Abstract
For about 2 decades, investigators have been comparing carotid endarterectomy with carotid artery stenting in regard to their effectiveness and safety in treating carotid artery stenosis. We conducted a systematic review to summarize and appraise the available evidence provided by randomized trials, meta-analyses, and registries comparing the clinical outcomes of the 2 procedures. We searched the MEDLINE, SciVerse Scopus, and Cochrane databases and the bibliographies of pertinent textbooks and articles to identify these studies.
The results of clinical trials and, consequently, the meta-analyses of those trials produced conflicting results regarding the comparative effectiveness and safety of carotid endarterectomy and carotid stenting. These conflicting results arose because of differences in patient population, trial design, outcome measures, and variability among centers in the endovascular devices used and in operator skills. Careful appraisal of the trials and meta-analyses, particularly the most recent and largest National Institutes of Healthsponsored trial (the Carotid Revascularization Endarterectomy vs Stenting Trial [CREST]), showed that carotid stenting and endarterectomy were associated with similar rates of death and disabling stroke. Within the 30-day periprocedural period, carotid stenting was associated with higher risks of stroke, especially for patients aged >70 years, whereas carotid endarterectomy was associated with a higher risk of myocardial infarction. The slightly higher cost of stenting compared with endarterectomy was within an acceptable range by cost-effectiveness standards. We conclude that carotid artery stenting is an equivalent alternative to carotid endarterectomy when patient age and anatomy, surgical risk, and operator experience are considered in the choice of treatment approach.
Key words: Carotid stenosis/stenting/surgery; embolic protection devices; endarterectomy, carotid; meta-analyses; randomized controlled trials as topic; registries; reviews as topic
Stroke is the third most common cause of death in the United States, and carotid artery stenosis is the cause of about 20% to 25% of strokes.1 The risk of stroke depends upon the severity of the carotid stenosis. According to the North American Symptomatic Carotid Endarterectomy Trial (NASCET), 75% to 94% stenosis is associated with a stroke risk of 27% in symptomatic patients and 18.5% in asymptomatic patients.2 The European Carotid Surgery Trial (ECST) produced similar results.3
Although carotid endarterectomy (CEA) (Fig. 1) emerged in 1954,5 only in the 1990s did a series of randomized controlled trials (RCTs) establish the superiority of CEA plus aspirin over aspirin alone in preventing stroke.6–11 Since the invention of endovascular techniques and devices for carotid artery revascularization, many RCTs have compared the safety and efficacy of CEA with that of carotid artery stenting (CAS) (Fig. 2) in treating carotid artery stenosis, producing somewhat conflicting results. However, the U.S. Food and Drug Administration (FDA) recently expanded the indications for CAS. Here, we review the current literature regarding the relative benefits and safety of CEA and CAS.
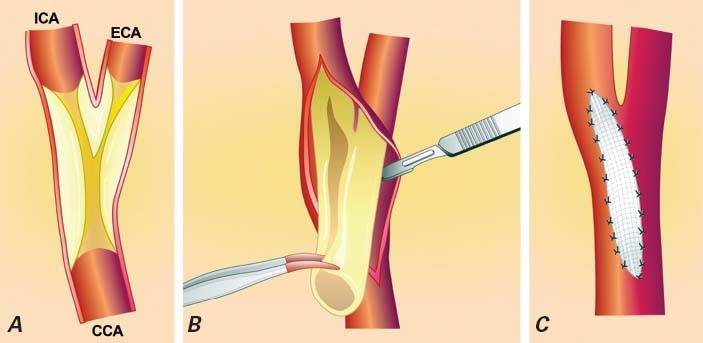
Fig. 1 Carotid endarterectomy. A) Dissection of the carotid artery. B) Removal of the atherosclerotic plaque. C) Closure of the carotid artery with a patch.
CCA = common carotid artery; ECA = external carotid artery; ICA = internal carotid artery
Reproduced with permission from: Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. endarterectomy. Eur Heart J 2009;30(22):2693–704.4
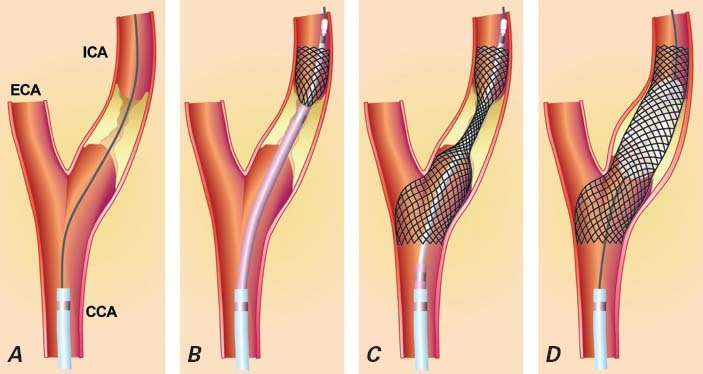
Fig. 2 Carotid artery stenting. A) A guidewire crosses the stenosis in the internal carotid artery; B, C) the stent is deployed; and D) balloon postdilation is performed to expand the stent.
CCA = common carotid artery; ECA = external carotid artery; ICA = internal carotid artery
Reproduced with permission from: Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. endarterectomy. Eur Heart J 2009;30(22):2693–704.4
Methods
Selection Criteria
We selected RCTs, meta-analyses, and registry studies that compared CEA and CAS and that were published from 1950 through August 2011.
Search Methods
We searched the MEDLINE, SciVerse Scopus, and Cochrane databases for these key words: carotid stent, carotid stenting, carotid angioplasty, and all 3 terms combined. Limiting the results to publications from 1950 through August 2011 resulted in 6,860 entries. Then the query was filtered by applying the following limits: randomized trials, meta-analyses, human, English language. This narrowed the results to 156 reported studies. Two of our investigators then independently applied the selection criteria to each study; 41 studies met these criteria and were included in the review.
Results
Randomized Controlled Clinical Trials
Mathias,12 in his 1977 report of the first canine carotid angioplasty, proposed the idea of performing carotid angioplasty in patients with carotid artery disease. The first carotid artery angioplasty performed in a human being was reported by Kerber and colleagues13 in 1980. Following the invention of stent technology and dedicated devices for the carotid artery, CAS emerged as a potential alternative to CEA in the 1990s. This inspired a large number of clinical trials14–32 that compared CAS to CEA (Table I).
