Abstract
BACKGROUND AND PURPOSE
Activation of cannabinoid receptors decreases emesis, inflammation, gastric acid secretion and intestinal motility. The ability to modulate intestinal permeability in inflammation may be important in therapy aimed at maintaining epithelial barrier integrity. The aim of the present study was to determine whether cannabinoids modulate the increased permeability associated with inflammation in vitro.
EXPERIMENTAL APPROACH
Confluent Caco-2 cell monolayers were treated for 24 h with IFNγ and TNFα (10 ng·mL−1). Monolayer permeability was measured using transepithelial electrical resistance and flux measurements. Cannabinoids were applied either apically or basolaterally after inflammation was established. Potential mechanisms of action were investigated using antagonists for CB1, CB2, TRPV1, PPARγ and PPARα. A role for the endocannabinoid system was established using inhibitors of the synthesis and degradation of endocannabinoids.
KEY RESULTS
Δ9-Tetrahydrocannabinol (THC) and cannabidiol accelerated the recovery from cytokine-induced increased permeability; an effect sensitive to CB1 receptor antagonism. Anandamide and 2-arachidonylglycerol further increased permeability in the presence of cytokines; this effect was also sensitive to CB1 antagonism. No role for the CB2 receptor was identified in these studies. Co-application of THC, cannabidiol or a CB1 antagonist with the cytokines ameliorated their effect on permeability. Inhibiting the breakdown of endocannabinoids worsened, whereas inhibiting the synthesis of endocannabinoids attenuated, the increased permeability associated with inflammation.
CONCLUSIONS AND IMPLICATIONS
These findings suggest that locally produced endocannabinoids, acting via CB1 receptors play a role in mediating changes in permeability with inflammation, and that phytocannabinoids have therapeutic potential for reversing the disordered intestinal permeability associated with inflammation.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: intestinal permeability, inflammation, Caco-2 cells, cytokines, transepithelial electrical resistance (TEER), endocannabinoid, cannabinoid CB1 receptor, Δ9-tetrahydrocannabinol and cannabidiol
Introduction
The pathogenesis of many intestinal disorders involves interactions between alterations in intestinal permeability and luminal exogenous agents, such as bacteria, toxins and foreign antigens, as well as secretory products of the mucosa itself, such as cytokines and growth factors (Madara and Pappenheimer, 1987; Hecht et al., 1992; Ma et al., 2004; Poritz et al., 2004). It is widely believed that the intestinal barrier becomes dysfunctional in certain disease states, potentially exposing the organism to lethal risk by permitting toxic material to enter the portal venous and lymphatic systems, and thus threaten the organism as a whole (Morehouse et al., 1986; Unno and Fink, 1998; Ammori et al., 1999). Inflammatory bowel disease (IBD) is accompanied by impaired epithelial barrier function in the small and large intestine (Gassler et al., 2001; Bruewer et al., 2006; Amasheh et al., 2009). This has two consequences; firstly contributing to diarrhoea by a leak flux mechanism, and secondly, perpetuating inflammation through increased luminal antigen and macromolecular uptake.
For many centuries, the plant Cannabis sativa has been used to treat various disorders of the gastrointestinal tract, such as vomiting, anorexia, abdominal pain, gastroenteritis, diarrhoea, intestinal inflammation and diabetic gastroparesis (Coutts and Izzo, 2004; Duncan et al., 2005; Sanger, 2007; Izzo and Camilleri, 2008). The presence of a functional endocannabinoid system has been identified in the gut. CB1 receptors are expressed in the gastrointestinal tract of many species, including rats, guinea-pigs and humans (Croci et al., 1998; Kulkarni-Narla and Brown, 2000; Coutts et al., 2002; Casu et al., 2003). Immunohistochemical studies indicate that the enteric nervous system is the main site of CB1 receptor expression and could be the main site of action for cannabinoids in the gastrointestinal tract (Coutts et al., 2002). In human colonic tissue, CB1 receptors are expressed in the epithelium, smooth muscle and the submucosal myenteric plexus (Wright et al., 2005). The CB2 receptor has been detected in rat peritoneal mast cells (Facci et al., 1995) and enteric neurons (Duncan et al., 2008). In human colonic tissue, CB2 is expressed in plasma cells and the lamina propria (Wright et al., 2005), and in the epithelium of colonic tissue characteristic of IBD (Wright et al., 2005; Izzo, 2007).
Recent studies have confirmed that the endocannabinoid system becomes activated during inflammatory conditions, both in animal models and in tissue samples from patients suffering from inflammatory disorders. In an experimental model of colitis, D'Argenio et al. found that the levels of the endogenously produced cannabinoids, anandamide (AEA), but not 2-arachidonylglycerol (2-AG), were significantly increased (D'Argenio et al., 2006). AEA levels are also increased in colon biopsies from patients with ulcerative colitis (D'Argenio et al., 2006), small bowel samples from patients with diverticular disease (Guagnini et al., 2006) and from individuals in the atrophic phase of coeliac disease (D'Argenio et al., 2007). During croton oil induced inflammation in murine small bowel, the expression of CB1 receptors and fatty acid amide hydrolase (FAAH), a membrane protein that metabolises AEA, are enhanced, and CB1 activation inhibits motility (Izzo et al., 2001). Colonic CB1 receptor expression has also been shown to be up-regulated in a murine colitis model, and genetic or pharmacological blockage of CB1 receptors worsens epithelial damage (Massa et al., 2004). However, pharmacological inhibition of the CB1 receptor has also been shown to inhibit ulcer formation and plasma TNF levels in an indomethacin-induced model of small intestinal inflammation (Croci et al., 2003). CB2 receptor expression is also increased in human intestinal epithelium in IBD (Wright et al., 2005; 2008). The role of CB2 receptors in inflammation is supported by the inhibition of TNFα-induced IL-8 release by CB2 receptor antagonists in human colonic epithelial cells (Ihenetu et al., 2003). CB2 receptor agonists have also been shown to offset LPS-induced inflammation in rats through COX-derived products (Mathison et al., 2004).
The ability to modulate intestinal permeability during the inflammatory process may be important in devising future therapeutic strategies to restore a ‘leaky’ tight junction paracellular barrier. Given the beneficial effects of cannabinoids in inflammatory conditions in the gut, and our recent findings that cannabinoids are capable of modulating intestinal permeability altered with EDTA (Alhamoruni et al., 2010), the aim of the present study was to determine whether cannabinoids modulate increased permeability associated with inflammation. To do this, carcinoma colon cell line (Caco-2) monolayers were used as an in vitro intestinal epithelial model system, and inflammatory conditions were mimicked by the co-application of the pro-inflammatory mediators IFNγ and TNFα. We found that endocannabinoids further worsen the increased permeability associated with cytokine application to Caco-2 cells, while phytocannabinoids or CB1 receptor antagonism speeded the recovery of permeability in inflammatory conditions. Inhibition of endocannabinoid degradation worsened the effects of inflammation on intestinal permeability, and inhibition of endocannabinoid synthesis ameliorated the increased permeability associated with inflammation. Our data suggest that locally produced endocannabinoids, acting via the CB1 receptor, play a role in mediating changes in permeability associated with inflammation.
