Abstract
We sought to determine whether skeletal myoblasts, wild-type or engineered to express relaxin, might improve myocardial viability and performance in a rat model of chronic myocardial infarction. Our purpose was to investigate a potential new therapy for heart failure.
From October 2005 through September 2009, we surgically induced acute myocardial infarction in 80 male Wistar rats. Thirty days after surgery, the rats underwent reoperation for the retrograde coronary venous infusion of skeletal myoblasts, relaxin, or both. The animals were randomly assigned to 4 experimental groups: R1 (the control group, which underwent saline-solution infusion), R2 (systemic relaxin therapy), R3 (myoblast infusion), and R4 (myoblast infusion and systemic relaxin therapy). Echocardiography, positron emission tomography, and cellular and histologic analysis were performed at 4 established time points.
Mortality rates were similar among the groups. Postinfarction echocardiographic evaluation revealed similar left ventricular dysfunction. Viable myocardium, evaluated with positron emission tomography, was analogous. After therapy, the echocardiographic values of cardiac function improved significantly (P<0.05) in all groups except R1. Myocardial viability volume increased significantly in groups R3 and R4 (P<0.05) but was unchanged in groups R2 and R1. In group R4, the echocardiographic and positron emission tomographic results improved significantly (P<0.001). Histologic analysis showed that myoblasts settled in regions of ischemic scarring, especially when combined with relaxin.
The retrograde venous route is safe, effective, and clinically feasible for cell delivery. Myoblasts and relaxin are better than either alone in terms of myocardial viability and performance improvement.
Key words: Cardiomyoplasty; cardiovascular system/drug effects; cell transplantation/methods; disease models, animal; myoblasts, skeletal/transplantation; myocardial ischemia/drug therapy; rats, Wistar; recovery of function; relaxin/metabolism/therapeutic use; ventricular remodeling
The loss of cardiomyocytes and contractile units after acute myocardial infarction (AMI) leads to acute dysfunction and eventually to congestive heart failure. Although the heart is not a completely post-mitotic organ, the regenerative potential of myocardium is not adequate to restore the dead tissue.1,2 The structural changes (remodeling) are accompanied by functional alteration that leads to deteriorating ventricular function and the worsening of congestive heart failure.
Cellular cardiomyoplasty—the replacement of dead muscle with autologous skeletal myoblasts—is a promising method of encouraging the regeneration of myocardial tissue after myocardial infarction. This approach has already yielded encouraging experimental results in terms of improved cardiac function and remodeling, although the mechanisms are not fully understood.1,3–5
Skeletal myoblasts are considered to be promising in remodeling,6 mainly because they are resistant to ischemia and hence could survive within the poorly vascularized myocardial scar tissue.7,8 Furthermore, these cells can be isolated and expanded ex vivo from skeletal muscle biopsies of the same patients who are scheduled for cell-grafting therapy. This presents obvious immunologic and ethical advantages. For these reasons, we chose to use skeletal myoblasts in our study.
Myoblasts appear to be capable of settling into host tissue and contributing to function; however, they do not completely differentiate into cardiomyocytes.9–12 The electrical properties of skeletal myoblasts differ from those of skeletal muscle cells and from those of cardiomyocytes; the resultant functional improvements are probably due to mechanisms other than electromechanical coupling with the host tissue.13 Skeletal myoblasts are thought to exert their beneficial effect by remodeling the extracellular matrix and secreting cytokines and growth factors that induce angiogenic stimuli.14–18
The route of administering these cells affects their actual delivery into the host. Many routes have been explored, with varying and often inconsistent results. Coronary venous retrograde delivery has seemed to be effective and safe.19,20
To overcome the short lifespans of transplanted cells, stem cells have been genetically manipulated to overexpress molecules that improve engraftment, survival, and paracrine action. One of the substances thus studied was relaxin, a cardiotropic hormone. Investigators in our research group have shown that relaxin can improve the contractile performance of postinfarcted swine hearts by positively influencing cardiac remodeling and neoangiogenesis.21 Relaxin is a member of a peptide hormone family that includes 3 distinct relaxin molecules (H1, H2, and H3) and insulin-like peptides 3 to 6.22,23 In human beings, 3 separate relaxin genes have been found: RLN1, RLN2, and RLN3.22,23 Relaxin-2 (H2), encoded by the RLN2 gene, is the major circulating form and accounts for most of the known biological effects of relaxin in human beings and experimental animals. Relaxin acts on both reproductive and nonreproductive tissues23–27 and exerts major effects on the cardiovascular system.23,26,27 Some of these effects, namely increased heart perfusion,28 neoangiogen-esis,29 and reduced cardiac fibrosis through increased extracellular matrix turnover,30 are potentially relevant to cell-grafting therapy. In fact, H2 relaxin might be used, both as a drug and as a functional transgene, for cellular or gene-based therapy of postinfarction cardiac disease. Besides its demonstrated protective role against ischemia-related myocardial damage,31 relaxin exerts a particular action on the connective-tissue milieu.30 This feature might make the extracellular matrix more receptive to administered myoblasts, and promote en-graftment and syncytial formation between myoblasts and resident cardiomyocytes.30,32 This in turn enables the replacement of dead tissue and promotes a beneficial paracrine effect. Adding relaxin to co-cultured myoblasts and cardiomyocytes has increased connex-in43 expression levels and exchanges of signals between cells.33
As have other investigators,34,35 we have used myoblasts from nonautologous sources. This is because using autologous skeletal myoblasts is time-consuming and less cost-effective, and it creates logistical concerns during large-scale clinical application. Furthermore, engrafted xenogeneic cells in injured myocardium have restored myocardial structure and improved cardiac function in animal models.36
In accordance with these concepts, we developed experimental models of postischemic congestive heart failure so that we could study the effects of retrograde venous infusion of myoblasts alone, relaxin alone, and myoblasts and relaxin concurrently. We sought to determine the safety of myoblast administration via retrograde coronary venous delivery, the effectiveness of cardiomyoplasty, the role ofrelaxin in functionality and myocardial viability as a factor independent of cardiomyoplasty, and the existence of synergy between myoblasts and relaxin.
