Abstract
We conducted a phase I-II study of transplantation conditioning with clofarabine-melphalan-alemtuzumab for patients with advanced hematologic malignancies. Ten patients were accrued to the phase I portion, which utilized an accelerated titration design. No dose-limiting toxicity was observed, and clofarabine 40 mg/m2 × 5, melphalan 140 mg/m2 × 1, and alemtuzumab 20 mg × 5 was adopted for the phase II study, which accrued 72 patients. Median age was 54 years. There were 44 patients with acute myelogenous leukemia or myelodysplastic syndromes, 27 with non-Hodgkin lymphoma, and nine patients with other hematologic malignancies. The largest subgroup of 35 patients had American Society for Blood and Marrow Transplantation high-risk, active disease. All evaluable patients engrafted with a median time to neutrophil and platelet recovery of 10 and 18 days, respectively. The cumulative incidence of treatment-related mortality was 26% at 1 year. Cumulative incidence of relapse was 29% at 1 year. Overall survival was 80% (95% confidence interval [CI], 71-89) at 100 days and 59% (95% CI, 47-71) at 1 year. Progression-free-survival was 45% (95% CI, 33-67) at 1 year. Rapid-onset renal failure was the main toxicity in the phase II study and more frequent in older patients and those with baseline decrease in glomerular filtration rate. Grade 3-5 renal toxicity was observed in 16 of 74 patients (21%) treated at the phase II doses. Clofarabine-melphalan-alemtuzumab conditioning yields promising response and duration of response, but renal toxicity poses a considerable risk particularly in older patients.
Keywords: Clofarabine, Conditioning, Phase II, Transplant
INTRODUCTION
Considerable effort has been devoted to the development of reduced-intensity conditioning (RIC) regimens for allogeneic stem cell transplantation. Unfortunately, the tradeoff for RIC has been an increase in disease recurrence and a high incidence of chronic graft-versus-host disease (cGVHD) with its considerable sequelae and ongoing risk for late transplantation-related mortality (TRM) [1-3]. For the past decade, we have used the reduced-intensity regimen of fludarabine melphalan and alemtuzumab. It has a low rate of acute and particularly chronic GVHD related to effective T cell depletion from alemtuzumab. It is well tolerated and effective in patients with leukemia and lymphoma entering transplantation with disease control, but has shown disappointing results in those with more advanced disease [4-7].
Clofarabine is a novel nucleoside analog, which, like other nucleoside analogs, requires intracellular phosphorylation. Clofarabine’s triphosphate inhibits DNA synthesis and repair [8,9]. Clofarabine also directly induces apoptosis by activation of caspase 9 and by a direct interaction with the mitochondrial membrane. It is the latter property that may explain its superior activity in treating acute leukemia [10]. Clofarabine is indicated in the United States for the treatment of pediatric patients (aged 1-21 years) with relapsed or refractory acute lymphoblastic leukemia (ALL) after at least two prior regimens at a recommended dose in monotherapy of 52 mg/m2 daily given over 2 hours for 5 days [11]. Hand-foot syndrome and usually reversible liver function abnormalities constitute the dose-limiting toxicities (DLT) [12]. Clofarabine also has activity in adult acute myelogenous leukemia (AML), although it is not approved for this use. Previous adult AML studies used clofarabine 40 mg/m2 given over 1 hour and reported hepatic and skin toxicities as being dose limiting [10]. More recently, clofarabine has also shown excellent activity in treating patients with lymphoma, in which case much lower doses are utilized [13]. Additionally, clofarabine has been combined with intermediate-dose cytarabine with promising results [14]. The combination with high-dose cyclophosphamide may be synergistic, but it was excessively myelosuppressive [15]. We conducted a phase I-II study of clofarabine, melphalan, and alemtuzumab for transplantation conditioning in patients with high-risk hematologic malignancies, the results of which are reported here. We conducted in parallel, a study of clofarabine-bridging cytoreduction before conditioning, reported elsewhere [16]. Only one patient who received clofarabine-bridge cytoreduction was also enrolled in the protocol described here.
PATIENTS AND METHODS
Eligibility
Eligibility was initially restricted to patients with intermediate and high-risk disease features as per American Society for Blood and Marrow Transplant criteria [17]. This included the following: acute leukemia beyond first remission, myelodysplastic syndromes, recurrent or refractory lymphoma (including Hodgkin lymphoma), chronic myelogenous leukemia beyond first chronic phase, and other myeloproliferative disorders with poor prognostic features. Patients with multiple myeloma or chronic lymphocytic leukemia (CLL) and poor prognostic features were also eligible, but no patients with myeloma were enrolled. As the study proceeded, several planned deviations were allowed for patients in first complete response who had high-risk disease by virtue of their karyotype or molecular characteristics but who were felt not to be appropriate candidates for standard myeloablative conditioning.
