Abstract
BACKGROUND AND PURPOSE
Apart from their effects on mood and reward, cannabinoids exert beneficial actions such as neuroprotection and attenuation of inflammation. The immunosuppressive activity of cannabinoids has been well established. However, the underlying mechanisms are largely unknown. We previously showed that the psychoactive cannabinoid Δ9-tetrahydrocannabinol (THC) and the non-psychoactive cannabidiol (CBD) differ in their anti-inflammatory signalling pathways.
EXPERIMENTAL APPROACH
To characterize the transcriptional effects of CBD and THC, we treated BV-2 microglial cells with these compounds and performed comparative microarray analysis using the Illumina MouseRef-8 BeadChip platform. Ingenuity Pathway Analysis was performed to identify functional subsets of genes and networks regulated by CBD and/or THC.
KEY RESULTS
Overall, CBD altered the expression of many more genes; from the 1298 transcripts found to be differentially regulated by the treatments, 680 gene probe sets were up-regulated by CBD and 58 by THC, and 524 gene products were down-regulated by CBD and only 36 by THC. CBD-specific gene expression profile showed changes associated with oxidative stress and glutathione depletion, normally occurring under nutrient limiting conditions or proteasome inhibition and involving the GCN2/eIF2α/p8/ATF4/CHOP-TRIB3 pathway. Furthermore, CBD-stimulated genes were shown to be controlled by nuclear factors known to be involved in the regulation of stress response and inflammation, mainly via the (EpRE/ARE)-Nrf2/ATF4 system and the Nrf2/Hmox1 axis.
CONCLUSIONS AND IMPLICATIONS
These observations indicated that CBD, but much less than THC, induced a cellular stress response in microglial cells and suggested that this effect could underlie its anti-inflammatory activity.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: cannabidiol, Δ9-tetrahydrocannabinol, gene expression, glutathione depletion, oxidative stress, nuclear factor-erythroid 2-related factor 2
Introduction
Preparations derived from Cannabis sativa (marijuana and hashish) are recognized nowadays as potentially addictive, as well as having wide medical applications (see Earleywine, 2002; Kogan and Mechoulam, 2007; Pertwee, 2009). Therapeutic uses of marijuana and its active constituents, the cannabinoids, range from treatment of nausea, vomiting and cachexia (in cancer chemotherapy and AIDS patients), to handling of chronic inflammatory pain, glaucoma, epileptic seizures, Parkinsonian tremor as well as multiple sclerosis (see Pertwee, 2002; Guzman, 2003; Di Marzo and De Petrocellis, 2006; Kogan and Mechoulam, 2007). Cannabinoids act as potent immunosuppressive and anti-inflammatory agents and have been reported to mediate modulatory activities on immune cell functions (Klein et al., 1998; McKallip et al., 2002; Cabral and Staab, 2005; Klein and Cabral, 2006; Kozela et al., 2010; Rieder et al., 2010). In addition, cannabinoids have pro-apoptotic, neuroprotective and anti-tumour properties (van der Stelt and Di Marzo, 2005; Massi et al., 2006; Galve-Roperh et al., 2000).
Two cannabinoid receptors have been characterized so far. The cannabinoid CB1 receptor, which is mainly present in neural cells, mediates the psychoactive and addictive activities of cannabinoids, and the CB2 receptor, which is expressed mainly in the immune system, is involved in cannabinoid immunomodulation (receptor nomenclature follows Alexander et al., 2011). The major psychoactive component of marijuana, Δ9-tetrahydrocannabinol (THC) is equally effective at either of these receptors (Rhee et al., 1997) and has effects on both the immune and the nervous systems (Cabral and Staab, 2005; Le Foll and Goldberg, 2005; Cabral and Griffin-Thomas, 2008; 2009; Woelkart et al., 2008).
One of the current pharmacological challenges is to elucidate the mechanisms underlying the beneficial properties ascribed to marijuana in order to develop cannabinoid-based therapeutics lacking the adverse psychotropic effects. Thus, another compound abundant in C. sativa extracts, cannabidiol (CBD), is under extensive investigation (Mechoulam et al., 2002, 2007; Pertwee, 2005; Izzo et al., 2009). CBD exhibits anti-inflammatory, antioxidant and neuroprotective properties, but, unlike THC, is devoid of psychotropic effects and has very low affinity for both CB1 and CB2 receptors (see Mechoulam et al., 2002, 2007; Izzo et al., 2009).
Cell-type specific induction of cell death is considered to be one of the primary actions of many plant-derived immunosuppressants and anticancer drugs (Ho and Lai, 2004; Fesik, 2005). Early investigations have postulated a role for cell death in the regulation of immune function by cannabinoids (Raz and Goldman, 1976; Davies et al., 1979). Both THC and CBD have been shown to induce apoptosis in leukaemia and primary lymphocytes (McKallip et al., 2002, 2006; Gallily et al., 2003; Do et al., 2004; Wu et al., 2008; Lee et al., 2008a). In addition, THC has been shown to induce apoptosis in macrophages, dendrocytes and glioma cells (Zhu et al., 1998; McKallip et al., 2002; Goncharov et al., 2005). Although the mechanisms by which these cannabinoids trigger apoptosis are not fully understood, a common feature of CBD and THC treatments in transformed and primary lymphocytes is the elevation of intracellular reactive oxygen species (ROS) accompanied by glutathione (GSH) depletion (McKallip et al., 2006; Wu et al., 2008; Lee et al., 2008a). Importantly, in U87MG human astrocytoma and in C6 glioma cells, induction of cell death by THC was reported to be associated with stress-related gene expression with a central role for the p8-ATF4-TRB3 pathway (Carracedo et al., 2006a,b; Salazar et al., 2009).
We have previously shown that CBD and THC have different effects on anti-inflammatory pathways in lipopolysaccharide (LPS)-treated BV-2 cells. CBD reduces the activity of the NF-κB pathway and up-regulates the activation of the STAT3 transcription factor. However, both CBD and THC decrease the activation of the LPS-induced STAT1 transcription factor, a key player in IFNβ-dependent pro-inflammatory processes (Kozela et al., 2010). Therefore, we decided to characterize the transcriptional effects of CBD and THC in surveillant (resting) BV-2 microglial cells. BV-2 cells exhibit morphological, phenotypic and functional properties associated with freshly isolated microglial cells (Blasi et al., 1990; Bocchini et al., 1992; Ulrich et al., 2001; Kim et al., 2004). Although BV-2 cells are used as a model of microglial cells, Horvath et al. (2008) and Pietr et al. (2009) reported that BV-2 cells did not exactly model the response of microglial cells in primary culture, when the reactive and inflammatory profiles of these cell cultures were compared, for instance, following treatment with LPS or IFNγ.
We have performed comparative gene profiling analyses of gene expression in BV-2 cells treated with either CBD or THC. The results of this study showed that CBD exerted a much greater effect on gene expression compared with that of THC. From the 1298 transcripts found to be differentially regulated by CBD and/or THC, 1204 gene probe sets were regulated by CBD and only 94 by THC. The Ingenuity Pathway Analysis (IPA) linked the CBD effects to activation of stress- and cell death-related signalling pathways, showing that CBD, and to a much lesser extent THC, activated the (EpRE/ARE)-Nrf2/ATF4 system and the GCN2/eIF2α/p8/ATF4/CHOP-TRIB3 pathway, known to be leading to autophagy and apoptotic cell death.
Methods
Microglial cell culture
The immortalized murine BV-2 microglial cell line was kindly provided by Prof. E.J. Choi from Korea University (Seoul, Korea). BV-2 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA) containing 4.5 g·L−1 glucose, supplemented with 5% fetal calf serum, penicillin (100 U·mL−1) and streptomycin (100 µg·mL−1) (Biological Industries Ltd, Kibbutz Beit Haemek, Israel), under a humidified 5% CO2 atmosphere at 37°C. Cells were treated with either THC or CBD (both at 10 µM) for 6 h, unless otherwise indicated.
Total RNA extraction
Total RNA was obtained using the PerfectPure RNA extraction kit (5Prime, Darmstadt, Germany) following the manufacture's instructions. Quantification of extracted RNA was performed using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA integrity was assessed by electrophoresis on ethidium bromide-stained 1% agarose gels. Purity of the RNA was analysed using the Agilent Bioanalyzer Nanochips (Agilent Technologies, Palo Alto, CA, USA).
Microarray transcript analysis
Total RNA (200 ng) was amplified and labelled, and then hybridized onto Illumina MouseRef-8 v1.1 Expression BeadChip (Illumina Inc., San Diego, CA, USA), querying the expression of >24 000 RefSeq-curated gene targets and 822 random sequences used for the assessment of background noise. Six independent preparations of mRNA were analysed on six independent BeadChips for each of the treatment conditions. Arrays were processed and scanned with Illumina BeadStation platform according to the manufacturer's protocol. Raw data were analysed using the Bioconductor packages (http://www.bioconductor.org; Gentleman et al., 2004). Quality-control analysis was performed using the inter-array Pearson correlation and clustering based on variance. Raw data were log2 transformed and normalized using quantile normalization. Analysis of differential expression was performed using a linear model fitting (LIMMA package, Smyth, 2005). Differentially expressed genes were classified according to their gene ontology (GO), using Bioconductor packages and online tools [Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources, http://david.abcc.ncifcrf.gov/; Huang et al., 2009; and WebGestalt, http://bioinfo.vanderbilt.edu/webgestalt/]. Significantly over-represented GO categories were defined using a threshold for statistical significance set at P < 0.05. Literature data mining for co-occurrence of gene names and keywords of interest (e.g. oxidative stress, mitochondria) was performed using Chilibot (http://www.chilibot.net/) search. Cellular pathway association was analysed according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/).
Ingenuity pathway analysis
Pathway and global functional analyses were performed using IPA 6.0 (Ingenuity® Systems, http://www.ingenuity.com/). A data set containing gene identifiers and corresponding expression values was uploaded into the application, and each gene identifier was mapped to its corresponding gene object using the Ingenuity Pathways Knowledge Base (IPKB). The functional and canonical pathways’ analyses identified the biological functions and the pathways from the IPA library that were most significant to the data set. Genes from the data set that met the P-value cut-off of 0.005 and were associated with biological functions or with a canonical pathway in the IPKB were considered for analysis. Fisher's exact test was used to calculate a P-value determining the probability that each biological function and/or canonical pathway assigned to this data set is not due to chance alone.
