Abstract
Purpose.
We investigated whether human limbal niche cells generate mesenchymal stem cells.
Methods.
Limbal niche cells were isolated from the limbal stroma by collagenase alone or following dispase removal of the limbal epithelium (D/C), and cultured on plastic in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), or coated or three-dimensional Matrigel in embryonic stem cell medium with leukemia inhibitory factor and basic fibroblast growth factor. Expression of cell markers, colony-forming units-fibroblast, tri-lineage differentiation, and ability of supporting limbal epithelial stem/progenitor cells were compared to limbal residual stromal cells.
Results.
Stromal cells expressing angiogenesis markers were found perivascularly, subjacent to limbal basal epithelial cells, and in D/C and limbal residual stromal cells. When seeded in three-dimensional Matrigel, D/C but not limbal residual stromal cells yielded spheres of angiogenesis progenitors that stabilized vascular networks. Similar to collagenase-isolated cells, D/C cells could be expanded on coated Matrigel for more than 12 passages, yielding spindle cells expressing angiogenesis and mesenchymal stem cells markers, and possessing significantly higher colony-forming units-fibroblast and more efficient tri-lineage differentiation than D/C and limbal residual stromal cells expanded on plastic in DMEM with 10% FBS, of which both lost the pericyte phenotype while limbal residual stromal cells turned into myofibroblasts. Upon reunion with limbal epithelial stem/progenitor cells to form spheres, D/C cells expanded on coated Matrigel maintained higher expression of p63α and lower expression of cytokeratin 12 than those expanded on plastic in DMEM with 10% FBS, while spheres formed with human corneal fibroblasts expressed cytokeratin 12 without p63α.
Conclusions.
In the limbal stroma, cells subjacent to limbal basal epithelial cells serve as niche cells, and generate progenitors with angiogenesis and mesenchymal stem cells potentials. They might partake in angiogenesis and regeneration during corneal wound healing.
In the limbal stroma, niche cells subjacent to limbal basal epithelial cells generate progenitors with angiogenesis and MSC potentials. They might partake in angiogenesis and regeneration during corneal wound healing.
Introduction
Mesenchymal stem cells refer to a group of multipotent stromal cells, which first were isolated and characterized from the bone marrow,1 but have now been isolated from nearly all adult tissues.2,3 A number of studies have disclosed that mesenchymal stem cells have a great potential in regenerative medicine due to their unique properties of self-renewal, high plasticity, modulation of immune responses, and flexibility for genetic modification.4–8
Present cumulative evidence indicates that in vivo mesenchymal stem cells are localized in a perivascular region, in which one prime candidate to generate mesenchymal stem cells is pericytes.2,3,9 Due to the lack of specific markers for pericytes and mesenchymal stem cells, it has been a great challenge to define the genuine in vivo ancestor for mesenchymal stem cells and pericytes. Nonetheless, one in vitro way of evaluating mesenchymal stem cells function is to measure their efficiency of generating colony-forming units-fibroblast.10 For example, bone marrow-derived colony-forming units-fibroblast has been placed in the same hierarchy with hematopoietic stem cells because it has an ability to replenish bone marrow hematopoietic stem cell niche in vivo.11,12 The frequency of colony-forming units-fibroblast does correlate with the incidence of progenitors in a given bone marrow sample.13 Furthermore, there is a subset of in vivo stromal cells that represents the ancestor of mesenchymal stem cells when cultured in vitro, shares the same perivascular niche with hematopoietic stem cell,11 and serves as the key component of hematopoietic stem cells niche by providing stem cell factor.14
Recently, we isolated human limbal niche cells successfully by digesting the entire limbal tissue with collagenase alone.15,16 We demonstrated that such limbal niche cells are a subset of mesenchymal cells immediately subjacent to limbal basal epithelial cells that have the cell size as small as 5 μm in diameter and heterogeneously express embryonic stem cells markers, such as Oct4, Sox2, SSEA4, and Nanog, as well as other stem cell markers, such as Nestin, N-Cadherin, and CD34.15 They could be expanded for up to 12 passages with 33 cell doubling times on coated Matrigel in the embryonic stem cell medium containing leukemia inhibitory factor and basic fibroblast growth factor.17 If re-seeded in three-dimensional Matrigel, they maintain the ability of reversibly expressing embryonic stem cell markers, support self-renewal of limbal epithelial progenitor cells with high clonal growth, and prevent corneal epithelial differentiation.16,17 Because they act as angiogenesis progenitors by differentiating into vascular endothelial cells and pericytes,17 we wonder whether they could be a better candidate giving rise to mesenchymal stem cells, although they are not in a perivascular location. To resolve this question, we devised a new strategy of enriching isolation of limbal niche cells and demonstrated that they expressed markers of angiogenesis progenitors and mesenchymal stem cells following expansion on coated Matrigel. They were a better candidate of supporting limbal epithelial progenitor cells than the residual stromal cells, and they generated mesenchymal stem cells with higher colony-forming units-fibroblast and tri-lineage differentiation than if they were expanded on plastic in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), which is the conventional method of generating mesenchymal stem cells. The significance of these findings in wound healing and regeneration is discussed further.
Materials and Methods
Cell Isolation and Culturing
Human limbal niche cells were isolated and cultured as prescribed previously.15–18 Corneoscleral rims from 18–60-year-old donors were obtained from the Florida Lions Eye Bank (Miami, FL) and managed in accordance with the Declaration of Helsinki. The limbal explants were digested with Dispase II at 4°C for 16 hours to generate intact epithelial sheets19 or with collagenase A (Coll) at 37°C for 18 hours to generate clusters containing the entire limbal epithelial sheet with subjacent stromal cells.15,16,18 To enrich isolation of stromal cells subjacent to limbal basal epithelial cells, we first removed the limbal epithelial sheet using Dispase II and then digested the remaining stroma with collagenase, in a manner termed D/C. This D/C method resulted in floating cell clusters (D/C clusters) and single cells adherent on plastic termed residual stromal cells (RSC). The D/C clusters were digested further with 0.25% trypsin and 1 mM EDTA (T/E) at 37°C for 15 minutes to yield single cells before being seeded at the density of 1 × 104 per cm2 in 6-well plates either on coated Matrigel in ESCM containing 10 ng/mL LIF and 4 ng/mL bFGF (MESCM) or on plastic in DMEM with 10% FBS. ESCM is made of knockout DMEM supplemented with 20% knockout serum, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, 1% nonessential amino acid, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. Upon 80–90% confluence, they were passaged serially at the density of 5 × 103 per cm2. Bone marrow-derived mesenchymal stem cells (BMMSC, PT-2501) obtained from LONZA (Allendale, NJ) and human corneal fibroblasts (HCF) obtained as reported previously20 were cultured on plastic in DMEM with 10% FBS as the controls. All materials used for cell isolation and culturing are listed in Supplementary Table S1 (http://www.iovs.org/content/53/9/5686/suppl/DC1).
