Abstract
BACKGROUND AND PURPOSE
Numerous studies have shown that N-arachidonoylethanolamine (AEA) can inhibit sperm motility and function but the ability of cannabinoids to inhibit sperm motility is not well understood. We investigated the effects of WIN 55,212-2, a CB1 cannabinoid receptor agonist, and Δ9-tetrahydracannabinol (Δ9-THC) on the ATP levels and motility of murine sperm in vitro. In addition, the effects of acute administration of Δ9-THC on male fecundity were determined.
EXPERIMENTAL APPROACH
Effects of Δ9-THC on basal sperm kinematics were determined using computer-assisted sperm analysis (CASA). Stop-motion imaging was performed to measure sperm beat frequency. The effect of Δ9-THC on sperm ATP was determined using a luciferase assay. Male fertility was determined by evaluating the size of litters sired by Δ9-THC-treated males.
KEY RESULTS
Pretreatment of sperm for 15 min with 1 µM Δ9-THC reduced their basal motility and attenuated the ability of bicarbonate to stimulate flagellar beat frequency. Treatment with 5 µM WIN 55,212-2 or 10 µM Δ9-THC for 30 min reduced sperm ATP levels. In sperm lacking CB1 receptors this inhibitory effect of WIN 55,212-2 on ATP was attenuated whereas that of Δ9-THC persisted. Administration of 50 mg·kg−1Δ9-THC to male mice just before mating caused a 20% decrease in embryonic litter size.
CONCLUSIONS AND IMPLICATIONS
Δ9-THC inhibits both basal and bicarbonate-stimulated sperm motility in vitro and reduces male fertility in vivo. High concentrations of WIN 55,212-2 or Δ9-THC inhibit ATP production in sperm; this effect of WIN 55,212-2 is CB1 receptor-dependent whereas that of Δ9-THC is not.
LINKED ARTICLES
This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
Keywords: sperm motility, cannabinoid, CB1 receptor, Δ9-THC, sperm energetics, male reproduction
Introduction
Sperm capacitation refers to ‘the change undergone by sperm in the female reproductive tract that enables them to penetrate and fertilize an egg’ (Chang et al., 1976). Capacitation occurring either in vitro or in the female reproductive tract involves a series of changes in sperm physiology including phospholipid remodelling of the plasma membrane, redistribution of membrane cholesterol, tyrosine phosphorylation of sperm proteins, increased motility, hyperactivation and the acrosome reaction. Early-stage capacitation events include increased synthesis of cAMP, which activates PKA, causing the onset of bicarbonate-stimulated sperm motility (Wennemuth et al., 2003; Nolan et al., 2004; Morgan et al., 2008). Sperm exhibit limited basal motility in the absence of bicarbonate. The presence of bicarbonate leads to the acquisition of rapid and progressive motility caused by elevated flagellar beat frequency (Wennemuth et al., 2003). Late-stage capacitation events such as hyperactivated motility, tyrosine phosphorylation and the acrosome reaction require prolonged exposure to bicarbonate and BSA in vitro (Byrd, 1981; Lee and Storey, 1986; Boatman and Robbins, 1991; Visconti et al., 1995a,b; Harrison, 1996).
Previous work has found a complete endocannabinoid signalling system in sperm. Sperm from mice, humans, pigs and frog express the CB1 receptor, while CB2 has been detected in boar and human sperm (Maccarrone et al., 2005; Rossato et al., 2005; Cobellis et al., 2006; Francavilla et al., 2009; Aquila et al., 2010b). However, CB2 protein appears absent from mouse sperm (Grimaldi et al., 2009). The TRPV1 channel, an ion-channel receptor for capsaicin that is also activated by N-arachidonoylethanolamine (AEA) is detected in boar and human sperm (Schuel et al., 2002a; Maccarrone et al., 2005; Francavilla et al., 2009; Grimaldi et al., 2009). The endocannabinoid AEA as well as NAPE-PLD, one of the enzymes responsible for its synthesis have been detected in human sperm (Francavilla et al., 2009). AEA has been detected in the female reproductive tract and seminal fluid at concentrations as high as 10–12 nM (Schuel et al., 2002a; Schuel, 2006). The enzymes, sn-1-diaglycerol (DAGL) and monoacylglycerol lipase (MAGL), that are responsible for the synthesis and degradation of 2-AG, respectively, are detected in epididymal sperm (Cobellis et al., 2010). The enzyme FAAH hydrolyzes AEA and is detected in sperm from frog, boar and human (Maccarrone et al., 2005; Cobellis et al., 2006; Francavilla et al., 2009). Consistent with an important role for FAAH in reproduction, male mice lacking FAAH exhibit decreased litter size (Sun et al., 2009). Sperm from these mice have reduced motility, decreased ability to undergo the acrosome reaction and lower capacity for in vitro fertilization (Sun et al., 2009). Previous work has shown that Δ9-THC and AEA inhibit the fertilizing capacity (capacitation) of sea urchin sperm (Chang et al., 1991; 1993; Schuel et al., 1991; 1994). More recent work has shown decreased progressive motility and a reduced ability to undergo the acrosome reaction in human sperm treated with sub-micromolar concentrations of Δ9-THC (Whan et al., 2006). Additional studies have found that AEA inhibits sperm motility, hyperactivation, mitochondrial function, plasma membrane voltage potential, as well as the zona pellucida-stimulated acrosome reaction (Schuel et al., 2002b; Maccarrone et al., 2005; Rossato et al., 2005). Exposure to 1 µM