Primer design for quantitative real time PCR validation assays
Primer sets for a selected collection of transcripts used in quantitative real time reverse transcription polymerase chain reaction (qPCR) validation were designed using the computer program Primer Express (Perkin Elmer/Applied Biosystems, Foster City, CA, USA) or Primer Quest, an online tool provided by Integrated DNA Technologies (http://test.idtdna.com) (Table 1). Wherever possible, designs with at least one of the primer sequences located on an intron–exon boundary were chosen, to avoid co-amplification of minor amounts of genomic DNA that could be present in the RNA samples. All primers were analysed using nucleotide blast to ensure primer specificity for the gene of interest (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1.
Primer sequences used for qPCR
| Sequence (5′→ 3′) | Length (bp)a | Accessionb | ||
|---|---|---|---|---|
| B2mg | FW | ATG GGA AGC CGA ACA TAC TG | 176 | NM_009735.3 |
| RV | CAG TCT CAG TGG GGG TGA AT | |||
| Trib3 | FW | AAG ACT TGG CTG TGG GAT TCA AGC | 190 | NM_175093.2 |
| RV | AGA ACA GGG CCT GAG ATT GTC TGT | |||
| Ddit3/Chop | FW | ACC AAG CAT GAA CAG TGG GCA TCA | 164 | NM_007837.3 |
| RV | ATG TAC CGT CTA TGT GCA AGC CGA | |||
| Sqstm1/p62 | FW | ACC CTC CAC CAT TGT GAT AGT GCT | 109 | NM_011018.2 |
| RV | AAT GCC AAG ACA CTG GGC CTA TCT | |||
| Aqp9 | FW | TGA AGG GAC AAG GTA GCC GTT TGA | 193 | NM_022026.2 |
| RV | AAA CAG TTG GCA GTG AAG GCA CAC | |||
| Slc7a11 | FW | AAA GCA GGT TCC ACA GCG AAG T | 227 | NM_011990.2 |
| RV | TGG CCA GCT CCG CAA ATG AAA T | |||
| Cdkn1a | FW | TGC CTG GTT CCT TGC CAC TTC TTA | 122 | NM_007669.4 |
| RV | TTC ACT GTC ATC CTA GCT GGC CTT | |||
| Scl40a1 | FW | AAC CAG AGT CAC TGT CAT CAG CCA | 153 | NM_016917.2 |
| RV | TCG GCC CAA GTC AGT GAA GGT AAA | |||
| Zfp472 | FW | TGG GAA AGC CTT CAT CCA ACG TGA | 198 | NM_153063.3 |
| RV | AGG TAT GTG GAA CAG GTG AAC GCT | |||
| Nupr1/p8 | FW | ACC CAG CAA TGG ATA CAG GAC CTT | 149 | NM_019738.1 |
| RV | CTT CTT GCT CCC ATC TTG CCC TTT | |||
| Atf4 | FW | TGA GGC TCT GAA AGA GAA GGC AGA | 130 | NM_009716.2 |
| RV | AGC ACA AAG CAC CTG ACT ACC CTA | |||
| Dusp1 | FW | ATT TGC TGA ACT CGG CAC ATT CGG | 154 | NM_013642.3 |
| RV | GGT GGG TGT GTC AAG CAT GAA GTT | |||
| Caspase 4 | FW | CCG GAA ACA TGC TTG CTC TTG TCA | 118 | NM_007609.2 |
| RV | TCT CGT CAA GGT TGC CCG ATC AAT | |||
| Hmox1 | FW | GTG GCC TGA ACT TTG AAA CCA GCA | 130 | NM_010442.2 |
| RV | ACA GCA GTC GTG GTC AGT CAA CAT | |||
| Ccl2 | FW | ACT GCA TCT GCC CTA AGG TCT TCA | 131 | NM_011333.3 |
| RV | TTC ACT GTC ACA CTG GTC ACT CCT | |||
| Gclm | FW | AAA GCA TCC CTG ACA TTG AAG CCC | 149 | NM_008129.3 |
| RV | TGT GGG TGT GAG CTG GAG TTA AGA | |||
| IL-1β | FW | TCA CCA TGG AAT CCG TGT CTT CCT | 173 | NM_008361.3 |
| RV | ATG TGC CAT GGT TTC TTG TGA CCC | |||
| IFNb1 | FW | TGA AGT ACA ACA GCT ACG CCT GGA | 163 | NM_010510.1 |
| RV | AGT CCG CCT CTG ATG CTT AAA GGT |
Amplicon length in base pairs.
Genbank accession number of cDNA and corresponding gene, available at http://www.ncbi.nlm.nih.gov
FW, forward primer; RV, reverse primer.
qPCR analysis
cDNA was generated by the QuantiTect Reverse Transcription kit containing gDNA ‘wipe out’ (to eliminate contamination with genomic DNA), according to the manufacturer's instructions (Qiagen, AG, Basel, Switzerland). qPCR was carried out in 0.1 mL tubes in the Rotor-Gene 3000 instrument (Corbett Research, Sydney, Australia). PCR reaction mixtures (20 µL) contained cDNA samples (in 3 µL), 5 pmol of each of the two primers and 10 µL of Blue SYBR Green PCR Rox Mix containing the DNA polymerase (Abgene House, Epsom, Surrey, UK) as detailed by Butovsky et al. (2006). For each of the examined mRNAs, normal and mock reverse transcribed samples, as well as no template control (total mix without cDNA) were run. In order to obtain the dilution curves for PCR amplification efficiencies, each set of duplicate PCR reactions was performed with five different concentrations of each of the cDNAs tested. The PCR reactions were subjected to the following conditions: 15 min at 95°C to activate the Termo-start DNA polymerase present in the PCR Master Mix, followed by 40 cycles consisting of: 15 s at 94°C, 30 s at 60°C and 30 s at 72°C. Fluorescence was measured at the end of each elongation step. A melting curve was generated at the end of each run to ensure product uniformity and to rule out primer–dimers and presence of splice variants. For each sample, an amplification plot was generated, showing the increase in the SYBR green fluorescence for each cycle of PCR. A threshold cycle value Ct was calculated from the exponential phase of each PCR sample and a standard curve for each gene was plotted (Ct vs. log DNA concentration). For each sample, the expression level of the gene of interest was normalized to the reference gene, β2-microglobulin (B2m), whose expression was found not to be affected by the various treatments. RNA expression levels are expressed as fold change using the calculation method described by Pfaffl (2001). The qPCR experiments were repeated four times using different mRNA batches from independent experiments and reactions were performed in duplicates for each cDNA sample.
Statistical analysis
qPCR data were plotted as the mean ± SEM of three or more independent experiments. Statistical significance was assessed using a one-way anova and followed by Bonferroni post hoc multiple comparison test as implemented in the version 6.1 (R2007b) Statistics Toolbox Software, MATLAB, MathWorks (http://www.mathworks.com/help/toolbox/stats/rn/brasjn_.html). A P-value < 0.05 was defined as statistically significant.
Materials
THC and CBD were obtained from the National Institute on Drug Abuse (Baltimore, MD, USA). Stock solutions of cannabinoids were prepared in ethanol and diluted into culture medium before experiments. Final ethanol concentration in the medium did not exceed 0.1%.
Results
Gene expression profile of BV-2 microglial cells treated with cannabinoids
Samples of mRNA were prepared from BV-2 microglial cells treated for 6 h with CBD or THC (both at 10 µM) or with vehicle. The time point of 6 h for gene array analysis was chosen, as it is known to cover the primary gene response (Lund et al., 2006). Moreover, our laboratory used this time point to study the signalling pathways involved in the anti-inflammatory effects of CBD and THC (both at 10 µM), in LPS-treated BV-2 microglial cells (Kozela et al., 2010). Six replicates of each experiment were carried out, resulting in six independent microarrays for each individual treatment or control (18 total arrays). The mRNA profiling was carried out using the MouseRef-8 v1.1 Expression BeadChip Illumina Array, which has >24 000 mouse targets based on the NCBI mouse Reference Sequence Database, including 16 287 constitutive exons/islands based on the splice variants in the mouse transcriptome (MouSDB3) and NCBI LocusLink databases. Of all transcripts, 32% were consistently ‘present’ across all the 18 arrays. Clustering based on inter-array Pearson correlation coefficient indicated no batch effects between the arrays used in these analyses. Gene classes constitutively expressed included metabolic enzymes, structural proteins, signalling molecules and transcription factors.
Microarray analysis based on a threshold of P≤ 0.005, revealed that 1298 transcripts out of the 24 000 targets of the Illumina gene set were differentially regulated across the various treatments. Of these, 680 gene probe sets were up-regulated after 6 h treatment with CBD (Figure 1), whereas a very low number of only 58 gene probe sets were observed to be increased by THC and only 22 of these genes were up-regulated by either CBD or THC. Thus 36 genes were up-regulated by THC and not by the CBD treatment; however, their relative levels did not exceed the twofold induction level in comparison to control. Moreover, CBD had also a much larger effect compared with THC on the number of gene products which were down-regulated: 524 gene products were down-regulated by CBD, 36 by THC and only seven gene products were down-regulated by either THC or CBD (P≤ 0.005). In all, 517 probe sets of the chip were down-regulated only by CBD and not affected by THC (Figure 1). When the fold change was set on two, we found that 123 gene products were up-regulated by CBD, and eight of them were up-regulated by either CBD or THC. Thirty-eight gene products were exclusively down-regulated by ≥twofold after CBD treatment while THC did not down-regulate any gene product to this extent.
Figure 1.