TABLE I. Clinical Trials of Carotid Artery Stenting and Carotid Endarterectomy
The Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS)29,30,33,34 was a multicenter RCT in which 504 patients with carotid stenosis were randomly assigned to carotid angioplasty (n=251) or CEA (n=253). This is the only RCT identified by our search that had restenosis as its primary endpoint. The median follow-up was 5 years. (Up to 11 years of follow-up data are available now.) In an intention-to-treat analysis, the primary endpoint (restenosis of ≥70%) was found more often in the angioplasty group (hazard ratio [HR]=3.17; P <0.0001). In the angioplasty and CEA groups, respectively, the cumulative incidences of the primary endpoint were 21.7% and 30.7% at 1 year and 7.5% and 10.5% at 5 years. It is important to note that stents were used in only 55 of the 250 angioplasty patients (26%). Patients who received stents had a significantly lower incidence of restenosis than did patients who received angioplasty alone (HR=0.43; P=0.04). During the 30-day perioperative period, there were 8 non-disabling strokes (defined as neurologic deficits that resolved completely within 7 days) in the angioplasty group and 1 in the CEA group, and the number of disabling strokes and deaths was the same in both groups (25 vs 25). After this period, the angioplasty and CEA groups did not significantly differ in the incidence of any stroke or transient ischemic attack (36.9% vs 30.2%) or of ipsilateral stroke or transient ischemic attack (19.3% vs 17.2%).
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial was a noninferiority RCT in which 334 patients, all with coexisting conditions that potentially increase the risks associated with surgery, were randomly assigned to CEA (n=167) or CAS (n=167).23 Both symptomatic patients with ≥50% stenosis and asymptomatic patients with ≥80% stenosis were enrolled. The primary endpoint was a composite of death, stroke, or myocardial infarction (MI) within 30 days or death or ipsilateral stroke from 31 days through 1 year. This endpoint was reached by 20 patients assigned to CAS and 32 patients assigned to CEA (cumulative incidence, 20.1%; absolute difference, −7.9%; P=0.004 for noninferiority). At 1 year, carotid revascularization was repeated in fewer patients who had undergone CAS than in patients who had undergone CEA (cumulative incidence, 0.6% vs 4.3%; P=0.04). In symptomatic patients, the incidence of the primary endpoint at 1 year was 16.8% in the CAS group and 16.5% in the CEA group (P=0.95). For asymptomatic patients, it was 9.9% in the CAS group and 21.5% in the CEA group (P=0.02).
The Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial was a noninferiority trial designed to compare CAS with CEA in symptomatic patients only.25 Patients were randomly assigned to CAS (n=605) or CEA (n=595) within 180 days of a transient ischemic attack or stroke. In contrast to all previous trials, the SPACE trial excluded patients with restenosis after a previous CEA. The primary endpoint of this study was ipsilateral ischemic stroke or death from the time of randomization to 30 days after the procedure. This endpoint was reached in 6.84% of CAS patients and 6.34% of CEA patients (absolute difference, 0.51%; 95% confidence interval [CI], −1.89% to 2.91%). The one-sided P value for non-inferiority was 0.09. The authors concluded that the trial had not proved the noninferiority of CAS to CEA.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial was a multicenter noninferiority RCT that compared CAS with CEA only in asymptomatic patients with carotid stenosis of at least 60%.17,35 The primary endpoint was the incidence of any stroke (not solely ipsilateral ischemic stroke, as in the SPACE trial) or death within 30 days after treatment. The relative risk of 30-day stroke or death associated with CAS was 2.5 (95% CI, 1.2–5.1) compared with CEA. At 6-month follow-up, the incidence of any stroke or death was 6.1% after CEA and 11.7% after CAS (P=0.02). Therefore, the trial was stopped prematurely (after 527 of the intended 872 patients were enrolled) for reasons of both safety and futility. However, it was noted that in the CAS patients, the risk of stroke or death was significantly lower when an embolic protection device (EPD) was used than when it was not (18/227 [7.9%] vs 5/20 [25%]; P=0.03). At the 4-year follow-up, the combined rate of periprocedural stroke or death and nonprocedural ipsilateral stroke was higher in CAS patients (11.1%) than in CEA patients (6.2%; HR=1.97; P=0.03).35 The HR for periprocedural disabling stroke or death and nonprocedural fatal or disabling ipsilateral stroke was 2.00 (95% CI, 0.75–5.33; P=0.17).
The Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) was sponsored by the National Institutes of Health and was by far the largest multicenter RCT with blinded adjudication.31 In CREST, 2,502 symptomatic and asymptomatic patients (treated at 108 centers in the United States and 8 centers in Canada) with at least 70% stenosis on ultrasound were randomly assigned to CAS or CEA. The 477 surgeons and 224 interventionalists who performed the procedures each met a set of standards for training and experience.36 Patients were considered symptomatic if, in the 6 months before enrollment, they had a transient ischemic attack, amaurosis fugax, or non-disabling stroke involving the index carotid artery. Patients were excluded if they had a disabling stroke, chronic atrial fibrillation, or paroxysmal atrial fibrillation within the prior 6 months or if they needed anticoagulation or had an MI or unstable angina in the 30 days before enrollment. The 2 groups were comparable at baseline except that hyperlipidemia was more prevalent in the CEA group (85.5% vs 82.9%; P=0.048). Open-cell stents (RX Acculink® Carotid Stent System; Abbott Vascular, part of Abbott Laboratories; Abbott Park, Ill) were used at all centers, and a distal EPD (RX Accunet® Embolic Protection System; Abbott Vascular) was deployed in 96.1% of patients. The primary endpoint was a composite of stroke, MI, or death of any cause during the periprocedural period (from treatment assignment to 30 days post-procedure) or any ipsilateral stroke during the 4-year follow-up. Myocardial infarction was identified by a combination of elevated levels of cardiac markers (at least twice the upper limit of their normal ranges) and signs or electrocardiographic evidence of MI. There was no difference in the composite primary endpoint rate between the CAS (7.2%) and CEA (6.8%) groups (HR=1.1; P=0.51). There was also no significant difference in the frequency of the primary endpoint between symptomatic and asymptomatic patients. The CAS and CEA groups had similar periprocedural mortality rates but significantly different rates of stroke (4.1% vs 2.3%; P=0.01) and MI (1.1% vs 2.3%; P=0.03). The 4-year rate of any ipsilateral stroke was 2.0% for CAS and 2.4% for CEA (P=0.85). The 4-year rate of death alone was very similar for CAS (11.3%) and CEA (12.6%) (P=0.45). For the first time in an RCT of CAS and CEA, a measure of quality of life, the SF-36 questionnaire, was included as a secondary endpoint. Major and minor strokes had a greater negative impact on patients' quality-of-life scores (−15.8 points) than did MI (−4.5 points). Of all patients, 872 were women (34.9%), and there was no difference in the primary endpoint rate between women and men with either treatment.37
The International Carotid Stenting Study (ICSS), which is ongoing, is also a multicenter RCT with blinded adjudication of outcomes. It enrolls only symptomatic patients with carotid stenosis. In 2010, the results of an interim analysis of 4-month follow-up data were published.32 There were 34 events of disabling stroke or death in the CAS group (n=855) and 27 events in the CEA group (n=858), making the HR 1.28 (95% CI, 0.77–2.11). Stroke, death, or periprocedural MI occurred in 72 CAS patients and 44 CEA patients (HR=1.69; 95% CI, 1.16–2.45; P=0.006). In contrast, the numbers of disabling strokes in the 2 groups were identical. It is crucial to mention that, in this trial, various stents and protection devices were used for CAS at the discretion of the interventionalist, and protection devices were used in only 593 (72%) of the 828 CAS patients.
