Abstract
BACKGROUND AND PURPOSE
Signalling networks that regulate the progression of cannabinoid CB1 receptor-mediated extracellular signal-regulated kinase (ERK) activation in neurons are poorly understood. We investigated the cellular mechanisms involved in CB1 receptor-stimulated ERK phosphorylation in a neuronal cell model.
EXPERIMENTAL APPROACH
Murine N18TG2 neuronal cells were used to analyse the effect of specific protein kinase and phosphatase inhibitors on CB1 receptor-stimulated ERK phosphorylation. The LI-COR In Cell Western assay and immunoblotting were used to measure ERK phosphorylation.
KEY RESULTS
The time-course of CB1 receptor-stimulated ERK activation occurs in three phases that are regulated by distinct cellular mechanisms in N18TG2 cells. Phase I (0–5 min) maximal ERK phosphorylation is mediated by CB1 receptor-stimulated ligand-independent transactivation of multiple receptor tyrosine kinases (RTKs). Phase I requires Gi/oβγ subunit-stimulated phosphatidylinositol 3-kinase activation and Src kinase activation and is modulated by inhibition of cAMP-activated protein kinase A (PKA) levels. Src kinase activation is regulated by the protein tyrosine phosphatases 1B and Shp1. The Phase II (5–10 min) rapid decline in ERK phosphorylation involves PKA inhibition and serine/threonine phosphatase PP1/PP2A activation. The Phase III (>10 min) plateau in ERK phosphorylation is mediated by CB1 receptor-stimulated, ligand-independent, transactivation of multiple RTKs.
CONCLUSIONS AND IMPLICATIONS
The complex expression of CB1 receptor-stimulated ERK activation provides cellular selectivity, modulation of sensitivity to agonists, and coincidence detection with RTK signalling. RTK and PKA pathways may provide routes to novel CB1-based therapeutic interventions in the treatment of addictive disorders or neurodegenerative diseases.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: CB1 receptor, cannabinoids, receptor tyrosine kinase, ERK, PTP1B, Src
Introduction
The cannabinoid CB1 receptor is a G protein-coupled receptor (GPCR; receptor nomenclature follows Alexander et al., 2011) that is stimulated by full agonists, functionally similar to Δ9-tetrahydrocannabinol (Δ9-THC) (e.g. CP55940, WIN55212-2), and the endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG) (Howlett et al., 2002). CB1 receptors are expressed predominantly in the nervous system and are responsible for many of the neuronal effects produced by endocannabinoids and cannabinoid drugs. The CB1 receptor associates with Pertussis toxin-sensitive Gi/o proteins to regulate a variety of signal transduction pathways including inhibition of adenylyl cyclase, inhibition of L-, N- and P/Q-type Ca2+ channels, activation of focal adhesion kinase, induction of immediate early gene expression, and stimulation of nitric oxide production (see Howlett, 2005 and Pertwee, 2006). CB1 receptors also activate members of the mitogen-activated protein kinase (MAPK) family including extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Bouaboula et al., 1995). These in vitro observations were confirmed by in vivo studies that showed acute Δ9-THC administration increased ERK1/2 activation in dorsal striatum, nucleus accumbens and hippocampus (Valjent et al. 2001; Derkinderen et al., 2003), whereas chronic Δ9-THC treatment increased phosphorylated ERK1/2 levels in prefrontal cortex and hippocampus (Rubino et al., 2004). Studies suggest alteration in ERK1/2 signalling in specific CB1 receptor-enriched brain regions is a key molecular adaptation that underlies the expression of cannabinoid tolerance and dependence (Rubino et al., 2005; Tonini et al. 2006).
The ERKs 1 and 2 are serine/threonine kinases that constitute the final component of the MAPK cascade [Raf/MAPK-ERK kinase (MEK)/ERK], which is considered a key junction point that mediates the integration and processing of information between signal transduction cascades in cells (Kyosseva, 2004). Dual phosphorylation of threonine and tyrosine residues that reside in the ERK1/2 activation loop is required for full activity (Chen et al., 2001). CB1 receptors regulate ERK1/2 phosphorylation/activation via several mechanisms that include Gi/o protein activation (Galve-Roperh et al., 2002; Davis et al. 2003), adenylate cyclase/protein kinase A (PKA) inhibition (Davis et al., 2003), receptor tyrosine kinase (RTK) transactivation (Bouaboula et al., 1997; Rubovitch et al. 2004; Korzh et al., 2008), phosphatidylinositol 3-kinase (PI-3K) activation (Galve-Roperh et al., 2002) and activation of the Src family kinase, Fyn (Derkinderen et al., 2003). Recent studies have demonstrated that CB1 receptor-mediated ERK activation is time-dependent (peak activation at 5 min followed by rapid inactivation) in HEK293 cells, and is regulated by CB1 receptor phosphorylation and desensitization, but not CB1 receptor internalization (Daigle et al., 2008).
The aim of the present study was to investigate the mechanisms that regulate the time-course of CB1 receptor-mediated ERK tyrosine phosphorylation in neuronal N18TG2 cells that express endogenous CB1 receptors. We found three phases of ERK phosphorylation: Phase I maximal ERK activation (0–5 min), Phase II decline in ERK activation (5–10 min) and Phase III plateau in ERK activation (>10 min). Cellular mechanisms responsible for each phase of CB1 receptor-mediated ERK activation differ, and include ligand-independent transactivation of multiple RTKs (Phase I and III), protein tyrosine phosphatase (PTP) activation (Phase I and III) and serine/threonine phosphatase activation/PKA inhibition (Phase II).
Methods
Cell culture
N18TG2 neuroblastoma cells (passage numbers 25–50) were maintained at 37°C under a 5% CO2 atmosphere in complete media comprising: Dulbecco's modified Eagle's medium : Ham's F-12 (1:1) complete with GlutaMax, sodium bicarbonate and pyridoxine–HCl, supplemented with penicillin (100 units·mL−1) and streptomycin (100 µg·mL−1) (Gibco Life Technologies, Gaithersburg, MD, USA) and 10% heat-inactivated bovine serum (JRH Biosciences, Lenexa, KS, USA). An aliquot of cannabinoid drug stocks (stored at −20°C as 10 mM solutions in ethanol) or ethanol (control) was air-dried under sterile conditions in trimethylsilyl-coated glass test tubes and taken up in 100 volumes of 5 mg·mL−1 fatty acid-free bovine serum albumin (BSA) and serially diluted before being added to cells. Where indicated, N18TG2 cells were pretreated with receptor antagonists or other inhibitors prior to addition of CB1 receptor agonists. Pertussis toxin (100 ng·mL−1; List Biological Laboratories, Campbell, CA, USA) was added to cells 16–20 h before addition of agonists.