Methods
The nomenclature for drugs and for their molecular targets conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Cell culture
Caco-2 cells (ECACC, Wiltshire, UK, passages 56–72) were cultured in Minimum Essential Medium Eagle supplemented with 10% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin. Cells were kept at 37°C in 5% CO2 and 95% humidity. Cells were grown in 12-well plates and seeded at 50 000 cells per insert on 12 mm diameter, 0.4 µm pore polycarbonate membrane inserts. Cells were grown for a minimum of 14 days and used for experimentation between days 14 and 21, when each insert had a transepithelial electrical resistance (TEER) value greater than 1000 Ω cm2.
TEER measurement
The TEER measurement was used to evaluate the paracellular permeability of cell monolayers (Madara et al., 1988). The TEER of the monolayer was determined using an EVOM™ voltohmmeter (World Precision Instruments, Sarasota, FL, USA) according to the methods of Wells and colleagues (Wells et al., 1998).
Inflammatory protocol
Initial TEER readings were made before the addition of 10 ng·mL−1 IFNγ (basolateral compartment). After 8 h, TEER was measured again, and 10 ng·mL−1 TNFα was added for another 16 h. TEER was measured again after a total of 24 h incubation with the cytokines, which caused an average fall in TEER of 20–25%, representing increased epithelial permeability.
Permeability studies
Intestinal permeability to fluorescein isothiocyanate (FITC)-dextran molecular mass 4 kDa (FD4), a tracer for the paracellular pathway, was evaluated by measuring the flux of FD4 across cell monolayers. Cannabinoids [cannabidiol (CBD, 1 µM), AM251 (100 nM) and methandamide, mAEA 100 Nm] were applied apically either concomitant with the cytokines (0 h) or following the inflammatory protocol (24 h), for a further 6 h. Cell layers (30 h) were then washed with HBSS/20 mM HEPES (pH 7.4) and left for 30 min at 37°C to equilibrate. FD4 (3 mg·mL−1) was applied apically and 100 µL aliquots were collected from the basolateral side of each insert after 30 min and 1 h. FD4 levels in the medium were measured using a fluorescence microplate reader at an excitation wavelength of 490 nm and emission wavelength of 520 nm (VICTOR, Perkin Elmer, USA). FD4 flux was calculated as the average fluorescence value of two samples taken from the same well, and expressed as a percentage of the FD4 permeability of vehicle control monolayers in the same experiment.
Cell viability (MTS) and membane integrity (lactate dehydrogenase release) assays
To show that the effect of cytokine application was not due to cellular damage and changes in transcellular permeability, we performed MTS (Promega, Madison, WI, USA) and lactate dehydrogenase (LDH) assays (Bio Vision, CA, USA), according to the manufacturer's instructions, on Caco-2 treated with 10 ng·mL−1 IFNγ and 10 ng·mL−1 TNFα for up to 72 h.
Effects of cannabinoids on Caco-2 cell monolayer integrity (apical application)
Fresh medium, with or without cannabinoids [Δ9-tetrahydrocannabinol (THC), CBD, AEA or 2-AG (all 10 µM)], was applied apically to plates where inflammation had been established (i.e. after 24 h). Vehicle (0.1% ethanol) was applied to control wells. TEER values were measured every 1 h for the next 8 h, and then again 48 and 72 h after cannabinoid administration. Our initial experiments showed that a single dose of THC or CBD (10 µM) ameliorated the fall in TEER caused by cytokines, while a single dose of AEA or 2-AG (10 µM) worsened this (see Figure 1). Therefore, we proceeded to perform concentration–response curves to THC, CBD, AEA and 2-AG by adding increasing concentrations of each drug to inserts. TEER values were monitored at all time points as described above.
Figure 1.
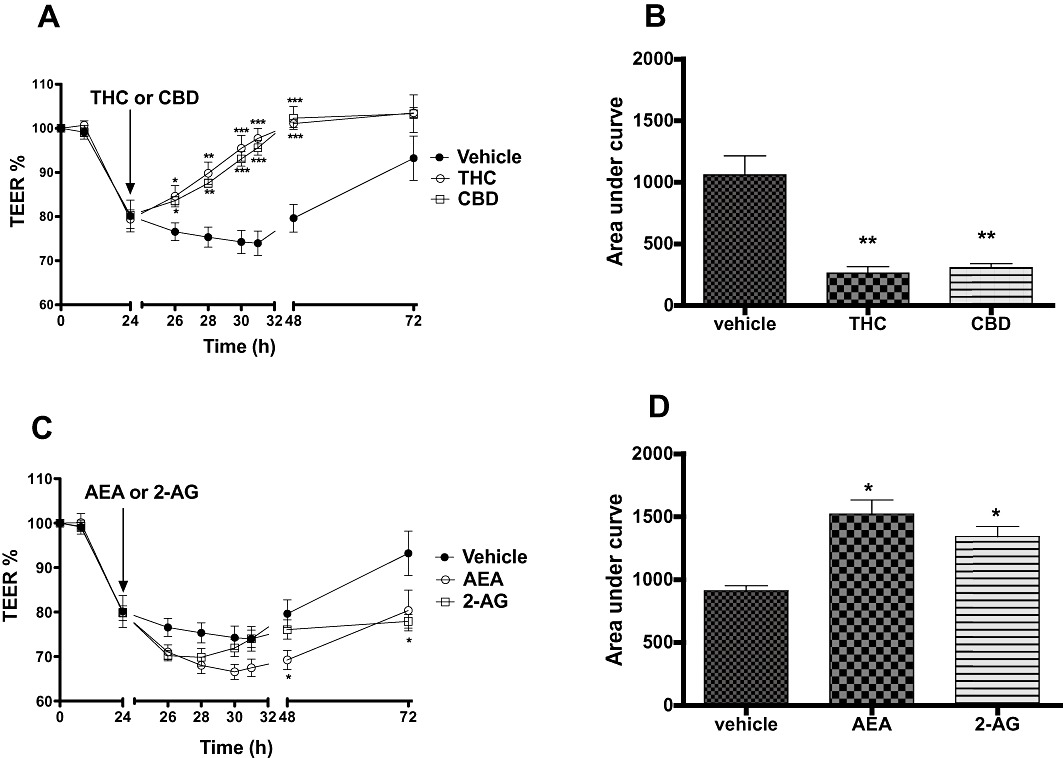
The effects of phytocannabinoids (THC and CBD, 10 µM, A) and endocannabinoids (AEA and 2-AG, 10 µM, C) applied apically on the fall in TEER values caused by the inflammatory cytokines (IFNγ and TNFα, 10 ng·mL−1). Integrated response over time (area under curve) to THC and CBD (C) and AEA and 2-AG (D) on the fall in TEER values caused by the inflammatory cytokines. Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, ***P < 0.001, anova).
In some experiments, 10 µM of either THC or CBD was applied at the apical compartment at 0 h (i.e. at the same time as the cytokines) or 48 h after cytokine application. TEER values were measured as above.
