrochloride[8] appeared generally acceptable, but we found the use of methylmagnesium iodide at elevated temperatures[9] to be the most simple and efficient.
We thus had a supply of large amounts of resveratrol. The previous methods for its 3-O-glycosylation involved either the low yielding direct glycosylation,[1] or statistically controlled TBS diprotection, separation, and glycosylation of the protected derivative.[1,4] Relatively high cost of TBS chloride and low melting point of the TBS protected derivative (prohibiting crystallization as a separation method) lead us to consider the acetate instead. Preparation of the selectively acetylated resveratrols was reported using enzymatic acetylation and deacetylation.[10] We attempted to develop a chemical method for this transformation. Our initial studies on the selective acetylation were not fruitful (although certain selectivity was observed in preferential monoacetylation of 4′-OH of resveratrol). That prompted us to explore the deacetylation route. The triacetylated resveratrol was prepared by treatment with triethylamine and acetic anhydride. To our satisfaction, selective removal of 3-O-acetate group over 4′ was observed using variety of reagents and conditions (catalytic K2CO3-EtOH, catalytic DBU-EtOH-CH2Cl2, catalytic HCl-EtOH, catalytic Ph3P-EtOH/reflux, and NH4OAc-MeOH-THF). We finally settled on using ammonium acetate in a mixture of THF and methanol as the most simple and convenient method for deacetylation, which resulted in preferential formation of 7 over the isomeric diacetate in about 6:1 ratio (by NMR). The resulting diacetate was crystalline, thus it was possible to use crystallization for its purification. We also found methylene chloride-ethyl acetate to be an effective system for separation of the isomers by flash chromatography. By employing the developed procedure we were able to achieve about 40% yield of 7 in a single run. Easy recycling of the by-products (by reacetylation) permitted recovery and reuse of the rest of the material (Scheme 2).
Scheme 2.
Deacetylation of triacetylresveratrol
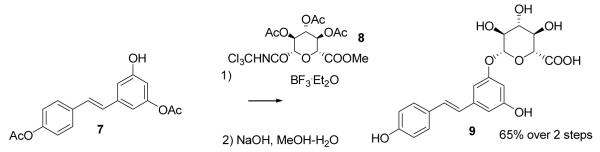
Thus obtained 3,4′-di-O-acetylresveratrol (7) was glycosylated using the trichloroimidate method[11] (attempts to use several other glycosylation agents were not effective). The requisite trichloroimidate, 8, was prepared as reported, from glucuronolactone,[12] using ammonium acetate method for selective monodeacetylation[13] and potassium carbonate as a base for formation of trichloroimidate.[14]
Hydrolysis of the resulting product was performed as reported, using NaOH-MeOH-H2O, providing resveratrol 3-O-β-D-glucuronide.[3]
CONCLUSION
We thus developed a practical way of preparation of resveratrol 3-O-β-D-glucuronide, utilizing the selective deacetylation of triacetyl resveratrol with ammonium acetate. This method can also be used for preparation of other 3-O-resveratrol derivatives. A simplified preparation of resveratrol from 3,5-dihydroxybenzoic acid and anisaldehyde, suitable for preparation of tens of grams of the compound, was also developed.
EXPERIMENTAL
All reactions were carried out under an inert atmosphere of dry nitrogen. Proton magnetic resonance spectra were recorded at 500 MHz on an Avance 500 Bruker spectrometer. Carbon magnetic resonance spectra were recorded at 125 MHz on an Avance 500 Bruker spectrometer. All chemical shifts were reported in δ units relative to tetramethylsilane. High resolution mass spectral data were obtained on the Agilent 61969A TOF high resolution mass spectrometer using electrospray ionization, direct infusion, 10ml/min in 50% MeOH 5mM ammonium formate. Melting points were determined on a MEL-TEMP melting point apparatus. Flash column chromatography was performed using 40-63 μm silica gel (Merck, Geduran, no. 11567-1) as the stationary phase. Tetrahydrofuran (THF) and ether were distilled over lithium aluminum hydride prior to use.