Other eligibility criteria included a Zubrod performance status ≤2, <75 years of age, adequate cardiac and pulmonary involvement, calculated creatinine clearance >50 mL/min, serum bilirubin <2.0 mg/dL, and serum glutamic pyruvic transaminase <3 × upper limit of normal. Patients with evidence of chronic active hepatitis or cirrhosis and those who were HIV positive were excluded. Pregnancy was an exclusion.
Donors
HLA-compatible sibling or eight of eight HLA-identical unrelated donors were used. One antigenmismatched related donors were acceptable. High-resolution HLA typing was used throughout this protocol. Donor peripheral blood stem cells were the preferred source of hematopoietic stem cells in all cases. Based on the donor’s preference, bone marrow stem cells were used in a few recipients of unrelated donor stem cells.
Study Design
Phase I
For the phase I portion of the trial, we used an accelerated titration design [18]. This has the advantage of treating fewer patients at subtherapeutic levels than the traditional “3 + 3” phase I design. Specifically, we enrolled one patient per dose level, beginning at dose level 1, until the first patient experienced a DLT or two patients experienced a grade 3 drug-related toxicity. At that point, we were to revert to a “3 + 3” design. That is, two additional patients were to be accrued at the dose that triggered the switch, and three to six patients were to be entered in that and each subsequent cohort. All patients in each cohort were to be followed for at least 28 days after transplantation before considering enrollment in a subsequent cohort. Under the “3+3” design, the maximally tolerated dose was defined as the highest dose level producing no more than one DLT in six patients.
Phase II
The primary endpoint for the phase II portion of the trial was the progression-free survival rate (PFS) at 1 year defined as the proportion of patients alive and without evidence of recurrence 1 year after the initiation of therapy. We tested the null hypothesis that the disease-free survival rate at 12 months is ≤25% versus the alternative that it is at least 40%. These boundaries were based on the 25% 1-year PFS previously observed with fludarabine-melphalan in high-risk patients [3]. A Simon two-stage design was employed in which 29 patients were enrolled in the first stage [19]. Because the results in the first 29 patients were acceptable, an additional 43 patients were enrolled for a total of 72. (Patients treated at the maximally tolerated dose during the phase I portion of the study were included in the phase II trial.) If 22 or fewer patients were progression free at 12 months, the regimen was to be rejected, whereas if 23 or more of the total 72 patients (≥32%) were progression free, the regimen was to be considered worthy of further evaluation in phase III trials. This design had an α-level of 10% and 90% power under the alternative hypothesis that the true 12-month PFS rate was 40%. The probability of early termination for a true 12-month PFS rate of 25% was 0.56.
Graft failure and liver toxicity were monitored, and early termination of the trial was to be considered if there was evidence that the graft failure rate exceeded 10% or the rate of irreversible liver toxicity was >15%. Chronic extensive GVHD over 40% would lead to consideration for early stopping. Early stopping guidelines used a Pocock group sequential monitoring boundary for a one-sided, overall α-level of 0.10 [20]. None of the early stopping guidelines were exceeded; therefore, the trial continued to completion.
Conditioning Regimen and GVHD Prophylaxis and Treatment
Alemtuzumab was administered at 20 mg i.v. on day −7 through day −3 over 1 hour. Clofarabine was initially administered i.v. over 1 hour on days −7 through −3; subsequently, the protocol was amended to infuse clofarabine over 3 hours. Melphalan was infused over 30 minutes on day −2 as previously described [4]. The doses of both clofarabine and melphalan were escalated as per the phase I design and are summarized in Table 2. Body surface area calculations were based on actual body weight. Tacrolimus i.v. was started on day 22 and converted to oral dosing after neutrophil engraftment as long as patients tolerated oral medications. Tacrolimus was adjusted to maintain levels of 5-15 ng/mL through day 100. Thereafter, tacrolimus was tapered by 20% every week. In recipients of mismatched or unrelated donor transplantations, tacrolimus was continued until day 180. No other GVHD prophylaxis was administered. First-line treatment for acute GVHD (aGVHD) or chronic GVHD (cGVHD) included steroids and resumption or continuation of calcineurin inhibitors. Second-line treatment varied and was often protocol driven.
Table 2. Hepatic, Renal, and Skin Toxicity (Day 7 until Day 30) CTC v. 3.0.
