Abstract
BACKGROUND AND PURPOSE
Cannabis and caffeine are two of the most widely used psychoactive substances. Δ9-Tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis, induces deficits in short-term memory. Caffeine, a non-selective adenosine receptor antagonist, attenuates some memory deficits, but there have been few studies addressing the effects of caffeine and THC in combination. Here, we evaluate the effects of these drugs using a rodent model of working memory.
EXPERIMENTAL APPROACH
Rats were given THC (0, 1 and 3 mg·kg−1, i.p.) along with caffeine (0, 1, 3 and 10 mg·kg−1, i.p.), the selective adenosine A1-receptor antagonist CPT (0, 3 and 10 mg·kg−1) or the selective adenosine A2A-receptor antagonist SCH58261 (0 and 5 mg·kg−1) and were tested with a delayed non-matching-to-position procedure in which behaviour during the delay was automatically recorded as a model of memory rehearsal.
KEY RESULTS
THC alone produced memory deficits at 3 mg·kg−1. The initial exposure to caffeine (10 mg·kg−1) disrupted the established pattern of rehearsal-like behaviour, but tolerance developed rapidly to this effect. CPT and SCH58261 alone had no significant effects on rehearsal or memory. When a subthreshold dose of THC (1 mg·kg−1) was combined with caffeine (10 mg·kg−1) or CPT (10 mg·kg−1), memory performance was significantly impaired, even though performance of the rehearsal-like pattern was not significantly altered.
CONCLUSION AND IMPLICATIONS
Caffeine did not counteract memory deficits induced by THC but actually exacerbated them. These results are consistent with recent findings that adenosine A1 receptors modulate cannabinoid signalling in the hippocampus.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: delayed spatial matching, mediating response, rehearsal, marijuana, adenosine receptor antagonist, caffeine, THC
Introduction
Cannabis is the most widely used illicit drug in the world, with about 160 million people using it at least once per year and over 22 million using it daily (Leggett, 2006). Efforts to decriminalize the recreational and medicinal use of marijuana have been gaining momentum in recent years. While cannabis and cannabinoid medications have the potential to produce some beneficial effects such as relief from pain and nausea, they also have the potential to produce adverse effects on physical and mental health, such as anxiety, psychosis and drug dependence. One adverse effect that is well documented is the ability of marijuana and Δ9-tetrahydrocannabinol (THC), the main psychoactive constituent of marijuana, to produce deficits in learning and memory (Lichtman et al., 2002; Castellano et al., 2003), especially short-term episodic and working memory (Ranganathan and D'Souza, 2006).
Caffeine is the most widely used licit drug with psychoactive effects, used daily by over 80% of the world's population (James, 1997). While coffee, tea and soft drinks containing caffeine and related compounds have long been popular, there has been a recent increase in the availability and use of ‘energy drinks’ containing higher levels of caffeine (Reissig et al., 2009). Caffeine is widely perceived as a cognitive enhancer (Glade, 2010), and there is evidence for this in certain situations (Cunha and Agostinho, 2010). Caffeine can increase arousal and attention, thereby enhancing performance in memory tasks when performance has been degraded by a wide variety of factors such as sleep deprivation (Alhaider et al., 2010) or the presence of distractors (Bain et al., 2003).
With caffeine and marijuana both used so widely, it is likely that the simultaneous use of both drugs is also common. Furthermore, with caffeine being perceived as a cognitive enhancer, some may be led to ingest caffeine in a specific attempt to counteract marijuana's effects on memory. Therefore, it would be valuable to determine whether caffeine alters the effects of THC on memory in a controlled laboratory setting. This question is also interesting because recent advances in our understanding of the effects of THC and caffeine in the brain indicate that caffeine may be capable of modulating the effects of THC in the hippocampus (Hoffman et al., 2010), an area known to be intimately involved in learning and memory. Moreover, a recent study demonstrates that a history of caffeine exposure can actually exacerbate the amnestic effects of THC (Sousa et al., 2011). However, to our knowledge, there have been no studies addressing the cognitive effects of THC and caffeine administered at the same time.
The goal of the present study was to assess the combined effects of acute THC and caffeine in a rodent model of working memory. We used a delayed non-matching-to-position procedure (Panlilio et al., 2011) that incorporates a measure of mediating behaviour – behaviour that enhances performance but is not explicitly required by the task (Pontecorvo et al., 1996) – that may be analogous to memory rehearsal in humans (Hasher and Zacks, 1979; Grant, 1998). This procedure measures spatial memory over a range of delay values (0–28 s) using nose poke holes that provide visual sample stimuli and also record behaviour during the delay period. We found earlier with this procedure that most responses during the delay occurred in a neutral hole (where responding was required to end the delay), but responses also occurred in the sample hole and the non-matching hole (i.e. the to-be-correct hole, where a response would be required at to receive a food pellet after the delay). Analysis of behaviour during the delay revealed that individual rats appeared to adopt one of two effective ‘rehearsal strategies’: responding in the to-be-correct hole during the delay, or responding in the sample hole during the delay. As in other studies with more conventional delayed non-matching-to-position procedures (i.e. with no recording of mediating behaviour; Heyser et al., 1993; Mallet and Beninger, 1998; Hampson and Deadwyler, 2000; see also Lane et al., 2005), THC decreased working-memory performance in a dose- and delay-dependent fashion. Notably, we found that this impairment did not result from a disruption in the performance of the mediating response but from a decrease in its effectiveness as a mediating strategy. That is, THC decreased the accuracy of the non-matching response even on trials when the rat had responded only in the ‘appropriate’ hole during the delay.
