Abstract
BACKGROUND AND PURPOSE
To evaluate the hypothesis that activation of somatodendritic 5-HT1A autoreceptors in the dorsal raphe nucleus (DRN) produces the anti-emetic/anti-nausea effects of cannabidiol (CBD), a primary non-psychoactive cannabinoid found in cannabis.
EXPERIMENTAL APPROACH
The potential of systemic and intra-DRN administration of 5-HT1A receptor antagonists, WAY100135 or WAY100635, to prevent the anti-emetic effect of CBD in shrews (Suncus murinus) and the anti-nausea-like effects of CBD (conditioned gaping) in rats were evaluated. Also, the ability of intra-DRN administration of CBD to produce anti-nausea-like effects (and reversal by systemic WAY100635) was assessed. In vitro studies evaluated the potential of CBD to directly target 5-HT1A receptors and to modify the ability of the 5-HT1A agonist, 8-OH-DPAT, to stimulate [35S]GTPγS binding in rat brainstem membranes.
KEY RESULTS
CBD suppressed nicotine-, lithium chloride (LiCl)- and cisplatin (20 mg·kg−1, but not 40 mg·kg−1)-induced vomiting in the S. murinus and LiCl-induced conditioned gaping in rats. Anti-emetic and anti-nausea-like effects of CBD were suppressed by WAY100135 and the latter by WAY100635. When administered to the DRN: (i) WAY100635 reversed anti-nausea-like effects of systemic CBD, and (ii) CBD suppressed nausea-like effects, an effect that was reversed by systemic WAY100635. CBD also displayed significant potency (in a bell-shaped dose–response curve) at enhancing the ability of 8-OH-DPAT to stimulate [35S]GTPγS binding to rat brainstem membranes in vitro. Systemically administered CBD and 8-OH-DPAT synergistically suppressed LiCl-induced conditioned gaping.
CONCLUSIONS AND IMPLICATIONS
These results suggest that CBD produced its anti-emetic/anti-nausea effects by indirect activation of the somatodendritic 5-HT1A autoreceptors in the DRN.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: cannabidiol, 5-HT1A, endocannabinoid, nausea, vomiting, shrew, rat, taste reactivity, gaping, conditioned disgust, emesis
Introduction
The cannabis plant has been used for centuries for the suppression of nausea and vomiting (for review, Russo, 2007). Recent research has revealed that among more than 80 cannabinoid compounds found in marihuana, both the intoxicant, Δ9-tetrahydrocannabinol (Δ9-THC; Darmani, 2001; Van Sickle et al., 2001; Kwiatkowska et al., 2004) and the non-intoxicant, cannabidiol (CBD; Kwiatkowska et al., 2004; Parker et al., 2004), suppress vomiting in animal models. CBD acts in a biphasic manner, such that low doses suppress toxin-induced vomiting but high doses potentiate (Kwiatkowska et al., 2004; Parker et al., 2004) or have no effect (Darmani et al., 2007) on vomiting. Both Δ9-THC and CBD also suppress the development of malaise-induced conditioned gaping reactions (Grill and Norgren, 1978a,b) in rats, a model of rat nausea-like behaviour (see Parker and Limebeer, 2008 for review). These conditioned gaping reactions in rats are only produced by drugs that also produce emesis in species capable of vomiting and are specifically prevented by pretreatment with anti-emetic drugs, such as the 5-HT3 receptor antagonist, ondansetron (Limebeer and Parker, 2000). This pattern contrasts with the more typically employed non-selective measure of conditioned taste avoidance (see Parker et al., 2008).
Considerable recent interest has been directed to the therapeutic potential of CBD. It has been shown to protect against cerebral ischaemia, inflammation, anxiety (see Mechoulam et al., 2007) and, most recently, depression (Zanelati et al., 2010) and even addiction (Ren et al., 2009). Several mechanisms of action have been identified for the various physiological effects of CBD (Mechoulam and Hanus, 2002; Pertwee et al., 2002; Mechoulam et al., 2007). Although CBD has very low affinity for both CB1 and CB2 cannabinoid receptors (Pertwee, 2004, 2008; receptor nomenclature follows Alexander et al., 2011), it has recently been shown to display unexpectedly high potency in vitro as an antagonist of CB1 agonists in mouse vas deferens (Pertwee et al., 2002) and brain (Thomas et al., 2007) tissues. Additionally, CBD displays inverse agonism at the human CB2 receptor (Thomas et al., 2007). CBD has also been reported to enhance adenosine signalling by inhibiting its re-uptake; in vivo treatment with a low dose of CBD decreased TNF-α production in lipopolysaccharide-treated mice (anti-inflammatory effect), an effect that was reversed with an A2A adenosine receptor antagonist and abolished in A2A receptor knockout mice (Carrier et al., 2006). CBD also acts as an antioxidant, potentially preventing damage in neurological disorders such as cerebral ischaemia (Hampson et al., 1998). The anti-arthritic potential of CBD may be the result of diminished IFN-γ release from lymph node cells in CBD-treated mice; subsequent in vitro experiments found that CBD suppressed the collagen type II-specific proliferation of lymph node cells from arthritic mice (Malfait et al., 2000).
Russo et al. (2005) recently reported that at a rather high concentration of 16 µM, CBD can bind to and activate human 5-HT1A receptors. Somatodendritic 5-HT1A autoreceptors in the raphe nuclei regulate the rate of firing of raphe 5-hydroxytryptaminergic afferents, and low doses of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT, a classical 5-HT1A agonist) reduce their firing rate, thereby reducing the release of 5-HT in terminal regions (Verge et al., 1985; Blier and de Montigny, 1987). CBD also reduces the volume of cerebral infarction in animal models of ischaemic injury (see Hampson et al., 1998). This neuroprotective effect of CBD is mediated by 5-HT1A receptors because it is reversed by the 5-HT1A receptor antagonist (S)-N-tert-butyl-3-(4-(2-methoxyphenyl)-piperazin-1-yl)-2-phenylpropanamide, WAY100135 (Hayakawa et al., 2004, 2007a,b; Mishima et al., 2005). Also, CBD-induced reversal of cognitive and motor function impairments in a mouse model of hepatic encephalopathy is prevented by a 5-HT1A receptor antagonist (Magen et al., 2010). When injected into the dorsolateral periaqueductal grey, the anxiolytic effects of CBD are prevented by pretreatment with WAY100135, but not by the CB1 receptor antagonist/inverse agonist AM251 (Campos and Guimaraes, 2008). Most recently, CBD has been reported to produce an antidepressant-like action which is 5-HT1A receptor mediated (Zanelati et al., 2010).
It is only recently that the mechanism of action for CBD's anti-emetic and anti-nausea effects has been evaluated (see Parker and Limebeer, 2008). Unlike Δ9-THC, which produces its effect on emetic behaviours by its action on the CB1 receptor (Darmani, 2001; Van Sickle et al., 2001; Parker et al., 2003, 2004), CBD has a very low affinity for the CB1 receptors (Mechoulam and Hanus, 2002) and its anti-emetic effect is not reversed by pharmacological blockade of these receptors (Parker et al., 2004). Like CBD, low doses of 8-OH-DPAT attenuate vomiting (Lucot and Crampton, 1989; Okada et al., 1994; Wolff and Leander, 1994; Andrews et al., 1996; Gupta and Sharma, 2002; Javid and Naylor, 2006) and conditioned gaping (Limebeer and Parker, 2003) in animal models. It is likely that the attenuation of nausea-like behaviour by low doses of 8-OH-DPAT is the result of action at somatodendritic 5-HT1A autoreceptors in the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN) because selective serotonin lesions of these nuclei also attenuate the establishment of lithium chloride (LiCl)-induced conditioned gaping reactions (Limebeer et al., 2004). Data reported in the literature show that 5-HT is able to displace fully [3H]8-OH-DPAT from specific binding sites both in rat cerebral cortex membranes (Carli et al., 1996) and in human 5-HT1A-expressing CHO cell membranes (Newman-Tancredi et al., 1998). These findings suggest that 5-HT and 8-OH-DPAT bind to the same 5-HT1A receptor binding site.
Here, we provide evidence that systemic pretreatment with a 5-HT1A receptor antagonist attenuated the anti-emetic and anti-nausea-like effects of CBD. Furthermore, when administered intracranially into the DRN, N-[2-[4-(2-methoxyphenyl)-1 piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate (WAY100635) attenuated the anti-nausea-like effects of systemic CBD in rats. When CBD was administered directly into the DRN, LiCl-induced conditioned gaping reactions were suppressed in rats, an effect that was reversed by systemic WAY100635. In vitro data also demonstrated that CBD augmented the effect of 8-OH-DPAT in rat brainstem tissues, and this effect was confirmed by a synergistic effect of combined subthreshold systemic doses of CBD and 8-OH-DPAT on LiCl-induced conditioned gaping in rats.