| Clo mg/m2. er Day × 5 |
Mel mg/m2 |
Enrolled | Hepatic | Renal | Sk | n | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Gr 1-2 | Gr3-4 | Gr 1-2 | Gr 3-4 | Gr 5 | Gr 1-2 | Gr 3-4 | Gr 1-2 | Gr 3-4 | Gr 5 | |||
| 10 | 100 | 1 | 1 | ||||||||||
| 20 | 100 | 2 | 0 | 1 seizure | |||||||||
| 30 | 100 | 2 | 2 | 3 | 1 cardiovascular | ||||||||
| 40 | 100 | 1 | 1 | ||||||||||
| 40 | 120 | 3 | 1 | 1 | 1 | 1 | |||||||
| Phase 2 | 40 | 140 | 24 | 14(58%) | 10(42%) | 9 (37%) | 4(17%) | 1 (4%) | 3(12%) | 6 (25%) | 1 confusion | 1 a.fib, | 1 cardiovascular |
| 1 a. flutter, | 1 mucositis | ||||||||||||
| 1 mucositis, | 1 mental status changes | ||||||||||||
| 1 pancreatitis | |||||||||||||
| 30 | 140 | 27 | 16 (59%) | 10(37%) | 9 (33%) | 7 (26%) | 2 (7%) | 1 (4%) | 1 pancreatitis, | 1 confusion, agitation | 1 irreversible mental status changes | ||
| 1 mucositis, | 2 severe mental status changes | 1 pulmonary hemorrhage, | |||||||||||
| 1 seizure, | 1 shock | ||||||||||||
| 1 anxiety, | |||||||||||||
| 1 pseudotumor cerebri | |||||||||||||
| 30 (3h) | 140 | 23 | 13 (56%) | 9 (39%) | 4(18%) | 2(10%) | 2(10%) | 1 (4%) | 1 mucositis, | 1 pulmonary embolism | 1 A fib with RVRand CHF | ||
| 1 diarrhea | 1 anaphylactic reaction | ||||||||||||
Clo indicates clofarabine.
Supportive Care
Patients were treated in rooms with high-efficiency, particulate-free (HEPA) air filters and with strict reverse isolation. They received filgrastim 5 μg/kg/day subcutaneously, starting on day 1 after transplantation until a neutrophil count of >10 × 109/L had been reached. Infection prophylaxis, transfusion support, and supportive care included high-dose acyclovir as well as pretransplantation trimethoprim/sulfamethoxazole. Patients also received quinolone prophylaxis until resolution of neutropenia and fluconazole 200 mg/day (or another broad-spectrum: azole) or echinocandin until day 180. Trimethoprim/sulfamethoxazole was given from engraftment until 1 year after transplantation.
Patients who were cytomegalovirus (CMV) seropositive or had a CMV-seropositive donor were given ganciclovir 5 mg/kg from day −8 until day −3. They then were given acyclovir 10 mg/kg every 8 hours i.v. until discharge. After engraftment, patients were converted to valacyclovir 2,000 mg four times a day until day 210 [21]. CMV negative donor/recipient pairs received routine acyclovir prophylaxis. All patients were screened weekly for CMV viremia until day 120 and treated with ganciclovir or valganciclovir on detection of CMV viremia. Irradiated and leuko-depleted blood products were administered to maintain a hemoglobin level greater than 8 g/dL and platelet count greater than 10 × 109/L. There was no routine screening for Epstein-Barr virus or adenovirus viremia.
Restaging and Outcome Definitions
Routine reevaluation was scheduled around day 28, day 100, day 180, and at the 1, 2, and 3 year anniversary and whenever warranted from signs or symptoms. The diagnosis of disease recurrence was based on clinical and pathological criteria. The molecular detection of minimal residual disease did not constitute relapse or disease persistence. Chimerism assays were performed at the same time points, using technology described elsewhere [22].
Toxicity was scored according to NCI/CTC version 3 [23]. Any grade 5 (fatal) toxicity was considered a DLT. In addition, grade 3 or 4 nonhematologic toxicities persisting for more than 24 hours was considered DLT with the exception of the following expected toxicities: alopecia or anorexia, grade 4 nausea, vomiting, diarrhea, or mucositis that resolved (with or without supportive care) to less than grade 2 within 48 hours. Grade 4 hematologic toxicities were not considered a DLT. Grade 4 or 5 infections and toxicities associated with infection were not considered a DLT. Grade 3 or 4 elevation in hepatic transaminases (alanine aminotransferase/serum glutamic pyruvic transaminase and aspartate aminotransferase/serum glutamic oxaloacetic transaminase) or alkaline phosphatase that returned to <grade 2 elevation within 14 days were anticipated and were not considered a DLT. Similarly, grade 4 elevations in amylase, lipase, or total bilirubin that were asymptomatic and that returned to less than grade 2 elevation within 7 days were not considered a DLT. For assessment of direct organ toxicity, we focused on the time period from the start of the conditioning regimen (day 27) until 28 days after transplantation.
aGVHD was scored according to the criteria proposed by Przepiorka et al [24]. cGVHD was scored according to the consensus criteria [25]. Engraftment was defined as per International Bone Marrow Transplant Registry and National Marrow Donor Program guidelines [26].
Statistical Analysis
The results of the phase I study are reported using descriptive statistics. Estimates of treatment-related mortality (TRM), PFS, overall survival (OS), and relapse rates refer to patients treated at phase II doses. PFS (time to relapse or death as a result of any cause) and OS were calculated using the Kaplan-Meier product-limit estimate and expressed as probabilities with a 95% confidence interval (CI) [27]. Cumulative incidence of disease progression with death before progression as the competing risk and cumulative incidence of TRM with relapse of the original disease as the competing risk were also calculated. In order to compare the cumulative incidence curves, we used Gray’s test. Log-rank test was used to compare the Kaplan-Meier curves. Cumulative incidence of aGVHD and cGVHD were also calculated with death or relapse as the competing risk.