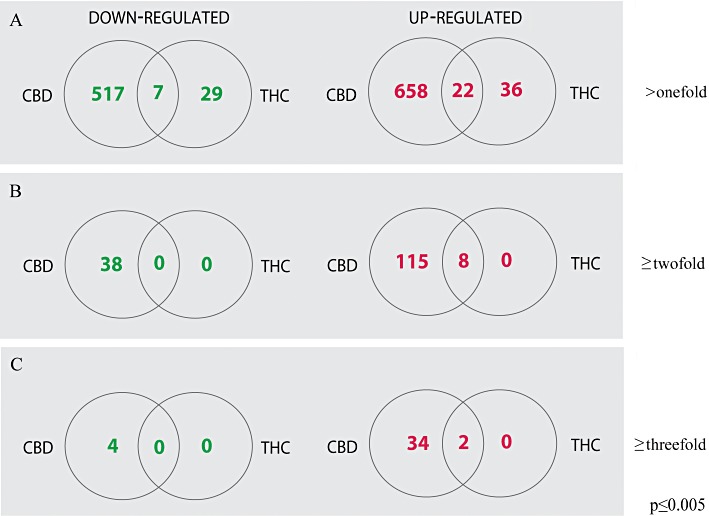
Venn diagrams comparing the number of gene products regulated by CBD and/or THC. The diagrams show the numbers of BV-2 genes that were selectively regulated by 6 h exposure to 10 µM CBD or 10 µM THC. (A) Gene products which were affected by >onefold, (B) by ≥twofold and (C) by ≥threefold. Numbers show the transcripts that were significantly (P≤ 0.005) either up- or down-regulated, relative to control untreated cells.
When the fold change was set on three, we found that 36 genes were up-regulated by CBD and only two of them were up-regulated by THC. Only four genes were exclusively down-regulated to this extent by CBD treatment. THC did not affect any gene to this level. Altogether, both CBD and THC had a greater effect on the number of gene products that were up-regulated than on the number of genes whose expression was repressed (as is best observed for gene products affected by ≥two- or ≥threefold), and the changes in gene expression after THC treatment were much more modest compared to those observed following exposure to CBD. As shown in Figure 1, a relatively small number of genes were selectively responsive to THC (36 up-regulated and 29 down-regulated; >onefold; P≤ 0.005) and not to CBD; however, none of these genes reached a level of twofold induction or 50% reduction, in comparison to control.
To identify functional subsets of genes and networks regulated by CBD and/or THC, IPA global functional analysis was performed. This detailed analysis revealed that the molecular and cellular functions associated with the CBD-up-regulated genes (≥twofold) included those related to amino acid metabolism, molecular transport, small molecule biochemistry, cell death and gene expression (Figure 2). Furthermore, the CBD-induced canonical metabolic and signalling pathways with the highest significance score include genes involved in Nrf2 (nuclear factor-erythroid 2-related factor 2)-mediated oxidative stress response and aminoacyl-tRNA biosynthesis, as well as genes related to metabolism of GSH, alanine and aspartate. These IPA canonical pathways are in agreement with those we found using the DAVID Bioinformatics Resources and the KEGG database (data not shown). On the other hand, CBD down-regulated the expression of numerous subsets of genes which are known to be associated with diverse biological functions (such as cell death as well as DNA replication, recombination and repair) and canonical metabolic and signalling pathways such as genes related to G1/S checkpoint regulation, metabolism of pyrimidine and methionine as well as ERK/MAPK signalling (Figure 2). As described above, THC affected very few genes; Figure 3 shows that some of the genes down-regulated by THC are related to cellular development, DNA replication, recombination and repair. However, the P-value for these effects as well as for other molecular and cellular functions is > 0.05 and did not allow the affected gene pathways to be accurately classified.
Figure 2.
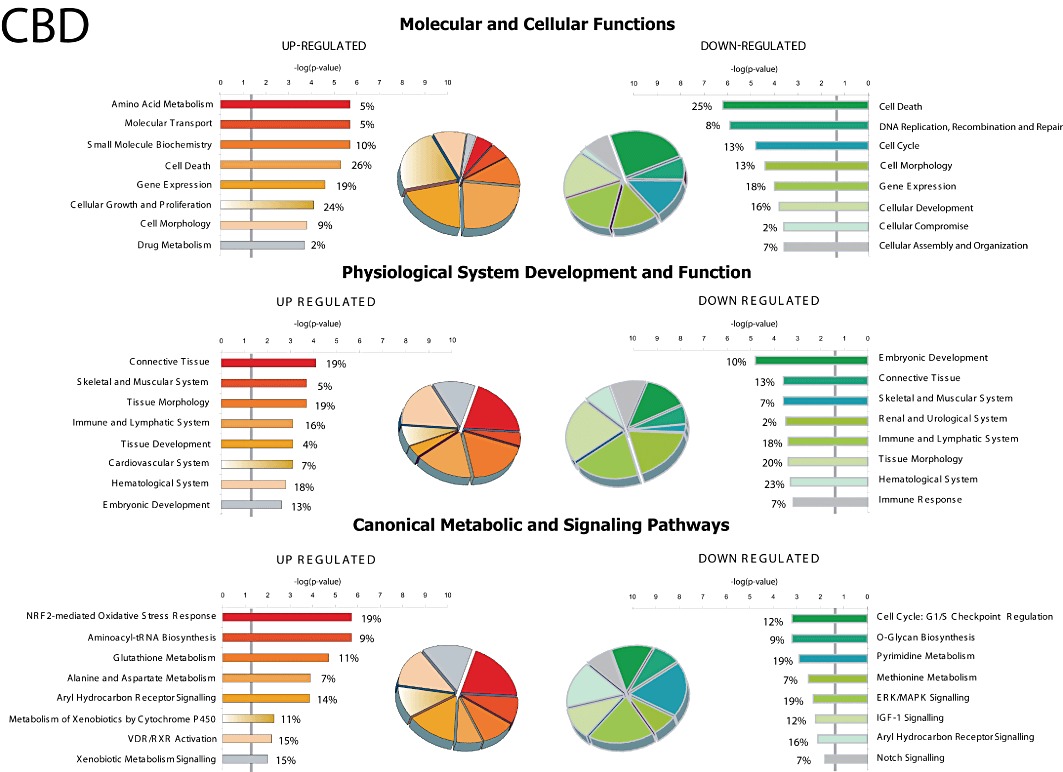
Ingenuity global functional and pathway analysis of the genes affected by CBD treatment. IPA analysis was used to examine the enriched functional classes of up-regulated (red and brown) and down-regulated (green, blue and yellow) genes. The y axis shows the top eight most high-level functions and canonical pathways associated with genes regulated in CBD-treated BV-2 microglial cells. The x axis displays the mean P-value for each associated high-level function and canonical pathway in a −log scale. Increasing value of −log (significance) indicates increased confidence for each category. The vertical gray line in each plot indicates P < 0.05. The percentage of genes in each category is shown next to the bars and corresponds to the share in the adjacent pie chart identified by the same colour.
Figure 3.
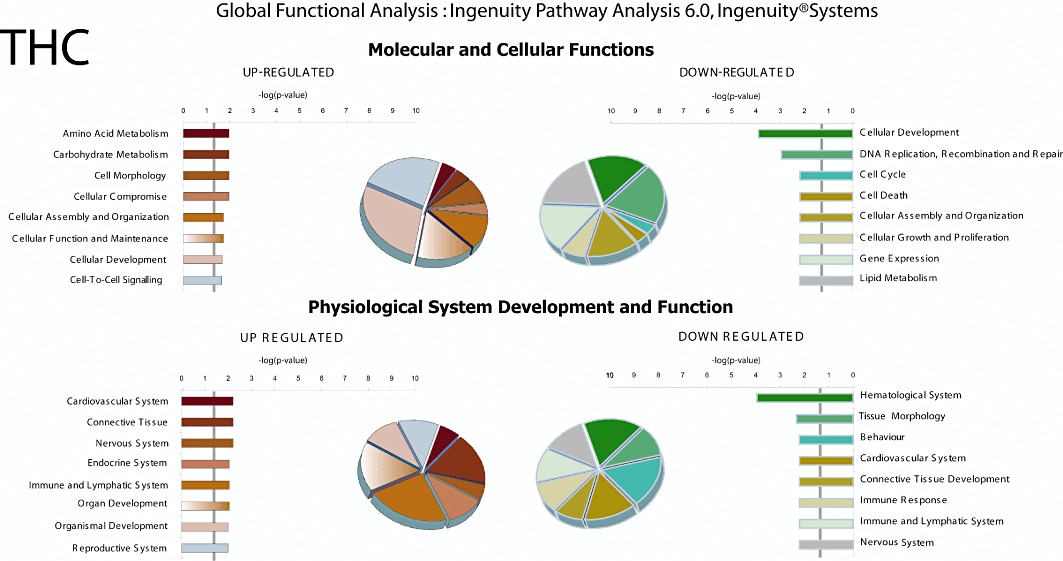
Ingenuity global functional and pathway analysis of the genes affected by THC treatment. IPA analysis was used to examine the enriched functional classes of up-regulated (red and brown) and down-regulated (green, blue and yellow) genes. Details are as indicated in Figure 2.
Gene-by-gene inspection revealed that genes whose products are known to be active in cellular stress response, regulation of transcription, lipid and amino acid metabolism, as well as membrane transport dominate the CBD-up-regulated transcripts (Table 2). Moreover, CBD treatment induced many transcripts of proteins known to participate in adhesion and migration. Genes highly up-regulated by CBD include tribbles homolog 3 (Trib3; 16-fold), aquaglyceroporin 9 (Aqp9; 14-fold), DNA-damage inducible transcript 3 (Ddit3; also known as Chop/Gadd153; 12-fold), cation ion transport regulator 1 (Chac1; 10-fold), growth differentiation factor 15 (Gdf15; eightfold), metallothionein 2 (Mt2; eightfold), sterol O-acyltransferase 2 (Soat2; eightfold), nuclear protein 1 (Nupr1; eightfold) and solute carrier family 7, member 11 (Slc7a11; sevenfold). THC increased the expression of some of these genes but to a significantly lower extent. THC-regulated transcripts that were increased by >twofold contained the following six gene transcripts: Trib3 (3.4-fold), Chac1 (3.1-fold), serum amyloid A3 (Saa3; 2.7-fold), lipocalin 2 (Lcn2; 2.5-fold), Gdf15 (2.4-fold) and homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (Herpud1; 2.3-fold) as well as two gene transcripts that were up-regulated by twofold, Aqp9 and Nupr1. As described above, the same genes were also up-regulated by CBD. Moreover, except for Lcn2, which was affected by both cannabinoids to a similar extent (2.5-fold), all the other genes were affected more strongly by CBD.