In the present study, this delayed non-matching-to-position procedure was used to assess possible interactions between THC (0, 1 and 3 mg·kg−1) and caffeine (0, 1, 3 and 10 mg·kg−1). We found that caffeine potentiated the effects of a subthreshold dose of THC, such that it produced short-term memory deficits comparable with those produced by higher doses of THC. Because caffeine is a non-selective adenosine receptor antagonist, we also examined the effects of combining a low dose of THC with selective adenosine receptor antagonists. As the adenosine A1 receptor antagonist CPT (receptor nomenclature follows Alexander et al., (2011) had effects similar to caffeine, but the A2A receptor antagonist SCH58261 did not, the effects of caffeine in this model are probably due to its actions at adenosine A1 receptors.
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the guidelines of the Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program and the National Research Council (1996). The animal facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Eight male Long–Evans hooded rats, about 15 months old, were maintained in individual cages with a 12 h light/dark cycle. Procedures were conducted Monday through Friday during the light phase. Rats were fed approximately 15 g of food per day to maintain stable body weights. The original training and testing of these rats was described previously (Panlilio et al., 2011). Two rats from that study were not used in the present study for health reasons, and one additional rat was added to the present study.
Apparatus
Each of eight individually enclosed training chambers (model MED-NPW-9L; MED Associates, St. Albans, VT) had three response holes (2 cm high × 2 cm wide × 2 cm deep) in a horizontal array on one wall, with the side holes 2.75 cm from the centre hole. Each hole could be illuminated from within by a yellow LED. Food pellets (45 mg; type F0021; Bio-Serv, Frenchtown, NJ) were dispensed into a food trough mounted on the wall opposite to the wall with the response holes.
Treatment schedules
THC (Research Triangle Institute, Research Triangle Park, NC) was given 40 min before the session in a vehicle of 40% cyclodextrin (Sigma-Aldrich, St. Louis, MO) and 60% saline. Caffeine (anhydrous base; Sigma-Aldrich) was given 30 min before the session in a vehicle of saline solution. CPT (8-cyclopentyltheophylline; Sigma Chemical Company, St. Louis, MO) was given 30 min before the session in a vehicle of saline solution with a few drops of NaOH. SCH58261 (2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine) was given 30 min before the session in a vehicle of 5% dimethyl sulphoxide and 5% Tween 80. Injections were given i.p. (1 mL·kg−1) up to two times per·week, usually on Tuesday or Friday. Doses and treatment times were chosen based on our previous work with these drugs using locomotor activity and drug discrimination procedures (Justinova et al., 2003; Karcz-Kubicha et al., 2003; Solinas and Goldberg, 2005; Solinas et al., 2005; Orru et al., 2011).
Non-matching-to-position task
Sessions were conducted once a day and lasted for 90 min or until 100 food pellets had been delivered. A discrete-trials procedure was used in which each trial started with the house light off and one of the two side holes being lit as the sample. Two responses in the sample hole were required to extinguish the side-hole light and turn on the centre-hole light, starting the delay period. After a delay of 0, 7, 14, 21 or 28 s the next response in the centre hole extinguished the centre-hole light and lit both side holes, starting the choice phase of the trial; responding in the centre hole was required to prevent the rat from simply waiting at the correct hole until the end of the delay. In the choice phase, a response in the correct hole (i.e. the side hole opposite to the sample hole) immediately produced a food pellet, extinguished the hole lights and turned on the house light for a 15 s inter-trial interval; a response in the incorrect hole (i.e. the side hole opposite to the sample hole) produced no pellet, extinguished the hole lights and caused the house light to flash (5 Hz) for 5 s, after which the house light remained on for the 15 s inter-trial interval. The delay value for each trial was selected by drawing without replacement from a list in which each of the five possible values appeared once. When each of the values had been used once, the list was replenished. The side used for the sample hole was chosen in a similar manner, using a list in which each side (left and right) appeared twice.
Drug testing
Drugs were tested only when a baseline performance criterion was met: >90% correct at the 0 s delay and <10 percentage points difference in accuracy at each given delay over the previous two baseline sessions. A dose–effect function for THC (0, 1, 3 and 5.6 mg·kg−1) had been obtained in the previous experiment (Panlilio et al., 2011), in which the two highest doses significantly decreased accuracy. In a preliminary dose-ranging phase of the present study, a dose–effect correlation for caffeine (0, 1, 3 and 10 mg·kg−1) was obtained, with a single injection given before each test session and the order of doses counterbalanced across rats. In the formal test phase of the study, to assess the effects of caffeine (0, 1, 3 and 10 mg·kg−1) and THC (0, 1 and 3 mg·kg−1) in combination, rats were tested with factorial combinations of these doses in counterbalanced order, given as two injections before the test session. Each combination was tested once, except the vehicle condition (i.e. 0 mg·kg−1 caffeine combined with 0 mg·kg−1 THC), which was given two to three times to each rat during this phase. Due to equipment malfunctions and health problems (i.e. unexplained loss of appetite in three of the rats), some rats were not tested under all conditions. The total number of rats in each condition was eight for all conditions in the THC–caffeine experiment except THC 0 + caffeine 10 (n= 7), THC 1 + caffeine 10 (n= 7), THC 3 + caffeine 3 (n= 6), THC 3 + caffeine 10 (n= 5). The number of rats was six in the THC–CPT experiment and five in the THC–SCH58261 experiment.