(3,5-dimethoxyphenyl)methanol (2)
Dihydroxybenzoic acid (10 g, 64.9 mmol) was dissolved in 95% ethanol (50 ml). Hexanes (40 ml), chloroform, (10 ml) and conc. H2SO4 (3 ml) were added. The mixture was refluxed for 10 h with a Dean-Stark trap. During this time the bottom layer collecting in the trap was drained several times (about 20 ml combined). After cooling down the mixture, saturated NaHCO3 solution (30 ml) and ethyl acetate (100 ml) were added. The layers were separated, the aqueous layer washed with ethyl acetate (3×30 ml). The combined organic layers were dried over MgSO4, and concentrated. The obtained crude ester was dissolved in acetone (50 ml), K2CO3 (19.7 g, 142.7 mmol) and iodomethane (8.9 ml, 142.7 mmol) were added, and the mixture was refluxed for 10 h. The volatiles were removed in vacuo, the remaining solid taken up in a mixture of ethyl acetate (100 ml) and water (100 ml). The layers were separated, the aqueous layer washed with ethyl acetate (2×30 ml). The combined organic layers were dried over MgSO4, and concentrated. The obtained crude ester was dissolved in THF (60 ml), and lithium aluminum hydride (2.7 g, 71 mmol) was added in portions, waiting for the reaction to subside after each portion. After stirring at rt for 3 h, were added dropwise with vigorous stirring: water (2.7 ml), then, in 5 minutes after completion of addition, 10% NaOH (2.7 ml), then in 5 more minutes, water again (2.7 ml). The mixture was allowed to stir for 1 h, filtered through celite, washing with ethyl acetate, and concentrated to produce (3,5-dimethoxyphenyl)methanol, 8.95 g (82%). The obtained product was directly used in the next step without purification. The physical and spectroscopic properties for the compound match those reported in the literature.[6]
Compound data
(3,5-dimethoxyphenyl)methanol (2)
1H NMR (500 MHz, CDCl3): δ 6.54 (d, J=2 Hz, 2H), 6.40 (t, J=2 Hz, 1H), 4.64 (d, J=4.5 Hz, 2H), 3.8 (s, 6H), 1.76 (br s, 1H).
Diethyl (3,5-dimethoxyphenyl)methylphosphonate (3)
HCl (37% in water, 15 ml) was added to (3,5-dimethoxyphenyl)methanol (3.2 g, 19 mmol) and stirred overnight. The mixture was extracted with hexanes (3×30 ml), washed with saturated NaHCO3, dried over MgSO4 and concentrated. To the resulting chloride was added to tiethylphosphite (10 ml, 58 mmol) and the mixture was heated at 140°C for 10 h. The mixture was cooled down, attached via a cooled trap to an oil pump vacuum line, and kept at 50°C in oil pump vacuum for 5 h. Thus obtained phosphonate (4.44 g, 81%) was used in the next step without purification. The physical and spectroscopic properties for the compound match those reported in the literature.[5]
Compound data
Diethyl (3,5-dimethoxyphenyl)methylphosphonate (3)
1H NMR (500 MHz, CDCl3): δ 6.47 (t, J=2 Hz, 2H), 6.36 (q, J=2 Hz, 1H), 3.99-4.09 (m, 4H), 3.78 (s, 6H), 3.1 (d, J=21.5 Hz, 2H), 1.27 (t, J=7 Hz, 6H).
1-(3,5-dimethoxystyryl)-4-methoxybenzene (4)
To the solution of diethyl (3,5-dimethoxyphenyl)methylphosphonate (2.03 g, 7 mmol) and 4-methoxybenzaldehyde (anisaldehyde, 1.44 g, 10.5 mmol) in THF (17 ml), KOH (1.4 g, 21 mmol) was added (the pellets were crushed into powder before addition). The mixture was vigorously stirred for 24 h. THF was removed in vacuo, water (30 ml), 1:1 mixture of hexanes and ethyl acetate (50 ml) was added, and the layers separated. The aqueous layer was washed with a 1:1 mixture of hexanes and ethyl acetate (2×20 ml). The combined organic layers were washed with 10% sodium bisulfite (NaHSO3) solution (3×30 ml, to remove excess anisaldehyde), brine, dried over MgSO4, and concentrated. Chromatography (Hexanes to Ethyl Acetate: Hexanes 1:10) provided 1-(3,5-dimethoxystyryl)-4-methoxybenzene in pure form, 1.61 g (85%). On larger scale, the product could be recrystallized by dissolving in minimal amount of ether and cooling down to −20°C. Alternatively, it could be used in the next step without purification, and purified afterwards. The physical and spectroscopic properties for the compound match those reported in the literature.[5]
Compound data
1-(3,5-dimethoxystyryl)-4-methoxybenzene (4)
1H NMR (500 MHz, CDCl3): δ 7.46 (d, J=8 Hz, 2H), 7.06 (d, J=16 Hz, 1H), 6.92 (d, J=16 Hz, 1H), 6.91 (d, J=8 Hz, 2H), 6.67 (d, J=2 Hz, 2H), 6.39 (t, J=2 Hz, 1H), 3.84 (s, 9H).