Table 2.
List of genes significantly up-regulated (>twofold) by CBD or THC in BV-2 cells
| Gene array (fold induction) | qPCR (fold induction)a | |||||
|---|---|---|---|---|---|---|
| Gene name | Description | Accession number | CBD | THC | CBD | THC |
| Stress response | ||||||
| Trib3 | Tribbles homolog 3 (Drosophila) | NM_175093.2 | 16.3 | 3.4 | 15.7 ± 0.3 | 3.6 ± 0.9 |
| Ddit3/Chop | DNA-damage inducible transcript 3 | NM_007837.3 | 12.0 | 1.7 | 12.9 ± 0.5 | 1.7 ± 0.2 |
| Gdf15 | Growth differentiation factor 15 | NM_011819.1 | 8.2 | 2.4 | ||
| Mt2 | Metallothionein 2 | NM_008630.1 | 7.9 | 1.3 | ||
| Ndrg1 | N-myc downstream regulated gene 1 | NM_010884.1 | 6.5 | 1.2 | ||
| Csprs | Component of Sp100-rs | NM_033616.2 | 6.5 | 1.5 | ||
| Ndrl | N-myc downstream regulated-like | NM_008681 | 5.5 | 1.1 | ||
| Cox6a2 | Cytochrome c oxidase, subunit VI a, polypeptide 2 | NM_009943.2 | 4.8 | 1.5 | ||
| Herpud1 | Homocysteine-inducible, ER stress-inducible, ubiquitin-like domain member 1 | NM_022331.1 | 3.3 | 2.3 | ||
| Sqstm1/p62 | Sequestosome 1 | NM_011018.1 | 3.2 | 1.2 | 4.8 ± 1.7 | 1.7 ± 0.1 |
| Ddit4 | DNA-damage-inducible transcript 4 | NM_029083.1 | 3.0 | 1.1 | ||
| Ctsg | Cathepsin G | NM_007800.1 | 2.7 | 0.9 | ||
| Hmox1 | Haem oxygenase (decycling) 1 | NM_010442.2 | 2.7 | 1.4 | 3.9 ± 1.2 | 2.1 ± 0.2 |
| Dnmt3l | DNA (cytosine-5-)-methyltransferase 3-like | NM_019448.2 | 2.7 | 1.5 | ||
| Vegfa | Vascular endothelial growth factor A | NM_009505.2 | 2.6 | 1.4 | ||
| Hp | Haptoglobin | NM_017370.1 | 2.6 | 1.9 | ||
| Npn3 | Sulphiredoxin 1 homologue (S. cerevisiae) | NM_029688.2 | 2.6 | 1.3 | ||
| Nqo1 | NAD(P)H dehydrogenase, quinone 1 | NM_008706.1 | 2.4 | 1.1 | ||
| Htatip2 | HIV-1 tat interactive protein 2, homologue (human) | NM_016865.2 | 2.1 | 1.0 | ||
| Regulation of transcription | ||||||
| Nupr1/p8 | Nuclear protein 1 | NM_019738.1 | 7.9 | 2.0 | 10.4 ± 1.5 | 2.2 ± 0.4 |
| Glrp1 | Glutamine repeat protein 1 | NM_008132.1 | 4.3 | 1.6 | ||
| IkB | NF-κB inhibitor β | NM_010908.4 | 3.5 | 1.4 | ||
| Neurl | Neuralized homolog (Drosophila) | NM_021360 | 3.2 | 1.0 | ||
| NF-kB | Nuclear factor of κ light polypeptide gene enhancer in B-cells 1 | NM_008689.2 | 3.1 | 1.4 | ||
| Jundm2 | Jun dimerization protein 2 | NM_030887.2 | 3.0 | 1.5 | ||
| Atf4 | Activating transcription factor 4 | NM_009716 | 2.8 | 1.2 | 3.5 ± 0.3 | 1.7 ± 0.1 |
| Chd2 | Chromodomain helicase DNA binding protein 2 | XM_145698.4 | 2.8 | 1.2 | ||
| Atf5 | Activating transcription factor 5 | NM_030693.1 | 2.5 | 1.3 | ||
| Spic | Spi-C transcription factor (Spi-1/PU.1 related) | NM_011461.2 | 2.5 | 1.2 | ||
| Relb | Avian reticuloendotheliosis viral (v-rel) oncogene related B | NM_009046.2 | 2.4 | 1.3 | ||
| Eif4ebp1 | Eukaryotic translation initiation factor 4E binding protein 1 | NM_007918.2 | 2.3 | 1.3 | ||
| Atf3 | Activating transcription factor 3 | NM_007498.2 | 2.3 | 1.2 | ||
| Hist1h3e | Histone 1, H3e | NM_178205 | 2.2 | 1.0 | ||
| Hist1h2ac | Histone 1, H2ac | NM_178189.2 | 2.2 | 1.1 | ||
| Hist1h1c | Histone 1, H1c | NM_015786 | 2.1 | 1.2 | ||
| Hist2h2be | Histone 2, H2be | NM_178214.1 | 2.1 | 1.0 | ||
| Mef2b | Myocyte enhancer factor 2B | NM_008578.1 | 2.1 | 1.1 | ||
| Sox4 | SRY-box containing gene 4 | NM_009238.2 | 2.1 | 0.9 | ||
| Hist1h3d | Histone1, H3d | NM_178204.1 | 2.0 | 1.0 | ||
| Nfe2l1 | Nuclear factor, erythroid derived 2,-like 1 | NM_008686.3 | 2.0 | 1.1 | ||
| Metabolic | ||||||
| Soat2 | Sterol O-acyltransferase 2 | NM_146064.1 | 8.0 | 1.7 | ||
| Cox6a2 | Cytochrome c oxidase, subunit VI a, polypeptide 2 | NM_009943.1 | 4.8 | 1.5 | ||
| Gpt2 | Glutamic pyruvate transaminase (alanine aminotransferase) 2 | NM_173866.1 | 4.7 | 1.3 | ||
| Gclm | Glutamate-cysteine ligase, modifier subunit | NM_008129.3 | 3.0 | 1.3 | 2.23 ± 0.06 | 1.5 ± 0.3 |
| Akr1b7 | Aldo-keto reductase family 1, member B7 | NM_009731.1 | 2.9 | 1.0 | ||
| Gstm6 | Glutathione S-transferase, µ6 | NM_008184.1 | 2.8 | 1.4 | ||
| Gstm1 | Glutathione S-transferase, µ1 | NM_010358.5 | 2.4 | 1.3 | ||
| Adfp | Adipose differentiation related protein | NM_007408.2 | 2.3 | 0.9 | ||
| Sars1 | Seryl-aminoacyl-tRNA synthetase | NM_011319.1 | 2.2 | 1.2 | ||
| Cars | Cysteinyl-tRNA synthetase | NM_013742.2 | 2.0 | 1.2 | ||
| Membrane transport and secretion | ||||||
| Aqp9 | Aquaglyceroporin 9 | NM_022026.2 | 14.3 | 2.0 | 14.6 ± 3.8 | 2.5 ± 0.5 |
| Chac1 | Cation ion transport regulator 1 | NM_026929 | 10.1 | 3.1 | ||
| Slc7a11 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 11 | NM_011990.1 | 7.1 | 1.7 | 9.2 ± 0.6 | 1.4 ± 0.2 |
| Slc40a1 | Solute carrier family 40 (iron-regulated transporter), member 1 | NM_016917.1 | 6.5 | 1.7 | 15.4 ± 0.6 | 1.6 ± 0.2 |
| Slc6a9 | Solute carrier family 6 (neurotransmitter transporter, glycine), member 9 | NM_008135.1 | 6.5 | 1.6 | ||
| Slc1a4 | Solute carrier family 1 (glutamate/neutral amino acid transporter), member 4 | NM_018861.2 | 5.9 | 1.9 | ||
| Slc3a2 | Solute carrier family 3 (activators of dibasic and neutral amino acid transport) member2 | NM_008577.2 | 3.8 | 1.1 | ||
| Slc39a4 | Solute carrier family 39 (zinc transporter), member 4 | NM_028064.2 | 3.5 | 1.6 | ||
| Stx11 | Syntaxin 11 | XM_203312.2 | 3.2 | 1.4 | ||
| Atp2a3 | ATPase, Ca++ transporting, ubiquitous | NM_016745.2 | 2.7 | 1.2 | ||
| Slc2a6 | Solute carrier family 2 (facilitated glucose transporter), member 6 | NM_172659.1 | 2.6 | 1.5 | ||
| Slc30a1 | Solute carrier family 30 (zinc transporter), member 1 | NM_009579.2 | 2.6 | 1.0 | ||
| Sfxn4 | Sideroflexin 4 | NM_053198.3 | 2.1 | 1.2 | ||
| Phosphatases | ||||||
| Dusp1 | Dual specificity phosphatase 1 | NM_013642.3 | 2.8 | 1.1 | 3.2 ± 1.5 | 0.8 ± 0.1 |
| Ptpn14 | Protein tyrosine phosphatase, non-receptor type 14 | NM_008976.1 | 2.7 | 1.1 | ||
| Ppap2b | Phosphatidic acid phosphatase type 2B | NM_080555.1 | 2.2 | 1.3 | ||
| Adhesion and migration | ||||||
| Angptl6 | Angiopoietin-like 6 | NM_145154 | 5.6 | 1.2 | ||
| Saa3 | Serum amyloid A 3 | NM_011315 | 4.4 | 2.7 | ||
| Col4a1 | Procollagen, type IV, α1 | NM_009931.1 | 3.5 | 1.0 | ||
| Sned1 | Sushi, nidogen and EGF-like domains 1 | NM_172463.3 | 2.8 | 1.2 | ||
| Clecsf9 | C-type (calcium dependent, carbohydrate recognition domain) lectin | NM_019948.1 | 2.3 | 1.7 | ||
| Mmp23 | Matrix metalloproteinase 23 | NM_011985.1 | 2.5 | 1.2 | ||
| Pcdha1 | Protocadherin α 1 | NM_054072 | 2.4 | 1.1 | ||
| Pcdha11 | Protocadherin α 11 | NM_009960 | 2.2 | 1.1 | ||
| Motility and morphogenesis | ||||||
| Avil | Advillin | NM_009635.2 | 2.6 | 1.0 | ||
| Vwf | Von Willebrand factor homolog ue | NM_011708.2 | 2.6 | 1.1 | ||
| Pfn2 | Profilin 2 | NM_019410.2 | 2.3 | 1.1 | ||
| Apoptosis | ||||||
| Tnfaip2 | Tumour necrosis factor α-induced protein 2 | NM_009396.1 | 3.3 | 1.8 | ||
| Lcn2 | Lipocalin 2 | NM_008491.1 | 2.5 | 2.5 | ||
| Traf1 | TNF receptor-associated factor 1 | NM_009421.2 | 2.3 | 1.2 | ||
| Casp4 | Caspase 4, apoptosis-related cysteine protease | NM_007609.1 | 2.1 | 1.2 | 3.2 ± 0.6 | 1.1 ± 0.3 |
| Pdcd1lg1 | Programmed cell death 1 ligand 1 | NM_021893.2 | 2.1 | 1.5 | ||
| Cell cycle and proliferation | ||||||
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) | NM_007669.4 | 3.7 | 0.7 | 3.24 ± 0.6 | 1.0 ± 0.1 |
| Cdkn2b | Cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | NM_007670.2 | 2.9 | 1.1 | ||
| Gadd45a | Growth arrest and DNA-damage-inducible 45 α | NM_007836.1 | 2.3 | 1.2 | ||
| Cdkn2a | Cyclin-dependent kinase inhibitor 2A | NM_009877.1 | 2.1 | 0.7 | ||
| G protein-coupled receptors | ||||||
| Ptgir | Prostaglandin I receptor (IP) | NM_008967.1 | 5.4 | 1.3 | ||
| Kinases | ||||||
| Plk2 | Polo-like kinase 2 | NM_152804.1 | 2.2 | 1.1 | ||
| Regulation of translation | ||||||
| Myd116 | Myeloid differentiation primary response gene 116 | NM_008654.1 | 2.7 | 1.1 | ||
| Cpeb1 | Cytoplasmic polyadenylation element binding protein 1 | NM_007755.1 | 2.2 | 1.2 | ||
Genes were validated by qPCR, using β2-microglobulin mRNA as a reference gene.