Culturing in Three-Dimensional Matrigel
Three-dimensional (3D) Matrigel was prepared by adding 150 μL of 50% Matrigel (diluted in MESCM) per chamber of an 8-well chamber slide following incubation at 37°C for 30 minutes. Single collagenase-isolated cells, D/C cells, and residual stromal cells were seeded in 3D Matrigel and cultured for 10 days in MESCM. Single cells from resultant spheres were released by digestion with 10 mg/mL dispase II at 37°C for 2 hours followed by T/E, and mixed with human umbilical vein endothelial cells at a ratio of 1:1, and seeded at the density of 105 cells per cm2 on the surface of 3D Matrigel prepared by adding 50 μL of 100% Matrigel into 24 well plates for 30 minutes before use, and cultured in endothelial cell growth medium 2 to elicit a vascular tube-like network as reported previously.21–23 The human umbilical vein endothelial cells were pre-labeled with red fluorescent nanocrystals, which were prepared by incubating 1 × 106 cells with 10 nM labeling solution at 37°C for 60 minutes before use (Qtracker cell labeling kits; Invitrogen, Carlsbad, CA). Human umbilical vein endothelial cells alone were seeded at the same density as the control. As reported previously,16–18 single limbal epithelial progenitor cells obtained from dispase-isolated limbal epithelial sheets were mixed at a ratio of 4:1 with the cells passaged serially on plastic or coated Matrigel and seeded at the total density of 5 × 104 per cm2 in 3D Matrigel. After 10 days of culture in MESCM, the resultant sphere growth was collected by digestion of Matrigel with 10 mg/mL dispase II at 37°C for 2 hours.
Colony-Forming Units-Fibroblast Assay
To determine the colony-forming units-fibroblast,24,25 each group of cells was seeded at the density of 50 cells per cm2 in 75 cm2 plastic dishes in DMEM with 10% FBS. After 12 days of culturing, cells were fixed with methanol (5 minutes, RT) and stained with 0.5% crystal violet in glacial acetic acid for 15 minutes. Resultant fibroblast-like clones were subdivided into three types according to the reported grading system, that is, micro (5–24 cells), small (>25 cells, <2 mm), or large (>2 mm) clones.25 The total numbers of clones was counted and expressed as the percentage of seeded cells (%) in triplicate.
Assays for Adipogenesis, Osteogenesis, and Chondrogenesis
For assays of adipogenesis or osteogenesis, single cells were seeded at the density of 1 × 104 cells per cm2 in 24-well plastic plates in DMEM with 10% FBS. After cells reached 90% confluence, the medium was switched to the Adipogenesis Differentiation Medium or the Osteogenesis Differentiation Medium (Invitrogen) and changed every 3 days. After 21 days of culturing, cells were fixed with 4% formaldehyde and stained with Oil Red O for adipocytes or with Alizarin Red for osteocytes following the manufacturer's protocol. Cells with positive Oil Red O were counted in a total of 2000 cells in triplicate cultures. Mineralized cells with positive Alizarin Red staining were quantified by the procedure following the manufacturer's protocol by measuring OD at 450 nm in triplicate cultures. For the chondrogenesis assay, pellets were prepared by spinning down 3 × 105 cells and incubated in the Chondrogenesis Differentiation Medium (Invitrogen) with the medium changed every 3 days. After 28 days of culturing, pellets were fixed with 4% formaldehyde, embedded in the Optimal Cutting Temperature Compound, prepared for 6 μm frozen cross-sections, stained with Alcian Blue and quantified following the manufacturer's protocol by measuring OD at 450 nm in triplicate cultures.
Immunofluorescence Staining
Single cells were prepared for cytospin using Cytofuge at 1000 rpm for 8 minutes (StatSpin, Inc., Norwood, MA), fixed with 4% formaldehyde for 15 minutes, permeabilized with 0.2% Triton X-100 in PBS for 15 minutes, and blocked with 2% BSA in PBS for 1 hour before being incubated with primary antibodies overnight at 4°C. After washing with PBS, cytospin preparations were incubated with corresponding secondary antibodies for 1 hour using appropriate isotype-matched nonspecific IgG antibodies as controls. The nucleus was counterstained with Hoechst 33342 before being analyzed with a Zeiss LSM 700 confocal microscope (LSM700; Carl Zeiss, Thornhood, NY). Detailed information about primary and secondary antibodies and agents used for immunostaining is listed in Supplementary Table S2 (http://www.iovs.org/content/53/9/5686/suppl/DC1).
Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
Total RNAs were extracted by RNeasy Mini RNA Isolation Kit (Qiagen, Valencia, CA). A total of 1–2 μg of total RNAs was reverse-transcribed to cDNA by the High Capacity cDNA Transcription Kit (Applied Biosystems, Foster City, CA). RT-qPCR was done in a 20 μL solution containing cDNA, TaqMan Gene Expression AssayMix, and universal PCR Master Mix (Applied Biosystems). The results were normalized by an internal control, that is glceraldehyde-3-phosphate dehydrogenase (GAPDH). All assays were performed in triplicate for each primer set. The relative gene expression was analyzed by the comparative CT method (ΔΔCT). All TagMan Gene Expression Assays with probe sequences are listed in Supplementary Table S3 (http://www.iovs.org/content/53/9/5686/suppl/DC1).
Western Blot
Proteins were extracted from day 10 spheres generated by limbal epithelial progenitor cells alone or mixed with other cells in RIPA buffer supplemented with proteinase inhibitors. Equal amounts of proteins determined by the BCA assay (Pierce, Rockford, IL) in total cell extracts were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes then were blocked with 5% (wt/vol) fat-free milk in TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% [vol/vol] Tween-20), followed by sequential incubation with specific primary antibodies and their respective secondary antibodies using β-actin as the loading control. The immunoreactive bands were visualized by a chemiluminescence reagent (Pierce). Antibodies used are listed in Supplementary Table S2 (http://www.iovs.org/content/53/9/5686/suppl/DC1).
Statistical Analysis
All assays were performed in triplicate, each with a minimum of three donors. The data were reported as means ± SD and compared using the appropriate version of Student's unpaired t-test. Test results were reported as two-tailed P values, where P < 0.05 was considered statistically significant.
Results
Distribution of Cells Expressing Angiogenesis Markers in Limbus Stroma
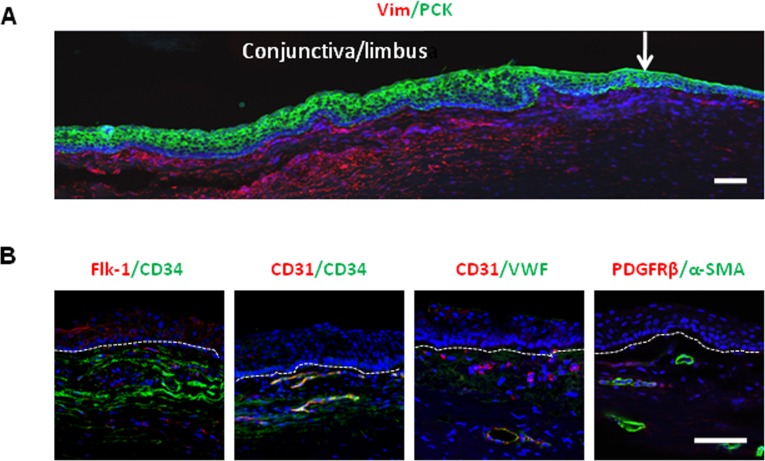
Previously, we reported that human limbal niche cells expanded on coated Matrigel give rise to angiogenesis progenitor cells, which can differentiate into vascular endothelial cells and pericytes.17 As a first step of localizing the origin of cells that carried such an angiogenesis potential, we performed double immunostaining of corneo-limbo-conjunctival sections between pan cytokeratin and vimentin to delineate limbal epithelial cells and underlying stromal cells, respectively (Fig. 1A). Subsequent double immunostaining between several pairs of angiogenesis markers, such as Flk-1/CD34, CD31/VWF, and α-SMA/PDGFRβ, also showed that some of vimentin+ stromal cells expressed these markers (Fig. 1B). A closer look disclosed that cells expressing these angiogenesis markers lay not only in the perivascular location but also immediately subjacent to limbal basal epithelial cells.