Materials and methods
Animals
All animal care and experimental procedures complied with the Canadian Council on Animal Care (CCAC) and the National Institutes of Health guidelines and were approved by the Institutional Animal Care Committee, which is accredited by the CCAC. Male (30–45 g) and female (20–37 g) Suncus murinus (house musk shrews) ranging from 40 days to 180 days of age were bred and raised at the University of Guelph colony. They were single-housed in cages in a colony room at an ambient temperature of 21°C on a 14/10 light dark schedule (lights off at 2100 h) as described in Parker et al. (2009). Shrews were tested during their light cycle, between 0800 h and 1700 h. The shrews had previous emetic experience with the limitation of a minimum of 3 weeks recovery between treatments. Because of its toxicity, cisplatin was always administered as the final treatment and shrews were killed thereafter.
Naïve male Sprague-Dawley rats, weighing between 275 and 350 g on the day of conditioning, obtained from Charles River Laboratories (St Constant, Quebec, Canada), were used for assessment of anti-nausea-like behaviour. They were single-housed in shoebox cages in the colony room at an ambient temperature of 21°C with a 12/12 light/dark schedule (lights off at 0800 h) and maintained on ad libitum food and water.
Drugs and materials
In vivo experiments
When systemically administered, CBD was prepared (2.5 mg·mL−1 or 0.5 mg·mL−1) in a vehicle of ethanol/Cremaphor (Sigma, St Louis, MO, USA)/saline (1:1:18) and administered s.c. 8-OH-DPAT HBr (8-OH-DPAT; Sigma) was prepared in saline (0.05 mg·mL−1 or 0.005 mg·mL−1) and administered s.c. The 5-HT1A receptor antagonist, WAY100135 (Sigma; 5 mg·mL−1; Mishima et al., 2005), and the more selective 5-HT1A receptor antagonist (Forster et al., 1995), WAY100635 (Sigma; 0.1 mg·mL−1; Campos and Guimaraes, 2008), were prepared in saline and administered i.p. LiCl (Sigma) was prepared in a 0.15 M solution with sterile water and was administered i.p. at a volume of 60 mL·kg−1 (390 mg·kg−1) in shrews (see Parker et al., 2004) and 20 mL·kg−1 (127.2 mg·kg−1) in rats. Nicotine (Sigma) bitartrate salt was prepared as a 2.5 mg·mL−1 solution (expressed as a salt) in saline and administered s.c. at a dose of 5 mg·kg−1 (2 mL·kg−1) (Parker et al., 2009). Cisplatin (Sigma) was prepared as a 1 mg·mL−1 solution in saline and was administered i.p. at doses of 20 mg·kg−1 (20 mL·kg−1) and 40 mg·kg−1 (40 mL·kg−1).
When administered intracranially into the DRN, WAY100635 was prepared in sterile saline at a concentration of 21 ng in 0.5 µL (Herges and Taylor, 1999) and intracranially microinfused at 0.5 µL·min−1 for 1 min. Intracranial CBD was prepared in 45% 2-hydroxypropyl-β-cyclodextrin at 10 µg·µL −1 and intracranially microinfused into the DRN at 1 µL·min−1 for 1 min (based on Murillo-Rodriguez et al., 2008).
In vitro experiments
CBD and 8-OH-DPAT HBr were supplied by Tocris (Bristol, UK). WAY100635 and fatty acid-free BSA were supplied by Sigma-Aldrich (Poole, Dorset, UK). For the binding experiments, [35S]GTPγS (1250 Ci·mmol−1) and [3H]8-OH-DPAT (187 Ci·mmol−1) were obtained from PerkinElmer Life Sciences Inc (Boston, MA, USA), GTPγS and adenosine deaminase from Roche Diagnostic (Indianapolis, IN, USA) and GDP from Sigma-Aldrich.
In vivo procedures
Effect of systemic injections of 5-HT1A receptor antagonists on CBD-induced suppression of nicotine- LiCl- and cisplatin-induced vomiting in shrews
The shrews were moved into the experimental room from the colony room and given four meal worms in an empty cage 15 min prior to receiving two pretreatment injections. The first pretreatment injection was 1 mL·kg−1 of saline or WAY100135, followed 15 min later by an injection of vehicle (2 or 4 mL·kg−1) or CBD [2 mL·kg−1 (5 mg·kg−1) or 4 mL·kg−1 (10 mg·kg−1), depending upon the emetic treatment]. Thirty minutes later, shrews were given an injection of nicotine (5 mg·kg−1), LiCl (390 mg·kg−1) or cisplatin (20 or 40 mg·kg−1). All shrews were then individually placed immediately into the clear Plexiglas observation chamber (22.5 × 26 × 20 cm) that sat on a table with a clear glass top. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the shrew to observe vomiting episodes. The duration of the test was determined by the duration of onset/action of the emetic effect of the drug: nicotine (15 min), LiCl (45 min) or cisplatin (60 min). The frequency of vomiting episodes (expulsion of fluids from the stomach) was counted by an observer unaware of the experimental conditions. Additionally, to evaluate the potential of the pretreatment drugs to produce vomiting on their own, six groups (n= 8/group) of shrews were injected with saline (60 mL·kg−1) following pretreatment with saline or WAY100135 (10 mg·kg−1) and, 15 min later, with vehicle, 5 mg·kg−1 or 10 mg·kg−1 of CBD. They were observed for 60 min. None of the saline-treated shrews displayed vomiting following the pretreatment injections; therefore, these groups were not included in the overall analyses.
The number of vomiting episodes elicited by nicotine or LiCl was entered into a one-way anova, with subsequent Bonferroni post hoc comparison tests of significant main effects. The number of vomiting episodes elicited by either 20 or 40 mg·kg−1 of cisplatin was entered into a 2 (saline or WAY100135) × 3 (vehicle, 5 mg·kg−1 CBD or 10 mg·kg−1 CBD) anova. For all analyses, significance is defined as P < 0.05.
Effect of systemic injections of 5-HT1A receptor antagonists on CBD-induced suppression of LiCl-induced conditioned gaping in rats
All rats were surgically implanted with an intra-oral cannula under isoflurane anaesthesia according to the procedures described by Limebeer et al. (2010). Following recovery from surgery (4 days), the rats received an adaptation trial in which they were placed in the taste reactivity (TR) chamber with their cannula attached to an infusion pump (KDS100, KD Scientific, Holliston, MA, USA) for fluid delivery. The TR chambers had the same specifications as the shrew observation chambers. Water was infused into their intra-oral cannula for 2 min at the rate of 1 mL·min−1. On the day following the adaptation trial, the rats received a conditioning trial in which they received two pretreatment injections. The first pretreatment injection was 2 mL·kg−1 of saline, 2 mL·kg−1 of WAY100135 (10 mg·kg−1) or 1 mL·kg−1 of WAY100635 (0.1 mg·kg−1). The second pretreatment injection given 15 min later was 2 mL·kg−1 of vehicle or CBD (5 mg·kg−1). The groups were as follows: saline-vehicle (n= 13), saline-CBD (n= 9), WAY100135-vehicle (n= 8), WAY100135-CBD (n= 8), WAY100635-vehicle (n= 7) and WAY100635-CBD (n= 8). Thirty minutes after the second pretreatment injection, the rats were individually placed in the chamber and intra-orally infused with 0.1% saccharin solution for 2 min at the rate of 1 mL·min−1 while the orofacial responses were video recorded from a mirror at a 45° angle beneath the chambers, with the feed from the video camera (Sony DCR-HC48, Henry's Cameras, Waterloo, ON, Canada) fire-wired into a computer. Immediately after the saccharin infusion, all rats were injected with 20 mL·kg−1 of 0.15 M LiCl and returned to their home cage. The videotapes were later scored (at ½ speed) by an observer unaware of the experimental conditions, using ‘The Observer’ (Noldus Information Technology Inc., Leesburg, VA, USA) for the gaping behaviour (large openings of the mouth and jaw, with lower incisors exposed).The mean number of gaping reactions elicited by the LiCl-paired saccharin solution was entered into a 3 (saline, WAY100135 or WAY100635) × 2 (vehicle or CBD) between-groups anova, with subsequent planned comparison tests.
Effect of intra-DRN WAY100635 and systemic CBD on conditioned gaping in rats
In all experiments, each rat was also permanently implanted unilaterally, entering from either the left or right hemisphere (counterbalanced across rats) with an intracranial cannula directed towards the DRN. Rats were anaesthetized with isoflurane and stabilized in the flat skull position (according to Paxinos and Watson, 1986) in the stereotaxic frame. A stainless steel guide cannula (22 G, 8 mm below pedestal; Plastics One, Roanoke, VA, USA) was implanted at an angle of 20° to the vertical so that the tip was located 2 mm dorsal to the DRN. Co-ordinates (relative to inter-aural zero) were: anterior-posterior (A-P) +1.2 mm, medial-lateral (M-L) 0.0 mm and ventral (V) +5.0 mm. The cannula was secured by three stainless steel screws and dental cement. At this time, the rat was given carprofen (0.1 mg·kg−1 i.p.; Pfizer, Kirkland, QC, Canada) as an analgesic, and a stainless steel obturator was inserted in the cannula to maintain patency. All rats were then surgically implanted with an intra-oral cannula under isoflurane anaesthesia according to the procedures described by Limebeer et al. (2010). The rats had at least 5 days recovery before behavioural testing.