The expression of several enzymes which are known to play a key role in cellular defence by increasing the removal of cytotoxic electrophiles and ROS, have been shown to be up-regulated by CBD and less so by THC. These include Herpud1 (3.3-fold), Gclm (threefold), Gstm6 (2.8-fold), Hmox1 (2.7-fold), Nqo1 (2.4-fold) and Gstm1 (2.4-fold). Results from our laboratory showed that incubation with 1 µM CBD for 4 h increased ROS formation in BV-2 cells by 215 ± 1% (P < 0.001). This assay was performed using the oxidation-sensitive non-fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate, whose oxidized form, 2′,7′-dichlorofluorescein is highly fluorescent, as previously described (Juknat et al., 2005). It is very likely that the up-regulation of these enzymes represents a compensatory response to the ROS formed by CBD.
As for the 38 gene transcripts whose expression was down-regulated by CBD (by 50% or more compared with the control values), they were mainly concentrated within categories of inflammatory chemokines, cell cycle and proliferation, host defence, and regulation of transcription. These transcripts include zinc finger protein 472 (Zfp472; reduced by 80.4%), chemokine ligand 2 (Ccl2; by 72%), chemokine ligand 7 (Ccl7; by 71.4%), complement component 1, q subcomponent, alpha polypeptide (C1qa; by 70%), cyclin D1 (Ccnd1; by 65.5%) and cyclin E1 (Ccne1; by 63%) (Table 3). As for the transcripts down-regulated by THC, none of them was decreased by 50% or more.
Table 3.
List of genes from gene arrays, down-regulated by CBD (by more than 50%)
| Fold change and percentage of reduction | ||||
|---|---|---|---|---|
| Gene name | Description | Accession number | CBD | THC |
| Inflammatory chemokines and receptors | ||||
| Ccl2 | CCL2 | NM_011333.1 | −3.6 (72%) | −1.0 (0%) |
| Ccl7 | CCL7 | NM_013654 | −3.5 (71.4%) | 1.2 |
| Cxcl14 | CXCL14 | NM_019568 | −2.6 (61.5%) | −1.4 (29%) |
| Cx3cr1 | CX3CR1 | NM_009987.2 | −2.1 (52.4%) | −1.3 (23%) |
| Ccl6 | CCL6 | NM_009139.1 | −2.1 (52.4%) | −1.0 (0%) |
| Ccl9 | CCL 9 | NM_011338 | −2.0 (50%) | −1.2 (17%) |
| Membrane transport and secretion | ||||
| Kctd12 | Potassium channel tetramerization domain containing 12 | NM_177715.2 | −2.5 (60%) | −1.0 (0%) |
| Slc25a22 | Solute carrier family 25 (mitochondrial carrier: glutamate), member 22 | NM_026646.1 | −2.1 (52.4%) | −1.0 (0%) |
| Slc39a10 | Solute carrier family 39 (zinc transporter), member 10 | NM_172653.2 | −2.1 (52.4%) | −1.2 (17%) |
| Slc5a6 | Solute carrier family 5 (sodium-dependent vitamin transporter), member 6 | NM_177870.2 | −2.1 (52.4%) | −1.1 (9%) |
| Metabolic | ||||
| Usp2 | Ubiquitin specific protease 2 | NM_198091.1 | −2.3 (57%) | −1.3 (23%) |
| Siat7d | Sialyltransferase 7 | NM_011373.1 | −2.2 (55%) | −1.2 (17%) |
| Chst10 | Carbohydrate sulphotransferase 10 | NM_145142.1 | −2.2 (55%) | −1.8 (44%) |
| Mat2a | Methionine adenosyltransferase II, α2 | NM_145569 | −2.0 (50%) | −1.3 (23%) |
| Cell cycle and proliferation | ||||
| Ccnd1 | Cyclin D1 | NM_007631.1 | −2.9 (65.5%) | −1.2 (17%) |
| Ccne1 | Cyclin E1 | NM_007633.1 | −2.7 (63%) | −1.2 (17%) |
| Idb1 | Inhibitor of DNA binding 1 | NM_010495.1 | −2.2 (54.5%) | −1.5 (33%) |
| Cdc6 | Cell division cycle 6 homologue (S. cerevisiae) | NM_011799.1 | −2.1 (52.4%) | −1.0 (0%) |
| Ccne2 | Cyclin E2 | NM_009830.1 | −2.0 (50%) | −1.2 (17%) |
| Cdc25a | Cell division cycle 25 homologue A (S. cerevisiae) | NM_007658 | −2.0 (50%) | −1.0 (0%) |
| Host defence and adaptive response | ||||
| C1qa | Complement component 1, q subcomponent, α polypeptide | NM_007572 | −3.3 (70%) | −1.3 (23%) |
| Ebi2 | EPSTEIN-Barr virus induced gene 2 | NM_183031.1 | −2.4 (58%) | −1.3 (23%) |
| Igfbp4 | Insulin-like growth factor binding protein 4 | NM_010517.2 | −2.4 (58%) | −1.3 (23%) |
| Tlr13 | Toll-like receptor 13 | NM_205820.1 | −2.3 (56.5%) | −1.3 (23%) |
| Ly86 | Lymphocyte antigen 86 (Ly86), mRNA. | NM_010745.1 | −2.0 (50%) | −1.7 (41%) |
| Dok2 | Docking protein 2 (Dok2), mRNA. | NM_010071.1 | −2.0 (50%) | −1.1 (9%) |
| Regulation of transcription | ||||
| Zfp472 | Zinc finger protein 472 | NM_153063.3 | −5.1 (80.4%) | 1.2 |
| Fos | FBJ osteosarcoma oncogene | NM_010234.2 | −2.5 (60%) | −1.2 (17%) |
| Mef2c | Myocyte enhancer factor 2C | NM_025282.3 | −2.1 (52.4) | −1.2 (17%) |
| Tle1 | Transducin-like enhancer of split 1, homologue of Drosophila E | NM_011599.2 | −2.0 (50%) | −1.3 (23%) |
| Ase1 | CD3E antigen, ε polypeptide associated protein | NM_145822.1 | −2.0 (50%) | −1.2 (17%) |
| Regulation of translation | ||||
| Eif5a | Eukaryotic translation initiation factor 5A | NM_181582.4 | −2.6 (61.5%) | −1.2 (17%) |
| Adhesion and migration | ||||
| Pscdbp | Pleckstrin homology, Sec7 and coiled-coil domains, binding protein | NM_139200.2 | −2.1 (52.4%) | −1.7 (41%) |
| Motility and morphogenesis | ||||
| Rab3il1 | RAB3A interacting protein (rabin3)-like 1 | NM_144538.1 | −2.1 (52.4%) | −1.3 (23%) |
| Rin2 | Ras and Rab interactor 2 | NM_028724.2 | −2.0 (50%) | −1.1 (9%) |
| Unidentified | ||||
| 4921501M20Rik | RIKEN cDNA 4921501M20 gene (4921501M20Rik), mRNA. | NM_028728.1 | −2.7 (63%) | −1.2 (17%) |
| 0910001A06Rik | RIKEN cDNA 0910001A06 gene (0910001A06Rik), mRNA. | NM_144846.2 | −2.1 (52.4%) | −1.1 (9%) |
| C85492 | expressed sequence C85492 (C85492), mRNA. | NM_153540.2 | −2.1 (52.4%) | −1.0 (0%) |
List of genes down-regulated at least twofold by CBD in resting BV-2 microglial cells. Fold decrease for each gene was calculated according to the formula: Fold change = (−1) × 2−(signal log ratio)
Observing the data presented in Tables 2 and 3, it seems that we can distinguish between two populations of genes: (i) those in which the effects by THC are in the same direction as by CBD (except that the effects with THC are smaller in size) and (ii) those which show CBD selective effects. The latter included genes in all of the groups listed, but especially in the stress response (Ndrg1, Ndrl, Sqstm1/p62, Ddit4, Cathepsin G, Nqo1 and Htatip2), motility and morphogenesis (Avil, Vwf and Pfn2) and cell cycle and proliferation (Cdkn1a, Cdkn2a, Cdkn2b and Gadd45a). This CBD selectivity is even better observed in the list of down-regulated genes where the THC-down-regulated genes never reached the twofold reduction (50% decrease). The gene products Chst 10 (reduced by 44%), Ly86 (by 41%) and Pscdbp (by 41%) were the main THC-down-regulated genes.
