Abstract
BACKGROUND AND PURPOSE
Activation of cannabinoid CB2 receptors protects against various forms of ischaemia-reperfusion (I/R) injury. Δ8-Tetrahydrocannabivarin (Δ8-THCV) is a synthetic analogue of the plant cannabinoid Δ9-tetrahydrocannabivarin, which exhibits anti-inflammatory effects in rodents involving activation of CB2 receptors. Here, we assessed effects of Δ8-THCV and its metabolite 11-OH-Δ8-THCV on CB2 receptors and against hepatic I/R injury.
EXPERIMENTAL APPROACH
Effects in vitro were measured with human CB2 receptors expressed in CHO cells. Hepatic I/R injury was assessed in mice with 1h ischaemia and 2, 6 or 24h reperfusion in vivo.
KEY RESULTS
Displacement of [3H]CP55940 by Δ8-THCV or 11-OH-Δ8-THCV from specific binding sites in CHO cell membranes transfected with human CB2 receptors (hCB2) yielded Ki values of 68.4 and 59.95 nM respectively. Δ8-THCV or 11-OH-Δ8-THCV inhibited forskolin-stimulated cAMP production by hCB2 CHO cells (EC50= 12.95 and 14.3 nM respectively). Δ8-THCV, given before induction of I/R, attenuated hepatic injury (measured by serum alanine aminotransferase and aspartate aminotransferase levels), decreased tissue protein carbonyl adducts, 4-hydroxy-2-nonenal, the chemokines CCL3 and CXCL2,TNF-α, intercellular adhesion molecule 1 (CD54) mRNA levels, tissue neutrophil infiltration, caspase 3/7 activity and DNA fragmentation. Protective effects of Δ8-THCV against liver damage were still present when the compound was given at the beginning of reperfusion. Pretreatment with a CB2 receptor antagonist attenuated the protective effects of Δ8-THCV, while a CB1 antagonist tended to enhance it.
CONCLUSIONS AND IMPLICATIONS
Δ8-THCV activated CB2 receptors in vitro, and decreased tissue injury and inflammation in vivo, associated with I/R partly via CB2 receptor activation.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: cannabinoids, oxidative stress, inflammation, ischaemia–reperfusion
Introduction
Transient ischaemia followed by reperfusion (I/R) is a principal mechanism of organ injury in common pathological conditions, such as myocardial infarction and stroke, and may accompany surgical interventions involving vascular occlusion. In particular, solid organ transplantation inherently exposes the graft to some degree of I/R injury, which considerably affects outcome. The deleterious effects of I/R arise from the acute generation of reactive oxygen (ROS) and nitrogen species (RNS) (Ferdinandy and Schulz, 2003; Pacher et al., 2007) subsequent to re-oxygenation upon vascular re-opening. These compounds cause direct tissue damage and initiate a chain of deleterious cellular responses leading to inflammation and cell death, and eventually to target organ failure (Liaudet et al., 2003; Pacher and Hasko, 2008).
Δ8-Tetrahydrocannabivarin (O-4395, Δ8-THCV) is a synthetic analogue of Δ9-tetrahydrocannabivarin (Δ9-THCV), a plant cannabinoid, which is a propyl homologue of Δ9-tetrahydrocannabinol, the main psychoactive ingredient of marijuana (Gill et al., 1970). Initial pharmacological experiments have already suggested that the plant-derived Δ9-THCV was a possible ligand for cannabinoid (CB) receptors (see Pertwee, 2008). It has been shown that Δ9-THCV may act as a CB1 receptor antagonist (Thomas et al., 2005; Pertwee et al., 2007; Ma et al., 2008; Pertwee, 2008; receptor nomenclature follows Alexander et al., 2011); however, it can also activate CB2 receptors (Bolognini et al., 2010), as well as non-CB1, non-CB2 receptor targets (see Pertwee, 2008). Studies using Δ8-THCV have shown that it has a similar pharmacological profile to Δ9-THCV. Thus, both compounds behave as CB1 receptor antagonists in vitro and in vivo at low doses. However, Δ8-THCV may also exhibit agonistic activity in vivo at higher doses (Pertwee et al., 2007). Regarding CB2 receptors, evidence from a very recent study implies that Δ9-THCV may behave as partial agonists at CB2 receptors, not only in vitro, but also in vivo, as shown by reduced inflammation and inflammatory pain in mouse models (Bolognini et al., 2010). The rationale behind the development of Δ8-THCV is that it is more stable than the plant-derived analogue Δ9-THCV, and it is easier to synthesize.
The protective role of CB2 receptor activation in a well-characterized mouse model of hepatic I/R injury has previously been demonstrated (Batkai et al., 2007; Rajesh et al., 2007; Moon et al., 2008). In the current study, we have investigated the effects of Δ8-THCV on the course of liver damage, oxidative stress, and acute and chronic inflammatory response and cell death. In our model, tissue damage is induced by the generation of reactive oxygen species (ROS) that is followed by a cascade of acute and chronic inflammatory response. Because a recent study has found that Δ9-THCV displayed high selectivity and potency as a CB2 receptor agonist (Bolognini et al., 2010), and CB2 receptors on endothelial, inflammatory and perhaps some parenchyma cells mediate protective effects in hepatic (Batkai et al., 2007; Rajesh et al., 2007), cardiac (Pacher and Hasko, 2008; Defer et al., 2009; Montecucco et al., 2009) and cerebral (Zhang et al., 2007; 2009a,b; Murikinati et al., 2010) I/R injury (Pacher and Mechoulam, 2011), we also explored the plausible role of CB2 receptors in the effects of Δ8-THCV on hepatic I/R injury. Our findings reinforce the potential of Δ8-THCV for prevention/treatment of hepatic, and perhaps other forms of, I/R injury.
Materials and methods
CHO cells
CHO cells stably transfected with cDNA encoding human cannabinoid CB2 receptors (Bmax= 72.6 pmol·mg−1 protein) were maintained at 37°C and in 5% CO2 in Dulbecco's modified Eagle's medium nutrient mixture F-12 HAM supplemented with 1 mM l-glutamine, 10% fetal calf serum, 0.6% penicillin–streptomycin, hygromycin B (300 µg·mL−1) and G418 (400 µg·mL−1). After reaching a confluent state, the cells were washed with PBS and passaged using non-enzymic cell dissociation fluid.