Target sites of action of cannabinoids
The following antagonists were co-applied with cannabinoids (24 h after inflammation was established); AM251 (CB1 receptor antagonist), AM630 (CB2 receptor antagonist), capsazepine (TRPV1 antagonist), GW9662 (PPARγ antagonist), GW6471 (PPARα antagonist) and O-1918 (proposed cannabinoid receptor antagonist). All antagonists were used at 1 µM except AM251, which was used at 100 nM (see Alhamoruni et al., 2010) and appropriate vehicles were applied to control inserts. TEER values were measured as above.
In some experiments, 100 nM of either AM251 or AM630 was applied at the apical compartment at 0 h (i.e. at the same time as the cytokines) or 24 h after cytokine application (when increased permeability was induced). TEER values for each group were monitored over time.
Effects of cannabinoids on Caco-2 cell monolayer permeability (basolateral application)
Fresh medium, with or without cannabinoids (THC, CBD, AEA or 2-AG, all 10 µM), was applied basolaterally to plates where inflammation had been established.
Effects of enzyme inhibitors on increased permeability induced by cytokines
To establish the role of the FAAH enzyme on the AEA effect on intestinal permeability, AEA (10 µM) was applied to the apical side of inserts in the absence or presence of an FAAH inhibitor 24 h after inflammation was established (URB597, 1 µM). Similarly, 2-AG (10 µM) was applied to the apical side of inserts either alone or together with a monoacylglycerol lipase (MGL) inhibitor (JZL 184, 1 µM). In both experiments, the vehicle [ethanol and dimethyl sulfoxide (DMSO)] was applied to control wells. TEER values were measured hourly for the next 8 h, and then again at 48, and 72 h after cannabinoid administration.
In some experiments, 1 µM of URB597 or JZL 184, alone or together with CB1 antagonist AM251 (100 nM), were applied at the apical compartment at the same time as the cytokines.
Orlistat (1 µM), a 2-AG synthesis inhibitor, alone or together with CB1 antagonist (AM251, 100 nM) was applied at the apical compartment at the same time as cytokine application.
Chemicals and reagents
All chemicals were purchased from Sigma-Aldrich (Poole, UK) unless otherwise stated. IFNγ and TNFα were purchased from Invitrogen (Paisley, UK), and further dilutions in BSA stored at −80°C for IFNγ and −20°C for TNFα. All cannabinoids and antagonists were purchased from Tocris Bioscience (Bristol, UK) except THC and capsazepine, which were obtained from Sigma UK. CBD, THC, capsazepine, AEA and 2-AG were dissolved in ethanol to a stock concentration of 10 mM with further dilutions made in distilled water. GW9662, AM251 and AM630 were dissolved in DMSO to 10 mM, with further dilutions made in distilled water. URB597, JZL 184 and Orlistat were dissolved in DMSO to 10 mM, with further dilutions made in fresh media.
Statistical analysis
In each protocol, values are expressed as mean ± SEM. Area under the curve (AUC) values were calculated using GraphPad Prism 5 software using the trapezoidal method. Data were compared, as appropriate, by Student's t-test or by anova with statistical significance between manipulations and controls determined by Dunnett's post hoc test.
Results
Cytokines increased permeability without affecting cell viability or membrane integrity
Combined application of IFNγ and TNFα (10 ng·mL−1) in Caco-2 cells caused a reversible decrease in TEER (i.e. increased permeability) over the 72 h measurement period. Application of IFNγ and TNFα to Caco-2 cells did not affect the Caco-2 cell mitochondrial activity at any point over the 72 h experimental period compared with the vehicle group, as indicated by the MTS assay (OD at 72 h; vehicle 0.54 ± 0.03, cytokine application, 0.52 ± 0.01, n= 4). The total LDH release from Caco-2 cells treated with cytokines was also not significantly different to vehicle at any point over the 72 h experimental period (OD at 72 h; vehicle 0.22 ± 0.01, cytokine application, 0.11 ± 0.01, n= 4).
Apical application of phytocannabinoids recovers cytokine-induced increased permeability
Twenty-four hours after exposure to IFNγ and TNFα, apical application of either THC or CBD (10 µM) accelerated the recovery of TEER values (see Figure 1A), and the total response over time (AUC) was significantly different to vehicle controls for both THC and CBD (P < 0.01, Figure 1B). Further experiments showed that the ability of THC and CBD to speed the recovery of TEER values after 24 h cytokine application was concentration-dependent (see Figure 2 and Table 1). When a sigmoidal concentration–response curve was plotted with the AUC data presented in Table 1, the logEC50 of THC and CBD were −6.03 and −5.68, respectively.
Figure 2.
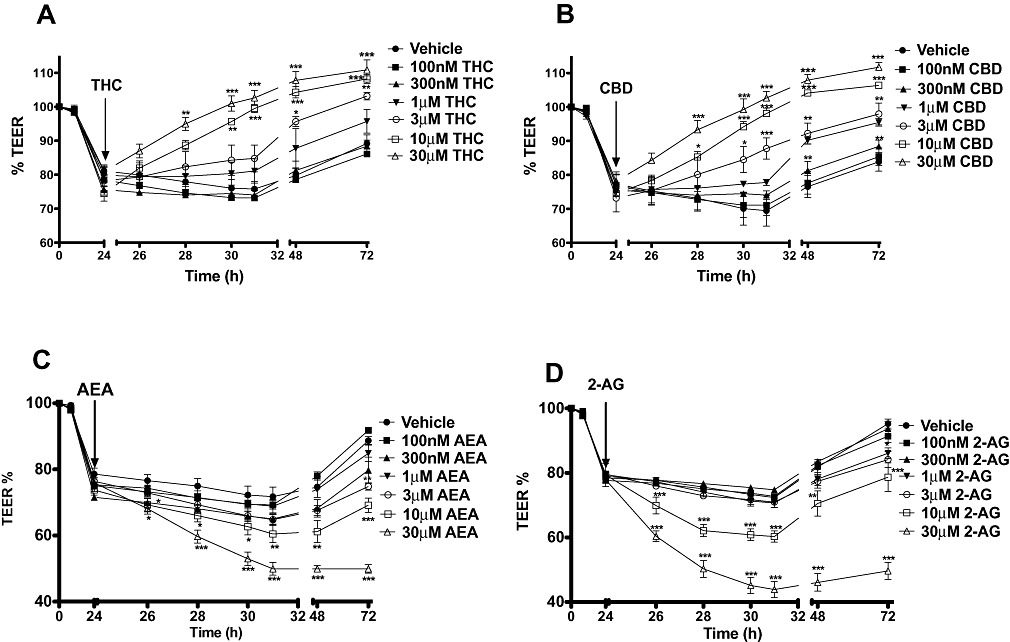
Concentration–response curves to THC (A), CBD (B), AEA (C) and 2-AG (D) applied apically on the fall in TEER caused by cytokine application. Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, ***P < 0.001, anova).
Table 1.