Immunoblot analysis
Because N18TG2 cells can produce 2-AG (Bisogno et al., 1997), cells at 90% confluency were serum-starved (20–24 h) and pretreated with the diacylglycerol lipase inhibitor tetrahydrolipstatin (THL; 1 µM, 2 h) prior to stimulation with cannabinoid agonists. This procedure reduced basal (i.e., in the absence of exogenous CB1 agonist) levels to 50% of ERK tyrosine phosphorylation in the absence of such pretreatment (data not shown). Following the indicated drug treatments, cells were harvested with PBS-EDTA (2.7 mM KCl, 138 mM NaCl, 10.4 mM glucose, 1.5 mM KH2PO4, 8 mM Na2HPO4, 0.625 mM EDTA, pH 7.4). Cells were resuspended for 20 min on ice in cold NP-40 lysis buffer that contained 50 mM Tris-HCl, pH 7.2, 150 mM NaCl, 2 mM EDTA, 1 mM sodium orthovanadate, 8 mM sodium fluoride, 1% NP-40, and a protease inhibitor cocktail (EMD Biosciences, La Jolla, CA, USA) with broad specificity for the inhibition of aspartic, cysteine and serine proteases as well as aminopeptidases. Lysates were clarified by centrifugation at 20 000×g at 4°C and supernatants were stored at −80°C. Protein concentrations were determined using the Bradford method with BSA as the standard (Bradford, 1976). Lysates were taken up in Laemmli's sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.002% bromophenol blue, 5% β-mercaptoethanol) and heated at 95°C for 5 min. Cell lysates were resolved by 10% SDS-polyacrylamide gel electrophoresis run at 55 volts for 30 min and then 120 volts for 90 min. Proteins were transferred to nitrocellulose membranes in Towbin's buffer (25 mM Tris base, 192 mM glycine, 0.1% SDS and 20% methanol; pH 8.3) overnight at 20 volts at 4°C using a Bio-Rad Trans-Blot Cell with an ice pack. Blots were rinsed once (10 min) with Tris-buffered saline (TBS) (20 mM Tris–HCl, pH 7.4, 137 mM NaCl), blocked with Odyssey® Blocking buffer, and then incubated simultaneously with anti-phospho-ERK1(p44)/ERK2(p42) (E-4, phosphotyrosine 204) and anti-ERK1(p44)/ERK2(p42) (K-23, Total ERK) primary antibodies overnight at 4°C. Blots were washed four times with TBST (TBS containing 0.1% Tween-20), incubated simultaneously with IRDye® 800CW goat anti-rabbit and IRDye® 680CW goat anti-mouse secondary antibodies (1:15 000) for 1 h at room temperature, followed by three washes with TBST and one wash with TBS. Immunoblots were imaged and bands were quantified by densitometry using Odyssey Infrared Imaging System software (LI-COR Biosciences, Lincoln, NE, USA).
In Cell Western analysis
Cells were seeded at a density of 25 × 103 cells per well in a 96-well microplate in complete media and incubated overnight at 37°C. Complete media was replaced with serum-free media for 20–24 h, and cells were pre-incubated with THL (1 µM) for 2 h prior to treatment with inhibitors or CB1 receptor agonists. Following drug treatments, cells were fixed in PBS/3.7% paraformaldehyde, permeabilized with PBS/0.3% NP-40, blocked with Odyssey® Blocking buffer and stained with anti-phospho-ERK1(p44)/ERK2(p42) (E-4, phosphotyrosine 204) primary antibodies overnight at 4°C. The following morning, plates were washed with PBST (PBS containing 0.1% Tween-20), incubated with IRDye® 800CW goat anti-mouse secondary antibodies for 1 h and washed with PBST. Nuclear staining with DRAQ5® was used to normalize for well-to-well differences in cell number. Plates were visualized and quantitated using Odyssey Imaging software. Data are reported as mean ± SEM from several experiments, each performed in triplicate unless otherwise indicated. Data were tested for statistically significant differences using one-way anova and Dunnett's post hoc test to compare samples to a selected control (GraphPad Prism V software, La Jolla, CA, USA).
Cell fractionation
Cells at 90% confluency were serum-starved for 20–24 h and pre-incubated with THL (1 µM) for 2 h prior to treatment with CB1 receptor agonists. Following drug treatments, cells were harvested in PBS-EDTA and centrifuged at 425× g for 5 min at 4°C. Cell pellets were resuspended in lysis buffer that contained 10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1% NP-40, 0.1 mM EDTA, 1 µM dithiothreitol (DTT), 1 mM sodium orthovanadate and protease inhibitor cocktail and incubated on ice for 20 min. Lysates were sedimented at 3000×g for 10 min at 4°C, after which the supernatants were sedimented at 100 000×g for 1 h at 4°C (100 000×g supernatant was the cytosolic fraction). Pellets from the 3000×g spin were washed three times in cold lysis buffer and resuspended in a second lysis buffer that contained 20 mM HEPES, pH 7.9, 500 mM NaCl, 0.1% NP-40, 1 mM EDTA, 10% glycerol, 1 µM DTT, 1 mM sodium orthovanadate and protease inhibitor cocktail and incubated on ice for 30 min, followed by sedimentation at 15 000×g for 10 min at 4°C to obtain the nuclear lysate (supernatant). Nuclear fractionation was confirmed via lamin B enrichment.
Immunoprecipitation of vascular endothelial growth factor receptor (Flk-1 VEGFR)
Cells at 90% confluency were serum-starved for 20 to 24 h and pre-incubated with THL (1 µM) for 2 h prior to treatment with CB1 receptor agonists. Following drug treatments, cells were harvested in PBS-EDTA and centrifuged at 425× g for 5 min at 4°C. Pellets were homogenized on ice in a glass homogenizer in ice-cold HME buffer (20 mM sodium-HEPES, pH 8.0, 2 mM MgCl2, 1 mM EDTA, 1 mM sodium orthovanadate, protease inhibitor cocktail). After sedimentation at 3000×g for 5 min at 4°C to remove unbroken cells and nuclei, supernatants were collected and sedimented at 100 000×g for 1 h at 4°C. The pellet (membrane fraction) was resuspended in ice-cold NP-40 lysis buffer, lysed on ice for 20 min, and the protein concentration was determined (Bradford, 1976). Membrane lysates (500 µg protein) were incubated overnight with a Flk-1 VEGFR antibody (1 µg) at 4°C, after which, protein A-sepharose beads (Sigma; 100 µL of a 50% slurry) were added for an additional 4 h at 4°C. The immune complexes were precipitated by centrifugation at 15 294× g for 5 min at 4°C, washed three times with ice-cold NP-40 buffer and boiled in Laemmli's sample buffer. Following centrifugation at 15 294× g for 5 min at 4°C, supernatants were collected and resolved by 7.5% SDS-PAGE.
Data analysis
Results are reported as mean ± SEM from multiple experiments, each performed in triplicate unless otherwise indicated. Data were tested for statistically significant differences using one-way ANOVA and Dunnett's post hoc test to compare samples to a selected control or Student's t-test. Graphs and statistical analyses were generated using GraphPad Prism V software (La Jolla, CA, USA).
Materials
Reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA), unless otherwise stated. Methanandamide [(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide] and tetrahydrolipstatin (THL, Orlistat) were from Cayman Chemical (Ann Arbor, MI, USA). Acrylamide, N,N,N′,N-tetramethylethylene diamine and sodium dodecyl sulphate (SDS) were from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Anti-phospho-ERK1(p44)/ERK2(p42) (E-4, phosphotyrosine 204 PY204), anti-ERK1(p44)/ERK2(p42) (K-23, Total ERK), anti-lamin B, anti-Flk-1 vascular endothelial growth factor receptor (VEGFR), anti-PY20, galardin (GM 6001) and PD98059 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). DRAQ5® was from Cell Signaling Technology (Danvers, MA, USA). Gallein, AG 1296, AG 1478, oxindole-1, PP2, LY294002, wortmannin, I-OMe-AG 538, PTP1B inhibitor, PTP inhibitor I (PTP1B/Shp1 inhibitor), NSC87877 (Shp1/Shp2 inhibitor), Rp-cAMPS (adenosine 3′,5′-cyclic phosphorothioate-Rp), Sp-cAMPS (adenosine 3′,5′-cyclic phosphorothioate-Sp), KT5720, okadaic acid and o-phenanthroline were purchased from EMD Biosciences (San Diego, CA, USA). Odyssey® Blocking buffer, nitrocellulose membranes, IRDye® 800CW goat anti-mouse secondary antibody, IRDye® 800CW goat anti-rabbit secondary antibody and IRDye® 680CW goat anti-mouse secondary antibody were purchased from LI-COR Biosciences (Lincoln, NE, USA). BD Falcon™ 96-well microplates were purchased from VWR International (Suwanee, GA, USA).
