Abstract
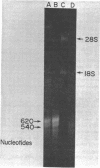
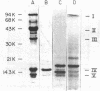
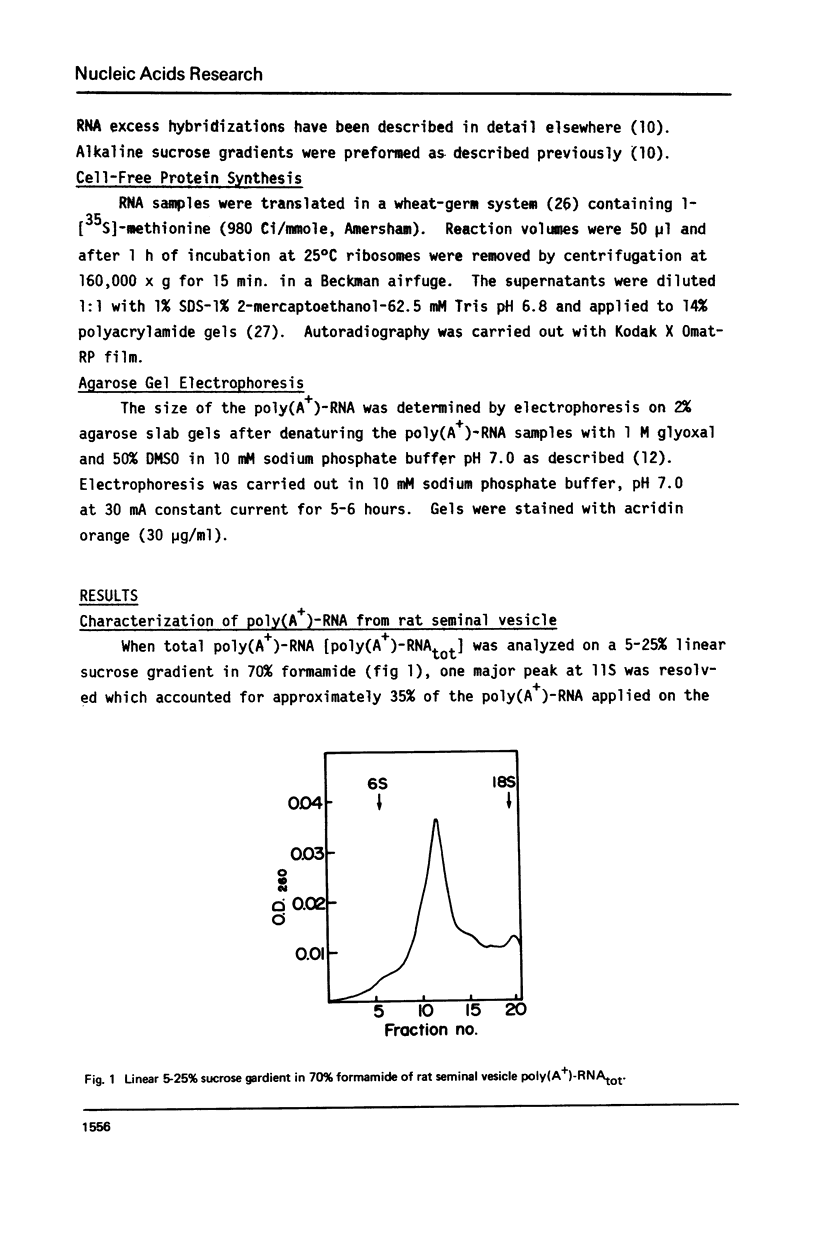
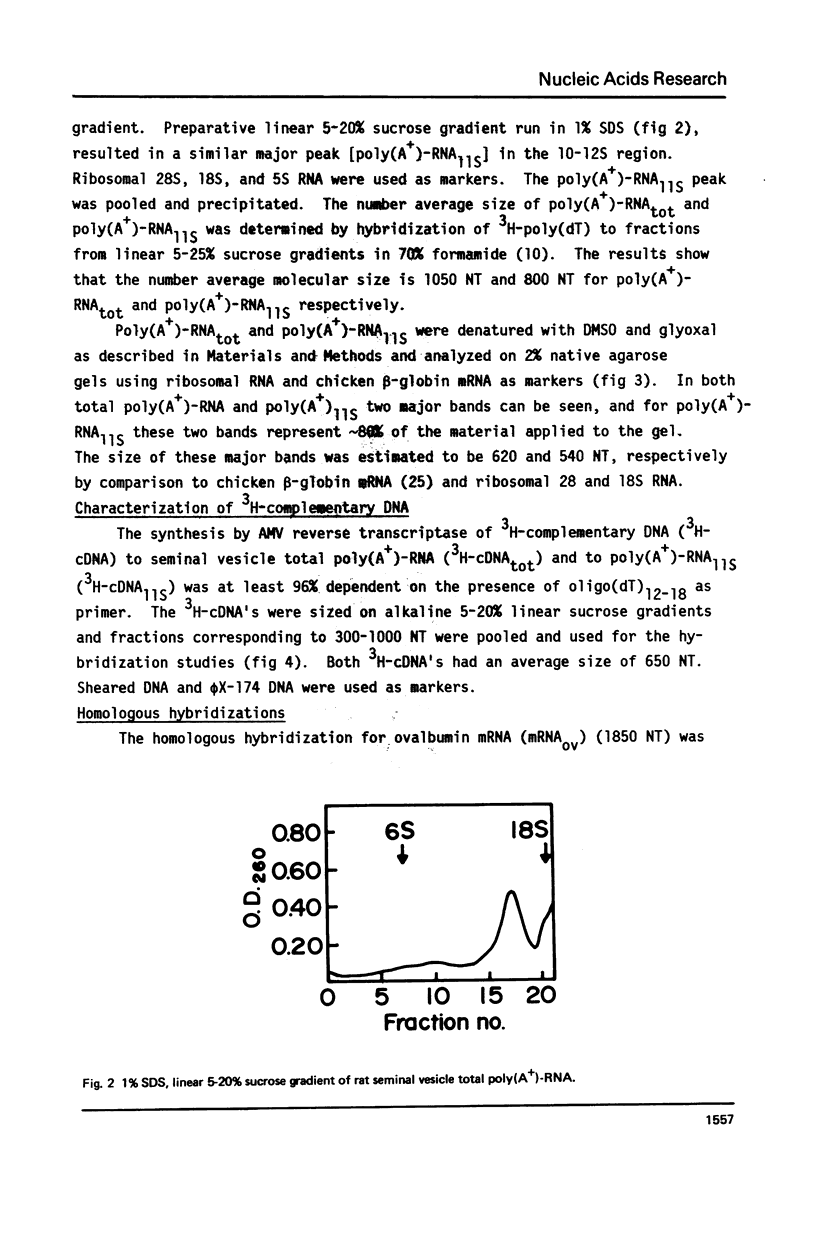
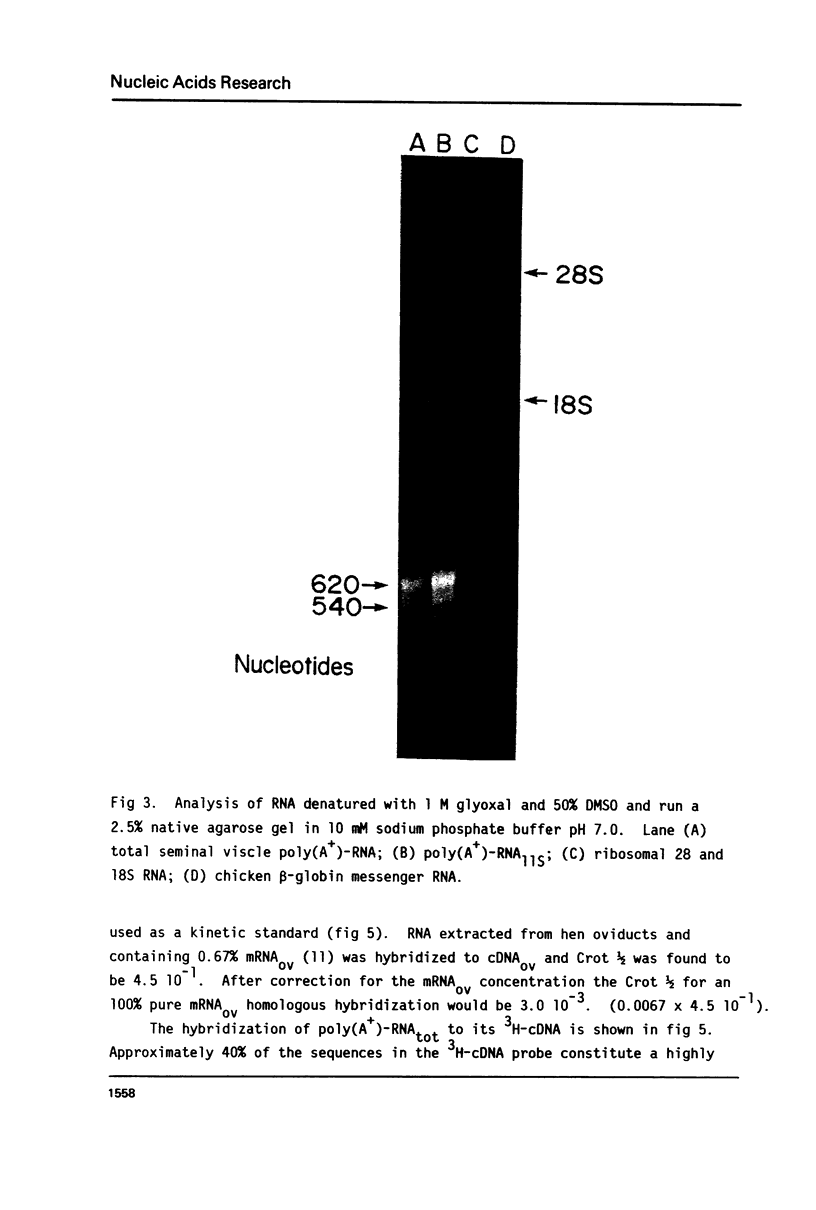
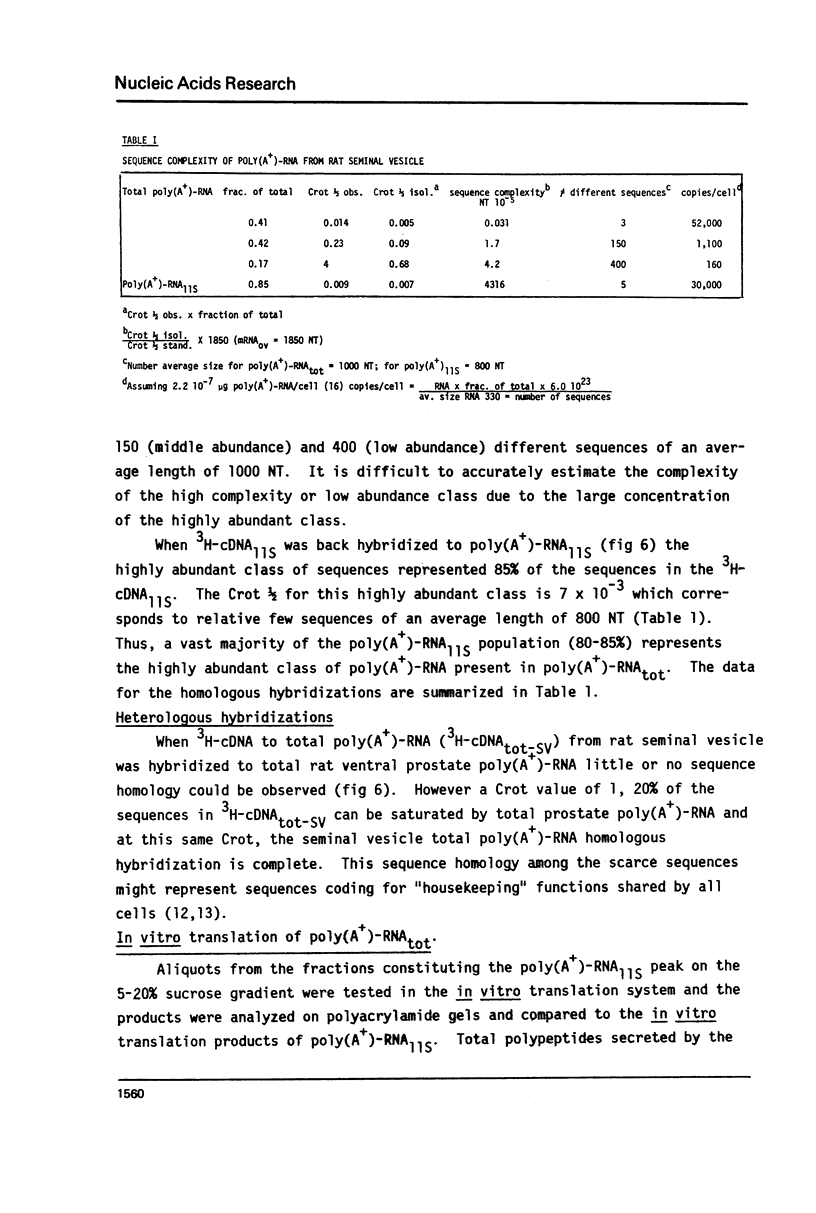
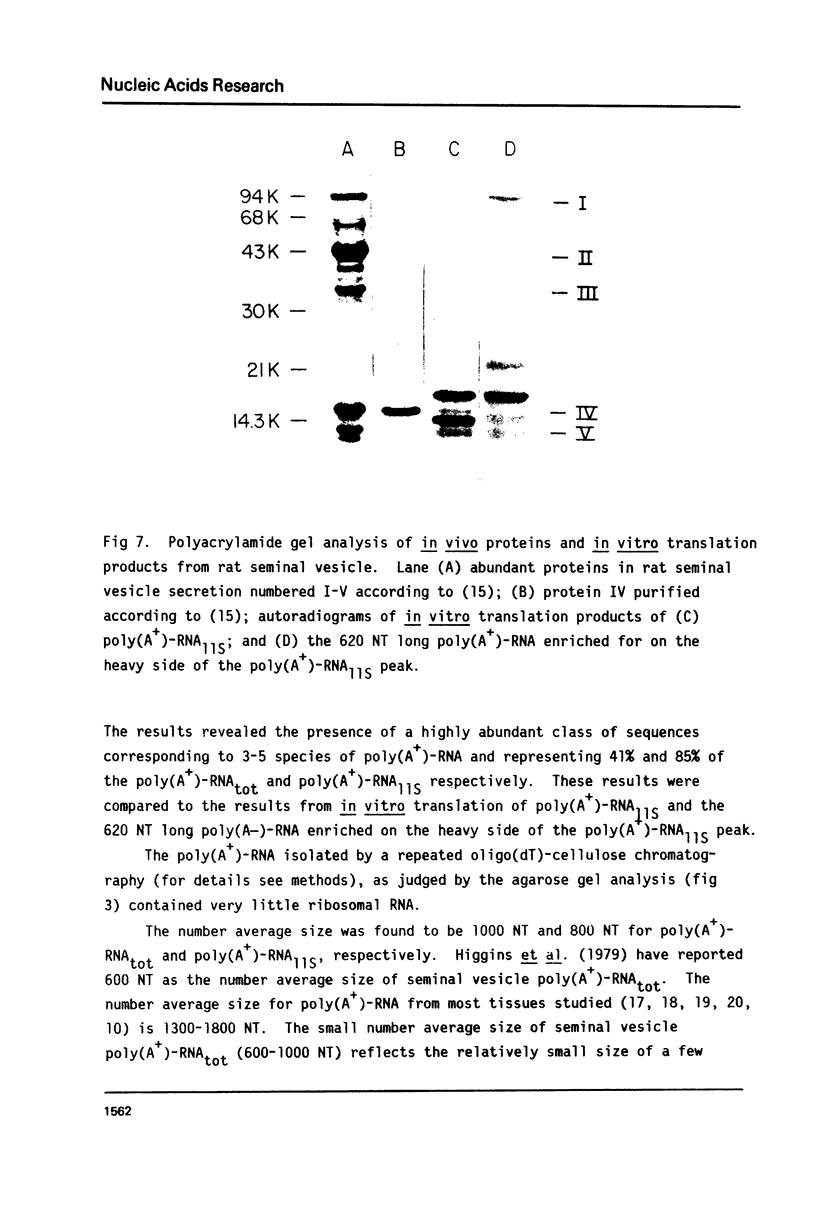
Total poly(A+)-RNA (poly(A+)-RNAtot) was isolated from rat seminal vesicle and its size distribution determined by 70% formamide 5-25% sucrose density analysis. One major peak was resolved in the 10-13 S region and accounted for ∼35% of the total poly(A+)-RNA applied. Preparative 1% SDS, 5-20% linear sucrose density gradients also resolved a single major peak in the 11S