Area under the curve values (%·min−1) for the concentration–responses to cannabinoids on TEER
| THC | CBD | AEA | 2-AG | |
|---|---|---|---|---|
| Vehicle | 1062 ± 96 | 1327 ± 210 | 1330 ± 162 | 1018 ± 72 |
| 100 nM | 1097 ± 113 | 1192 ± 92 | 1258 ± 41 | 1059 ± 45 |
| 300 nM | 868 ± 67 | 1134 ± 81 | 1353 ± 73 | 985 ± 57 |
| 1 µM | 726 ± 168 | 843 ± 40* | 1663 ± 132 | 1224 ± 81 |
| 3 µM | 519 ± 130** | 665 ± 177** | 1671 ± 76 | 1265 ± 86 |
| 10 µM | 315 ± 20*** | 336 ± 14*** | 1763 ± 84* | 1622 ± 103** |
| 30 µM | 226 ± 12*** | 263 ± 49*** | 2519 ± 65*** | 2694 ± 129*** |
Data are given as means with error bars representing SEM. Significant difference between vehicle and drug responses,
P < 0.05,
P < 0.01,
P < 0.001, anova with Dunnett's post hoc test.
Apical application of endocannabinoids further increases permeability after cytokine application
Twenty-four hours after exposure to IFNγ and TNFα, apical application of endocannabinoids (10 µM of either AEA or 2-AG) caused a further and sustained drop in TEER in addition to the effects of cytokines (P < 0.05, Figure 1C and D). Further experiments showed that this effect was concentration-dependent (see Figure 2 and Table 1). When a sigmoidal concentration–response curve was plotted with the AUC data presented in Table 1, the logEC50 of AEA and 2-AG were −3.95 and −3.78, respectively.
The effects of both phytocannabinoids and endocannabinoids are CB1 mediated
The effects of THC and CBD were only significantly inhibited by the cannabinoid CB1 receptor antagonist, AM251. Similarly, the effects of the endocannabinoids AEA and 2-AG were also only sensitive to AM251 (Figure 3 and Table 2).
Figure 3.
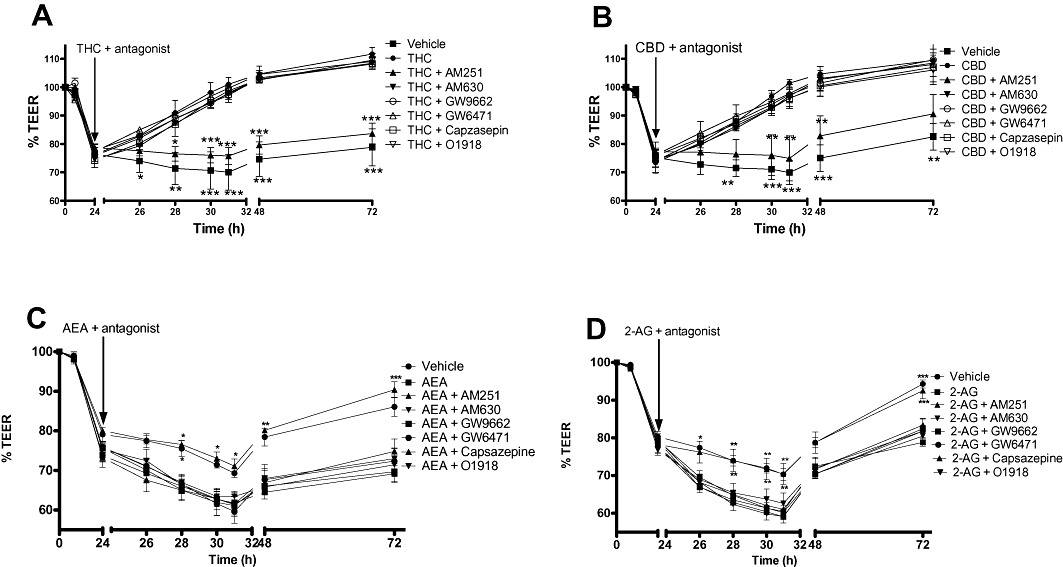
The effects of various receptor antagonists on the effects of THC (10 µM, A), CBD (10 µM, B), AEA (10 µM, C) and 2-AG (10 µM, D) applied apically on the fall in TEER caused by cytokine application. Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, ***P < 0.001, anova).
Table 2.
Area under the curve values (%·min−1) for the effects of cannabinoids on TEER in the presence of various receptor antagonists
| THC | CBD | AEA | 2-AG | |
|---|---|---|---|---|
| Vehicle | 1442 ± 334 | 1386 ± 247 | 1232 ± 47 | 1100 ± 34 |
| Cannabinoid (10 µM) | 531 ± 85** | 555.5 ± 62*** | 1886 ± 62** | 1561 ± 71** |
| & AM251 | 1152 ± 157 | 1351 ± 30 | 1112 ± 17 | 1137 ± 121 |
| & AM630 | 513 ± 50** | 519 ± 4*** | 1787 ± 77** | 1627 ± 61** |
| & GW9662 | 477 ± 69*** | 531 ± 4*** | 1834 ± 121** | 1591 ± 28** |
| & GW6471 | 519 ± 50** | 586 ± 5** | 1772 ± 163** | 1591 ± 57** |
| & Capsazepine | 499 ± 25** | 579 ± 55*** | 1784 ± 156** | 1528 ± 60** |
| & O-1918 | 491 ± 39** | 547 ± 28*** | 1749 ± 71* | 1538 ± 134** |
Data are presented as means with error bars representing SEM. Significant difference between vehicle and drug responses,
P < 0.05,
P < 0.01,
P < 0.001, anova with Dunnett's post hoc test.
Basolateral application of cannabinoids and permeability after cytokine application
When applied to the basolateral membrane after cytokine application, neither THC, CBD, AEA or 2-AG had any significant effect on TEER (data not shown).
Phytocannabinoids prevented increased permeability associated with cytokine application
When inserts were treated with cytokines (basolateral) and THC or CBD (apical) at the same time (0 h), THC and CBD (10 µM) completely inhibited the fall in TEER caused by the cytokines (see Figure 4A). However, when THC or CBD were applied 48 h after cytokine application, they had no effect on the response to these cytokines (Figure 4B).
Figure 4.
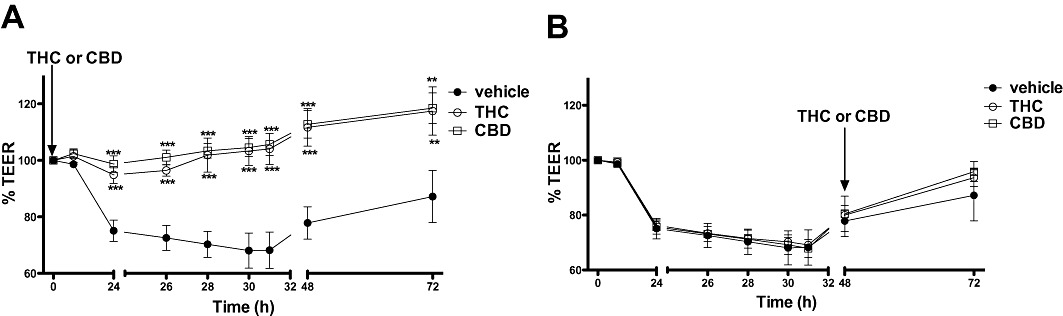
The effect of phytocannabinoids (THC and CBD, 10 µM) applied apically at time 0 h (A), or after 48 h (B) on the fall in TEER caused by cytokine application. Data are given as means with error bars representing SEM. (n= 3, *P < 0.01, ***P < 0.001, anova).
