Abstract
Objective
Understanding triggers is important for managing asthma particularly for patients who seek emergency department (ED) care for exacerbations. The objectives of this analysis were to delineate self-reported triggers in ED patients and to assess associations between triggers and asthma knowledge, severity, and quality of life.
Methods
At the time of an ED visit, 296 patients were asked what were their usual asthma triggers based on a checklist of 25 potential items, and what they thought specifically precipitated their current ED visit. Using standardized scales, patients also were asked about asthma knowledge, severity and quality of life.
Results
Mean age was 44 years and 72% were women. Patients cited a mean of 12 triggers; most patients had diverse triggers spanning respiratory infections, environmental irritants, emotions, allergens, weather, and exercise. Patients with more triggers were more likely to be women (OR 2.0, CI 1.3, 3.2, p=.002), obese (OR 1.7, CI 1.1, 2.5, p=.01), and to not have a smoking history (OR 1.9, CI 1.3, 2.9, p=.001). There were no associations between number of triggers and current age, age at diagnosis, education, socioeconomic status or race/ethnicity. Patients who cited more triggers had more frequent flares (OR 1.1, CI 1.1, 1.2, p<.0001), worse quality of life scores (OR 1.6, CI 1.1, 2.4, p=.02), and were more likely to have been previously hospitalized for asthma (OR 1.9, CI 1.3, 2.9, p=.003) and to have previously required oral corticosteroids (OR 2.9, CI 1.6, 5.1, p=.003). There was little clustering of specific triggers according to the variables we considered except for more frequent animal allergy in patients diagnosed at a younger age (OR 2.8, CI 1.7, 4.5, p<.0001) and worse quality of life in patients citing emotional stress as a trigger (OR 2.5, CI 1.5, 4.0, p=.0002). Patients attributed their current ED visit to multiple precipitants, particularly respiratory infections and weather, and these were concordant with what they reported were known triggers.
Conclusions
Patients presenting to the ED for asthma reported multiple triggers spanning diverse classes of precipitants and having more triggers was associated with worse clinical status. ED patients should be instructed that although it may not be possible to eliminate all triggers, mitigating even some triggers can be helpful. (ClinicalTrials.gov NCT00110409)
Keywords: flares, exacerbations, precipitants, irritants, emotional stress
Introduction
Asthma is a chronic inflammatory condition with symptoms worsened by diverse triggers.1,2 Triggers include environmental irritants such as air pollution, strong smells and smoke; allergens from pollen, animals, dust mites and molds; and weather characteristics such hot and cold temperatures, changes in weather, humidity and wind.1,2 In addition, asthma symptoms can be precipitated by other distinct triggers, such as respiratory infections, emotional stress, exercise, medications, menstruation, and gastroesophageal reflux.1,2
A cornerstone of asthma treatment is instructing patients in identifying and self-managing triggers. Many patients will note that several different triggers will precipitate their asthma whereas others will state that only one major trigger poses problems.1 However, patients may not realize that triggers can change with time and something that was not bothersome before can gradually and inconspicuously become a trigger. Therefore, learning to identify triggers should be an ongoing process. This is important for all asthma patients, but particularly for those requiring emergency care.
Some triggers are easy to identify because they cause symptoms acutely and dominate the patient’s attention, such as abrupt exposure to concentrated and potent smoke. Most triggers, however, are not acute but are still noticeable and cause distressing respiratory symptoms that limit function and require timely intervention. In addition, some triggers are ubiquitous and may be difficult to avoid and control, such as chronic emotional stress, and cause persistent low grade symptoms that may decrease asthma-related quality of life.2,3
The goals of this analysis were to delineate self-reported history of triggers in patients presenting to the emergency department (ED) for asthma. Additional objectives were to assess the prevalence of triggers according to demographic and clinical characteristics and to assess associations between triggers and clinical status, such as asthma knowledge, severity, and quality of life.
Methods
Enrollment
This is a secondary analysis of enrollment data from a previous randomized clinical trial of self-management education for patients presenting to the ED for asthma.4 The education module was developed to increase asthma knowledge and self-efficacy. The design and results of the trial have been reported elsewhere.4 Briefly, patients were recruited from two New York City EDs - New York Methodist Hospital in Brooklyn and New York Presbyterian Hospital in Manhattan - if they presented to the ED between 8 AM and 5 PM Monday through Friday or if they had been admitted to the hospital from the ED within the previous 24 hours. Patients were enrolled by research assistants who were college graduates and had an interest in health education. Patients were eligible if they were 18 years of age or older, fluent in English, had a known diagnosis of asthma, came to the ED for respiratory symptoms, and had a telephone which was required for the education module. Patients were approached when the treating physician considered them stable enough to be interviewed. The trial was approved by the Institutional Review Boards at New York Methodist Hospital and Weill Cornell Medical College/New York Presbyterian Hospital and all patients provided written informed consent for themselves (surrogate consent was not used).