Membrane preparation
Binding assays with [3H]CP55940 were performed with CHO–hCB2 cell membranes as described by Ross et al. (1999). The hCB2-transfected cells were removed from flasks by scraping and then frozen as a pellet at −20°C until required. Before using in a radioligand binding assay, the cells were defrosted, diluted in 50 mM Tris binding buffer and homogenized with a 1 mL handheld homogenizer. Protein assays were performed using a Bio-Rad Dc kit (Bio-Rad, Hercules, CA, USA).
Radioligand displacement assay
The assays were carried out with [3H]CP55940, Tris binding buffer (50 mM Tris–HCl, 50 mM Tris–base, 0.1% BSA), total assay volume 500 µL, using the filtration procedure described previously by Ross et al. (1999). Binding was initiated by the addition of CHO–hCB2 cell membranes (50 µg protein per well). All assays were performed at 37°C for 60 min before termination by the addition of ice-cold Tris binding buffer and vacuum filtration using a 24-well sampling manifold (cell harvester, Brandel Inc., Gaitherburg, MD, USA) and GF/B filters (Whatman, Maidstone, UK) that had been soaked in wash buffer at 4°C for at least 24 h. Each reaction well was washed six times with a 1.2 mL aliquot of Tris binding buffer. The filters were oven-dried for 60 min and then placed in 5 mL of scintillation fluid (Ultima Gold XR, PerkinElmer Life Sciences Inc., Waltham, MA, USA). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 µM unlabelled CP55940. The concentration of [3H]CP55940 used in our displacement assays was 0.7 nM. Δ8-THCV and 11-OH-Δ8-THCV were stored as stock solutions of 10 mM in DMSO, the vehicle concentration in all assay wells being 0.1% DMSO. These were diluted with Tris binding buffer before use. Drug additions were made in a volume of 10 µL to produce a final vehicle concentration in all assay wells of 0.1% DMSO. The binding parameters for [3H]CP55940, determined by fitting data from saturation binding experiments to a one-site saturation plot using GraphPad Prism (San Diego, CA, USA), were 72 570 fmol·mg−1 protein (Bmax) and 1.043 nM (Kd) as reported previously (Thomas et al., 2005).
cAMP assay
Adherent CHO–hCB2 cells were washed once with Dulbecco's PBS and detached using non-enzymic cell dissociation solution. After centrifugation, the cells were resuspended (2 × 106 cells·mL−1) in buffer containing PBS (calcium and magnesium free), 1% BSA and 10 µM rolipram. The cells were incubated for 30 min at 37°C with the cannabinoid under investigation. A further 30 min of incubation was carried out with 10 µM of forskolin in a total volume of 500 µL. The reaction was terminated by the addition of 500 µL of 0.1 M HCl, followed by centrifugation to remove cell debris. The pH was then adjusted to between 8.0 and 9.0 by the addition of 1 M of NaOH, and cyclic AMP content was measured using a radioimmunoassay kit (GE Healthcare, Amersham Ltd., Little Chalfont, Buckinghamshire, UK). Forskolin and rolipram were dissolved in DMSO and stored at −20°C as 10 mM stock solutions.
Metabolism of AEA and 2-AG in vitro
The synthesis of AEA through the activity of N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) was assayed using brain homogenates (50 µg per test) and 100 µM [3H]NArPE as reported (Maccarrone et al., 2008). The hydrolysis of AEA by fatty acid amide hydrolase (FAAH) was assessed in brain homogenates (20 µg per test), by measuring the release of [3H]arachidonic acid from 10 µM [3H]AEA, as reported (Maccarrone et al., 2008). NAPE-PLD activity was expressed as pmol of AEA released per min per mg of protein, whereas FAAH activity was expressed as pmol of arachidonate released per min per mg of protein. The synthesis of 2-AG through the activity of diacylglycerol lipase (DAGL) was assayed with 10 µM [14C]DAG as substrate, and that of the 2-AG hydrolase, monoacylglycerol lipase (MAGL), was determined using 10 µM [3H]2-OG as substrate, as reported (Maccarrone et al., 2008). Both DAGL and MAGL activities were expressed as pmol product per min per mg protein. In all cases, the effect of Δ8-THCV on enzyme activity was ascertained by adding this substance directly to the assay buffer.
Hepatic I/R
All animal care and experimental procedures in this study complied with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committees of NIAAA. Male C57BL/6J mice (25–30 g; Jackson Laboratories, Bar Harbor, ME, USA) were anaesthetized with pentobarbital sodium (65 mg·kg−1 i.p.). In our study, the model of segmental (70%) hepatic ischaemia has been used, as described (Batkai et al., 2007; Rajesh et al., 2007; Moon et al., 2008). Briefly, the liver was exposed by midline laparotomy, and the hepatic artery and the portal vein were clamped using an atraumatic micro-serrefine. This method of partial ischaemia prevents mesenteric venous congestion by allowing portal decompression throughout the right and caudate lobes of the liver. The duration of hepatic ischaemia was 60 min, after which the vascular clips were removed and liver was reperfused for 2, 6 or 24 h, as indicated. Sham surgeries were identical except that hepatic blood vessels were not clamped with a micro-serrefine. The liver was kept moist at 37°C with gauze soaked in 0.9% saline. Body temperature was monitored with a rectal temperature probe and was maintained at 37°C by a heating blanket. Treatment with Δ8-THCV 3 and 10 mg·kg−1 or vehicle i.p., started 2 h before IR or were given post-ischaemia at the moment of reperfusion, as indicated in the text. CB1 and CB2 antagonists were given 90 min prior to ischaemia or Δ8-THCV pretreatment. At the experimental end points, blood was collected and liver samples were removed and snap-frozen in liquid nitrogen for determining biochemical parameters or fixed in 4% buffered formalin for histopathological evaluation (Batkai et al., 2007; Rajesh et al., 2007; Moon et al., 2008).
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels
The activities of AST and ALT, indicators of liver cellular damage, were measured in serum samples using a clinical chemistry analyser system (VetTest 8008, IDEXX laboratories, Westbrook, ME, USA) (Batkai et al., 2007; Rajesh et al., 2007; Moon et al., 2008).
Histological examination of liver sections
Liver samples were fixed in 4% buffered formalin. After embedding and cutting 5 µm slices, all sections were stained with haematoxylin/eosin (HE). Myeloperoxidase (MPO) in neutrophils was stained with an anti- MPO antibody according to the manufacturer's protocol (Biocare Medical, Concord, CA, USA), and samples were counter-stained with nuclear fast red as described earlier (Batkai et al., 2007; Rajesh et al., 2007). Histological evaluation was performed without knowledge of the treatments.