Figure 1. .
Cells expressing angiogenesis markers in human limbal stroma. (A) Double immunostaining of corneo-limbo-conjunctival sections with pan cytokeratin (PCK) and vimentin (Vim) delineated the epithelium and the stroma in the limbal region. White arrow: the border between the cornea and limbus. (B) In the stroma, double immunostaining of Flk-1/CD34, CD31/CD34, CD31/VWF, and α-SMA/PDGFRβ pairs showed cells expressing potential angiogenesis markers. Although the majority of these cells were present in the perivascular location, some were found subjacent to limbal basal epithelial cells (white lines). Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 100 μm.
Preferential Isolation of Stromal Cells Subjacent to Limbal Basal Epithelial Cells
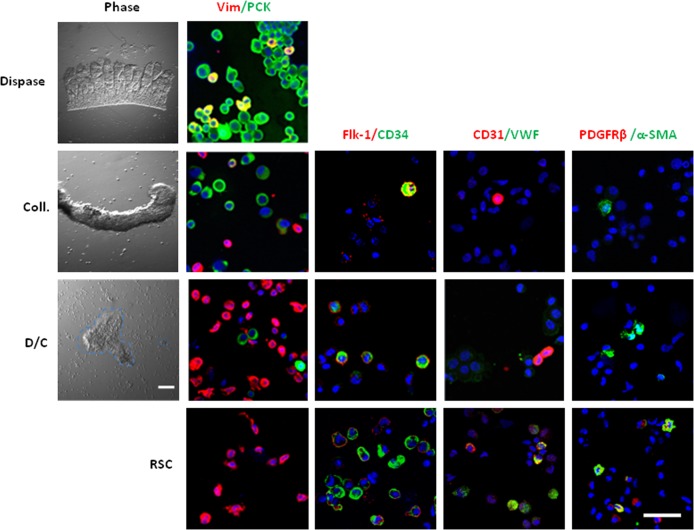
As reported previously,19,26 digestion with dispase alone removed the entire limbal epithelial sheet that consisted of pan cytokeratin+ epithelial cells, of which some also co-expressed vimentin (Fig. 2, Dispase).26,27 In contrast, digestion with collagenase alone successfully removed pan cytokeratin+ epithelial cells together with subjacent vimentin+ mesenchymal cells (Fig. 2, Coll).16–18,26 To enrich the isolation of stromal cells subjacent to limbal basal epithelial cells, we first removed the limbal epithelial sheet by dispase digestion and then subjected the remaining stroma to collagenase digestion. This method, termed D/C digestion, yielded clusters of cells floating in the medium and single residual stromal cells adherent on the plastic dish (Fig. 2). Double immunostaining between pan cytokeratin and vimentin showed that approximately 80% pan cytokeratin+ epithelial cells and 20% vimentin+ stromal cells were present in collagenase-isolated clusters, consistent with our previous reports.18,26 In contrast, approximately 5% pan cytokeratin+ epithelial cells and 95% vimentin+ stromal cells were in D/C clusters, while all limbal residual stromal cells were vimentin+ (Fig. 2). Double immunostaining of several angiogenesis markers and counting a total of 2000 cells in each condition revealed that less than 1% of collagenase- or D/C-isolated vimentin+ cells expressed Flk-1, CD34, CD31, or α-SMA. In residual stromal cells, however, more than 10% did so. Furthermore, VWF+ cells and PDGFRβ+ cells were detected only in limbal residual stromal cells (Fig. 2). These results suggested that cells expressing potential angiogenesis markers were found in D/C-isolated vimentin+ cells subjacent to limbal basal epithelial cells as well as in vimentin+ cells in the remaining limbal stroma.
Figure 2. .
Isolation of limbal stromal cells by enzymatic digestion. Dispase digestion of the limbal segment isolated an intact epithelial sheet, which contained exclusively pan cytokeratin (PCK)+ cells, of which few co-expressed vimentin (Vim). Collagenase digestion (Coll) isolated clusters consisting of 80% pan cytokeratin+ cells and 20% vimentin+ cells. Following removal of the epithelial sheet by dispase, the residual stroma was digested with collagenase, resulting in D/C cell clusters floating in the medium and single RSC adherent on the plastic dish. D/C clusters contained 95% vimentin+ cells and 5% pan cytokeratin+ epithelial cells, while limbal residual stromal cells contained only vimentin+ cells. Double immunostaining of Flk-1/CD34, CD31/VWF, and α-SMA/PDGFRβ pairs revealed that cells expressing angiogenesis markers were present in the above three stromal fractions. Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 50 μm.
D/C but Not Limbal Residual Stromal Cells Form Spheres Containing Angiogenesis Progenitors in 3D Matrigel
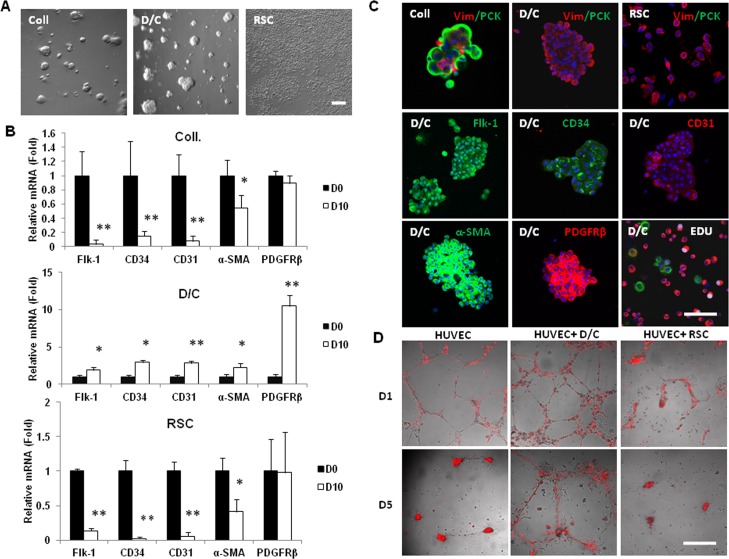
Previously, we found that collagenase-isolated limbal niche cells expanded on coated Matrigel turn into angiogenesis progenitor cells when reseeded in 3D Matrigel in MESCM.17 To determine whether D/C and limbal residual stromal cells, of which both expressed angiogenesis markers in vivo (Fig. 2), could have the potential of differentiating into angiogenesis progenitors, we seeded them directly in 3D Matrigel immediately after isolation in MESCM. As reported,16–18 single cells from collagenase-isolated clusters generated sphere growth during 10 days of culturing in embryonic stem cell medium. Herein, we noted that they also formed spheres during 10 days of culturing in MESCM (Fig. 3A). As a comparison, single cells from D/C clusters also generated spheres, but single limbal residual stromal cells did not (Fig. 3A). When compared to cells immediately isolated at Day 0 (D0), spheres formed by collagenase-isolated cells at Day 10 expressed significantly less Flk-1, CD34, CD31, and α-SMA transcripts (Fig. 3B, P < 0.05, n = 3). A similar expression level was noted in single limbal residual stromal cells cultured at Day 10. In contrast, expression levels of the aforementioned markers and that of PDGFRβ transcript were upregulated significantly in spheres formed by D/C isolated cells (Fig. 3B, P < 0.05, n = 3). As reported,18 spheres formed by collagenase-isolated cells consisted of predominantly pan cytokeratin+ epithelial cells and few vimentin+ cells (Fig. 3C). Nonetheless, cells in D/C spheres and single limbal residual stromal cells were exclusively vimentin+ (Fig. 3C), suggesting that vimentin+ cells could be enriched in D/C clusters by culturing in 3D Matrigel. Immunostaining confirmed that vimentin+ cells in D10 D/C spheres in 3D Matrigel expressed Flk-1, CD34, CD31, α-SMA, and PDGFRβ (Fig. 3C), but not SMMHC, which is a marker of smooth muscle cells,27 and not S100A4, which is a marker of myofibroblasts28 (not shown). These findings suggested that D10 D/C spheres in 3D Matrigel consisted of angiogenesis progenitors. The notion that these angiogenesis progenitors could serve as pericytes was confirmed by 5-day co-culturing with human umbilical vein endothelial cells on the surface of 100% Matrigel. Single cells from day 10 D/C spheres could, but single limbal residual stromal cells could not, stabilize the vascular network formed by human umbilical vein endothelial cells (Fig. 3D).