Results
The kinetics and dose-dependence of CB1 receptor-mediated ERK tyrosine phosphorylation in N18TG2 cells
The dose-dependence of CB1 receptor-stimulated ERK tyrosine phosphorylation in N18TG2 cells was examined using antibodies that detect ERK1(p44)/2(p42) phosphorylation at tyrosine residue 204. A 5 min treatment with the synthetic CB1 receptor agonist WIN55212-2 (WIN, 0.01 µM) produced the greatest increase in ERK tyrosine phosphorylation (Table 1). WIN55212-2-stimulated ERK activation exhibited a convex bell-shaped dose–response curve (i.e. ERK inhibition at higher agonist concentrations) which may result from CB1 receptor desensitization. The CB1 receptor antagonist SR141716 blocked WIN55212-2-stimulated ERK activation, which indicates this is a CB1 receptor-dependent process. Pretreatment with Pertussis toxin eliminated the effect of WIN55212-2 on ERK tyrosine phosphorylation, indicating the requirement for CB1 stimulation of Pertussis toxin-sensitive Gi/o proteins. Kinetic analysis revealed CB1 receptor-mediated ERK tyrosine phosphorylation occurs in three phases in N18TG2 cells (Figure 1A,B). Maximally effective concentrations of WIN55212-2 and methanandamide produced a robust and transient increase in ERK tyrosine phosphorylation that reached maximal levels in 2 to 5 min (Phase I), declined to a low level by 10 min (Phase II) and reached a plateau after 10 min (Phase III). Studies in our laboratory have confirmed Phase III ERK activation is present in N18TG2 cells following CB1 receptor agonist treatment for 24 h (data not shown). Furthermore, immunoblotting experiments demonstrate ERK1 and ERK2 exhibit identical trends of activation upon different treatments, which is noteworthy as the In Cell Western assay does not distinguish between ERK1 and ERK2 when utilized to quantify changes in ERK phosphorylation (Figure 1B).
Table 1.
The pharmacological specificity and dose-dependence of WIN55212-2 (WIN)-stimulated ERK tyrosine phosphorylation in N18TG2 cells
| Control | % Change over basal |
|---|---|
| 0.001 µM WIN | 68.7 ± 9.0 |
| 0.01 µM WIN | 117.7 ± 12.2 |
| 0.1 µM WIN | 87.0 ± 12.0 |
| 1 µM WIN | 61.7 ± 11.6 |
| 0.1 µM WIN + 1 µM SR | −2.8 ± 7.2 |
| Pertussis toxin | % Change over basal |
|---|---|
| 0.001 µM WIN | 15.1 ± 5.5 |
| 0.01 µM WIN | −5.6 ± 6.2 |
| 0.1 µM WIN | −1.9 ± 11.1 |
| 1 µM WIN | −8.0 ± 5.5 |
| 0.1 µM WIN + 1 µM SR | 7.8 ± 1.5 |
Values are mean ± SEM.
Determination of the pharmacological specificity and dose-dependence of WIN55212-2 (WIN)-stimulated ERK tyrosine phosphorylation in N18TG2 cells using the LI-COR In Cell Western assay. Following serum starvation in the absence (control) or presence of Pertussis toxin (100 ng·mL−1, 20 h), cells were treated with the indicated concentrations of WIN for 5 min at 37°C with or without the CB1 receptor antagonist SR141716 (SR, 1 µM, 30 min). Cells stained with anti-PY204-ERK1/2 antibodies were imaged, and densities were normalized using DRAQ5®. Normalized data are reported as mean ± SEM of the % change over basal PY204-ERK1/2 levels from three separate experiments, each performed in quadruplicate.
Figure 1.

Time-course analysis of CB1 receptor-stimulated ERK tyrosine phosphorylation in N18TG2 cells. (A) Cells were treated with 0.01 µM WIN55212-2 (WIN) or 1 µM methanandamide at 37°C for the indicated times. In Cell Western analysis was performed and data are reported as mean ± SEM of the % change over basal PY204-ERK1/2 levels, normalized to DRAQ5® at each time point from three separate experiments performed in triplicate. (B) Immunoblot analysis of the time-course of 0.01 µM WIN-stimulated ERK1 and ERK2 tyrosine phosphorylation in N18TG2 cells. Data are reported as mean ± SEM of the % change over basal PY204-ERK1/2 (pERK) levels, normalized to ERK1/2 at each time point, from three separate experiments. (C) Immunoblot analysis of the time-course of 0.01 µM WIN-stimulated ERK1 and ERK2 tyrosine phosphorylation in N18TG2 cell nucleus and cytosol. Cellular fractionation was confirmed by lamin B enrichment. Data are reported as mean ± SEM of PY204-ERK1/2 levels (normalized to ERK2) at each time point from three separate experiments.
Cell fractionation experiments were performed to assess the effect of CB1 receptor stimulation on ERK nuclear translocation in N18TG2 cells. The purity of nuclear and cytosolic fractions was verified by immunoblotting for nuclear lamin B. As shown in Figure 1C, the kinetics of WIN55212-2-stimulated ERK1 and ERK2 tyrosine phosphorylation are similar in N18TG2 cytosol and nucleus, with ERK1/2 levels being seven times greater in the cytosol than in the nucleus. However, the increase in ERK1/2 tyrosine phosphorylation was not accompanied by a disproportionate increase in nuclear ERK1/2, which indicates CB1 receptor activation does not promote ERK1/2 nuclear accumulation in N18TG2 cells.
CB1 receptor-mediated maximal ERK tyrosine phosphorylation (Phase I) involves transactivation of multiple receptor tyrosine kinases in N18TG2 cells
CB1 receptors have been shown to transactivate a variety of RTKs in non-neuronal and neuronal cell lines that include the Flk-1 VEGFR, insulin-like growth factor 1 receptor (IGF-1R), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR) and tyrosine receptor kinase B (TrkB) receptor (Bouaboula et al., 1997; Rueda et al. 2000; Hart et al., 2004; Rubovitch et al. 2004; Berghuis et al., 2005; Korzh et al. 2008). In order to determine if CB1 receptors transactivate these RTKs, N18TG2 cells were pretreated with selective RTK inhibitors at concentrations that were based on published IC50 values (Kovalenko et al., 1994; Levitzki and Gazit, 1995; Sun et al., 1998; Blum et al. 2000; Shushan et al., 2004). TrkB receptors were not examined in this study, because real-time reverse transcriptase-quantitative polymerase chain reaction analysis indicated N18TG2 cells do not express TrkB receptors (data not shown). In Cell Western analyses revealed the Flk-1 VEGFR inhibitor oxindole-1, the EGFR inhibitor AG 1478 and the IGF-1R inhibitor I-OMe-AG 538 attenuated WIN55212-2-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells (Figure 2A). In contrast, WIN55212-2-stimulated maximal ERK tyrosine phosphorylation was not inhibited by the PDGFR inhibitor AG 1296. Basal ERK activity was not altered by any of the RTK inhibitors (Figure 2A), or the dimethylsulfoxide vehicle for these inhibitors (data not shown). Dose–response studies determined that the RTK inhibitors inhibited WIN55212-2-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells with IC50 values in the range of 100 to 500 nM (Figure 2B). The combination of sub-maximal concentrations of AG 1478 plus I-OMe-AG 538 produced an additive inhibitory effect on WIN55212-2-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells (Figure 2C). The non-classical cannabinoid agonist CP55940 also stimulated Phase I ERK tyrosine phosphorylation via VEGF (Flk-1), IGF-1 and EGF receptors (data not shown). WIN55212-2 increased the tyrosine phosphorylation of the mature, membrane-bound 230 kDa Flk-1 VEGF receptor in N18TG2 cells under the same conditions that evoked Phase I ERK tyrosine phosphorylation (Figure 2D).