region (poly(A+)11S. Analysis of poly(A+)-RNAtot and poly(A+)-RNA11S under denaturing conditions on 2% agarose gel electrophoresis demonstrated two major components in both poly(A+)-RNA populations. Size estimations for these components are 620 and 540 NT respectively. 3H-cDNA was made to both poly(A+)-RNAtot and poly(A+)-RNA11S. Back-hybridization of poly(A+)-RNAtot and poly(A+)-RNA11S to their respective 3H-cDNA revealed a highly abundant class representing 41% and 85% of the sequences in their respective 3H-cDNA's. The highly abundant class corresponded to 3-5 sequences present in 30,000-50,000 copies/cell. Invitro translation of poly(A+)-RNA11S resulted in two major polypeptides coded for by the 620 NT long and 540 NT long poly(A+)-RNA respectively.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Elder P. K., Benz E. W., Jr, Stephens R. E., Moses H. L. Effect of cell proliferation on levels and diversity of poly(A)-containing mRNA. Cell. 1976 Feb;7(2):255–65. doi: 10.1016/0092-8674(76)90025-8. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M. Effects of testosterone on messenger ribonucleic acid and protein synthesis in rat seminal vesicle. Biochem J. 1978 Aug 15;174(2):543–551. doi: 10.1042/bj1740543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. Biochem J. 1976 Aug 15;158(2):271–282. doi: 10.1042/bj1580271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