Identification of triggers
At enrollment patients were asked what triggered their asthma. They were given a list of 25 potential triggers compiled from literature review and reports from patients in prior studies.5–7 Triggers included environmental irritants, allergens, weather, emotional stress, and exercise. Patients were asked to cite which items on the list generally precipitated their asthma and then to volunteer any additional items. Patients also were asked what they thought precipitated the current exacerbation that brought them to the ED.
Identification of variables potentially associated with triggers
Variables potentially associated with triggers were obtained from patients. These included demographic characteristics, such as age, gender, race, ethnicity, and socioeconomic status, and clinical characteristics such as age at diagnosis, obesity, and smoking history. Possible associations between triggers and clinical status were assessed using the following valid and standardized questionnaires. Asthma knowledge was measured with the Asthma Self-Management Questionnaire, a 16-item multiple choice survey that includes questions about triggers; scores range from 0 to 100, higher is more knowledge.8 Long-term asthma status was measured with the Severity of Asthma Scale, a 13-item weighted scale that includes history of medications and resource utilization; scores range from 0 to 28, higher is more severe asthma.9 The effect of asthma on daily life was measured with the Asthma Quality of Life Questionnaire, a 32-item scale with 4 domains measuring activity limitations, symptoms, and the emotional and environmental impact of asthma; scores range from 1 to 7, higher is better status.10 Patients also were asked if their asthma symptoms were worse during specific seasons, and how often they have an asthma flare with six response options ranging from every day to once every few months.
Data analysis
Data analysis was primarily descriptive. Frequencies of triggers were compared to demographic and clinical characteristics using chi-square and Fisher’s exact tests, and odds ratios (OR) and 95% confidence intervals (CI) were calculated. Continuous variables were dichotomized according to group mean scores with scores above the group mean representing more asthma knowledge, scores above the group mean representing more severe asthma, and scores below the group mean representing worse asthma-related quality of life. Analyses were carried out in SAS.11
Results
Demographic and clinical characteristics
In total 296 patients were enrolled from April 2005 to January 2009. The mean age of the sample was 44 years, 72% were women, and most were not college graduates (Table 1). Patients had diverse ethnic and racial backgrounds and most had health care coverage. Eighteen percent lived alone, 35% lived with 1 other person, 20% lived with 2 other people, and 27% lived with 3 or more people. As a measure of socioeconomic status, total annual household income was divided by number of household members, and ranged from less than $10,000 for 17% of patients to greater than $40,000 for 15%.
Table 1.
Demographic characteristics (N=296)
| Characteristic
|
Value
|
|---|---|
| Age, years (mean±SD) | 44±13 |
| Women | 72% |
| College graduate | 26% |
| Latino ethnicity | 44% |
| Race | |
| White | 63% |
| African American | 32% |
| Other | 5% |
| Insurance status | |
| Private | 58% |
| Medicare | 7% |
| Medicaid | 26% |
| Uninsured | 9% |
| Annual income/household member a | |
| < $10,000 | 17% |
| ≥ $10,000, < $20,000 | 35% |
| ≥ $20,000, < $30,000 | 25% |
| ≥ $30,000, < $40,000 | 8% |
| ≥ $40,000 | 15% |
calculated from total annual household income and number of household members, n=271
More than half of patients were obese and almost half had a history of smoking, with 25% currently smoking (Table 2). Seventeen percent had major comorbidity (mostly diabetes mellitus) and 6% (19 patients) reported gastroesophageal reflux. The median age at asthma diagnosis was 14 years and 25% reported a diagnosis before age 5. Most patients (81%) reported having a physician for asthma, mostly a generalist (55%) or a pulmonologist or allergist (24%). Over 90% had been previously treated in the ED and 67% had been hospitalized for asthma. Over 30% were not taking any asthma maintenance medications although 86% had taken oral corticosteroids in the past.
Table 2.