Figure 2.

Cannabinoid-stimulated maximal ERK tyrosine phosphorylation (Phase I) involves CB1 receptor transactivation of multiple RTKs in N18TG2 cells. (A) Cells were pretreated with SR141716 (SR, 1 µM, 30 min), oxindole-1 (Ox, 2 µM, 15 min), AG 1478 (10 µM, 15 min), I-OMe-AG 538 (IOMe, 10 µM, 15 min) and AG 1296 (10 µM, 15 min) prior to treatment with 0.01 µM WIN55212-2 (WIN) or vehicle for 5 min at 37°C and subsequent In Cell Western analysis. Data are mean ± SEM of PY204-ERK1/2 levels normalized to WIN or basal (expressed as 100%) from three separate experiments performed in triplicate. *P < 0.01, significantly different from WIN (control) values (anova, Dunnett's method). (B) Cells were pretreated for 15 min with increasing concentrations of oxindole-1, AG 1478 and I-OMe-AG 538 prior to treatment with 0.01 µM WIN for 5 min at 37°C and the dose-response of each RTK inhibitor for the inhibition of WIN-stimulated ERK2 tyrosine phosphorylation was assessed by immunoblotting. Data are mean ± SEM of % ERK2 tyrosine phosphorylation (pERK), normalized to ERK2 at each inhibitor concentration, from three separate experiments; 100% ERK2 tyrosine phosphorylation represents WIN-stimulated ERK2 activation in the absence of RTK inhibitor. (C) Cells were pretreated for 15 min with AG 1478 (0.1 µM or 1 µM), I-OMe-AG 538 (IOMe, 0.1 µM or 1 µM) and AG 1478 + I-OMe-AG 538 (Add, 0.1 µM or 1 µM) prior to treatment with 0.01 µM WIN for 5 min at 37°C and the inhibition of WIN-stimulated ERK2 tyrosine phosphorylation was assessed by immunoblotting (B, Basal; W, WIN; 14, AG 1478; IO, I-OMe-AG 538; Ad, AG 1478 + I-OMe-AG 538). Data are mean ± SEM of PY204-ERK2 levels normalized to WIN (expressed as 100%) from two separate experiments. *P < 0.05, significantly different from AG 1478 + I-OMe-AG 538 (Add, 0.1 µM) values (anova, Dunnett's method): +P < 0.05; #P < 0.01, significantly different from WIN values (anova, Dunnett's method). (D) Immunoblot of WIN-stimulated Flk-1 VEGFR tyrosine phosphorylation in N18TG2 cell membranes. Cells were treated as in (A) with 0.01 µM WIN (W5) or vehicle (V) for 5 min at 37°C. Membrane lysates were immunoprecipitated using a Flk-1 VEGFR antibody and resolved by 7.5% SDS-PAGE, and stained simultaneously with antibodies to the Flk-1 VEGFR and tyrosine phosphorylated proteins (PY20 antibody). Data are the % change from basal pTyr-Flk-1 VEGFR levels, normalized to total Flk-1 VEGFR, in N18TG2 cell membranes from one experiment.
CB1 receptor-mediated RTK transactivation occurs in a ligand-independent fashion to stimulate maximal ERK tyrosine phosphorylation (Phase I) in N18TG2 cells
The extracellular signal-regulated MAPK pathway (Raf/MEK/ERK) is a three-kinase cascade that begins when phosphorylated Raf directly phosphorylates and activates MEK1/2, which subsequently phosphorylate and activate their only known biological target ERK1/2, respectively (Chen et al., 2001). The Raf/MEK/ERK cascade in CB1 receptor-mediated Phase I is illustrated in Figure 3A, in which pretreatment of N18TG2 cells with PD98059 (30 µM) at a concentration that inhibits ERK activation (Dudley et al., 1995) abolished WIN55212-2-stimulated maximal ERK tyrosine phosphorylation.
Figure 3.

CB1 receptors transactivate RTKs in a ligand-independent manner to stimulate maximal ERK tyrosine phosphorylation (Phase I) in N18TG2 cells. (A) Cells were pretreated with PD98059 (PD, 30 µM, 15 min), Src kinase inhibitor PP2 (2 µM, 15 min), the PI-3K inhibitors LY294002 (LY, 20 µM, 15 min) and wortmannin (Wort, 500 nM, 15 min) and the matrix metalloproteinase inhibitors o-phenanthroline (o-Phen, 200 µM, 15 min) and galardin (10 µM, 15 min) prior to treatment with 0.01 µM WIN55212-2 (WIN) or vehicle for 5 min at 37°C and subsequent In Cell Western analysis. Data are mean ± SEM of PY204-ERK1/2 levels normalized to WIN or basal (expressed as 100%) from three separate experiments performed in triplicate. *P < 0.01, significantly different from WIN (control) values (anova, Dunnett's method). (B) Immunoblot analysis of the effect of the Gβγ inhibitor gallein (10 µM, 15 min) on WIN (0.01 µM, 5 min)-stimulated maximal ERK1/2 tyrosine phosphorylation (Phase I) in N18TG2 cells [(1) Basal, (2,3) WIN 5 min, (4) 10 µM gallein, (5) 10 µM gallein + WIN 5 min]. Data are mean ± SEM of PY204-ERK1/2 (pERK) levels, normalized to ERK2 at each time point, from three separate experiments, with WIN-treated expressed as 100%. *P < 0.05, significantly different from WIN (Student's t-test).
The pathway to Raf activation via RTK transactivation by GPCRs can occur via ligand-dependent or ligand-independent mechanisms (Wetzker and Bohmer, 2003; Werry et al. 2005). Ligand-dependent RTK transactivation involves GPCR-mediated matrix metalloproteinase activation and matrix metalloproteinase-mediated cleavage of membrane-bound precursor proteins, such as heparin-binding epidermal growth factor, which bind to and activate their cognate RTKs (e.g. EGFR) (Wetzker and Bohmer, 2003). In contrast, ligand-independent RTK transactivation occurs in the absence of a cleaved RTK cognate ligand, and can involve GPCR association with RTKs, as well as GPCR-mediated activation of protein kinases, such as Src kinase, PI-3K, and second messengers (Ca2+) that mediate RTK phosphorylation and activation (Hawes et al., 1996; Luttrell et al. 1997; Werry et al., 2005). To investigate if CB1 receptor-mediated RTK transactivation involves a ligand-independent mechanism, N18TG2 cells were pretreated with selective inhibitors of Src kinase (PP2) and PI-3K (wortmannin, LY294002) at concentrations that were based on published IC50 values (Powis et al. 1994; Vlahos et al., 1994; Hanke et al., 1996). In Cell Western analyses revealed inhibition of both Src kinase and PI-3K significantly reduced Phase I, WIN55212-2-stimulated, maximal ERK tyrosine phosphorylation in N18TG2 cells (Figure 3A). Alternatively, N18TG2 cells were pretreated with the broad spectrum matrix metalloproteinase inhibitor galardin (GM 6001) and the zinc chelating matrix metalloproteinase inhibitor o-phenanthroline at concentrations that inhibit ligand-dependent GPCR-mediated RTK transactivation (Belcheva et al., 2001; Santiskulvong and Rozengurt, 2003). Matrix metalloproteinase inhibition had no effect on Phase I WIN55212-2-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells. None of these inhibitors altered the basal ERK phosphorylation state (Figure 3A).