Figure 3. .
Spheres of angiogenesis progenitors in 3D Matrigel. Single cells from collagenase-isolated (Coll) clusters, D/C clusters, and limbal residual stromal cells were seeded in 3D Matrigel containing MESCM for 10 days. (A) Sphere growth was noted only from collagenase-isolated clusters (Coll) and D/C cells, but not limbal residual stromal cells. Compared to the expression level by cells immediately isolated (D0) set as 1, those of Flk-1, CD34, CD31, and α-SMA transcripts were reduced significantly in collagenase-isolated cells spheres and limbal RSC. (B) However, those of the aforementioned transcripts and PDGFRβ transcript were upregulated significantly in D/C spheres (*P < 0.05 and **P < 0.01, n = 3). (C) Collagenase-isolated cells spheres consisted of predominately pan cytokeratin (PCK)+ cells, while cells in D/C spheres and single limbal residual stromal cells were all vimentin (Vim)+. Cells in D/C spheres uniformly expressed Flk-1, CD34, CD31, α-SMA, and PDGFRβ with low EdU nuclear labeling (5%, white). (D) In 5 days co-culturing experiments on 100% Matrigel, single D10 D/C cells, but not D10 limbal residual stromal cells, stabilized the vascular network formed by human umbilical vein endothelial cells (HUVEC, prelabeled with red Q-tracker). Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 100 μm.
Cells Expanded by Serial Passage on Coated Matrigel Express Pericyte and Mesenchymal Stem Cells Markers
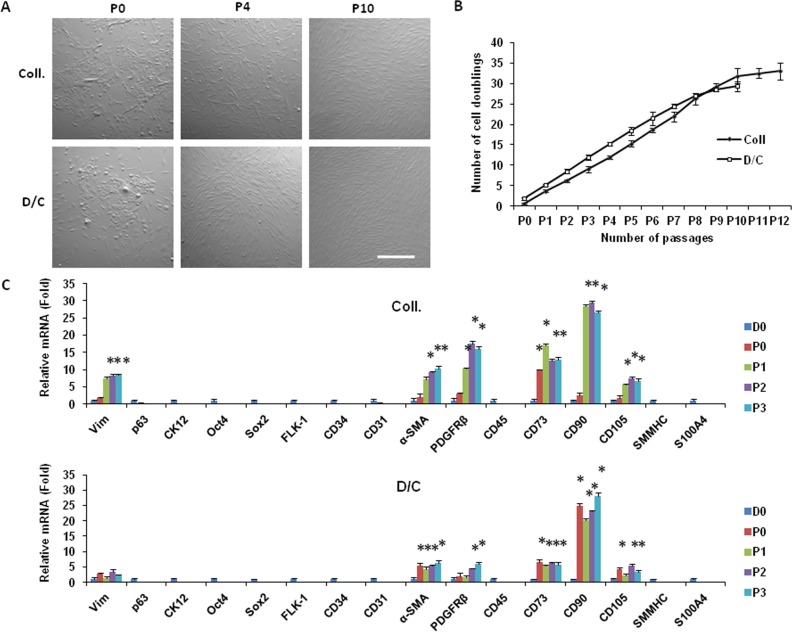
As noted previously,16 cells from collagenase-isolated clusters exhibited poor proliferation if seeded in 3D Matrigel immediately after isolation. Herein, we also noted that cells from D/C-isolated clusters exhibited poor proliferation as evidenced by low (5%) labeling by EdU, a thymidine analogue, when seeded immediately in 3D Matrigel to generate spheres (Fig. 3C, white merged nuclear fluorescence). To circumvent this limitation, we discovered previously that collagenase-isolated cells could be expanded by a total of 12 passages if seeded on coated Matrigel in MESCM, resulting in 33 cell doublings and 1 × 1010 cells.17 Herein, we also found that D/C-isolated cells similarly could be expanded to yield spindle cells (Fig. 4A) and a growth potential for more than 10 passages (Fig. 4B). Similar to what we reported for collagenase-isolated cells,16 compared to the expression level by D0 D/C-isolated cells, RT-qPCR revealed rapid extinction of p63 and cytokeratin 12 transcripts during serial passages to the third passage (Fig. 4C), indicating successful elimination of epithelial cells. Also similar to collagenase-isolated cells,16,17 expanded spindle cells from D/C-isolated cells also lost the expression of such embryonic stem cell markers as Oct4 and Sox2, and such markers for endothelial progenitor cells as Flk-1, CD34, and CD31. Also similar to collagenase-isolated cells,17 the expression levels of vimentin, α-SMA, and PDGFRβ transcripts were upregulated by an average of 2.5-, 6.4-, and 6-fold, respectively (Fig. 4C). Expanded spindle cells from collagenase- and D/C-isolated cells did not express CD45, but upregulated expression of such mesenchymal stem cells markers as CD73, CD90, and CD105 by an average of 5.8-, 28-, and 3.5-fold, respectively (Fig. 4C, n = 3, P < 0.05). They did not express SMMHC and S100A4 transcript, suggesting that they were neither smooth muscle cells nor myofibroblasts. Taken together, the above data suggested that limbal stromal cells isolated by the D/C method could be expanded on coated Matrigel in MESCM in a manner similar to those isolated by collagenase, and that both expanded cells exhibited a similar growth potential and adopted a similar phenotype with features of pericytes and mesenchymal stem cells.
Figure 4. .
Serial passages on coated Matrigel in MESCM. Single cells from collagenase-isolated clusters (Coll), D/C, or limbal RSC were seeded at a density of 1 × 104 per cm2 and passaged serially on coated Matrigel in MESCM, resulting in spindle cells (A) with a steady growth up to P10 and a total of more than 1 × 1010 cells (B). In contrast, limbal residual stromal cells did not grow. Compared to the expression level by cells immediately isolated (D0), spindle cells expanded from collagenase-isolated clusters and D/C exhibited a similar expression pattern up to P3, that is with more expression of vimentin, CD73, CD90, CD105, α-SMA, and PDGFRβ transcripts (C). Scale bar: 200 μm.
