Abstract
We implanted a continuous-flow total heart replacement device in a 55-year-old man who had severe end-stage heart failure due to amyloidosis and no other options for treatment. The device was composed of 2 modified HeartMate II ventricular assist pumps. After the implantation, our patient recovered normal neurologic function and was able to converse with his family and work on his computer. He died of multisystem organ failure caused by severe amyloidosis 5 weeks after the implantation.
During the past 6 years, we have been developing and testing various technological iterations for total heart replacement in our animal laboratory and have achieved survival periods as long as 90 days in calves. We describe the development, preclinical trials, and adaptation for human use of the modified HeartMate II apparatus, as well as its role in our patient's survival.
Key words: Amyloidosis/therapy; biomedical engineering; blood flow velocity; heart, artificial; prosthesis design
During the past 6 years, we have been developing a continuous-flow device to totally replace the failing heart. The device, composed of 2 continuous-flow ventricular assist devices, has been subjected to extensive in vivo testing, with survival periods as long as 90 days in calves. In March 2011, we first used a similar device to replace the heart of a man who had refractory heart failure due to amyloidosis. The patient had no other treatment options. We describe this device's development, preclinical trials, adaptation for human use, and role in the patient's survival.
Case Report
A 55-year-old man was admitted to our institution in February 2011. He had rapidly progressive end-stage heart failure due to amyloidosis, which was confirmed histologically (Fig. 1). Intra-aortic balloon pump counterpulsation and intravenous inotropic therapy were unsuccessful in restoring adequate circulation. The patient had to be intubated because of progressive respiratory distress and was undergoing continuous hemodialysis for anuric renal failure. At that point, we decided to try extra-corporeal circulatory assistance with use of a TandemHeart® (CardiacAssist, Inc.; Pittsburgh, Pa). A transseptal 21F left atrial cannula was placed percutaneously via the right femoral vein, and a 17F cannula was placed via the left femoral artery. Because of severe right ventricular compromise, only 3 L/min of flow was attainable with the device. Despite multiorgan failure, the patient's neurologic function remained intact. An echocardiogram obtained after 2 weeks of TandemHeart support showed a small, akinetic-restricted heart and no evidence of myocardial recovery.
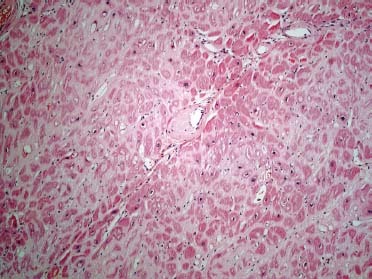
Fig. 1 Photomicrograph. A section of left ventricular wall shows eosinophilic deposits of amyloid surrounding individual myocytes, interstitial spaces, and blood vessel walls (H & E, orig. × 10).
Options were limited for this gravely ill patient, partially because of his small physical stature (height, 175.3 cm; weight, 63.2 kg). However, we thought that he might be a candidate for heart and bone marrow transplantation if his other organs could recover enough function.1 Emergent cardiac transplantation was not considered, not only because of the logistical unlikelihood of finding a donor but also because the patient's critical condition would have made post-transplantation survival unlikely.
Use of a left or right ventricular assist device was considered for intermediate-term circulatory support. However, the patient's extremely thickened and stiff left ventricular (LV) wall and small, contracted LV cavity were contraindications to LV assist device placement. In such patients, obtaining an unobstructed inlet-cannula position is extremely difficult. In addition, the patient's small body habitus and hepatomegaly greatly limited the space available for an implantable subdiaphragmatic pump, necessitating paracorporeal device placement. The Syn Cardia CardioWest® pneumatically actuated total artificial heart (SynCardia Systems, Inc.; Tucson, Ariz) was also not an option: the patient had a narrow chest cavity, and suturing the device to the stiff amyloid-infiltrated rim of ventricular muscle would have been a technical challenge. For this second reason, advanced cardiac amyloidosis is a contraindication to the use of the SynCardia pump. We finally decided to replace his heart with 2 HeartMate® II axial-flow pumps (Thoratec Corporation; Pleasanton, Calif) that would function as a continuous-flow total heart replacement. To achieve this, we used customized atrial adapters that we had developed for use with the HeartMate II blood pumps during the preceding 6 years of extensive in vitro and in vivo preclinical studies.