Validation of microarray results
Several genes that were identified by microarray analysis as differentially regulated were subjected to validation by qPCR using β2-microglobulin (B2m) as a reference gene. Individual sets of genes were selected to be validated according to their association with the amino acid deprivation pathway and cyst(e)ine uptake as well as with the oxidative stress response. The qPCR assays were repeated four times using at least three mRNA preparations from independent experiments. The results are expressed as fold change relative to control levels. We found that in almost all cases, there is a very good agreement between the microarray and the qPCR results in terms of direction of change as well as of its magnitude. For example, the qPCR data (Table 2) show that CBD up-regulated the expression of Trib3, Slc40a1, Aqp9, Ddit3/Chop/Gadd153, Nupr1/p8, Slc7a11, Sqstm1, Atf4, Hmox1, Cdkn1a, Dusp1 and Casp4. The qPCR data show that THC treatment up-regulated the mRNA expression of Trib3, Aqp9, Nupr1 and Hmox1. qPCR results also show that CBD down-regulated the expression of Zfp472 and Ccl2 . All these results are in complete agreement with the gene array data. The latter result (regarding Ccl2) differs from the data reported by Kozela et al. (2010) due to a change to a better set of primers oriented toward a different part of the sequence using a newer gene accession number.
Numerous molecular targets (some overlapping and some are not) have been reported for CBD and for THC (De Petrocellis and Di Marzo, 2010; De Petrocellis et al., 2011), including, but not limited to, CB1 and CB2 receptors, GPR55, GPR18, the transient receptor potential of vanilloid type-1 and type-2 (TRPV1; TRPV2) channels, the peroxisome proliferator-activating receptors (PPARs) and fatty acid amide hydrolase (FAAH). We and others found that unstimulated BV-2 microglial cells express CB1 and CB2 receptors, GPR55, GPR18 and TRPV2 channels (Pietr et al., 2009; McHugh et al., 2010; Stella, 2010; Rimmerman et al., 2012). In contrast to the relatively low abundance of CB1 mRNA, we found that both CB2 and GPR55 mRNAs are present at relatively high levels in BV-2 cells as well as in unstimulated primary microglia in culture (Pietr et al., 2009). Using the gene array analysis in this study, we have observed that the relative levels of CB1, CB2, GPR18, TRPV2 and FAAH did not exceed the twofold induction or 50% reduction by the cannabinoid treatment and were not affected to a significant extent. Only the effect of CBD on PPARγ2 (+40%) was found to be statistically significant (P < 0.01). qPCR studies strengthened this observation showing that the changes observed in most of these gene products (CB1, CB2, GPR55 and GPR18) were small and did not reach a statistical significance.
In a recent paper from our group, Kozela et al. (2010) described the effects of CBD and THC on LPS-activated BV-2 cells, showing differential anti-inflammatory activities and mechanisms for these cannabinoids. The signalling pathways involved in these activities are composed of different factors and regulators, like IL-1β, IL-6, IRAK1, IFN-β, suppressors of cytokine signalling 3 (SOCS3) as well as signal transducers and activators of transcription 1 and 3 (STAT1 and STAT3). Using gene array analysis, we found that CBD and THC did not statistically significantly affect the expression of these genes in surveillant (resting) cells; their relative levels did not exceed the twofold induction or 50% reduction. Analysing the expression of these genes by qPCR, we found that indeed in most cases, they were not significantly affected by either CBD or THC. On the other hand, the qPCR data showed that IL-1β was highly down-regulated by CBD and THC (80 ± 3% and 74 ± 10% respectively; n= 5; P < 0.001) and IFNb1 was up-regulated by CBD (120 ± 30%; n= 3; P < 0.005) and down-regulated by THC by 65 ± 8% (n= 3; P < 0.05).
Discussion
Cannabinoids have antioxidant, neuroprotective, proapoptotic and anti-tumour properties (Klein et al., 1998; Galve-Roperh et al., 2000; McKallip et al., 2002; Cabral and Staab, 2005; van der Stelt and Di Marzo, 2005; Klein and Cabral, 2006; Massi et al., 2006; Kozela et al., 2010; Rieder et al., 2010). Several cannabinoids were effective in treating pain, glaucoma, wasting, nausea and vomiting as well as AIDS, and spasticity in multiple sclerosis (Pertwee, 2002; Guzman, 2003; Di Marzo and De Petrocellis, 2006; Kogan and Mechoulam, 2007). Various cannabinoids also have immunomodulatory and anti-inflammatory effects which are probably mediated via their activity on various types of immune cells (such as B cells, T cells, macrophages, NK cells, dendritic cells) and microglia (Klein et al., 1998; 2000; Croxford and Yamamura, 2005; Kozela et al., 2010; Rieder et al., 2010). These cannabinoids exert their immunosuppressive activity by induction of apoptosis, inhibition of cell proliferation, down-regulation of cytokines and chemokine production and release, and induction of regulatory T cells (Croxford and Yamamura, 2005; Klein and Cabral, 2006; Kozela et al., 2010; Rieder et al., 2010). Moreover, both THC and CBD have anti-inflammatory activities, even though CBD does not seem to be active on either CB1 or CB2 receptors, suggesting a CB1/CB2 receptor-independent mechanism and the involvement of other cell targets (Kozela et al., 2010; O'Sullivan and Kendall, 2010).
However, none of these studies addressed the effects of cannabinoids and especially of the non-psychotropic CBD, at the level of genome-wide expression. The present study shows the effect of treatment with the two major cannabinoids present in Cannabis, CBD and THC, on genome-wide mRNA levels in resting murine BV-2 microglial cells. We identified 1204 differentially expressed genes in response to CBD treatment, which were associated with many different functions, including stress, inflammatory response, membrane transport, adhesion and migration, cell cycle and proliferation. Moreover, we show that CBD affects the expression of many more gene transcripts (by about 18-fold) in comparison to THC and that the changes in the level of transcription are also much higher with CBD. Only 36 genes were up-regulated and 29 down-regulated exclusively by THC and not by CBD. Moreover, the effects on the expression of these genes were small, compared with the effects of CBD.
We show here, that CBD, and less so THC, repressed the expression of an important subset of pro-inflammatory genes, especially the chemokine ligands, CCL2, CCL7, CXCL14, CCL6, CCL9 and the chemokine receptor CX3CR1. These effects are in agreement with reports showing that cannabinoids can modulate the functional activities of immune cells and exert immunosuppressive effects mainly by inhibition of cytokine and chemokine production, induction of apoptosis and inhibition of cell proliferation (Klein and Cabral, 2006; Kozela et al., 2010; Rieder et al., 2010). Among the chemokines found to be down-regulated, the chemokine-receptor network, involving the chemokine CCL2(MCP-1/JE) and its receptor CCR2, is by far the best known for its involvement in (i) neuroprotection (such as reduction in CCL2-mediated immune cell infiltration after stroke or traumatic brain injury); (ii) neurotransmission (such as increase of cell excitability, dopamine release and locomotor activity in CCL2-injected rats); and (iii) neurogenesis (by CCL2 induction of stem-cell migration into sites of damage in the injured brain, and directing differentiation of precursor cells into neural cells) (Chintawar et al., 2009; Magge et al., 2009; Semple et al., 2010). The CCL2/CCR2 signalling is involved not only in these physiological processes but also in regulating immune cell infiltration into injured areas of the CNS (Semple et al., 2010). Moreover, the observation that CBD treatment down-regulates the expression of CCL2 mRNA is in agreement with our previous results showing that CBD decreased by 58% the LPS-up-regulated CCL2 mRNA expression in BV-2 cells (Kozela et al., 2010).
The list of CBD-up-regulated genes and the IPA analysis reveal that the CBD-specific gene expression profile is showing changes associated with oxidative stress and GSH depletion which normally occur under nutrient limiting conditions or P450-dependent biotransformation of xenobiotics. Furthermore, these CBD-up-regulated genes are known to be controlled by nuclear factors usually involved in the regulation of stress response and cell death, mainly Nrf2 and the activating transcription factor 4 (ATF4). These observations indicate that CBD, but less so THC, induces a cellular stress response and that this response seems to underlie its immunosuppressive activity. Indeed, the gene expression profile induced by treatment with CBD on resting BV-2 microglial cells, shows changes in several families of genes known to be regulated/activated by ATF4, including those encoding amino acid biosynthetic enzymes, amino acid transporters and aminoacyl-tRNA synthetases. Of the subset of genes that were significantly up-regulated in response to CBD, five genes (Trib3, Ddit3/Chop/Gadd153, Atf4, Atf3 and Slc7a11) are known to contain amino acid response elements (AAREs), and to respond to amino acid deprivation, suggesting an effect of CBD on the GCN2/eIF2α/ATF4 pathway (Wek et al., 2006; Kilberg et al., 2009). The amino acid deprivation pathway is initiated by the amino acid sensor GCN2 [general control non-derepressible 2; also known as eukaryotic initiation factor-2α (eIF2α) kinase 4], which leads to phosphorylation and inactivation of eIF2α, suppressing global mRNA translation but allowing translation of ATF4 with the subsequent induction of genes for amino acid biosynthesis and transport (Taylor, 2009). Moreover, Gclm (glutamate-cysteine ligase, modifier subunit), Slc1a4[solute carrier family 1 (glutamate/neutral amino acid transporter), member 4], Slc3a2[solute carrier family 3 (activator of dibasic and neutral amino acid transporter), member 2] and Cars (cysteinyl-tRNA synthetase) are additional related genes found to be up-regulated by CBD. These genes are involved in cyst(e)ine transport and its metabolism. Up-regulation of these genes (including Slc7a11) would facilitate cysteine uptake by the cells and lead to increased GSH synthesis as part of the normal response of the cell to amino acid deficiency or to other stress stimuli (such as proteasome inhibition) that activates GCN2/eIF2α/ATF4 pathway. Other amino acid-related genes found to be up-regulated by CBD are Slc6a9[solute carrier family 6 (neurotransmitter transporter, glycine), member 9], Sars (seryl-aminoacyl-tRNA synthetase), Chac1 and Gadd45a (growth arrest and DNA-damage-inducible 45α). The latter gene product is known to encode a protein that inhibits proliferation and suppresses growth, functions that serve as protecting events during amino acid deficiency. Our present results are, thus, in agreement with reports that show up-regulation of genes of the amino acid response pathway leading to eIF2α phosphorylation and increased ATF4 translation, in response to cysteine deprivation or amino acid-deficient media in HepG2 human hepatocarcinoma cells (Lee et al., 2008c; Palii et al., 2009).