Phenotypic Change by Serial Passages on Plastic in DMEM with 10% FBS
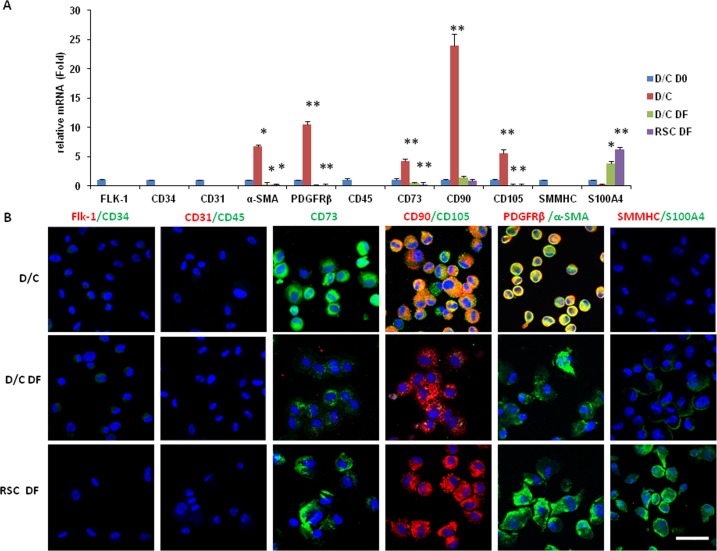
We then determined whether D/C cells and limbal residual stromal cells also could generate mesenchymal stem cells by serial passages on plastic in DMEM with 10% FBS (DF), which is the conventional method of generating mesenchymal stem cells. Similar to D/C cells expanded on coated Matrigel up to the third passage (Fig. 4), D/C cells at P4 did not express Flk-1, CD34, CD31, and CD45 (Fig. 5A). The same result was noted for D/C cells and limbal residual stromal cells expanded on plastic in DMEM with 10% FBS at the fourth passage. Also similar to D/C cells cultured up to the third passage (Fig. 4), D/C cells at the fourth passage still upregulated expression of CD73, CD90, CD105, α-SMA, and PDGFRβ transcripts by 4.3-, 24.0-, 5.6-, 6.8-, and 10.5-fold, respectively (Fig. 5A, P < 0.05 for CD73, but P < 0.01 for all others, n = 3). In contrast, except for CD90, of which a comparable level was expressed, significant downregulation of CD73, CD105, α-SMA, and PDGFRβ transcripts was noted in D/C DF and limbal residual stromal cells DF cells at passage 4 (Fig. 5A, P < 0.05, n = 3). As noted in Figure 4, D/C cells expanded on coated Matrigel at passage 4 still did not express SMMHC and S100A4 transcripts. Although D/C and limbal residual stromal cells cultured in DMEM with 10% FBS at passage 4 did not express SMMHC transcript, both significantly upregulated expression of the S100A4 transcript, with limbal residual stromal cells cultured in DMEM with 10% FBS being more than D/C cells cultured in DMEM with 10% FBS (Fig. 5A, P < 0.05, n = 3). The above expression pattern of different markers by D/C cells, D/C cells cultured in DMEM with 10% FBS, and limbal residual stromal cells cultured in DMEM with 10% FBS was confirmed by immunostaining (Fig. 5B). Unlike D/C cells expanded on coated Matrigel, D/C cells cultured in DMEM with 10% FBS lost expression of CD105 and PDGFRβ. Limbal residual stromal cells cultured in DMEM with 10% FBS exhibited a similar phenotype to D/C cells cultured in DMEM with 10% FBS except that they expressed even more S100A4 (Fig. 5A, P < 0.05, n = 3). These data indicated that D/C cells and limbal residual stromal cells expressed mesenchymal stem cell markers, but lost the pericyte phenotype, while limbal residual stromal cells adopted the myofibroblast phenotype when they were expanded on plastic in DMEM with 10% FBS.
Figure 5. .
Phenotypic change by serial passage on plastic in DMEM with 10% FBS. The phenotype was determined by marker expression using RT-qPCR (A) and immunostaining (B) among D/C cells expanded on coated Matrigel (D/C) or on plastic in DMEM with 10% FBS (D/C DF), and limbal RSC expanded on plastic in DMEM with 10% FBS (RSC DF) at P4. All three expanded cells did not express Flk-1, CD34, CD31, and CD45 transcripts. D/C cells expressed the highest level of CD73, CD90, CD105, α-SMA, and PDGFRβ transcripts and proteins, but did not express SMMHC and S100A4 transcripts and proteins (Fig. 5, *P < 0.05 and **P < 0.01, n = 3 ). D/C DF and RSC DF cells did not express CD105 and PDGFRβ transcripts and proteins, while the latter expressed more S100A4 transcripts and protein than the former (Fig. 5, P < 0.05, n = 3). Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 50 μm.
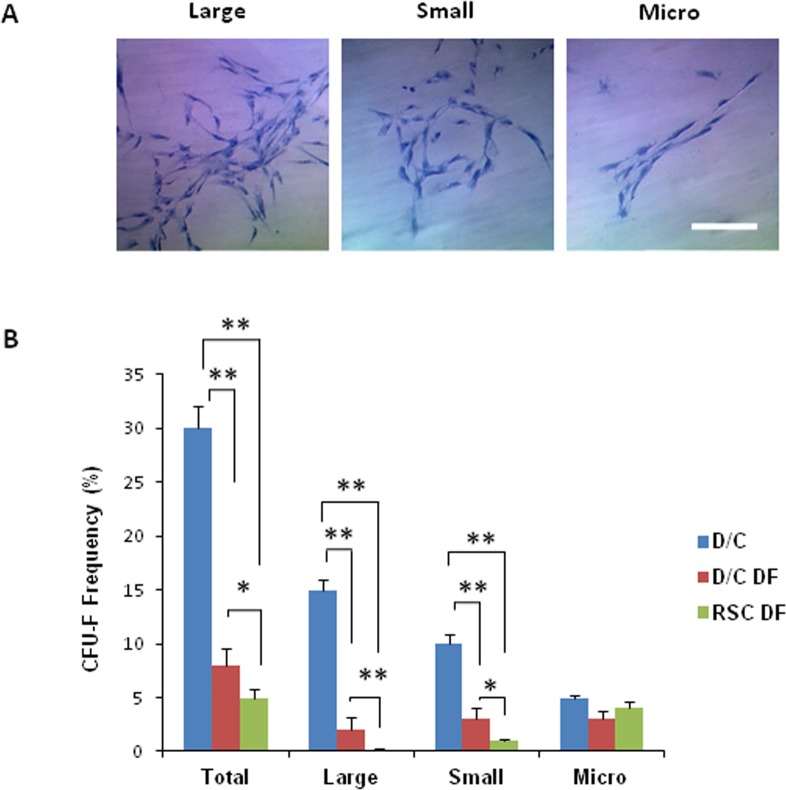
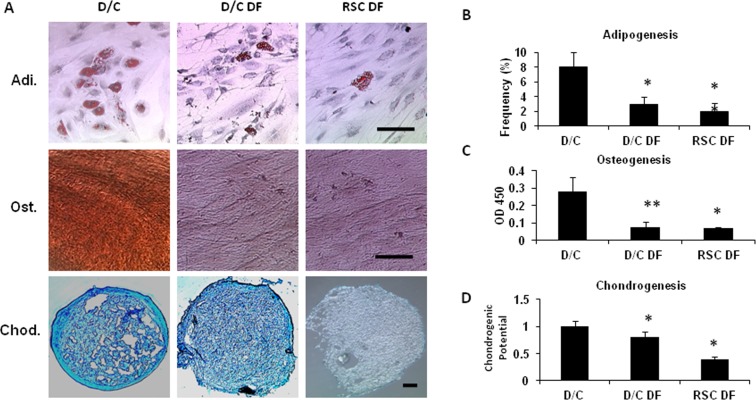
Higher Colony-Forming Units-Fibroblast and Tri-lineage Differentiation by Cells Expanded on Coated Matrigel
To demonstrate further that above cells expressing mesenchymal stem cells markers phenotype were, indeed, mesenchymal stem cells, we compared colony-forming units-fibroblast, an in vitro way of evaluating mesenchymal stem cells function,10 and differentiation into osteogenic, chondrogenic, and adipogenic lineages.29 Our results showed that D/C cells expanded on coated Matrigel in MESCM at passage 4 (D/C) exhibited the highest colony-forming units-fibroblast, judged by either a total or by three different clones when compared to D/C or RSC cells cultured in DMEM with 10% FBS expanded at passage 4 (Fig. 6B, P < 0.05, n = 3). The colony-forming units-fibroblast of D/C cells cultured in DMEM with 10% FBS was significantly higher than that of the limbal residual stromal cells cultured in DMEM with 10% FBS (Fig. 6B, P < 0.05, n = 3). When these three cells were cultured in the medium designated for adipogenesis, osteogenesis, and chondrogenesis, respectively, we noted that although all of them could differentiate into adipocytes, osteocytes, and chondrocytes (Fig. 7A), D/C cells were significantly more potent than D/C and the limbal residual stromal cells cultured in DMEM with 10% FBS (Figs. 7B–D). There was no difference in adipogenesis and osteogenesis between D/C and the limbal residual stromal cells cultured in DMEM with 10% FBS, but D/C cells were more potent than the limbal residual stromal cells in chondrogenesis when cultured in DMEM with 10% FBS.