CB1 antagonism reduces the increased permeability associated with cytokines
To determine whether the effect of cytokines can be prevented by cannabinoid receptor antagonism, AM251 or AM630 (both 100 nM, apical application) were added at the same time as cytokine application (0 h) or after cytokine-induced increases in TEER were induced (24 h). When applied at time 0, AM251 significantly reduced the fall in TEER caused by cytokines. However, when AM251 was applied after 24 h, there was no effect of this compound (Figure 5A). AM630 did not affect TEER values when co-applied with cytokines, or when applied after inflammation was induced (Figure 5B), indicating no role for CB2 receptor activation.
Figure 5.
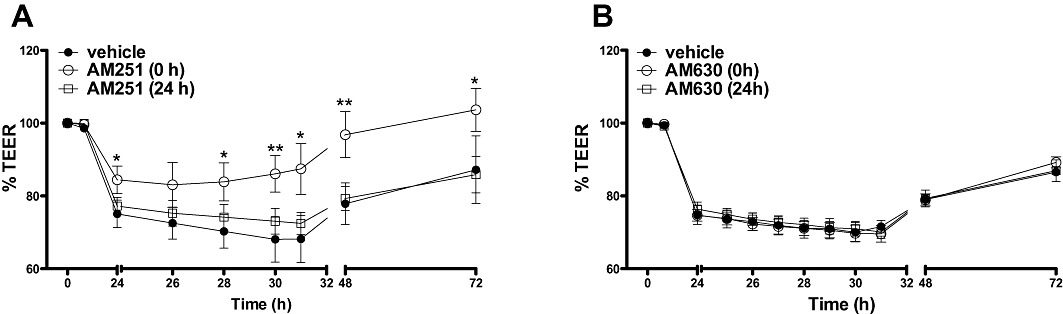
The effects of the CB1 receptor antagonist, AM251 (100 nM, A) on TEER applied apically at the same time as (0 h), or 24 h after cytokine application. The effects of the CB2 receptor antagonist, AM630 (100 nM, B) on TEER applied at the same time as (0 h), or 24 h after cytokine application. Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, anova).
FAAH and MGL inhibition worsened endocannabinoids effects on increased permeability after cytokine application
URB597 alone caused no significant change in the recovery of TEER compared with the vehicle (see Figure 6A and B). As previously shown, AEA alone caused a significant drop in TEER in addition to the effects of cytokines compared with vehicle. However, application of URB597 together with AEA caused a significantly greater drop in TEER than AEA alone (Bonferroni's multiple comparison test, Figure 6A and B). JZL 184 alone also caused no significant change in the recovery of TEER compared with vehicle. 2-AG alone caused a significant decrease in TEER as compared with vehicle group, and application of JZL 184 with 2-AG caused a significantly greater drop in TEER than 2-AG alone (Figure 6C and D).
Figure 6.
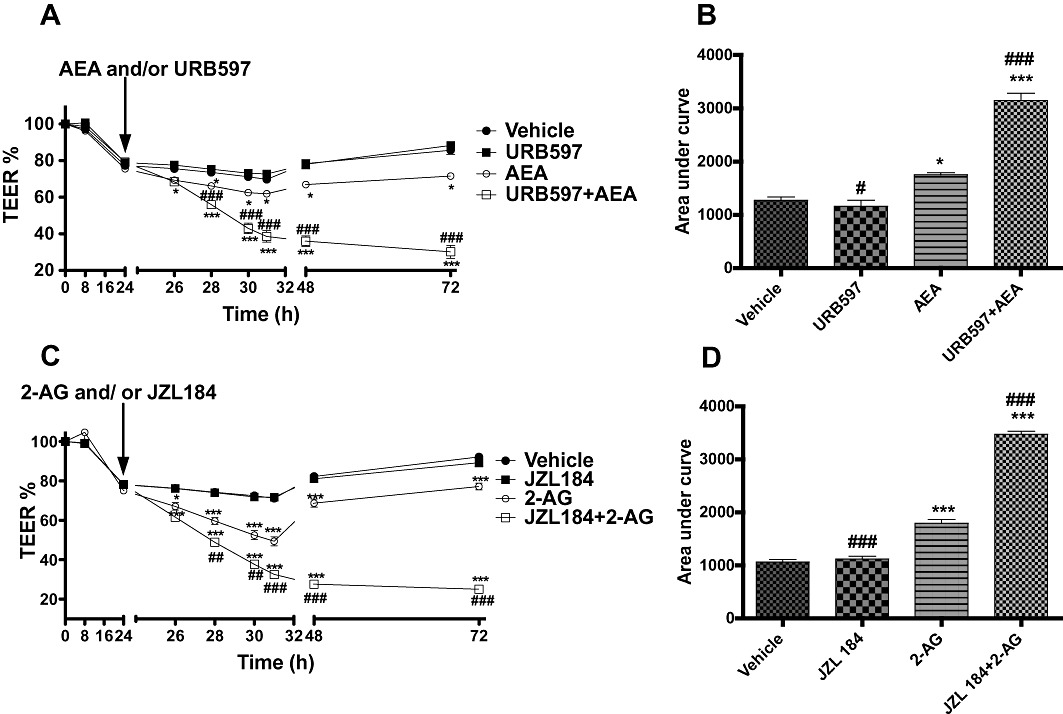
The effect of the FAAH inhibitor URB597 (1 µM, A) and MGL inhibitor JZL 184 (1 µM, C) applied apically alone, or in combination with AEA or 2-AG on the fall in TEER values caused by inflammatory cytokines. (B, D) Integrated response over time (area under curve). Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle group; ##P < 0.01, ###P < 0.001, compared with endocannabinoid alone, anova).
To test the hypothesis that locally produced AEA and 2-AG partly mediates the increase in permeability caused by cytokines, and whether these effects, if any, are mediated by CB1 receptors, URB597 or JZL 184 (1 µM each) were applied apically at the same time as cytokine application either alone or with AM251 (100 nM). When applied at the same time as cytokines, URB597 alone caused a further drop in TEER (i.e. increased permeability) than cytokine application alone, and this effect was inhibited by AM251 (Figure 7A and B). Similarly, JZL 184 application led to a decrease in TEER, and this effect was also inhibited by AM251 (Figure 7C and D).
Figure 7.
