Abstract
BACKGROUND AND PURPOSE
The prevalence of heart disease continues to rise, particularly in subjects with insulin resistance (IR), and improved therapies for these patients is an important challenge. In this study we evaluated cardiac function and energy metabolism in IR JCR:LA-cp rat hearts before and after treatment with an inotropic compound (glucagon), a glucagon-like peptide-1 (GLP-1) receptor agonist (ZP131) or a glucagon-GLP-1 dual-agonist (ZP2495).
EXPERIMENTAL APPROACH
Hearts from IR and lean JCR:LA rats were isolated and perfused in the working heart mode for measurement of cardiac function and metabolism before and after addition of vehicle, glucagon, ZP131 or ZP2495. Subsequently, cardiac levels of nucleotides and short-chain CoA esters were measured by HPLC.
KEY RESULTS
Hearts from IR rats showed decreased rates of glycolysis and glucose oxidation, plus increased palmitate oxidation rates, although cardiac function and energy state (measured by ATP/AMP ratios) was normal compared with control rats. Glucagon increased glucose oxidation and glycolytic rates in control and IR hearts, but the increase was not enough to avoid AMP and ADP accumulation in IR hearts. ZP131 had no significant metabolic or functional effects in either IR or control hearts. In contrast, ZP2495 increased glucose oxidation and glycolytic rates in IR hearts to a similar extent to that of glucagon but with no concomitant accumulation of AMP or ADP.
CONCLUSION AND IMPLICATIONS
Whereas glucagon compromised the energetic state of IR hearts, glucagon-GLP-1 dual-agonist ZP2495 appeared to preserve it. Therefore, a glucagon-GLP-1 dual-agonist may be beneficial compared with glucagon alone in the treatment of severe heart failure or cardiogenic shock in subjects with IR.
Keywords: glucagon, GLP-1, glucagon-GLP-1 dual-agonist, insulin-resistance, cardiac metabolism, cardiac function, cardiac energetics, inotropic compounds, working hearts, diabetic cardiomyopathy
Introduction
Insulin resistance (IR) is extremely common in our society; 150 million people worldwide and an estimated 43 million people in the USA are currently affected by type 2 diabetes mellitus or metabolic syndrome (Cook et al., 2005). Heart disease is prevalent in subjects with metabolic syndrome and type 2 diabetes, and many of these patients do not survive their first cardiovascular event (Buse et al., 2007). Despite significant improvements in the management of heart disease in the general population, specific therapies for subjects with IR remain elusive. A decrease in the metabolism of glucose accompanied by an increased fatty acid oxidation in the heart appears to be an important link between IR and cardiac dysfunction (Lopaschuk, 2002). Therefore, a possible therapeutic approach for the treatment of heart disease in subjects with the metabolic syndrome or type 2 diabetes could be to increase cardiac glucose uptake and metabolism.
Glucagon-like peptide-1 (GLP-1), a 30-amino-acid hormone produced in the intestinal epithelial endocrine L cells by differential processing of proglucagon (Holst, 2007), has been found to increase myocardial glucose uptake in an insulin-independent manner in normal and post-ischaemic rat hearts (Zhao et al., 2006), isolated mouse hearts (Ban et al., 2008) as well as in conscious dogs with dilated cardiomyopathy (Nikolaidis et al., 2004b; 2005). Moreover, GLP-1 reduces infarct size following global ischaemia in isolated rat hearts (Ossum et al., 2009). Preliminary clinical studies have shown that GLP-1 infusion can improve regional and/or global left ventricular function in patients with acute myocardial infarction (Nikolaidis et al., 2004a), and that GLP-1 infusion improves left ventricular function, functional status and quality of life in subjects with severe heart failure (Sokos et al., 2006). (For a recent review on cardiovascular actions of GLP-1, see Grieve et al., 2009).
Positive inotropic agents are often used clinically to improve haemodynamic parameters and relieve symptoms in patients with acute decompensated heart failure or cardiogenic shock. However, both acute and continuous administration of inotropic agents have also been associated with increased mortality (White, 1999; Hamad et al., 2007). Glucagon has well-documented inotropic and chronotropic effects (Farah and Tuttle, 1960; Levey and Epstein, 1969; Mayer et al., 1970; Buse et al., 1973), although the effects of glucagon on cardiac substrate metabolism in the diseased heart have not been fully elucidated.
We hypothesized that increased mortality following treatment with positive inotropic agents could at least partly be explained by a decreased ability of the diseased hearts to increase their energy production in response to increases in energy demand. Therefore, a drug with both inotropic and metabolic effects could potentially have beneficial effects compared with those of inotropic compounds currently used in the clinic. Accordingly, two groups of peptides (GLP-1 receptor agonists and glucagon-GLP-1 dual-agonists) have been developed and are being evaluated as putative strategies to improve cardiac metabolism during IR or other pathophysiological conditions, such as severe heart failure or cardiogenic shock, where an acute increase in cardiac performance is needed.
As a model for IR, we used JCR:LA-cp rats; these rats contain a mutation in the leptin receptor gene, and homozygous (cp/cp) rats are characterized by the absence of any apparent leptin signalling, which results in obesity, hyperlipidaemia and impaired systemic insulin signalling (Russell et al., 2007). Furthermore, the hearts of cp/cp rats require high doses of insulin to maintain mechanical function (Lopaschuk and Russell, 1991). In contrast, heterozygous and homozygous normal JCR:LA rats are lean and metabolically normal.
Here we showed that the inotropic compound, glucagon and a glucagon-GLP-1 dual-agonist (ZP2495) have similar effects on cardiac function and metabolic rates in hearts from both lean and IR JCR:LA rats. However, whereas the glucagon compromised the energetic state of IR hearts, the glucagon-GLP-1 dual-agonist ZP2495 preserved cardiac energy.
Methods
Experimental animals
Male JCR:LA-cp rats, cp/cp (IR) and +/? (controls; a 2:1 mix of cp/+ and +/+), were bred and maintained in our established rat colony (Russell and Amy, 1986) and housed in an isolated HEPA-filtered caging system (Techniplast S.p.a., Buguggiate, Italy). All food was Lab Diet 5001 (PMI Nutrition International Inc., Brentwood, MO). Rats used for this study were all 12–14 weeks of age. All animal care and experimental procedures complied with the guidelines of the Canadian Council on Animal Care and were approved by the Institute's Ethical Committee.
Peptide synthesis
The peptides (ZP131: H-HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGASKKKKKK-NH2, glucagon: H-HSQGTFTSDYSKYLDSRRAQDFVQWLMNT-OH, ZP2495: H-HSQGTFTSDYSKYLDRARADDFVAWLKST-NH2) were synthesized using standard Fmoc chemistry. The peptides were purified by preparative HPLC using trifluoroacetic acid–water/acetonitrile as buffers. Characterization of the peptides was done using analytical HPLC and mass spectrometry. The theoretic peptide content of the trifluoroacetic acid salts was used when calculating concentrations.