Verification of cannula placement into the DRN was determined by histological evaluation of tissue. Rats were injected with 85 mg·kg−1 sodium pentobarbital (Euthansol, Intervet Canada Corp., Kirkland, QC, Canada) and were transcardially perfused with PBS (0.1 M) and 4% formalin. The brains were removed and stored at 4°C in 4% formalin solution for 24–48 h, after which they were placed in a 20% sucrose solution overnight at room temperature. The brains were then sliced in 60 µm sections using a CM1850 Leica cryostat (Leica Microsystems Inc., Concord, ON, Canada), and relevant sections were mounted on glass microscope slides. The tissue was later stained with cresyl violet and examined for accurate injector tip placement using a Leica MZ6 Stereomicroscope with a Leica DFC420 Digital Camera and Leica Application Suite software.
Following recovery from surgery, the rats received an adaptation trial in which they were placed in the TR chamber with their cannula attached to the infusion pump for fluid delivery. Water was infused into their intra-oral cannula for 2 min at the rate of 1 mL·min−1. On the day following the adaptation trial, the rats received a conditioning trial in which they received two pretreatment injections. The first pretreatment injection, saline or WAY100635, was infused into the DRN at 0.5 µL·min−1 for 1 min (with the injector tip protruding 2 mm below the tip of the cannula). The injector remained in place for an additional 1 min. Fifteen minutes later, the rats received a 2 mL·kg−1 pretreatment injection of either vehicle or CBD (5 mg·kg−1, s.c.). Thirty minutes later, the rats were individually placed in the chamber and intra-orally infused with 0.1% saccharin solution for 2 min at the rate of 1 mL·min−1 while the orofacial responses were video recorded. Immediately after the saccharin infusion, all rats were injected with 20 mL·kg−1 of 0.15 M LiCl and returned to their home cages. The final groups (with proper placement) were as follows: saline-vehicle (n= 7), saline-CBD (n= 7), WAY100635-vehicle (n= 9) and WAY100635-CBD (n= 6). Additionally, rats in group WAY100635-CBD (n= 5) with placements outside of the DRN were included in the analysis as a separate group. The mean number of gaping reactions elicited by the LiCl-paired saccharin solution was entered into a one-way anova, with subsequent planned comparison tests.
Effect of intra-DRN CBD and systemic WAY100635 on conditioned gaping in rats
The rats were treated exactly as those receiving intra-DRN WAY100635, except as indicated. On the conditioning trial, they were injected i.p. with 1 mL·kg−1 of saline or WAY100635 (0.1 mg·kg−1). Fifteen minutes later, vehicle or CBD (0.21 ng) was infused into the DRN at 0.5 µL·min−1 for 1 min (with the injector tip protruding 2 mm below the tip of the cannula). The injector remained in place for one additional minute. Immediately after microinfusion the rats were infused in the TR chamber. The final groups (with proper placement) were as follows (DRN drug/systemic drug): vehicle-saline (n= 6), CBD-saline (n= 7), vehicle-WAY100635-vehicle (n= 6) and CBD-WAY100635-CBD (n= 5). The mean number of gaping reactions elicited by the LiCl-paired saccharin solution during the drug-free test was entered into a one-way anova, with subsequent planned comparison tests.
Effect of subthreshold doses of systemic injections of CBD and 8-OH-DPAT on LiCl-induced conditioned gaping in rats
In Experiment A, the doses of CBD (2.5 mg·kg−1) and 8-OH-DPAT (0.05 mg·kg−1) initially tested were the half-optimal doses of each of these compounds previously demonstrated to interfere with conditioned gaping (Parker et al., 2002; Limebeer and Parker, 2003). However, at these doses, both CBD and 8-OH-DPAT each suppressed LiCl-induced conditioned gaping on their own; therefore, in Experiment B, lower (subthreshold) doses of each compound (0.5 mg·kg−1 of CBD and 0.005 mg·kg−1 8-OH-DPAT) were then evaluated.
Twenty-four hours following adaptation, the rats received a conditioning trial in which they were administered a pretreatment and a treatment injection. The pretreatment injection was 1 mL·kg−1 of vehicle or CBD (2.5 mg·mL−1 in Experiment A and 0.5 mg·mL−1 in Experiment B), followed 15 min later by a treatment injection of 1 mL·kg−1 of saline or 8-OH-DPAT (0.05 mg·mL−1 in Experiment A or 0.005 mg·mL−1 in Experiment B). This design resulted in the following groups for each of Experiments A and B: vehicle-saline (n= 7), CBD-8-OH-DPAT (n= 9), CBD-saline (n= 8) and vehicle-8-OH-DPAT (n= 8). Thirty minutes after the treatment injection, the rats were individually placed in the chamber and intra-orally infused with a 0.1% saccharin solution for 2 min at a rate of 1 mL·min−1 while their orofacial and somatic responses were video recorded. Immediately following the saccharin infusion, the rats were injected with 20 mL·kg−1 of 0.15 M LiCl and returned to their home cages. Ninety-six hours following the conditioning trial, the rats individually received a single drug-free test trial in which they were returned to the chamber and intra-orally infused with the 0.1% saccharin solution for 2 min (1 mL·min−1) while their orofacial and somatic responses were video recorded. For each of Experiment A and B, the mean number of gaping reactions during the test trial was entered into a one-way anova, with subsequent planned comparison tests.
In vitro procedures
Preparation of cell membranes from rat brainstem
Rat brainstem tissues were homogenized in ice-cold Choi lysis buffer (Tris–HCl 20 mM, sucrose 0.32 M, EDTA 0.2 mM, EGTA 0.5 mM, pH 7.5) containing Roche© protease inhibitor cocktail (1:40 v/v; Roche Diagnostics, Mannheim, Germany) and phenylmethylsulphonyl fluoride (1 mM). The homogenate was centrifuged at 13 500×g for 15 min, and the resulting pellet was kept in −80°C for at least 2 h. The pellet was then resuspended in TME buffer (50 mM Tris–HCl; EDTA 1.0 mM; MgCl2 3.0 mM; pH 7.4), homogenized and stored at −80°C.
Radioligand displacement assay
The assays were carried out with [3H]-8-OH-DPAT and Tris-binding buffer (50 mM Tris–HCl, 50 mM Tris–base, 0.1% BSA; pH 7.4), total assay volume 500 µL, using the filtration procedure described previously by Ross et al. (1999). Binding was initiated by the addition of rat brainstem membranes (500 µg protein per well). All assays were performed at 37°C for 60 min before termination by addition of ice-cold Tris-binding buffer and vacuum filtration using a 24-well sampling manifold (Brandel Cell Harvester; Brandel Inc., Gaithersburg, MD, USA) and Brandel GF/B filters that had been soaked in wash buffer at 4°C for at least 24 h. Each reaction well was washed six times with a 1.2 mL aliquot of Tris-binding buffer. The filters were oven-dried for 60 min and then placed in 5 mL of scintillation fluid (Ultima Gold XR, PerkinElmer). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 µM unlabelled 8-OH-DPAT. The concentration of [3H]-8-OH-DPAT used in our displacement assays was 0.7 nM. The compounds under investigation were stored at −20°C as stock solutions of 10 mM in DMSO, the vehicle concentration in all assay wells being 0.1% DMSO.
[35S]GTPγS binding assay
The assays were carried out with GTPγS binding buffer (50 mM Tris-HCl, 100 mM NaCl, 3 mM MgCl2, 0.2 mM EGTA and 0.1% BSA fatty acid free; pH 7.4) in the presence of [35S]GTPγS and GDP, in a final volume of 500 µL. Binding was initiated by the addition of [35S]GTPγS to the wells. Non-specific binding was measured in the presence of 30 µM GTPγS. Rat brainstem membranes were pre-incubated for 30 min at 30°C with 0.5 U·mL−1 adenosine deaminase to remove endogenous adenosine. The drugs were incubated in the assay for 60 min at 30°C. The reaction was terminated by a rapid vacuum filtration method using Tris-binding buffer, and the radioactivity was quantified by liquid scintillation spectrometry. In all the [35S]GTPγS-binding assays, we used 0.1 nM [35S]GTPγS, 30 µM GDP and a protein concentration of 100 µg per well.