Clinical characteristics (N=296)
| Characteristic
|
Value
|
|---|---|
| Obese a | 52% |
| Current smoker | 25% |
| Ever smoked | 46% |
| Age at diagnosis, years (mean±SD) | 20±18 |
| Duration of asthma, years (mean±SD) | 24±16 |
| Ever hospitalized for asthma | 67% |
| Asthma medications | |
| No medications | 6% |
| Only inhaled beta agonists | 25% |
| Any maintenance medication b | 69% |
| Ever took oral corticosteroids for asthma | 86% |
| Frequency of flares | |
| Once every few months/once a month | 70% |
| A few times a month/every week | 18% |
| Several times a week/every day | 12% |
| Seasonal exacerbation of symptoms | |
| Spring | 9% |
| Summer | 17% |
| Autumn | 6% |
| Winter | 43% |
| Change in season | 30% |
| No, the same throughout the year | 30% |
| Asthma knowledge, score (mean±SD) c | 58±20 |
| Asthma severity, score (mean±SD) d | 12±4 |
| Asthma quality of life, score (mean±SD) e | 3.5±1.0 |
body mass index ≥30 kilograms/meter2
includes long-acting beta agonists, inhaled corticosteroids, leukotriene modifiers, mast cell stabilizers, theophylline, oral corticosteroids
Asthma Self-Management Questionnaire, range 0–100, higher is more knowledge, n=291
Severity of Asthma scale, range 0–28, higher is more severe
Asthma Quality of Life Questionnaire, range 1–7, higher is better status
Most patients (56%) reported an asthma flare once every few months, with an equal distribution between men and women, and 7% reported a flare every day (Table 2). Seventy percent reported certain seasons were worse for asthma, particularly winter, and 13% reported more than one season. Only 30% presented to the ED and were enrolled in this study during a corresponding season. Thirty percent reported their asthma was worse during change of season, but enrollment in this study did not correspond to seasonal changes within a 2 week window. It should be noted, however, that 36% of patients had been in the ED for asthma during the 3 months prior to enrollment in this study.
Scores on the Asthma Self-Management Questionnaire were low (29% scored less than 50) and patients had moderately severe disease based on the Severity of Asthma Scale (Table 2). Asthma Quality of Life Questionnaire scores reflected marked impairment, as would be expected in an ED population.
Types of triggers
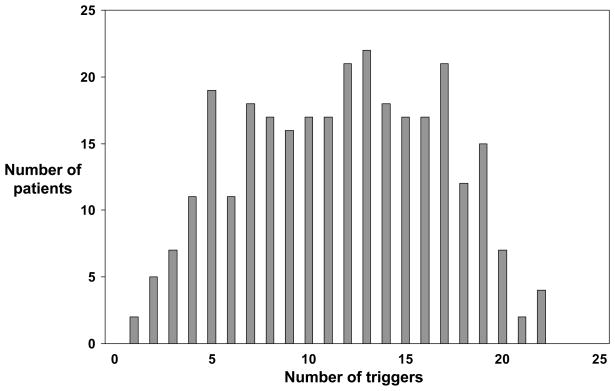
Patients cited a variety of triggers (Table 3). Of the 25 specific triggers offered, the most frequently endorsed items were dust (79%), upper respiratory tract infection (76%) and cold weather (74%). The items endorsed least often were aspirin (7%) and menstruation (4% of women under age 51, the mean age of menopause in the US12). Sixty-three patients (21%) volunteered additional triggers. Most of these were detailed items related to the other 25 triggers, such as strong smells (e.g. bleach, ammonia, incense, paint) and emotional distress (e.g. anxiety, being in small spaces). Other distinct items included cockroaches, mold and mildew, and certain foods (e.g. milk, tea, orange juice). The total number of triggers per patient ranged from 1 to 22 (Figure 1). The mean and median values were 12 and the distribution was relatively flat between 5 and 19 triggers. Two patients cited only 1 trigger, one patient cited tobacco smoke and the other cited change in weather. Among the 7 women who cited menstruation, the median number of triggers was 17 (range 5 to 21).
Table 3.
Frequency of triggers
| Triggers
|
Percent
|
|---|---|
| Dust | 79% |
| Upper respiratory tract infection | 76% |
| Cold weather | 74% |
| Tobacco smoke | 69% |
| Strong smells | 67% |
| Change in weather | 66% |
| Humidity | 61% |
| Bronchitis | 61% |
| Stress | 60% |
| Pollen | 55% |
| Allergies | 54% |
| Exercise | 54% |
| Pollution | 51% |
| Certain animals | 48% |
| Hot weather | 46% |
| Wind | 45% |
| Grass | 37% |
| Trees | 35% |
| Laughing | 35% |
| Flowers | 32% |
| Certain foods | 24% |
| Crying | 20% |
| Alcoholic beaverages | 7% |
| Aspirin | 7% |
| Menstruation a | 4% |
for women < 51 years old
Figure 1.
Number of triggers and number of patients
Cause of exacerbation and triggers
Patients were asked what they thought caused the exacerbation that brought them to the ED. Most patients (87%) were able to propose a cause and 40% listed several possible causes. Their suppositions were compared to items they had cited as general triggers. Some frequently proposed causes were an upper respiratory tract infection by 76 patients (72 had cited this as a general trigger); cold weather by 38 patients (35 had cited this as a general trigger); hot weather by 20 patients (17 had cited this as a general trigger); and change in weather by 33 patients (30 had cited this as a general trigger). Overall, 61% of patients proposed causes that coincided with triggers they had cited.