methananamide (Me-AEA), a non-hydrolyzable analogue of AEA, also inhibits sperm motility and mitochondrial membrane potential in a CB1-dependent manner (Barbonetti et al., 2010). Treatment of human sperm with either Δ9-THC or Δ8-THC reduces mitochondrial O2 production indicating that both endocannabinoids as well as phytocannabinoids such as Δ9-THC can impair mitochondrial respiration (Badawy et al., 2009). Antagonism of CB1 with rimonabant has been shown to enhance sperm motility, sperm energy metabolism, survival, protein tyrosine phosphorylation and the capacity to undergo the acrosome reaction (Aquila et al., 2010a). Inhibition of the TRPV1 receptor increases the incidence of spontaneous acrosome reaction in human and boar sperm suggesting that TRPV1-mediated AEA signalling is important for correct timing of the acrosome reaction (Maccarrone et al., 2005; Francavilla et al., 2009). However, despite the large number of recent studies, the effects of cannabinoids on basal and bicarbonate-stimulated flagellar beat frequency, male fertility and sperm energetics are still not well understood. In particular, very little is known about the possible effects of Δ9-THC on the bicarbonate-stimulated motility that occurs within the female reproductive tract. Therefore, in this study, we have investigated whether Δ9-THC inhibits sperm ATP levels as well as basal and bicarbonate-stimulated motility in vitro. We have also given male mice a single injection of 50 mg·kg−1Δ9-THC, just prior to mating, to determine whether acute exposure to Δ9-THC inhibits litter size in vivo.
Methods
Animals
All animal care and experimental procedures were approved by the institutional animal care and use committees at the University of Washington or Indiana University and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. CD1 male mice were obtained from Charles River (Wilmington, MA). CB1 knockout mice in a CD1 background were generously provided by Catherine Ledent and bred in our facility (Ledent et al., 1999). Nomenclature for receptors follows BJP's Guide to Receptors and Channels (Alexander et al., 2011). All mice used in these experiments were housed under a 12:12 h light–dark cycle (lights on 06h 00min, lights off 18h 00min) and provided with standard mouse chow ad libitum. In order to harvest sperm, mice were killed by CO2 asphyxiation followed by cervical dislocation.
Sperm preparation
The caudal epididymides and vasa deferentia were excised and cleaned in HS medium containing: 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 1 mM pyruvic acid, 20 mM lactic acid, 5 mM glucose and 20 mM HEPES (pH 7.4). Sperm were harvested by a 15 min ‘swim out’ in medium HS supplemented with 5 mg BSA mL−1. Released sperm were sedimented, then resuspended in BSA-free HS medium. Bicarbonate stimulation of motility was measured in HS medium supplemented with 15 mM NaHCO.
Analysis of sperm motility
Flagellar beat frequency was examined in individual sperm as previously described (Wennemuth et al., 2003; Nolan et al., 2004; Morgan et al., 2008). Motility was analysed for sperm bathed with HS medium alone or HS medium containing Δ9-THC for 15 min. No sperm possessing a well-defined sinusoidal waveform necessary for estimation of beat frequency were observed for sperm treated with 10 µM Δ9-THC for 15 min. Therefore, data for sperm treated with 10 µM Δ9-THC for 13 min, the longest period of treatment for which beat frequency could be determined, were shown in Supporting Information Figure S1. Bicarbonate-stimulated motility was analysed in sperm perfused with HS medium containing 15 mM bicarbonate for 60 s. Briefly, stop-motion images were collected at 20–40 ms intervals for sperm loosely tethered to a glass surface at the head. A solenoid-controlled gravity-driven local perfusion device produced rapid changes in medium composition. Images were processed, and motility was determined using MetaMorph (Universal Imaging, Downington, PA). Computer-assisted sperm analysis (CASA) was performed using a Hamilton-Thorne Research IVOS sperm motility analysis system with version 10 software as previously described (Hamilton-Thorne, Danvers, MA) (Burton et al., 1999). Sperm were treated with a range of Δ9-THC concentrations (0.001, 0.01, 0.1, 1 and 10 µM) in HS medium. For each Δ9-THC concentration examined, sperm were pretreated with HS medium containing the appropriate concentration of Δ9-THC for 30 min prior to CASA. In separate experiments, sperm were incubated with 10 µM Δ9-THC for 15, 30, 60 or 90 min prior to CASA. CASA was performed on sperm placed in 20 µm-deep Leja Standard Count fixed-coverslip slides (Leja Products B.V., Nieuw-Vennep, the Netherlands). Analysis was restricted to 15–100 track points at a 60 Hz frame rate using Olympus ‘negative phase’ optics. Standard kinematics were calculated by the CASA programme. Cells exhibiting less than 10 µm·s−1 average path velocity were considered to be non-motile. Sperm velocity was measured as straight-line velocity (VSL; the straight-line distance from beginning to end of track divided by the elapsed time), average path velocity (VAP; the five-point smoothed average path distance divided by time elapsed) and curvilinear velocity (VCL or track speed; the total distance between all detected head centroids divided by the elapsed time).