Real-time PCR analyses of mRNA
Total RNA was isolated from liver homogenate using TRIzol reagents (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The isolated RNA was treated with RNase-free DNase (Ambion, Austin, TX, USA) to remove traces of genomic DNA contamination. One microgram of total RNA was reverse-transcribed to cDNA using the SuperScript II (Invitrogen). The target gene expression was quantified with Power SYBER Green PCR Master Mix using an ABI HT7900 real-time PCR instrument (Applied Biosystems, Foster City, CA, USA). Each amplified sample in all wells was analysed for homogeneity using dissociation curve analysis. After denaturation at 95°C for 2 min, 40 cycles were performed at 95°C for 10 s, and at 60°C for 30 s. Relative changes in mRNA levels were calculated using the comparative Ct method. This method involves comparing the Ct values of the samples of interest with a control (2-ΔΔCt method: ΔΔCt=ΔCt sample −ΔCt reference). Lower ΔCt values and lower ΔΔCt reflect a relatively higher amount of gene transcript (Schmittgen and Livak, 2008). Statistical analyses were carried out for at least 6 to 15 replicate experimental samples in each set.
The primers used were as follows:
CCL3, 5′-TGCCCTTGCTGTTCTTCTCTG-3′ and 5′-CAACGATGAATTGGCGTGG-3′; CXCL2, 5′-AGTGAACTGCGCTGTCAATGC-3′ and 5′-AGGCAAACTTTTTGACCGCC-3′; TNF-α, 5′-AAGCCTGTAGCCCACGTCGTA-3′ and 5′-AGGTACAACCCATCGGCTGG-3′; ICAM-1, 5′-AACTTTTCAGCTCCGGTCCTG-3′ and 5′-TCAGTGTGAATTGGACCTGCG-3′; and actin, 5′-TGCACCACCAACTGCTTAG-3′ and 5′-GGATGCAGGGATGATGTTC-3′
Hepatic 4-hydroxy-2-nonenal (4-HNE) content
Lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as 4-HNE, which has been shown to be capable of binding to proteins and forming stable HNE adducts. HNE in hepatic tissues was determined using a kit (Cell Biolabs, San Diego, CA, USA). In brief, BSA or hepatic tissue extracts (10 µg·mL−1) were adsorbed onto a 96-well plate for 12 h at 4°C. HNE adducts present in the sample or standard were probed with anti-HNE antibody, followed by an HRP conjugated secondary antibody. The HNE protein adduct content in an unknown sample was determined by comparing with a standard curve (Mukhopadhyay et al., 2010).
Detection of hepatic carbonyl adducts
Carbonyl content in liver tissues was determined by OxiSelect Protein Carbonyl elisa Kit (Cell Biolabs) (Rajesh et al., 2010; Mukhopadhyay et al., 2011). In brief, BSA standards or protein samples (10 µg·mL−1) were adsorbed onto a 96-well plate for 2 h at 37°C. The protein carbonyls present in the sample or standard were derivatized to DNP hydrazone and probed with an anti-DNP antibody, followed by an HRP conjugated secondary antibody. The protein carbonyl content in the unknown sample was determined by comparing with a standard curve that was prepared from predetermined reduced and oxidized BSA standards.
Detection of apoptosis by caspase 3/7 activity assays
Caspase 3/7 activity in hepatic tissue lysate was measured using the Apo-One Homogenous Caspase-3/7 assay kit (Promega Corp., Madison, WI, USA). An aliquot of caspase reagent was added to each well and mixed on a plate shaker for 1 h at room temperature shielded from light, and the fluorescence was measured (Mukhopadhyay et al., 2009; Rajesh et al., 2009).
Hepatic DNA fragmentation elisa
The quantitative determinations of cytoplasmic histone-associated DNA fragmentation (mono- and oligonucleosomes) due to cell death in liver homogenates were measured using elisa kit (Roche Diagnostics Corp., Indianapolis, IN, USA) (Mukhopadhyay et al., 2009; Pan et al., 2009).
Statistical analysis
Values have been expressed as means and variability as SEM or as 95% confidence limits. The concentrations of Δ8-THCV and 11-OH-Δ8-THCV that produced a 50% displacement of radioligand from specific binding sites (IC50 values) were calculated using GraphPad Prism 5. Their dissociation constants (Ki values) were calculated using the equation of Cheng and Prusoff (1973). GraphPad Prism 5 was also used to calculate EC50 and Emax values from data obtained in the binding or cyclic AMP assays. Statistical significance among groups was determined by one-way anova followed by Newman–Keuls post hoc analysis using GraphPad Prism 5 software. Probability values of P < 0.05 were considered significant.
Materials
Δ8-THCV (6aR,10aR)-6,6,9-trimethyl-3-propyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol or O-4395) and 11-OH-Δ8-THCV were synthesized by Dr Anu Mahadevan (Organix Inc, Woburn, MA, USA). Δ8-THCV has been synthesized as follows. p-Menth-2-ene-1,8-diol (Figure 1A, compound 1) (3.98 g, 23.5 mmol) and 5-propylbenzene-1,3-diol (Figure 1A, compound 2) (3.2 g, 21.3 mmol) were dissolved in dry benzene (300 mL) and p-toluene sulphonic acid (200 mg, 1.1 mmol) was added. The reaction mixture was refluxed for 4 h using a Dean–Stark apparatus, cooled to room temperature and stirred overnight. The reaction mixture was concentrated under vacuum, and the crude material was purified by silica gel column chromatography eluting with 7% ethyl acetate/hexane to yield Δ8-THCV as a light brown solid (Figure 1A).
Figure 1.