Dissociation kinetics
Dissociation kinetic assays were performed with the 5-HT1A receptor agonist [3H]-8-OH-DPAT (0.7 nM) and Tris-binding buffer, total assay volume 500 µL. We used the ‘isotopic dilution’ method to measure the dissociation rate constant for [3H]-8-OH DPAT from brainstem membranes (Christopoulos, 2000; Price et al., 2005). [3H]-8-OH-DPAT (0.7 nM) was incubated with rat brainstem membranes (500 µg protein per well) for 60 min at 25°C. Dissociation was initiated by the addition of 1 µM unlabelled ligand in the presence and absence of test compounds. Dissociation times of 0.5 to 120 min at 25°C were used. To determine the non-specific binding, experiments were also performed in the presence of a 1 µM concentration of the unlabelled ligand. Binding was terminated by addition of ice-cold wash buffer (50 mM Tris–HCl, 50 mM Tris–base and 0.1% BSA) followed by vacuum filtration.
Analysis of in vitro data
Values have been expressed as means and variability as SEM or as 95% confidence limits. The concentrations of the compounds under investigation that produced a 50% displacement of radioligand from specific binding sites (IC50 values) were calculated using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) and the corresponding Ki values were calculated using the equation of Cheng and Prusoff (1973). Values for EC50, maximal effect (Emax) and SEM or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism). The dissociation rate constant for [3H]8-OH-DPAT was calculated using a one-phase exponential decay equation (GraphPad Prism).
Results
CBD-induced suppression of toxin-induced vomiting in shrews was reversed by pretreatment with 5-HT1A receptor antagonist
CBD suppressed nicotine- and LiCl-induced vomiting in S. murinus, effects which were reversed by pretreatment with the 5-HT1A receptor antagonist WAY100135. Figure 1 shows the mean number of vomiting episodes elicited by nicotine (top half) and LiCl (bottom half) for each pretreatment group. The pretreatment group effect was significant for both the nicotine-treated shrews, F (3, 36) = 5.2, P < 0.01, and the LiCl-treated shrews, F (3, 50) = 5.6, P < 0.01. In each experiment, Group saline-CBD displayed significantly less vomiting than any other group (P < 0.05), which did not differ among themselves.
Figure 1.
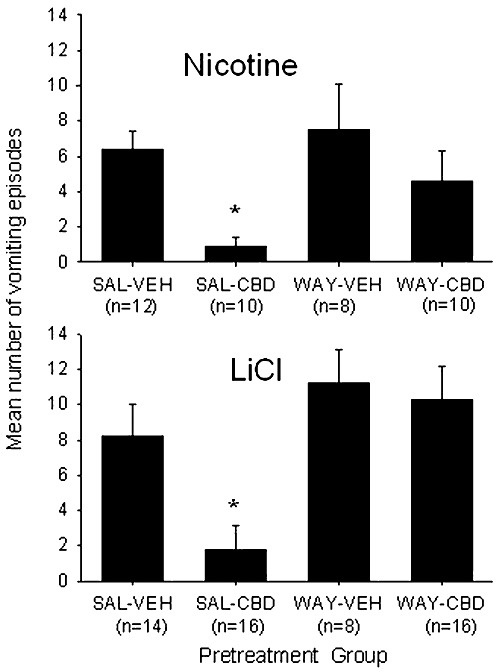
Mean (±SEM) number of vomiting episodes elicited by nicotine (5 mg·kg−1, s.c.) and LiCl (390 mg·kg−1, i.p.), among shrews pretreated with saline (SAL) or WAY100135 (10 mg·kg−1, i.p.) and vehicle (VEH) or CBD (5 mg·kg−1, sc). *P < 0.05, significantly different from all other groups.. The number of shrews in each condition is indicated in parentheses below pretreatment group name. The male (M) to female (F) (M : F) ratio within each treatment group for nicotine treated groups is saline-vehicle (7:5), saline-CBD (6:4), WAY-vehicle (4:4), WAY-CBD (6:4) and shrew age at the time of experimentation ranged from 44 days to 178 days with a mean of 96 days. The M : F ratio within each treatment group for LiCl treated groups is: saline-vehicle (6:8), saline-CBD (8:8), WAY-vehicle (4:4) and WAY-CBD (8:8).
CBD also suppressed vomiting produced by 20 mg·kg−1 of cisplatin, but not 40 mg·kg−1 of cisplatin, with the former effect prevented by pretreatment with WAY100135. Figure 2 presents the mean number of vomiting episodes elicited by 20 mg·kg−1 of cisplatin (top panels A) and 40 mg·kg−1 cisplatin (bottom panels B) displayed by the shrews pretreated with saline (left-hand sections) or WAY100135 (right-hand sections) prior to an injection of vehicle, 5 mg·kg−1 CBD or 10 mg·kg−1 CBD. Among shrews administered 20 mg·kg−1 cisplatin, but not 40 mg·kg−1, the 3 × 2 anova revealed a significant interaction, F (2, 47) = 4.3, P < 0.05. For the group treated with 20 mg·kg−1 cisplatin, among the saline-pretreated groups but not the WAY100135 pretreated groups, both groups pretreated with CBD (5 or 10 mg·kg−1) displayed fewer vomiting episodes (P < 0.05) than the vehicle-pretreated group. CBD did not interfere with vomiting produced by the higher dose of cisplatin (40 mg·kg−1).
Figure 2.
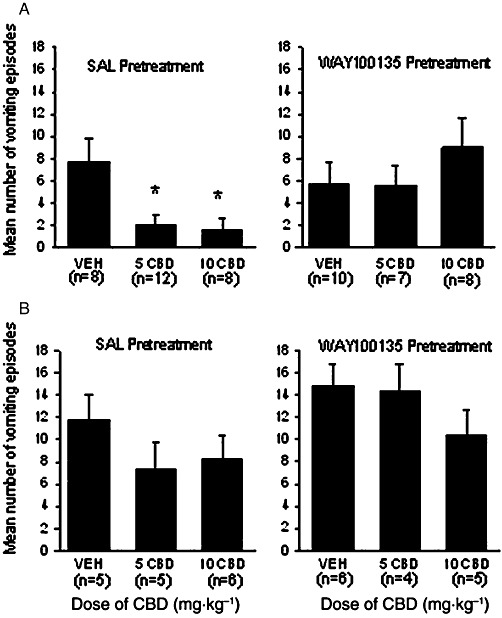
Mean (±SEM) number of vomiting episodes elicited by 20 mg·kg−1 cisplatin (Panel A) or 40 mg·kg−1 cisplatin (Panel B) by shrews pretreated with saline (SAL; right-hand sections) or WAY100135 (10 mg·kg−1, i.p.; left-hand sections) prior to the second pretreatment of vehicle (VEH), 5 mg·kg−1 CBD or 10 mg·kg−1 CBD. *P < 0.05, significant difference between other groups. The number of shrews in each group is indicated in parentheses. The M : F ratio within each treatment group for 20 mg·kg−1 cisplatin treated shrews in Panel A is: saline-vehicle (4:4), saline-5 CBD (6:6), saline-10 CBD (5:3), WAY100135-vehicle (5:5), WAY100135-5 CBD (4:3), WAY100135-10 CBD (3:5). For Panel B the M : F ratio is: saline-vehicle (3:2), saline-5 CBD (2:3), saline-10 CBD (3:3), WAY100135-vehicle (3:3), WAY100135-5 CBD (2:2) and WAY100135-10 CBD (2:3).
Systemic CBD-induced suppression of LiCl-induced conditioned gaping in rats was reversed by systemic pretreatment with 5-HT1A receptor antagonists
Systemic administration of the 5-HT1A receptor antagonists, WAY100135 and WAY100635, prevented the anti-nausea-like effect of CBD in rats, just as WAY100135 prevented the anti-emetic effects of CBD in shrews. As seen in Figure 3, the 2 × 3 anova revealed a significant interaction, F (2, 47) = 3.4, P < 0.05; only rats that were pretreated with saline-CBD displayed a suppression of LiCl-induced conditioned gaping reactions during the drug-free test (P < 0.05). Groups pretreated with either antagonist before CBD did not display suppressed conditioned gaping reactions.
Figure 3.
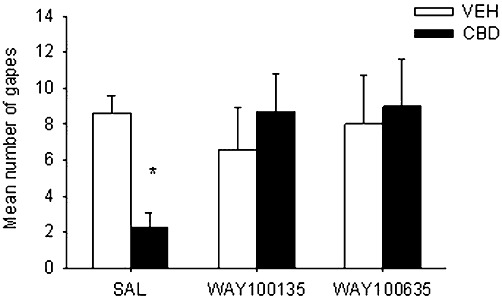
Mean (±SEM) number of gapes elicited by LiCl-paired saccharin solution during the drug-free test trial. During conditioning, rats were pretreated with systemic saline (SAL), WAY100135 (10 mg·kg−1, i.p.) or WAY100635 (0.1 mg·kg−1, i.p.) 15 min prior to systemic vehicle (VEH) or CBD (5 mg·kg−1, s.c.). *P < 0.05, significant difference.