Gβγ subunits bind to and activate PI-3K, which is a known mediator of Gβγ-stimulated ERK activation (Hawes et al., 1996). We examined whether inhibition of Gβγ-dependent activation of PI-3K could preclude WIN55212-2-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells. Gallein (Lehmann et al., 2008) suppressed CB1-mediated ERK1 and ERK2 tyrosine phosphorylation (Figure 3B).
Phase I CB1 receptor-stimulated maximal ERK tyrosine phosphorylation involves activation of protein tyrosine phosphatases in N18TG2 cells
Protein dephosphorylation is a post-translational modification catalysed by specific protein phosphatases that reverse the action of protein kinases. Src kinase is 90–95% phosphorylated at Tyr527 under basal conditions, such that a key mechanism in Src kinase activation is Tyr527 dephosphorylation (Roskoski, 2005). Both PTP1B, which recognizes tyrosine phosphorylated proteins associated with the plasma membrane and membranous organelles, and Shp1, which targets cytosolic proteins, can catalyse Src kinase Tyr527 dephosphorylation (Somani et al. 1997; Roskoski, 2005). To investigate whether PTP1B and Shp1 are involved in CB1-mediated Phase I ERK phosphorylation, N18TG2 cells were pretreated with a PTP1B/Shp1 inhibitor, a selective PTP1B inhibitor or the Shp1/Shp2 inhibitor NSC87877 at concentrations that were based on reported IC50 values (Arabaci et al., 2002; Wiesmann et al., 2004; Chen et al. 2006). As shown in Figure 4, the simultaneous inhibition of PTP1B and Shp1 reduced ERK1 and ERK2 tyrosine phosphorylation nearly completely at both 2 min and 5 min. The PTP1B inhibitor reduced ERK1 tyrosine phosphorylation effectively at 2 min, but with limited efficacy at 5 min. Inhibition of Shp1/Shp2 reduced ERK1 and ERK2 tyrosine phosphorylation somewhat more effectively at 2 min than at 5 min. In addition to regulating Src kinase, PTP1B also regulates growth factor signalling events in cells by dephosphorylating/inactivating RTKs that include the insulin receptor, EGFR, IGF-1R and PDGFR (Bourdeau et al., 2005). It is possible that the recovery of ERK phosphorylation at 5 min compared with 2 min of PTP1B inhibition can be attributed to attenuation of RTK inactivation by dephosphorylation.
Figure 4.

Regulation of Phase I CB1 receptor-stimulated maximal ERK tyrosine phosphorylation in N18TG2 cells by the protein tyrosine phosphatases PTP1B and Shp1. Cells were pretreated with a PTP1B/Shp1 inhibitor (80 µM, 15 min), a PTP1B inhibitor (40 µM, 15 min) and a Shp1/Shp2 inhibitor NSC87877 (1 µM) prior to treatment with 0.01 µM WIN55212-2 (WIN) for 2 min or 5 min at 37°C. Data are mean ± SEM of PY204-ERK1/2 levels (pERK), normalized to ERK2, from three separate experiments, with WIN-treated expressed as 100%. *P < 0.05, significantly different from WIN (Student's t-test).
Phase I and II CB1 receptor-stimulated ERK tyrosine phosphorylation in N18TG2 cells requires a reduction in PKA activity, and the presence of a serine/threonine phosphatase
Cannabinoid inhibition of adenylyl cyclase type 6 has been demonstrated in N18TG2 cells (Howlett and Fleming, 1984; McVey et al. 1999). The inhibition of cAMP production is predicted to yield a net reduction in phosphorylation of PKA target proteins such as Raf, the initial component of the Raf/MEK/ERK cascade. Phosphorylation of Raf-1 inhibits Raf-1 activity and subsequently inhibits ERK phosphorylation (Chen et al., 2001). To determine the contribution of CB1 receptor-mediated inhibition of the adenylyl cyclase/PKA signalling, PKA modulators were utilized that increase (the PKA activator Sp-cAMPS, the adenylyl cyclase activator forskolin, the phosphodiesterase IV inhibitor Ro 20-1724) or decrease (the PKA inhibitors Rp-cAMPS or KT5720) PKA activity (Seamon et al., 1981; Van Haastert et al. 1984; Kase et al., 1987; Dent et al. 1994). The latter do not appear to contribute a greater response than that observed with CB1-mediated inhibition of adenylyl cyclase (Figure 5A). PKA activators precluded Phase I CB1-mediated maximal ERK tyrosine phosphorylation in N18TG2 cells in the In Cell Western assay (Figure 5A), with the greatest decrease in Phase I maximal ERK activation produced by Sp-cAMPS, or forskolin in combination with Ro 20-1724.
Figure 5.

Phases I and II of CB1 receptor-stimulated ERK tyrosine phosphorylation in N18TG2 cells involve PKA inhibition and activation of serine/threonine phosphatases. (A) Cells were pretreated for 15 min with forskolin (10 µM, For), Ro 20-1724 (30 µM, Ro), forskolin (10 µM) + Ro 20-1724 (30 µM, Ro + For), KT5720 (0.1 µM, KT), Sp-cAMPS (10 µM, Sp) or Rp-cAMPS (50 µM, Rp) prior to treatment with 0.01 µM WIN55212-2 (WIN) for 5 min at 37°C and subsequent In Cell Western analysis. Data are mean ± SEM of PY204-ERK1/2 levels (normalized to DRAQ5®) from three separate experiments performed in triplicate, with WIN-treated expressed as 100%. #P < 0.05, *P < 0.01, significantly different from WIN (control) values (anova, Dunnett's method). (B) Cells were pretreated for 15 min with forskolin (10 µM) plus Ro 20-1724 (30 µM) prior to treatment with 0.01 µM WIN for the indicated times at 37°C and subsequent immunoblot analysis. Data are mean ± SEM of PY204-ERK1/2 (pERK) levels, normalized to ERK1/2 at each time point, from three separate experiments. (C) Cells were pretreated for 15 min with 0.15 µM okadaic acid prior to treatment with 0.01 µM WIN for the indicated times at 37°C and subsequent immunoblot analysis. Data are mean ± SEM of PY204-ERK1/2 (pERK), normalized to ERK1/2 at each time point from three separate experiments. Values were normalized to the time point that produced maximal PY204-ERK1/2 levels (expressed as 100%). *P < 0.05, significantly different from Control/WIN 10 min (Student's t-test).
Immunoblotting experiments revealed the importance of PKA to Phase II, as PKA activation by forskolin plus Ro 20-1724 prevented the decline in ERK1 and ERK2 tyrosine phosphorylation that characterizes Phase II (Figure 5B). This finding suggests that the Phase II decline in CB1 receptor-mediated ERK tyrosine phosphorylation also involves the activation of serine/threonine phosphatases in N18TG2 cells. This was demonstrated by pretreatment with the PP1/PP2A serine/threonine phosphatase inhibitor, okadaic acid (Haystead et al., 1989), which attenuated the decline in ERK tyrosine phosphorylation in Phase II and resulted in an increase in net ERK tyrosine phosphorylation following WIN55212-2 treatment for 10 min (Figure 5C).