Figure 6. .
Comparison of colony-forming units-fibroblast among expanded cells. (A) After seeding at the density of 50 cells per cm2 for 12 days on plastic in DMEM with 10% FBS (DF), single cell-derived clones were stained by crystal violet. Three clones, that is large, small, and micro, were identified. (B) Colony-forming units-fibroblast (%) in D/C cells was significantly higher than those of D/C DF cells and RSC DF cells; colony-forming units-fibroblast (%) of D/C DF cells was significantly higher than that of RSC DF cells (*P < 0.05 and **P < 0.01, n = 3). Scale bar: 100 μm.
Figure 7. .
Comparison of tri-lineage differentiation among expanded cells. D/C, D/C DF, and RSC DF cells at P4 were cultured in the standard adipogenesis (Adi), osteogenesis (Ost), or chondrogenesis (Chod) medium. D/C cells had a significantly higher frequency of adipocytes stained by Oil Red O (A, Adi, B), osteocytes stained based on matrix mineralization by Alizarin Red (A, Ost; C), and chondrocytes stained by Alcian Blue (A, Chod) than D/C DF and RSC DF cells (B, C, D, *P < 0.05 and **P < 0.01, n = 3). Scale bar: 50 μm.
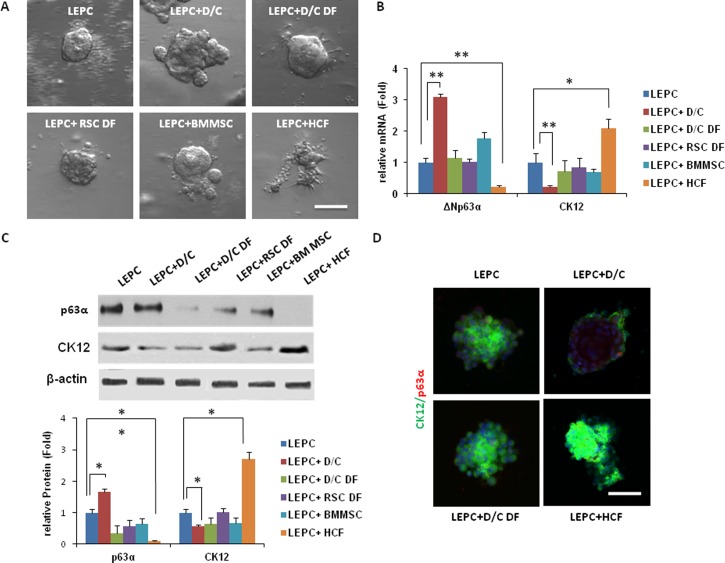
Corneal Differentiation and Stemness of Limbal Epithelial Stem/Progenitor Cells Are Affected by Different Mesenchymal Cells
Previously, we noted that collagenase-isolated cells expanded on coated Matrigel in MESCM prevent corneal epithelial differentiation of dispase-isolated limbal epithelial stem/progenitor cells judged by expression of cytokeratin 12 when both single cells were recombined to form spheres in 3D Matrigel.16,17 To determine whether similarly expanded D/C cells also could serve as niche cells to support limbal epithelial stem/progenitor cells, we performed the same assay and compared to D/C and the limbal residual stromal cells cultured in DMEM with 10% FBS, all expanded up to passage 4. We also compared to bone marrow-derived mesenchymal stem cells and human corneal fibroblasts cells that had been cultured on plastic in DMEM with 10% FBS. All these mesenchymal cells could form reunion quickly with limbal epithelial stem/progenitor cells to yield spheres in 10 days of culturing in 3D Matrigel (Fig. 8A). Compared to spheres formed by limbal epithelial stem/progenitor cells alone, expression level of the ΔNp63α transcript was upregulated significantly in limbal epithelial stem/progenitor cells + D/C spheres and limbal epithelial stem/progenitor cells + bone marrow-derived mesenchymal stem cells spheres (to a lesser extent), but significantly downregulated in limbal epithelial stem/progenitor cells + human corneal fibroblasts spheres (Fig. 8B, *P < 0.05, **P < 0.01, n = 3). In contrast, expression of the cytokeratin12 transcript was downregulated significantly in limbal epithelial stem/progenitor cells + D/C spheres but upregulated significantly in limbal epithelial stem/progenitor cells + human corneal fibroblasts spheres, while not changed significantly in limbal epithelial stem/progenitor cells + D/C when cultured in DMEM with 10% FBS, limbal epithelial stem/progenitor + limbal residual stromal cells when cultured in DMEM with 10% FBS, and limbal epithelial stem/progenitor cells + bone marrow-derived mesenchymal stem cells spheres (Fig. 8B, *P < 0.05, **P < 0.01, n = 3). The above transcript expression pattern was consistent with the protein level of p63α and cytokeratin 12 using β-actin as a loading control in Western blots (Fig. 8C, P < 0.01, n = 3), and consistent with the extent of double immunostaining between cytokeratin 12 and p63α (Fig. 8D). These results indicated that D/C-isolated limbal stromal cells expanded on coated Matrigel also served as niche cells to prevent corneal epithelial differentiation of limbal epithelial stem/progenitor cells more efficiently than their counterparts and limbal residual stromal cells expanded on plastic in DMEM with 10% FBS. As a contrast, human corneal fibroblasts expanded on plastic in DMEM with 10% FBS stimulated full-blown corneal epithelial differentiation with the loss of p63α expression.