Meta-Analyses
Our search identified 12 meta-analyses of RCTs (Table II).38–51 A meta-analysis of 5 trials involving 1,154 patients was performed by Qureshi and colleagues.51 The frequency of the composite endpoint of 30-day stroke (either minor or disabling) or death was not different between patients treated with CAS and those treated with CEA. Moreover, no significant differences were observed in 1-year rates of ipsilateral stroke (relative risk [RR] = 0.8; 95% CI, 0.5–1.2; P=0.2). However, the 1-month rates of MI (RR=0.3; 95% CI, 0.1–0.9) and cranial nerve injury (RR=0.05; 95% CI, 0.01–0.3) were significantly lower for CAS patients.
TABLE II. Meta-Analyses of Trials Comparing Carotid Artery Stenting and Carotid Endarterectomy
Similarly, another meta-analysis of 5 clinical trials with 2,122 patients by Gurm and colleagues47 revealed no difference in risk of 30-day mortality, stroke, disabling stroke, combined death and stroke, or combined death and disabling stroke among patients randomly assigned to CAS versus CEA.
The results of a meta-analysis of 7 trials involving 2,973 patients48 suggested that CAS carries a slightly higher 30-day risk of stroke or death than does CEA (8.2% vs 6.2%; P=0.04; odds ratio [OR] = 1.35), but the difference was not significant when non-disabling strokes were excluded. In contrast, CEA posed a significantly higher risk of cranial nerve palsy (4.7% vs 0.2%; P <0.0001; OR=0.17) and MI (2.3% vs 0.9%; P=0.03; OR=0.37) than did CAS.
Brahmanandam and associates46 performed a meta-analysis of 10 trials, using an intention-to-treat approach to compare the effects of CAS and CEA on the primary outcome of 30-day stroke or death. Patients who underwent CAS had a higher risk of 30-day stroke or death than did patients who underwent CEA (RR=1.30; 95% CI, 1.01–1.67). A sensitivity analysis of only the RCTs (n=8) produced similar results. Subgroup analysis of trials that enrolled only symptomatic patients also showed a higher risk of 30-day stroke or death (RR=1.63; 95% CI, 1.18–2.25) in CAS patients. Interestingly, no between-trial heterogeneity was found in this meta-analysis.
Jeng and colleagues' meta-analysis of 9 trials involving 3,138 patients found totally different results when using random-effects and fixed-effects models.45 When a random-effects model was used for meta-analysis, there was no significant difference between treatment groups in 30-day event rates for any stroke (OR for CAS = 1.46; 95% CI, 0.91–2.36), death or any stroke (OR=1.37; 95% CI, 0.9–2.1), or death, any stroke, or MI (OR=1.02; 95% CI, 0.49–2.11). There was also no significant difference in the rates of death or any stroke at 6 months (OR=1.50; 95% CI, 0.69–3.23) or 1 year (OR=1.25; 95% CI, 0.59–2.63). In contrast, when a fixed-effects model was used for meta-analysis, the 30-day event rate for death or any stroke was significantly higher after CAS than after CEA (OR=1.37; 95% CI, 1.04–1.81), and the risk of cranial nerve injury was much lower in CAS patients than in CEA patients (OR=0.12; 95% CI, 0.05–0.29). In addition, there was significant heterogeneity among the trials (P=0.04).
In a meta-analysis of 10 randomized clinical trials that included 3,182 patients, Murad and co-authors49 associated CAS with a nonsignificant reduction in the risk of death (RR=0.6; 95% CI, 0.27–1.37), nonfatal MI (RR=0.43; 95% CI, 0.17–1.11), and a nonsignificant increase in any stroke (RR=1.29; 95% CI, 0.732.26), and major/disabling stroke (RR=1.06; 95% CI, 0.73–2.26). The authors examined each component of composite outcomes individually, rather than the composite outcomes as reported by each trial, because the treatment effect was not uniform across the individual components of the composite outcomes (death, stroke, MI, or cranial nerve palsy).
Ringleb and colleagues44 conducted a meta-analysis of 8 RCTs with 2,985 patients, ofwhom 89% were symptomatic. This meta-analysis, which used a fixed-effects model, showed that the risk of stroke or death within 30 days was higher after CAS than after CEA (OR=1.38; 95% CI, 1.04–1.83; P=0.024). Although there was significant heterogeneity among the trials with regard to this outcome (P=0.03), the authors did not report the results of using the random-effects model. Regarding the odds of disabling stroke or death, the difference between the CAS and CEA groups was not significant, and no heterogeneity was found among the trials.
In the most recent meta-analysis of studies that compared CEA with CAS, Meier and associates42 examined 11 trials that included 4,796 patients. Compared to CAS, CEA was associated with significantly less risk of periprocedural death or stroke (OR=0.67; 95% CI, 0.47–0.95; P=0.025), mainly because of a lower risk of stroke (OR=0.65; 95% CI, 0.43–1.00; P=0.049). The risk of death and the composite endpoint of death or disabling stroke did not differ significantly, whereas CEA was associated with a significantly higher risk of periprocedural MI (OR=2.69; 95% CI, 1.06–6.79; P=0.036) and cranial nerve injury (OR=10.2; 95% CI, 4.0–26.1; P <0.001).
Registries
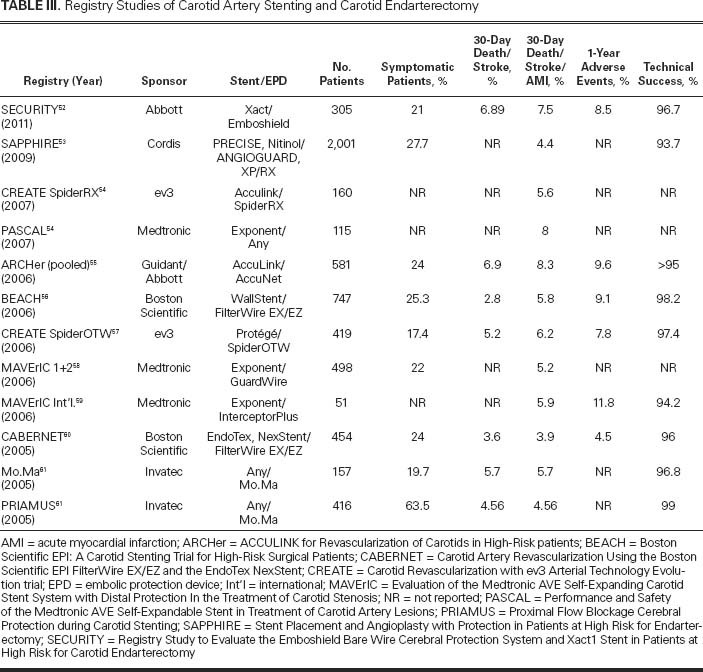
After the initial reports of case series and trials of CAS were published in the 1990s, a large number of single-center and multicenter registries were established across Europe and North America to record the outcomes of carotid revascularization procedures, mainly angioplasty and stenting. Registries of high-risk patients who underwent CAS are listed in Table III.52–61 These registries have many limitations, such as inconsistent inclusion criteria, incomplete follow-up of large percentages of patients, various definitions of adverse events, and variable levels of operator experience. However, registries provide data regarding real, everyday cases, and one can compare their results with the results of RCTs. Here, we review the results of some large registry studies.