Multivariate analyses used Cox proportional hazards regressions for OS and PFS [28]. Regression modeling for relapse and TRM incorporated competing risk [29]. For the multivariate models, independent variables with P > .1 were excluded sequentially from the models. Data were updated until June 30, 2011.
RESULTS
Patient Characteristics
Eighty-two patients were enrolled. Ten patients received the phase I doses of clofarabine and melphalan, and 72 were treated at phase II doses. Patient characteristics are summarized in Table 1. Median age was 54 years, and approximately half of the donors were unrelated. Forty-four AML or myelodysplastic syndrome patients, 27 lymphoma patients, five myeloproliferative disorder patients, four CLL patients, and three patients with ALL were enrolled. The largest subgroup of 35 patients had American Society for Blood and Marrow Transplant high-risk, active disease. Seven had failed previous autologous transplantation. Five had failed previous allogeneic transplantation; in all of these, the same donor was used for the second allogeneic transplantation. One had failed a prior syngeneic transplantation and an HLA identical allogeneic sibling was used for the second transplantation. Median serum creatinine on admission was 0.8 mg/dL (range, 0.5-1.4), and the median estimated glomerular filtration rate (GFR) on admission was 96 mL/min/ 1.73m2 (range, 25-≥120) based on the four variable Modification of Diet in Renal Disease equation [30].
Table 1. Patient Characteristics.
| N | 82 |
|---|---|
| Age, median (range) | 54 (21-73) |
| Gender: male/female | 34/48 |
| Diagnosis | |
| ALL | 3 |
| AML | 34 |
| CLL | 4 |
| CML | 3 |
| Myelofibrosis | 2 |
| Lymphoma | 27 |
| MDS | 9 |
| ASBMT high/int/low | 34/26/21 |
| ECOG PS 0/1/2/missing | 42/31/4/6 |
| HCTCI ≥3 | 28 (33%) |
| Prior transplantation | |
| Auto | 7 |
| Allo | 6 |
| MRD/MUD | 42/40 |
| Gender match | 42 |
| GFR mg/mL/1.73 m2 (range) | 96 (25->120) |
CML indicates chronic myeloid leukemia; ASBMT, American Society for Blood and Marrow Transplant; MDS, myelodysplastic syndromes; ECOG, Eastern Cooperative Oncology Group; HCT, hematopoietic transplantation; MRD, matched related donor; MUD, matched unrelated donor.
Engraftment and Chimerism
The first two patients had undergone a previous allogeneic transplantation. They recovered their counts promptly after transplantation. Because they had residual donor chimerism at the time of conditioning and the same donor was used for the second transplantation, the impact of the regimen on donor engraftment could not be assessed. All subsequent evaluable patients at all dose levels engrafted, and there were no cases of primary or secondary graft rejection. The median time to neutrophil and platelet recovery was 10 days and 18 days, respectively. Only one patient had neutrophil engraftment that was delayed beyond day 28. She was reconditioned but recovered cell counts with full-donor chimerism before the second stem cell infusion. Chimerism studies showed that all but one patient achieved full-donor chimerism in unsorted cells, and 84% achieved full-donor chimerism in T cells at day 30. Donor chimerism slowly declined over time, so that by day 180, only approximately half of the patients were full-donor chimeras (Figure 1). Such mixed chimerism is common after alemtuzumab-based conditioning, and in our experience is protective of GVHD but does not predict for disease recurrence [22]. Clofarabine at phase II dose levels appears to be more immunosuppressive than fludarabine, based on higher levels of donor chimerism, achieving statistical significance by day 180.
Figure 1.
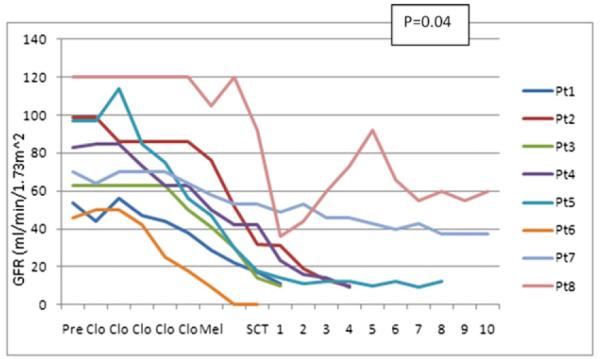
Patterns of decline in GFR in patients with renal toxicity. Patients with grade 4-5 renal toxicity. Clo, day of administration of clofarabine; Mel, day of administration of melphalan; SCT, day of stem cell transplantation. Alemtuzumab was given on the same days as clofarabine. Tacrolimus was started on day 2 after melphalan.
GVHD
The cumulative incidence of aGVHD grade II-IV was 22% (95% CI, 15-29). The cumulative incidence of grade III-IV aGVHD was 5% (95% CI, 1-9). The incidence of aGVHD was similar among related and unrelated donor recipients. The cumulative incidence of cGVHD was 5% (95% CI, 2-8), all cases occurring in unrelated donor recipients.