5-(4-hydroxystyryl)benzene-1,3-diol (resveratrol, 5)
Iodomethane (1.15 ml, 18.2 mmol) was added in portions to magnesium turnings (490 mg, 20 mmol) in ether (18 ml). After completion of reaction, the solids in the solutions were allowed to settle, and the liquid was canulated to a flask containing 1-(3,5-dimethoxystyryl)-4-methoxybenzene (820 mg, 3 mmol). The outlet of the flask was attached though a trap to controlled vacuum, and ether was gradually evaporated with vigorous stirring. When evaporation slowed down, the flask was placed in a warm (45°C) oil bath. When no more ether was visibly evaporating, the flask was filled with nitrogen and the bath temperature was gradually increased to 190°C, maintaining stirring while possible (particular care should be taken at ~ 160°C when gas evolution starts. Excessively fast heating with insufficient stirring may lead to formation of foam that solidifies and prevents thorough heating, resulting in incomplete conversion). After 10 minutes at 190°C, the reaction flask was allowed to cool down to room temperature. Ether (20 ml) was added, followed by slow dropwise addition of 1N HCl (30 ml). After all solids reacted, the mixture was diluted with ethyl acetate (50 ml), water (20 ml), and stirred vigorously for 10 minutes. The layers were separated, the aqueous layer washed with ethyl acetate (2×20 ml). The combined organic layers were washed with water, dried over MgSO4, and concentrated, providing resveratrol, 650 mg (94%). The obtained resveratrol was typically used in the next step without purification. If purification was necessary, the crude product could be recrystallized from a mixture of isopropanol and water. The obtained compound was identical by physical data to the commercial sample.
Compound data
5-(4-hydroxystyryl)benzene-1,3-diol (resveratrol, 5)
1H NMR (500 MHz, CD3COCD3): δ 7.41 (d, J=8.5 Hz, 2H), 7.01 (d, J=16 Hz, 1H), 6.87 (d, J=16 Hz, 1H), 6.83 (d, J=8.5 Hz, 2H), 6.53 (d, J=2 Hz, 2H), 6.26 (t, J=2 Hz, 1H).
1-(3,5-diacetoxystyryl)-4-acetoxybenzene (resveratrol triacetate, 6)
To the suspension of resveratrol (707 mg, 3.1 mmol) in dichloromethane (10 ml), acetic anhydride (1.03 ml, 10.8 mmol) and triethylamine (1.51 ml, 10.8 mmol) were added with stirring. Resveratrol gradually dissolved. Upon completion of the reaction (by TLC, approximately 4 h), the reaction mixture was washed with 1N HCl (20 ml), sat NaHCO3 (20 ml), dried over MgSO4, and concentrated. The obtained resveratrol triacetate (1.055 g, 96%) can be used in the next step without purification. If purification was needed (such as when recycling of the partially deacetylated products), it could be purified by chromatography or by dissolving in minimal amount of ethyl acetate, and careful dilution with hexanes. The spectroscopic properties for the compound match those reported in the literature.[15]
Compound data
1-(3,5-diacetoxystyryl)-4-acetoxybenzene (resveratrol triacetate, 6)
1H NMR (500 MHz, CDCl3): δ 7.50 (d, J = 8.5 Hz, 2H) 7.12 (d, J=2 Hz, 2H), 7.10 (d, J = 8.5 Hz, 2H), 7.07 (d, J=16 Hz, 1H), 6.97(d, J=16 Hz, 1H) 6.83 (t, J = 2 Hz, 1H), 2.32 (s, 9H).