The GCN2/eIF2α/ATF4 pathway has been also found to be activated by proteosome inhibition (Wek et al., 2006). In this regard, we found that CBD up-regulates Sqstm1/p62 expression (sequestosome 1; 3.2-fold), a multifunctional ubiquitin-binding protein, which has been implicated in a variety of processes including cell signalling, receptor internalization, protein turnover (Seibenhener et al., 2007) and autophagic clearance of ubiquitinated protein aggregates (Pankiv et al., 2007; Ding and Yin, 2008). Recent reports showed that Sqstm1/p62 can be employed as an autophagic marker as there is a correlation between inhibition of autophagy and increased levels of Sqstm1/p62 (Pankiv et al., 2007).
Another gene up-regulated by CBD is Nupr1/p8 (eightfold). Nupr1 was found to be induced by ATF4 in response to various cellular stressors. Nupr1 expression has been reported to be associated with enhanced transcriptional activation of genes downstream of ATF4 (like CHOP and TRIB3), suggesting that Nupr1/p8 promotes the transcription of stress-regulated genes via positive feedback on the ATF4 pathway (Jin et al., 2009). According to these results, CBD and to a lesser extent THC, activates the GCN2/eIF2α/p8/ATF4/CHOP-TRIB3 pathway, known to lead to autophagy as well as to apoptotic cell death. It is important to recall that the group of Velasco reported induction by THC of cell death associated with stress-related gene expression via the p8-ATF4-TRIB3 pathway, in U87MG human astrocytoma and C6 glioma cells (Carracedo et al., 2006a,b; Salazar et al., 2009).
As mentioned above, the CBD-induced pathways include genes involved in Nrf2-mediated oxidative stress. Nrf2 is a redox-sensitive transcription factor that plays a central role in cellular defence against oxidative stress- and cytotoxic electrophile-induced insults through transcriptional activation of an array of genes, including phase II detoxifying enzymes, antioxidants and transporters. Nrf2 binds to the antioxidant response element/electrophile response element (ARE/EpRE), located in the promoter region of genes encoding phase II detoxifying or antioxidant enzymes and related stress-response proteins (Kobayashi and Yamamoto, 2006). These include among others, haem oxygenase-1 (Hmox1), GSH S-transferase (GST), GSH peroxidase, NAD(P)H:quinone oxidoreductase (Nqo1) and glutamate-cysteine ligase (Gcl), which play key roles in cellular defence by enhancing the removal of cytotoxic electrophiles and ROS, and by maintaining GSH homeostasis. From the list of up-regulated genes (Table 2) Herpud1, Hmox1, Nqo1 and Gclm are genes known to be regulated in response to oxidative stress via the (ARE/EpRE)/Nrf2 system. One of the mechanisms of defence against the toxicity of ROS is the GST-catalysed conjugation of xenobiotics with endogenous GSH (Shih et al., 2003). Indeed, we found up-regulation by CBD of GST mu6 and GST mu1 mRNA expression by 2.8- and 2.4-fold respectively.
Nrf2 also regulates the expression of two cellular transporters, the cysteine glutamate exchange transporter (that buffers cysteine influx against GSH efflux) and the Mrp1 transporter (functions to exclude the entry of xenobiotic metabolites) (Cullinan and Diehl, 2006). Nrf2 belongs to a subclass of transcription factors that are incapable of self-dimerization. Thus, sequence-specific DNA binding and subsequent induction of target gene transcription requires association of Nrf2 with other transcription factors like ATF4 that has been identified as an Nrf2 interacting protein (He et al., 2001). Indeed, Nrf2 is a potential activator at the ATF4 promoter (Cullinan and Diehl, 2006).
Among the mediators up-regulated by Nrf2, the antioxidant enzyme Hmox1 (whose gene is up-regulated by CBD) has been shown to modulate inflammation and innate immunity (see Kim et al., 2010). Interestingly, Nrf2-dependent Hmox1 was found to inhibit CCL2 (shown to be down-regulated by CBD) suggesting that activation of Nrf2 negatively regulates the expression of pro-inflammatory chemokines, via Hmox1 up-regulation. This up-regulation of Hmox1 and down-regulation of Ccl2 mRNAs suggest the possible involvement of CBD in the regulation of the Nrf2/Hmox1 axis, a pathway relevant for the restoration of the redox homeostasis and for the modulation of inflammatory responses (Innamorato et al., 2008, 2009; Singh et al., 2010). Tzima et al. (2009) reported that myeloid Hmox1 regulates IFN-β production establishing Hmox1 as a critical early mediator of the innate immune response in mice. Moreover, aside from our findings on the up-regulation of Hmox1 by CBD, we found (by qPCR) that CBD enhanced the expression of IFNb1 mRNA. This is a very significant result, due to the fact that IFN-β is currently used in the treatment of multiple sclerosis, a chronic inflammatory disease of the CNS (Bates, 2011; Rudick and Goelz, 2011) and was shown to diminish symptoms of experimental autoimmune encephalomyelitis (EAE), an multiple sclerosis model in mice (Galligan et al., 2010). These results could explain the effect of CBD in ameliorating the EAE disease symptoms as previously reported (Kozela et al., 2011).
As reported by Kozela et al. (2010), STAT molecules have a role in CBD-induced anti-inflammatory effects. In this regard, it was reported that the down-regulation of the expression of Ccl2 mRNA by CBD (but not THC) in LPS-treated cells is in agreement with the increase in STAT3 activation and with the decrease in STAT1 phosphorylation after CBD treatment. Accordingly, our current results show down-regulation of Ccl2 mRNA by CBD and not by THC. As CBD down-regulates the LPS-up-regulated SOCS3 expression, the inducible effect of CBD on the activation of STAT3 could be mediated via the CBD effect on SOCS3. These effects of CBD on STAT3 and on SOCS3 could underlie the potential benefits of CBD in the treatment of neuroinflammatory diseases.
We found that CBD increases ROS formation in BV-2 cells. This result is in agreement with our gene array analysis, where we found up-regulation of the expression of Herpud1, Gclm, Gstm6, Hmox, Nqo1 and Gstm1 by CBD. These are key enzymes which are involved in cellular defence by increasing the removal of cytotoxic electrophiles and ROS, leading to GSH homeostasis. These results are in agreement with previous reports showing the ability of CBD to induce ROS production as well as enhancing apoptosis via an oxidative stress-dependent mechanism (Massi et al., 2006; Wu et al., 2008; Lee et al., 2008a). In our previous work, we reported that THC (0.1–1.0 µM) is able to enhance cellular damage from oxidative stress in C6 glioma cells, when incubated in the presence of the synthetic cell permeable quinone Qcb (2-phenyl-4-[butylamino]-naphtholquinoline-7,12-dione), a reagent that generates ROS. No effect of THC on release of LDH was observed in the absence of this ROS generator, demonstrating that THC by itself is a weak generator of ROS. Moreover, THC was found to be a radical scavenger and is likely to serve as a trap for ROS (Goncharov et al., 2005).
Lee et al. (2008b) reported that induction of ROS by cocaine treatment of AF5, a neural progenitor cell line, contributes to eIF2α-ATF4-mediated cyclin A2 down-regulation, leading to cocaine-induced inhibition of proliferation. We have found that CBD, and less so THC, down-regulates the expression of cyclins D1 and E1 in resting BV-2 microglial cells. Moreover, CBD induces the expression of cyclin-dependent kinase inhibitor 1A (Cdkn1a, also called p21; Table 2), a gene product that regulates many cellular processes such as cell cycle arrest, DNA replication and repair, cell differentiation, senescence and apoptosis. Interestingly, Gadd45a, a gene product that binds directly to Cdkn1a/p21, is also up-regulated by CBD. In this regard, Cdkn1a/p21 has been shown to inhibit apoptosis signal-regulating kinase-1 (ASK-1)-mediated activation of JNK and to induce resistance to cell death under conditions of oxidative stress both in vivo and in vitro (Langley et al., 2008). Recent evidence suggests that p21-dependent cell survival under oxidative stress is mediated through the activation of the Nrf2 signalling pathway, showing that Nrf2 (and its downstream genes) is up-regulated by p21 via a direct interaction between these two proteins (Chen et al., 2009). The mRNA and protein expression of p21 was also reported to be up-regulated in response to amino acid deprivation in HepG2 human hepatoma cells (Leung-Pineda et al., 2004).
Regulated water transport is important for mitochondrial homeostasis. It is known that changes in mitochondrial volume are associated with normal physiological processes as well as with pathological conditions such as those involved in ROS formation and cell injury. Aquaporin water channels are responsible for water transport (import and export) through mitochondrial and cell membranes (see Gena et al., 2009). In this regard, we found that CBD up-regulated genes related to water, iron and zinc transport, including Aqp9 (14-fold), Slc40a1 (iron-regulated transporter; 6.5-fold) and the zinc transporters Slc39a4 and Slc30a1 (3.5-fold and 2.6-fold respectively) (Table 2). In addition to water, Aqp9 contributes to the transport of small solutes (such as glycerol, lactate and urea), and participates in osmotic swelling induced by apoptotic stimuli (Lee and Thevenod, 2006). It is interesting to note that, following metabolic stress, Aqp9 expression is induced in pyramidal neurons, which do not express this channel under normal physiological conditions (see Badaut, 2010).