ATP assay
Sperm ATP levels were determined using a luciferase-based ATP Determination Kit from Molecular Probes (Eugene, OR) and a Lmax II microplate reader (Molecular Devices, Sunnyvale, CA). Prior to assaying for ATP, sperm were treated with HS medium containing Δ9-THC, WIN 55,212-2 or the inactive enantiomer, WIN 55,212-3. The concentration curve examining the effects of Δ9-THC on ATP was determined in sperm treated with 0, 0.001, 0.01, 0.1, 1 and 10 mM Δ9-THC for 30 min. Experiments investigating the amount of time required for 10 µM Δ9-THC or 5 µM WIN 55,212-2 to reduce ATP levels in sperm were performed using sperm treated with HS medium containing drug for 15, 30, 60 or 90 min. Quantification of ATP was determined by measuring luminescence.
Analysis of Δ9-THC effects on litter size
Sexually mature wild-type and CB1 knockout (KO) male CD1 mice (12–18 weeks) were given i.p. injections of either 50 mg·kg−1Δ9-THC (n= 9 wild-type males and 17 litters) or 18:1:1 vehicle containing 0.9% saline, 5% cremaphor and 5% ethanol (n= 7 wild-type males and 20 litters). Litter size was also examined for CB1 KO males treated with 50 mg·kg−1Δ9-THC (n= 5 males and 6 litters) or vehicle (n= 5 males and 6 litters). The volume of vehicle or 5 mg·mL−1Δ9-THC injected was 10 µL·g−1 of body weight. Injections were administered just prior to the onset of the dark cycle, and injected males were bred overnight with wild-type CD1 females. Plug-positive CD1 females were removed from breeding cages the following morning. New cages of wild-type male and female breeders were set up daily due to possible desensitization of sperm CB1 receptors. Males treated with Δ9-THC were reused after being allowed to recover for 2 weeks. Plug-positive females were killed by CO2 asphyxiation on the 12th day of gestation, and litter size was determined by counting the number of e12.5 embryos present.
Data analysis
Data are presented as means ± SEM. Statistical analyses were performed using Microsoft Excel (unpaired t-tests) (Redmond, WA, USA) or GraphPad Prism 4 (La Jolla, CA, USA) (two-way anova with Bonferroni's post hoc test). Unpaired t-tests were used to analyse data shown in Figures 1–3. A two-way repeated-measures anova with Bonferroni's post hoc test was used to analyse the data in Figure 4.
Figure 1.
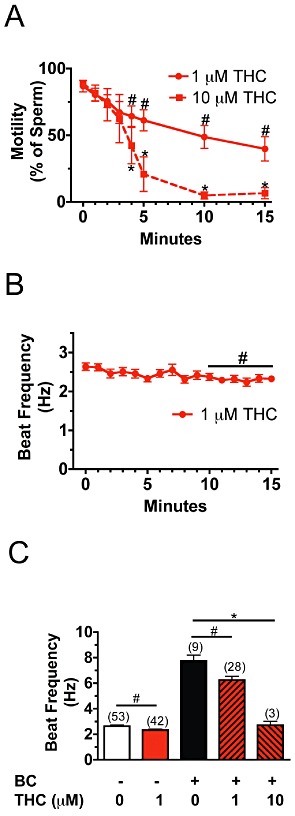
Basal and bicarbonate-stimulated motility is inhibited by Δ9-THC. (A) Treatment with 1 µM Δ9-THC or 10 µM Δ9-THC for 15 min progressively reduced the percentage of motile sperm. (n= 111–138 cells). At least eight cells were examined from each animal in two to three independent experiments. #P < 0.05 (untreated vs. 1 µM THC), *P < 0.05 (untreated vs. 10 µM THC). (B) Averaged flagellar beat frequency was determined for wild-type sperm that were bathed in HS medium containing 1 µM Δ9-THC (THC) for 15 min. (n= 22–47 cells). At least eight cells were examined from each animal in two to three independent experiments. #P < 0.05 (untreated vs. 1 µM THC). (C) Sperm were bathed in HS medium alone or HS medium containing 1 µM or 10 µM Δ9-THC (THC) for 15 min and subsequently perfused with HS medium containing 15 mM HCO3- (BC) for 1 min. Bicarbonate-stimulated beat frequency was reduced in sperm treated with 1 µM Δ9-THC relative to sperm treated with HS medium containing bicarbonate. #P < 0.05 (BC vs. BC+1 µM THC). Treatment of sperm with 10 µM Δ9-THC completely blocked the stimulating effect of bicarbonate on beat frequency. *P < 0.001 (BC vs. BC +10 µM THC). The number of sperm used for each condition is designated in parentheses. Unpaired t-tests were used to calculate P values. Error bars represent SEM.
Figure 3.