Intra-DRN WAY100635 reversed the suppressive effect of systemic CBD on conditioned gaping in rats
The accurate injector tip placements (circles) are presented in Figure 4A. The tips of the injectors were located in the DRN between 2.04 and 0.84 mm anterior to the inter-aural line for a total of 29 rats. A total of 20 rats had placements outside the DRN. The rats in group WAY-CBD with inaccurate placements (triangles) were included in the analysis as a separate group (WAY-CBD-OUT; n= 5) for comparison with those receiving the antagonist in the DRN. Figure 4B presents a representative photomicrograph of the tip/track of the injector in the DRN.
Figure 4.
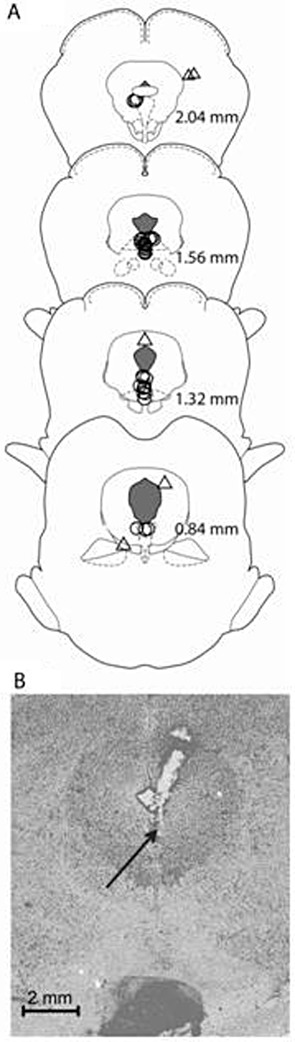
(A) Traces of infusion sites in the DRN (circles) and out of the DRN for animals treated with WAY-CBD (triangles) on drawings of coronal sections. Numbers indicate sections relative to inter-aural zero. (B) A representative photomicrograph of the tip/track of the injector in the DRN.
When administered intracranially to the DRN, the 5-HT1A receptor antagonist WAY100635 prevented the suppression of LiCl-induced gaping by CBD. As seen in Figure 5, there was a significant effect of pretreatment group, F (4, 28) = 4.4; Groups saline-CBD and WAY-CBD-OUT displayed fewer gaping reactions than all other groups (P < 0.05).
Figure 5.
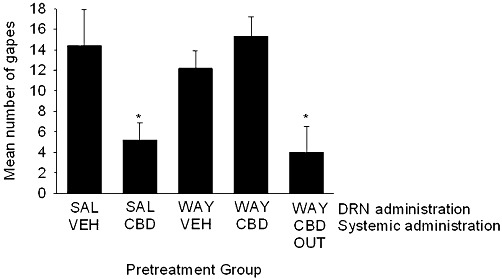
Mean (±SEM) number of gapes elicited by LiCl-paired saccharin solution during the drug-free test. During conditioning, rats were pretreated with intracranially administered saline or WAY100635 (21 ng) into the DRN 15 min prior to systemic vehicle or CBD (5 mg·kg−1, s.c.) *P < 0.05, significant difference.
Intra-DRN administration of CBD-induced suppression of LiCl-induced conditioned gaping, reversal by systemic administration of 5-HT1A antagonist
The injector tip placements (circles) are presented in Figure 6A. The tips of the injectors were located in the DRN between 2.04 and 0.84 mm anterior to the inter-aural line for a total of 24 rats. Only one rat in the CBD-saline group had his guide cannula placed outside of the DRN and this rat did not show the CBD-induced suppression of gaping but was not included in the analysis. Figure 6B presents a representative photomicrograph of the tip/track of the injector in the DRN.
Figure 6.
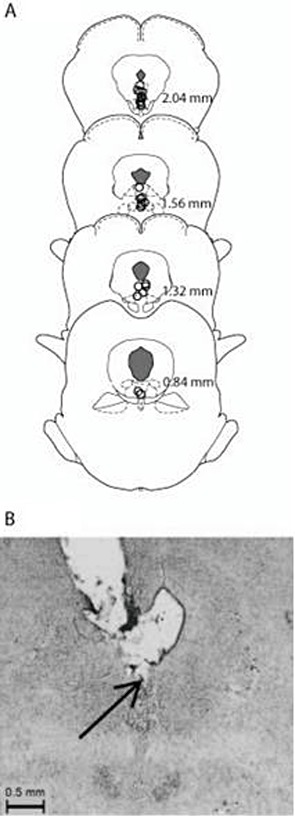
(A) Traces of infusion sites in the DRN for all groups (circles). Numbers indicate sections relative to inter-aural zero. (B) A representative photomicrograph of the tip/track of the injector in the DRN.
When administered intracranially to the DRN, CBD suppressed LiCl-induced gaping and this effect was blocked by systemic administration of the 5-HT1A receptor antagonist WAY100635. As seen in Figure 7, there was a significant effect of pretreatment group, F (3,20) = 11.0, P < 0.001; Group CBD-saline displayed fewer gaping reactions than all other groups (P < 0.001).
Figure 7.
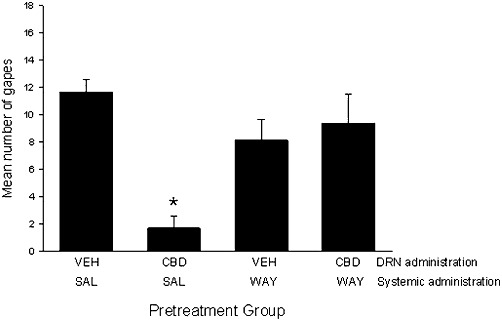
Mean (±SEM) number of gapes elicited by LiCl-paired saccharin solution during the drug-free test. During conditioning, rats were pretreated with systemic saline (SAL) or WAY100635 (0.1 mg·kg−1, i.p.) 15 min before intracranially administered vehicle (VEH) or CBD (10 µg) into the DRN. *P < 0.001, significant difference.
CBD enhanced the ability of a 5-HT1A receptor agonist to stimulate [35S]GTPγS binding to rat brainstem membranes
There is already evidence that CBD, albeit at the rather high concentration of 16 µM, can directly bind to and activate human 5-HT1A receptors that have been transfected into Chinese hamster ovary cells (Russo et al., 2005). However, the ability of lower concentrations of this cannabinoid to activate 5-HT1A receptors in vitro when they are expressed naturally at physiological levels in rat brainstem membranes has not been investigated before. Accordingly, we sought for evidence that CBD can directly target 5-HT1A receptors in rat brainstem when administered in vitro at concentrations ranging from 1 nM to 10 µM.
First, we compared the abilities of CBD and the 5-HT1A receptor-selective agonist, 8-OH-DPAT, to displace [3H]8-OH-DPAT from specific binding sites on rat brainstem membranes. These experiments showed that at concentrations of up to 10 µM, CBD does not share the ability of 8-OH-DPAT to induce such displacement (Figure 8). Since 5-HT1A receptors signal through Gi/o proteins (Alexander et al., 2008), we also compared the abilities of 8-OH-DPAT and CBD to stimulate [35S]GTPγS binding to rat brainstem membranes in a concentration-related manner. We found that 8-OH-DPAT can indeed induce such stimulation and, also, that this effect could be potently antagonized by the 5-HT1A receptor-selective antagonist, WAY100635 (Figure 9). In contrast, no detectable stimulation was observed in response to any of the concentrations of CBD used (Figure 9). We therefore decided to investigate whether the stimulatory effect of 8-OH-DPAT on [35S]GTPγS binding could be enhanced by CBD. This we did to explore the possibility that 5-HT1A receptor antagonists reduce CBD-induced anti-nausea like-effects because CBD augments activation of 5-HT1A receptors in the brainstem by endogenously released 5-HT. As shown in Figure 10, we found that at 100 nM, CBD produced an upward shift in the log concentration response curve of 8-OH-DPAT that resulted in a statistically significant increase in the Emax but not the EC50 of this 5-HT1A receptor-selective agonist. However, CBD did not increase the Emax of 8-OH-DPAT at 1, 10 or 31.6 nM or at 1 µM (Figure 10). We also found that 100 nM CBD caused [35S]GTPγS binding to rise significantly above the basal level (0) in the presence of 10−14 and 10−12 M 8-OH-DPAT, but not in the presence of 10−16 M 8-OH-DPAT (P < or >0.05; 1-sample t-test; n = 7). The mean values for percent stimulation of [35S]GTPγS binding by 100 nM CBD in the presence of 10−16, 10−14 and 10−12 M 8-OH-DPAT were 3.3 ± 1.5%, 9.5 ± 1.8% and 12.2 ± 2.7%, respectively.
Figure 8.