TABLE III. Registry Studies of Carotid Artery Stenting and Carotid Endarterectomy
The CAPTURE Registry. The Carotid Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE) is a prospective, multicenter registry created to evaluate the outcomes of CAS after FDA device approval.62–64 It is, in fact, a post-marketing survey in which 144 clinical sites with 353 physicians participate. The investigators reported the results in 3,500 patients.63 The 30-day event rate of the primary endpoint—death, stroke, or MI—was 6.3% (95% CI, 5.5%-7.1%). It is of interest that this event rate did not differ among operators with different levels of experience.63 Stroke occurred in 4.8% of patients (3.9% ipsilateral, 0.9% contralateral). Forty-four percent of all ipsilateral strokes and 26% of all contralateral strokes were major and disabling. Most strokes (57.7%) were noted after the procedure but before discharge, whereas 22.3% were noted during the procedure and 20% were identified after discharge. These proportions were similar for patients who were symptomatic and those who were asymptomatic before the procedure. However, the incidence of major strokes was significantly greater among symptomatic patients (4.6%; 22/482) than asymptomatic patients (1.6%; 47/3,018). 0verall, 23% of the major strokes were hemorrhagic, and 94% of these strokes were ipsilateral to the stented carotid artery. There was a tendency toward more major hemorrhagic strokes in symptomatic patients (36%) than in asymptomatic ones (17%; P=0.07).
ELOCAS Registry. From 1993 through 2004, 2,172 patients treated by CAS were recorded in the European Long-term Carotid Artery Stenting Registry (ELOCAS) in 4 centers in Europe.65 Of all patients, 95.6% received stents. Direct stenting was performed in 1,455 (70.3%) and predilation in 614 (29.7%) of patients. 0ne of several different EPDs was deployed in 85.9% of patients.65 The major stroke or death rate was 1.2% at 30-day follow-up, and the stroke or death rate was 4.1%, 10.1%, and 15.5% at 1-, 3-, and 5-year follow-up. The stroke or death event rates were not significantly different between symptomatic and asymptomatic patients. Restenosis was evaluated periodically by duplex ultrasonography during follow-up. Restenosis of >50% was noted in 1%, 2%, and 3.4% of patients after 1, 3, and 5 years of follow-up.
Society for Vascular Surgery Vascular Registry. The Vascular Registry was developed by the Society for Vascular Surgery to register the outcomes of carotid procedures and report to the Centers for Medicare and Medicaid Services' National Coverage Decision on CAS. In December 2007, the results of2,763 CAS and 3,259 CEA patients were reported.66 The rate of the primary endpoint of death or stroke or MI at 30 days was reported to be higher for symptomatic CAS patients (7.13%) than for asymptomatic ones (4.6%; P=0.04); likewise, this rate was higher for symptomatic CEA patients than for asymptomatic ones (3.75% vs 1.97%; P=0.05). After risk-adjustment for age, history of stroke, and diabetes mellitus, the results of a logistic regression analysis associated CEA with better outcomes (that is, a lower rate of endpoints) than those of CAS. The volume of cases had no significant effect on CAS outcomes.
ALKKRegistry: Results of CAS in Octogenarians. The Arbeitsgemeinschaft Leitende Kardiologische Kranken-hausärzte (ALKK) registry67 included 2,780 CAS patients, whose median age was 70.8 years (interquartile range, 64.7–73.3 yr) and 11.2% of whom were octogenarians. A comparison with younger patients showed that in octogenarians, symptomatic stenosis was a more common indication for CAS (60.7% vs 48%; P <0.001), the CAS procedure was aborted more frequently (6.9% vs 2.2%; P <0.001), the duration of intervention was longer (median, 45 vs 40 min; P=0.008), and the residual stenosis after CAS was greater (10% vs 5%; P=0.006). The in-hospital death or stroke rate was also higher in octogenarians than in younger patients (5.5% vs 3.2%; P <0.032).
In this cohort, octogenarians were slightly more likely to have arterial hypertension (93.4% vs 90.7%; P=0.18) and significantly more likely to have atrial fibrillation (16.4% vs 7.9%; P <0.001) than were younger patients. However, it is of interest that the prevalences of other comorbidities, including coronary artery disease and heart failure, were comparable between octogenarians and younger patients.67
The Impact of Embolic Protection Devices
The use of EPDs is recommended by expert consensus68 to reduce the risk of stroke associated with CAS. Three main types of EPDs have been used to address this risk: distal occlusion balloons, distal filter devices, and proximal protection devices (Fig. 3). Distal occlusion balloons, which were developed first, were rapidly replaced by distal filters because some patients could not tolerate the balloons and because stopping blood flow in the carotid artery made stent deployment more difficult.
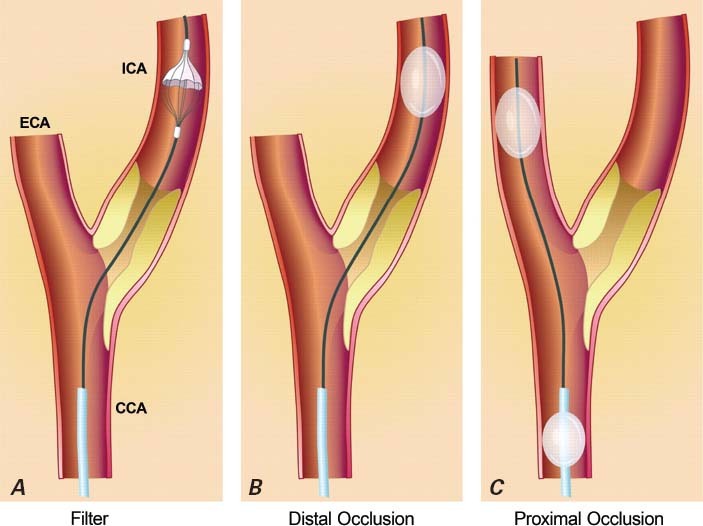
Fig. 3 Embolic protection devices. A) A filter is deployed in the internal carotid artery (ICA). B) A balloon is inflated in the ICA. C) Balloons are inflated in the external carotid (ECA) and common carotid (CCA) arteries.