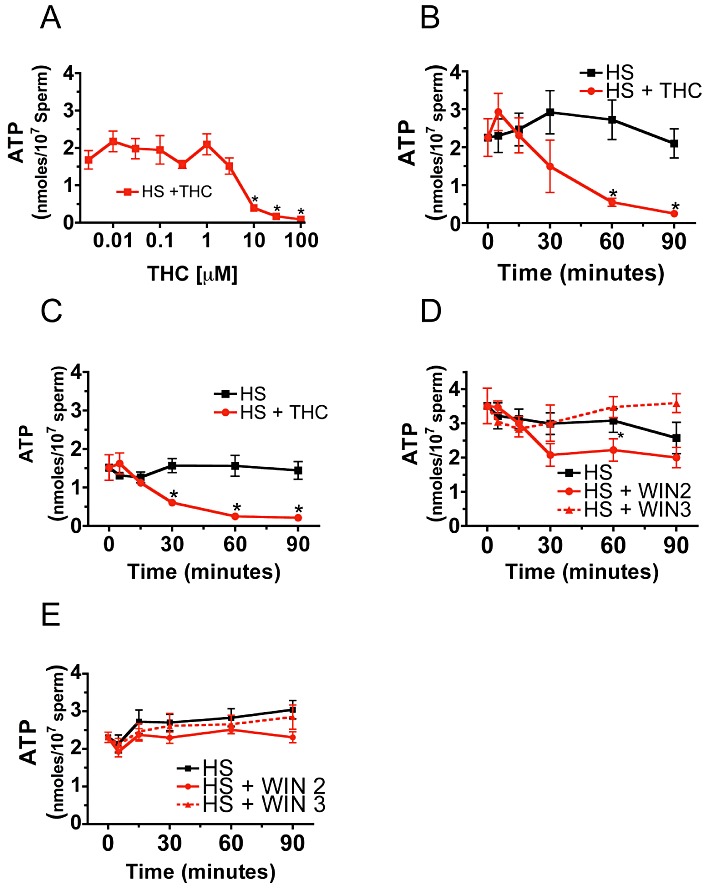
Δ9-THC and WIN 55,212-2 reduce sperm ATP levels. Treatment of sperm for 60 min with 10, 30 and 100 µM Δ9-THC reduced ATP levels (A). 10 µM Δ9-THC reduced ATP levels in sperm from wild-type mice relative to untreated controls (HS) in a time-dependent fashion (B). Significantly, the effect of Δ9-THC on ATP levels was present in sperm lacking CB1 receptors, suggesting that the inhibitory effect of Δ9-THC on ATP levels was not CB1 mediated (C). Treatment with 5 µM WIN 55,212-2 (WIN2) causes a 35% reduction in ATP levels in wild-type sperm. WIN 55,212-3 (WIN3), which does not bind with high affinity to CB1 receptors, had no effect on sperm ATP levels (D). The effect of WIN 55,212-2 on sperm ATP was absent in sperm lacking the CB1 cannabinoid receptor (E). Student's unpaired t-test was used to calculate significance (*P < 0.05). Error bars represent SEM.
Figure 4.
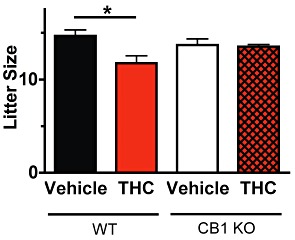
Acute administration of Δ9-THC reduces male fertility. The acute effect of Δ9-THC on male fertility was determined by measuring embryonic (e12.5) litter sizes sired by vehicle treated CD1 wild-type (WT) males (n= 7 males with 20 litters) or CD1 wild-type males treated with 50 mg·kg−1Δ9-THC (n= 9 males with 17 litters). The effect of vehicle (n= 5 males with 6 litters) and 50 mg·kg−1Δ9-THC (n= 5 males with 6 litters) on litter size was also examined in mice lacking CB1 receptors (KO). Error bars indicate SEM and P-values were calculated using two-way repeated measures anova with Bonferroni's post test (*P < 0.01).
Results
Activation of sperm motility
We examined the effects of 1 and 10 µM Δ9-THC on basal and bicarbonate-stimulated motility in sperm from wild-type CD1 mice. The percentage of motile wild-type sperm decreased from 82% (untreated sperm) to 35% when sperm were bathed in HS medium containing 1 µM Δ9-THC for 15 min (Figure 1A). However, the slow resting beat frequency of motile sperm (2.63 ± 0.09 Hz) was only slightly decreased to 2.33 ± 0.08 Hz (P < 0.01) during 15 min of exposure to 1 µM Δ9-THC (Figure 1B). While perfusion of HS medium containing 15 mM NaHCO3 for 1 min caused a threefold increase in beat frequency (7.8 ± 0.6 Hz) in wild-type sperm, perfusion of sperm exposed to 1 µM Δ9-THC in the same medium increased their beat frequency significantly less (6.24 ± 0.29 Hz; P < 0.01) (Figure 1C). Treatment of sperm with 10 µM Δ9-THC for 15 min reduced the percentage of motile sperm to 5% (Figure 1A). The basal beat frequency of sperm treated with 10 µM Δ9-THC for 13 min was reduced to 1.02 ± 0.06 Hz (Supporting Information Figure S1). Bicarbonate-stimulated motility was completely abolished in the few 10 µM Δ9-THC-treated sperm that did possess a sinusoidal waveform (Figure 1C). Thus, 15 min of treatment with 1 µM Δ9-THC reduces the activating effects of bicarbonate on beat frequency by 20%, while 10 µM Δ9-THC completely abolishes this form of motility (Figure 1C).