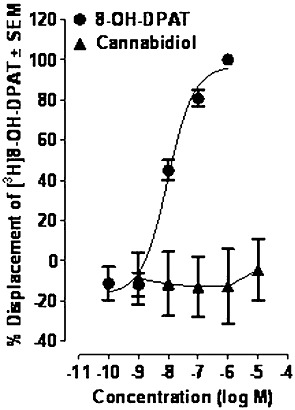
Effects of 8-OH-DPAT and CBD on specific binding of [3H]8-OH-DPAT to rat brainstem membranes (n= 6). The IC50 and Emax values of 8-OH-DPAT for its displacement of [3H]8-OH-DPAT, with 95% confidence limits shown in parentheses, were 9.6 nM (5.6 and 16.3 nM) and 96.6% (86.8 and 106.5%), respectively. Symbols represent mean values ± SEM.
Figure 9.
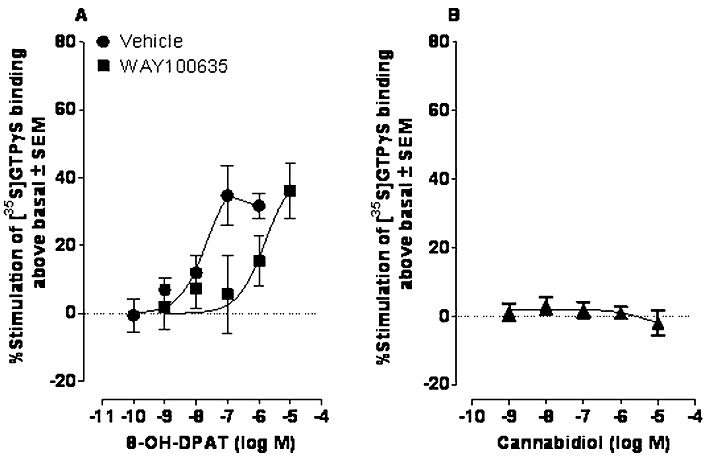
Effects of (A) 8-OH-DPAT in the presence of DMSO (vehicle; n= 7) or 100 nM WAY100635 (n= 7) and (B) CBD (n= 4) on [35S]GTPγS binding to rat brainstem membranes. Mean EC50 values for 8-OH-DPAT, with 95% confidence limits shown in parentheses were 21.7 nM (7.6 and 61.9 nM), in the presence of vehicle and 1565 nM (390 and 6277 nM) in the presence of WAY100635. Symbols represent mean values ± SEM.
Figure 10.
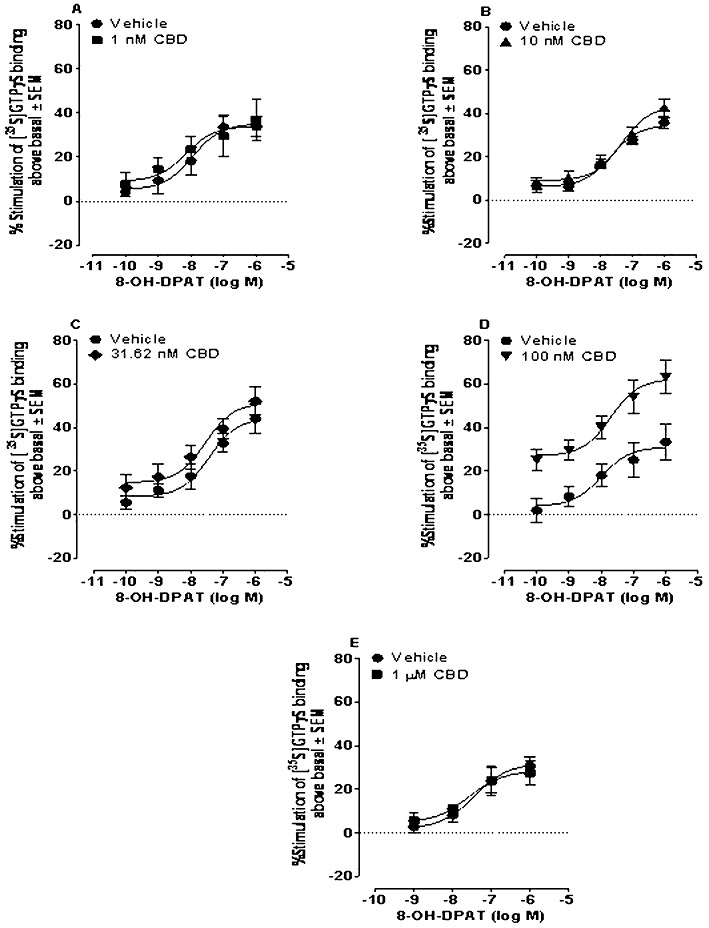
Effect of 8-OH-DPAT on [35S]GTPγS binding to rat brainstem membranes in the presence of DMSO (VEH) or CBD. Mean Emax values for 8-OH-DPAT in panels A, B, C, D and E with 95% confidence limits shown in parentheses were 35.1% (26.6 and 43.5%; n= 8), 34.8% (30.3 and 39.3%; n= 8), 44.3% (33.4 and 55.1%; n= 6), 32.4% (22.8 and 41.9%; n= 10) and 32.0% (22.9 and 41.1%; n= 9), respectively, in the presence of vehicle and 34.0% (22.2 and 45.9%; n= 8), 43.3% (35.5 and 51.0%; n= 6), 51.3% (39.3 and 63.3%; n= 6), 62.3% (51.0 and 73.6%; n= 10) and 28.4% (18.5 and 38.3%; n= 9), respectively, in the presence of 1nM, 10 nM, 31.6 nM, 100 nM or 1 µM CBD. Corresponding mean EC50 values for 8-OH-DPAT were 11.2 nM (1.9 and 66.7 nM), 22.0 nM (9.5 and 50.9 nM), 37.3 nM (8.1 and 173 nM), 12.7 nM (1.2 and 139 nM) and 37.4 nM (6.3 and 222 nM), respectively, in the presence of vehicle and 7.1 nM (0.4 and 126 nM), 45.7 nM (14.1 and 149 nM), 28.6 nM (40.5 and 180 nM), 19.7 nM (2.8 and 139 nM) and 26.2 nM (2.1 and 332 nM), respectively, in the presence of 1nM, 10 nM, 31.6 nM, 100 nM or 1 µM CBD. Symbols represent mean values ± SEM.
There is good evidence that some compounds that enhance the ability of agonists to activate certain G protein-coupled receptors do so by targeting allosteric sites on these receptors in a manner that slows the rate at which these agonists dissociate from their receptors (Christopoulos and Kenakin, 2002). Accordingly, because there is also evidence for the presence of an allosteric site on the 5-HT1A receptor (Barrondo and Salles, 2009), we investigated the ability of an 8-OH-DPAT-potentiating concentration of CBD (100 nM) to alter the rate at which [3H]8-OH-DPAT dissociated from specific binding sites in rat brainstem membranes. Our experiments showed that the mean dissociation rates of [3H]8-OH-DPAT in the presence of vehicle or of 100 nM CBD were not significantly different. More specifically, these values, with their 95% confidence limits shown in parentheses, were 10.0 min (7.1 and 17.0) and 6.0 min (4.4 and 9.4), respectively (n= 6).
CBD and 8-OH-DPAT acted synergistically to suppress LiCl-induced conditioned gaping in rats
Because the in vitro data suggested that at 100 nM CBD potentiated the stimulation of [35S]GTPγS binding of 8-OH-DPAT in rat brainstem tissue, we next evaluated the potential of subthreshold doses of CBD to potentiate the anti-nausea-like effect of 8-OH-DPAT. When given at half the optimal dose during conditioning (Parker et al., 2002; Limebeer and Parker, 2003), both pretreatments suppressed LiCl-induced conditioned gaping reactions. The upper half of Figure 11 presents the mean number of conditioned gaping reactions elicited on the test trial for each group in Experiment A. There was a significant effect of group F (3, 28) = 3.9, P < 0.025. All groups displayed fewer gaping reactions than vehicle-saline, with no other significant group differences.
Figure 11.
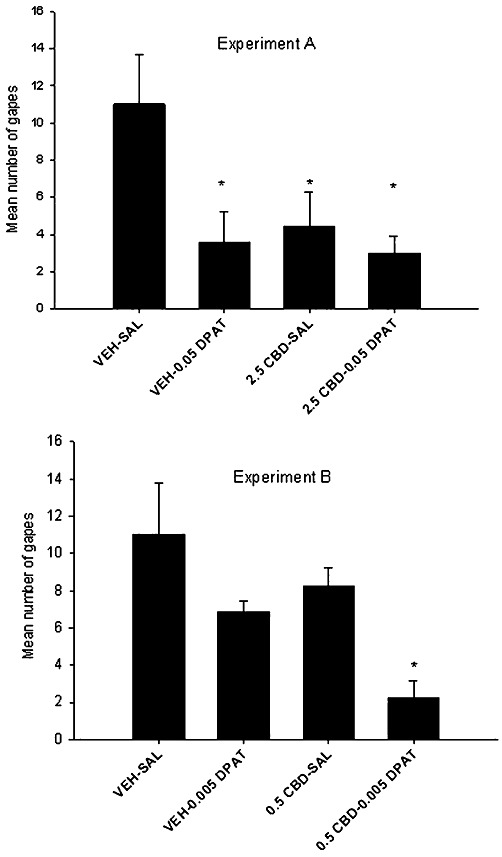
Mean (±SEM) number of gapes elicited by LiCl-paired saccharin solution during the drug-free test trial in Experiments A and B. During conditioning, rats were pretreated with systemic CBD (20.5 mg·kg−1 in Experiment A or 00.5 mg·kg−1 in Experiment B) 15 min prior to saline or 8-OH-DPAT (0.05 mg·kg−1 in Experiment A or 0.005 mg·kg−1 in Experiment B). Thirty minutes later, all rats were conditioned with 0.1% saccharin, followed immediately by LiCl. *P < 0.05., significant difference.