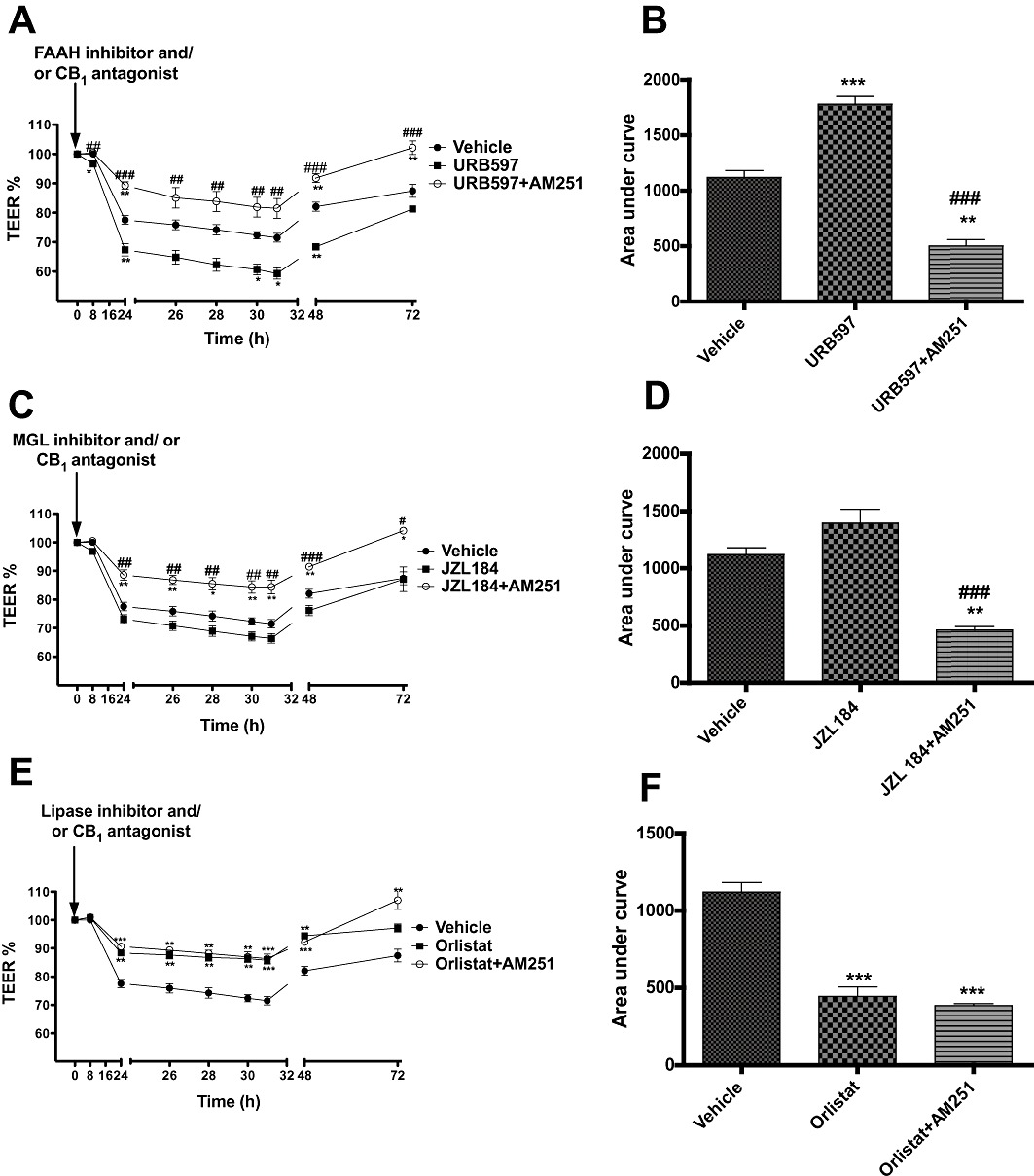
The effect of endocannabinoid enzyme inhibitors (URB597, 1 µM, A; JZL 184, 1 µM, C; Orlistat, 1 µM, E) applied apically at the same time as cytokines, either alone or together with the CB1 antagonist AM251 (100 nM) on the fall in TEER values caused by inflammatory cytokines. (B, D and F) Integrated response over time (area under curve). Data are given as means with error bars representing SEM. (n= 3, *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle group; ##P < 0.01, ###P < 0.001, compared with inhibitors alone, anova).
To further investigate the possible role of locally produced 2-AG on the TEER reduction caused by cytokines, Orlistat (1 µM), a 2-AG synthesis inhibitor was applied either alone or together with AM251 (100 nM). It was observed that Orlistat inhibited the drop in TEER caused by cytokines as compared with vehicle group (Figure 7E and F). This was not further affected by AM251.
FD4 flux was increased by cytokines and modulated by cannabinoids
To support our TEER data, we performed key experiments to measure the functional outcome of junctional change, that is, paracellular permeability, by using FITC-conjugated dextran (FD4) as a tracer. Cytokine application [interferon gamma and TNF alpha (IT)] induced an increase of 22 ± 4% in permeability to FD4 when compared with basal flux (Figure 8A). CBD both reversed (IT + CBD) and prevented (CBD + IT) this increase, as previously observed in the TEER experiments. As before, the CB1 antagonist AM251 (100 nM) blocked the CBD effect on cytokine-induced FD4 flux.
Figure 8.
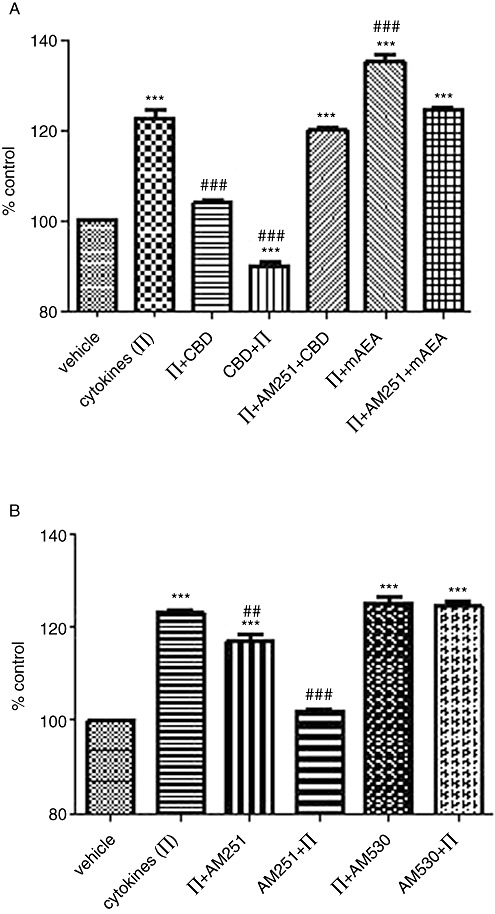
The effect of cannabinoids on cytokine-induced FD4 flux. (A) CBD (1 µM) or mAEA (100 nM) was applied apically at time zero together with the cytokines (IFNγ and TNFα, 100 ng·mL−1) or after 24 h of cytokine application (basal application). AM251 (100 nM) was applied apically with CBD or mAEA after 24 h cytokine application. (B) AM251 or AM630 (both at 100 nM) were applied apically either with cytokines at time zero or after 24 h of cytokine application. Data are given as means with error bars representing SEM. (n= 3, ***P < 0.001, as compared with vehicle group; ##P < 0.01, ###P < 0.001, as compared with cytokine-treated group, anova).
In addition, reflecting the AEA effect on cytokine-induced TEER changes, mAEA (100 nM) further enhanced the increased permeability to FD4 to 35.2 ± 3.1% (an enhancement of approximately 12%), which was also blocked by AM251.