Figure 8. .
Comparison of sphere growth by reunion between limbal epithelial stem/progenitor cells and expanded cells. (A) Limbal epithelial stem/progenitor cells (LEPC) derived from dispase-isolated limbal epithelial sheets were mixed with D/C, D/C DF, and RSC DF (all at P4), as well as bone marrow-derived mesenchymal stem cells and human corneal fibroblasts to generate sphere growth on Day 10 in 3D Matrigel containing MESCM. (B) Compared to limbal epithelial stem/progenitor cells alone, expression of the ΔNp63α transcript by limbal epithelial stem/progenitor cells +D/C and limbal epithelial stem/progenitor cells + BMMSC spheres to a lesser extent was upregulated significantly, while that by limbal epithelial stem/progenitor cells + human corneal fibroblasts cells was downregulated significantly (*P < 0.05, **P < 0.01, n = 3). In contrast, expression of the cytokeratin 12 transcript was downregulated significantly in limbal epithelial stem/progenitor cells + D/C but significantly upregulated in limbal epithelial stem/progenitor cells + human corneal fibroblasts cells. The above finding of transcript expression was consistent with the protein level of p63α andcytokeratin12 based on Western blots using β-actin as a loading control (C; *P < 0.05, **P < 0.01, n = 3) and with double immunostaining between cytokeratin 12 and p63α (D).
Discussion
It commonly is believed that one major source of mesenchymal stem cells in a number of tissues is pericytes that are located perivascularly (reviewed previously3,30–33). Using the human limbus as an example, our study demonstrated that another source of mesenchymal stem cells could be located immediately subjacent to limbal basal epithelial cells. Although those cells expressing potential angiogenesis markers, such as Flk-1, CD34, CD31, VWF, α-SMA, and PDGFRβ, were found perivascularly, they also were found elsewhere in the limbal stroma (Fig. 1). As shown in Figure 2, cells expressing these potential angiogenesis markers were found subjacent to limbal basal epithelial cells because they could be isolated by collagenase alone and the D/C method, of which both spare the basement membrane, but not by dispase alone, which specifically cleaves the basement membrane. Relatively speaking, more such cells actually were found in limbal residual stromal cells adherent onto the plastic after D/C digestion. Using the conventional method, that is culturing on plastic in DMEM with 10% FBS, to expand adherent cells,28 we confirmed that mesenchymal stem cells, which are characterized by the expression of positive (CD73, CD90, and CD105) and negative (CD34, CD31, and CD45) markers,1,29 could indeed be obtained from D/C-isolated cells and limbal residual stromal cells (Fig. 5). According to the extent of CFU-F (Fig. 6) and tri-lineage differentiation (Fig. 7), we concluded that mesenchymal stem cells generated from D/C-isolated cells are more potent than mesenchymal stem cells generated from limbal residual stromal cells. We suggested that mesenchymal cells located immediately subjacent to limbal basal epithelial cells could be a better source of mesenchymal stem cells.
Interestingly, D/C cells but not limbal residual stromal cells formed spheres when seeded immediately in 3D Matrigel after digestion in MESCM (Fig. 3). Cells of these D10 D/C spheres significantly upregulated expression of Flk-1, CD34, CD31, VWF, α-SMA, and PDGFRβ transcripts and proteins, suggesting that they turned into angiogenesis progenitors similar to what we have reported for collagenase-isolated Vim+ cells.17 Because single cells from D10 D/C spheres, but not single limbal residual stromal cells, could adhere and stabilize the vascular network formed by human umbilical vein endothelial cells when seeded on the surface of 3D Matrigel (Fig. 3), we concluded that D/C-isolated cells, but not limbal residual stromal cells, can turn into angiogenesis progenitors with the pericyte phenotype. Although pericytes are believed to be the common origin of mesenchymal stem cells in almost all adult tissues (reviewed previously3,30–33), the lack of specific markers for pericytes and mesenchymal stem cells makes it difficult to define the hierarchy relationship between mesenchymal stem cells and pericytes. Because few cells in D/C spheres at day 10 were labeled by EdU, resembling the situation we reported in spheres formed by collagenase-isolated cells in 3D Matrigel,18 they could not be used to resolve this question. Recently, we have circumvented this difficulty by expanding collagenase-isolated cells on coated Matrigel in MESCM.16,17 Herein, we found that D/C-isolated cells could be expanded similarly into Vim+ spindle cells by serial passages on coated Matrigel in MESM (Fig. 4). The resultant spindle cells from collagenase-isolated cells have been characterized as angiogenesis progenitors because they can differentiate further into vascular endothelial cells and pericytes.17 Our present study further showed that spindle cells expanded from collagenase-isolated cells and D/C-isolated cells also expressed three key mesenchymal stem cells markers (Fig. 5) with high colony-forming units-fibroblast (Fig. 6) and tri-lineage differentiation (Fig. 7). Thus, we concluded that angiogenesis progenitors with the pericyte phenotype predated mesenchymal stem cells, suggesting that this subset of vimentin+ mesenchymal cells subjacent to limbal basal epithelial cells were an origin of mesenchymal stem cells.
Using the same criteria of colony-forming units-fibroblast and tri-lineage differentiation, mesenchymal stem cells with an angiogenesis potential derived from D/C-isolated cells were more potent than mesenchymal stem cells derived from the counterpart expanded by the conventional method of culturing mesenchymal stem cells, that is, on plastic in DMEM with 10% FBS (Figs. 6, 7). We attributed such a dramatic difference to the use of coated Matrigel as the substrate and MESCM as the medium. Previously, we reported that collagenase-isolated cells irreversibly lost expression of embryonic stem cell markers if they were expanded in DMEM with 10% FBS even if they were seeded on coated Matrigel.16 We noted further that they lost expression of angiogenesis markers and gained myofibroblast markers, such as S100A4, if they were seeded on plastic even if they were in MESCM.17 Herein, we demonstrated that D/C-isolated cells cultured on plastic in DMEM with 10% FBS also lost the expression of angiogenesis markers and turned into myofibroblasts expressing α-SMA and S-100A4, but not PDGFRβ and SMMHC (Fig. 5). This finding is in agreement with prior studies showing that basement membrane components improve proliferation and differentiation capacity of human bone marrow-derived mesenchymal stem cells,34,35 and that Matrigel helps retain the undifferentiated state of human embryonic stem cells.36 Because cells possessing the phenotype of angiogenesis progenitor cells and mesenchymal stem cells perform better in cardiovascular repair after injury,36,37 the expansion method described herein based on coated Matrigel in MESCM could be adopted for such cell-based therapies where vascularization is desired.