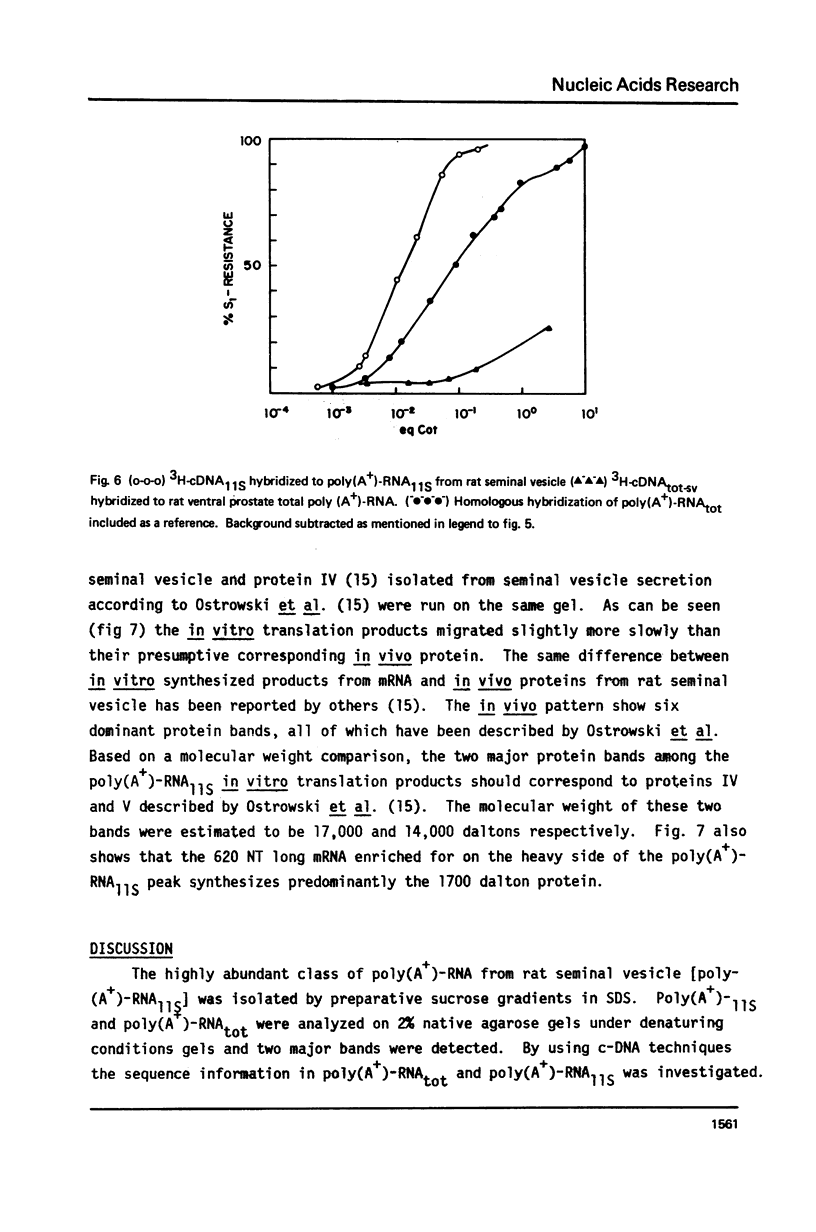
- Higgins S. J., Burchell J. M., Parker M. G., Herries D. G. Effects of testosterone on sequence complexity of polyadenylated RNA from rat seminal vesicle. Eur J Biochem. 1978 Nov 15;91(2):327–334. doi: 10.1111/j.1432-1033.1978.tb12683.x. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy B., Johnson C. B., McCarthy B. J. Diversity of sequences in total and polyadenylated nuclear RNA from Drosophila cells. Nucleic Acids Res. 1976 Jul;3(7):1777–1789. doi: 10.1093/nar/3.7.