Sperm kinematics
Sperm motility was also evaluated by CASA in wild-type mouse sperm treated with increasing concentrations of Δ9-THC for 30 min. We found that Δ9-THC at 1 µM and above inhibited curvilinear velocity (Figure 2E), while only 10 µM Δ9-THC inhibited VSL (Figure 2A) and VAP (Figure 2C). Additional CASA analyses were performed to determine the amount of time required for 10 µM Δ9-THC to inhibit sperm motility. In these experiments, treatment of wild-type sperm with 10 µM Δ9-THC for 15 min or more generally decreased the VSL (Figure 2B), VAP (Figure 2D) and VCL (Figure 2F).
Figure 2.
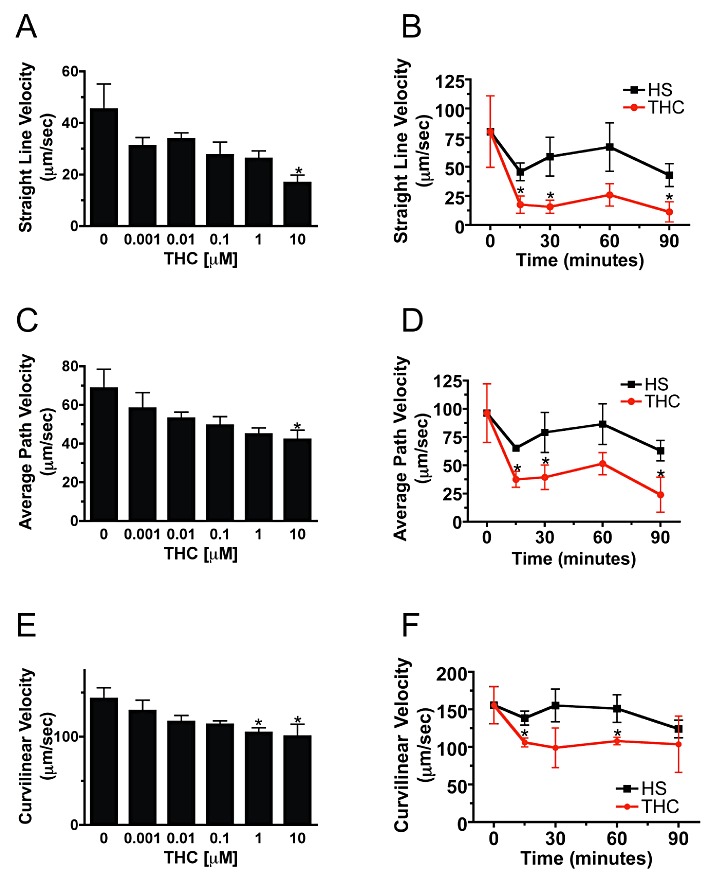
Sperm kinematics are rapidly inhibited by 10 µM Δ9-THC. Dose–response curves over a range of Δ9-THC concentrations were constructed for CASA kinematic analysis (A, C, E). Treatment of sperm with 10 µM Δ9-THC for 30 min significantly inhibited VSL (A) and average path velocity (C), while VCL (E) was inhibited by both 1 and 10 µM Δ9-THC. (n= 2113–4498 cells for A, C, and E.) *, P < 0.05 (untreated vs. 10 µM THC and 1 µM THC). Onset of the Δ9-THC effect was rapid, with treatment of wild-type sperm with 10 µM Δ9-THC decreasing the VSL (B), VAP (D) and VCL (F) within 15 min. (n= 791–1603 cells for HS in B, D, and F). (n= 1240, 886, 252, 113 and 620 cells at 0, 15, 30, 60 and 90 min for HS +THC in B, D and F) *P < 0.05 (HS vs. THC). Unpaired t-tests were used to calculate P-values. Error bars represent SEM.
Sperm ATP
In multiple studies, AEA or Me-AEA has been shown to inhibit sperm motility (Schuel et al., 2002b; Maccarrone et al., 2005; Rossato et al., 2005; Barbonetti et al., 2010). Therefore, we hypothesized that decreased ATP production due to mitochondrial dysfunction might account for the reduced basal motility of Δ9-THC-treated sperm. We found that treatment with either 5 µM WIN 55,212-2 (high-efficacy CB1 agonist) or 10 µM Δ9-THC (low-efficacy CB1 agonist) reduced sperm ATP levels (Figure 3). A concentration-effect curve for Δ9-THC inhibition of sperm ATP levels was determined, and 10, 30 and 100 µM Δ9-THC significantly reduced sperm ATP (Figure 3A). Treatment of wild-type sperm with 10 µM Δ9-THC reduced ATP levels by 91% relative to untreated controls (Figure 3B) within 60 min. The inhibitory effect of Δ9-THC on ATP persisted in sperm lacking CB1 receptors, suggesting this effect was not mediated by CB1 receptors (Figure 3C). Exposure to 5 µM WIN 55,212-2 for 30 min caused a 35% decrease in sperm ATP (Figure 3D). The inhibitory effect of 5 µM WIN 55,212-2 on sperm ATP was attenuated in sperm lacking the CB1 receptor (Figure 3E). The non-CB1 interacting enantiomer, WIN 55,212-3, had no effect on sperm ATP levels in either wild-type or CB1 deficient sperm (Figure 3D, E), suggesting that the effects of 5 µM WIN 55,212-2 on sperm ATP levels are mediated by CB1 receptors, while those of Δ9-THC are not.