Glucagon and GLP-1 receptor potency and efficacy assay
HEK293 cells expressing the human glucagon receptor, or human GLP-1 receptor were seeded at 40 000 cells per well in 96-well microtitre plates coated with 0.01% poly-l-lysine and grown for 1 day in culture in 100 µL growth medium (Dulbecco's Modified Eagles Medium, Gibco/Invitrogen,Taastrup, Denmark). On the day of analysis, growth medium was removed, and the cells were washed once with 200 µL Tyrode buffer (Sigma-Aldrich, Broendby, Denmark) + 0.1% casein (Casein Hammarsten Utrapure, USB Corporation, Cleveland, OH). Cells were incubated in 100 µL Tyrode buffer + 0.1% casein containing increasing concentrations (0.01–100 nM) of test peptides, 100 µM IBMX (Sigma-Aldrich) and 6 mM glucose for 15 min at 37°C. The reaction was stopped by addition of 25 µL 0.5 M HCl and incubated on ice for 60 min. The cAMP content was estimated using a [125I]-cAMP FlashPlate® assay kit (Perkin Elmer Life Sciences, Skovlunde, Denmark). EC50 values were estimated by computer aided curve fitting according to the following algorithm:
where cAMPMAX was the maximum amount of cAMP formed in presence of receptor agonist, cAMP0 was the amount of cAMP formed in absence of receptor agonist, s was the concentration of test substance and EC50 was the concentration of test substance at which 50% of the maximum amount of cAMP was obtained. The log means of EC50 values were calculated, because the statistical scattering follows a logarithmic distribution (Kenakin, 1981; 2001; 2004). Thus, the variances of EC50 values were estimated as the log ratio: 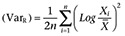 , where Xi is the ith value, and
, where Xi is the ith value, and 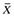 is the mean value. The VarR is equivalent to the square of the SD of the log ratio (SDR2) and the SEM log ratio:
is the mean value. The VarR is equivalent to the square of the SD of the log ratio (SDR2) and the SEM log ratio: 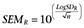 .
.
Isolated working hearts
Rats were anaesthetized with sodium pentobarbital (60 mg·kg−1 i.p.) (Bimeda®-MTC Animal Health, Inc., Cambridge, Ontario, Canada), and hearts were then quickly excised and immersed in ice-cold Krebs–Henseleit solution (118 mM NaCl, 25 mM NaHCO3, 5.9 mM KCl, 1.2 mM MgSO4·7H2O, 1.25 mM CaCl2·2H2O and 11 mM glucose). Spontaneously beating hearts were perfused in the working heart mode as previously described (Lopaschuk and Barr, 1997), with a preload pressure of 11.5 mmHg, an afterload pressure of 80 mmHg and oxygenated Krebs–Henseleit solution containing 11 mM glucose, 2000 µU·mL−1 insulin (Novo Nordisk A/S, Bagsvaerd, Denmark), 1.25 mM free Ca2+ and 0.8 mM palmitate (Sigma, St. Louis, MO) bound to 3% BSA (Equitech-Bio, Inc. Kerrville, TX).
Measurement of cardiac function
Heart rate and aortic pressure were measured with a Gould P21 pressure transducer (Harvard Apparatus, Saint-Laurent QC, Canada) connected to the aortic outflow line. Cardiac output and aortic flow were measured with Transonic T206 flow probes (Ithaca, NY, USA) in the preload and afterload line, respectively. Cardiac work was calculated as the product of aortic peak systolic pressure and cardiac output. Data were collected using a MP100 system from AcqKnowledge (BIOPAC Systems, Goleta, CA, USA). At the end of the perfusion, hearts were quick frozen with tongs cooled to the temperature of liquid N2. Frozen hearts were powdered, and approximately 100 mg (wet weight) was dried at 60°C overnight to remove all water (dry weight). The ratio of this sample (dry/wet weight) was used to calculate the total dry mass of the heart.
Perfusion protocols
Protocol 1: 65 min of aerobic perfusion for measurements of cardiac function, glucose oxidation and palmitate oxidation. The hearts were allowed to stabilize for the first 10 min, followed by 10 min of baseline measurements. Vehicle (Krebs–Henseleit solution without glucose, BSA and fatty acid), ZP131, glucagon or ZP2495 were then added at increasing concentrations (10, 50 and 100 nM) at 20, 35 and 50 min of perfusion, respectively. Following drug administration, cardiac metabolism and function were allowed to stabilize for 5 min and thereafter measured over a 10 min period.
Protocol 2: 55 min of aerobic perfusion for measurements of cardiac function, glycolysis and glucose oxidation. Following a 10 min stabilization period, baseline measurements were performed for 20 min. Subsequently, 100 nM of glucagon or ZP2495 was added to the perfusate. Hearts were allowed to stabilize for 5 min, and metabolism and function were measured for the following 20 min.
Glucose oxidation rates
Glucose oxidation rate was determined by measuring 14CO2 released from the metabolism of [U-14C]- glucose (Amersham Radiochemicals, GE Healthcare, UK), as previously described (Lopaschuk and Barr, 1997). In brief, released 14CO2 was collected by continuously bubbling outflow gases from the perfusion apparatus through 15 mL of 1 M hyamine hydroxide (Mallinckrodt Baker Inc., Phillipsburg, NJ). Two 150 µL samples of hyamine hydroxide and a 2 mL perfusion buffer sample were collected at each sampling point. The 14CO2 trapped in the perfusion buffer was released by adding 1 mL of perfusion buffer into two sealed test tubes containing 1 mL of 9 M H2SO4. Test tubes were vortexed for 20 s and left overnight. The released 14CO2 was trapped in a hyamine hydroxide saturated filter paper. All hyamine hydroxide samples were counted with the use of Ecolite scintillation fluid (MB Biomedicals, Solon, OH) using standard counting procedures.
Glycolysis and palmitate oxidation rates
Glycolysis and palmitate oxidation were measured by perfusing hearts with [5-3H]-glucose or [9,10-3H]-palmitate (NEN Radiochemicals, Perkin Elmer Inc., Boston, MA), respectively. The total amount of myocardial 3H2O produced was measured by collecting 1 mL samples of the perfusion buffer at each sampling point and storing the buffer sample under mineral oil (Fisher Scientific, Fair Lawn, NJ) until further use. At the end of experiments, 3H2O from the perfusion buffer samples were separated from [3H]-glucose or [3H]-palmitate using the evaporation method: 0.2 mL of the buffer samples was added in duplicates into capless microfuge tubes placed into 7 mL scintillation vials containing 500 µL of ddH2O. For each assay, a standard 3H Krebs buffer containing approximately 0.01 mCi·mL−13H2O (Sigma) was prepared and added into capless microfuge tubes as described above and used to determine the percentage of 3H2O transferred to the 500 µL of ddH2O. All vials were capped and placed in a 50°C incubator for 24 h. Subsequently, all vials were transferred to a 4°C cold room overnight. Finally, the capless microfuge tubes were disposed of after dragging the tubes against the edge of the vial to remove excess 3H2O droplets leaving the remaining 3H2O in the scintillation vials. 200 µL of the standard 3H Krebs buffer and 200 µL of non-metabolized buffer were also placed into scintillation vials in duplicates. All vials were filled with Ecolite scintillation fluid and counted using standard counting procedures.
Calculation of ATP production
ATP production rates from glucose and fatty acid metabolism were calculated by using values of 2 and 36 mol of ATP produced mol−1 of glucose utilized for glycolysis and glucose oxidation, respectively. For palmitate oxidation, a value of 129 mol ATP produced mol−1 of palmitate oxidized was used.
Measurement of short chain CoA esters
Frozen rat heart tissue (∼30 mg) was homogenized in a 6% perchloric acid/1 mM 1,4-dithiothreitol (DTT) solution. The homogenate was incubated on ice for 10 min and centrifuged at 12 000×g for 5 min. The supernatant was collected and incubated on ice for 4–5 h. Short-chain CoA (acetyl CoA, malonyl CoA, succinyl CoA and free CoA) concentrations were determined by HPLC as described elsewhere (Ally and Park, 1992).