The Continuous-Flow Total Heart Replacement Device
The 2 atrial adapters used in this case were fabricated from knitted polyester Vascutek® Gelseal™ cardiovascular patches (Terumo Cardiovascular Systems Corporation; Ann Arbor, Mich) that were reinforced with 4 layers of 10 × 14-in polypropylene hernia repair mesh (C.R. Bard, Inc.; Murray Hill, NJ). The adapters were impregnated with medical-grade silicone adhesive and subjected to both ethylene-oxide and steam sterilization. Each polyester patch was fashioned into a cone of proper dimension (20° at the apex) by using a single hand-sewn line of 5-0 polypropylene suture. Each polyester cone was then placed over a solid plastic mandrel to hold the conical geometry while the outer layers were reinforced with the polypropylene hernia mesh and medical silicone adhesive. When the adhesive had dried, the cones were removed from the mandrels, and the apex of each cone was excised at a sharp angle. The resulting elliptical opening was then measured, and an elliptical patch of the same dimensions was fashioned from mesh-reinforced polyester. A round hole, 14 mm in diameter, was then cut in each elliptical patch close to one end to accommodate the HeartMate II inflow after removal of the inflow cannulas.
Implantation Procedure
After extensive discussion with the patient and his family about the procedure, they decided to proceed, and written informed consent was obtained. We implanted the pumps through a median sternotomy, placing a right-angled, wire-reinforced venous cannula in both the superior and inferior venae cavae. The circulation was transferred from the TandemHeart to full cardio-pulmonary bypass, after which circumferential caval tapes were placed and tightened. The heart was excised as if for transplantation, including the removal of all ventricular muscle, all 4 valves, and both atrial appendages. The 2 layers of the atrial septum were carefully teased apart, thereby separating the left and right atria. The fossa ovalis, the only tissue held in common by both atria, was preserved on the left atrial side.2 By separating the atria, we were able to displace the right atrium laterally and the left atrium caudally, which greatly facilitated anatomic placement of the 2 pumps.
The 2 previously constructed cones were tailored by beveling the bases, leaving an opening in each base approximately the same size as the perimeter of each atrial remnant. The bevels were cut so as to direct the apex of each cone inferiorly and to the left, so that the Heart-Mate II inlets would be as far as possible from the transected ends of the aorta and pulmonary artery. The cones were then sutured to the cut edges of the left and right atrial remnants with running 2-0 polypropylene sutures and felt strips for reinforcement.
The inlets of the 2 HeartMate II pumps were removed from the pump housing by unscrewing the blue anodized collets. The inlets were customized by excising the white silicone-elastic bellows. After the bellows were removed, the 14-mm, silicone-coated polyester grafts were visible. These grafts were transected approximately 3 mm from the curved titanium tube that attached the inlets to the pump housing. The 3-mm rim of the 14-mm graft was then sutured to the 14-mm hole in the elliptical patch with a 5-0 polypropylene suture. The elliptical patches were sutured to the elliptical holes at the apex of each cone, completing the construction of the atrial adaptors. Each adaptor was then pressurized with a dedicated system that allowed the suture lines to be inspected for hemostasis. The curved titanium tubes, now attached to the atrial adaptors, were reattached to the inlet end of the HeartMate II pumps.
The outflow grafts of each pump were cut to the appropriate length and were sutured in end-to-end fashion to the cut ends of the aorta and pulmonary artery with use of 5-0 polypropylene sutures. The curved titanium tube attached to the outlet of the right pump was rotated to direct the outflow graft toward the pulmonary artery anterior to the aorta. A customized straight titanium outflow adaptor was used on the left pump. Tailored anastomoses were performed to accommodate the size discrepancy between the outflow grafts and the 2 large vessels (Fig. 2). Hemostasis was ensured, and deairing maneuvers were performed. Full mechanical ventilation was then resumed, and the patient was weaned from cardiopulmonary bypass while the left and right pump flows were progressively increased. All cannulas were removed, and protamine was administered to the patient. After undergoing temporary chest closure, the patient was taken to the intensive care unit in guarded condition but with an excellent hemodynamic status. Several hours later, however, he was taken back to the operating room because of excessive bleeding. A leak in the pulmonary anastomosis was found and repaired. The next day, he underwent definitive chest closure. The pericardial sac was reconstructed with polytetra-fluoroethylene to facilitate a possible future heart transplant (Fig. 3).
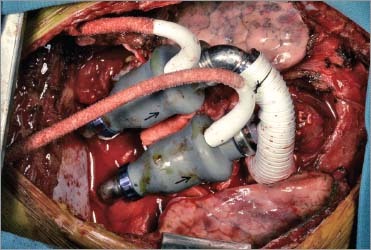
Fig. 2 Photograph shows operative placement of the pumps. Arrows indicate the direction of blood flow.