Data analysis
All analyses were performed using restricted maximum likelihood estimation (Proc Mixed; SAS Institute, Cary, NC). Tukey–Kramer correction was used to maintain a 0.05 significance level for paired comparisons. Bonferroni-corrected simultaneous confidence intervals were constructed to determine whether mean levels of accuracy differed from chance (50%). For each rat, data from all vehicle sessions were pooled. Arcsine-root transformation was used for analysis of percentage measures. To assess accuracy of choice responding, the percentage of trials with a correct response was analysed using THC dose, caffeine dose and delay value as factors. Under the nominal 0 s delay (which actually lasted until the first response in the centre hole during the delay), accuracy was consistently high in all rats under all conditions, and there was very little mediating behaviour, so data from this delay value were excluded from all analyses.
To assess the effects of mediating behaviour, side-hole responses during the delay period were characterized as occurring in either the hole that would be correct at choice time or the hole that would be incorrect at choice time. In the previous study (Panlilio et al., 2011), logistic regression was performed for each rat, relating trial outcome (correct vs. incorrect) to three factors: whether there was at least one response in the to-be-correct hole, whether there was at least one response in the to-be-incorrect hole and the length of the delay. This analysis provided odds ratios for each rat describing the influence of side-hole responding, taking the effect of delay into account. In some rats, a response in the to-be-correct hole during the delay increased the odds of a correct outcome, but in other rats, a response in the opposite hole (the to-be-incorrect hole) increased the odds of a correct outcome. Thus, the ‘appropriate’ hole was defined for each rat as the hole associated with the higher odds ratio, and the ‘inappropriate’ hole was defined as the hole associated with the lower odds ratio. The labels ‘appropriate’ and ‘inappropriate’, which are defined by the subject's established pattern of behaviour, were chosen to be distinct from ‘correct’ and ‘incorrect’, which are defined by the procedure. The individual mediating-response patterns observed in the previous study remained consistent in the present study. The ‘appropriate’ hole was the to-be-correct hole for three rats and the to-be-incorrect hole in five rats in the present study. To determine whether drug treatments altered the performance of mediating behaviour, the effects of THC dose, adenosine antagonist dose and delay value were assessed on response rates in the three holes and on the relative frequency of the four ‘trial types’: trials in which there were at least one response during the delay (i) in the appropriate hole only, (ii) in the inappropriate hole only, (iii) in both side holes or (iv) in neither side hole. To determine whether drugs altered the effectiveness of mediating behaviour, the accuracy of choice responding was analysed as a function of trial type, drug treatment and delay value. For clarity of presentation, mediating-behaviour data were averaged across delay values in the figures.
Results
Initial exposure to caffeine
During preliminary testing with of caffeine, it was found that the first exposure to the 10 mg·kg−1 dose significantly reduced the percentage of correct trials in the non-matching-to position task (see Figure 1; main effect of caffeine: F2,10= 35.2, P < 0.0001; main effect of delay: F4,20= 54.62, P < 0.0001). However, tolerance to this effect developed rapidly, and there was no significant decrease in performance when this dose was given the second time or in any subsequent test of caffeine in the absence of THC for the remainder of the study.
Figure 1.
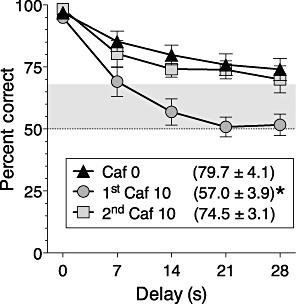
Accuracy of delayed non-matching-to-position performance after treatment with vehicle (Caf 0), 10 mg·kg−1 caffeine the first time it was given and 10 mg·kg−1 caffeine the second time it was given. Data are presented as percentage (mean ± SEM) of trials with a correct choice response, as a function of delay value. Values in parentheses indicate overall percent correct for delays greater than 0 s (for comparison with data in Figure 2). * (P < 0.05), significantly different from vehicle control. Points above the grey band are significantly different (P < 0.05) from chance (50%).
Combined effects of THC and adenosine antagonists on memory performance
As in our earlier study, THC given alone significantly decreased accuracy at 3 mg·kg−1, but not at 1 mg·kg−1 (see Figure 2A). The combination of subthreshold doses of THC and caffeine (1 and 10 mg·kg−1, respectively) produced a deficit comparable with that produced by the higher dose of THC (3 mg·kg−1). The interaction of THC and caffeine was significant (F6,32= 2.54, P < 0.05), as was the main effect of delay (F3,21= 11.99, P < 0.0001). Paired comparisons indicated that the overall level of accuracy (i.e. accuracy averaged across delays) when rats received the THC 1 + caffeine 10 combination was significantly lower than when they received either THC 1 or caffeine 10 alone. Overall levels of accuracy were significantly better than chance (50%) under all doses of caffeine in the absence of THC but did not differ from chance under the combination of THC 1 + caffeine 10 or any combination that included THC 3. The selective adenosine A1 receptor antagonist CPT had no effect when given alone, but the combination of subthreshold doses of THC and CPT (1 and 10 mg·kg−1, respectively) impaired accuracy; in these tests, the main effects of THC (F1,5= 30.54, P < 0.003), CPT (F2,10= 7.84, P < 0.01) and delay (F3,15= 8.79, P < 0.002) were significant, and planned comparisons revealed that the only treatment in Figure 2B that significantly affected accuracy was the combination of 10 mg·kg−1 CPT with 1 mg·kg−1 THC. In contrast, the adenosine A2A receptor antagonist SCH58261 did not significantly affect accuracy when given alone or in combination with 1 mg·kg−1 THC (Figure 2C).