Reproduced with permission from: Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. endarterectomy. Eur Heart J 2009;30(22):2693–704.4
Iyer and colleagues69 retrospectively studied CAS databases at 4 centers and analyzed a total of 3,160 CAS procedures in which 9 different EPDs were used. An EPD was used in 3,030 patients (95.9%). Adverse events were defined as death, stroke, or transient ischemic attack, and their timing was considered to be procedural (during the procedure) or 30-day (during the procedure and up to 30 days afterward). Use of any protection device was associated with a nonsignificant reduction in procedural adverse event rates (0.9% vs 2.3%, P=0.12). Comparison of occlusion balloons versus filters and comparison of proximal versus distal occlusion balloons revealed no significant differences in the risk of procedural or 30-day adverse events. However, the 30-day adverse event risk was higher with the Accunet filter than with the FilterWire EZ™ Embolic Protection System (Boston Scientific Corporation; Natick, Mass) (RR=2.67; 95% CI, 1.41–5.04; P=0.005).
In CREST, the use of an EPD (the RX Acculink) was mandatory when feasible, and this device was used in 96% of patients. The systematic use of EPDs in CREST has been proposed to be a factor in the relatively low rate of adverse events in CREST compared with other trials.70
In a systematic review of literature from 1999 through 2002, Kastrup and co-authors71 compared the stroke and death rates associated with 2,537 CAS procedures performed without EPDs and 896 CAS procedures performed with EPDs. The authors found that the 30-day combined stroke and death rate was 5.5% in patients treated without EPDs and 1.8% in patients treated with EPDs (P <0.001) regardless of the patients' symptom status, whereas the rates of death alone were almost identical (approximately 0.8%). The results of the Global Carotid Artery Registry also favored the use of EPDs; as in EVA-3S, the risk of stroke or death in CAS patients was significantly lower if an EPD was used.72 However, a subgroup analysis of data from the SPACE RCT showed that EPDs had no impact on event rate (ipsilateral stroke or ipsilateral stroke or death rate within 30 days).73 In a small RCT in which 30 patients underwent CAS with or without an EPD (Emboshield® NAV6 Embolic Protection System; Abbott Vascular), transcranial Doppler surprisingly showed significantly more signals—suggestive of more particulate emboli—in the protected group. However, there was no difference in clinical event rates during the 30 days after the procedure.74
In theory, proximal protection systems (Fig. 3) are more effective than any distal device because the carotid lesion does not need to be crossed first (to deploy the balloon or filter) and because the external carotid artery is occluded during the procedure.75 The feasibility and efficacy of using proximal EPDs have been reported in the past few years.76–78 In 2010, Stabile and colleagues79 reported data from a large, single-center registry in which a proximal EPD (the Mo.Ma® Ultra Proximal Cerebral Protection Device; Invatec S.p.a.; Roncadelle, Italy) was used in 1,300 patients. The device was successfully deployed in 99.7% of cases. In-hospital adverse events were 5 deaths (0.38%), 6 major strokes (0.46%), 5 minor strokes (0.38%), and no MIs. Within the 30-day follow-up period, there were 2 additional deaths and 1 minor stroke. Symptomatic patients had a higher 30-day incidence of death or stroke (3.04% vs 0.82%; P <0.05) than did asymptomatic patients. In this registry, patients over 80 years of age did not have worse outcomes than did younger patients. Such a low adverse event rate has not been reported in any other registry or RCT.
Discussion
Randomized trials of CAS and CEA have produced somewhat conflicting results, probably because they drew their cohorts from heterogeneous patient populations, used different endpoints and endovascular devices, and involved operators with various levels of experience in performing endovascular techniques. Likewise, the results of meta-analyses conflict because these analyses included different combinations of trials and used different statistical methods. Of the meta-analyses we reviewed, all but one46 showed significant heterogeneity between clinical trials. The meta-analyses performed by Jeng and coworkers45 and by Murad and colleagues49 appear to be more accurate than other meta-analyses we reviewed, because the methods used in these 2 meta-analyses were more appropriate given the significant heterogeneity among trials and the nonuniform treatment effect on the individual components of the composite endpoints. When heterogeneity was taken into account, meta-analyses performed with the random-effects model always showed comparable outcomes between CAS and CEA. Of note, in the most recent meta-analysis, performed by Meier and associates,42 the inferiority of CAS to CEA disappeared as newer trials were added sequentially to the analysis. Better trial design, the maturation of endovascular techniques, and improvements in interventionals' skills might explain this finding.
The CREST trial was designed to overcome these confounding factors. It enrolled both symptomatic and asymptomatic patients and excluded patients with previous disabling stroke or with atrial fibrillation. Also, CREST included standard-risk CEA candidates, in contrast to SAPPHIRE, which enrolled only high-risk patients. Therefore, CREST's results can be applied to a broader patient population. Patients in the CEA and CAS groups were comparable in terms of age, sex, and comorbidities, except for hyperlipidemia, which was more prevalent in the CEA group. In contrast to other trials, only one kind of stent and embolic protection filter was allowed, and rigorous training criteria were used to standardize operator skill.31,36 Overall, in CREST, the rates of stroke, death, and MI were lower than or equal to corresponding rates in previous trials for both CAS and CEA procedures. This finding could be the result of controlling for confounders (mentioned above) in the study design, the devices used, and operator experience. The rates of any periprocedural stroke or death associated with CAS and CEA were 2.5% versus 1.4% for asymptomatic patients and 6.0% versus 3.2% for symptomatic patients; all of these rates are less than or equal to current American Heart Association “acceptable risk” guidelines for patients who undergo these procedures.31,80–82 The 30-day risk of any stroke was higher for CAS than for CEA in EVA-3S (HR=1.97; P=0.03), in ICSS (HR=1.92; P=0.002), and in CREST (HR=1.74; P=0.04). As we mentioned, in EVA-3S, this risk was significantly lower in patients who were treated with EPD (18/227 [7.9%] vs 5/20 [25%]). In ICSS, only 72% of patients were treated with an EPD, and the final results of ICSS have not yet been reported. The lower rate of stroke in CREST than in similar trials is probably due to the use of EPDs in 96% of patients, the use of the same EPD and stent system in all patients, and a higher standard for interventionalist training. However, in future studies, we must continue to improve CAS by refining the stent design and stenting techniques and by investigating the role of proximal EPDs.
Age could be an important predictor of clinical outcome for patients who undergo CAS. Patients younger than 70 years had a significantly better outcome (lower stroke rates) with CAS than with CEA in the CREST, SPACE, and ICSS (interim results) trials. In a pooled analysis of data from the EVA-3S, SPACE, and ICSS trials, the risk of stroke or death within a 4-month follow-up period was 5.8% and 5.7% in the CAS and CEA patients who were younger than 70 years of age. However, this risk was 12% versus 5.9% (RR=2.04; P=0.0053) in CAS and CEA patients who were 70 years of age or older.83 More carotid artery tortuosity and calcification could be the reasons for the increased risk of stroke in elderly CAS patients.