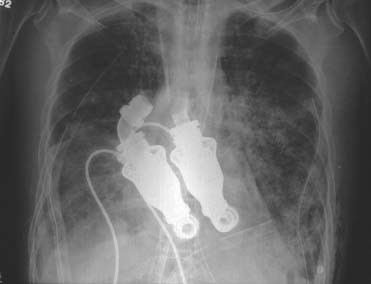
Fig. 3 Postoperative radiograph shows the modified HeartMate II pumps totally replacing the patient's heart.
Postoperative Results
The patient's hemodynamic values remained stable for the next 5 weeks with little adjustment of the left or right pump speed. Only his central venous pressure and arterial pressure were continuously monitored during the postoperative period; pulmonary pressure was not monitored, nor was left or right atrial pressure. The central venous pressure was maintained between 5 and 10 cm H2O and the arterial pressure between 70 and 90 mmHg. The calculated flow through the left pump was between 5 and 6 L/min. In the immediate postoperative period, the pump speed was altered as necessary to obtain these values. By the 3rd postoperative day, we were able to keep the 2 pumps at constant speeds: the right pump at 8,390 rpm and the left at 10,590 rpm (Fig. 4).
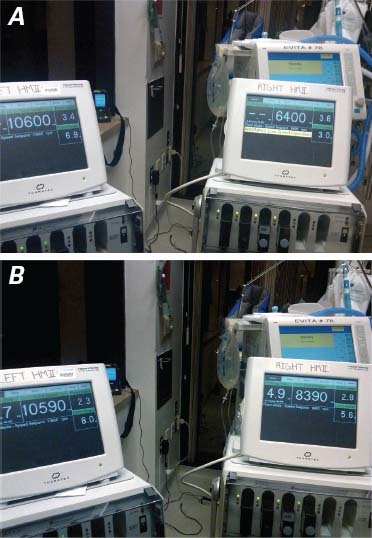
Fig. 4 Photographs show automaticity of response of the continuous-flow pumps, with direct correlation of inlet pressure to flow at constant revolutions per minute (rpm). A) The right flow is at 6,400 rpm, the left at 10,600 rpm. The power generated by the pump is directly correlated to blood flow through the pump. B) The right pump has been increased manually to 8,390 rpm. The concomitant increase in the input power of the pump indicates an increase in flow. In the left pump, there is an immediate response in power from 6.9 to 8 W with no change in the rpm. The intrinsic characteristic of continuous-flow pumps to respond automatically to inlet pressure, analogous to the mammalian heart, would enable left- and right-sided heart flow imbalances to be corrected automatically.
Although the patient had been intubated for 18 days before implantation, his blood gas values improved quickly, and he was extubated successfully on the 4th postoperative day. After extubation, he was alert, oriented, and conversant with his family. However, on day 9, he aspirated and had to be reintubated. A therapeutic bronchoscopy was performed, and a lung biopsy revealed extensive amyloidosis. Subsequently, a trache-ostomy was performed. However, prolonged respiratory support was not required, and the patient was maintained on a tracheostomy collar thereafter. The preoperative anuria persisted throughout the postoperative course, necessitating continued hemodialysis. His liver function continued to worsen slowly. Laboratory evaluation revealed a normal acid-base balance and no evidence of hemolysis, but the bilirubin level progressively rose, and by the 4th postoperative week, liver failure was impairing the patient's prior alert mental status. At that point (5 weeks after implantation), after extensive discussion with the family, we withdrew care, because of the multisystemic amyloid involvement and the extremely poor prognosis.
Autopsy results confirmed the presence of advanced multisystemic amyloidosis (heart, liver, lung, and kidney). The atrial adaptors of the total heart replacement device were lined with a smooth, shiny layer of neointima, and both pumps were free of thrombus.
Discussion
We implanted a continuous-flow total heart replacement device in a patient who had severe end-stage heart failure and no other options for treatment. After the implantation, the patient recovered normal neurologic function and was able to communicate with his family and work on his computer. Unfortunately, after 5 weeks of support, he died of progressive hepatic failure caused by severe amyloidosis.
Development of a self-contained, robust, and reliable mechanical pump for replacing the failing human heart has heretofore posed a prohibitive technical challenge. To date, completely implantable pulsatile-output, volume-displacement devices have been limited by cyclic fatigue. Moreover, size constraints have made these pumps useful in only the physically largest of patients. Externally actuated devices, such as the pneumatic Syn-Cardia pump, are smaller but remain susceptible to cyclic fatigue and require an external drive unit, which limits their application to patients who are awaiting transplantation. The need remains for a durable, reliable artificial heart with a useful life of 10 years or longer.