Figure 2.
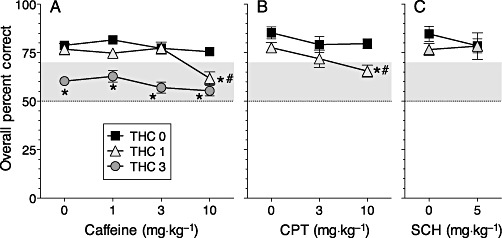
Accuracy of delayed non-matching-to-position performance after treatment with THC (0, 1 or 3 mg·kg−1) in combination with (A) caffeine, (B) the specific adenosine A1-receptor antagonist CPT or (C) the specific adenosine A2A-receptor antagonist SCH58261. Data are presented as percentage of trials (mean ± SEM) with a correct choice response, averaged across trials with 7, 14, 21 and 28 s delays. Zero doses refer to vehicles. *P < 0.05, significantly different from THC 0 at the same dose of caffeine, CPT or SCH58261. # P < 0.05, significantly different from caffeine 0, CPT 0 or SCH 0 at the same dose of THC. Points above the grey band are significantly different (P < 0.05) from chance (50%).
To further analyse the effects of combining THC with caffeine, the data in Figure 2A were plotted in Figure 3 as a function of delay. This plot shows that accuracy was always high at the 0 s delay but decreased at longer delays, where accuracy is presumably more dependent on working memory. Paired comparisons focusing on the main effect of delay indicated that accuracy was significantly higher at the 7 s delay than at the three longer delays. Simultaneous confidence intervals comparing each point in Figure 3 to chance level indicated that performance was significantly better than chance under 1 mg·kg−1 of THC alone (Figure 3A) and all doses of caffeine alone (Figure 3B) at all delay values. In contrast, THC 1 + caffeine 10 (Figure 3E) and each treatment combination that included THC 3 (Figure 3D,F) produced deficits, such that accuracy was not significantly better than chance, at all delay values of 7 s or more. In the THC–CPT experiment (Figure 4A), accuracy was better than chance at all delay values when CPT was given alone, and it was also better than chance at all but the longest delay under 1 mg·kg−1 THC given alone or in combination with 3 mg·kg−1 CPT. But when 1 mg·kg−1 THC was given along with 10 mg·kg−1 CPT (Figure 4D), the level of accuracy was not better than chance at any delay greater than 7 s (Figure 4D).
Figure 3.

Accuracy of delayed non-matching-to-position performance after treatment with combinations of THC and caffeine, as a function of delay value. Panels show the effects of (A) THC in the absence of caffeine, (B) caffeine in the absence of THC, (C) 1 mg·kg−1 THC in combination with 1 or 3 mg·kg−1 caffeine, (D) 3 mg·kg−1 THC in combination with 1 or 3 mg·kg−1 caffeine, (E) 1 mg·kg−1 THC in combination with 10 mg·kg−1 caffeine and (F) 3 mg·kg−1 THC in combination with 10 mg·kg−1 caffeine. Points above the grey band are significantly different (P < 0.05) from chance (50%).
Figure 4.
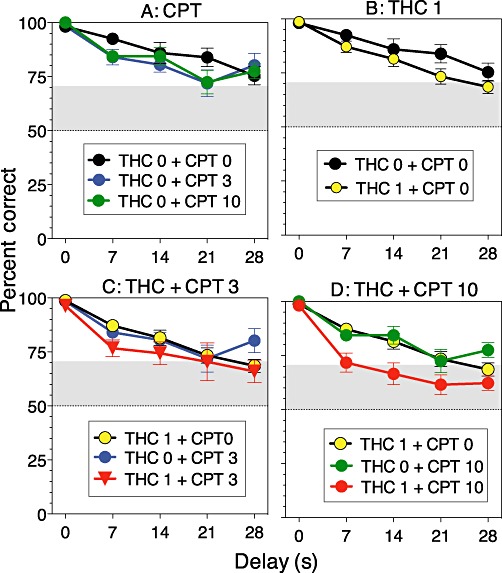
Accuracy of delayed non-matching-to-position performance after treatment with combinations of THC (0 or 1 mg·kg−1) and CPT (0, 3 or 10 mg·kg−1), as a function of delay value. Panels show the effects of (A) CPT in the absence of THC, (B) THC in the absence of CPT, (C) THC in combination with 3 mg·kg−1 CPT and (D) THC in combination with 10 mg·kg−1 CPT. Points above the grey band are significantly different (P < 0.05) from chance (50%).
Actual delay and number of trials and pellets per session
The length of the actual delay (i.e. the amount of time between termination of the sample cue and performance of the choice response) was analysed because (i) delay strongly influences the accuracy of memory performance, and (ii) the length of the actual delay could be sensitive to general changes in locomotor activity. However, the mean actual delay was within a few seconds of the nominal delay at all delay values under vehicle conditions, and this measure was not altered significantly by any drug treatment. The number of trials per session was also not altered significantly by drug treatments (mean ± SEM = 101.4 ± 6.0 under vehicle conditions in the caffeine–THC experiment, 99.9 ± 2.6 in the CPT–THC experiment and 94.2 ± 7.2 in the SCH58261–THC experiment).