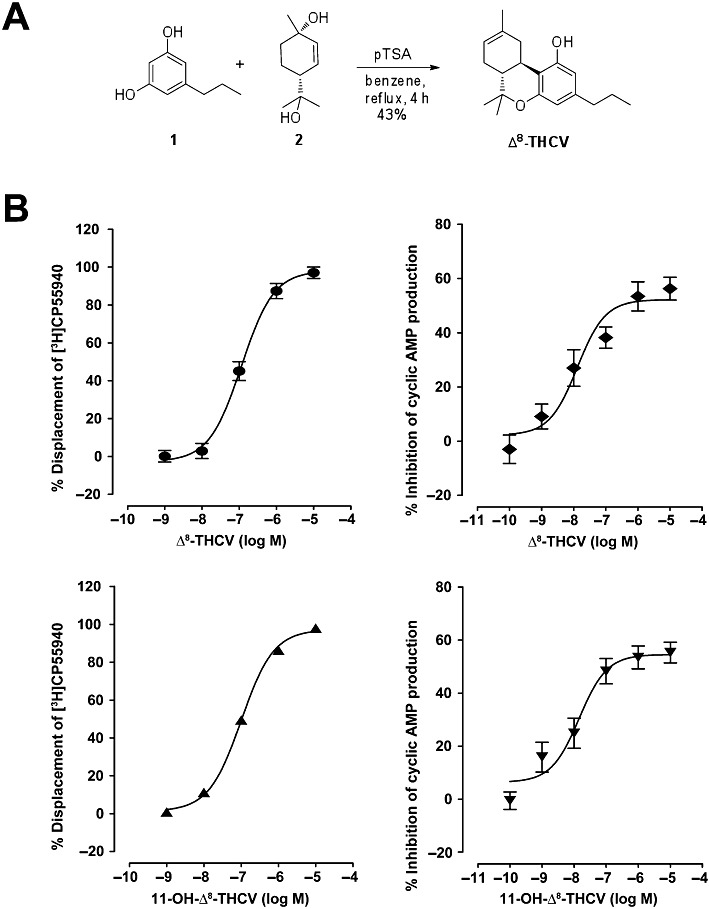
Synthesis and in vitro effects of Δ8-THCV on CB2 receptors. (A) Scheme of the synthesis of Δ8-THCV from 5-propylbenzene-1,3-diol (1) and p-menth-2-ene-1,8-diol (2). (B) Left-hand panels: displacement of [3H]CP55940 from specific binding sites in CHO hCB2 cell membranes. Each symbol represents the mean percent displacement ± SEM (n= 6). Right-hand panels: inhibition of forskolin-induced stimulation of cyclic AMP production in CHO hCB2 cells. Each symbol represents the mean percent inhibition ± SEM (n= 7). The derived Ki, EC50 and Emax values are given in the Results.
Forskolin and rolipram were purchased from Sigma-Aldrich (Poole, Dorset, UK), CP55940 (–)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol from Tocris (Bristol, UK) and [3H]CP55940 from PerkinElmer Life Sciences Inc. (Boston, MA, USA). CB1 and CB2 antagonists/inverse agonists SR141716A and SR144528 were obtained from the NIDA Drug Supply Program. For in vivo administration, all drugs were dissolved in vehicle solution (1 drop of Tween-80 in 3 mL 2.5% DMSO in saline). Vehicle solution was used in control experiments.
Anandamide (N-arachidonoyl ethanolamine, AEA) was purchased from Sigma Chemical Co. (St. Louis, MO, USA), and 2-arachidonoyl glycerol (2-AG) was from Alexis Corporation (San Diego, CA, USA). [3H]AEA (205 Ci·mmol−1) was from PerkinElmer Life Sciences. [3H]N-arachidonoyl phosphatidylethanolamine ([3H]NArPE, 200 Ci·mmol−1), and [3H]2-oleoyl glycerol ([3H]2-OG, 20 Ci·mmol−1) were from ARC (Arlington, VA, USA), whereas [14C]sn-1-stearoyl-2-arachidonoyl glycerol ([14C]DAG, 56 mCi·mmol−1) was from Amersham (Glattbrugg, Switzerland).
Results
Δ8-THCV and 11-OH-Δ8-THCV activate CB2 receptors in vitro
Mean Ki values for displacement of [3H]CP55940 from specific binding sites in CHO hCB2 cell membranes with 95% confidence limits shown in brackets were 68.4 nM (46.2 and 101.3 nM; n= 6) for Δ8-THCV, and 59.95 nM (49.1 and 73.2 nM; n= 6) for 11-OH-Δ8-THCV (Figure 1B). Corresponding Emax values were 98.0% (91.4 and 104.7%) and 97.3% (94.2 and 100.4%) respectively. Δ8-THCV and 11-OH-Δ8-THCV inhibited forskolin-induced stimulation of cyclic AMP production in CHO hCB2 cells with mean EC50 values of 12.95 nM (4.5 and 37.6 nM; n= 7) for Δ8-THCV, and 14.3 nM (5.3 and 38.7 nM; n= 7) for 11-OH-Δ8-THCV (Figure 1B). Corresponding Emax values were 52.2% (45.6 and 58.8%) and 54.6% (48.2 and 61.0%) respectively.
Δ8-THCV does not affect the activity of enzymes involved in endocannabinoid synthesis and hydrolysis
In a set of in vitro experiments, 10 µM Δ8-THCV almost halved NAPE-PLD activity (466 ± 56 vs. 833 ± 33 pmol·min−1·mg−1 protein, P < 0.001), whereas at the same concentration, it did not change the FAAH activity (125 ± 5 pmol·min−1·mg−1 protein in both Δ8-THCV-treated and control samples). In addition, 2-AG synthesis via DAGL was not significantly affected by 10 µM Δ8-THCV (396 ± 126 vs. 273 ± 103 pmol·min−1·mg−1 protein in both Δ8-THCV-treated and control samples; P > 0.05), neither was 2-AG degradation via MAGL (689 ± 16 vs. 653 ± 19 pmol·min−1·mg−1 protein in Δ8-THCV-treated and control samples; P > 0.05). Taken together, these data demonstrate that Δ8-THCV does not affect the metabolism of AEA or 2-AG.
Δ8-THCV attenuates markers of hepatic I/R injury (ALT, AST)
For assessments of hepatocellular damage of the post-ischaemic liver, the serum transaminase activities (AST and ALT) were measured. After 1 h of ischaemia and a subsequent 2 or 6 h of reperfusion, a dramatic increase in liver enzyme activities was observed in vehicle-treated C57BL/6J mice as compared with sham-operated controls (Figure 2). Pretreatment with Δ8-THCV (3 or 10 mg·kg−1) 2 h before the induction of the ischaemia dose-dependently attenuated the serum transaminase elevations at 2 and 6 h of reperfusion compared to vehicle. At 2 h reperfusion with 10 mg·kg−1Δ8-THCV, ALT level decreased by ∼46% and AST by ∼54%. At 6 h reperfusion with 3 or 10 mg·kg−1Δ8-THCV, ALT decreased by 41 and 58%, respectively, whereas AST levels decreased by 48 and 69%, respectively (Figure 2). Pretreatment with the CB2 receptor antagonist/inverse agonist SR144528 (3 mg·kg−1) alone had no statistically significant effect on post-ischaemic transaminase levels; however, it attenuated the beneficial effect of 10 mg·kg−1Δ8-THCV at the time of the peak serum ALT/AST elevations (Figure 2), implying a role for CB2 receptors in the protective effects of Δ8-THCV. On the other hand, the CB1 antagonist/inverse agonist SR141716 pretreatment alone at the doses of 3 and 10 mg·kg−1 attenuated the injury (Figure 2). Also SR141716 in combination with 10 mg·kg−1Δ8-THCV produced a stronger protective effect, although it did not reach statistical significance. Δ8-THCV, SR141716 or SR144528 alone had no effects on ALT and AST levels in the sham animals compared to the vehicle-treated group.