More than a decade of clinical experience has clearly revealed that rotary blood pumps have benefits over volume-displacement pumps, especially with respect to size and, more important, durability. Little is known, however, about the long-term effects of true pulseless circulation. Many patients who have received axial-flow or centrifugal-flow rotary blood pumps as LV assist devices no longer have a palpable pulse. In nearly every case, however, the arterial pressure still rises and falls with each cardiac contraction in response to inflow pressure sensitivity, which is characteristic of rotary blood pumps. When a rotary blood pump is used to replace the heart rather than to assist it, there is no time variation in the arterial pressure. In this setting, the pump can be said to provide steady-state, pulseless perfusion, in contrast with the perfusion that results when such a pump is used as a ventricular assist device.
For 6 years before encountering the above-described patient, we had been working in our large-animal laboratory to develop a fully implantable total heart replacement device and to characterize the mammalian physiologic response to steady-state, pulseless perfusion. To that end, we implanted various prototypes of the device in more than 40 calves. Each prototype consisted of a pair of rotary blood pumps configured so that they could be readily sutured to the atrial remnant after excision of the entire heart. The iterative experience gained in this research enabled us to develop adaptors that would meet the requirements of total heart replacement. With progressive refinement of our custom cuffs and surgical technique, we were routinely able to implant the device successfully in the bovine model: the animals generally stood upright within the first 12 hours postoperatively and survived for at least 1 or 2 months. The longest survivor was humanely killed at 90 days. The animals were able to exercise on a treadmill and had normal end-organ function and neuro-hormonal responses throughout their postoperative courses. Most important, we were able to demonstrate the automaticity of these pumps to variations in inflow pressure. This important characteristic of continuous-flow pumps enables correction of the natural imbalance of right and left ventricular output due to the bronchial circulation, which has been a barrier to totally implantable pulsatile heart pump replacements.3 When our calves were placed on treadmill exercise, this inflow re-sponsivity also permitted increased blood flow and oxygen consumption without a need to change the speed. This automatic balancing in response to increased inflow pressure was shown repeatedly in our patient (Fig. 4).
Although other pumps used in our preclinical experiments might have been technically easier to implant than the HeartMate II as a clinical cardiac replacement device and would have required less complex atrial adaptors, only the HeartMate II was approved for human use at the time of this implantation. Indeed, we had specifically developed HeartMate II adaptors that would be suitable for use if the opportunity arose for clinical implantation.
In this clinical experience, we demonstrated the feasibility of total nonpulsatile circulatory replacement after the removal of a patient's diseased heart. Continuous-flow pumps have a number of advantages for use as a total heart, including durability and small size, making them feasible for people of all sizes—even infants and children. Patients have lived more than 8 years thus far with continuous-flow ventricular assist devices, without evidence of pump wear, and we anticipate that these pumps will last for 10 to 15 years. Of note, the automaticity of response that is characteristic of continuous-flow pumps greatly simplifies the application of this technology to total heart replacement. However, this technology does not confine us to total nonpulsa-tile flow. If future experience shows that some pulsatility is optimal, continuous-flow pumps can be rendered pulsatile. We have previously induced pulsatility in our experimental models4 and continue to conduct research experiments to test the relative role of pulsatility in experimental animals.
Acknowledgments
The authors acknowledge Pranav Loyalka, MD, for his clinical care of the patient described in this case; and Diana Kirkland and Marianne Mallia, ELS, of the Department of Scientific Publications at the Texas Heart Institute, for their editorial assistance in the preparation of the manuscript.
Footnotes
Address for reprints: William E. Cohn, MD, Center for Cardiac Support, Texas Heart Institute at St. Luke's Episcopal Hospital, 6770 Bertner Ave, Houston, TX 77030
E-mail: wcohn@texasheart.org
References
- 1.Thaunat O, Alyanakian MA, Varnous S, Bors V, Damaj G, Rerolle JP, et al. Long-term successful outcome of sequential cardiac and allogeneic bone marrow transplantations in severe AL amyloidosis. Bone Marrow Transplant 2005;35(4):419–20. [DOI] [PubMed]
- 2.Parnis SM, Yu LS, Ochs BD, Macris MP, Frazier OH, Kung RT. Chronic in vivo evaluation of an electrohydraulic total artificial heart. ASAIO J 1994;40(3):M489–93. [DOI] [PubMed]
- 3.Frazier OH, Cohn WE, Tuzun E, Winkler JA, Gregoric ID. Continuous-flow total artificial heart supports long-term survival of a calf. Tex Heart Inst J 2009;36(6):568–74. [PMC free article] [PubMed]
- 4.Bourque K, Dague C, Farrar D, Harms K, Tamez D, Cohn W, et al. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artif Organs 2006;30(8):638–42. [DOI] [PubMed]