Toxicity (Table 2)
Phase I study
Three nonrelapse deaths occurred during the phase I portion of the study because of sepsis (n = 2) and a cardiac event in a patient with coronary artery disease (n = 1). The latter patient had a history of breast cancer and had undergone multiple prior chemotherapy regimens as well as chest wall irradiation resulting in therapy-related AML. On autopsy, there was advanced coronary stenosis and an incidental finding of residual breast cancer. None of the three deaths was attributed to the conditioning regimen, and these patients were replaced.
Table 2 details the number of patients per dose level and the toxicities observed. Except for transient liver function abnormalities and occasional cases of skin toxicity, no grade 3-5 toxicities occurred during the phase I portion of the study that were attributed to the conditioning regimen. Six patients were enrolled at the final dose level of clofarabine 40 mg/m2 × 5 and melphalan 140 mg/m2 × 1, and no DLTs were observed. This dose level combines the recommended dose of clofarabine in adults with a commonly used transplantation dose of melphalan and was adopted for further phase II study.
Phase II study
Twenty-four patients were treated at the initial phase II level (clofarabine 40 mg/m2 × 5 and melphalan 140 mg/m2). Grade 3-5 renal toxicity was observed in five of them. Overall, grade 3-5 renal toxicity was observed in 16 of 74 patients (21%) treated at the phase II doses. Sepsis was associated in only two of these cases. Grade 1-2 elevations of creatinine were observed in another 20 patients. Grade 2-5 renal failure was often irreversible. Figure 2 shows the rate of decline of GFR in the patients with grade 4-5 toxicity. The two patients with concomitant sepsis are excluded from this figure. In the majority of cases, the onset of decline in GFR occurred within days of the start of conditioning. There was a significant correlation between age and the occurrence of renal toxicity (r = .26, P = .018) and between baseline GFR and occurrence of renal toxicity. As expected, increasing age and lower baseline GFR were themselves highly correlated.
Figure 2.
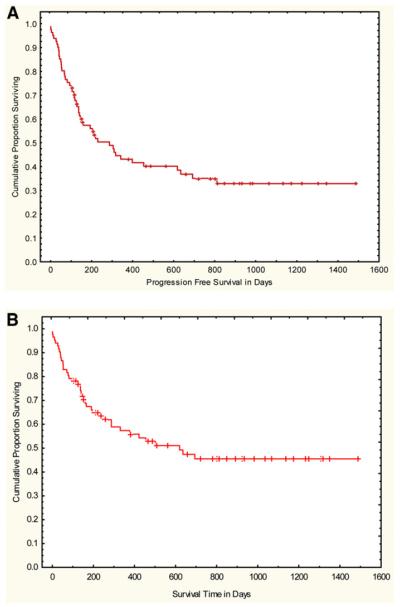
(A) PFS and (B) OS.
Transient liver function abnormalities were common and occasionally reached CTC level 3 or 4, but they were always rapidly reversible. No cases of sinusoidal obstruction disease/veno-occlusion disease were observed. Seven cases of grade 3 hand-foot syndrome were observed, six of which were at the 40 mg/m2 level.
Other toxicities are also documented in Table 2 and were uncommon. Of note, we did observe four cases of severe and prolonged mental status changes, which in one case was irreversible. There were also three cases of very early fatal heart failure during the phase II study. A 67-year-old patient undergoing second allogeneic transplantation developed pneumonia and intractable arrhythmias and died on day 15. A 51-year-old AML patient with preexisting cardiomyopathy died on day −1 from a combination of renal failure and chronic heart failure. A similar event occurred in a 61-year-old female with AML, diabetes, hypertension, and obesity, who died on day +1.
OS, PFS, TRM, and Relapse (Table 3)
Table 3. Multivariate Analysis of OS, PFS, TRM, and Relapse Based on Pretransplantation Characteristics.
| Variables | Relative Risk | P Value |
|---|---|---|
| OS | ||
| ASBMT risk group | 0.59 | .015 |
| Age | 2.6 | .004 |
| PFS | ||
| ASBMT risk group | 0.59 | .01 |
| TRM | ||
| Age | 0.17 | .002 |
| Relapse | ||
| Age | 5 | .003 |
ASBMT indicates American Society for Bone Marrow Transplant.
With a median follow-up of 25 months (range, 3-43 months), 27 patients treated at phase II levels remain alive and free of disease. In addition, two participants in the phase I part of the study remain alive and free of recurrence after 44 and 49 months.
In the phase II study, the cumulative incidence of TRM was 19% (95% CI, 10-28) at 100 days and 26% (95% CI, 16-36) at 1 year. Cumulative incidence of relapse was 29% (95% CI, 18-40) at 1 year. OS was 80% (95% CI, 71-89) at 100 days and 59% (95% CI, 47-71) at 1 year. PFS was 60% (95% CI, 48-72) at 100 days and 45% (95% CI, 33-67) at 1 year (Figure 2).