Triggers and demographic/clinic characteristics
Several triggers were compared to demographic and clinical characteristics (Table 4). These included distinct triggers as well as groups of triggers that were highly correlated in our sample and have been reported to be related.1 Specifically, grass, flowers and trees were grouped as plants; laughing and crying were grouped as intense emotional expression; and pollution, tobacco, and strong smells were grouped as environmental irritants. Pollen was maintained as a distinct item to acknowledge that it is often seasonal. There were notable differences in the prevalence of triggers according to sex with women citing more triggers (OR 2.0, CI 1.3, 3.2, p=.002) and greater frequency of emotion-related triggers, such as stress (OR 2.7, CI 1.6, 4.4, p=.0002) and crying and laughing (OR 2.2, CI 1.3, 3.9, p=.004). Patients diagnosed with asthma at an earlier age were more likely to cite animals as a trigger (OR 2.8, CI 1.7, 4.5, p<.0001) but not to cite other triggers. Obese patients cited more triggers (median 13, range 1 to 22) than patients who were not obese (median 11, range 1 to 20) (OR 1.7, CI 1.1, 2.5, p=.01), and patients who never smoked cited more triggers (median 13, range 2 to 22) than patients who were current or past smokers (median 10, range 1 to 22) (OR 1.9, CI 1.3, 2.9, p=.001) We considered both current and past smokers together because many patients who reported they quit often continued to be around others who smoked. There were no specific types of triggers based on obesity or smoking. Regarding other characteristics, there were no differences in the frequency of triggers based on age, education, or income/household members. However, African American patients were more likely to cite hot weather as a trigger compared to non-African American patients (58% versus 40%, OR 2.0, CI 1.2, 3.4, p=.005).
Table 4.
Triggers and demographic and clinical characteristics
| Triggers | Gender
|
Age at diagnosis
|
Obese
|
Ever smoked
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women n=212 |
Men n=84 |
OR a 95% CI |
≤ 14 n=148 |
> 14 n=148 |
OR a 95% CI |
Yes n=153 |
No n=143 |
OR a 95% CI |
Yes n=137 |
No n=159 |
OR a 95% CI |
|
| Number (median) b | 13 | 10 | 2.0 (1.3, 3.2)f | 12 | 12 | 1.0 (0.7, 1.5) | 13 | 11 | 1.7 (1.1, 2.5)g | 10 | 13 | 1.9 (1.3, 2.9)f |
|
| ||||||||||||
| Dust | 79% | 79% | 1.0 (0.6, 1.9) | 80% | 78% | 1.1 (0.6, 1.9) | 80% | 78% | 1.1 (0.6, 1.9) | 74% | 84% | 1.8 (1.0, 3.2)h |
|
| ||||||||||||
| Upper respiratory infection | 81% | 64% | 2.4 (1.4, 4.2)f | 80% | 72% | 1.6 (0.9, 2.7) | 82% | 71% | 1.9 (1.1, 3.2)h | 80% | 74% | 0.7 (0.4, 1.2) |
|
| ||||||||||||
| Cold weather | 77% | 66% | 1.8 (1.0, 3.1)h | 75% | 72% | 1.2 (0.7, 1.9) | 76% | 71% | 1.3 (0.8, 2.1) | 66% | 81% | 2.2 (1.3, 3.7)f |
|
| ||||||||||||
| Environmental irritants c | 90% | 77% | 2.7 (1.4, 5.3)f | 86% | 87% | 0.9 (0.5, 1.7) | 91% | 82% | 2.2 (1.1, 4.4)h | 82% | 91% | 2.1 (1.1, 4.3)h |
|
| ||||||||||||
| Stress | 67% | 43% | 2.7 (1.6, 4.4)f | 53% | 66% | 0.6 (0.4, 0.9)h | 65% | 54% | 1.6 (1.0, 2.6)h | 56% | 64% | 1.4 (0.9, 2.2) |
|
| ||||||||||||
| Pollen | 57% | 51% | 1.3 (0.8, 2.1) | 53% | 58% | 0.8 (0.5, 1.3) | 63% | 48% | 1.9 (1.2, 3.0)g | 51% | 59% | 1.4 (0.9, 2.2) |
|
| ||||||||||||
| Exercise | 56% | 50% | 1.3 (0.8, 2.1) | 49% | 59% | 0.7 (0.4, 1.1) | 60% | 48% | 1.7 (1.1, 2.6)h | 53% | 55% | 1.1 (0.7, 1.8) |
|
| ||||||||||||
| Animals | 50% | 44% | 1.3 (0.8, 2.1) | 61% | 36% | 2.8 (1.7, 4.5)f | 50% | 46% | 1.2 (0.8, 1.9) | 44% | 52% | 1.4 (0.9, 2.2) |
|
| ||||||||||||
| Hot weather | 49% | 39% | 1.5 (0.9, 2.4) | 45% | 47% | 0.9 (0.6, 1.4) | 48% | 44% | 1.2 (0.7, 1.8) | 41% | 50% | 1.5 (0.9, 2.3) |
|
| ||||||||||||
| Plants d | 46% | 39% | 1.3 (0.8, 2.2) | 41% | 47% | 0.8 (0.5, 1.2) | 48% | 39% | 1.5 (0.9, 2.3) | 35% | 52% | 2.0 (1.2, 3.2)f |
|
| ||||||||||||
| Expression of strong emotion e | 46% | 27% | 2.2 (1.3, 3.9)f | 41% | 41% | 1.0 (0.6, 1.6) | 44% | 37% | 1.3 (0.8, 2.1) | 37% | 43% | 1.3 (0.8, 2.1) |
|
| ||||||||||||