When the doses of CBD and 8-OH-DPAT were reduced such that each was ineffective in suppressing LiCl-induced conditioned gaping alone, the combined effect of these two doses interfered with the establishment of conditioned gaping reactions. The lower half of Figure 11 presents the mean number of conditioned gaping reactions elicited on the test trial for each group in Experiment B. There was a significant pretreatment effect of group F (3,26) = 5.9, P < 0.01. As is apparent, group 0.5 CBD-0.005 8-OH-DPAT gaped significantly (P < 0.05) less than all other groups, with no other significant group differences.
Discussion
Consistent with previous work, a low dose of systemically administered CBD suppressed nicotine-, LiCl- and cisplatin-induced vomiting in S. murinus (Kwiatkowska et al., 2004; Parker et al., 2004) and LiCl-induced conditioned gaping in rats (Parker et al., 2002). The CBD-induced suppression of vomiting and conditioned gaping was attenuated by pretreatment with 5-HT1A receptor antagonists (WAY100135 and WAY100635). Most interestingly, when WAY100635 was infused directly into the DRN, but not in adjacent structures, the CBD-induced suppression of gaping was also attenuated. The effectiveness of CBD itself to suppress nausea-like behaviours when administered to the DRN and the reversal of this effect by systemic administration of WAY100635 provide additional evidence that the anti-nausea-like action of CBD is produced by its action on 5-HT1A receptors in the DRN. As the DRN is a site of the somatodendritic 5-HT1A autoreceptors, stimulation of which reduces the firing rate of 5-HT afferents to terminal regions (Verge et al., 1985; Sotelo et al., 1990), these results suggest that CBD may exert its anti-nausea-like effects by reducing the firing rate of 5-HT afferents to terminal forebrain regions. It is likely that these effects were mediated by CBD-induced augmentation of the action of endogenous 5-HT on the 5-HT1A receptor because CBD enhanced the action of 8-OH-DPAT in both in vitro (% stimulation of [35S]GTPγS binding above basal) and in vivo (conditioned gaping) experiments, but did not have a direct agonist action on the 5-HT1A receptor in the brainstem preparation.
Although rats (unlike shrews) are not capable of vomiting, they do exhibit conditioned gaping reactions in response to oral infusion of a flavour that has previously been paired with illness (Grill and Norgren, 1978a,b). These gaping reactions are only produced by drugs that induce vomiting in other emetic species, such as shrews (Parker, 2003; Parker et al., 2008). In fact, Travers and Norgren (1986) suggest that the muscular movements involved in the gaping response mimic those seen in species capable of vomiting. Previously, we found that the anti-emetic drug, ondansetron (a 5-HT3 receptor antagonist), suppressed LiCl-induced conditioned gaping in rats, presumably by reducing the nausea produced by the emetic treatment (Limebeer and Parker, 2000). Consistent with the findings of the present study, the 5-HT1A receptor agonist, 8-OH-DPAT, also interfered with LiCl- (Limebeer and Parker, 2003) and fluoxetine-induced gaping in rats (Limebeer et al., 2009) as well as toxin-induced vomiting in other species (see. Lucot and Crampton, 1989; Andrews et al., 1996; Javid and Naylor, 2006). Of most relevance, depletion of forebrain 5-HT induced by 5,7-dihydroxytryptamine (5,7-DHT) lesions of the MRN and the DRN also prevented the LiCl-induced conditioned gaping reactions (Limebeer et al., 2004) that rely on an intact forebrain (Grill and Norgren, 1978a). Forebrain 5-HT may therefore be critical for the establishment of these nausea-like behaviours in rats.
The therapeutic effects of CBD on ischaemic injury (Hayakawa et al., 2004, 2007a; Mishima et al., 2005), hepatic encephalopathy (Magen et al., 2010), anxiety (Campos and Guimaraes, 2008; Gomes et al., 2011) and depression (Zanelati et al., 2010) are each attenuated by pretreatment with 5-HT1A antagonists. Here, we report that the CBD-induced suppression of vomiting in shrews and conditioned gaping in rats is also reversed by 5-HT1A antagonists. Furthermore, the site of action of the anti-nausea-like effect of CBD appears to be the somatodendritic 5-HT1A autoreceptors in the DRN, which have been reported to reduce the firing rate of 5-HT afferents to terminal forebrain regions (Verge et al., 1985; Sotelo et al., 1990). These results suggest that CBD may exert its anti-nausea-like effects by reducing the release of 5-HT in terminal forebrain regions (as yet to be identified).
Because we found that CBD enhances the ability of the 5-HT1A receptor-selective agonist, 8-OH-DPAT, to stimulate [35S]GTPγS binding to rat brain membranes with significant potency, CBD may suppress LiCl-induced conditioned gaping in rats by augmenting activation of 5-HT1A receptors in the brainstem produced by endogenously released 5-HT. Indeed, this hypothesis was supported by the synergistic effects of subthreshold doses of 8-OH-DPAT and CBD on suppression of LiCl-induced conditioned gaping in rats. Also, this hypothesis is strengthened further by our finding that the concentration–response curve of CBD for the production of its in vitro effect on [35S]GTPγS binding is bell-shaped, consistent with the 5-HT1A-mediated bell-shaped dose–response curves of its effects on emesis (Kwiatkowska et al., 2004), nausea-like-behaviour (Rock et al., 2008), ischaemic injury (Mishima et al., 2005), anxiety (Campos and Guimaraes, 2008) and depression (Zanelati et al., 2010).
Our finding that the concentration of CBD (100 nM) that potentiated 8-OH-DPAT in the [35S]GTPγS binding assay performed with rat brainstem membranes did not significantly alter the rate at which [3H]8-OH-DPAT dissociates from specific binding sites in these membranes does not support the hypothesis that 100 nM CBD potentiates 8-OH-DPAT-induced activation of 5-HT1A receptors in an allosteric manner. However, this hypothesis cannot yet be entirely excluded since it is also possible that the action of an agonist at its receptor could be enhanced by compounds that act on an allosteric site to increase the rate at which the agonist binds to its receptor and/or the intensity of receptor signalling that is induced by such binding (Christopoulos and Kenakin, 2002). That CBD might act in this way to potentiate 5-HT1A receptor activation by 8-OH-DPAT, or indeed by 5-HT, merits further investigation. It will also be important to establish whether CBD produces such potentiation by directly targeting 5-HT1A receptors/allosteric sites or by acting on a different target which then somehow augments 5-HT1A receptor activation by 8-OH-DPAT through an indirect mechanism. There is, therefore, a need for further experiments directed at investigating whether CBD can potentiate 8-OH-DPAT in a cell line that expresses only 5-HT1A receptors.
The anti-emetic potential of CBD not only depends upon its dose, but also upon the nature of the inducing stimulus. In Experiment 3, CBD suppressed vomiting produced by 20 mg·kg−1 cisplatin, but the suppression of vomiting was surmounted by the greater emetic efficiency of the higher dose of 40 mg·kg−1 of cisplatin, suggesting that CBD may not be as effective in reducing nausea produced by highly emetogenic therapies. Furthermore, CBD does not appear to attenuate vomiting produced by activation of the vestibular system by motion (Cluny et al., 2008). This inconsistency may be due to differing neuronal pathways involved in the induction of emesis by these emetogenic stimuli, with the vagal (cisplatin) and blood-borne (LiCl and nicotine) activation of the area postrema (Leslie and Reynolds, 1992) which is not essential to the development of motion sickness.
Interestingly, Yang et al. (2010) have reported that CBD acts as an allosteric inhibitor of 5-HT3A receptor-mediated currents in Xenopus laevis oocytes. Since 5-HT3 antagonists are highly effective anti-emetic/anti-nausea agents (Limebeer and Parker, 2003; Kwiatkowska et al., 2004), it is also likely that the allosteric inhibition of the 5-HT3 receptor may be important in the potential of CBD to regulate nausea and vomiting.