In multivariate analysis, age >55 years predicted for an increased risk of TRM as well as a decreased risk for disease relapse (Table 3). Age >55 years and disease risk category were the determinants of survival. Disease risk category was the only significant predictor of PFS.
We also analyzed an alternative model that incorporated the GFR on the day of transplantation. A GFR of <80 mL/min/1.73 m2 on the day of transplantation reflected an early decline in GFR and was by far the best predictor of TRM, survival, and relapse. Those with a GFR <80 mL/min/1.73 m2 had a 19-fold increase in TRM and corresponding decreases in OS and PFS. Early renal impairment was therefore the major determinant of long-term outcome.
DISCUSSION
Allogeneic transplantation remains the most effective treatment in many cases of hematologic malignancy but is beset by a high incidence of disease recurrence and toxicity. Efforts at reducing toxicity by the use of RIC and/or alemtuzumab have been successful in patients with chemotherapy-responsive disease, but high relapse rates remain a major obstacle for patients with more advanced disease. With the purpose of improving outcomes in patients with advanced hematologic malignancies, we replaced fludarabine in our conditioning regimen with clofarabine, a novel nucleoside analog with better activity in leukemia and lymphoma. At the time of this study’s design, we were aware of pharmacodynamic studies showing that higher doses of clofarabine were associated with more sustained inhibition of replication of leukemic blasts and better accumulation of clofarabine triphosphate in CLL cells [31].
The phase I portion showed that 40 mg/m2 for = days and melphalan 140 mg/m2 was a tolerable dose with the primary toxicity of reversible transaminitis. The completion of the phase II study allowed us to establish estimates of the immunosuppressive potential, efficacy, and toxicity of this regimen. Engraftment occurred promptly with durable engraftment in all evaluable patients. We therefore established in agreement with others’ observations that clofarabine has immunosuppressive properties that are equal or superior to those of fludarabine [32].
The regimen met the phase II primary endpoint of PFS of 32% or more, as 28 (39%) patients have survived free of disease for more than a year after transplantation, and the estimated 1-year PFS is 45%. We therefore believe the regimen warrants additional study. The 1-year PFS of 31% for patients with active disease is similar to the 25% PFS we previously reported for similar patients treated with fludarabine-melphalan-alemtuzumab [4]. However, only one out of seven patients with active disease has relapsed beyond 1 year, and six of 35 high-risk patients remain in remission with a median follow-up of 2 years (range, 1.2-3.0 years). By contrast with fludarabine melphalan high risk patients continued to relapse beyond one year and there were very few with durable remissions. These data suggest that clofarabine-alkylator combinations may have greater antileukemic activity and result in better long-term disease control. Other studies provide additional support for high activity of clofarabine alklaytor combinations. Farag et al. [32] used busulfan and clofarabine in 15 patients, mostly with refractory disease and found that seven patients survived in remission for longer than 1 year. Mineishi et al. [33] treated 46 patients with busulfan and clofarabine, none of them in remission. In this study, 94% of AML patients achieved remission, and the median 18-month survival for AML patients was 50%. Andersson et al. [34] report on 51 patients with AML or chronic myeloid leukemia, many with refractory disease who underwent conditioning with a combination of busulfan clofarabine and varying doses of fludarabine. Seventeen of 32 patients with active leukemia were in remission with median follow-up of 14 months. Similar outcomes were described in other preliminary reports including some pediatric studies [35-38]. Whether busulfan, melphalan, or total-body irridiation are better for combination with clofarabine requires further study, as do the relative benefits and disadvantages of in vivo T cell depletion [39,40].
Although efficacy and long-term PFS were promising, unexpected acute renal toxicity affected long-term outcomes. Renal toxicity occurred early and predicted for TRM and survival. This prompted us to reduce the dose of clofarabine to 30 mg/m2, and subsequently, based on pediatric data, to lengthen the infusion time of clofarabine. These modifications did not appear to reduce renal toxicity. Recipient age and baseline GFR were the only parameters that correlated with renal toxicity, and they may be surrogates for each other.
Our data on renal injury contrast with other transplantation reports. Kirshbaum et al. [32] in a study of 16 patients combined clofarabine with melphalan, and Farag et al. [38] combined it with busulfan in a 15-patient study. Each reported only one case of irreversible renal toxicity. Others have not reported any evidence of renal toxicity [33,34], and we cannot completely explain this discrepancy between studies. It is of course possible that the limited number of patients in some other studies and in the phase I part of our own study precluded detection of renal toxicity. It is unlikely that our policy of pretransplantation administraton of ganciclovir, trimethoprim/sulfamethoxazole, and an oral quinolone should be implicated as a cause of renal failure because it has been used as a standard in all our protocols, and we have not observed similar deteriorations in previous studies [4,41]. Neither melphalan nor alemtuzumab are associated with nephrotoxicity and both can even be safely administered to patients with renal failure [42]. Furthermore, fludarabine, melphalan, and alemtuzumab or fludarabine, busulfan, and alemtuzumab combinations rarely invoke early renal injury [4,41]. Renal deterioration, often occurred early, after one or two doses of clofarabine and alemtuzumab but before administration of melphalan, tacrolimus, or other renal-damaging drugs. Unless one believes there is a hitherto unknown interaction between clofarabine and alemtuzumab, our findings suggest that clofarabine has intrinsic nephrotoxicity, at least in susceptible patients. Patients with impaired baseline renal function and/or older adults may be particularly prone to it. Our data are consistent with those of Faderl et al. [43], who in a study of elderly adults with leukemia observed elevations in creatinine in up to 36% of patients who received clofarabine 30 mg/m2 × = days, all in the setting of serious adverse events and hypotension.