Measurement of nucleotides
Frozen rat heart tissue (∼30 mg) was homogenized in a 6% perchloric acid/1 mM DTT solution. The homogenate was incubated on ice for 10 min and centrifuged at 12 000×g for 5 min. 0.5 mM EGTA was added to each sample, and the pH was adjusted to 5–7 with 5 M K2CO3. The homogenate was incubated for an additional 30 min and centrifuged at 10 000×g for 2 min. The supernatant was collected, and nucleotide (AMP, ADP, ATP and GTP) concentrations were determined by HPLC as described elsewhere (Ally and Park, 1992).
Statistical analyses
Comparisons between control and IR JCR:LA-cp rats were performed using Student's unpaired t-tests. For a subset of data, where variances were significantly different between the two groups, an unpaired two-tailed t-test with Welch's correction was performed. Comparisons between different drug concentrations within a given group in protocol 1 were done with one-way anova for repeated measures followed by Dunnett's multiple comparison test, comparing all columns with the baseline column. Analysis of drug effects in protocol 2 was performed by use of Student's paired t-test. Differences were considered to be statistically significantly different at P < 0.05.
Results
General characteristics of control (+/?) and insulin-resistant (cp/cp) JCR:LA-cp rats
At 12 to 14 weeks of age, IR JCR:LA-cp rats were markedly obese compared with their control counterparts (+/?), and dry heart weight was also significantly increased (Table 1). This was a compensatory hypertrophy, as heart rate, cardiac output and cardiac power at a normal workload was not different between the two groups, and left ventricular developed pressure was actually slightly increased in hearts from IR rats compared with controls (Table 1). Both glycolysis and glucose oxidation were significantly decreased in hearts from IR rats, and a tendency towards an increased palmitate oxidation rate was seen in these hearts compared with control hearts (Table 1).
Table 1.
General characteristics of control (+/?) and IR (cp/cp) JCR:LA rats
| Control (+/?) | n | IR (cp/cp) | n | P | |
|---|---|---|---|---|---|
| Body weight (g) | 331 ± 9 | 28 | 517 ± 10 | 31 | <0.0001 |
| Dry heart weight (mg) | 0.234 ± 0.01 | 28 | 0.298 ± 0.01 | 31 | <0.0001 |
| Heart rate (beats min−1) | 262 ± 6 | 28 | 261 ± 5 | 31 | NS |
| Cardiac output (mL·min−1) | 44.0 ± 2.7 | 28 | 47.1 ± 1.8 | 31 | NS |
| Developed pressure (mmHg) | 48.7 ± 1.8 | 28 | 55.0 ± 2.5 | 31 | <0.05 |
| Cardiac power (J·min−1) | 632 ± 38 | 28 | 720 ± 38 | 31 | NS |
| Glycolysis (nmol·g−1 dry weight·min−1) | 2,528 ± 211 | 8 | 1,456 ± 203 | 11 | <0.01 |
| Glucose oxidation (nmol·g−1 dry weight·min−1) | 814 ± 75 | 28 | 486 ± 41 | 31 | <0.001 |
| Palmitate oxidation (nmol·g−1 dry weight·min−1) | 576 ± 60 | 16 | 742 ± 68 | 16 | 0.077 |
Data are presented as mean ± SEM. The glucose oxidation data showed significantly different variances between the two groups and were analysed by Student's unpaired two tailed t-test with Welch's correction. All other data were analysed by Student's unpaired t-test (NS: not statistically significantly different).
Effects of glucagon, ZP131 and ZP2495 on cardiac function
Glucagon is a known inotropic compound, which activates the glucagon receptor (MacLeod et al., 1981; White, 1999). In contrast, ZP131 is a full GLP-1 receptor agonist with similar potency as exendin-4 (Table 2), whereas ZP2495 is a dual-agonist, which is able to fully activate both the GLP-1 and glucagon receptors (as measured by cAMP formation), but with less potency than GLP-1 and glucagons, respectively (Table 2).
Table 2.
In vitro glucagon, ZP131 and ZP2495 potency and efficacy estimates
| GluR | GLP-1R | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peptide | n | EC50 (nM) | SEMR | EMAX (cAMP) (pmol·per well) | SEM | EC50 (nM) | SEMR | EMAX (cAMP) (pmol·per well) | SEM |
| Glucagon | 4 | 0.032 | 1.2 | 54.1 | 1.7 | 1.666 | 1.1 | 53.5 | 1.7 |
| GLP-1 | 4 | ∞ | 0 | 0.034 | 1/2 | 52.7 | 1.6 | ||
| Exendin-4 | 4 | ∞ | 0 | 0.027 | 1/1 | 53.1 | 2.0 | ||
| ZP131 | 4 | ∞ | 0 | 0.029 | 1.1 | 49.6 | 1.5 | ||
| Oxyntomodulin | 4 | 0.697 | 1.1 | 50.2 | 1.6 | 1.090 | 1.0 | 55.1 | 0.9 |
| ZP2495 | 4 | 0.794 | 1.0 | 50.2 | 0.9 | 0.412 | 1.0 | 54.9 | 0.6 |
Potency (EC50) and efficacy (EMAX) estimates of ZP131 and ZP2495 as well glucagon, two GLP-1 standard agonists (GLP-1 and exendin-4) and oxyntomodulin as standard glucagon-GLP-1 dual-agonist measured by glucagon receptor- and GLP-1 receptor- mediated cAMP formation. (SEMR: SEM log ratio; for further details, see Methods).
Figure 1 shows the effects of acute administration of glucagon, ZP131 and ZP2495 on cardiac function. During perfusions with vehicle, heart rate increased modestly in hearts from control rats, whereas cardiac output, developed pressure and cardiac power gradually decreased during the 65 min of perfusion (Figure 1). In hearts from IR rats, heart rate and cardiac output were stable throughout the perfusion, whereas developed pressure and cardiac power decreased over time (Figure 1).
Figure 1.