The main effect of THC on the number of pellets received per session was significant (F2,13= 6.59, P < 0.05). Comparisons revealed that the mean (±SEM) number of pellets received under 3 mg·kg−1 THC (69.7 ± 3.0) was significantly lower (P < 0.05) than under 1 mg·kg−1 THC (85.3 ± 3.1) or vehicle (84.5 ± 3.4). The number of pellets per session was not affected by any drug treatment in the CPT or SCH58261 experiments, where the 3 mg·kg−1 dose of THC was not tested (mean ± SEM under vehicle = 89.8 ± 3.1 in the CPT experiment and 84.7 ± 3.9 in the SCH58261 experiment).
Mediating behaviour
Response rates during the delay
Responding in the centre hole – which was required to end the delay period – occurred at a much higher rate than responding in either side hole (Figure 5A). For the preliminary caffeine testing phase, the THC–caffeine experiment and the THC–CPT experiment (Figure 5A,B,C, respectively), the main effect of hole was significant (preliminary caffeine: F2,10= 82.16, P < 0.0001; caffeine: F2,14= 88.13, P < 0.0001; CPT: F2,10= 147.16, P < 0.0001). For all experiments, paired comparisons indicated that the only significant differences were between the centre hole and each side hole; there were no significant differences between response rates in the two side holes under any condition.
Figure 5.
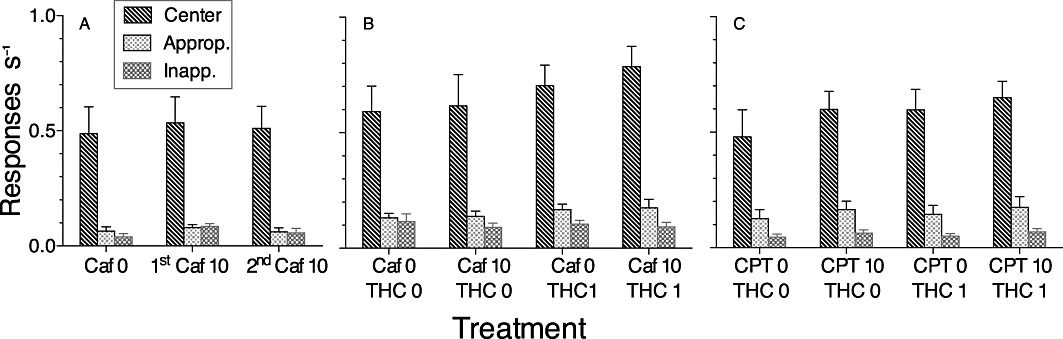
Response rates during the delay period. Panels show responses per second (mean ± SEM) during the delay period after: (A) the first and second treatments with 10 mg·kg−1 caffeine; (B) treatment with 0 or 1 mg·kg−1 THC in combination with 0 or 10 mg·kg−1 caffeine and (C) treatment with 0 or 1 mg·kg−1 THC in combination with 0 or 10 mg·kg−1 CPT. Data were averaged across trials with 7, 14, 21 and 28 s delays. For each rat, one of the two side holes was defined as ‘appropriate’ and the other as ‘inappropriate’ based on the previously established relationship between responding in that hole and the odds of a correct choice at the end of the trial. Response rates in the centre hole were significantly higher than in either side hole under all drug conditions.
Distribution of responding during the delay
In the THC–caffeine and THC–CPT experiments (Figure 6B,C), drug treatments did not significantly alter the distribution of the four trial types, classified by what kind of responses occurred during the delay (appropriate only, inappropriate only, both or none). The profile after treatment with THC 1 + caffeine 10 – the combination that produced a significant deficit in accuracy – was similar to the profiles seen after the THC 0 + caffeine 10 and THC 1 + caffeine 0 treatments, which did not affect accuracy. Notably, the initial exposure to 10 mg·kg−1 caffeine (Figure 6A) significantly altered the distribution of responding during the delay (interaction of caffeine and trial type: F6,30= 5.31, P < 0.0008). Specifically, there was a significant decrease in the percentage of trials in which there were no responses in either side hole during the delay, the trial type that was predominant under vehicle conditions. As with the performance deficit produced by the first exposure to 10 mg·kg−1 caffeine (Figure 1), tolerance to the effect of 10 mg·kg−1 caffeine on trial-type distribution developed rapidly, and caffeine did not significantly alter the distribution of trial types in subsequent testing.
Figure 6.
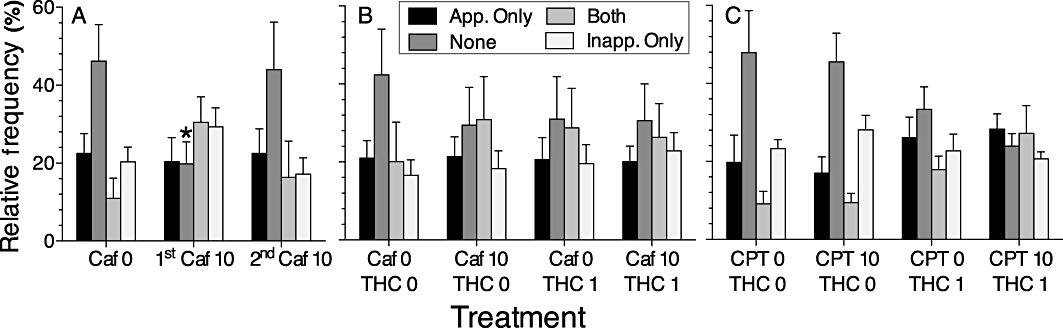
Distribution of trial types, defined by behaviour during the delay period. Panels show percentage of trials (mean ± SEM) with at least one response in the appropriate hole only, neither side hole (‘none’), both side holes, or the inappropriate hole only. Panels show distributions after: (A) the first and second treatments with 10 mg·kg−1 caffeine; (B) treatment with 0 or 1 mg·kg−1 THC in combination with 0 or 10 mg·kg−1 caffeine and (C) treatment with 0 or 1 mg·kg−1 THC in combination with 0 or 10 mg·kg−1 CPT. *P < 0.05, significantly different from vehicle control.