Materials and Methods
This investigation conformed with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (NIH publication no. 85–23, revised 1996), and it was approved by the institutional animal welfare committee at the University of Florence.
Animals
Eighty male Wistar rats (weight, 200–300 g) were used from October 2005 through September 2009. All were first quarantined for 7 days, at 22 to 24°C and with 12-hour alternating cycles of light and dark. Standard laboratory chow and water were freely available. The experimental protocol complied with recommendations from the European Economic Community (86/609/CEE) for the care and use of laboratory animals and was approved by the Animal Care Committee of the University of Florence. Figure 1 summarizes the study's diagnostic and surgical steps.
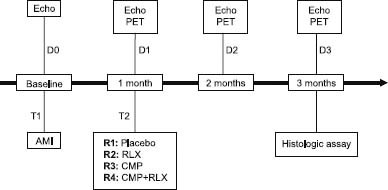
Fig. 1 Study protocol.
AMI = acute myocardial infarction; CMP = cardiomyoplasty; D = distinct time point; Echo = echocardiography; PET = positron emission tomography; R = rat group; RLX = relaxin; T = thoracotomy
The animals were randomly assigned in equal numbers to 4 groups: R1 (the control group, consisting of rats undergoing saline-solution infusion), R2 (rats undergoing systemic relaxin therapy), R3 (rats undergoing myoblast infusion), and R4 (rats undergoing myoblast infusion and systemic relaxin therapy).
Myoblast Cultures and Gene Transfection
Heterologous mouse myoblasts C2C12 were grown in Dulbecco's modified Eagle's medium, which contained 10% fetal bovine serum and 100 U/mL of gentamicin-streptomycin, preserved in a 5% carbon dioxide atmosphere at 37°C. All myoblasts were transfected with the enhanced green fluorescent protein (eGFP) gene (C2C12-GFP) for immunohistochemistry. Only the myoblasts used in group R4 were genetically engineered through a lentiviral carrier to express the human gene RLN2 coding for the H2 relaxin hormone (C2C12-relaxin cells). These cells were able to produce adequate local concentrations of relaxin.7 When required for transplantation, the cells were detached with use of EDTA 0.1% in phosphate-buffered saline solution; they were mechanically scraped, centrifuged, washed twice in phosphate-buffered saline solution, and resuspended in complete culture medium. Cell concentration was determined in a Burker counting chamber and was adjusted to the amount required for individual injections.
Surgical Induction of Acute Myocardial Infarction and Cardiomyoplasty
Figure 1 shows the experimental protocol. The rats were placed under general anesthesia by means of the intraperitoneal administration of pentobarbital (50 mg/kg), and mechanical ventilation was established. They then underwent thoracotomy (T1) at the left 5th intercostal space. To induce AMI, the left anterior descending coronary artery was directly ligated just beyond the first diagonal branch. The rats were then caged, under controlled temperature and high Po2 to facilitate postoperative recovery, until the resumption of normal respiratory activity and heart rate (350–450 beats/min). They were then housed under standard conditions until the next experimental step.
Thirty days after surgery, the rats underwent another left thoracotomy (T2) for retrograde coronary vein infusion.19,20 A 24G catheter was inserted into the left cardiac vein, and a purse-string suture was used to control bleeding from the insertion site. During infusion, a temporal snare suture was placed at the distal left cardiac vein to prevent injected solution from being flushed back into the coronary sinus. With the snare tied, and over a period of 1 minute, the rats in groups R3 and R4 were injected with 1 mL of saline solution that contained 2 × 106 cells/100 g body weight. This occlusion was maintained for 10 minutes. The R3 rats were infused with myoblasts C2C12-GFP, and the R4 rats were infused with myoblasts plus C2C12-relaxin. Groups R2 and R4 underwent intraperitoneal infusion of relaxin through a mini-osmotic pump for constant release (daily relaxin dose, 1 μg for 28 d). Group R1 received only an injection of 1 mL of saline solution (placebo).
In most studies of myoblast transplantation, immu-nosuppressive therapy has been used to improve cell acceptance and survival. In particular, cyclosporine prevents donor-cell rejection.37,38 Similar results have been reported for allo- and xenomyoblast transplantation.39
Therefore, because of the nonisogenic cell origins and immunogenic eGFP expression, the rats all received immunosuppressive therapy: a daily dose of 15 mg/kg of cyclosporine with food, beginning on day 1 of the administration of cells or placebo.13
Deaths
Twenty-five rats (31.3%) died: 14 at T1 (4 in group R1, 5 in R2, 3 in R3, and 2 in R4) and 11 at T2 (4 in group R1, 3 in R2, 2 in R3, and 2 in R4). The final population consisted of 55 animals (R1 = 12, R2 = 12, R3 = 15, R4 = 16). After the last functional assay, the surviving rats were humanely killed with injected pentothal.
Cardiac Function Analysis
We established 4 distinct time points (D) for echocar-diographic and positron emission tomographic (PET) evaluations: before AMI as baseline (D0), 1 month after the induction of AMI (D1), and 1 month (D2) and 2 months (D3) after reoperation.
Echocardiographic Analysis
At each time point, all the rats were anesthetized (30 mg/kg phenobarbital intraperitoneally) and underwent transthoracic 2-dimensional and M-mode echocardio-graphic studies with use of an ACUSON Sequoia 512 system (Siemens Medical Solutions USA, Inc.; Mountain View, Calif) with a 15-MHz probe. Local and overall left ventricular contractility20 was evaluated as follows: left ventricular ejection fraction (LVEF), left ventricular end-systolic and end-diastolic diameters (LVESD and LVEDD), and shortening fraction (SF). Myocardial performance index (MPI) was calculated as the sum of isovolumetric contraction and relaxation times/ejection time.
Positron Emission Tomographic Analysis
We evaluated myocardial viability with use of a GE eXplore® Vista microPET scan device (GE/Suinsa; Madrid, Spain) for the study of small animals (maximum weight, 350–450 g). Radiopharmaceutical 18F-deoxyglucose (18FDG) was taken up by living and metabolically active cells, which enabled us to calculate and compare the volume of myocardium that remained viable at each time point as an expression of treatment results.