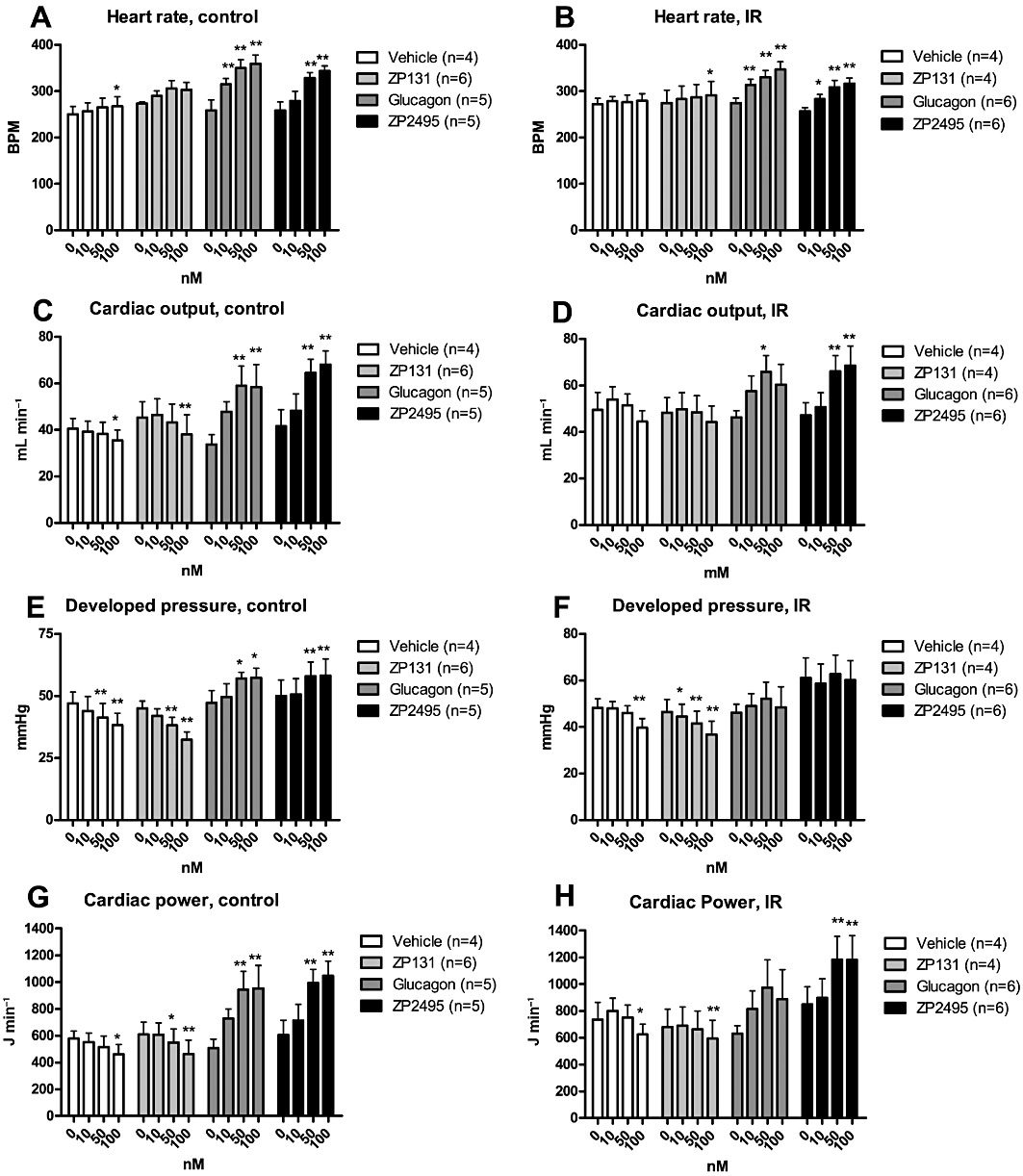
Effects of vehicle, a GLP-1 receptor agonist (ZP131), glucagon and a glucagon-GLP-1 dual-agonist (ZP2495) on (A) heart rate in control JCR:LA rat hearts; (B) heart rate in insulin-resistant (IR) JCR:LA rat hearts; (C) cardiac output in control hearts; (D) cardiac output in IR hearts; (E) developed pressure in control hearts; (F) developed pressure in IR hearts; (G) cardiac power in control hearts; and (H) cardiac power in IR hearts. Values are presented as mean + SEM. *P < 0.05; **P < 0.01 compared with baseline by one-way anova for repeated measures followed by Dunnett's multiple comparison test.
Adding 10, 50 or 100 nM of ZP131 to the perfusion buffer did not change cardiac function in hearts from either control or IR rats compared with vehicle-perfused hearts. In contrast, glucagon and ZP2495 increased heart rate, cardiac output, developed pressure and cardiac power in a concentration-dependent manner in control hearts. By comparison, in hearts from IR rats, both glucagon and ZP2495 increased heart rate and cardiac output; however, only ZP2495 significantly increased cardiac power, whereas both glucagon and ZP2495 prevented the decrease in developed pressure and cardiac power observed over time in hearts perfused with vehicle (Figure 1).
Effects of glucagon, ZP131 and ZP2495 on cardiac energy metabolism
Isolated working hearts in this study were perfused with energy substrates and insulin that mimicked the fatty acid, glucose and insulin conditions seen in the plasma of IR rats (i.e. 0.8 mM palmitate, 11 mM glucose and 2000 µU·mL−1 insulin). The perfusion condition resulted in stable glucose and palmitate oxidation rates throughout the 65 min of perfusion in hearts from both control and IR rats (Figure 2). ZP131 had no effect on glucose or palmitate oxidation in hearts from control rats (Figure 2A,C), but 100nM ZP131 tended to increase glucose oxidation in hearts from IR hearts, but this was not significant (P= 0.080) (Figure 2B). At higher concentrations (50 and 100 nM), both glucagon and ZP2495 increased glucose oxidation and palmitate oxidation rates in control hearts (Figure 2A,C). However, in hearts from IR rats, 50 nM glucagon, and 50 and 100 nM ZP2495 significantly increased the glucose oxidation rates (Figure 2B), whereas only ZP2495 was found to increase palmitate oxidation rates (Figure 2D).
Figure 2.
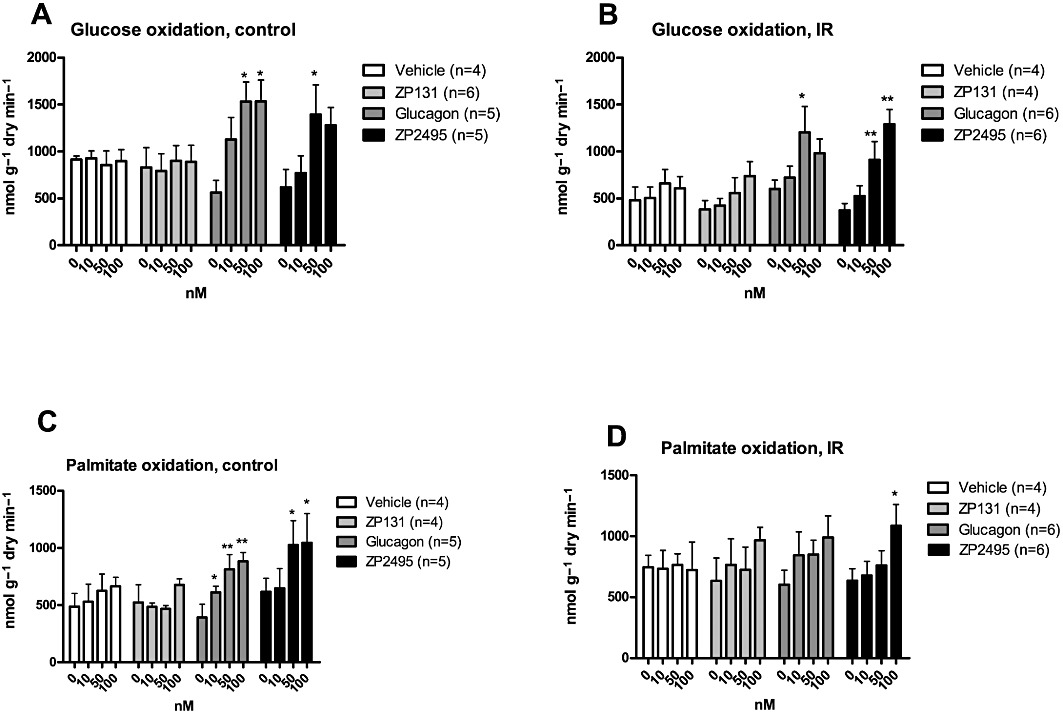
Effects of vehicle, a GLP-1 receptor agonist (ZP131), glucagon and a glucagon-GLP-1 dual-agonist (ZP2495) on (A) glucose oxidation rates in control hearts; (B) glucose oxidation rates in IR hearts; (C) palmitate oxidation rates in control hearts; and (D) palmitate oxidation rates in IR hearts. Values are presented as mean + SEM. *P < 0.05; **P < 0.01 compared with baseline by one-way anova for repeated measures followed by Dunnett's multiple comparison test.