Accuracy of choice responding as a function of behaviour during the delay
The drug-induced deficits in accuracy of the delayed non-matching performance were associated with a decrease in the effectiveness of the mediating response (Figure 7). In the THC–caffeine experiment (Figure 7B), the type of responding during the delay (i.e. trial type; F3,21= 6.98, P < 0.002) and the interaction of THC and caffeine (F1,6= 7.84, P < 0.05) significantly affected accuracy. The combination of THC 1 and caffeine 10 decreased the mean level of accuracy in all four trial types, making them closer to 50%. The most prominent change was evident in trials where only appropriate side-hole responses occurred during the delay. In the absence of drug treatments, accuracy was highest (averaging >90%) in these ‘appropriate-only’ trials. But when THC 1 was combined with caffeine 10, accuracy in appropriate-only trials was significantly decreased relative to the vehicle condition and the THC 1 + caffeine 0 condition (although not relative to the THC 0 + caffeine 10 condition, P= 0.09). Similarly, in the THC–CPT experiment (Figure 7C), accuracy in appropriate-only trials (and also in trials with no side-hole responses) was substantially reduced after combined treatment with CPT (10 mg·kg−1) and THC (1 mg·kg−1). In this experiment, the main effects of THC (F1,5= 14.8. P < 0.05) and trial type (F3,15= 3.4, P < 0.05) were significant, and the main effect of CPT approached significance (F1,5= 5.3, P= 0.065). The level of accuracy in appropriate-only trials under THC 1 + CPT 10 did not differ significantly from the level under THC 0 + CPT 0, but accuracy in appropriate-only trials under THC 1 + CPT 10 (unlike the other treatments in Figure 7C) was not significantly better than chance. During the initial exposure to 10 mg·kg−1 caffeine (Figure 7A), performance was significantly affected by caffeine (F2,10= 12.66, P < 0.002) and trial type (F3,15= 3.14, P= 0.057), and accuracy in appropriate-only trials was significantly decreased compared with the vehicle condition.
Figure 7.
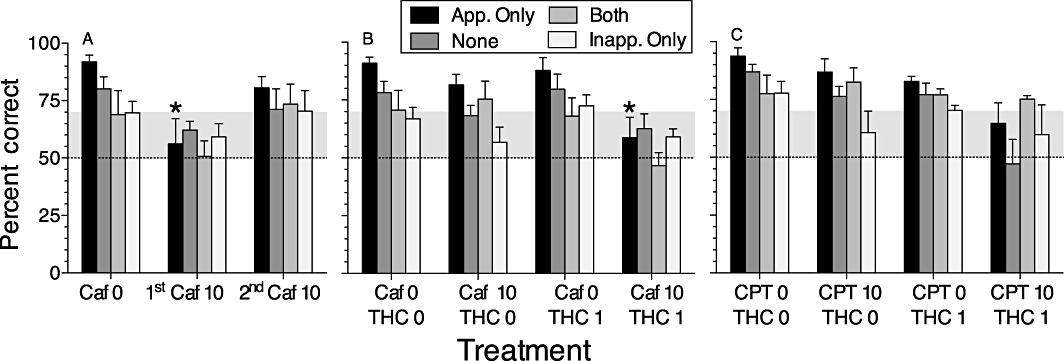
Accuracy of delayed non-matching-to-position performance as a function of behaviour during the delay period. Panels show percentage of trials (mean ± SEM) with a correct outcome as a function of trial type (appropriate only, none, both and inappropriate only) after treatment with (A) 10 mg·kg−1 caffeine in the first and second exposures, (B) combination of 0 or 1 mg·kg−1 THC with 0 or 10 mg·kg−1 caffeine or (C) combination of 0 or 1 mg·kg−1 THC with 0 or 10 mg·kg−1 CPT. **P < 0.05, significantly different from appropriate-only trials under vehicle conditions. Bars that extend above the grey band are significantly different (P < 0.05) from chance (50%).
Discussion
These results indicate that when a subthreshold dose of THC was combined with caffeine, the amnestic effects of THC were potentiated, creating deficits in working memory comparable with those produced by a higher dose of THC. None of the treatments decreased accuracy at the 0 s delay, suggesting that THC and the combination of THC and caffeine selectively impaired working memory rather than affecting procedural memory for the matching task or producing a general disruption of behaviour. Although caffeine did not shift the entire THC dose–effect function, this may be due to a floor effect; in the absence of caffeine, the 3 mg·kg−1 dose of THC already produced near-maximal deficits, with accuracy near chance level at delays greater than 0 s.