AM251 (100 nM) was able to both partially inhibit and prevent the cytokine-induced increase in FD4 flux, whereas the CB2 receptor antagonist/inverse agonist, AM630, had no effect (see Figure 8B), again in support of the previous TEER data.
Discussion
Cannabinoids have been used to treat various disorders of the gastrointestinal tract, such as vomiting, anorexia, abdominal pain, gastroenteritis, diarrhoea, intestinal inflammation and diabetic gastroparesis (Coutts and Izzo, 2004,Duncan et al., 2005; Sanger, 2007; Izzo and Camilleri, 2008). Many of these digestive disorders are associated with acute or chronic inflammatory processes, and with alterations in intestinal permeability. Our data show that cannabinoids have the ability to both positively and negatively modulate permeability through the CB1 receptor. Specifically, endocannabinoids seem to be involved in the increase in permeability associated with the development of inflammation, while phytocannabinoids can inhibit or restore increased permeability after cytokine application.
In our model, basolateral application of 10 ng·mL−1 IFNγ and TNFα led to increased permeability in confluent Caco-2 monolayers, as reflected by a fall in TEER of around 20%. In our study, the effect of cytokines was reversible, as TEER values normalized after washing. Furthermore, LDH levels in media after Caco-2 monolayers were treated with IFNγ and TNFα for 3 days were comparable to those within a non-treated control group. Cell proliferation was also not negatively affected by cytokine application in our study. This indicates that the effect of cytokine application on TEER was not due to cellular damage and changes in transcellular permeability.
Our first main finding was that during inflammatory conditions, the phytocannabinoids THC and CBD both enhanced TEER recovery over time in a concentration-dependent fashion. Cannabinoids have previously been shown to reverse increases in permeability in other models. For example, in a co-culture of endothelial cells and astrocytes, CP55940 and ACEA, both synthetic CB1 receptor agonists, inhibited HIV-1-induced or substance P-induced decreases in epithelial permeability (Lu et al., 2008). Rajesh et al. (2007) also found that CBD attenuates the effects of high glucose-associated increased cellular permeability in human coronary endothelial cells. Furthermore, CBD treatment has been shown to significantly reduce vascular hyperpermeability in the diabetic retina (El-Remessy et al., 2006), improve type I diabetes-induced cardiac dysfunction and inflammation (Rajesh et al., 2010a) and attenuates TNFα signalling, inflammation and kidney dysfunction in a nephropathy model (Pan et al., 2009).
We found that the effects of THC and CBD in reversing the increase in permeability were sensitive to antagonism of the CB1 receptor, but not the CB2 receptor. We also examined a number of other potential sites of action at which cannabinoids are known to act, such as TRPV1 (see Di Marzo and De Petrocellis, 2010) and the PPAR nuclear receptors (see O'Sullivan, 2007), but did not find any contribution from these target sites. Our TEER data were supported by FD4 flux data, demonstrating that CBD reversed increase flux associated with cytokines, and that this was inhibited by a CB1 receptor antagonist. The effect of THC and CBD on permeability is in agreement with our previous study showing phytocannabinoid-mediated changes in intestinal epithelial permeability and tight junction protein expression were brought about through activation of the CB1 receptor (Alhamoruni et al., 2010). It should be noted that received wisdom is that CBD is a poor/ineffective agonist at CB1 receptors (Pertwee, 2008). However, Capasso et al. (2008) and de Filippis et al. (2008) have similarly both shown that the effects of CBD in inhibiting hypermotility in mice were sensitive to CB1 antagonism, which might suggest that CBD agonizes CB1 in the gut. However, another explanation for the effects of CBD in the present study could be that CBD is antagonizing CB1-mediated increases in permeability mediated by locally produced endocannabinoids.
Our data suggest that there may be a therapeutic role for THC or CBD in reversing abnormally increased permeability associated with intestinal inflammation. A prophylactic role was also suggested by our finding that applying THC or CBD at the same time as cytokines completely abolished their deleterious effects on permeability. Similarly, CBD could prevent the increased flux of FD4 if applied at the same time as cytokines. However, if applied 48 h after inflammation was established, the positive effects of phytocannabinoids were no longer observed, suggesting there is a therapeutic window for the use of these compounds in reversing increased permeability. However, this may be different in vivo, as the inflammatory insult may not be reversible as was the case in our current experiments.
Our second main finding was that the endocannabinoids AEA and 2-AG further increased Caco-2 permeability in addition to the effects of the cytokines, and that this effect was concentration-dependent and mediated by the CB1 receptor. This is in agreement with our previous work showing that endocannabinoid application to Caco-2 cells was associated with increased permeability (Alhamoruni et al., 2010). In another cell model, Wang and colleagues have also demonstrated that mAEA (a non-hydrolysable analogue of AEA) increased paracellular permeability in alveolar cells (Wang et al., 2003). We similarly showed in the present study that mAEA further increases the flux of FD4 in addition to the effects of cytokines, and that this effect is mediated by the CB1 receptor.
Several studies have demonstrated increased AEA levels in biopsies from untreated ulcerative colitis patients (D'Argenio et al., 2006), coeliac disease (D'Argenio et al., 2007) and diverticular disease (Guagnini et al., 2006). 2-AG also has been found to be elevated in samples from patients with active coeliac disease, with direct correlations observed between endocannabinoids levels and the most active disease manifestations (D'Argenio et al., 2007). It is therefore possible that overproduction of endocannabinoids plays a role in increased gut permeability in these conditions. We performed a series of experiments examining the potential role of the endocannabinoid system in changes in permeability associated with inflammation. In the first experiment, we showed that a CB1 receptor antagonist (but not a CB2 receptor antagonist) was able to limit the fall in TEER associated with cytokines, and that a CB1 receptor antagonist (but not a CB2 receptor antagonist) limited the increased FD4 flux associated with inflammatory conditions. This suggests that CB1 activation at least partially underlies increased permeability, and we have previously shown that both AEA and 2-AG change the expression of certain tight junction proteins via CB1 activation (Alhamoruni et al., 2010). In an experimental model of diabetic nephropathy in mice, CB1 receptors were found to be overexpressed within the glomeruli, and i.p. injection of AM251 for 14 weeks was found to ameliorate albuminuria by a restoration of the glomeruli junction complex (Barutta et al., 2010). Furthermore, in the small intestine, CB1 receptor antagonism has been shown to inhibit ulcer formation and plasma TNF levels in an indomethacin-induced model of small intestinal inflammation (Croci et al., 2003). CB1 activation is increasingly being shown to be pro-inflammatory in several conditions, including nephropathy (see Mukhopadhyay et al., 2010) and in endothelial and cardiac dysfunction (Rajesh et al., 2010b), supporting our suggestion that endocannabinoid-mediated activation of the CB1 receptor may play a role in mediating the effects of inflammation in our Caco-2 cell model.