1-(3-hydroxy-5-acetoxystyryl)-4-acetoxybenzene (resveratrol 3,4′-di-O-acetate, 7)
To the solution of 6 (743 mg, 2.1 mmol) in THF (2.7 ml) and MeOH (2.7 ml), was added ammonium acetate (162 mg, 2.1 mmol). The mixture was stirred at rt for 24 h, while monitored by TLC. Following that the reaction mixture was concentrated in vacuo, diluted with water (10ml) and extracted with ethyl acetate (3×20 ml). The organic layer was dried over MgSO4 and concentrated. The resulting viscous oil was triturated with dichloromethane; the formed precipitate (monoacetates and resveratrol) was filtered off, while the solution was put on a silica gel column and separated by a quick chromatography (ethyl acetate-dichloromethane, 7:93). Thus obtained were unreacted triacetate, a mixture of 3,5-diacetate and 3,4′-diacetate, and a mixture of monoacetates. The mixture of diacetates was dissolved in a minimal amount of chloroform, and cooled to −20°C (in the refrigerator). The formed crystals of 3,4′-di-O-acetate were separated, and washed with cold chloroform. The combined mother liquor was concentrated, and the remaining mixture was subjected to chromatography on silica gel eluting with 7:93 ethyl acetate-dichloromethane mixture. Thus obtained 3,4′-di-O-acetate was combined with the portion obtained by crystallization, giving 268 mg of the product (41%). Additionally, a small amount of pure resveratrol 3,5-di-O-acetate was isolated. Resveratrol, the mixture of monoacetates, and 3,5-diacetate were combined and reconverted to resveratrol triacetate by reacetylation using the procedure described for resveratrol. The spectral data match for resveratrol 3,4′-di-O-acetate are a very good match for the reported values[10a] except for a single unexpected mismatch in 13C spectrum – the signal for C3′/C5′ is observed at 122.1, while the reported value is 115. Therefore, full data for the compound are provided. The spectroscopic properties for the resveratrol 3,5-di-O-acetate match those reported in the literature.[10b]
Compound data
1-(3-hydroxy-5-acetoxystyryl)-4-acetoxybenzene (resveratrol 3,4′-di-O-acetate, 7)
Rf 0.3 (7:93 EtOAc:Dichloromethane). m.p. 134-135°C. 1H NMR (500 MHz, CD3SOCD3): δ 9.79 (s, 1H), 7.63 (d, J=8.5 Hz, 2H), 7.20 (d, J=16 Hz, 1H), 7.13 (d, J= 8.5 Hz, 2H), 7.12 (d, J=16 Hz, 1H), 6.87 (t, J=1.5 Hz, 1H), 6.83 (t, J=1.5 Hz, 1H), 6.46 (t, J=2 Hz, 1H), 2.27 (s, 3H), 2.26 (s, 3H). 1H NMR (500 MHz, CDCl3): δ 7.47 (d, J=8.5 Hz, 2H), 7.09 (d, J=8.5 Hz, 2H), 7.01 (d, J=16 Hz, 1H), 6.91 (d, J=16 Hz, 1H), 6.82 (br s, 1H), 6.79 (br s, 1H), 6.51 (t, J=2 Hz, 1H), 5.25 (br s, 1H), 2.32 (s, 3H), 2.31 (s, 3H). Note: in CDCl3 the 1H signals tend to move around slightly on concentration change. 13C NMR (125 MHz, CD3SOCD3): δ 169.2, 169.1, 158.4, 151.7, 150.0, 139.1, 134.5, 128.2, 127.9, 127.6, 122.1, 111.1, 110.2, 108.5, 20.9, 20.8. 13C NMR (125 MHz, CDCl3): δ 170.0, 169.9, 157.0, 151.9, 150.4, 139.8, 135.0, 129.1, 128.0, 127.8, 122.0, 112.0, 111.4, 108.7, 21.4, 21.37. HRMS calcd for C18H17O5[M+H] 313.1075, found 313.1099.