In protocol 2, the effect of a single concentration (100 nM) of glucagon or ZP2495 was used to examine not only glucose and palmitate oxidation but also glycolytic rates. Similar to what was observed in hearts perfused with lower doses, both glucagon and ZP2495 significantly increased glucose oxidation rates in hearts from both control and IR rats (Figure 3A,B). Glucagon and ZP2495 also increased glycolytic rates in hearts from both control and IR rats (Figure 3C,D), although the effect of ZP2495 in control hearts did not reach statistical significance (P= 0.058).
Figure 3.
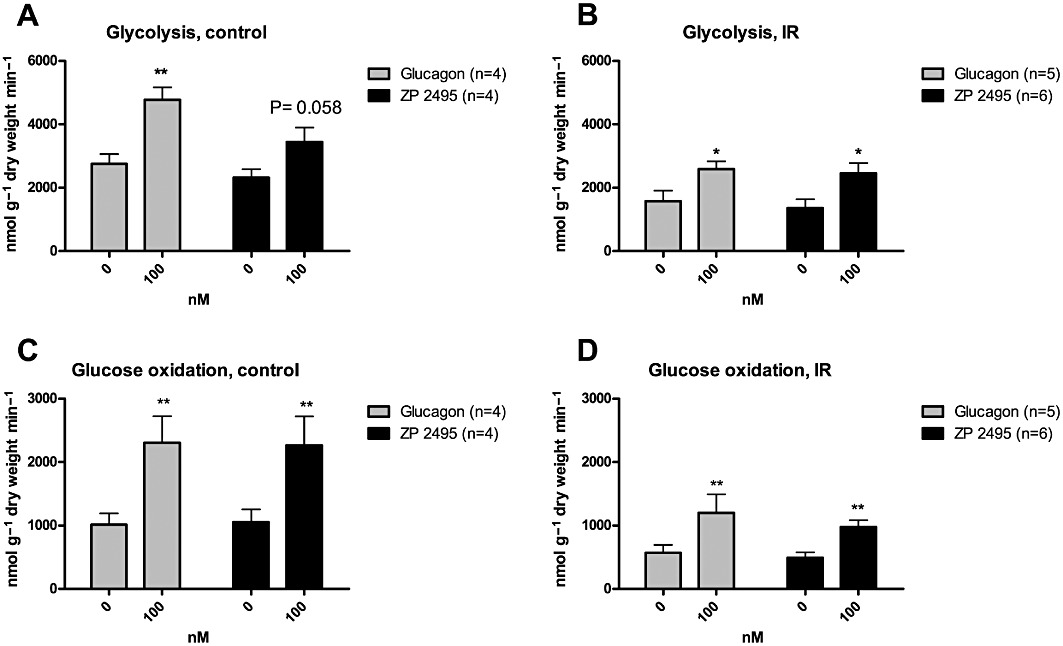
Effects of glucagon and a glucagon-GLP-1 dual-agonist (ZP2495) on (A) glucose oxidation rates in control hearts; (B) glucose oxidation rates in IR hearts; (C) glycolysis rates in control hearts; and (D) glycolysis rates in IR rat hearts. Values are presented as mean + SEM. *P < 0.05; **P < 0.01 compared with baseline by Student's paired t-test.
Based on the measured rates of glucose oxidation, glycolysis and palmitate oxidation, we calculated the amount of ATP produced from each metabolic pathway (Table 3). Under baseline conditions (absence of compounds), hearts from control rats obtained roughly two-thirds of their ATP production from fatty acid oxidation; whereas in IR rat hearts, the proportion was closer to three-quarters, suggesting an increased (but not statistically significant) contribution of fatty acid oxidation to overall energy production. Perfusion of hearts with 100 nM glucagon or ZP2495 increased the total ATP production in both control and IR hearts compared with baseline levels. However, in the IR hearts, glucagon caused a further increase in the proportion of ATP obtained from fatty acid oxidation, whereby the proportional energy contribution from fatty acid oxidation became significantly different between control and IR hearts (P < 0.04). The same tendency was observed for ZP2495, but this did not reach statistical significance.
Table 3.
ATP production in control (+/?) and insulin-resistant (IR) (cp/cp) JCR:LA rats
| Control (+/?) | n | IR (cp/cp) | n | |
|---|---|---|---|---|
| Glucose oxidation | ||||
| Baseline | 24 ± 2.9 | 18 | 22 ± 3.1 | 23 |
| Glucagon | 57 ± 7.6*** | 9 | 29 ± 4.8 | 11 |
| ZP2495 | 52 ± 8.3** | 9 | 30 ± 2.7* | 12 |
| Glycolysis | ||||
| Baseline | 5.1 ± 0.4 | 8 | 2.9 ± 0.4 | 11 |
| Glucagon | 9.5 ± 0.8*** | 4 | 5.2 ± 0.5* | 5 |
| ZP2495 | 6.9 ± 0.9 | 4 | 4.9 ± 0.6* | 6 |
| FFA oxidation | ||||
| Baseline | 53 ± 9.1 | 10 | 65 ± 7.6 | 12 |
| Glucagon | 93 ± 8.1 | 5 | 104 ± 19 | 6 |
| ZP2495 | 110 ± 27* | 5 | 114 ± 18* | 6 |
ATP production calculated in µmol·g−1 dry weight·min−1 and presented as mean ± SEM. Data were analysed by one-way anova followed by Dunnett's multiple comparison test comparing the glucagon and ZP2495 groups to baseline (
P < 0.05,
P < 0.01,
P < 0.001).
Effects of glucagon, ZP131 and ZP2495 on cardiac energy status
The effects of glucagon, ZP131 and ZP2495 on short-chain CoA esters and high energy phosphates are shown in Figures 4 and 5, respectively. Concentrations of malonyl-CoA (an important endogenous regulator of cardiac fatty acid oxidation) and acetyl-CoA (a product of both fatty acid and glucose oxidation) were not significantly different between vehicle-, ZP131-, glucagon- or ZP2495-treated hearts (Figure 4A,B). The free CoA concentration in hearts from control rats and the succinyl-CoA concentration in hearts from both control and IR rat hearts were significantly decreased following perfusion with glucagon, whereas these remained unchanged following perfusion with ZP131 and ZP2495 compared with vehicle-perfused hearts (Figure 4C,D).
Figure 4.