Litter size
To determine if acute exposure of sperm to Δ9-THC inhibited male fertility, 50 mg·kg−1Δ9-THC was administered to male CD1 mice just prior to breeding at the onset of the dark cycle. Litter size from vehicle- or drug-treated males was determined by counting the number of embryos from plug-positive CD1 females on the 12th day of gestation. Acute administration of 50 mg·kg−1Δ9-THC reduced litter size from 14.7 ± 0.6 (vehicle-treated wild-type males, n= 7) to 11.8 ± 0.8 (THC treated wild-type males, n= 9) embryos per litter (P < 0.01). Litter size was also examined in male CB1 knockout mice to determine whether the reduction in litter size observed in wild-type mice treated with 50 mg·kg−1Δ9-THC was CB1-mediated. Acute administration of 50 mg·kg−1Δ9-THC had no effect on the sizes of litters sired by CB1 knockout males (13.5 ± 0.2, n= 6 males) when compared with vehicle-treated CB1 knockout males (13.7 ± 0.7, n= 6 males).
Discussion and conclusions
Basal motility
The primary objective of this study was to employ multiple methodological approaches to better understand the effects of Δ9-THC on sperm ATP levels and motility. Numerous previous studies have demonstrated that AEA inhibits basal sperm motility as well as other sperm functions such as the acrosome reaction that are required for fertilization of the oocyte. However, the ability of exogenous cannabinoids such Δ9-THC to inhibit sperm motility is not well understood. Earlier studies investigating the role of cannabinoid signalling in sperm motility have focused mostly on the ability of AEA, Me-AEA or Δ9-THC to reduce the percentage of motile sperm. However, a recent study using CASA demonstrated that the motility of human sperm is inhibited by 5 and 10 µM Me-AEA (Barbonetti et al., 2010). Our study confirms previous work showing that activation of cannabinoid signalling increases the percentage of immotile sperm. However, extending previous studies, we have used CASA and stop-motion videos to determine whether Δ9-THC reduces the beat frequency and swimming speed (kinematics) of the remaining fraction of sperm that are motile. Interestingly, we find that while 1 µM Δ9-THC dramatically reduces the percentage of sperm that are motile, the beat frequency of sperm that retain their motility is only slightly affected (12% reduction). Measurement of sperm motility using CASA indicates 1 µM Δ9-THC reduces sperm ‘swimming speed’ by 46% (VSL), 42% (VAP) and 30% (VCL).
Sperm ATP
Inhibition of sperm ATP production is one way that Δ9-THC might reduce basal motility. Recent work has demonstrated the ability of Me-AEA to disrupt mitochondrial function in sperm (Rossato et al., 2005; Barbonetti et al., 2010). However, blockade of electron transport with the respiratory chain complex I inhibitor, rotenone, does not significantly impair sperm motility when glucose is present, and glycolysis is able to occur (Barbonetti et al., 2010). Sperm motility and ATP levels were also normal when oxidative phosphorylation was inhibited using carbonyl cyanide m-chlorophenylhydrazone (Mukai and Okuno, 2004). In contrast, sperm from mice lacking glyceraldehyde-3-phosphate dehydrogenase-S, an enzyme required for glycolysis in sperm, fail to exhibit progressive motility (Miki et al., 2004). Cumulatively, these earlier studies suggest that glycolysis rather than oxidative phosphorylation produces most of the ATP needed to sustain motility in sperm. In order to determine whether Δ9-THC impairment of mitochondrial function might disrupt energy production, we investigated ATP levels in sperm treated with Δ9-THC. In this study we find that 10 µM Δ9-THC severely decreases sperm ATP levels in a CB1 receptor-independent manner. Since this effect is present in CB1−/−sperm it is likely that 10 µM Δ9-THC reduces ATP via a non-CB1-mediated mechanism. Previous work has shown that 10 µM AEA reduces sperm viability, raising the possibility that 10 µM Δ9-THC might be reducing ATP levels in our study via CB1-independent cytotoxicity (Barbonetti et al., 2010). In contrast, treatment with 1 µM Δ9-THC does not decrease sperm ATP levels despite the ability of this concentration to inhibit basal motility. These results suggest that the inhibition of basal motility by 1 µM Δ9-THC is not caused by THC-induced decreases in ATP availability. However, treatment with 5 µM WIN 55,212-2 does cause moderate reductions in sperm ATP levels that are absent in CB1 knockout sperm or sperm treated with the inactive enantiomer WIN 55,212-3, suggesting an efficacious CB1 agonist can inhibit ATP production.