4-(3,5-diacetoxystyryl)phenol (resveratrol 3,5-di-O-acetate)
Rf 0.4 (7:93 EtOAc:Dichloromethane). 1H NMR (500 MHz, CDCl3): δ 7.36 (d, J=8.5 Hz, 2H), 7.09 (d, J=2 Hz, 2H), 7.00 (d, J=16 Hz, 1H), 6.84 (d, J=16 Hz, 1H), 6.79-6.83 (m, 3H), 5.18 (br s, 1H), 2.32 (s, 6H). 13C NMR (125 MHz, CDCl3): δ 169.7, 156.3, 151.4, 140.4, 130.6, 129.3, 128.3, 124.6, 116.9, 115.9, 113.9, 21.3.
(E)-1-(3-Acetoxy-5-O-2,3,4-triacetyl-β-D-glucuronopyranosidophenyl)-2-(4′-acetoxyphenyl)ethene Methyl Ester
Resveratrol 3,4′-di-O-acetate (7) (460 mg, 1.47 mmol) and trichloroimidate 8 (1.03 g, 2.2 mmol) were dissolved in anhydrous dichloromethane (12 ml), the solution cooled to −10°C. Boron trifluoride etherate (45 μl, 0.37 mmol) was added while stirring. After 5 minutes, the stirring was stopped and the reaction mixture was kept for 1.5 h at −10°C (in the refrigerator). Saturated aqueous NaHCO3 (10 ml) was added, and the reaction mixture was warmed up. The mixture was washed with ethyl acetate (3×30 ml), the organic layer was dried over MgSO4 and concentrated. Chromatography (toluene-ethyl acetate, 100:0 to 80:20) provided the 657 mg of (71%). The spectroscopic properties for the compound match those reported in the literature.[3]
Compound data
(E)-1-(3-Acetoxy-5-O-2,3,4-triacetyl-β-D-glucuronopyranosidophenyl)-2-(4′-acetoxyphenyl)ethene Methyl Ester (S1)
1H NMR (500 MHz, CDCl3): δ 7.50 (d, J=8.5 Hz, 2H), 7.10 (d, J=8.5 Hz, 2H), 7.05 (d, J=16 Hz, 1H), 7.00 (t, J =1.5 Hz, 1H), 6.98 (t, J=1.5 Hz, 1H), 6.95 (d, J=16 Hz, 1H), 6.65 (d, J = 2 Hz, 1H), 5.26-5.40 (m, 3H), 5.20 (d, J=7 Hz, 1H), 4.23 (d, J =9 Hz, 1H), 3.74 (s, 3H), 2.31 (s, 3H), 2.31 (s, 3H), 2.08 (s, 3H), 2.06 (s, 3H), 2.05 (s, 3H).
Resveratrol 3-O-β-D-glucuronide (9)
Hydrolysis of S1 was performed as described.[3] We additionally found that resveratrol 3-O-β-D-glucuronide, if necessary, can be purified by chromatography on silica gel using ethyl acetate-acetic acid (94:6) system. The spectroscopic properties for the compound match those reported in the literature.[3]
Compound data
Resveratrol 3-O-β-D-glucuronide (9)
1H NMR (500 MHz, CD3SOCD3): δ 9.57 (s, 1H), 9.49 (s, 1H), 7.40 (d, J=8.5 Hz, 2H), 7.01 (d, J=16 Hz, 1H), 6.87 (d, J=16 Hz, 1H), 6.76 (d, J=8.5 Hz, 2H), 6.66 (br t, 1H), 6.58 (br t, 1H), 6.32 (t, J=2 Hz, 1H), 5.40 (d, J=5.5 Hz, 1H), 5.33 (br s, 1H), 5.21 (br d, J=4.5 Hz, 1H), 4.98 (d, J=7.5 Hz, 1H), 3.88 (d, J=9.5 Hz, 1H), 3.40 (t, J=9 Hz, 1H, overlapped with water peak), 3.32 (t, J=9 Hz, 1H), 3.25 (t, J=9 Hz, 1H).
Scheme 3.
Preparation of resveratrol 3-O-β-D-glucuronide
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health under grant No. GM085645. We thank Alena Kubatova for HRMS analyses. The work on TOF MS was supported by the National Science Foundation under grant No. CHE-0216038.