Figure 2.
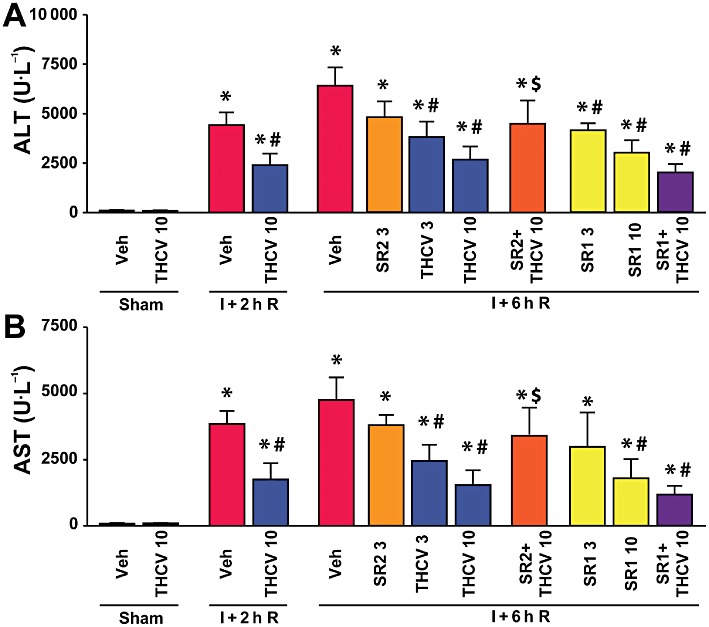
Δ8-THCV pretreatment decreases liver I/R injury. (A and B) Serum transaminase ALT (A) and AST (B) levels in sham-operated mice treated with vehicle (veh) or Δ8-THCV (n= 5 per group) or in mice exposed to 1 h of hepatic ischaemia followed by 2h (I + 2h R) or 6 h (I + 6h R) of reperfusion pretreated with vehicle, Δ8-THCV (3 or 10 mg·kg−1) or SR144528 (SR2, 3 mg·kg−1), SR141716A (SR1, 3 and 10 mg·kg−1) and 10 mg·kg−1Δ8-THCV in combination with SR1 or SR2 (n= 6–10 per group). *P < 0.05 versus vehicle; #P < 0.05 versus corresponding mice exposed to I/R; $P < 0.05 versus corresponding mice exposed to I/R and pretreated with 10 mg·kg−1Δ8-THCV.
Post-ischaemic Δ8-THCV treatment, i.e., given at the moment of reperfusion attenuated (Figure 3), although to a lesser extent than in the pretreatment experiments, the hepatic injury measured at 6 h of reperfusion.
Figure 3.
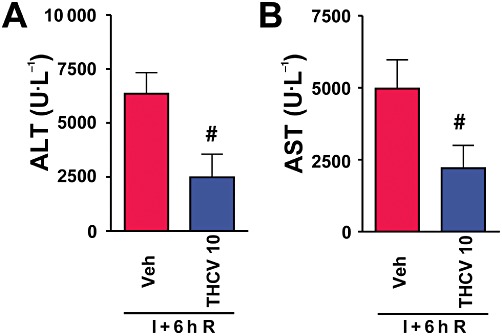
Δ8-THCV treatment at the moment of reperfusion decreases liver I/R injury. (A and B) Serum transaminase ALT (A) and AST (B) levels in mice exposed to 1 h of hepatic ischaemia followed by 6 h of reperfusion (I+6h R) treated with vehicle or Δ8-THCV (10 mg·kg−1, n= 7–8 per group) at the start of reperfusion. #P < 0.05 versus corresponding mice exposed to I/R.
Δ8-THCV improves I/R-induced histological damage
HE staining of representative liver sections after 24 h of reperfusion showed (Figure 4) that I/R induced marked coagulation necrosis (lighter areas, with marked inflammatory cell infiltration), which was dramatically reduced and became more focal in Δ8-THCV-treated mice. Vehicle or Δ8-THCV treatment in the sham animals had no effect on liver histopathology. A similar histological profile was seen throughout the groups (n = 3–5).
Figure 4.
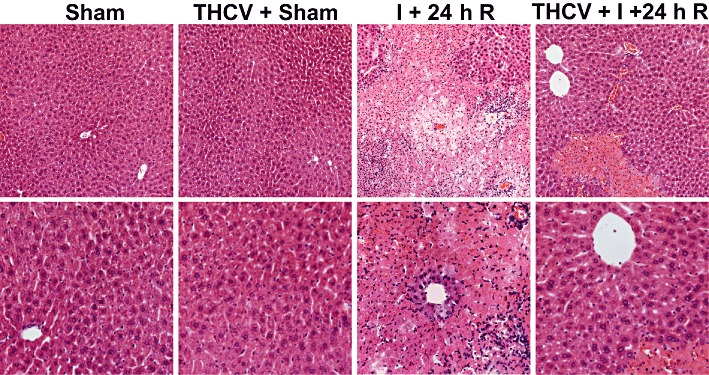
Δ8-THCV decreases histological damage at 24 h following ischaemia. HE staining of representative liver sections of sham mice treated with vehicle (sham) or Δ8-THCV (THCV), and mice exposed to 1 h of ischaemia followed by 24 h of reperfusion treated with vehicle (I + 24 h R) or Δ8-THCV (THCV + I + 24 h R). Upper row of images depicts 200× magnification, while the lower one 400× magnification. A similar histological profile was seen throughout each group (n = 3–5).
Δ8-THCV attenuates the I/R-induced marked neutrophil infiltration
Neutrophils are important mediators of the delayed tissue injury following I/R. An indicator of neutrophil infiltration is the tissue MPO activity. In sham mice, MPO staining was barely detectable (Figure 5). In contrast, there was a marked increase in infiltrating MPO-positive immune cells (brown staining), after 24 h of reperfusion in vehicle-treated animals, which was significantly attenuated by Δ8-THCV. A similar histological profile was seen throughout the groups (n = 3–5).