In spatial delayed matching and non-matching tasks like the one used here, efforts are made to prevent the animal from simply waiting by the to-be-correct choice until the end of the delay. For example, in the present study, rats were required to respond in the centre hole to end the delay. However, such requirements do not prevent the development of mediating behaviour (Bushnell, 1988; Herremans et al., 1996; Chudasama and Muir, 1997). Therefore, the present procedure was developed to automatically record mediating behaviour and study it as an analogue of memory rehearsal. Although it is possible that other, unrecorded, forms of mediating behaviour could also have occurred, nose poke responding in the side holes during the delay period was a prominent and highly effective strategy. Most notably, in the absence of drug treatment, accuracy was consistently high in trials in which mediating responses occur only in the appropriate hole. Within the range of delays used here, accuracy does not even decrease at the longest delay (28 s) in these trials (Panlilio et al., 2011).
Conceivably, caffeine-induced increases in general locomotor activity could disrupt the performance of mediating behaviour and thereby decrease accuracy in the delayed non-matching-to sample task. At a dose of 10 mg·kg−1 (but not at doses of 3 mg·kg−1 or less), acute caffeine administration stimulates locomotor activity in drug-naïve rats, but tolerance to this effect develops rapidly (Holtzman and Finn, 1988; Lau and Falk, 1995; Karcz-Kubicha et al., 2003). In the present study, 10 mg·kg−1 caffeine did have a disruptive effect on mediating behaviour the first time it was administered. However, this effect was not seen in subsequent testing. Most likely, the rats became tolerant to the motor effects of this dose, or learned to perform the task despite the motor effects (i.e. developed behavioural tolerance). But after the initial exposure to 10 mg·kg−1 caffeine, no drug treatment had a significant effect on the performance of the established mediating response during the delay (either response rate or the distribution of responding across trials) for the remainder of the study. This finding is consistent with our previous study (Panlilio et al., 2011), where doses of 3 or 5.6 mg·kg−1 THC impaired working memory but did not disrupt the mediating response. In contrast with THC and caffeine, the amnestic muscarinic receptor antagonist scopolamine did have a disruptive effect on the mediating response in our earlier study, producing a 36% decrease in the incidence of trials in which there were mediating responses only in the appropriate hole (see also Chudasama and Muir, 1997).
Caffeine can facilitate human working-memory performance in some situations, primarily through its effects on attention, arousal, mood and concentration (Smith, 2002; Nehlig, 2010). In animals, caffeine has been found to counteract certain kinds of memory impairments, such as those associated with sleep deprivation (Alhaider et al., 2010) and models of Alzheimer's disease (Arendash et al., 2009) or attention deficit disorder (Pires et al., 2009). In rhesus monkeys, caffeine enhanced performance in a delayed matching-to-sample procedure with task-relevant distractors (Bain et al., 2003), but not in procedures without distractors (Buffalo et al., 1993; Hudzik and Wenger, 1993). It has been suggested that caffeine acts as a ‘cognitive normalizer’ in humans and animals, since its enhancing effects are most prominent when memory is perturbed by stressful or noxious stimuli (Cunha and Agostinho, 2010).
However, the present results indicate that caffeine is ineffective at reversing memory deficits induced by THC and can even potentiate the effects of a subthreshold dose of THC. This combined effect of THC and caffeine was due at least in part to a decrease in the effectiveness of mediating behaviour, such that rats performed at chance level even on trials where the most propitious mediating pattern occurred. This same pattern – impaired memory performance without disruption of the mediating response – was produced by THC at 3 and 5.6 mg·kg−1 in our earlier study (Panlilio et al., 2011).
The results obtained here with selective adenosine antagonists suggest that the combined effects of THC and caffeine on memory are due to caffeine's actions at adenosine A1 receptors. Like the non-selective adenosine antagonist caffeine, the selective A1 receptor antagonist CPT produced significant deficits in the non-matching-to-position task when combined with a low dose of THC, without producing significant alterations in mediating behaviour. In contrast, the selective A2A receptor antagonist SCH58261 did not affect memory performance when given alone or in combination with a low dose of THC. The fact that combining THC with CPT did not produce as strong a decrement as combining THC with caffeine could be due to the shorter duration of effect of CPT [about 1 h, compared with several hours for caffeine (Antoniou et al., 2005) and SCH58261 (Monopoli et al., 1998)]. In addition, selective adenosine antagonists such as CPT (Karcz-Kubicha et al., 2003) and SCH58261 (Orru et al., 2011) are more potent but less efficacious than caffeine at stimulating locomotor activity in naïve rats, and CPT is also more potent but less efficacious than caffeine at producing a caffeine-like interoceptive cue in caffeine-experienced rats (Solinas et al., 2005). Although only one dose of SCH58261 (5 mg·kg−1) was tested, this dose was well above the lowest dose (1 mg·kg−1) that increased locomotor activity in drug-naïve rats (Orru et al., 2011).
The finding that the amnestic effects of THC can be potentiated by antagonism of adenosine A1 receptors is consistent with and extends the findings of recent behavioural and ex vivo studies from other laboratories. Sousa et al. (2011) studied the combined effects of chronic caffeine exposure and acute THC administration on water-maze performance in mice. They found that 5 mg·kg−1 THC had no significant effect in vehicle-exposed mice but induced significant short-term memory deficits in mice that had been chronically exposed to caffeine (3 mg·kg−1·day−1, discontinued 22 h before testing with THC). This potentiation of the amnestic effects of THC by chronic caffeine exposure was mediated by adenosine A1 receptors. Sousa et al. (2011) also found evidence for an antagonistic interaction between A1 receptors and cannabinoid CB1 receptors, in which stimulation of A1 receptors decreased CB1-dependent signalling and inhibitory control of GABA and glutamate neurotransmission in a hippocampal synaptosome preparation. Similarly, Hoffman et al. (2010) showed that in hippocampal slices, activation of A1 receptors regulated CB1-mediated inhibition of glutamatergic transmission. Thus, caffeine may potentiate the effects of THC on memory by blocking the effects of endogenous adenosine.