In summary, in the present study we show that CBD affected the expression of many more genes, than those affected by THC. We found that CBD induced a robust response related to oxidative stress and GSH deprivation, which seems to be controlled by Nrf2 and ATF4 transcription factors. Concerning the mechanism underlying the CBD actions, it seems that CBD treatment leads to depletion of intracellular GSH, activating the GCN2/eIF2α/p8/ATF4/CHOP-TRIB3 pathway, accompanied by generation of ROS via the (EpRE/ARE)-Nrf2/ATF4 system, and to regulation of the Nrf2/Hmox1 axis, involved in modulation of redox homeostasis and inflammatory responses. The anti-inflammatory effects of CBD seem to correlate with up-regulations of the expression of Hmox1 and IFNb1, and down-regulation of the expression of Ccl2, via the IFN-β-STAT pathway.
Acknowledgments
To the memory of Maciej Pietr. This work was supported by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation and by the Dr Miriam and Sheldon G. Adelson Center for the Biology of Addictive Diseases. A.J and N.R. are supported by the Israeli Ministry for Absorption in Science.
Glossary
- CBD
cannabidiol
- GSH
glutathione
- Nrf2
nuclear factor-erythroid 2-related factor 2
- THC
Δ9-tetrahydrocannabinol
Conflicts of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaut J. Aquaglyceroporin 9 in brain pathologies. Neuroscience. 2010;168:1047–1057. doi: 10.1016/j.neuroscience.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Bates D. Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials. Neurology. 2011;76(Suppl. 1):S14–S25. doi: 10.1212/WNL.0b013e3182050388. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, Kettenmann H. An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res. 1992;31:616–621. doi: 10.1002/jnr.490310405. [DOI] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Elbaz J, Shabat-Simon M, Eilam R, Zangen A, et al. Chronic exposure to Delta9-tetrahydrocannabinol downregulates oxytocin and oxytocin-associated neurophysin in specific brain areas. Mol Cell Neurosci. 2006;31:795–804. doi: 10.1016/j.mcn.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Cannabinoids as therapeutics agents for ablating neuroinflammatory disease. Endocr Metab Immune Disord Drug Targets. 2008;8:159–172. doi: 10.2174/187153008785700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Staab A. Effects on the immune system. Handb Exp Pharmacol. 2005;168:385–423. doi: 10.1007/3-540-26573-2_13. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006a;66:6748–6755. doi: 10.1158/0008-5472.CAN-06-0169. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006b;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang X-J, Jiang T, Huang Z, Fang D, et al. Direct interaction between Nrf2 and p21Cip1/WAF1 upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintawar S, Cayrol R, Antel J, Pandolfo M, Prat A. Blood-brain barrier promotes differentiation of human fetal neural precursor cells. Stem Cells. 2009;27:838–846. doi: 10.1002/stem.25. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Davies P, Sornberger GC, Huber GL. Effects of experimental marijuana and tobacco smoke inhalation on alveolar macrophages. A comparative stereologic study. Lab Invest. 1979;41:220–223. [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids and synthetic cannabimimetics: focus on G-protein coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Plant, synthetic and endogenous cannabinoids in medicine. Ann Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- Ding W-X, Yin X-M. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Understanding Marijuana: A New Look at the Scientific Evidence. Oxford: Oxford University Press; 2002. [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Galligan CL, Pennell LM, Murooka TT, Baig E, Majchrzak-Kita B, Rahbar R, et al. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult Scler. 2010;16:1458–1473. doi: 10.1177/1352458510381259. [DOI] [PubMed] [Google Scholar]
- Gallily R, Even-Chena T, Katzavian G, Lehmann D, Dagan A, Mechoulam R. Gamma-irradiation enhances apoptosis induced by cannabidiol, a non-psychotropic cannabinoid, in cultured HL-60 myeloblastic leukemia cells. Leuk Lymphoma. 2003;44:1767–1773. doi: 10.1080/1042819031000103917. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, del Pulgar TG, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Gena P, Fanelli E, Brenner C, Svelto M, Calamita G. News and views on mitochondrial water transport. Front Biosci. 2009;14:4189–4198. doi: 10.2741/3522. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov I, Weiner L, Vogel Z. Delta9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience. 2005;134:567–574. doi: 10.1016/j.neuroscience.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, et al. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- Ho LJ, Lai JH. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Curr Drug Metab. 2004;5:181–192. doi: 10.2174/1389200043489081. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, Nutile-McMenemy N, Alkaitis MS, DeLeo JA. Differential migration, LPS-induced cytokine, chemokine and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem. 2008;107:557–569. doi: 10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Innamorato NG, Lastres-Becker I, Cuadrado A. Role of microglial redox balance in modulation of neuroinflammation. Curr Opin Neurol. 2009;22:308–314. doi: 10.1097/WCO.0b013e32832a3225. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jin H-O, Seo S-K, Woo S-H, Choe T-B, Hong S-I, Kim J-I, et al. Nuclear protein 1 induced by ATF4 in response to various stressors acts as a positive regulator on the transcriptional activation of ATF4. IUBMB Life. 2009;61:1153–1158. doi: 10.1002/iub.271. [DOI] [PubMed] [Google Scholar]
- Juknat AA, Méndez M, del V, Quaglino A, Fameli CI, Mena M, et al. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005;38:84–92. doi: 10.1111/j.1600-079X.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglia pro-inflammatory cytokine IL-1β production. Neurobiol Aging. 2004;25:431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- Kim J, Cha Y-N, Surh Y-J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol. 2006;1:50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Immunol Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. Review. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids Δ9 -tetrahydrocannabinol and cannabidiol differentially inhibit the LPS-activated NF-κB and IFNβ/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616–1625. doi: 10.1074/jbc.M109.069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, et al. Cannabidiol inhibits pathogenic T-cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in mice. Br J Pharmacol. 2011;163:1507–1519. doi: 10.1111/j.1476-5381.2011.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley B, D'Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Lee W-K, Thevenod F. A role for mitochondrial aquaporins in cellular life-and-death decisions? Am J Physiol Cell Physiol. 2006;291:C195–C202. doi: 10.1152/ajpcell.00641.2005. [DOI] [PubMed] [Google Scholar]
- Lee CY, Wey SP, Liao MH, Hsu WL, Wu HY, Jan TR. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int Immunopharmacol. 2008a;8:732–740. doi: 10.1016/j.intimp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL, et al. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008b;5:e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-I, Dominy JR, Jr, Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics. 2008c;33:218–229. doi: 10.1152/physiolgenomics.00263.2007. [DOI] [PubMed] [Google Scholar]
- Leung-Pineda V, Pan YX, Chen H, Kilberg MS. Induction of p21 and p27 expression by amino acid deprivation of HepG2 human hepatoma cells involves mRNA stabilization. Biochem J. 2004;379:79–88. doi: 10.1042/BJ20031383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Christensen KV, Hedtjarn M, Mortensen AL, Hagberg H, Falsig J, et al. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS. Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther. 2002;302:451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- Magge SN, Malik SZ, Royo NC, Chen HI, Yun L, Snyder EY, et al. Role of monocyte chemoattractant protein-1 (MCP-1/CCL2) in migration of neural progenitor cells toward glial tumors. J Neurosci Res. 2009;87:1547–1555. doi: 10.1002/jnr.21983. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Bianchessi S, Costa B, Macchi P, Parolaro D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell Mol Life Sci. 2006;63:2057–2066. doi: 10.1007/s00018-006-6156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol-recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids. 2009;37:79–88. doi: 10.1007/s00726-008-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabidiol as a potent medicine. In: Mechoulam R, editor. Cannabinoids as Therapeutics. 1st edn. Basel: Birkhäuser Basel; 2005. pp. 49–66. [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, et al. Differential changes in GPR55 during microglial activation. FEBS Lett. 2009;583:2071–2076. doi: 10.1016/j.febslet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Raz A, Goldman R. Effect of hashish compounds on mouse peritoneal macrophages. Lab Invest. 1976;34:69–76. [PubMed] [Google Scholar]
- Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, et al. Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem. 1997;40:3228–3233. doi: 10.1021/jm970126f. [DOI] [PubMed] [Google Scholar]
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215:598–605. doi: 10.1016/j.imbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmerman N, Bradshaw HB, Kozela E, Levy R, Juknat A, Vogel Z. Compartmentalization of endocannabinoids into lipid rafts in a microglial cell line devoid of caveolin-1. Br J Pharmacol. 2012;165:2436–2449. doi: 10.1111/j.1476-5381.2011.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Goelz SE. Beta interferon for multiple sclerosis. Exp Cell Res. 2011;317:1301–1311. doi: 10.1016/j.yexcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva IJ, Hernández-Tiedra S, Lorente M, Egia A, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, Geetha T, Wooten MW. Sequestosome 1/p62 – More than just a scaffold. FEBS Lett. 2007;581:175–179. doi: 10.1016/j.febslet.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the Ccl2/Ccr2 and Cxcl8/Cxcr2 networks. J Cereb Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res. 2010;44:1267–1288. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Cannabinoid receptors and their role in neuroprotection. Neuromolecular Med. 2005;7:37–50. doi: 10.1385/NMM:7:1-2:037. [DOI] [PubMed] [Google Scholar]
- Taylor PM. Amino acid transporters: éminences grises of nutrient signalling mechanisms? Biochem Soc Trans. 2009;37:237–241. doi: 10.1042/BST0370237. [DOI] [PubMed] [Google Scholar]
- Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-β production. J Exp Med. 2009;206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich O, Diestel A, Eyupoglu IY, Nitsch R. Regulation of microglial expression of integrins by poly(ADP-ribose)polymerase-1. Nat Cell Biol. 2001;3:1035–1042. doi: 10.1038/ncb1201-1035. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang H-Y, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Woelkart K, Salo-Ahen OM, Bauer R. CB receptor ligands from plants. Curr Top Med Chem. 2008;8:173–186. doi: 10.2174/156802608783498023. [DOI] [PubMed] [Google Scholar]
- Wu HY, Chu RM, Wang CC, Lee CY, Lin SH, Jan TR. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol Appl Pharmacol. 2008;226:260–270. doi: 10.1016/j.taap.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286:1103–1109. [PubMed] [Google Scholar]