In addition to renal toxicity, there were also four cases of early fatal shock, including one during the phase I portion of the study. These events tended to occur during or immediately after completion of conditioning. They are reminiscent of a syndrome of a cytokine release event originally described by Jeha and are associated with hypotension, respiratory distress, and multiorgan failure [44]. We cannot therefore rule out that clofarabine contributed to these events, but high-dose melphalan, particularly in combination with nucleoside analogs [45,46], and even rarely alemtuzumab [47], have also been associated with rare cases of fulminant cardiac failure.
The only other unexpected toxicity was encephalopathy, which occurred in four patients and was irreversible in one. Nucleoside analogs, and in particular fludarabine, have been associated with encephalopathy [48]. Other causes of neurologic toxicity including toxicity from calcineurin inhibitors, antivirals, or antifungals, however, cannot be excluded.
In contrast to the severity of renal problems, liver function abnormalities were always rapidly reversible, and no cases of sinusoidal obstruction syndrome/veno-occlusive disease were observed throughout the course of this study. No fatal or irreversible hepatic toxicity was observed with our clofarabine-melphalan combination in contrast to the studies of clofarabine and busulfan where occasional cases occurred [32-34]. The hepatic toxicity of clofarabine therefore is of limited consequence, at least in the regimen studied. Hand-foot syndrome occurred but was not dose limiting. The only study in which clofarabine hand-foot syndrome appeared limiting was in combination with cytarabine, a known skin toxin [49].
In summary, our phase II trial yielded promising PFS, and further investigaton of clofarabine-melphalan combinations is warranted. However, we newly identified renal toxicity as a considerable risk, particularly in older patients. Ongoing pharmacokinetic analysis will help better characterize this toxicity and to design future studies that mitigate renal toxicity, potentially by reducing or individualizing clofarabine doses.
ACKNOWLEDGMENTS
Financial disclosure: This work was supported in part by Grant K24 CA116471 and supported in part by an unrestricted grant from Genzyme Corp. Drs. van Besien and Stock have received research support from Genzyme Corporation and participated in Advisory Boards.
REFERENCES
- 1.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2011 Mar 28; doi: 10.1038/bmt.2011.69. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared with myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignanciesundergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 4.van Besien K, Artz A, Smith S, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 5.Kenkre VP, Horowitz S, Artz AS, et al. T-cell-depleted allogeneic transplant without donor leukocyte infusions results in excellent long-term survival in patients with multiply relapsed lymphoma. Predictors for survival after transplant relapse. Leuk Lymphoma. 2011;52:214–222. doi: 10.3109/10428194.2010.538777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris E, Mackinnon S. Outcome following alemtuzumab (CAMPATH-1H)-containing reduced intensity allogeneic transplant regimen for relapsed and refractory non-Hodgkin’s lymphoma (NHL) Transfus Apher Sci. 2005;32:73–83. doi: 10.1016/j.transci.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 8.Xie KC, Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine. Cancer Res. 1996;56:3030–3037. [PubMed] [Google Scholar]
- 9.Genini D, Adachi S, Chao Q, et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96:3537–3543. [PubMed] [Google Scholar]
- 10.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 11.Jeha S, Razzouk B, Rytting M, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Oncol. 2009;27:4392–4397. doi: 10.1200/JCO.2008.18.8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 13.Nabhan C, Davis N, Bitran JD, et al. Efficacy and safety of clofarabine in relapsed and/or refractory non-Hodgkin lymphoma, including rituximab-refractorypatients. Cancer. 2011;117:1490–1497. doi: 10.1002/cncr.25603. [DOI] [PubMed] [Google Scholar]
- 14.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karp JE, Ricklis RM, Balakrishnan K, et al. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood. 2007;110:1762–1769. doi: 10.1182/blood-2007-03-081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locke FL, Artz A, Rich E, et al. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant. 2010;45:1692–1698. doi: 10.1038/bmt.2010.32. [DOI] [PubMed] [Google Scholar]
- 17.ASBMT RFI Disease classifications. Retrieved September 27, 2011 from http://asbmt.org/displaycommon.cfm?an51&sub articlenbr=35. [Google Scholar]
- 18.Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Pocock S. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 21.Kline J, Pollyea DA, Stock W, et al. Pre-transplant ganciclovir and post transplant high-dose valacyclovir reduce CMV infections after alemtuzumab-based conditioning. Bone Marrow Transplant. 2006;37:307–310. doi: 10.1038/sj.bmt.1705249. [DOI] [PubMed] [Google Scholar]
- 22.van Besien K, Dew A, Lin S, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–1817. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 23.Common terminology criteria for adverse events (CTCAE) and common toxicity criteria (CTC) Retrieved on September 28, 2011 from http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 24.Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.CIBMTR data management manual. Retrieved on September 28, 2011 from http://www.cibmtr.org/DataManagement/Training Reference/Manuals/DataManagement/pages/index.aspx.