In an analysis of a multicenter Italian/German registry of CAS patients (n=695), Schluter and colleagues84 found that diabetes and age were predictors of the 30-day incidence of any stroke and death. Compared with nondiabetic patients, diabetic patients aged 75 years or older had a 4.3-fold greater risk of stroke or death (95% CI, 1.3–12.3; P=0.016) and a 12.0-fold greater risk of major stroke or death (95% CI, 2.1–66.5; P=0.005). It is worth noting that diabetes was not a risk factor for stroke or death in patients younger than 75 years. Similarly, age was not a predictor of complications in nondiabetic patients.
Acute MI has been consistently shown to be more prevalent after CEA than after CAS. The rates of organ dysfunction and death due to MI were not reported in any of the trial reports we identified. In 2010, Illuminati and associates85 evaluated the effectiveness of elective coronary angiography and percutaneous coronary intervention (PCI) before CEA in reducing the incidence of postoperative MI. They randomly assigned 426 CEA candidates either to coronary angiography with possible PCI before CEA or to CEA without an-giography or PCI. The primary endpoint was the combined rate of postoperative MI and complications of coronary angiography and PCI. No postoperative MI was observed in the PCI group, but 9 myocardial events, including one fatal MI, were observed in the no-PCI group. There were no postoperative complications due to aspirin or clopidogrel use. Although this treatment approach needs more investigation, it could be considered as a means of minimizing the risk of postoperative MI for patients who are not candidates for CAS and who need to be treated with CEA.
In January 2011, the FDA's Circulatory System Device Panel expanded the indications for CAS in standard-risk patients to include stenosis of ≥70% by ultrasound or ≥50% by angiogram combined with neurologic symptoms, and stenosis of ≥70% by ultrasound or ≥60% by angiogram in the absence of symptoms. These are FDA-approved indications for using the RX Acculink stent and the RX Accunet embolic protection filter. The panel members emphasized the significance of adequate operator training and experience. Vigorous accreditation requirements for operators and hospitals are to be launched soon. In future trials, the efficacy of proximal EPDs (which appears promising) needs to be compared with that of filters such as the RX Acculink, because proximal devices might make CAS procedures much safer. In a cost-effectiveness analysis of the SAPPHIRE trial, it was found that CAS with EPD was more costly overall (in terms of both the procedure and hospitalization) than was CEA ($559 more per patient); however, by accepted economic standards, CAS is still a viable alternative, particularly for patients at high surgical risk.86
Conclusions
Our systematic review focused on the results of randomized trials, meta-analyses, and registries. Our purpose was to cover not only clinical trials but also real-world cases to increase the external validity of our conclusions. However, the possibility of publication bias still exists.
We conclude that if performed by experienced hands at experienced centers, CAS is an acceptable alternative to CEA, particularly for patients who are at high surgical risk, and is probably preferable for patients younger than 70 years of age. However, patients' preferences and anatomy must also be taken into consideration.
Acknowledgment
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript.
Footnotes
Address for reprints: Guilherme Sllva, MD, 6624 Fannin St, Suite 2220, Houston, TX 77030
E-mail: gsilva@texasheart.org
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association [published errata appear in Circulation 2011;123(6):e240 and Circulation 2011;124(16):e426]. Circulation 2011;123(4):e18–e209. [DOI] [PMC free article] [PubMed]
- 2.Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, Barnett HJ. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000;342(23):1693–700. [DOI] [PubMed]
- 3.MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, White H. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group [published erratum appears in Circulation 1998;97(24):2479]. Circulation 1998;97(18):1784–90. [DOI] [PubMed]
- 4.Roffi M, Mukherjee D, Clair DG. Carotid artery stenting vs. endarterectomy. Eur Heart J 2009;30(22):2693–704. [DOI] [PubMed]
- 5.Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet 1954;267(6846):994–6. [DOI] [PubMed]
- 6.Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke, Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke 1991;22(6):816–7. [DOI] [PubMed]
- 7.Carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. National Institute of Neurological Disorders and Stroke. J Neurol Sci 1995;129(1):76–7. [DOI] [PubMed]
- 8.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351(9113):1379–87. [PubMed]
- 9.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial [published erratum appears in Lancet 2004;364(9432):416]. Lancet 2004;363 (9420):1491–502. [DOI] [PubMed]
- 10.Hobson RW 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993;328(4):221–7. [DOI] [PubMed]
- 11.Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991;266(23):3289–94. [PubMed]
- 12.Mathias K. A new catheter system for percutaneous transluminal angioplasty (PTA) of carotid artery stenosis [in German]. Fortschr Med 1977;95(15):1007–11. [PubMed]
- 13.Kerber CW, Cromwell LD, Loehden OL. Catheter dilatation of proximal carotid stenosis during distal bifurcation endarterectomy. AJNR Am J Neuroradiol 1980;1(4):348–9. [PMC free article] [PubMed]
- 14.Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg 1998;28(2):326–34. [DOI] [PubMed]
- 15.Alberts MJ. Results of a multicenter prospective randomized trial of carotid artery stenting vs carotid endarterectomy [abstract]. Stroke 2001;32:325–d.
- 16.Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol 2001;38(6):1589–95. [DOI] [PubMed]
- 17.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006;355(16):1660–71. [DOI] [PubMed]
- 18.Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004;54(2):318–25. [DOI] [PubMed]
- 19.CaRESS Steering Committee. Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg 2005;42(2):213–9. [DOI] [PubMed]
- 20.Diethrich E, Fogarty TJ, Zarins CK, Hopkins LN, Roubin GS, Wholey MH, et al. CaRESS: carotid revascularization using endarterectomy or stenting systems [published erratum appears in Tech Vasc Interv Radiol 2005;8(2):103]. Tech Vasc Interv Radiol 2004;7(4):194–5. [DOI] [PubMed]
- 21.Ling F, Jiao LQ. Preliminary report of trial of endarterectomy versus stenting for treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chin J Cerebrovasc Dis 2006;3(1):4–8.