| Aspirin | 8% | 4% | 2.4 (0.7, 8.3) | 5% | 9% | 0.5 (0.2, 1.3) | 8% | 6% | 1.4 (0.6, 3.6) | 6% | 8% | 1.3 (0.5, 3.3) |
referent variables are women, age ≤ 14 years, obese, never smoked
based on possible total of 24 triggers, omitting menstruation
includes pollution, smoke, strong smells
includes grass, trees, flowers
includes laughing, crying
p ≤ .005
p ≤ .01
p ≤ .05
Triggers and asthma status
We also considered how triggers may be associated with clinical asthma status. Patients with more triggers had better scores on the self-management knowledge questionnaire (OR 2.0, CI 1.3, 3.0, p=.0007). Six of the 16 questions in the questionnaire address triggers and greater familiarity with triggers and their effects probably contributed to more knowledge and better scores. Patients who cited more triggers had more frequent flares (OR 1.1, CI 1.1, 1.2, p<.0001), were more likely to have been previously hospitalized for asthma (OR 1.9, CI 1.3, 2.9, p=.003), and to have previously required oral corticosteroids (OR 2.9, CI 1.6, 5.1, p=.003). Patients who cited more triggers also reported worse overall asthma-related quality of life scores (OR 1.6, CI 1.1, 2.4, p=.02). Emotional stress was particularly important and was associated with worse overall quality of life score (OR 2.5, CI 1.5, 4.0, p=.0002) (Table 5) as well as worse scores for the activities domain (OR 1.8, CI 1.1, 2.8, p=.02), symptoms domain (OR 2.4, 1.5, 3.8, p=.0004), and environmental domain (OR 2.4, CI 1.5, 3.8, p=.0005). There also were associations between worse environmental domain scores and related triggers, notably environmental irritants (OR 3.6, CI 1.6, 7.8, p=.002) and dust (OR 1.8, CI 1.0, 3.3, p=.04). Finally, patients who required oral corticosteroids more often reported animals (OR 3.1, CI 1.5, 6.3, p=.003), environmental irritants (OR 3.2, CI 1.5, 7.0, p=.003), and dust (OR 3.2, CI 1.6, 6.3, p=.001) as triggers.
Table 5.
Frequency of triggers and clinical asthma status
| Triggers | More asthma knowledge a
|
Frequent flares b
|
Worse asthma severity c
|
Worse asthma quality of life d
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| % | OR 95% CI | % | OR 95% CI | % | OR 95% CI | % | OR 95% CI | ||
| Dust | Has trigger | 56% | 1.6 (0.9, 2.9) | 24% | 1.8 (0.8, 3.9) | 59% | 1.6 (0.9, 2.8) | 49% | 1.0 (0.6, 1.8) |
| Doesn’t have trigger | 44% | 15% | 47% | 48% | |||||
|
| |||||||||
| Upper respiratory infection | Has trigger | 58% | 2.3 (1.3, 4.1)h | 23% | 1.4 (0.7, 2.9) | 54% | 0.8 (0.4, 1.3) | 45% | 0.6 (0.3, 1.0)j |
| Doesn’t have trigger | 37% | 17% | 61% | 60% | |||||
|
| |||||||||
| Cold weather | Has trigger | 53% | 1.0 (0.6, 1.7) | 26% | 3.0 (1.4, 6.7)i | 60% | 1.9 (1.1, 3.1)j | 51% | 0.9 (0.5, 1.5) |
| Doesn’t have trigger | 53% | 10% | 45% | 48% | |||||
|
| |||||||||
| Environmental irritants e | Has trigger | 56% | 2.1 (1.1, 4.1)j | 24% | 6.1 (1.4, 26)j | 58% | 1.7 (0.9, 3.3) | 50% | 1.7 (0.9, 3.4) |
| Doesn’t have trigger | 38% | 5% | 45% | 38% | |||||
|
| |||||||||
| Stress | Has trigger | 59% | 1.8 (1.1, 2.8)j | 25% | 1.6 (0.9, 3.0) | 61% | 1.7 (1.0, 2.6)j | 58% | 2.5 (1.5, 4.0)h |
| Doesn’t have trigger | 45% | 17% | 49% | 35% | |||||
|
| |||||||||
| Pollen | Has trigger | 57% | 1.4 (0.9, 2.2) | 24% | 1.3 (0.8, 2.4) | 57% | 1.1 (0.7, 1.8) | 51% | 1.2 (0.8, 1.9) |
| Doesn’t have trigger | 49% | 19% | 55% | 46% | |||||
|
| |||||||||
| Exercise | Has trigger | 59% | 1.7 (1.0, 2.6)j | 24% | 1.3 (0.8, 2.3) | 58% | 1.2 (0.8, 1.9) | 52% | 1.3 (0.8, 2.1) |
| Doesn’t have trigger | 46% | 19% | 54% | 45% | |||||
|
| |||||||||
| Animals | Has trigger | 61% | 1.8 (1.2, 2.9)i | 23% | 1.2 (0.7, 2.1) | 59% | 1.2 (0.8, 2.0) | 51% | 1.2 (0.8, 1.9) |
| Doesn’t have trigger | 46% | 20% | 54% | 46% | |||||
|
| |||||||||
| Hot weather | Has trigger | 57% | 1.3 (0.8, 2.1) | 28% | 2.0 (1.1, 3.5)j | 66% | 2.2 (1.4, 3.5)h | 52% | 1.3 (0.8, 2.1) |
| Doesn’t have trigger | 50% | 16% | 48% | 46% | |||||
|
| |||||||||
| Plants f | Has trigger | 52% | 1.0 (0.6, 1.5) | 27% | 1.7 (1.0, 3.0)j | 62% | 1.5 (0.9, 2.4) | 57% | 1.8 (1.1, 2.9)i |
| Doesn’t have trigger | 54% | 18% | 52% | 42% | |||||