Figure 5.
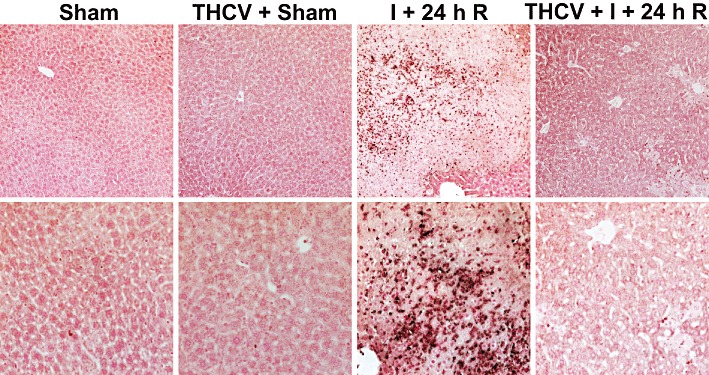
Δ8-THCV decreases neutrophil infiltration. MPO staining (brown) of representative liver sections of sham mice treated with vehicle (sham) or Δ8-THCV (THCV), and mice exposed to 1 h of ischaemia followed by 24 h of reperfusion treated with vehicle (I + 24 h R) or Δ8-THCV (THCV + I + 24 h R). Slides were counterstained by nuclear fast red. Upper row of images depicts 200× magnification, while the lower one 400× magnification. A similar histological profile was seen throughout each group (n = 3–5).
Δ8-THCV attenuates the I/R-induced hepatic pro-inflammatory chemokine, cytokines and adhesion molecule expression
I/R greatly increased the expression of mRNAfor the chemokines CCL3 and CXCL2 in liver tissue as shown by real-time PCR, which was attenuated by Δ8-THCV pretreatment (Figure 6A,B). Hepatic CCL3 mRNA increased to the highest level at 2 h of reperfusion and gradually decreased by 24 h of reperfusion almost to control levels. Δ8-THCV pretreatment decreased hepatic peak CCL3 mRNA level at 2 and 6 h of reperfusion (Figure 6A). mRNA for CXCL2 increased to its peak at 2 h of reperfusion and gradually decreased by 24 h of reperfusion (Figure 6B). Δ8-THCV treatment significantly decreased peak levels of mRNA for CCL3 and CXCL2 at 2 h of reperfusion, and also markedly attenuated the I/R values at 6 h of reperfusion (Figure 6A,B).
Figure 6.
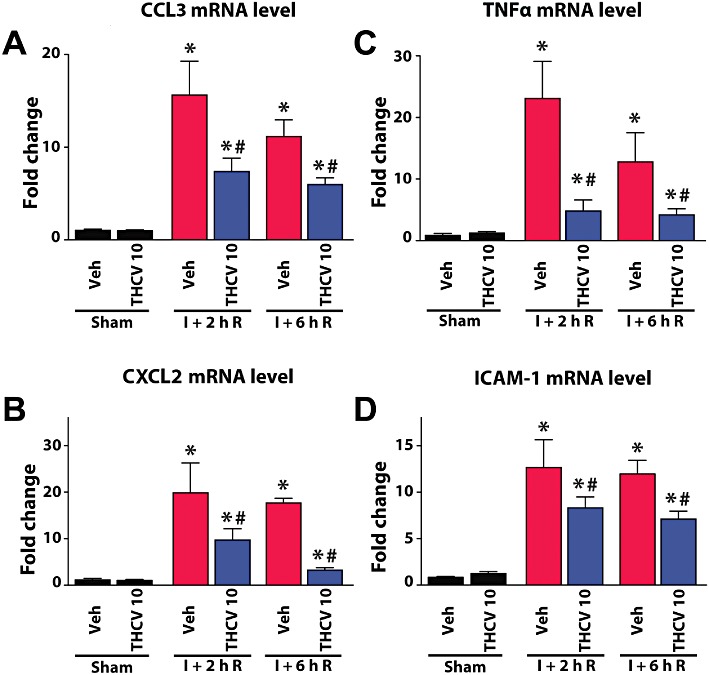
Δ8-THCV attenuates the I/R-induced acute pro-inflammatory response in the liver. Real-time PCR shows significant increase of mRNA for the chemokine CCL3 (A), the chemokine CXCL2 (B), the pro-inflammatory cytokine TNF-α (C) and adhesion molecule ICAM-1 (D) at 2 h of reperfusion (I + 2 hR), and a gradual decrease at 6 h (I + 6 hR). Pretreatment with Δ8-THCV at 10 mg·kg−1 significantly attenuates the I/R-induced increased levels of these inflammatory markers at the time points of the reperfusion studied (2 and 6 h). Results are mean ± SEM; n= 6–12. *P < 0.05 versus vehicle; #P < 0.05 versus corresponding I/R mice.
Real-time PCR analyses of cytokine TNF-1α (Figure 6C) and adhesion molecule ICAM-1 (Figure 6D) demonstrated markedly increased mRNA expression in liver tissue after I/R, peaking at 2 h of reperfusion and gradually declining thereafter. Δ8-THCV pretreatment significantly decreased the I/R-induced increased TNF-α and ICAM-1 mRNA expression, and also markedly attenuated these values at 6 h of reperfusion (Figure 6C,D).
Δ8-THCV decreases the I/R-induced increased oxidative stress
The rate of oxidative post-translational modification of proteins and lipid peroxidation was negligible in sham livers, as indicated by the low level of carbonyl adducts and HNE. HNE increased by 2.5- and 4-fold after 6 and 24 h of reperfusion, respectively, and these increases were significantly attenuated by Δ8-THCV (10 mg·kg−1) (Figure 7A). The hepatic content of carbonyl adducts increased by 3.2- and 7.1-fold after 6 and 24 h of reperfusion, respectively, which were significantly attenuated by Δ8-THCV (10 mg·kg−1) (Figure 7B).
Figure 7.