The rats were placed in a transparent, closed room so that anesthesia could be induced with use of 4% sevoflurane gas. Subsequently, they were intraperitoneally injected with 5% glucose solution to standardize the circulating insulin level and to favor the 18FDG uptake. After 20 minutes, we administered 45 MBq of 18FDG into the tail vein. Thirty minutes later, redistribution images were acquired while the rats were under continuous general anesthesia and were breathing spontaneously. After the scan, the rats were placed in a heated environment and were weaned from anesthesia. No complications were observed after the procedure in any of the animals.
The PET images of 18FDG-positive heart tissue areas were reconstructed iteratively by means of a 2-dimensional ordered-subsets expectation maximization algorithm, with use of Emory Cardiac Toolbox 3.1 (ECTb) software (Philips Healthcare; Best, The Netherlands). This software enables calculation of the myocardial viability volume (MVV) from the coaptation of viable cells, which indicates the viability in the myocardial region. In this manner, myocardial metabolic function and its treatment and time-related changes were semiquantitatively evaluated.
Histologic Analysis
To study the distribution and differentiation of myoblasts, we examined the eGFP and sarcomeric α-actin expression by immunofluorescence (10p-thick myocardial samples fixed in formaldehyde vapors for 10 min and then incubated with rhodamine conjugated monoclonal antibody anti-eGFP and anti-sarcomeric α-actin). Other sections were examined for immunore-action by means of incubation with polyclonal antibodies anti-VCAM-1 and ICAM-1. The immunoreaction was detected with use of a confocal laser scanner microscope that could detect secondary antibodies. Ultra-thin sections of myocardial tissue samples fixed in glutaral-dehyde and osmium tetroxide of epoxy resin were put on a slide with uranyl acetate and citrate and examined under electron microscopy. Some fragments not fixed in osmium were used for immunoelectron microscopy to reveal the relaxin produced in the grafted C2C12-relaxin cells by anti-H2-relaxin antibodies.
Fibrosis in the cardiac tissue was studied by means of morphologic analysis of samples of tissue fixed in para-formaldehyde and paraffin. Some sections were stained in accordance with the Van Gieson method for evaluation of collagen (0.1% fuchsin in hydrogen peroxide containing 0.08% hydrochloric acid for 4 min, then washed in 95% ethanol for 5 min) and analyzed by means of optical microscopy.
Statistical Analysis
Randomization was carried out with use of StatsDirect for Windows release 2.3.8 (StatsDirect Ltd.; Cheshire, UK). The allocation sequence was generated by a laboratory assistant who, in blinded fashion, assigned animals to the groups. The power analysis was determined with use of GraphPad StatMate software release 2.00 (GraphPad Software, Inc.; La Jolla, Calif) on the basis of the following assumptions: type I error of 0.05 (2-sided) and difference in LVEF of 0.04. The calculated statistical power was 0.85 with a sample size of 40 animals (10/group). Data were compared for statistical significance by means of the paired t test, and multiple comparisons underwent analysis of variance with the Tukey post hoc test and the Mantel-Haenszel test, where appropriate. Significance was assumed when the P value was <0.05; SPSS software version 12.0 (IBM Corporation; Armonk, NY) was used for these calculations.
Results
Surgical Results
After AMI induction (T1), the operative mortality rate was below 30% on average (chiefly within 24 hr after induction), with no statistically significant differences among the groups. When autopsies were performed, the areas of ischemic scarring were found to be similar in all the treated rats.
During and after the surgery, we found no life-threatening bleeding or fatal respiratory complications. Reoperation (T2) was associated with an average mortality rate below 20%, with no statistically significant differences among the groups. The overall mortality rate was similar within all groups. No arrhythmias were detected in the rats that survived the experimental period.
Echocardiographic Results
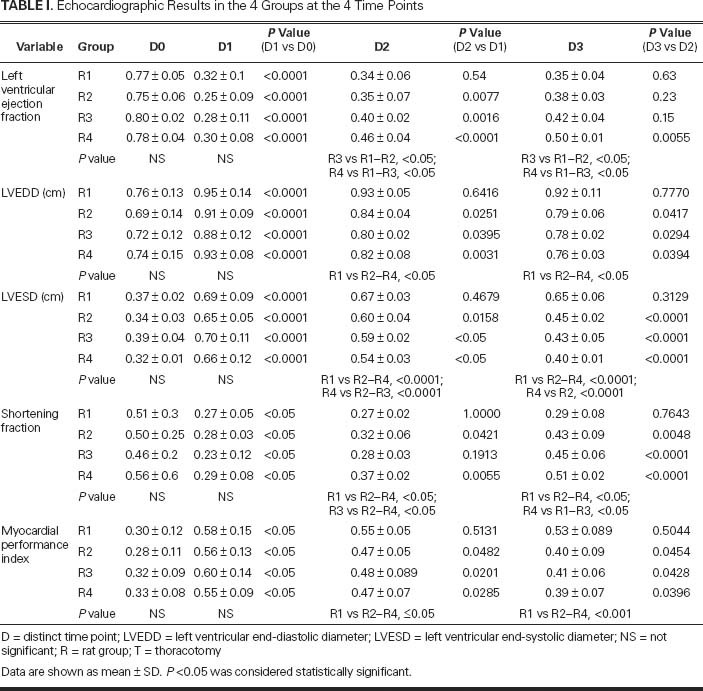
Table I shows the average echocardiographic values in each group at each time point and quantifies the statistical significance of differences. In all animals, the surgical induction of AMI caused a marked decline in the assayed echocardiographic values of cardiac function.
TABLE I. Echocardiographic Results in the 4 Groups at the 4 Time Points
Left Ventricular Ejection Fraction. The average LVEF (Fig. 2A) dropped from approximately 0.77 in the healthy animals to 0.29 at D1 (P<0.0001), without significant intergroup differences.