|
| |||||||||
| Expression of strong emotion g | Has trigger | 57% | 1.3 (0.8, 2.0) | 28% | 1.8 (1.0, 3.1)j | 63% | 1.7 (1.0, 2.7)j | 54% | 1.5 (0.9, 2.3) |
| Doesn’t have trigger | 51% | 18% | 51% | 45% | |||||
|
| |||||||||
| Aspirin | Has trigger | 60% | 1.4 (0.5, 3.4) | 20% | 0.9 (0.3, 2.8) | 85% | 4.8 (1.4, 17)j | 75% | 3.4 (1.2, 9.7)j |
| Doesn’t have trigger | 53% | 22% | 54% | 47% | |||||
Asthma Self-Management Questionnaire score above group mean score
flares at least once per week
Severity of Asthma Scale score above group mean score
Asthma Quality of Life Questionnaire score below group mean score
includes pollution, smoke, strong smells
includes grass, trees, flowers
includes laughing, crying
p ≤ .005
p ≤ .01
p ≤ .05
Discussion
We measured self-reported asthma triggers for adult patients presenting to the ED for asthma exacerbations. This study is the first to focus on the identification and prevalence of triggers in the high risk ED population. Most patients reported a variety of triggers that encompassed various physiological mechanisms, such as direct immunological reaction to allergens and bronchospasm from exercise.13 Except for women who reported more triggers, particularly more stress and emotion-related triggers, there were few systematic associations between specific triggers and demographic and clinical characteristics. Reporting more triggers, however, was uniformly associated with worse clinical status, including more asthma flares, worse asthma severity, and worse quality of life. Reporting dust, environmental irritants, plants, emotions and stress were particularly associated with these clinical manifestations of asthma.
Although triggers are important for diagnosing asthma and in gauging success of treatment, only a few recent reports have focused on describing their prevalence and characteristics in adults.2,3,14,15 In a study of 205 ambulatory patients in the Epidemiological Study on the Genetics and Environment of Asthma, of 22 possible triggers, the most frequently cited triggers were exercise, smoke, emotional stress and dust.14 When dichotomized into subgroups based on skin testing, patients with a negative skin test more often reported environmental irritants and patients with a positive test more often reported dust and pollen. Similar percentages reported other common triggers, such as weather and infections. Recently Ritz et al developed and validated the Asthma Trigger Inventory to measure triggers in a standardized way.2 Developed in 247 general practice patients, the inventory asks patients how often 32 specific triggers cause asthma symptoms. Triggers are grouped into seven main categories: psychological factors, physical activity, air pollution/irritants, infection, general allergens, and specific animal and pollen allergens. Our results are similar to theirs in that they also found that earlier onset of asthma was associated with animal allergy, and fewer smokers reported air pollution was a trigger. They also found that triggers were associated with clinical status with more frequent triggers associated with more asthma medications and resource utilization, and more psychological triggers associated with worse daily function. In a follow-up study using this inventory with 370 ambulatory and hospitalized patients in Germany, fewer triggers were reported by men, infectious triggers were associated with more resource utilization, and psychological triggers were associated with worse mental functional status.3 Our study also parallels the findings of another study from Turkey with 131 ambulatory patients in an allergy clinic.15 In the Turkish study, the mean number of triggers per patient was 12, the most common being environmental irritants, dust, emotional stress, and infections, and there were few associations with demographic and clinical characteristics except that more women reported stress as a trigger. Similar to our findings, more severe asthma was associated with emotional stress, weather, environmental irritants and aspirin-related triggers.