Phase III CB1 receptor-stimulated ERK tyrosine phosphorylation involves CB1 receptor-mediated ligand-independent transactivation of the Flk-1 VEGFR and EGFR in N18TG2 cells
To evaluate the signalling mechanisms involved in Phase III CB1 receptor-stimulated ERK tyrosine phosphorylation, N18TG2 cells were allowed to complete Phases I and II by treatment with WIN55212-2 for 10 min prior to the addition of specific inhibitors (Figure 6A–D). Phase III WIN55212-2-stimulated ERK2 tyrosine phosphorylation was rapidly reversed by the CB1 antagonist/inverse agonist SR141716, which indicates that Phase III is a CB1 receptor-dependent process (Figure 6A). In addition, the Flk-1 VEGFR antagonist oxindole-1 and the EGFR antagonist AG 1478 inhibited Phase III WIN-stimulated ERK2 tyrosine phosphorylation (Figure 6B). As in Phase I, the PDGFR antagonist AG 1296 had no influence on WIN-stimulated ERK2 tyrosine phosphorylation, while the IGF-1R antagonist I-OMe-AG 538 appeared to suppress initial Phase III phosphorylated ERK2 levels (Figure 6B,C). The Src kinase inhibitor PP2 and the PI-3K inhibitor LY29400 also reduced Phase III CB1 receptor-stimulated ERK2 tyrosine phosphorylation (Figure 6A). Phase III CB1 receptor-mediated ERK2 tyrosine phosphorylation was ligand-independent because it was not reversed by either of the matrix metalloproteinase inhibitors, o-phenanthroline and galardin (data not shown). Finally, the selective PTP1B inhibitor rapidly reversed Phase III CB1 receptor-stimulated ERK2 tyrosine phosphorylation, while Shp1/Shp2 inhibition by NSC87877 (Chen et al., 2006) slowly reversed Phase III (Figure 6D).
Figure 6.
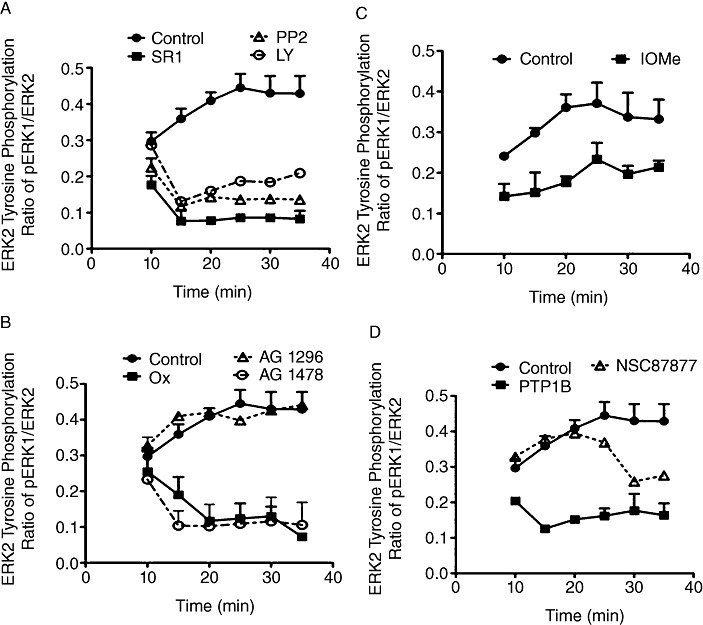
Phase III CB1 receptor-stimulated ERK tyrosine phosphorylation in N18TG2 cells involves CB1 receptor-mediated ligand-independent transactivation of the Flk-1 VEGFR and EGFR. Cells were treated with 0.01 µM WIN55212-2 (WIN) at 37°C for 10 min before addition of specific inhibitors at 5 min intervals until 25 min had elapsed and data were analysed by immunoblotting. Inhibitors were: (A) SR141716 (SR1, 1 µM), PP2 (2 µM) and LY294002 (LY, 20 µM); (B) AG 1478 (10 µM), oxindole-1 (Ox, 2 µM) and AG 1296 (10 µM); (C) I-OMe-AG 538 (IOMe, 10 µM); (D) PTP1B inhibitor (40 µM), Shp1/Shp2 inhibitor NSC87877 (1 µM). Data are mean ± SEM of PY204-ERK2 (pERK) levels, normalized to ERK2, at each time point from three separate experiments.
Discussion
The principal finding of this study is that CB1 receptor-stimulated ERK tyrosine phosphorylation/activation occurs in three phases that are regulated by distinct cellular mechanisms. Phase I (0–5 min) initiates maximal ERK activation, Phase II involves a rapid decline in ERK activation (5–10 min), and Phase III maintains a plateau in ERK activation (>10 min). Phase I is mediated by CB1 receptor-stimulated transactivation of the Flk-1 VEGFR, IGF-1R and EGFR in a ligand-independent fashion mediated by Gi/oβγ-mediated activation of PI-3K and tyrosine phosphatase (PTP1B and Shp1) – regulation of Src kinase (Figure 7). Phase I is under permissive regulation by inhibition of adenylyl cyclase/PKA, while Phase II requires adenylyl cyclase/PKA inhibition plus serine/threonine phosphatase activation. The plateau in ERK phosphorylation in Phase III involves CB1 regulation of RTKs and is regulated via signalling mechanisms, similar to those used in Phase I. Blocking the CB1 receptor stimulus during Phase I or Phase III by the competitive antagonist SR141716 was sufficient to terminate ERK phosphorylation, indicating the stimulus must be continuously applied, rather than initially applied as a one-time trigger for an ensuing process. These results are in agreement with the time-course observed for exogenous CB1 receptors expressed in HEK293 cells, in which the agonist CP55940 induced rapid, transient ERK activation peaking at 5 min and rapidly decaying by 15–30 min, but with no apparent establishment of a sustained Phase III in these cells (Daigle et al., 2008). Expression of an S426A/S430A CB1 receptor mutant in HEK293 cells established that phosphorylation at this domain is required for the development of Phase II ‘desensitization’ (Daigle et al., 2008), possibly by attenuating Gi/o stimulation.
Figure 7.

Signalling pathways utilized by the CB1 receptor to stimulate Phase I maximal ERK tyrosine phosphorylation in N18TG2 cell nucleus and cytosol. CB1 receptor-stimulated maximal ERK activation occurs via ligand-independent transactivation of the Flk-1 VEGF, EGF and IGF-1 receptor tyrosine kinases (RTK). The components involved in the transactivation process include Gi/oβγ subunit-mediated activation of PI-3K and Src kinase activation. A key event in Src kinase activation may be the dephosphorylation of Tyr527 by the tyrosine phosphatases PTP1B and Shp1. CB1 receptor-stimulated maximal ERK activation involves the traditional Raf/MEK/ERK cascade and results in an increase in ERK activation in both nucleus and cytosol. However, Phase I CB1 receptor-stimulated ERK activation does not involve net nuclear translocation of ERK in N18TG2 cells.
Our findings expand upon previous studies showing CB1 receptor transactivation of VEGFRs to regulate ERK activation in N18TG2 cells (Rubovitch et al., 2004). Those studies reported that the CB1 receptor agonist desacetyllevonantradol potentiated Ca2+ influx into N18TG2 cells via VEGFR transactivation and the subsequent activation of ERK (Rubovitch et al., 2004). Desacetyllevonantradol-mediated ERK phosphorylation was attenuated by inhibition of matrix metalloproteinases and protein kinase C (Rubovitch et al., 2004), both of which can play a role in ligand-dependent RTK transactivation (Belcheva and Coscia, 2002). In contrast, our studies indicate that both Phase I and Phase III CB1 receptor-mediated ERK activations occur via ligand-independent transactivation of multiple RTKs, a discrepancy that may stem from methodological differences. In those studies (Rubovitch et al., 2004), N18TG2 cells were treated with desacetyllevonantradol for 10 min, which our studies demonstrate coincides with Phase II Gi/o protein desensitization. It is possible that the response to matrix metalloproteinase-mediated release of RTK-stimulating ligands may become evident as Gi/o protein regulation is suppressed.