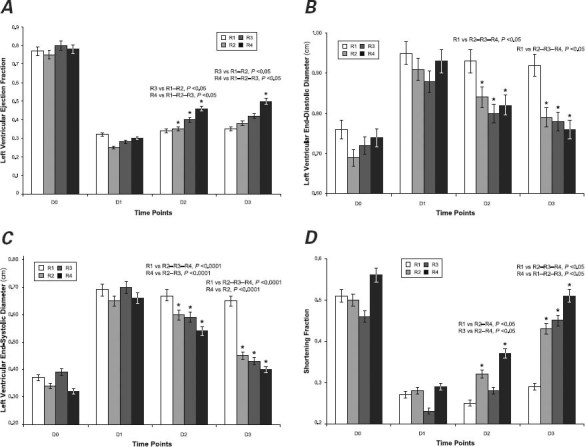
Fig. 2 Graphs show echocardiographic measurements of cardiac function: A) left ventricular ejection fraction, B) left ventricular end-diastolic diameter, C) left ventricular end-systolic diameter, and D) shortening fraction. Mean values were calculated for each distinct time point (D) in each rat group (R). Asterisks indicate a statistically significant difference within the same group in comparison with earlier measurements.
The LVEF at D2 revealed a statistically significant increase over D1 values in groups R2 (P=0.0077), R3 (P=0.0016), and R4 (P<0.0001). Significant improvement was not observed in group R1. In particular, the comparisons between groups R1 or R2 and R3 or R4 showed remarkable improvement in R3, and R4 achieved the best statistically significant result among all groups (LVEF, 0.46; P <0.05).
The LVEF values at D3 were similar to those at D2 in groups R1, R2, and R3; only R4 had a further increase in LVEF (to 0.50; P=0.0055).
At D2 and D3, the LVEFs in group R3 showed significant improvement in comparison with R1 and R2 (P<0.05), and improvement in group R4 was significantly higher in comparison with R1, R2, and R3 (P<0.05).
Left Ventricular End-Diastolic Diameter. At D1, the LVEDD values (Fig. 2B) showed a statistically significant increase in all groups (P<0.05) over D0, without significant differences between groups.
In comparison with D1, values at D2 showed statistically significant reduced diameters in groups R2 (P=0.0251), R3 (P=0.0395), and R4 (P=0.0031); conversely, in group R1, LVEDD values remained similar to those observed at D1.
At D3, the LVEDD values showed further significant reduction in groups R2 (P=0.0417), R3 (P=0.0294), and R4 (P=0.0394) versus values at D2; in contrast, the D3 numbers in group R1 overlapped with previous measurements. In R1, LVEDD was statistically greater than all the others at both D2 and D3 (P <0.05).
Left Ventricular End-Systolic Diameter. Analysis of LVESD at D1 showed a statistically significant increase over preoperative values (D0) in all groups (P<0.0001), without notable differences among groups (Fig. 2C).
At D2, analysis yielded statistically significant reductions in LVESD in groups R2 (P<0.0158), R3 (P<0.05), and R4 (P<0.05) in comparison with D1. In group R1, however, LVESD remained unchanged. The intergroup comparison at this stage suggested a statistically significant increase in the average diameter in group R1 in comparison with the other groups (P<0.0001). In group R4, LVESD compared to that in R2 and R3 showed a significant reduction (P<0.0001), reaching the lowest value among all groups.
At D3, groups R2, R3, and R4 showed a further statistically significant LVESD reduction from D2 values (P=0.0001). In group R1, just as with the D2 values, average LVESD at D3 was significantly higher than in the other groups. The LVESD in group R4 was also significantly lower than the D2 value (P<0.0001).
Shortening Fraction. The SF at D1 versus D0 was reduced in all groups (P<0.05), without significant intergroup differences (Fig. 2D).
At D2 versus D1, the SF increased in groups R2 (P<0.0421) and R4 (P<0.0055), whereas the values in groups R1 and R3 were similar. There was also a statistically significant difference in groups R1 and R2 when compared with R3 and R4 (R1 vs R2–R4, P<0.05; and R3 vs R2–R4, P <0.05). Group R4 achieved the best value among all groups.
At D3 versus D2, SF values increased significantly in groups R2 (P=0.0048), R3 (P<0.0001), and R4 (P<0.0001).
At D3, the SF in group R1 showed a statistically significant reduction from D2 in comparison with groups R2, R3, and R4 (P<0.05), and SF in R4 reached the best value. These values differed significantly from those in the other groups (R4 vs R1-R2-R3, P <0.05).
In the MPI analysis, D1 values increased significantly over D0 values in all the groups (P<0.05) without any remarkable intergroup difference (Fig. 3).
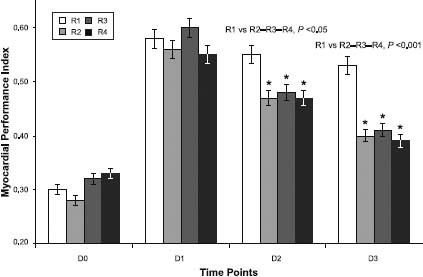
Fig. 3 Graph shows myocardial performance index calculated for each distinct time point (D) in each rat group (R). Asterisks indicate a statistically significant difference within the same group in comparison with earlier measurements.
The D2-versus-D1 determination showed significant MPI reductions in groups R2 (P<0.0482), R3 (P<0.0201), and R4 (P<0.0285), whereas the values in group R1 were similar. The MPI value differed significantly between this group and the others (R1 vs R2–R4, P <0.05).
In groups R1, R2, and R3, the MPI values at D3 were significantly reduced in comparison with those at D2 (P=0.0254, P=0.0428, and P=0.0396, respectively). The D3 values in group R1 remained similar to the 2 previous determinations and were significantly different in comparison with the other groups (P<0.0001). The MPI recovery in group R4 was stronger than that in the other groups.
Results of Positron Emission Tomography
The PET results were different between groups but were homogeneous within the same group (Table II). At D1, MVV in each group was similar, suggesting that the ischemic lesion areas were similar in all the rats (Fig. 4).