It was interesting to find in our study the increased rate of reporting triggers by obese patients and the decreased rate reported by those with a smoking history. Obesity alters respiratory physiology and causes dyspnea in most individuals, but the reason why it leads to airway inflammation in some individuals is not apparent.1 Among those affected, obesity is associated with more persistent and severe asthma, and is independent of diet and physical activity.16 In our study, obese patients reported greater susceptibility to diverse triggers with no systematic pattern, thus obesity most likely is detrimental through varied mechanisms. In addition, although weight loss is associated with reductions in exacerbations17, it is not known if this is due to decreased sensitivity to any particular trigger or group of triggers. It was surprising that patients with a smoking history reported fewer triggers. It is possible that smoking is so detrimental to asthma that it dwarfs effects of other triggers and leads patients to under-rate and under-report other triggers. Conversely, it may be that patients who smoke are less inclined to acknowledge external precipitants and instead attribute asthma to internal physiological conditions beyond their control. It is unlikely that patients with a smoking history are truly less sensitive to many of the triggers we considered, and therefore it is particularly important to encourage this subgroup to identify and avoid triggers.
Triggers are major considerations in characterizing asthma and may be useful in defining phenotypes.13 Traditionally, asthma has been described as extrinsic allergy-driven or intrinsic non-allergy driven. However, most patients have multiple triggers that exceed the boundaries of these classifications. Therefore, more information is needed if clinicians are to advise patients about recognizing triggers and whether costly home-based initiatives to manage triggers should be implemented by public health services.18–23 In addition, certain triggers may be similar, such as grass and trees, while others are dissimilar and require different avoidance strategies.24 In fact, it may not be practical to target all triggers, but rather to focus on those that are most salient or amenable to change. Most patients in our ED study volunteered that several triggers came together and caused their current exacerbations; therefore patients can be encouraged that there is benefit to modifying even some triggers.
This study has several limitations. First, this study was a secondary data analysis conducted with patients from urban inner-city hospitals and may not be generalizable to patients in other emergency department settings. Second, we did not assess physiological impact of triggers but focused on patients’ perceptions which may have depended on factors we did not measure, such as intensity and frequency of triggers.3 Third, all our patients were in the midst of an exacerbation and thus had great incentive to explain their current situation. While this potentially is a strength of our study in that temporal associations were more reliable, it also may have led to over-reporting. Fourth, we used ad hoc questions to measure triggers instead of open-ended questions or the validated scale that is now available.2 Fifth, we attempted to provide novel and preliminary information about triggers in an ED sample, therefore our analyses were descriptive. Multivariate analyses controlling for covariates in larger samples would be required to clearly discern associations between specific triggers and clinical characteristics. In addition, our comparisons of triggers and asthma severity did not include new classifications of severity which incorporate additional features, such as intensity of exacerbations and responsiveness to treatment.25
Conclusions
We found that patients presenting to the ED for asthma reported multiple triggers spanning diverse classes of precipitants. There was no systematic clustering of most triggers according to demographic characteristics. Having more triggers was associated with more severe disease and worse asthma-related quality of life. Our preliminary findings support instructing ED patients to identify their triggers, to actively avoid those they can, and to find ways to mitigate unavoidable exposures.
Footnotes
Declaration of Interest
None of the authors has a conflict of interest to disclose. This manuscript was written exclusively by the authors. The work described in this manuscript was funded by R01 HL075893 from the National Heart Lung and Blood Institute.
Contributor Information
Margaret G. E. Peterson, Research Division, Hospital for Special Surgery, New York, NY, USA.
Theodore J. Gaeta, Department of Emergency Medicine, New York Methodist Hospital, Weill Cornell Medical College, New York, NY, USA.
Robert H. Birkhahn, Department of Emergency Medicine, New York Methodist Hospital, Weill Cornell Medical College, New York, NY, USA.
José L. Fernández, Department of Medicine, Division of Emergency Medicine, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA.
Carol A. Mancuso, Department of Medicine, Hospital for Special Surgery, Weill Cornell Medical College, New York, NY, USA.