- 22.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. Long-term results of carotid stenting versus endar-terectomy in high-risk patients. N Engl J Med 2008;358(15):1572–9. [DOI] [PubMed]
- 23.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351 (15):1493–501. [DOI] [PubMed]
- 24.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial [published erratum appears in Lancet Neurol 2009;8(2):135]. Lancet Neurol 2008;7(10):893–902. [DOI] [PubMed]
- 25.SPACE Collaborative Group, Ringleb PA, Allenberg J, Bruck-mann H, Eckstein HH, Fraedrich G, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial [published erratum appears in Lancet 2006; 368(9543):1238]. Lancet 2006;368(9543):1239–47. [DOI] [PubMed]
- 26.Steinbauer MG, Pfister K, Greindl M, Schlachetzki F, Borisch I, Schuirer G, et al. Alert for increased long-term follow-up after carotid artery stenting: results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J Vasc Surg 2008;48(1):93–8. [DOI] [PubMed]
- 27.Stehr A, Scodacek D, Wustrack H, Steinbauer M, Topel I, Pfister K, Kasprzak PM. Retrojugular versus ventrojugular approach to carotid bifurcation for eversion endarterectomy: a prospective randomized trial. Eur J Vasc Endovasc Surg 2008; 35(2):190–7. [DOI] [PubMed]
- 28.Steinbauer MG, Stehr A, Pfister K, Herold T, Zorger N, Topel I, et al. Endovascular repair of proximal endograft collapse after treatment for thoracic aortic disease. J Vasc Surg 2006;43(3):609–12. [DOI] [PubMed]
- 29.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357(9270):1729–37. [PubMed]
- 30.Ederle J, Bonati LH, Dobson J, Featherstone RL, Gaines PA, Beard JD, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol 2009;8(10):898–907. [DOI] [PMC free article] [PubMed]
- 31.Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis [published errata appear in N Engl J Med 2010;363(5):498 and N Engl J Med 2010;363 (2):198]. N Engl J Med 2010;363(1):11–23. [DOI] [PMC free article] [PubMed]
- 32.International Carotid Stenting Study Investigators, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010;375(9719):985–97. [DOI] [PMC free article] [PubMed]
- 33.Bonati LH, Ederle J, McCabe DJ, Dobson J, Featherstone RL, Gaines PA, et al. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or end-arterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol 2009;8(10):908–17. [DOI] [PMC free article] [PubMed]
- 34.McCabe DJ, Pereira AC, Clifton A, Bland JM, Brown MM; CAVATAS Investigators. Restenosis after carotid angioplasty, stenting, or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). Stroke 2005;36(2):281–6. [DOI] [PubMed]
- 35.Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol 2008;7(10):885–92. [DOI] [PubMed]
- 36.Hopkins LN, Roubin GS, Chakhtoura EY, Gray WA, Ferguson RD, Katzen BT, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: credentialing of inter-ventionalists and final results of lead-in phase. J Stroke Cere-brovasc Dis 2010;19(2):153–62. [DOI] [PMC free article] [PubMed]
- 37.Howard VJ, Lutsep HL, Mackey A, Demaerschalk BM, Sam AD 2nd, Gonzales NR, et al. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol 2011;10(6):530–7. [DOI] [PMC free article] [PubMed]
- 38.Yavin D, Roberts DJ, Tso M, Sutherland GR, Eliasziw M, Wong JH. Carotid endarterectomy versus stenting: a meta-analysis of randomized trials. Can J Neurol Sci 2011;38(2):230–5. [DOI] [PubMed]
- 39.Economopoulos KP, Sergentanis TN, Tsivgoulis G, Mariolis AD, Stefanadis C. Carotid artery stenting versus carotid end-arterectomy: a comprehensive meta-analysis of short-term and long-term outcomes. Stroke 2011;42(3):687–92. [DOI] [PubMed]
- 40.Bangalore S, Kumar S, Wetterslev J, Bavry AA, Gluud C, Cut-lip DE, Bhatt DL. Carotid artery stenting vs carotid endarter-ectomy: meta-analysis and diversity-adjusted trial sequential analysis of randomized trials. Arch Neurol 2011;68(2):172–84. [DOI] [PubMed]
- 41.Carotid Stenting Trialists' Collaboration, Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 2010;376(9746):1062–73. [DOI] [PubMed]
- 42.Meier P, Knapp G, Tamhane U, Chaturvedi S, Gurm HS. Short term and intermediate term comparison of endarterec-tomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials [published erratum appears in BMJ 2010;340:c1798]. BMJ 2010;340:c467. [DOI] [PMC free article] [PubMed]
- 43.Liu Z, Shi Z, Wang Y, Chen B, Zhu T, Si Y, Fu W. Carotid artery stenting versus carotid endarterectomy: systematic review and meta-analysis. World J Surg 2009;3(3):586–96. [DOI] [PubMed]
- 44.Ringleb PA, Chatellier G, Hacke W, Favre JP, Bartoli JM, Eckstein HH, Mas JL. Safety of endovascular treatment of carotid artery stenosis compared with surgical treatment: a meta-analysis. J Vasc Surg 2008;47(2):350–5. [DOI] [PubMed]
- 45.Jeng JS, Liu HM, Tu YK. Carotid angioplasty with or without stenting versus carotid endarterectomy for carotid artery stenosis: a meta-analysis. J Neurol Sci 2008;270(1–2):40–7. [DOI] [PubMed]
- 46.Brahmanandam S, Ding EL, Conte MS, Belkin M, Nguyen LL. Clinical results of carotid artery stenting compared with carotid endarterectomy. J Vasc Surg 2008;47(2):343–9. [DOI] [PubMed]
- 47.Gurm HS, Nallamothu BK, Yadav J. Safety of carotid artery stenting for symptomatic carotid artery disease: a meta-analysis. Eur Heart J 2008;29(1):113–9. [DOI] [PubMed]
- 48.Wiesmann M, Schopf V, Jansen O, Bruckmann H. Stent-pro-tected angioplasty versus carotid endarterectomy in patients with carotid artery stenosis: meta-analysis of randomized trial data. Eur Radiol 2008;18(12):2956–66. [DOI] [PubMed]
- 49.Murad MH, Flynn DN, Elamin MB, Guyatt GH, Hobson RW 2nd, Erwin PJ, Montori VM. Endarterectomy vs stenting for carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg 2008;48(2):487–93. [DOI] [PubMed]
- 50.Ederle J, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev 2007;(4):CD000515. [DOI] [PubMed]
- 51.Qureshi AI, Kirmani JF, Divani AA, Hobson RW 2nd. Carotid angioplasty with or without stent placement versus carotid endarterectomy for treatment of carotid stenosis: a meta-analysis. Neurosurgery 2005;56(6):1171–81. [DOI] [PubMed]
- 52.Xact Carotid Stent System—P040038. Summary of safety and effectiveness data.
- 53.Massop D, Dave R, Metzger C, Bachinsky W, Solis M, Shah R, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv 2009;73 (2):129–36. [DOI] [PubMed]
- 54.CAS clinical trial and registry update. Endovasc Today 2007 Sept;9:76–9.
- 55.Gray WA, Hopkins LN, Yadav S, Davis T, Wholey M, Atkinson R, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results [published erratum appears in J Vasc Surg 2007;45(1):226]. J Vasc Surg 2006;44(2):258–68. [DOI] [PubMed]
- 56.White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME; BEACH Trial Investigators. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv 2006;67(4):503–12. [DOI] [PubMed]
- 57.Safian RD, Bresnahan JF, Jaff MR, Foster M, Bacharach JM, Maini B, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol 2006;47(12):2384–9. [DOI] [PubMed]
- 58.Jokhi PP, Saw J. Carotid stenting registries and randomized trials. In: Saw J, editor. Carotid artery stenting: the basics. Totowa (NJ): Humana Press; 2009. p. 37–65.