REFERENCES
- 1.Wang L, Heredia A, Song H, Zhang Z, Yu B, Davis C, Redfield R. Resveratrol Glucuronides as the Metabolites of Resveratrol in Humans: Characterization, Synthesis, and Anti-HIV Activity. J. Pharm. Sci. 2004;93:2448–2457. doi: 10.1002/jps.20156. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2a).Das S, Das DK. Anti-inflammatory Responses of Resveratrol. Inflamm. Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]; b) Hao HD, He LR. Mechanisms of Cardiovascular Protection by Resveratrol. J. Med. Food. 2004;7:290–298. doi: 10.1089/jmf.2004.7.290. [DOI] [PubMed] [Google Scholar]
- 3.Learmonth DA. A Concise Synthesis of the 3-O-β-D- and 4′-O-β-D-Glucuronide Conjugates of trans-Resveratrol. Bioconj. Chem. 2003;14:262–267. doi: 10.1021/bc020048x. [DOI] [PubMed] [Google Scholar]
- 4.Lucas R, Alcantara D, Morales JC. A Concise Synthesis of Glucuronide Metabolites of Urolithin-B, Resveratrol, and Hydroxytyrosol. Carbohydr. Res. 2009;344:1340–1346. doi: 10.1016/j.carres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Murias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, Szekeres T, Jaeger W. Resveratrol Analogues as Selective Cyclooxygenase-2 Inhibitors: Synthesis and Structure-activity Relationship. Bioorg. Med. Chem. Lett. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Denmark SE, Kobayashi T, Regens CS. Total Synthesis of (+)-Papulacandin D. Tetrahedron. 2010;66:4745–4759. doi: 10.1016/j.tet.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McOmie JFW, West DE. 3,3′-Dihydroxybiphenyl. Org. Syn. Collect. 1977;Vol. 5:412. [Google Scholar]
- 8.Gates M, Tschudi G. The Synthesis of Morphine. J. Am. Chem. Soc. 1956;78:1380–1393. [Google Scholar]
- 9a).Carnduff J, Miller JA. Synthesis of (+/−)-Bakuchiol. J. Chem. Soc. (C) 1968:2671–2673. More recently: Hoye TR, Humpal PE, Moon B. Total Synthesis of (−)-Cylindrocyclophane A via a Double Horner-Emmons Macrocyclic Dimerization Event. J. Am. Chem. Soc. 2000;122:4982–4983. Gu Z, Zakarian A. Studies toward the Synthesis of Maoecrystal V. Org. Lett. 2011;13:1080–1082. doi: 10.1021/ol1031238.
- 10a).Nicolosi G, Spatafora C, Tringali C. Regioselective Lipase-Catalyzed Synthesis of 3-O-Acyl Chemo-enzymatic Preparation of Resveratrol Derivatives. Mol. Catal. B: Enzym. 2002;16:223–229. [Google Scholar]; b) Torres P, Poveda A, Jimenez-Barbero J, Ballesteros A, Plou FJ. Regioselective Lipase-Catalyzed Synthesis of 3-O-Acyl Derivatives of Resveratrol and Study of Their Antioxidant Properties. J. Agric. Food Chem. 2010;58:807–813. doi: 10.1021/jf903210q. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt RR. New Methods for the Synthesis of Glycosides and Oligosaccharides—Are There Alternatives to the Koenigs-Knorr Method? Angew. Chem. Int. Ed. 1986;25:212–235. [Google Scholar]
- 12.Bollenback GN, Long JW, Benjamin DG, Lindquist JA. The Synthesis of Aryl-D-glucopyranosiduronic Acids. J. Am. Chem. Soc. 1955;77:3310–3315. [Google Scholar]
- 13.Chittaboina S, Hodges B, Wang Q. A Facile Route for the Regioselective Deacetylation of Peracetylated Carbohydrates at Anomeric Position. Lett. Org. Chem. 2006;3:35–38. [Google Scholar]
- 14.Harding JR, King CD, Perrie JA, Sinnott D, Stachulski AV. Glucuronidation of Steroidal Alcohols Using Iodo-sugar and Imidate Donors. Org. Biomol. Chem. 2005;3:1501–1507. doi: 10.1039/b412217h. [DOI] [PubMed] [Google Scholar]
- 15.Ruan B, Huang X, Ding H, Xu C, Ge H, Zhu H, Tan R. Synthesis and Cytotoxic Evaluation of a Series of Resveratrol Derivatives. Chem. Biodiversity. 2006;3:975–981. doi: 10.1002/cbdv.200690106. [DOI] [PubMed] [Google Scholar]