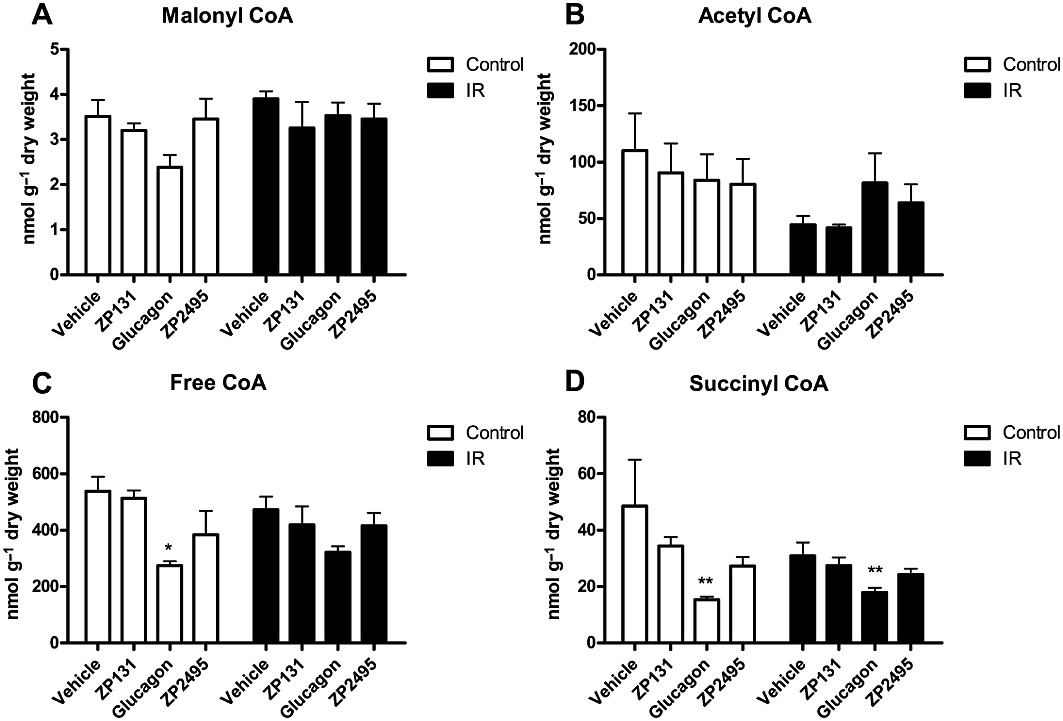
Short chain CoA concentrations in control and IR JCR:LA rat hearts after perfusion with vehicle, a GLP-1 receptor agonist (ZP131), glucagon and a glucagon-GLP-1 dual-agonist (ZP2495). (A) Malonyl CoA levels, (B) acetyl CoA levels, (C) free CoA levels and (D) succinyl CoA levels. Values are presented as mean + SEM. *P < 0.05; **P < 0.01 compared with vehicle by one-way anova for repeated measures followed by Dunnett's multiple comparison test. (For specific group sizes, see Figures 1 and 2).
Figure 5.
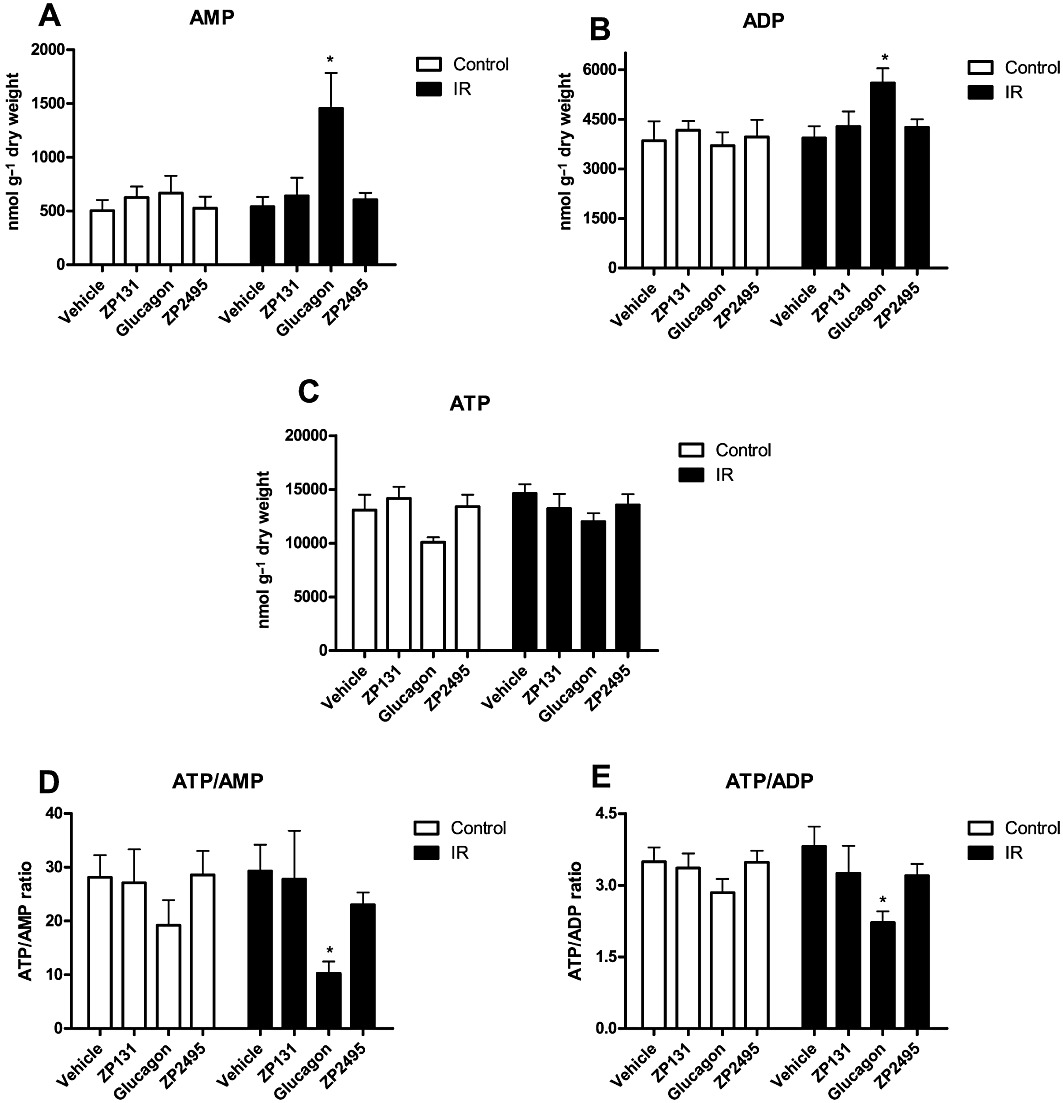
Measured energy state in hearts from control and IR JCR:LA rats after perfusion with vehicle, a GLP-1 receptor agonist (ZP131), glucagon and a glucagon-GLP-1 dual-agonist (ZP2495). (A) AMP levels following a total of 65 min perfusion with increasing concentrations of vehicle, ZP131, glucagon or ZP2495. (B) ADP levels following perfusion with increasing concentrations of vehicle, ZP131, glucagon or ZP2495. (C) ATP levels following perfusion with increasing concentrations of vehicle, ZP131, glucagon or ZP2495. (D) ATP/AMP ratios following perfusion with increasing concentrations of vehicle, ZP131, glucagon or ZP2495. (E) ATP/ADP ratios following perfusion with increasing concentrations of vehicle, ZP131, glucagon or ZP2495. Values are presented as mean + SEM. *P < 0.05; **P < 0.01 compared with vehicle by one-way anova for repeated measures followed by Dunnett's multiple comparison test. (For specific group sizes, see Figure 1).
In hearts from IR rats, AMP and ADP levels were significantly increased following perfusion with glucagon (Figure 5A,B), suggesting that cardiac energy status was compromised. Glucagon also caused a modest, but not statistically significant decrease in ATP levels in both IR and control hearts (Figure 5C). Collectively, these changes resulted in decreased ATP/AMP and ATP/ADP ratios in hearts from IR rats perfused with glucagon compared with vehicle (Figure 5D,E). Furthermore, the ratio of ATP/AMP and ATP/ADP was also significantly lower in hearts perfused with 100 nM glucagon compared with 100 nM ZP2495 in hearts from both control and IR rats when perfused according to protocol 2 (data not shown). In contrast, the ATP, ADP and AMP levels were not significantly different between ZP131-, ZP2495- and vehicle-perfused hearts (Figure 5A–E).