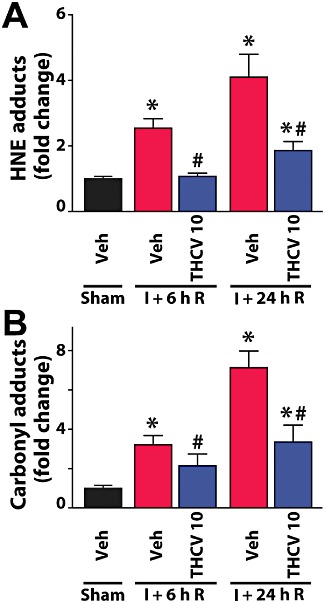
Δ8-THCV decreases I/R-induced increased oxidative stress. (A) HNE adducts, a marker for lipid peroxidation/oxidative stress, increase with time following I/R injury. 10 mg·kg−1Δ8-THCV pretreatment attenuates these increases at the time points of reperfusion studied, 6h (I + 6h R) and 24 h (I+24h R). (B) Oxidative modification of proteins, measured by carbonyl adducts, increases with time following I/R injury. 10 mg·kg−1Δ8-THCV pretreatment attenuates these increases at the time points of reperfusion studied, 6h (I + 6h R) and 24 h (I+24h R). Results are mean ± SEM for both panels and n= 8 per group. *P < 0.05 versus vehicle; #P < 0.05 versus corresponding I/R mice.
Δ8-THCV decreases the I/R-induced hepatocyte cell death
Apoptotic cell death in the liver following I/R was evaluated by monitoring caspase-3/7 activity and DNA fragmentation (Figure 8). All markers of cell death in the liver were increased at 24 h of reperfusion following 60 min ischaemia. Δ8-THCV significantly attenuated the increase of both markers (Figure 8).
Figure 8.
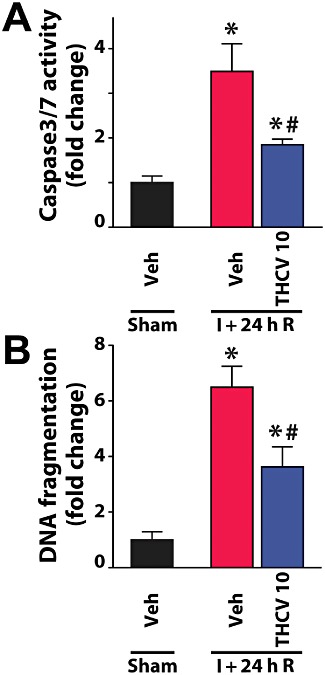
Δ8-THCV attenuates the I/R-induced enhanced hepatic cell death. (A) Changes in hepatic caspase3/7 activity following I/R, and attenuation of the observed increases by pretreatment with 10 mg·kg−1Δ8-THCV. Results are mean ± SEM of n= 8 per group. *P < 0.05 versus vehicle; #P < 0.05 versus corresponding I/R mice. (B) Increase of hepatic DNA fragmentation demonstrated following I/R injury and attenuation by pretreatment with 10 mg·kg−1Δ8-THCV. Results are mean ± SEM of n= 8 per group. *P < 0.05 versus vehicle; #P < 0.05 versus corresponding I/R mice.
Discussion
In the present study, we have demonstrated that Δ8-THCV exerted protective effects against liver I/R reperfusion damage by attenuating tissue injury, oxidative stress and inflammatory response. Hepatic I/R injury may occur in a number of clinical settings, such as those associated with low flow states, surgical procedures and transplantation, affecting morbidity and mortality. The damage to the liver caused by I/R represents a continuum of processes that culminate in tissue injury. These processes are triggered when the liver is transiently deprived of oxygen and subsequently re-oxygenated. The destructive effects of I/R are induced by the rapid generation of ROS from the activation of various cellular sources, such as xanthine oxido-reductases (Engerson et al., 1987; Pacher et al., 2006) and impairment of the mitochondrial respiratory chain (Jaeschke, 2003; Moon et al., 2008), resulting in increased lipid peroxidation, oxidative modification of proteins and DNA damage, impairing pivotal cellular functions. The increased ROS during early reperfusion also leads to activation of Kupffer cells (the resident inflammatory cells of the liver) and endothelial cells with consequent increased production of pro-inflammatory chemokines (this response peaks 2 h following reperfusion) and cytokines in these cells, leading to the priming and recruitment of neutrophils and other inflammatory cells into the liver vasculature upon reperfusion. These inflammatory cells attach to the activated endothelium and generate ROS and RNS, and pro-inflammatory mediators leading to endothelial dysfunction, followed by transmigration through the injured endothelium, attachment to hepatocytes and further activation to release oxidants and proteolytic enzymes. These changes in turn trigger intracellular oxidative/nitrative stress and mitochondrial dysfunction in hepatocytes (the oxidative/nitrative stress peaks at 24 h of reperfusion), culminating in cell death (both apoptotic and necrotic) (Jaeschke, 2006; Pacher and Hasko, 2008).
In agreement with this cascade of pathological events and previous reports using the same model (Batkai et al., 2007; Rajesh et al., 2007; Moon et al., 2008), we have found increased hepatic carbonyl and 4-HNE adduct levels (markers of lipid peroxidation and oxidative stress), and increased serum ALT and AST activities (markers of liver injury/necrosis) at 2 h of reperfusion, accompanied by peak increases in markers of acute inflammatory response and endothelial activation [mRNA for TNF-α, CCL3, CXCL2, ICAM-1 (CD54)] in the liver. The peak levels of serum ALT and AST could be detected at 6 h of reperfusion in our model, which gradually returned to almost normal levels by 24 h of reperfusion, indicating that the predominant type of cell death at the earlier time points of reperfusion is necrotic. As the inflammatory reactions progress, the histological picture of post-ischaemic liver morphology at 24 h of reperfusion is characterized by marked coagulation necrosis (lighter areas) with massive inflammatory cell infiltration (Figures 4 and 5). In this model of I/R, MPO-positive neutrophil recruitment starts from 6 h of reperfusion and peaks during 12–24 h of reperfusion (Moon et al., 2008) (Figure 5), which coincides with the peak of oxidative stress and apoptotic cell death (caspase 3/7 activity and DNA fragmentation) 24 h following reperfusion (Figures 7 and 8).
Δ8-THCV is a synthetic analog of Δ9-THCV, a natural component of marijuana. The latter has been shown to exert protective effects against seizure activity (Hill et al., 2010); has also hypophagic properties; and is in phase I development as a potential treatment for obesity, diabetes and related metabolic disorders (Riedel et al., 2009). On the other hand, Δ9-THCV also exhibits significant potency and efficacy as a CB2 receptor agonist in vivo, and exerts anti-oedema and anti-hyperalgesic activity in a model of local inflammation (Bolognini et al., 2010). Based on these data, we hypothesized that Δ8-THCV may also activate CB2 receptors and mediate beneficial effects in hepatic I/R.