There is still much about adenosine–cannabinoid interactions that is not well understood. For example, in the study of Sousa et al. (2011), it is not clear why a history of chronic caffeine exposure potentiated cannabinoid-induced memory impairments when this exposure also increased the density of A1 receptors and decreased the density of CB1 receptors in the cortex and hippocampus, effects that might be expected to attenuate rather than potentiate the effects of THC on memory. However, there is no question that the cannabinoid and adenosine systems interact (Ferréet al., 2010). The present results are consistent with recent demonstrations of antagonistic interactions between adenosine A1 receptors and cannabinoid CB1 receptors in the hippocampus, and they indicate that the combined administration of THC and caffeine can have a deleterious effect on cognitive function.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse.
Glossary
- CPT
8-cyclopentyltheophylline
- SCH58261
2-(2-furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine)
- THC
Δ9-tetrahydrocannabinol
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010;33:437–444. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, et al. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- Bain JN, Prendergast MA, Terry AV, Jr, Arneric SP, Smith MA, Buccafusco JJ. Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology. 2003;169:150–160. doi: 10.1007/s00213-003-1483-1. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Gillam MP, Allen RR, Paule MG. Acute effects of caffeine on several operant behaviors in rhesus monkeys. Pharmacol Biochem Behav. 1993;46:733–737. doi: 10.1016/0091-3057(93)90570-j. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Effects of delay, intertrial interval, delay behavior and trimethyltin on spatial delayed response in rats. Neurotoxicol Teratol. 1988;10:237–244. doi: 10.1016/0892-0362(88)90023-2. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis. 2010;20(Suppl. 1):S95–116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, et al. Adenosine-cannabinoid receptor interactions. Implications for striatal function. Br J Pharmacol. 2010;160:443–453. doi: 10.1111/j.1476-5381.2010.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade MJ. Caffeine – not just a stimulant. Nutrition. 2010;26:932–938. doi: 10.1016/j.nut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Grant DS. Directed forgetting in pigeons. In: Golding JM, MacLeod C, editors. Intentional Forgetting: Interdisciplinary Approaches. Hillsdale, NJ: Erlbaum; 1998. pp. 239–264. [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen. 1979;108:356–388. [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264:294–307. [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG, Finn IB. Tolerance to behavioral effects of caffeine in rats. Pharmacol Biochem Behav. 1988;29:411–418. doi: 10.1016/0091-3057(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Wenger GR. Effects of drugs of abuse and cholinergic agents on delayed matching-to-sample responding in the squirrel monkey. J Pharmacol Exp Ther. 1993;265:120–127. [PubMed] [Google Scholar]
- James JE. Understanding Caffeine: A Biobehavioral Analysis. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- Justinova Z, Ferré S, Segal PN, Antoniou K, Solinas M, Pappas LA, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, Tcheremissine OV. Marijuana effects on human forgetting functions. J Exp Anal Behav. 2005;83:67–83. doi: 10.1901/jeab.2005.22-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CE, Falk JL. Dose-dependent surmountability of locomotor activity in caffeine tolerance. Pharmacol Biochem Behav. 1995;52:139–143. doi: 10.1016/0091-3057(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Leggett T. A review of the world cannabis situation. Bull Narc. 2006;58:1–155. [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, Martin BR. Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology. 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Monopoli A, Casati C, Lozza G, Forlani A, Ongini E. Cardiovascular pharmacology of the A2A adenosine receptor antagonist, SCH 58261, in the rat. J Pharmacol Exp Ther. 1998;285:9–15. [PubMed] [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington, DC: The National Academies Press; 1996. [PubMed] [Google Scholar]
- Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20(Suppl. 1):S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- Orru M, Bakešová J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, et al. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS ONE. 2011;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Thorndike EB, Goldberg SR, Schindler CW. Automatic recording of mediating behavior in delayed matching- and nonmatching-to-position procedures in rats. Psychopharmacology. 2011;214:495–504. doi: 10.1007/s00213-010-2057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RD, Takahashi RN. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: a rodent model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2009;20:134–145. doi: 10.1097/FBP.0b013e32832a80bf. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Steckler T. Further developments in the measurement of working memory in rodents. Brain Res Cogn Brain Res. 1996;3:205–213. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks – a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology. 2005;179:804–812. doi: 10.1007/s00213-004-2118-x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Ferré S, Antoniou K, Quarta D, Justinova Z, Hockemeyer J, et al. Involvement of adenosine A1 receptors in the discriminative-stimulus effects of caffeine in rats. Psychopharmacology. 2005;179:576–586. doi: 10.1007/s00213-004-2081-6. [DOI] [PubMed] [Google Scholar]
- Sousa VC, Assaife-Lopes N, Ribeiro JA, Pratt JA, Brett RR, Sebastião AM. Regulation of hippocampal cannabinoid CB(1) receptor actions by adenosine A(1) receptors and chronic caffeine administration: implications for the effects of δ(9)-tetrahydrocannabinol on spatial memory. Neuropsychopharmacology. 2011;36:472–487. doi: 10.1038/npp.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