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., Woo S. L., Robberson D. L., O'Malley B. W. The synthesis and properties of the complete complementary DNA transcript of ovalbumin mRNA. Biochemistry. 1976 Jan 13;15(1):223–233. doi: 10.1021/bi00646a034. [DOI] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler M. K., Kistler W. S. Purification and cell-free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem. 1979 Jan 25;254(2):383–390. [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Parker M. G., Mainwaring W. I. Effects of androgens on the complexity of poly(A) RNA from rat prostate. Cell. 1977 Oct;12(2):401–407. doi: 10.1016/0092-8674(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T. The androgenic regulation of abundant mRNA in rat ventral prostate. Eur J Biochem. 1978 Apr 17;85(2):399–406. doi: 10.1111/j.1432-1033.1978.tb12252.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Land H., Lindenmaier W., Nguyen-Huu M. C., Wurtz T., Timmis K. N., Giesecke K., Schütz G. Cloning of chicken lysozyme structural gene sequences synthesized in vitro. Nucleic Acids Res. 1978 Sep;5(9):3275–3294. doi: 10.1093/nar/5.9.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Woo S. L., Means A. R., O'Malley B. W. Molecular cloning of ovomucoid gene sequences from partially purified ovomucoid messenger RNA. Biochemistry. 1978 Dec 26;17(26):5763–5772. doi: 10.1021/bi00619a025. [DOI] [PubMed] [Google Scholar]