ZP131, glucagon and ZP2495 did not cause significant changes in cardiac GTP levels compared with vehicle-perfused hearts (data not shown).
Discussion and conclusions
The data from this study provide some important insights into the effects of glucagon, GLP-1 agonists and combined glucagon and GLP-1 agonists on cardiac function and metabolism in IR rats. In IR JCR:LA-cp rats, cardiac substrate metabolism shifted from glucose towards increased fatty acid metabolism. The shift of utilization from glucose to fatty acids created a situation where exposure of IR hearts to the inotropic agent glucagon caused cardiac energy metabolism to be lower than the energy demand, and AMP and ADP accumulated in the cardiomyocytes. While the GLP-1 agonist per se had no effect on cardiac function or metabolism, the glucagon-GLP-1 dual-agonist ZP2495 maintains the energetic state of IR hearts, despite having similar inotropic effects as glucagon. Collectively, the data suggest that dual-agonism of glucagon and GLP-1 receptors may offer simultaneous inotropic and energy preserving effects in IR hearts.
Insulin-resistant JCR:LA-cp rats maintain cardiac function despite marked alterations in cardiac metabolism
Our finding that hearts from IR rats had decreased glycolytic rates is consistent with previous studies in both JCR:LA-cp rats (Atkinson et al., 2003) and Zucker rats (Morabito et al., 2002). The apparent decrease in glycolytic rates occurred in the presence of a high concentration of insulin (2000 µU·mL−1), which verifies marked cardiac IR. In contrast to other studies (Lopaschuk and Russell, 1991; Atkinson et al., 2003), we observed an increase in cardiac fatty acid oxidation rates, as well as a modest decrease in cardiac glucose oxidation in IR JCR:LA-cp rats. These differences probably reflect different perfusion conditions; here we used a higher palmitate concentration (0.8 mM vs. 0.4 mM in previous studies), which could account for the increased palmitate oxidation rate found in IR hearts. In turn, we propose that an increased fatty acid oxidation suppresses glucose oxidation in IR hearts.
In spite of the marked metabolic changes observed in hearts from IR rats, we found no changes in the energetic state, and cardiac function was almost identical to that of control rats during normal workloads. The only difference was a modest increase in developed pressure in the IR hearts, which is in accordance with previous studies using JCR:LA-cp rats (Lopaschuk and Russell, 1991; Atkinson et al., 2003). Collectively, these data suggest that despite metabolic alterations, at normal workloads IR hearts are not energetically compromised.
ZP131 had minor effects on cardiac metabolism
The GLP-1 receptor agonist ZP131 had no effects on cardiac function and only a minor non-significant effect on glucose oxidation in IR hearts. Furthermore, the energetic state (ATP/AMP and ATP/ADP ratios and short-chain CoA levels) of ZP131-perfused hearts was unchanged compared with vehicle-perfused hearts.
GLP-1 has previously been found to increase glucose uptake in normal and post-ischaemic Langendorff-perfused rat hearts, in a fashion similar to that of insulin (Zhao et al., 2006). However, this effect was found in the absence of fatty acid and insulin, and the metabolic fate of the increased glucose uptake was not determined. The data presented here suggest that GLP-1 agonism does not have acute effects on cardiac metabolism in hearts provided with high insulin and fatty acids. It is also noteworthy that recombinant GLP-1 has been found to increase myocardial glucose uptake in dogs with pacing-induced dilated cardiomyopathy (Nikolaidis et al., 2004b; 2005), but that glucose uptake was not significantly increased until 6 h after initiation of the GLP-1 infusion (Nikolaidis et al., 2005). These data further support the concept that stimulation of the GLP-1 receptor does not have acute effects on glucose or fatty acid metabolism in the heart.
According to the amino acid composition, ZP131 is an exendin-4-like GLP-1 agonist. Pretreatment with GLP-1 and exendin-4 have a similar level of protection against ischaemia–reperfusion injury in mice isolated hearts (Ban et al., 2008). However, the cardioprotective effects of GLP-1 were still evident in GLP-1 receptor-knockout mice, whereas the salutary effects of exendin-4 were reduced (Ban et al., 2008). This suggests that native GLP-1 have GLP-1 receptor-independent cardioprotective effects. Based on the available data, it cannot be determined whether the lack of ZP131 effect on cardiac glucose oxidation is due to (1) reduced effect on glucose uptake compared with native GLP-1, (2) changed effect due to the presence of high insulin and/or fatty acids, (3) increased glucose uptake does not reflect an increased glucose oxidation and/or (4) the fact that the cardiac energy state was uncompromised.
The inotropic effect of glucagon is accompanied by marked changes in cardiac metabolism and energy state
The inotropic and chronotropic effects of glucagon are well described (Farah and Tuttle, 1960; Buse et al., 1973). It is also known that glucagon activates adenylate cyclase, which in turn increases the amount of cAMP in the heart (Levey and Epstein, 1969; Mayer et al., 1970). However, the effects of glucagon on cardiac substrate metabolism are less well described. Here we found that the inotropic and chronotropic effects of glucagon are accompanied by increases in glycolytic rates, glucose oxidation rates and palmitate oxidation rates in both control and IR rat hearts. These data are in agreement with the study of Buse et al. who found that glucagon increases pyruvate oxidation and insulin stimulated glucose oxidation in normal isolated rat hearts (Buse et al., 1973).
In control hearts, the overall increase in ATP production following perfusion with glucagon did not alter the respective proportions of ATP produced from glycolysis, glucose- and palmitate oxidation. However, in hearts from IR rats, the proportion from palmitate oxidation increased, whereas the proportion obtained from glucose oxidation decreased. Furthermore, a significant accumulation of AMP and ADP was found in hearts from IR rats perfused with glucagon compared with hearts perfused with vehicle alone, which resulted in a significant decrease in ATP/AMP and ATP/ADP ratios. These data show that IR hearts have a reduced capacity to raise their glucose oxidation in response to increased workload, which creates a mismatch between energy supply and demand.
We found that glucagon caused a modest non-significant decrease in malonyl CoA concentrations in control hearts, which, at least in part, may be responsible for the increased fatty acid oxidation rates. However, the concentration of malonyl CoA remained unchanged following perfusion with glucagon in the IR rat hearts. Therefore, the increase in fatty acid oxidation rates in the glucagon-treated IR hearts may be mediated through a malonyl CoA-independent mechanism. Glucagon also induced a decrease in succinyl CoA and free CoA levels in both control and IR rat hearts. The exact explanations for these changes are currently unclear.