Bicarbonate-stimulated motility
To date, the ability of endo- or exo- cannabinoids to inhibit bicarbonate-stimulated motility has not been studied. This type of motility is best characterized by an increase in progressive forward motility due to increased flagellar beat frequency. Studies of mutant sperm lacking either the sperm-specific PKA catalytic subunit (Cα2) (Nolan et al., 2004) or the soluble form of adenylyl cyclase (SACY) (Esposito et al., 2004; Hess et al., 2005; Xie et al., 2006) have provided definitive evidence that both proteins are required for the acceleration of the flagellar beat frequency that characterizes the rapid activation of motility by the HCO3- anion. However, neither cAMP production (SACY) nor PKA activation in sperm (Cα2) are required for the maintenance of a slow basal flagellar beat. Mice possessing sperm that are unable to synthesize cAMP in response to bicarbonate are infertile demonstrating the necessity of this signalling pathway for fertility (Esposito et al., 2004; Hess et al., 2005; Xie et al., 2006). The ability of sperm treated with Δ9-THC to undergo bicarbonate-stimulated motility was investigated in this study. We find that 15 min of treatment with 1 µM Δ9-THC attenuates bicarbonate enhancement of beat frequency by 20%. However, despite the slightly reduced response to bicarbonate these sperm do respond to bicarbonate by substantially increasing their beat frequency from 2.64 Hz (resting basal motility) to 6.24 Hz. Previous work has shown that activation of the Gi/o-coupled CB1 inhibits the production of cAMP by transmembrane adenylyl cyclases (tmACs) (Howlett et al., 1986; 1990; 2004). However, the increase in cAMP synthesis that drives bicarbonate-stimulated motility in sperm is catalysed by SACY rather than tmAC. The finding that 10 µM Δ9-THC does not appear to block bicarbonate-stimulated motility supports the conclusion that bicarbonate-stimulated cAMP signalling via SACY is not substantially modulated by CB1 receptors.
In vivo male reproduction
Previous work demonstrated that chronic treatment with cannabinoids causes a reduction in spermatogenesis, circulating testosterone and male fertility (Dalterio et al., 1982). This early study raised the possibility that chronic exposure to cannabinoids might inhibit male fertility via endocrine mediated down-regulation of spermatogenesis. In order to determine whether Δ9-THC might inhibit male fertility via an acute, non-endocrine mechanism on sperm function, male mice were treated with 50 mg·kg−1Δ9-THC just before mating (onset of the dark phase of the light–dark cycle). Treatment of wild-type CD1 males with 50 mg·kg−1Δ9-THC reduced their litter size by 20% (11.8 ± 0.8) relative to vehicle-treated males (14.7 ± 0.6). The effect of 50 mg·kg−1Δ9-THC on decreased litter size was absent in CB1 knockout males, suggesting that effects of Δ9-THC on litter size is CB1 mediated. Interestingly, the 20% reduction in litter size from males treated acutely with 50 mg·kg−1Δ9-THC is similar in magnitude to the reduced litter size for FAAH−/−males that has been previously reported (Sun et al., 2009). Our result raises the possibility that acute administration of Δ9-THC inhibits male fertility by a mechanism involving reduced sperm function.
Taken together, the results of the current study provide significant new insight into the ability of cannabinoid signalling to partially inhibit bicarbonate-stimulated motility while providing additional evidence that cannabinoids can inhibit basal motility. We found that 10 µM Δ9-THC inhibits ATP levels in sperm through a non-CB1 mechanism since the reduction of ATP by 10 µM Δ9-THC is retained in CB1 knockout sperm. In contrast, treatment with 5 µM WIN 55,212-2, an efficacious CB1 agonist, caused a more modest 42% decrease in sperm ATP levels that was absent in CB1 knockout sperm or sperm treated with the inactive analogue WIN 55,212-3. This finding suggests that CB1 activation can disrupt sperm energetics and ATP levels under certain conditions. Finally, we also determined that a single acute injection of 50 mg·kg−1Δ9-THC to male mice just prior to mating can the reduce size of litters sired by those males.
Acknowledgments
This research was supported by NIH grants DA11322 and DA021696, the Indiana University MetaCyt Initiative (funded in part by a grant from the Lilly Foundation), and the Linda and Jack Gill Center for Biomolecular Science. We would like to thank Donner Babcock for assistance capturing stop-motion images for determining sperm flagellum beat frequency.