TABLE II. Percentages of Myocardial Viable Volume Measured by Positron Emission Tomography
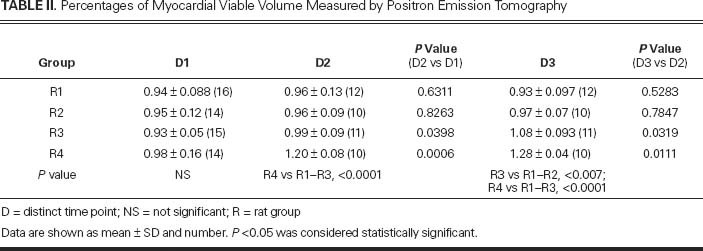
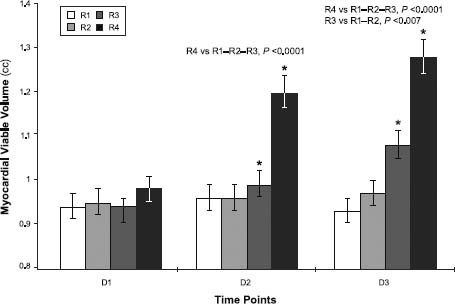
Fig. 4 Graph shows myocardial viability as measured by micro-positron emission tomographic analysis: 1 month after surgical induction of acute myocardial infarction (D1), and at 1 month (D2) and 2 months (D3) after the therapeutic treatments in each rat group (R).
D = distinct time point
At D2 versus D1, MVV values remained approximately unchanged in groups R1 (0.94 ± 0.088 vs 0.96 ± 0.13 cc; P=0.6311) and R2 (0.95 ± 0.12 vs 0.96 ± 0.09 cc; P=0.8263). In contrast, groups R3 and especially R4 showed a stronger and more significant increase in viability: 0.99 ± 0.09 vs 0.93 ± 0.05 cc in R3 (+6%; P=0.0398) and 1.2 ± 0.08 vs 0.98 ± 0.16 cc in R4 (+22%; P=0.0006). At this stage, the difference between R4 and the other groups was significant (P<0.0001).
This trend was confirmed by images acquired at D3: group R1 expressed a mean 0.93 ± 0.097 cc of MVV and group R2 expressed 0.97 ± 0.07 cc of MVV, both without significant differences compared with the previous scan (P=0.5283 and P=0.7847, respectively). Conversely, mean MVV in group R3 rose to 1.08 ± 0.093 cc, and group R4 reached a mean MVV of 1.28 ± 0.04 cc. This means that MVV in group R3 increased at D3 over D2 (P=0.0319), with an average MVV increase of 16% compared with the D1 value. At D3 versus D2, group R4 also showed a significant increase (P=0.0111), reaching an average increase of 31% at D3 in comparison with D1. In terms of intergroup differences at D3, group R3 was statistically better than R1 or R2 (P<0.007), and R4 was better than R1 through R3 (P<0.0001).
Histologic Results
Two months after re-intervention, we humanely killed the rats and performed histologic analysis of infarcted tissue samples. Inspection of the external and internal heart chambers showed ischemic lesions of the left ventricular front wall and the interventricular septum, with no apparent differences between samples; this confirmed the in vivo morphologic and functional data.
We used antibodies for GFP, sarcomeric α-actin, and relaxin to identify and locate the implanted cells in the myocardium. Confocal microscopic immunofluorescence analysis revealed numerous GFP-positive cells, either C2C12-GFP or C2C12-relaxin, in the scar tissue in groups R3 and R4 (Fig. 5A). Upon visual observation, the hearts of the C2C12-GFP-treated animals contained lower amounts of immunoreactive cells compared with those that had been treated with C2C12-relaxin plus relaxin. The engrafted cells were mainly located in the proximity of blood vessels (Fig. 5B), whereas they were not detected in the viable myocardium and not involved in the postinfarction remodeling process. In small blood vessels within the infarcted area beside myoblast colonies, we found endothelial cell adherence markers—intercellular cell adhesion molecules (ICAM) and vascular cell adhesion molecules (VCAM)—which are not expressed in nonischemic myocardial tissue. Analysis of vascular endothelial growth factor (VEGF) expression enabled us to evaluate the effects of cardiomyoplasty on scar tissue vascularization. We found that microvascular density (MVD) increased in the myocardial tissue of the treated animals in comparison with the control group, especially among those in group R4, which had been given relaxin. Through Van Gieson staining of postinfarction cardiac scar tissue from the untreated control rats, we found densely packed bundles of collagen fibers. Conversely, the samples from the rats that had been subjected to the therapeutic treatments displayed less collagen fiber, confirmed by morphometric analysis of tissue collagen content expressed as a sclerosis index (data not shown). Under electron microscopic examination, fibroblasts in the tissue samples from the untreated rats appeared as activated cells. In the rats given C2C12-re-laxin plus relaxin, and in those that were given relaxin alone, the fibroblasts featured predominantly quiescent cells. These cells displayed no evidence of pericellular collagen microfibril assembly and were immersed in a loose, electronlucent extracellular matrix with few and thin collagen fibrils. In contrast, the samples from the rats that had been given C2C12-GFP showed ultrastructural features that did not differ substantially from those of the untreated rats. The engrafted myoblasts could be distinguished from the activated fibroblasts in the scar tissue because they never showed any signs of collagen secretion and fiber buildup. None of the C2C12 cells showed any tendency to fuse into multinucleated myotubes or to develop myofibrils; this suggested that they were unable to regenerate contractile tissue at the grafting site, at least under our experimental conditions.
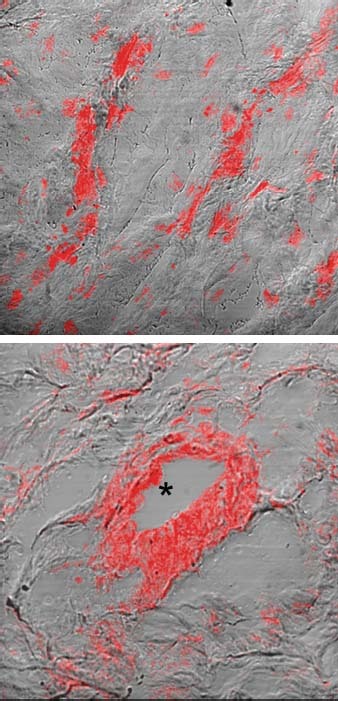
Fig. 5 Photomicrographs show C2C12-GFP and C2C12-relaxin myoblasts grafted in the postinfarction cardiac scar tissue. A) Sections were stained with anti-GFP antibodies (red) to identify the engrafted myoblasts. B) A myoblast-injected heart shows immunoreactive cells in the postinfarcted zone, mainly located around blood vessel lumina (asterisk) and scattered within the extracellular matrix.