Although our studies identified an absolute requirement for Flk-1 VEGFR transactivation in CB1 receptor-mediated ERK phosphorylation in N18TG2 cells, the use of RTK inhibitors designed to inhibit EGFRs and IGF-1Rs inhibited CB1 receptor-mediated ERK phosphorylation. The combination of EGFR and IGF-1R inhibitors produced additive inhibition of CB1 receptor-stimulated ERK phosphorylation in N18TG2 cells. One explanation is that EGF and IGF-1 receptors are transactivated by Flk-1 VEGFRs, as there is a precedent for crosstalk between RTKs to regulate ERK. For example, Shc-EGFR complexes were responsible for IGF-1-stimulated, ligand-dependent EGFR-driven ERK phosphorylation in a COS-7 cell model (Roudabush et al., 2000). In addition, PDGF stimulation of PDGFR–EGFR heterodimers resulted in EGFR transactivation and EGFR-mediated ERK phosphorylation in rat aortic vascular smooth muscle cells (Saito et al., 2001).
The ramifications of CB1 receptor signalling that depends entirely upon RTKs are (i) selectivity in cellular response based upon specific RTKs that are expressed; and (ii) either additivity, synergism or competition with growth factors to which RTKs would otherwise respond. Crosstalk between CB1 receptors and RTKs was first reported in Chinese hamster ovary cells expressing recombinant human CB1 receptors (Bouaboula et al., 1997). In that model system, the CB1 antagonist SR141716 blocked MAPK activation in response to endogenously expressed insulin and IGF-1 receptors, suggesting the requirement for functional coupling of CB1 receptors to these RTKs (Bouaboula et al., 1997). In U373MG glioblastoma and NCI-H292 lung carcinoma cells, cannabinoid agonists induced activation of protein kinase B and ERK1/2 via ligand-dependent transactivation of EGFRs (Hart et al., 2004), suggesting the response to endocannabinoids might not be observed if the EGFRs were fully stimulated by matrix metalloproteinase-mediated release of EGF ligands catalysed by other stimuli. 2-AG and anandamide evoked TrkB–CB1 receptor complex formation and tyrosine phosphorylation of TrkB in PC12 pheochromocytoma cells co-expressing exogenous TrkB and CB1 receptors, suggesting that these receptors form a complex or are held in close proximity in a common membrane domain (such as a lipid raft structure) (Berghuis et al., 2005). Migration of developing, CB1-expressing interneurons in response to anandamide occurred through Src kinase-mediated TrkB transactivation and was additive with the effects of brain-derived neurotrophic factor (BDNF) (Berghuis et al., 2005). However, CB1 agonists inhibited BDNF-induced morphological differentiation, which also occurred via a Src kinase-dependent mechanism. In other transactivation studies, anandamide precluded nerve growth factor-stimulated TrkA-induced PC12 cell differentiation via CB1 receptor-mediated inhibition of Rap1/B-Raf/ERK (Rueda et al., 2002). These investigations of RTK downstream functions seem to suggest a competitive interaction exists such that CB1 agonists can stimulate RTKs in the absence of other signals. However, if the cognate growth factor is available, CB1 receptor-mediated RTK transactivation appears to be competitive or not observed at all.
We propose that during Phase I, Gi/oβγ subunits mediate the sequential activation of PI-3K and Src kinase to stimulate CB1 receptor-mediated RTK transactivation in N18TG2 cells. Evidence from other cellular systems supports a pathway by which Gi/oβγ subunits bind to and activate PI-3K (Lopez-Ilasaca et al., 1997; Stephens et al. 1997), while Src kinase functions as a mediator of Gi/oβγ subunit-stimulated RTK phosphorylation and ERK activation (Luttrell et al., 1996; 1997). Studies using U373MG human astrocytoma cells indicated that CB1 receptors can activate ERK via Gi/oβγ subunit-mediated PI-3K activation (Galve-Roperh et al., 2002). Although CB1 receptors were not coupled to RTKs or a Src kinase in U373MG cells (Galve-Roperh et al., 2002), CB1 receptor-stimulated TrkB transactivation in PC12 cells was mediated by a Src kinase (Berghuis et al., 2005), and CB1 receptor-regulated hippocampal ERK activity was mediated by the Src kinase Fyn (Derkinderen et al., 2003). Our studies suggest that Phases I and III of CB1 receptor-stimulated ERK activation are regulated by specific tyrosine phosphatases that activate Src kinase (Somani et al., 1997; Roskoski, 2005). These studies demonstrate the complex interplay that tyrosine phosphorylation/dephosphorylation can exert on the endpoint of ERK activation, allowing for multiple points of intervention by concurrent signal transduction pathways.
CB1 receptor-mediated inhibition of the adenylyl cyclase/PKA pathway is a critical modulator in the regulation of Phase I and II ERK activation. This regulatory mechanism is predicted to exert a dominant effect under circumstances of concurrent neuromodulator-mediated stimulation of types 5/6 adenylyl cyclase or Ca2+ influx-mediated regulation of types 1/3/8 adenylyl cyclase (Rhee et al., 1998). PKA-mediated phosphorylation/inhibition of Raf is a well-defined mechanism for regulation of ERK activity (Kikuchi and Williams, 1996; Mischak et al. 1996). This mechanism played the predominant role in anandamide-stimulated ERK phosphorylation in N1E-115 neuroblastoma cells, in which net Raf dephosphorylation resulted in activation of the Raf/MEK/ERK cascade (Davis et al., 2003). Hippocampal ERK phosphorylation was also significantly influenced by modification of cAMP levels (Derkinderen et al., 2003). Other reports have attributed the sustained phase of the Raf/MEK/ERK cascade in neuronal cells to PKA-mediated activation of Rap-1, a Ras superfamily member that mediates inhibition of Raf-1 and activation of B-Raf (Vossler et al., 1997; Bouschet et al. 2003). Thus, CB1 receptor-mediated inhibition of adenylyl cyclase/PKA may be an important regulatory mechanism in Phase II by precluding PKA-Rap-1-mediated ERK activation.
Studies of recombinant WT and desensitization-deficient CB1 receptors expressed in HEK293 cells implicated phosphorylation of Ser426 and Ser430 in the Phase II off-rate (t1/2 = 3 min) of CB1-stimulated ERK phosphorylation (Daigle et al., 2008). When phosphorylated by GPCR kinase 3, Ser426 and Ser430 were responsible for the uncoupling of the CB1 receptor from G protein-mediated ion channel regulation in an oocyte model (Jin et al., 1999), suggesting a similar desensitization mechanism for CB1 receptor uncoupling to the ERK phosphorylation process (Daigle et al., 2008). Pretreatment of N18TG2 cells with the serine/threonine phosphatase inhibitor okadaic acid at concentrations that inhibit both PP1 and PP2A activity prevented the decline in Phase II ERK activation and resulted in an increase in net ERK1 and ERK2 tyrosine phosphorylation compared to control values following WIN55212-2 treatment for 10 min. PP1 and PP2A typically inhibit MAPK signalling by catalysing the dephosphorylation/inactivation of Raf, MEK or ERK (Zhou et al., 2002; Junttila et al. 2008).