Glossary
- Δ9-THC
Δ9-tetrahydrocannabinol
- AEA
N-arachidonoylethanolamine
- CASA
computer-assisted sperm analysis
- CB1
cannabinoid receptor 1
- DAGL
sn-1-diaglycerol
- KO
knockout
- MAGL
monoacylglycerol lipase
- Me-AEA
methanadamide
- SACY
soluble adenylyl cyclase
- tmAC
transmembrane adenylyl cyclase
- VAP
average path velocity
- VCL
curvilinear velocity
- VSL
straight-line velocity
- WIN
55,212-2 or WIN-2, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1, 4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
- WIN
55,212-3 or WIN-3, (S)-(–)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1, 4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Treatment with 10 μMΔ9-THC reduces basal beat frequency. Averaged flagellar beat frequency was determined for wild type sperm that were bathed in HS medium containing 1 μM Δ9-THC (red circles and solid line) or 10 μM Δ9-THC (red squares and dashed line) (THC) for up to 15 min. (n = 3–47 cells from 2–3 independent experiments). *P< 0.05 (Untreated vs. + 10 μM THC). #P < 0.05 (untreated vs. 1 μM THC). Error bars represent the SEM and P-values were calculated by unpaired Student's t-tests.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, Gazzerro P, Laezza C, Baffa MF, et al. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br J Pharmacol. 2010a;159:831–841. doi: 10.1111/j.1476-5381.2009.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, Perrotta I, Laezza C, Bifulco M, et al. Human sperm anatomy: ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec (Hoboken) 2010b;293:298–309. doi: 10.1002/ar.21042. [DOI] [PubMed] [Google Scholar]
- Badawy ZS, Chohan KR, Whyte DA, Penefsky HS, Brown OM, Souid AK. Cannabinoids inhibit the respiration of human sperm. Fertil Steril. 2009;91:2471–2476. doi: 10.1016/j.fertnstert.2008.03.075. [DOI] [PubMed] [Google Scholar]
- Barbonetti A, Vassallo MR, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151:5882–5892. doi: 10.1210/en.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman DE, Robbins RS. Bicarbonate: carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol Reprod. 1991;44:806–813. doi: 10.1095/biolreprod44.5.806. [DOI] [PubMed] [Google Scholar]
- Burton KA, Treash-Osio B, Muller CH, Dunphy EL, McKnight GS. Deletion of type IIalpha regulatory subunit delocalizes protein kinase A in mouse sperm without affecting motility or fertilization. J Biol Chem. 1999;274:24131–24136. doi: 10.1074/jbc.274.34.24131. [DOI] [PubMed] [Google Scholar]
- Byrd W. In vitro capacitation and the chemically induced acrosome reaction in bovine spermatozoa. J Exp Zool. 1981;215:35–46. doi: 10.1002/jez.1402150105. [DOI] [PubMed] [Google Scholar]
- Chang MC, Austin CR, Brown J. Mammalian fertilization. Res Reprod. 1976;8:chart. [PubMed] [Google Scholar]
- Chang MC, Berkery D, Laychock SG, Schuel H. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. III. Activation of phospholipase A2 in sperm homogenate by delta 9-tetrahydrocannabinol. Biochem Pharmacol. 1991;42:899–904. doi: 10.1016/0006-2952(91)90051-6. [DOI] [PubMed] [Google Scholar]
- Chang MC, Berkery D, Schuel R, Laychock SG, Zimmerman AM, Zimmerman S, et al. Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev. 1993;36:507–516. doi: 10.1002/mrd.1080360416. [DOI] [PubMed] [Google Scholar]
- Cobellis G, Cacciola G, Scarpa D, Meccariello R, Chianese R, Franzoni MF, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol Reprod. 2006;75:82–89. doi: 10.1095/biolreprod.106.051730. [DOI] [PubMed] [Google Scholar]
- Cobellis G, Ricci G, Cacciola G, Orlando P, Petrosino S, Cascio MG, et al. A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biol Reprod. 2010;82:451–458. doi: 10.1095/biolreprod.109.079210. [DOI] [PubMed] [Google Scholar]
- Dalterio S, Badr F, Bartke A, Mayfield D. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science. 1982;216:315–316. doi: 10.1126/science.6801767. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, Antonangelo C, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150:4692–4700. doi: 10.1210/en.2009-0057. [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci USA. 2009;106:11131–11136. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod Fertil Dev. 1996;8:581–594. doi: 10.1071/rd9960581. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, et al. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Howlett AC, Champion TM, Wilken GH, Mechoulam R. Stereochemical effects of 11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacology. 1990;29:161–165. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl. 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Barboni B, Paradisi A, Bernabo N, Gasperi V, Pistilli MG, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci. 2005;118(Pt 19):4393–4404. doi: 10.1242/jcs.02536. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Weisenhaus M, Shum S, Su T, Zheng R, Zhang C, et al. Tissue-specific PKA inhibition using a chemical genetic approach and its application to studies on sperm capacitation. Proc Natl Acad Sci USA. 2008;105:20740–20745. doi: 10.1073/pnas.0810971105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab. 2005;90:984–991. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- Schuel H. Tuning the oviduct to the anandamide tone. J Clin Invest. 2006;116:2087–2090. doi: 10.1172/JCI29424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuel H, Chang MC, Berkery D, Schuel R, Zimmerman AM, Zimmerman S. Cannabinoids inhibit fertilization in sea urchins by reducing the fertilizing capacity of sperm. Pharmacol Biochem Behav. 1991;40:609–615. doi: 10.1016/0091-3057(91)90371-8. [DOI] [PubMed] [Google Scholar]
- Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci USA. 1994;91:7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Forester E, Piomelli D, et al. N-Acylethanolamines in human reproductive fluids. Chem Phys Lipids. 2002a;121:211–227. doi: 10.1016/s0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, et al. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev. 2002b;63:376–387. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- Sun X, Wang H, Okabe M, Mackie K, Kingsley PJ, Marnett LJ, et al. Genetic loss of Faah compromises male fertility in mice. Biol Reprod. 2009;80:235–242. doi: 10.1095/biolreprod.108.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995a;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995b;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, Carlson AE, Harper AJ, Babcock DF. Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development. 2003;130:1317–1326. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]
- Whan LB, West MC, McClure N, Lewis SE. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil Steril. 2006;85:653–660. doi: 10.1016/j.fertnstert.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