Discussion
Ischemic heart disease often leads to cardiac failure due to contractile tissue loss and its replacement with fibrous scar tissue. In fact, over time, a critical lesion induces adaptive mechanisms that are associated with progressive molecular, cellular, and geometric changes. The remodeling process correlates closely with ventricular dysfunction and promotes disease progression toward end-stage heart failure. None of the various therapeutic approaches proposed for this condition has proved to be feasible for all patients, or completely effective. Cardiomyoplasty therapy might enable ischemic scar tissue to be enriched with viable cells, leading to functional benefits. This approach prompts questions: How does it improve cardiac performance? Which way to administer cells is safer and more effective? What type of cell is most suitable? Do adjuvant substances increase success rates? Our study was designed to investigate these issues and yield answers where possible.
Although other experimental models are easier than ours for surgical and diagnostic management, we chose the rat. Doing so enabled us to perform our protocol on a high-enough number of animals to yield extensive case numbers and reliable results, consistent with those of other authors.3,9,19,20,40,41
Several types of cells have been proposed for cardiomyoplasty.42–47 As did others,3,7,9,15,19,20,40,41,48,49 we chose heterologous mouse myoblasts because they are more resistant to ischemia than most other cells7 and are easy to harvest and expand in cultures.8 To avoid any possible host immune response to heterologous myoblasts, we gave our rats immunosuppressive therapy. After we reviewed several studies in which various infusion routes had been used,7,14,40,41,50–54 we decided to investigate the safety and efficiency of retrograde coronary venous infusion.19,20 When we evaluated our surgical results after re-intervention, we saw that the mortality rates in the treated groups overlapped with the mortality rate in the control group (P<0.05). It follows that our chosen cell administration route was safe.
We selected 5 echocardiographic characteristics by which to evaluate the functional results of cardiomyo-plasty. As expected, because of the homogeneous population, our baseline and postinfarction evaluations showed no intergroup differences.
Overall evaluation of the LVEF results suggests that, without therapy, functional myocardial recovery would not have been significant. After 1 month, the separate administration of relaxin and myoblasts yielded relevant functional effects that remained constant over time. In particular, cardiomyoplasty led to better results than did relaxin alone, and cardiomyoplasty combined with relaxin therapy achieved the highest, most stable level within 2 months.
Although the results from the separate administration of relaxin and myoblasts separately confirm those previously reported,8,20,31,40 the combined therapy that we investigated clearly achieved superior results. This suggests synergy between the 2 therapies, not previously reported.
The LVEDD results suggest that, without therapy, the ventricular chamber reaches a diameter that does not improve in subsequent determinations. The expansion progressively decreased in both of the groups that were treated with relaxin and cardiomyoplasty, reaching final values not much different from those at baseline. The LVEDD measurements showed that the effects of relaxin were not distinguishable from those of cardiomyoplasty or combined therapy.
Echocardiographic evaluation revealed a post-AMI increase in LVESD that did not decrease significantly in the absence of therapy. Either relaxin or cell administration led to notable results after a month, and these improved further within the month thereafter. Combined therapy led to better results than did either approach separately. After the 2nd month, the difference was still significant in comparison with the administration of relaxin alone, thereby suggesting synergy between the 2 therapies.
Shortening fraction did not change after AMI in the absence of treatment. One month after therapy administration, only relaxin therapy or combined therapy produced significant results that kept improving through the next month. Myoblast-only treatment required 2 months to achieve significant effects. This trend suggests that relaxin has a faster onset that explains the early results recorded in groups R2 and R4, whereas myoblasts alone took a longer time to reach full effectiveness. Group R4, which received both, attained the best results, and earlier than did the other groups.
The MPI was inversely proportional to left ventricular functionality and almost doubled after AMI. It then remained constant over time in group R1, so there was not a substantial recovery in myocardial function in the absence of therapy. Either myoblast or relaxin administration resulted in a clear and steady improvement, especially in group R4; however, there were no significant statistical differences between the 2 therapies or in comparisons with their combined administration.
The echocardiographic results showed that the therapies (relaxin, cardiomyoplasty, and combined) resulted in left ventricular geometric global and segmental kinetic improvement, probably with a positive influence on remodeling. Systemic administration of relaxin or myoblasts improved functional and anatomic values. The combined administration of the two usually produced better results. Further investigation is required to confirm these effects of relaxin and myoblasts.
To investigate whether functional and anatomic echocardiographic changes achieved through the use of relaxin and cardiomyoplasty were related to a real recovery of viable myocardial tissue in scarred areas or to changes in matrix composition or scar vascularization, we used microPET scanning in vivo. To our knowledge, this is the first PET myocardial viability evaluation to have been performed in this particular animal model.
The PET analyses showed that cardiomyoplasty increased the amount of viable myocardial tissue and led to the functional outcomes that were detected echocar-diographically. This result can be explained by assuming that myoblasts administered via retrograde venous delivery overcome the endothelial barrier, settle in the extracellular matrix, and survive. The evolution of myocardial viability suggests a myoblast replication,19,20,55 or, alternatively, myoblast homing could encourage resident cells to proliferate through a paracrine action.4,7,9 Relax-in-only administration yielded results very similar to those in the control group; relaxin itself does not act on myocardial viability. Perhaps relaxin's proven effectiveness is linked to a positive effect on the extracellular matrix composition or on scar vascularization. Combined therapy yielded viability improvement that exceeded the myoblast-only therapy in terms of the rapidity and amount of myocardial viability recovery, thereby explaining the morphologic and functional improvements apparent on echocardiography. In this context, relaxin appears to be a cardiomyoplasty-improving factor. In combined therapy, we can reason that relaxin administered through a systemic route and in loco secreted by engineered myoblasts makes the matrix more permeable and receptive to administered cells. The myoblasts can settle into scar tissue and proliferate faster and better. Relaxin also influences the injured tissue's microvascular circulation, improving the blood supply to the myoblasts and supporting their survival and proliferation.