References
- 1.Expert Panel Report (EPR-3): Guidelines for the Diagnosis and Management of Asthma. National Heart Lung and Blood Institute, National Asthma Education and Prevention Program, US Department of Health and Human Services; Aug 28, 2007. [Google Scholar]
- 2.Ritz T, Steptoe A, Bobb C, Harris AHS, Edwards M. The Asthma Trigger Inventory: validation of a questionnaire for perceived triggers of asthma. Psychosom Med. 2006;68:956–965. doi: 10.1097/01.psy.0000248898.59557.74. [DOI] [PubMed] [Google Scholar]
- 3.Ritz T, Kullowatz A, Kanniess F, Dahme B, Magnussen H. Perceived triggers of asthma: evaluation of a German version of the Asthma Trigger Inventory. Respir Med. 2008;102:390–398. doi: 10.1016/j.rmed.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Mancuso CA, Peterson MGE, Gaeta TJ, Fernandez JL, Birkhahn RH, Melniker LA, Allegrante JP. A randomized controlled trial of self-management education for asthma patients in the emergency department. Annals Emerg Med. 2011;57:603–612. doi: 10.1016/j.annemergmed.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel Report (EPR-2): Guidelines for the Diagnosis and Management of Asthma. National Heart Lung and Blood Institute, National Asthma Education and Prevention Program, US Department of Health and Human Services; Apr, 1997. [Google Scholar]
- 6.Mancuso CA, Rincon M, McCulloch CE, Charlson ME. Self-efficacy, depressive symptoms, and patients’ expectations predict outcomes in asthma. Medical Care. 2001;39:1326–1338. doi: 10.1097/00005650-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso CA, Sayles W, Robbins L, Phillips EG, Ravenell K, Duffy C, Wenderoth S, Charlson ME. Barriers and facilitators to healthy physical activity in asthma patients. J Asthma. 2006;43:137–143. doi: 10.1080/02770900500498584. [DOI] [PubMed] [Google Scholar]
- 8.Mancuso CA, Sayles W, Allegrante JP. Development and testing of an asthma self-management questionnaire. Ann Allergy Asthma Immunol. 2009;102:294–302. doi: 10.1016/S1081-1206(10)60334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisner MD, Katz PP, Yelin EH, Henke J, Smith S, Blanc PD. Assessment of asthma severity in adults with asthma treated by family practitioners, allergists, and pulmonologists. Medical Care. 1998;36:1567–1577. doi: 10.1097/00005650-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SAS User’s Guide: Statistics. Cary, NC: SAS Institute; 1985. Version 5 ed. [Google Scholar]
- 12.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin N Am. 2004;33:637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 14.Charpin D, Ramadour M, Lanteaume A, Vervloet D. Triggers in intrinsic asthma in the EGEA study. J Asthma. 2003;40:87–91. doi: 10.1081/jas-120017211. [DOI] [PubMed] [Google Scholar]
- 15.Goksel O, Celik GE, Erkekol FO, Gullu E, Mungan D, Misirligil Z. Triggers in adult asthma: are patients aware of triggers and doing right? Allergol Immunopathol (Madr) 2009;37:122–128. doi: 10.1016/S0301-0546(09)71723-9. [DOI] [PubMed] [Google Scholar]
- 16.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 17.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund E, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, Hopkins DP, Lawrence BM, Sipe TA. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity. A community guide systematic review. Am J Prev Med. 2011;41(2 Suppl 1):S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T, Lurie M, Gomez M, Redy A, Pandya K, Medvesky M. The National Asthma Survey – New York State: association of the home environment with current asthma status. Public Health Reports. 2010 Nov-Dec;125:877–887. doi: 10.1177/003335491012500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton A, Basham M, Foy C, Buckingham K, Somerville M. The Watcombe Housing Study: the short term effect of improving housing condition on the health of residents. J Epidemiol Community Health. 2007;61:771–777. doi: 10.1136/jech.2006.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JR, Mildenhall S, Noble MJ, Shepstone L, Koutantji M, Mugford M, Harison BEW. The Coping with Asthma Study: a randomized controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax. 2005;60:1003–1011. doi: 10.1136/thx.2005.043877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MD, Reeves MJ, Meyerson K, Korzeniewski SJ. Randomized trial of a comprehensive asthma education program after an emergency department visit. Ann Allergy Asthma Immunol. 2006;97:44–51. doi: 10.1016/S1081-1206(10)61368-3. [DOI] [PubMed] [Google Scholar]
- 23.Largo TW, Borgialli M, Wisinski CL, Wahl RL, Priem WF. Health Homes University: A home-based environmental intervention and education program for families with pediatric asthma in Michigan. Public Health Reports. 2001 May-Jun;126:14–26. doi: 10.1177/00333549111260S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washington D, Yeatts K, Sleath B, Ayala GX, Gillette C, Williams D, Davis S, Tudor G. Communication and education about triggers and environmental control strategies during pediatric asthma visits. Pat Educ Counsel. 2011;86:63–69. doi: 10.1016/j.pec.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bousquet J, Anto JM, Demoly P, Schunemann HJ, Togias A, Akdis M, et al. Severe chronic allergic (and related) diseases: a uniform approach – a MeDALL-GA2LEN-ARIA position paper. Int Arch Allergy Immunol. 2012;158:216–231. doi: 10.1159/000332924. [DOI] [PubMed] [Google Scholar]