In further experiments, we showed that inhibition of the enzymes that degrade either AEA or 2-AG in combination with AEA and 2-AG application caused a very large and irreversible increase in permeability (within our time frame), in addition to the effects of cytokines. More importantly, we also found that application of these enzyme inhibitors alone at the same time as cytokine application worsened the effect cytokines on cell permeability, and this could be antagonized by a CB1 receptor antagonist. This suggests that endocannabinoids may be produced by intestinal epithelial cells during inflammation, and that their activation of the CB1 receptor contributes to tight junction disruption and thus increased permeability. Interesting, the FAAH and MGL inhibitors only worsened the fall in permeability when they were applied at the same time as cytokines (Figure 7), and not when applied after inflammation had been established (Figure 8). This suggests that it is in the development of inflammation that endocannabinoid production may play a role in modulating permeability. It is of note that enhanced tissue inflammation has been observed in FAAH knockout mice in models of inflammation and tissue damage in the liver and cardiac tissue (Siegmund et al., 2006; Mukhopadhyay et al., 2011), again supporting our theory that under pathological conditions, endocannabinoid activation of CB1-dependent mechanisms may contribute to injury in inflammation.
Finally, we found that inhibiting 2-AG synthesis significantly reduced the increased permeability associated with cytokines, demonstrating a role for the local production of 2-AG during inflammation. Unfortunately, no commercially available inhibitor of AEA synthesis exists, so we were unable to test whether a similar reduction might be observed. However, taken together, our data strongly suggest that local release of endocannabinoids, acting via the CB1 receptor, and potentially via changes in tight junction proteins (Alhamoruni et al., 2010) underlie the changes in intestinal epithelial permeability associated with inflammation.
Finally, we did not find that basolateral application of either phytocannabinoids or endocannabinoids influenced the changes in permeability after cytokine application. These findings may reflect differential expression of target sites of action for cannabinoids across epithelial cells in inflammatory conditions, and indicate that it is the apical (luminal) membrane that it is more important in the regulation of permeability in these circumstances. In the light of our findings regarding the potential role for endocannabinoid release during inflammation causing changes in permeability, it also suggests that it is endocannabinoid production at the luminal membrane that may play a role.
In conclusion, our study demonstrates for the first time that cannabinoids are capable of modulating intestinal permeability in an in vitro model of inflammation. In particular, endocannabinoids caused further increases in Caco-2 cell permeability, whereas phytocannabinoids restored increased permeability induced by cytokines. The effects of cytokines on increased permeability were inhibited by a CB1 receptor antagonist and a 2-AG synthesis inhibitor, and were enhanced by inhibitors of the degradation of AEA or 2-AG, suggesting that local production of endocannabinoids activating CB1 may play a role in the modulation of gut permeability during inflammation. Our study also suggests that cannabis-based medicines may possess therapeutic benefit in inflammatory intestinal disorders associated with abnormal intestinal permeability.
Acknowledgments
We would like to thank the technical support of Mrs Averil Warren and Mr Andrew Lee.
Glossary
- AEA
arachidonyl-ethanolamide, anandamide
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM630
6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl) methanone
- Caco-2
carcinoma colon cell line
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- CBD
cannabidiol
- EVOM
epithelial tissue volt-ohm-meter
- FAAH
fatty acid amide hydrolase
- GW6471
[(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- IBD
inflammatory bowel disease
- JZL 184
4-nitrophenyl-4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- MGL
monoacylglycerol lipase
- O-1918
1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene
- TEER
transepithelial electrical resistance
- THC
Δ9-tetrahydrocannabinol
- URB597
3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhamoruni A, Lee AC, Wright KL, Larvin M, O'Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1149–1156. doi: 10.1007/s00384-009-0737-8. [DOI] [PubMed] [Google Scholar]
- Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg. 1999;3:252–262. doi: 10.1016/s1091-255x(99)80067-5. [DOI] [PubMed] [Google Scholar]
- Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, et al. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59:1046–1054. doi: 10.2337/db09-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001–1008. doi: 10.1038/bjp.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu MA, Porcella A, Ruiu S, Saba P, Marchese G, Carai MA, et al. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003;459:97–105. doi: 10.1016/s0014-2999(02)02830-3. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- Croci T, Manara L, Aureggi G, Guagnini F, Rinaldi-Carmona M, Maffrand JP, et al. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br J Pharmaco. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci T, Landi M, Galzin AM, Marini P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br J Pharmacol. 2003;140:115–122. doi: 10.1038/sj.bjp.0705412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Petrosino S, Gianfrani C, Valenti M, Scaglione G, Grandone I, et al. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J Mol Med. 2007;85:523–530. doi: 10.1007/s00109-007-0192-3. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Filippis D, Iuvone T, d’amico A, Esposito G, Steardo L, Herman AG, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil. 2008;20:919–927. doi: 10.1111/j.1365-2982.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- Guagnini F, Valenti M, Mukenge S, Matias I, Bianchetti A, Di Palo S, et al. Neural contractions in colonic strips from patients with diverticular disease: role of endocannabinoids and substance P. Gut. 2006;55:946–953. doi: 10.1136/gut.2005.076372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- Ihenetu K, Molleman A, Parsons ME, Whelan CJ. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur J Pharmacol. 2003;458:207–215. doi: 10.1016/s0014-2999(02)02698-5. [DOI] [PubMed] [Google Scholar]
- Izzo AA. The cannabinoid CB(2) receptor: a good friend in the gut. Neurogastroenterol Motil. 2007;19:704–708. doi: 10.1111/j.1365-2982.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Brown DR. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- Lu TS, Avraham HK, Seng S, Tachado SD, Koziel H, Makriyannis A, et al. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J Immunol. 2008;181:6406–6416. doi: 10.4049/jimmunol.181.9.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Madara JL, Stafford J, Barenberg D, Carlson S. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am J Physiol. 1988;254(3 Pt 1):G416–G423. doi: 10.1152/ajpgi.1988.254.3.G416. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehouse JL, Specian RD, Stewart JJ, Berg RD. Translocation of indigenous bacteria from the gastrointestinal tract of mice after oral ricinoleic acid treatment. Gastroenterology. 1986;91:673–682. doi: 10.1016/0016-5085(86)90638-4. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;3:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–195. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Sur Res. 2004;116:14–18. doi: 10.1016/s0022-4804(03)00311-1. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610–H619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010a;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Mackie K, Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol. 2010b;160:688–700. doi: 10.1111/j.1476-5381.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ. Endocannabinoids and the gastrointestinal tract: what are the key questions? Br J Pharmacol. 2007;152:663–670. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund SV, Seki E, Osawa Y, Uchinami H, Cravatt BF, Schwabe RF. Fatty acid amide hydrolase determines anandamide-induced cell death in the liver. J Biol Chem. 2006;281:10431–10438. doi: 10.1074/jbc.M509706200. [DOI] [PubMed] [Google Scholar]
- Unno N, Fink MP. Intestinal epithelial hyperpermeability. Mechanisms and relevance to disease. Gastroenterol Clin North Am. 1998;27:289–307. doi: 10.1016/s0889-8553(05)70004-2. [DOI] [PubMed] [Google Scholar]
- Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, et al. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- Wells CL, van de Westerlo EM, Jechorek RP, Haines HM, Erlandsen SL. Cytochalasin-induced actin disruption of polarized enterocytes can augment internalization of bacteria. Infection Immunity. 1998;66:2410–2419. doi: 10.1128/iai.66.6.2410-2419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]