- 59.Hill MD, Morrish W, Soulez G, Nevelsteen A, Maleux G, Rogers C, et al. Multicenter evaluation of a self-expanding carotid stent system with distal protection in the treatment of carotid stenosis. AJNR Am J Neuroradiol 2006;27(4):759–65. [PMC free article] [PubMed]
- 60.Rogers C, Huynh R, Seifert PA, Chevalier B, Schofer J, Edelman ER, et al. Embolic protection with filtering or occlusion balloons during saphenous vein graft stenting retrieves identical volumes and sizes of particulate debris. Circulation 2004; 109(14):1735–40. [DOI] [PubMed]
- 61.Reimers B, Sievert H, Schuler GC, Tubler T, Diederich K, Schmidt A, et al. Proximal endovascular flow blockage for cerebral protection during carotid artery stenting: results from a prospective multicenter registry. J Endovasc Ther 2005;12(2):156–65. [DOI] [PubMed]
- 62.Fairman R, Gray WA, Scicli AP, Wilburn O, Verta P, Atkinson R, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg 2007;246 (4):551–8. [DOI] [PubMed]
- 63.Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv 2007;70(7):1025–33. [DOI] [PubMed]
- 64.Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Car-diovasc Interv 2007;69(3):341–8. [DOI] [PubMed]
- 65.Bosiers M, Peeters P, Deloose K, Verbist J, Sievert H, Sugita J, et al. Does carotid artery stenting work on the long run: 5-year results in high-volume centers (ELOCAS Registry). J Cardiovasc Surg (Torino) 2005;46(3):241–7. [PubMed]
- 66.Sidawy AN, Zwolak RM, White RA, Siami FS, Schermer-horn ML, Sicard GA; Outcomes Committee for the Society for Vascular Surgery. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: results from the SVS Vascular Registry. J Vasc Surg 2009;49 (1):71–9. [DOI] [PubMed]
- 67.Zahn R, Ischinger T, Hochadel M, Zeymer U, Schmalz W, Treese N, et al. Carotid artery stenting in octogenarians: results from the ALKK Carotid Artery Stent (CAS) Registry. Eur Heart J 2007;28(3):370–5. [DOI] [PubMed]
- 68.American College of Cardiology Foundation; American Society of Interventional & Therapeutic Neuroradiology; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology, Bates ER, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (ACCF/SCAI/SVMB/SIR/ASITN Clinical Expert Consensus Document Committee on Carotid Stenting) [published erratum appears in J Am Coll Cardiol 2007;49(8):924]. J Am Coll Cardiol 2007;49(1):126–70. [DOI] [PubMed]
- 69.Iyer V, de Donato G, Deloose K, Peeters P, Castriota F, Cre-monesi A, et al. The type of embolic protection does not influence the outcome in carotid artery stenting. J Vasc Surg 2007; 46(2):251–6. [DOI] [PubMed]
- 70.Abbott receives FDA approval to expand use of the RX AC-CULINK® Carotid Stent System to patients at standard surgical risk.
- 71.Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke 2003;34(3):813–9. [DOI] [PubMed]
- 72.Wholey MH, Al-Mubarek N. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv 2003; 60(2):259–66. [DOI] [PubMed]
- 73.Jansen O, Fiehler J, Hartmann M, Bruckmann H. Protection or nonprotection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE Trial [published erratum appears in Stroke 2009;40 (6):e477]. Stroke 2009;40(3):841–6. [DOI] [PubMed]
- 74.Macdonald S, Evans DH, Griffiths PD, McKevitt FM, Venables GS, Cleveland TJ, Gaines PA. Filter-protected versus unprotected carotid artery stenting: a randomised trial. Cerebrovasc Dis 2010;29(3):282–9. [DOI] [PubMed]
- 75.White CJ. Proximal embolic protection: a “game changer” for carotid stents. J Am Coll Cardiol 2010;55(16):1668–70. [DOI] [PubMed]
- 76.Diederich KW, Scheinert D, Schmidt A, Scheinert S, Reimers B, Sievert H, et al. First clinical experiences with an endovas-cular clamping system for neuroprotection during carotid stenting. Eur J Vasc Endovasc Surg 2004;28(6):629–33. [DOI] [PubMed]
- 77.Parodi JC, Ferreira LM, Sicard G, La Mura R, Fernandez S. Cerebral protection during carotid stenting using flow reversal. J Vasc Surg 2005;41(3):416–22. [DOI] [PubMed]
- 78.Schmidt A, Diederich KW, Scheinert S, Braunlich S, Olen-burger T, Biamino G, et al. Effect of two different neuropro-tection systems on microembolization during carotid artery stenting. J Am Coll Cardiol 2004;44(10):1966–9. [DOI] [PubMed]
- 79.Stabile E, Salemme L, Sorropago G, Tesorio T, Nammas W, Miranda M, et al. Proximal endovascular occlusion for carotid artery stenting: results from a prospective registry of 1,300 patients. J Am Coll Cardiol 2010;55(16):1661–7. [DOI] [PubMed]
- 80.Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Har-baugh RE, Dempsey RJ, et al. Guidelines for carotid end-arterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation 1998;97(5):501–9. [DOI] [PubMed]
- 81.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published erratum appears in Circulation 2006;114(25):e617]. Circulation 2006;113(24):e873–923. [DOI] [PubMed]
- 82.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006;113(10):e409–49. [PubMed]
- 83.Bonati LH, Fraedrich G; Carotid Stenting Trialists' Collaboration. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis–a pooled analysis of EVA-3S, SPACE and ICSS. Eur J Vasc Endovasc Surg 2011;41(2):153–8. [DOI] [PubMed]
- 84.Schluter M, Reimers B, Castriota F, Tubler T, Cernetti C, Cremonesi A, et al. Impact of diabetes, patient age, and gender on the 30-day incidence of stroke and death in patients undergoing carotid artery stenting with embolus protection: a post-hoc subanalysis of a prospective multicenter registry. J Endovasc Ther 2007;14(3):271–8. [DOI] [PubMed]
- 85.Illuminati G, Ricco JB, Greco C, Mangieri E, Calio F, Cec-canei G, et al. Systematic preoperative coronary angiography and stenting improves postoperative results of carotid endar-terectomy in patients with asymptomatic coronary artery disease: a randomised controlled trial. Eur J Vasc Endovasc Surg 2010;39(2):139–45. [DOI] [PubMed]
- 86.Mahoney EM, Greenberg D, Lavelle TA, Natarajan A, Ber-ezin R, Ishak KJ, et al. Costs and cost-effectiveness of carotid stenting versus endarterectomy for patients at increased surgical risk: results from the SAPPHIRE trial. Catheter Cardio-vasc Interv 2011;77(4):463–72. [DOI] [PubMed]