It is not clear which forebrain regions are critical for the effects of CBD on conditioned gaping reactions, but a likely candidate is the insular cortex; the site of convergence of gustatory and interoceptive information (Cechetto and Saper, 1987). Indeed, ablation of the rat insular cortex prevents the establishment of LiCl-induced gaping (Kiefer and Orr, 1992), unlike lesions of the basolateral or central amygdala (Rana and Parker, 2008). Electrical stimulation of the insular cortex produces vomiting in cats (Kaada, 1951) and humans (Catenoix et al., 2008), as well as a sensation of nausea in humans (Penfield and Faulk, 1955). Reversible lesions (lidocaine) of the rat insular cortex interfere with the unconditioned behaviour of lying on belly (see Parker, 1984) produced by LiCl (Contreras et al., 2007). In order to determine the role of 5-HT availability in the insular cortex on nausea, future studies will examine the potential of 5,7-DHT lesions of the insular cortex to prevent conditioned gaping reactions in rats.
Acknowledgments
The authors would like to thank Katharine Tuerke and Martin Sticht for assistance with the experiments and Linda Groocock for maintaining the shrew colony. The research reported here was supported by a grant from the Natural Sciences and Engineering Research Council to LAP, a scholarship from NSERC to ER, funding from the National Institute of Drug Abuse (US) to RM and RGP (DA-03672 and DA-09789) and funding from GW Pharmaceuticals to DB, MGC, SAG and RGP.
Glossary
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- CCAC
Canadian Council on Animal Care
- CBD
cannabidiol
- Δ9-THC
Δ9-tetrahydrocannabinol
- DRN
dorsal raphe nucleus
- MRN
median raphe nucleus
- TR
taste reactivity
- WAY100135
(S)-N-tert-butyl-3-(4-(2-methoxyphenyl)-piperazin-1-yl)-2-phenylpropanamide
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1 piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate
Conflicts of interest
None.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur J Pharmacol. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- Barrondo S, Salles J. Allosteric modulation of 5-HT(1A) receptors by zinc: binding studies. Neuropharmacology. 2009;56:455–462. doi: 10.1016/j.neuropharm.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Carli M, Afkhami-Dastjerdian S, Reader TA. 3H]8-OH-DPAT binding and serotonin content in rat cerebral cortex after acute fluoxetine, desipramine, or pargyline. J Psychiatry Neurosci. 1996;21:114–122. [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenoix H, Isnard J, Guenot M, Petit J, Remy C, Mauguiere F. The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Quantification of allosteric interactions at G protein-coupled receptors using radioligand binding assays. In: Enna SJ, editor. Current Protocols in Pharmacology. New York: John Wiley & Sons; 2000. pp. 1.22.1–1.22.40. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Naylor RJ, Whittle BA, Javid FA. The effects of cannabidiol and tetrahydrocannabinol on motion-induced emesis in Suncus murinus. Basic Clin Pharmacol Toxicol. 2008;103:150–156. doi: 10.1111/j.1742-7843.2008.00253.x. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Darmani NA. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol Biochem Behav. 2001;69:239–249. doi: 10.1016/s0091-3057(01)00531-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Crim J, Ramirez J. Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur J Pharmacol. 2007;563:187–196. doi: 10.1016/j.ejphar.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, et al. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimaraes FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl) 2011;213:465–473. doi: 10.1007/s00213-010-2036-z. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978a;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978b;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Sharma SS. Involvement of 5-HT1A and 5-HT2 receptor in cisplatin induced emesis in dogs. Indian J Physiol Pharmacol. 2002;46:463–467. [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Abe K, Hasebe N, Takamatsu F, Yasuda H, et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–2385. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem. 2007a;102:1488–1496. doi: 10.1111/j.1471-4159.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Ogata A, Hazekawa M, Liu AX, et al. Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology. 2007b;52:1079–1087. doi: 10.1016/j.neuropharm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Herges S, Taylor DA. Modulation of cocaine-induced locomotor activity, rears and head bobs by application of WAY100635 into the dorsal and median raphe nuclei of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:129–134. doi: 10.1007/s002109900058. [DOI] [PubMed] [Google Scholar]
- Javid FA, Naylor RJ. The effect of the 5-HT1A receptor agonist, 8-OH-DPAT, on motion-induced emesis in Suncus murinus. Pharmacol Biochem Behav. 2006;85:820–826. doi: 10.1016/j.pbb.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24:1–262. [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;174:254–259. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJM. Functional anatomy of the emetic circuitry in the brainstem. In: Bianchi AL, Grelot L, Miller AD, King GL, editors. Mechanisms and Control of Emesis. London: Colloqu INSERM; 1992. pp. 19–27. [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The 5-HT1A agonist 8-OH-DPAT dose-dependently interferes with the establishment and the expression of lithium-induced conditioned rejection reactions in rats. Psychopharmacology (Berl) 2003;166:120–126. doi: 10.1007/s00213-002-1309-6. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA, Fletcher PJ. 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Neurosci. 2004;118:1391–1399. doi: 10.1037/0735-7044.118.6.1391. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Litt DE, Parker LA. Effect of 5-HT3 antagonists and a 5-HT(1A) agonist on fluoxetine-induced conditioned gaping reactions in rats. Psychopharmacology (Berl) 2009;203:763–770. doi: 10.1007/s00213-008-1421-3. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri V, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, et al. Peripheral inverse agonism of CB1 receptors potentiates LiCl-induced nausea: evidence from the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucot JB, Crampton GH. 8-OH-DPAT suppresses vomiting in the cat elicited by motion, cisplatin or xylazine. Pharmacol Biochem Behav. 1989;33:627–631. doi: 10.1016/0091-3057(89)90399-7. [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 2010;159:950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Hanus L. Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects. Chem Phys Lipids. 2002;121:35–43. doi: 10.1016/s0009-3084(02)00144-5. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol – recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Millan-Aldaco D, Palomero-Rivero M, Mechoulam R, Drucker-Colin R. The nonpsychoactive cannabis constituent cannabidiol is a wake-inducing agent. Behav Neurosci. 2008;122:1378–1382. doi: 10.1037/a0013278. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Chaput C, Gavaudan S, Verriele L, Millan MJ. Agonist and antagonist actions of (-)pindolol at recombinant, human serotonin1A (5-HT1A) receptors. Neuropsychopharmacology. 1998;18:395–398. doi: 10.1016/S0893-133X(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Okada F, Torii Y, Saito H, Matsuki N. Antiemetic effects of serotonergic 5-HT1A-receptor agonists in Suncus murinus. Jpn J Pharmacol. 1994;64:109–114. doi: 10.1254/jjp.64.109. [DOI] [PubMed] [Google Scholar]
- Parker LA. Behavioral conditioned responses across multiple conditioning/testing trials elicited by lithium- and amphetamine-paired flavors. Behav Neural Biol. 1984;41:190–199. doi: 10.1016/s0163-1047(84)90569-7. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL. Cannabinoids in the management of nausea and vomiting. In: Köfalvi A, editor. Cannabinoids and the Brain. New York: Springer; 2008. pp. 259–273. [Google Scholar]
- Parker LA, Mechoulam R, Schlievert C. Cannabidiol, a non-psychoactive component of cannabis and its synthetic dimethylheptyl homolog suppress nausea in an experimental model with rats. Neuroreport. 2002;13:567–570. doi: 10.1097/00001756-200204160-00006. [DOI] [PubMed] [Google Scholar]
- Parker LA, Mechoulam R, Schlievert C, Abbott L, Fudge ML, Burton P. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology (Berl) 2003;166:156–162. doi: 10.1007/s00213-002-1329-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;171:156–161. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rock EM, Litt DL, Kwiatkowska M, Piomelli D. The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew) Physiol Behav. 2009;97:121–124. doi: 10.1016/j.physbeh.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 2nd edn. New York: Academic Press; 1986. [Google Scholar]
- Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology and therapeutic potential of cannabidiol. In: Di Marzo V, editor. Cannabinoids. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 32–83. [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA, Craib SJ, Thomas A. (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Rana SA, Parker LA. Differential effects of neurotoxin-induced lesions of the basolateral amygdala and central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behav Brain Res. 2008;189:284–297. doi: 10.1016/j.bbr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764–14769. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology. 2008;196:389–395. doi: 10.1007/s00213-007-0970-1. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007;4:1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci. 1986;100:544–555. doi: 10.1037//0735-7044.100.4.544. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. Antiemetic effects of 5-HT1A agonists in the pigeon. Pharmacol Biochem Behav. 1994;49:385–391. doi: 10.1016/0091-3057(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Yang KH, Galadari S, Isaev D, Petroianu G, Shippenberg TS, Oz M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydrosytryptamine 3A receptor-mediated currents in Xenopus laevis oocytes. J Pharmacol Exp Ther. 2010;222:547–554. doi: 10.1124/jpet.109.162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelati TV, Biojone C, Moreira FA, Guimaraes FS, Joca SR. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