ZP2495 has positive inotropic effects without compromising cardiac energetics
The effect of increasing concentrations of the glucagon-GLP-1 dual-agonist ZP2495 on cardiac function and metabolism in both control and IR rats was very similar to that of glucagon. In contrast to glucagon, ZP2495 was able to maintain free CoA and succinyl CoA concentrations, and ZP2495 did not compromise the ATP/AMP and ATP/ADP ratios of either control or IR hearts. Given the fact that there were no major differences in glycolysis, glucose or fatty acid oxidation rates between glucagon- and ZP2495-perfused hearts, other factors seem to influence the final energetic state. One explanation could be differences in ATP production efficiency caused by uncoupling of the electron transport chain, which may be upregulated by increased oxidative stress (Echtay et al., 2002). Both exendin-4 (Kim et al., 2010) and GLP-1 (Oeseburg et al., 2010) have in other pathophysiological settings been found to protect against oxidative stress. Potentially, the difference in energy state following perfusion with glucagon and ZP2495 might be explained by differences in oxidative stress-induced uncoupling of the electron transport chain. However, improved insulin signalling or increased insulin-independent glucose uptake may also be potential explanations for the improved energetic state. Clearly, future studies are needed in order to address the potential mechanism further.
Pharmacological perspectives
Glucagon is used as a first-line treatment for acute β-blocker overdose (White, 1999), and the positive inotropic and chronotropic effects of glucagon are potentially beneficial in disorders such as congestive heart failure and circulatory shock. However, clinical studies with glucagon have failed to show beneficial effects on survival rates in heart failure and shock patients, despite temporary symptomatic improvements (White, 1999). It is plausible that the failure of glucagon to improve survival in subjects with heart failure or shock might be due to a compromised energetic state following glucagon treatment. However, since we find here that ZP2495 has equivalent beneficial effects on cardiac function to that of glucagon without any compromises to the energy state, we propose that a glucagon- GLP-1 dual-agonist, may be a more attractive option for subjects with severe heart failure and shock, especially in those with IR. Further studies on the effects of ZP2495 in the setting of heart failure are clearly needed.
Conclusions
The GLP-1 receptor agonist ZP131 had no apparent effects on either cardiac function or metabolism in hearts from normal or IR JCR:LA-cp rats. Whereas glucagon compromised the energetic state of IR hearts, the glucagon-GLP-1 dual-agonist ZP2495 appears to preserve the energetic state. Therefore, a combined glucagon-GLP-1 dual-agonist may be beneficial compared with glucagon alone in the treatment of severe heart failure or cardiogenic shock in subjects with IR.
Acknowledgments
The authors want to thank Sandra Kelly and Panakkezhum Thomas for their outstanding technical assistance to the project. The studies were funded by a grant from the Canadian Institutes for Health Research to GDL, a grant from Zealand Pharma, and a Grant-in-Aid from the Heart and Stroke Foundation of Canada to SDP. SDP is further supported by a New Investigator Award from the Heart and Stroke Foundation of Canada. LNA was supported by a Studentship award from Zealand Pharma, a student-grant from Danish Cardiovascular Research Academy and The Danish National Research Foundation.
Glossary
- AC
adenylate cyclase
- GLP-1
glucagon-like peptide-1
- IR
insulin resistance
Conflicts of interest
At the time of the study, HDP, EM, DR, ALK and JSP were employed by Zealand Pharma A/S. LNA was supported by a Studentship award by Zealand Pharma A/S. W Keung, SDP, N-HH-R and GDL have no conflict of interest to disclose.
References
- Ally A, Park G. Rapid determination of creatine, phosphocreatine, purine bases and nucleotides (ATP, ADP, AMP, GTP, GDP) in heart biopsies by gradient ion-pair reversed-phase liquid chromatography. J Chromatogr. 1992;575:19–27. doi: 10.1016/0378-4347(92)80499-g. [DOI] [PubMed] [Google Scholar]
- Atkinson LL, Kozak R, Kelly SE, Onay-Besikci A, Russell JC, Lopaschuk GD. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am J Physiol Endocrinol Metab. 2003;284:E923–E930. doi: 10.1152/ajpendo.00360.2002. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- Buse MG, Biggers JF, Drier C, Buse JF. The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem. 1973;248:697–706. [PubMed] [Google Scholar]
- Cook SA, Aitman T, Naoumova RP. Therapy insights: heart disease and the insulin-resistant patient. Nat Clin Pract Cardiovasc Med. 2005;2:252–260. doi: 10.1038/ncpcardio0194. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel DS, Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Farah A, Tuttle R. Studies on the pharmacology of glucagon. J Pharmacol Exp Ther. 1960;129:49–55. [PubMed] [Google Scholar]
- Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol. 2009;157:1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad E, Mather PJ, Srinivasan S, Rubin S, Whellan DJ, Feldman AM. Pharmacological therapy of chronic heart failure. Am J Cardiovasc Drugs. 2007;7:235–248. doi: 10.2165/00129784-200707040-00002. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. A pharmacological method to estimate the pKi of competitive inhibitors of agonist uptake processes in isolated tissues. Naunyn Schmiedebergs Arch Pharmacol. 1981;316:89–95. doi: 10.1007/BF00505300. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Quantitation in receptor pharmacology. Receptors Channels. 2001;7:371–385. [PubMed] [Google Scholar]
- Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lim DM, Moon CI, Jo KJ, Lee SK, Baik HW, et al. Exendin-4 protects oxidative stress-induced β-cell apoptosis through reduced JNK and GSK3β activity. J Korean Med Sci. 2010;25:1626–1632. doi: 10.3346/jkms.2010.25.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey GS, Epstein SE. Activation of adenyl cyclase by glucagon in cat and human heart. Circ Res. 1969;24:151–156. doi: 10.1161/01.res.24.2.151. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev. 2002;7:149–159. doi: 10.1023/a:1015328625394. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Barr RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem. 1997;172:137–147. [PubMed] [Google Scholar]
- Lopaschuk GD, Russell JC. Myocardial function and energy substrate metabolism in the insulin-resistant JCR:LA corpulent rat. J Appl Physiol. 1991;71:1302–1308. doi: 10.1152/jappl.1991.71.4.1302. [DOI] [PubMed] [Google Scholar]
- MacLeod KM, Rodgers RL, McNeill JH. Characterization of glucagon-induced changes in rate, contractility and cyclic AMP levels in isolated cardiac preparations of the rat and guinea pig. J Pharmacol Exp Ther. 1981;217:798–804. [PubMed] [Google Scholar]
- Mayer SE, Namm DH, Rice LUCI. Effect of glucagon on cyclic 3′,5′-AMP, phosphorylase activity and contractility of heart muscle of the rat. Circ Res. 1970;26:225–233. doi: 10.1161/01.res.26.2.225. [DOI] [PubMed] [Google Scholar]
- Morabito D, Montessuit C, Rosenblatt-Velin N, Lerch R, Vallotton M, Lang U. Impaired glucose metabolism in the heart of obese Zucker rats after treatment with phorbol ester. Int J Obes. 2002;26:327–334. doi: 10.1038/sj.ijo.0801881. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004a;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004b;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HHW. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- Ossum A, van Deurs U, Engstrøm T, Jensen JS, Treiman M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9-36)a in an isolated rat heart. Pharmacol Res. 2009;60:411–417. doi: 10.1016/j.phrs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Russell JC, Amy RM. Early atherosclerotic lesions in a susceptible rat model the LA/N-corpulent rat. Atherosclerosis. 1986;60:119–129. doi: 10.1016/0021-9150(86)90004-3. [DOI] [PubMed] [Google Scholar]
- Russell JC, Kelly SE, Proctor SD. The JCR:LA-cp rat: animal model of the metabolic syndrome exhibiting micro- and macrovascular disease. In: Shafrir E, editor. Animal Models of Diabetes. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2007. pp. 159–183. [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- White CM. A review of potential cardiovascular uses of intravenous glucagon administration. J Clin Pharmacol. 1999;39:442–447. [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]