Histologic evaluation showed that the administered myoblasts were located selectively in affected myocar-dial tissue, as documented by immunofluorescence distribution of their markers. As did others,19,20 we noticed that myoblasts delivered via the retrograde venous route colonized the infarcted tissue effectively. Histologic studies clarify that myoblasts tend to settle mainly in post-capillary venulae at the edge of the scar—where the endothelium expresses markers of cell adherence, which are absent in the healthy myocardium. This suggests a role of activated endothelium in promoting the adhesion and diapedesis of administered cells, encouraging their location in the injured tissue. Similarly to previous studies,3,12 our histologic evaluation of VEGF expression showed a higher capillary density in sections of treated myocardium than in untreated regions, especially in groups R2 and R4, proving that relaxin has a relevant role. The Van Gieson staining method showed that collagen in the ischemic scar tissue was substantially reduced in samples of the heart that underwent cellular therapy, particularly in group R4. As with a previous study,10 our study failed to prove a clear formation of new contractile tissue as an outcome of morphologic and functional integration of myoblasts with native tissue, by means of gap-junction formation or myoblast-driven regeneration of injured myocardium.
Study Limitations
In our study, no data on infarct size were presented. Furthermore, we did not take into account the number or percentage of myoblasts retained in the infarcted myocardium. This will be the object of our future study.
Conclusions
Our study provides data in support of the following issues: the venous retrograde delivery route is safe and effective; cardiomyoplasty provides a significant improvement in function and viability; relaxin seems to resist postischemic cardiac remodeling and encourages myocardial functional recovery but has no effects on myocardial viability; and combined therapy consisting of skeletal myoblasts and relaxin seems to have more of a synergistic effect in counteracting postischemic remodeling than does either therapy separately. Further studies are necessary to confirm a possible role of this therapy in the treatment of chronic, postischemic heart failure.
Acknowledgment
We gratefully acknowledge Dr. Orlando Parise for performing the statistical analysis.
Footnotes
Address for reprints: Gabriella Di Lascio, MD, Cardiac Surgery Unit, University of Florence, Viale Morgagni 85, 50141 Florence, Italy
E-mail: gdilascio@tosnet.it
References
- 1.Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soon-paa MH, de la Riviere AB, et al. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res 2008;78(1):18–25. [DOI] [PMC free article] [PubMed]
- 2.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bus-sani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001;344(23): 1750–7. [DOI] [PubMed]
- 3.Schuldt AJ, Rosen MR, Gaudette GR, Cohen IS. Repairing damaged myocardium: evaluating cells used for cardiac regeneration. Curr Treat Options Cardiovasc Med 2008;10(1): 59–72. [DOI] [PubMed]
- 4.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation 2005;112(20): 3174–83. [DOI] [PubMed]
- 5.Ghostine S, Carrion C, Souza LC, Richard P, Bruneval P, Vilquin JT, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation 2002;106(12 Suppl 1):I131–6. [PubMed]
- 6.Ye L, Haider HK, Sim EK. Adult stem cells for cardiac repair: a choice between skeletal myoblasts and bone marrow stem cells. Exp Biol Med (Maywood) 2006;231(1):8–19. [DOI] [PubMed]
- 7.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation 2006;114(1 Suppl):I108–13. [DOI] [PubMed]
- 8.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomy-opathy: four-year follow-up. Circulation 2005;112(12): 1748–55. [DOI] [PubMed]
- 9.Tamaki T, Akatsuka A, Okada Y, Uchiyama Y, Tono K, Wada M, et al. Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS One 2008;3(3):e1789. [DOI] [PMC free article] [PubMed]
- 10.Minami E, Reinecke H, Murry CE. Skeletal muscle meets cardiac muscle. Friends or foes? J Am Coll Cardiol 2003;41 (7):1084–6. [DOI] [PubMed]
- 11.Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 2006;113(15):1832–41. [DOI] [PubMed]
- 12.Menasche P, Alfieri O, Janssens S, McKenna W, Reichen-spurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 2008;117(9):1189–200. [DOI] [PubMed]
- 13.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci U S A 2003;100(13):7808–11. [DOI] [PMC free article] [PubMed]
- 14.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol 2001;37(6):1726–32. [DOI] [PubMed]
- 15.Murtuza B, Suzuki K, Bou-Gharios G, Beauchamp JR, Smo-lenski RT, Partridge TA, Yacoub MH. Transplantation of skeletal myoblasts secreting an IL-1 inhibitor modulates adverse remodeling in infarcted murine myocardium. Proc Natl Acad Sci U S A 2004;101(12):4216–21. [DOI] [PMC free article] [PubMed]
- 16.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131(5):861–72. [DOI] [PubMed]
- 17.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: patho-physiological mechanisms. Annu Rev Pathol 2007;2:307–39. [DOI] [PubMed]
- 18.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, et al. Cardiac stem cells in the real world. J Thorac Cardio-vasc Surg 2008;135(3):673–8. [DOI] [PubMed]
- 19.Suzuki K, Murtuza B, Smolenski RT, Yacoub MH. Selective cell dissemination into the heart by retrograde intracoronary infusion in the rat. Transplantation 2004;77(5):757–9. [DOI] [PubMed]
- 20.Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela-Carver A, Coppen SR, Yacoub MH. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation 2004;110(11 Suppl 1):II225–30. [DOI] [PubMed]
- 21.Formigli L, Perna AM, Meacci E, Cinci L, Margheri M, Nis-tri S, et al. Paracrine effects of transplanted myoblasts and relaxin on post-infarction heart remodelling. J Cell Mol Med 2007;11(5):1087–100. [DOI] [PMC free article] [PubMed]
- 22.Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tre-gear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab 2003;14(5):207–13. [DOI] [PubMed]