- 27.Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant. 2001;28:909–915. doi: 10.1038/sj.bmt.1703260. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life tables (with discussions) J R Statist Soc B. 1972;34:187–220. [Google Scholar]
- 29.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 30.Cirillo M, Lombardi C, Luciano MG, et al. Estimation of GFR: a comparison of new and established equations. Am J Kidney Dis. 2010;56:802–804. doi: 10.1053/j.ajkd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Farag SS, Wood LL, Schwartz JE, et al. Phase I trial and pharmacokinetic study of high-dose clofarabine and busulfan and allogeneic stem cell transplantation in adults with high-risk and refractory acute leukemia. Leukemia. 2011;25:599–605. doi: 10.1038/leu.2010.319. [DOI] [PubMed] [Google Scholar]
- 33.Mineishi S, Magenau J, Tobai H, et al. Allogeneic hematopoietic stemcell transplantation with clofarabine/busulfan × 4 conditioning exhibits significant anti-tumor activity in non-remission hematologic malignancies, especially in AML. Blood. 2010;116:a35. [Google Scholar]
- 34.Andersson BS, Valdez BC, de Lima M, et al. Clofarabine ± fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011;17:893–900. doi: 10.1016/j.bbmt.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci AM, Geyer MB, Harrison LA, et al. Preliminary results of phase I/II study of clofarabine incombination with cytarabine and total body irradiation followed by allogeneic stem cell transplantation in children, adolescents and young adults with poor-risk acute leukemia. Biol Blood Marrow Transplant. 2011;17:S186. [Google Scholar]
- 36.Teltschik H, Mueller I, Pfeiffer M, et al. Clofarabine versus fludarabine for haploidentical stem cell transplantation in paediatric patients with refractory leukaemias. Bone Marrow Transplant. 2009;43:S224. [Google Scholar]
- 37.Agura E, Berryman RB, Luis P, et al. Preliminary results of a phase II trial of clofarabine with parenteral busulfan followed by allogeneic transplantation for the treatment of hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:S298. [Google Scholar]
- 38.Kirschbaum MH, Stein AS, Popplewell L, et al. A phase I study in adults of clofarabine combined with high-dose melphalan as reduced intensity conditioning for allogeneic transplantation. Biol Blood Marrow Transplant. 2011 Jul 27; doi: 10.1016/j.bbmt.2011.07.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.van Besien K, Kunavakkam R, Rondon G, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on GvHD prophylaxis with or without anti-T-cell globulin ATG-fresenius. Blood. 2011;117:6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell PH, Artz AS, Undevia SD, et al. Phase I study of dose-escalated busulfan with fludarabine and alemtuzumab as conditioning for allogeneic hematopoietic stem cell transplant: reduced clearance at high doses and occurrence of late sinusoidal obstruction syndrome/veno-occlusive disease. Leuk Lymphoma. 2010;51:2154–2156. doi: 10.3109/10428194.2010.520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Besien K, Schouten V, Parsad S, et al. Allogeneic stem cell transplantation in renal failure. engraftment and prolonged survival, but high incidence of neurologic toxicity. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.604756. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 44.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 45.Morandi P, Ruffini PA, Benvenuto GM, Raimondi R, Fosser V. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant. 2005;35:323–334. doi: 10.1038/sj.bmt.1704763. [DOI] [PubMed] [Google Scholar]
- 46.van Besien K, Devine S, Wickrema A, et al. Regimen-related toxicity after fludarabine-melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transplant. 2003;32:471–476. doi: 10.1038/sj.bmt.1704166. [DOI] [PubMed] [Google Scholar]
- 47.Lenihan DJ, Alencar AJ, Yang D, et al. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome. Blood. 2004;104:655–658. doi: 10.1182/blood-2003-07-2345. [DOI] [PubMed] [Google Scholar]
- 48.Beitinjaneh A, McKinney AM, Cao Q, Weisdorf DJ. Toxic leukoencephalopathy following fludarabine-associated hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:300–308. doi: 10.1016/j.bbmt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Martin MG, Uy GL, Procknow E, et al. Allo-SCT conditioning for myelodysplastic syndrome and acute myeloid leukemia with clofarabine, cytarabine and ATG. Bone Marrow Transplant. 2009;44:13–17. doi: 10.1038/bmt.2008.423. [DOI] [PubMed] [Google Scholar]