Indeed, we found that Δ8-THCV and one of its metabolites, 11-OH-Δ8-THCV (Harvey and Brown, 1988), behaved as potent CB2 receptor agonists in vitro. In our binding assay, Δ8-THCV caused agonist displacement in hCB2-transfected CHO cells and also inhibited forskolin-induced stimulation of cAMP production (with an EC50 of 13 nM and Emax of 52%) in these cells, which were not significantly different from the recently reported values for Δ9-THCV (EC50= 38 nM and Emax= 40%; Bolognini et al., 2010).
Various cannabinoid ligands may also interact with the synthetic and metabolic enzymes of endocannabinoids, eliciting indirect effects mediated by changes of the levels of these bioactive substances, with potential relevance to tissue injury (Pacher and Mechoulam, 2011). However, we found that Δ8-THCV in relevant concentrations had no effect on the activities of the major enzymes involved in endocannabinoid synthesis (NAPE-PLD and DAGL) or degradation (FAAH and MAGL). Although in this study we have not tested the effect of Δ8-THCV on the putative endocannabinoid transporter(s), on the basis of the data described earlier, it is unlikely that Δ8-THCV would directly lead to major changes in tissue endocannabinoid levels in normal livers. However, it is very likely that by decreasing inflammation and oxidative stress during I/R, which are involved in modulating endocannabinoid production/degradation (Pacher and Hasko, 2008), Δ8-THCV may indirectly affect tissue endocannabinoid levels in damaged tissues, an interesting issue which should be explored in future studies.
In our in vivo model of liver I/R injury, Δ8-THCV, given prior to the induction of I/R, significantly attenuated the elevations of serum liver transaminases (ALT/AST), decreased tissue oxidative stress (carbonyl adducts and HNE), attenuated acute and chronic hepatic inflammatory response (TNF-α, CCL3, CXCL2, ICAM-1/CD54 mRNA levels and tissue neutrophil infiltration), and necrotic (ALT/AST levels and coagulation necrosis) and apoptotic (caspase 3/7 activity, DNA fragmentation) cell death. The protective effects of Δ8-THCV were largely abolished by pretreatment with the CB2 receptor antagonist SR144528, indicating that these were, at least in part, mediated by CB2 receptor activation (CB2 antagonist in tested dose had no significant effect on I/R injury by itself). Pretreatment with the CB1 receptor antagonist SR141716 did not prevent the beneficial effect of Δ8-THCV, in fact it showed protective effects by itself, which tended to be more pronounced when Δ8-THCV was given in combination with SR141716. The protective effect of the CB1 receptor antagonist observed in our study is consistent with the protection observed in rat liver I/R complicated with endotoxemia recently (Caraceni et al., 2009) and in other models of I/R injury (Pacher and Hasko, 2008; Zhang et al., 2009b), likewise with the proposal of combining CB2 receptor agonists with CB1 receptor antagonists for the treatment of reperfusion damage (Pacher and Hasko, 2008; Zhang et al., 2009b). From this perspective, it would be interesting to see in future studies if Δ8-THCV could inhibit CB1 receptors in in vitro and in vivo models of injury. Importantly, the protective effects of Δ8-THCV against liver damage were also preserved when it was given after the ischaemia, at the moment of reperfusion, which is very important from a clinical point of view.
In summary, our results indicate that Δ8-THCV, a more stable and more readily synthesized analogue of the naturally occurring Δ9-THCV, may represent a novel protective strategy against I/R injury by attenuating oxidative stress, acute and chronic inflammatory response, and cell death.
Acknowledgments
This study was supported by the National Institutes of Health (DA-03672, DA-005488 and DA-009789) and Intramural Research Program of NIH/NIAAA. Dr Horváth is a recipient of a Hungarian Research Council Scientific Research Fund Fellowship (NKTH-OTKA-EU, MB08-80238). The authors are indebted to Dr George Kunos for providing key resources and support.
Glossary
- 4-HNE
4-hydroxy-2-nonenal
- Δ8-THCV
Δ8-tetrahydrocannabivarin
- AEA
anandamide (N-arachidonoyl ethanolamine)
- CB1
or CB2 receptor, cannabinoid 1 or 2 receptor
- ICAM-1
intercellular adhesion molecule 1 (CD54)
- I/R
ischaemia/reperfusion
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
Conflict of interest
R.G.P. receives funding from GW Pharmaceuticals; other authors have no conflicts.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160:677–687. doi: 10.1111/j.1476-5381.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraceni P, Pertosa AM, Giannone F, Domenicali M, Grattagliano I, Principe A, et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia–reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135–1143. doi: 10.1136/gut.2007.147652. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Defer N, Wan J, Souktani R, Escoubet B, Perier M, Caramelle P, et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. FASEB J. 2009;23:2120–2130. doi: 10.1096/fj.09-129478. [DOI] [PubMed] [Google Scholar]
- Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987;79:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill EW, Paton WD, Pertwee RG. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Brown NK. Metabolism of delta-8- and delta-9-tetrahydrocannabinol homologues in mice. In: Chesher G, Consroe P, Musty R, editors. Marijuana: An International Research Report, Monograph Series No. 7. Canberra: Australian Gov. Publ. Service; 1988. pp. 253–258. [Google Scholar]
- Hill AJ, Weston SE, Jones NA, Smith I, Bevan SA, Williamson EM, et al. Delta-tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia. 2010;51:1522–1532. doi: 10.1111/j.1528-1167.2010.02523.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Role of reactive oxygen species in hepatic ischemia–reperfusion injury and preconditioning. J Invest Surg. 2003;16:127–140. [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia–reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- Liaudet L, Szabo G, Szabo C. Oxidative stress and regional ischemia–reperfusion injury: the peroxynitrite-poly(ADP-ribose) polymerase connection. Coron Artery Dis. 2003;14:115–122. doi: 10.1097/00019501-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid delta(9)-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol. 2008;154:204–215. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, et al. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–H1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–195. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murikinati S, Jüttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia–reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog Lipid Res. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, et al. The psychoactive plant cannabinoid, delta9-tetrahydrocannabinol, is antagonized by delta8- and delta9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Pan H, Mukhopadhyay P, Batkai S, Osei-Hyiaman D, Hasko G, et al. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol. 2007;82:1382–1389. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Mukhopadhyay B, Patel V, Hasko G, et al. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:2330–2341. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RK, Jallo JI, Tuma RF. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009a;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, et al. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009b;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