- 23.Samuel CS, Du XJ, Bathgate RA, Summers RJ. ‘Relaxin’ the stiffened heart and arteries: the therapeutic potential for relax-in in the treatment of cardiovascular disease. Pharmacol Ther 2006;112(2):529–52. [DOI] [PubMed]
- 24.Conrad KP, Novak J. Emerging role of relaxin in renal and cardiovascular function. Am J Physiol Regul Integr Comp Physiol 2004;287(2):R250–61. [DOI] [PubMed]
- 25.Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev 2004;25(2):205–34. [DOI] [PubMed]
- 26.Dschietzig T, Bartsch C, Baumann G, Stangl K. Relaxin-a pleiotropic hormone and its emerging role for experimental and clinical therapeutics. Pharmacol Ther 2006;112(1):38–56. [DOI] [PubMed]
- 27.Nistri S, Bigazzi M, Bani D. Relaxin as a cardiovascular hormone: physiology, pathophysiology and therapeutic promises. Cardiovasc Hematol Agents Med Chem 2007;5(2):101–8. [DOI] [PubMed]
- 28.Bani-Sacchi T, Bigazzi M, Bani D, Mannaioni PF, Masini E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol 1995;116(1):1589–94. [DOI] [PMC free article] [PubMed]
- 29.Unemori EN, Lewis M, Constant J, Arnold G, Grove BH, Normand J, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen 2000;8(5):361–70. [DOI] [PubMed]
- 30.Samuel CS, Unemori EN, Mookerjee I, Bathgate RA, Layfield SL, Mak J, et al. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 2004;145(9):4125–33. [DOI] [PubMed]
- 31.Perna AM, Masini E, Nistri S, Briganti V, Chiappini L, Ste-fano P, et al. Novel drug development opportunity for relaxin in acute myocardial infarction: evidences from a swine model. FASEB J 2005;19(11):1525–7. [DOI] [PubMed]
- 32.Bonacchi M, Nistri S, Nanni C, Gelsomino S, Pini A, Cinci L, et al. Functional and histopathological improvement of the post-infarcted rat heart upon myoblast cell grafting and relax-in therapy. J Cell Mol Med 2009;13(9B):3437–48. [DOI] [PMC free article] [PubMed]
- 33.Formigli L, Francini F, Tani A, Squecco R, Nosi D, Polidori L, et al. Morphofunctional integration between skeletal myoblasts and adult cardiomyocytes in coculture is favored by direct cell-cell contacts and relaxin treatment. Am J Physiol Cell Physiol 2005;288(4):C795–804. [DOI] [PubMed]
- 34.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xeno-transplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg 2002;74(1):19–24. [DOI] [PubMed]
- 35.Haider HK, Jiang SJ, Ye L, Teh M, Chua T, Fang G, et al. Transient immunosuppression is effective for xenotransplantation of human myoblasts for cardiac repair in a porcine heart model [abstract]. Circulation 2002;106(19 Suppl):II–15.
- 36.Min JY, Sullivan MF, Yang Y, Zhang JP, Converso KL, Morgan JP, Xiao YF. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg 2002;74(5):1568–75. [DOI] [PubMed]
- 37.Pavlath GK, Rando TA, Blau HM. Transient immunosup-pressive treatment leads to long-term retention of allogeneic myoblasts in hybrid myofibers. J Cell Biol 1994;127(6 Pt 2):1923–32. [DOI] [PMC free article] [PubMed]
- 38.Vilquin JT, Asselin I, Guerette B, Kinoshita I, Lille S, Roy R, Tremblay JP. Myoblast allotransplantation in mice: degree of success varies depending on the efficacy of various immunosuppressive treatments. Transplant Proc 1994;26(6):3372–3. [PubMed]
- 39.Peduto G, Rinsch C, Schneider BL, Rolland E, Aebischer P. Long-term host unresponsiveness to encapsulated xenogeneic myoblasts after transient immunosuppression. Transplantation 2000;70(1):78–85. [PubMed]
- 40.Suzuki K, Murtuza B, Suzuki N, Smolenski RT, Yacoub MH. Intracoronary infusion of skeletal myoblasts improves cardiac function in doxorubicin-induced heart failure. Circulation 2001;104(12 Suppl 1):I213–7. [DOI] [PubMed]
- 41.Suzuki K, Brand NJ, Smolenski RT, Jayakumar J, Murtuza B, Yacoub MH. Development of a novel method for cell transplantation through the coronary artery. Circulation 2000;102 (19 Suppl 3):III359–64. [DOI] [PubMed]
- 42.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Van-denwijngaert S, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol 2010;55(20):2232–43. [DOI] [PubMed]
- 43.Pozzobon M, Bollini S, Iop L, De Gaspari P, Chiavegato A, Rossi CA, et al. Human bone marrow-derived CD133(+) cells delivered to a collagen patch on cryoinjured rat heart promote angiogenesis and arteriogenesis. Cell Transplant 2010;19(10): 1247–60. [DOI] [PubMed]
- 44.Gao LR, Zhang NK, Bai J, Ding QA, Wang ZG, Zhu ZM, et al. The apelin-APJ pathway exists in cardiomyogenic cells derived from mesenchymal stem cells in vitro and in vivo. Cell Transplant 2010;19(8):949–58. [DOI] [PubMed]
- 45.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: a study of gender-mismatched bone marrow transplantation patients. Circulation 2003;107(9):1247–9. [DOI] [PubMed]
- 46.Zhang YM, Hartzell C, Narlow M, Dudley SC Jr. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation 2002;106(10):1294–9. [DOI] [PubMed]
- 47.Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, et al. Percutaneous transvenous cellular cardio-myoplasty. A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol 2003;41(11):1964–71. [DOI] [PubMed]
- 48.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003;41(7):1078–83. [DOI] [PubMed]
- 49.Tamaki T, Uchiyama Y, Okada Y, Tono K, Masuda M, Nitta M, et al. Clonal differentiation of skeletal muscle-derived CD34(−)/45(−) stem cells into cardiomyocytes in vivo. Stem Cells Dev 2010;19(4):503–12. [DOI] [PubMed]
- 50.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106(15):1913–8. [DOI] [PubMed]
- 51.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population ofmultipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest 2010;120(4):1125–39. [DOI] [PMC free article] [PubMed]
- 52.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kit-tleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 2004;363 (9411):783–4. [DOI] [PubMed]
- 53.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 2004;363(9411):751–6. [DOI] [PubMed]
- 54.Oettgen P, Boyle AJ, Schulman SP, Hare JM. Cardiac stem cell therapy. Need for optimization of efficacy and safety monitoring. Circulation 2006;114(4):353–8. [DOI] [PubMed]
- 55.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 2008;451(7181):937–42. [DOI] [PubMed]