Phase III CB1 receptor-mediated ERK activation may occur as signal components relocate from the plasma membrane to sub-cellular loci. Moreover, studies in neurons have suggested that sustained ERK activation is necessary for ERK nuclear translocation and regulation of gene expression by transcription factors such as Elk-1 (Traverse et al., 1992; Roux and Blenis, 2004). In the present study, the kinetics of CB1 agonist-mediated ERK phosphorylation were identical in N18TG2 cytosol and nucleus. However, CB1 receptor-mediated ERK phosphorylation appears to be dissociated from the process of ERK nuclear translocation in N18TG2 cells, inasmuch as ERK is present in the nucleus in the absence of exogenously applied CB1 receptor agonists and sustained CB1 receptor-mediated ERK activation did not evoke ERK nuclear accumulation. Nevertheless, Phase III sustained ERK activation during chronic cannabinoid exposure may underlie the cellular modifications necessary for expression of cannabinoid tolerance (Rubino et al., 2004; 2005; Tonini et al., 2006).
In conclusion, the information gained regarding the cellular regulation of CB1 receptor-stimulated ERK activation reveals how protein kinases, protein phosphatases and CB1 receptor-mediated RTK transactivation play a role in the complex signalling networks that regulate cellular function. A thorough analysis of how each of these signalling processes participate in CB1 receptor regulation of the MAPK cascade can provide targets for modification of cellular behaviour in either specific cell types or states of disease. At present, there is a growing body of evidence that CB1 receptor agonists and antagonists have therapeutic benefits in modulating cellular processes that involve synaptic plasticity and neuronal remodelling in pathologies such as substance abuse and neurodegenerative diseases. Targeting the cellular signalling mechanisms utilized by CB1 receptors may provide new intervention strategies that can maximize benefits and reduce risks associated with the therapeutic use of cannabinoid ligands.
Acknowledgments
This work was supported by NIH grants R01-DA03690 to ACH and F32-DA026295 to GDD. GDD was an RJR-Leon Golberg Postdoctoral Scholar in Pharmacology at Wake Forest University.
Glossary
- Δ9-THC
Δ9-tetrahydrocannabinol
- BSA
fatty acid-free bovine serum albumin
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- IGF-1R
insulin-like growth factor 1 receptor
- MAPK
mitogen-activated protein kinase
- MEK
MAPK-ERK kinase
- PDGFR
platelet-derived growth factor receptor
- PI-3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PTP
protein tyrosine phosphatases
- RTK
receptor tyrosine kinase
- SDS
sodium dodecyl sulfate
- THL
tetrahydrolipstatin
- TrkB
tyrosine receptor kinase B
- VEGFR
vascular endothelial growth factor receptor
Conflict of interest
The authors have no conflict of interest to declare.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabaci G, Yi T, Fu H, Porter ME, Beebe KD, Pei D. alpha-bromoacetophenone derivatives as neutral protein tyrosine phosphatase inhibitors: structure-Activity relationship. Bioorg Med Chem Lett. 2002;12:3047–3050. doi: 10.1016/s0960-894x(02)00681-9. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Coscia CJ. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals. 2002;11:34–44. doi: 10.1159/000057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Szucs M, Wang D, Sadee W, Coscia CJ. mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322:671–677. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum G, Gazit A, Levitzki A. Substrate competitive inhibitors of IGF-1 receptor kinase. Biochemistry. 2000;39:15705–15712. doi: 10.1021/bi001516y. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Dube N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L. Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem. 2003;278:4778–4785. doi: 10.1074/jbc.M204652200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, et al. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, et al. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, Ronesi J, Lovinger DM. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J Biol Chem. 2003;278:48973–48980. doi: 10.1074/jbc.M305697200. [DOI] [PubMed] [Google Scholar]
- Dent G, Giembycz MA, Evans PM, Rabe KF, Barnes PJ. Suppression of human eosinophil respiratory burst and cyclic AMP hydrolysis by inhibitors of type IV phosphodiesterase: interaction with the beta adrenoceptor agonist albuterol. J Pharmacol Exp Ther. 1994;271:1167–1174. [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, et al. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I, Rueda D, Gomez del Pulgar T, Velasco G, Guzman M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol. 2002;62:1385–1392. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004;64:1943–1950. doi: 10.1158/0008-5472.can-03-3720. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. Phosphatidylinositol 3-kinase is an early intermediate in the G beta gamma-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- Haystead TA, Sim AT, Carling D, Honnor RC, Tsukitani Y, Cohen P, et al. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989;337:78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 1984;26:532–538. [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, et al. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Williams LT. Regulation of interaction of ras p21 with RalGDS and Raf-1 by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:588–594. doi: 10.1074/jbc.271.1.588. [DOI] [PubMed] [Google Scholar]
- Korzh A, Keren O, Gafni M, Bar-Josef H, Sarne Y. Modulation of extracellular signal-regulated kinase (ERK) by opioid and cannabinoid receptors that are expressed in the same cell. Brain Res. 2008;1189:23–32. doi: 10.1016/j.brainres.2007.10.070. [DOI] [PubMed] [Google Scholar]
- Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin CH, et al. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73:410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gbetagamma subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- McVey M, Hill J, Howlett A, Klein C. Adenylyl cyclase, a coincidence detector for nitric oxide. J Biol Chem. 1999;274:18887–18892. doi: 10.1074/jbc.274.27.18887. [DOI] [PubMed] [Google Scholar]
- Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, et al. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol Cell Biol. 1996;16:5409–5418. doi: 10.1128/mcb.16.10.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(Suppl. 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Modulation of extracellular signal-regulated kinases cascade by chronic delta 9-tetrahydrocannabinol treatment. Mol Cell Neurosci. 2004;25:355–362. doi: 10.1016/j.mcn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Forlani G, Vigano D, Zippel R, Parolaro D. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J Neurochem. 2005;93:984–991. doi: 10.1111/j.1471-4159.2005.03101.x. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Gafni M, Sarne Y. The involvement of VEGF receptors and MAPK in the cannabinoid potentiation of Ca2+ flux into N18TG2 neuroblastoma cells. Brain Res Mol Brain Res. 2004;120:138–144. doi: 10.1016/j.molbrainres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Rueda D, Galve-Roperh I, Haro A, Guzman M. The CB(1) cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol Pharmacol. 2000;58:814–820. doi: 10.1124/mol.58.4.814. [DOI] [PubMed] [Google Scholar]
- Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- Saito Y, Haendeler J, Hojo Y, Yamamoto K, Berk BC. Receptor heterodimerization: essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol. 2001;21:6387–6394. doi: 10.1128/MCB.21.19.6387-6394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiskulvong C, Rozengurt E. Galardin (GM 6001), a broad-spectrum matrix metalloproteinase inhibitor, blocks bombesin- and LPA-induced EGF receptor transactivation and DNA synthesis in rat-1 cells. Exp Cell Res. 2003;290:437–446. doi: 10.1016/s0014-4827(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shushan A, Rojansky N, Laufer N, Klein BY, Shlomai Z, Levitzki R, et al. The AG1478 tyrosine kinase inhibitor is an effective suppressor of leiomyoma cell growth. Hum Reprod. 2004;19:1957–1967. doi: 10.1093/humrep/deh355. [DOI] [PubMed] [Google Scholar]
- Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, et al. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, et al. Synthesis and biological evaluations of 3-substituted indolin-2-ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem. 1998;41:2588–2603. doi: 10.1021/jm980123i. [DOI] [PubMed] [Google Scholar]
- Tonini R, Ciardo S, Cerovic M, Rubino T, Parolaro D, Mazzanti M, et al. ERK-dependent modulation of cerebellar synaptic plasticity after chronic Delta9-tetrahydrocannabinol exposure. J Neurosci. 2006;26:5810–5818. doi: 10.1523/JNEUROSCI.5469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Van Driel R, Jastorff B, Baraniak J, Stec WJ, De Wit RJ. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984;259:10020–10024. [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Werry TD, Sexton PM, Christopoulos A. ‘Ins and outs’ of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11:730–737. doi: 10.1038/nsmb803. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277:31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]