Besides possessing the aforementioned capability of generating mesenchymal stem cells with an angiogenesis potential, this subset of vimentin+ mesenchymal cells also served as niche cells to support subjacent limbal epithelial stem/progenitor cells. Judged by the expression level of cytokeratin 12,38,39 corneal epithelial differentiation of collagenase-isolated limbal epithelial stem/progenitor cells is promoted when reunion with niche cells is prevented by AMD3100 that disrupts the chemokine axis of SDF-1/CXCR4.18 Among a number of mesenchymal cells tested, the expression level of cytokeratin 12 also was significantly promoted by reunion with human corneal fibroblasts (Fig. 8C). Thus, corneal epithelial differentiation limbal epithelial stem/progenitor cells is promoted by dissociation from their niche cells as well as by association with human corneal fibroblasts, a process also revealed in vivo. On the contrary, the cytokeratin 12 expression level was significantly downregulated by collagenase-isolated cells16 and D/C cells (Fig. 8C) expanded on coated Matrigel, and it was abolished completely by collagenase-isolated cells that were expanded on coated Matrigel followed by reseeding in 3D Matrigel.17 These results suggested that corneal epithelial differentiation of limbal epithelial stem/progenitor cells is downregulated by reunion with niche cells expressing angiogenesis/pericyte markers (our study), but prevented completely by reunion with niche cells expressing additional embryonic stem cell markers.17 Judged by the expression level of ΔNp63α, a marker for limbal basal epithelial progenitors including stem cells,40,41 stemness of limbal epithelial stem/progenitor cells was demoted significantly by reunion with collagenase-isolated cells expanded on coated Matrigel in DMEM with 10% FBS,16 and with D/C-isolated cells, limbal residual stromal cells, and bone marrow-derived mesenchymal stem cells, but abolished completely by reunion with human corneal fibroblasts, of which all cells were expanded on plastic in DMEM with 10% FBS (Fig. 8C). These findings suggested that stemness of limbal epithelial stem/progenitor cells also can be influenced by neighboring mesenchymal cells that adopt different phenotypes, a notion that also has been suggested by our prior tissue recombinant experiments42 and by studies based on co-culturing with different feeder cell layers. Because mesenchymal stem cells, especially those with angiogenesis potential, can differentiate into a number of stromal components and serve as niche cells to support other types of adult stem cells,43–46 we also believe that limbal niche cells described herein might also partake in stromal wound healing and tissue regeneration. Further studies on how the niche cells phenotype expressing embryonic stem cell-angiogenesis/pericyte-mesenchymal stem cells might control the aforementioned stemness and corneal fate decision are warranted so as to shed new light on how angiogenesis and fibrosis might arise in the limbal niche in several corneal diseases characterized by limbal stem cell deficiency.
Supplementary Material
Acknowledgments
Angela Y. Tseng prepared this manuscript.
Footnotes
Supported by RO1 EY06819 Grant from The National Eye Institute, National Institutes of Health, Bethesda, Maryland (SCGT), partially by the Nature Science Foundation of Hubei Province (No. 2010CDB09802), and Wuhan Chen-Guang Plan Grant (No. 201150431124), Wuhan, People's Republic of China (GGL).
Disclosure: G.-G. Li, None; Y.-T. Zhu, None; H.-T. Xie, None; S.-Y. Chen, None; S.C.G. Tseng, None
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 2.da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230 [DOI] [PubMed] [Google Scholar]
- 4.Motaln H, Schichor C, Lah TT. Human mesenchymal stem cells and their use in cell-based therapies. Cancer. 2010;116:2519–2530 [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther. 2011;11:893–909 [DOI] [PubMed] [Google Scholar]
- 6.Reinshagen H, uw-Haedrich C, Sorg RV, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2009;89:741–748 [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321 [DOI] [PubMed] [Google Scholar]
- 8.Zajicova A, Pokorna K, Lencova A, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19:1281–1290 [DOI] [PubMed] [Google Scholar]
- 9.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313 [DOI] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403 [DOI] [PubMed] [Google Scholar]
- 11.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelekanos RA, Li J, Gongora M, et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac MSC-like populations supports strong congruence with bone marrow MSC despite maintenance of distinct identities. Stem Cell Res. 2012;8:58–73 [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding L, Saunders TL, Enikolopov G, Morrison SM. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie HT, Chen SY, Li GG, Tseng SC. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li GG, Chen SY, Xie HT, Zhu YT, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012;53:3357–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie HT, Chen SY, Li GG, Tseng SC, et al. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29:1874–1885 [DOI] [PubMed] [Google Scholar]
- 19.Espana EM, Romano AC, Kawakita T, Di Pasquale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281 [DOI] [PubMed] [Google Scholar]
- 20.Espana EM, Kawakita T, Liu CY, Tseng SC. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci. 2004;45:2985–2991 [DOI] [PubMed] [Google Scholar]
- 21.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85 [DOI] [PubMed] [Google Scholar]
- 23.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatlapatka T, Moretti P, Lavrentieva A, et al. Optimization of culture conditions for the expansion of umbilical cord-derived mesenchymal stem or stromal cell-like cells using xeno-free culture conditions. Tissue Eng Part C Methods. 2011;17:485–493 [DOI] [PubMed] [Google Scholar]
- 25.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011;17:537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashida Y, Li W, Chen YT, et al. Heterogeneity of limbal basal epithelial progenitor cells. Cornea. 2010;29:11. [DOI] [PubMed] [Google Scholar]
- 28.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317 [DOI] [PubMed] [Google Scholar]
- 30.Crisan M, Corselli M, Chen CW, Péault B. Multilineage stem cells in the adult: a perivascular legacy? Organogenesis. 2011;7:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernardo ME, Cometa AM, Pagliara D, et al. Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol. 2011;24:73–81 [DOI] [PubMed] [Google Scholar]
- 32.Corselli M, Chen CW, Crisan M, Lazzari L, Péault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109 [DOI] [PubMed] [Google Scholar]
- 33.Chen CW, Montelatici E, Crisan M, et al. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20:429–434 [DOI] [PubMed] [Google Scholar]
- 34.Lindner U, Kramer J, Behrends J, et al. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005 [DOI] [PubMed] [Google Scholar]
- 35.Matsubara T, Tsutsumi S, Pan H, et al. A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun. 2004;313:503–508 [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara H, Hayashi Y, Sanzen N, et al. Regulation of mesodermal differentiation of mouse embryonic stem cells by basement membranes. J Biol Chem. 2007;282:29701–29711 [DOI] [PubMed] [Google Scholar]
- 37.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210 [DOI] [PubMed] [Google Scholar]
- 38.Chen WY, Mui MM, Kao WW, Liu CY, Tseng SC. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Curr Eye Res. 1994;13:765–778 [DOI] [PubMed] [Google Scholar]
- 39.Liu C-Y, Zhu G, Converse R, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–24636 [PubMed] [Google Scholar]
- 40.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeon F. p63 and the epithelial stem cell: more than status quo? Genes Dev. 2004;18:465–469 [DOI] [PubMed] [Google Scholar]
- 42.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135 [DOI] [PubMed] [Google Scholar]
- 43.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084 [DOI] [PubMed] [Google Scholar]
- 44.Hardy SA, Maltman DJ, Przyborski SA. Mesenchymal stem cells as mediators of neural differentiation. Curr Stem Cell Res Ther. 2008;3:43–52 [DOI] [PubMed] [Google Scholar]
- 45.Yoo SW, Kim SS, Lee SY, et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008;40:387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter MN, Wright KT, Fuller HR, MacNeil S, Johnson WE. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316:1271–1281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.