Abstract
AIM
The functional significance of the autonomic nerves in the anterior mitral valve leaflet (AML) is unknown. We tested the hypothesis that remote stimulation of the vagus nerve (VNS) reduces AML stiffness in the beating heart.
METHODS
Forty-eight radiopaque-markers were implanted into eleven ovine hearts to delineate left ventricular and mitral anatomy, including an AML array. The anesthetized animals were then taken to the catheterization laboratory and 4-D marker coordinates obtained from biplane videofluoroscopy before and after VNS. Circumferential (Ecirc) and radial (Erad) stiffness values for three separate AML regions, Annulus, Belly and Edge, were obtained from inverse finite element analysis of AML displacements in response to trans-leaflet pressure changes during isovolumic contraction (IVC) and isovolumic relaxation (IVR).
RESULTS
VNS reduced heart rate: 94±9 vs. 82±10 min−1, (mean±SD, p<0.001). Circumferential AML stiffness was significantly reduced in all three regions during IVC and IVR (all p<0.05). Radial AML stiffness was reduced from control in the annular and belly regions at both IVC and IVR (P<0.05), while the reduction did not reach significance at the AML edge.
CONCLUSION
These observations suggest that one potential functional role for the parasympathetic nerves in the AML is to alter leaflet stiffness. Neural control of the contractile tissue in the AML could be part of a central control system capable of altering valve stiffness to adapt to changing hemodynamic demands.
Keywords: Intrinsic heart valve physiology, Heart valve innervation, Vagus nerve stimulation, Mitral valve
INTRODUCTION
The mitral valve (MV) leaflets, chordae tendinae, and papillary muscles have been shown to be densely innervated(Ellison and Hibbs, 1973; Hibbs and Ellison, 1973; De Biasi et al., 1984; Kawano et al., 1993; Marron et al., 1996), with both sympathetic and parasympathetic nerve fibers observed in multiple species, including man(Ehinger et al., 1968; Williams et al., 1990a; Ahmed et al., 1997; Williams and Jew, 2004). The functional significance of these nerves, however, has not been elucidated.
Mitral leaflets, previously considered as passive flaps of connective tissue, are now known to contain at least three populations of cells with contractile properties(Krishnamurthy et al.; Swanson et al.; Swanson et al.; Cooper et al., 1966; Curtis and Priola, 1992; Itoh et al., 2009; Krishnamurthy et al., 2009b). Bundles of cardiac myocytes, containing networks of nerve fibers and nerve terminals (Cooper et al., 1966; Sonnenblick et al., 1967), extend from the annulus into the anterior mitral leaflet (AML) in electrophysiological continuity with the rest of the heart(Swanson et al.; Montiel, 1970; Fenoglio et al., 1972; Wit et al., 1973; Boucek et al., 1978). Smooth muscle cells are present just below the valve’s endocardial investment throughout the length of the leaflet(Hibbs and Ellison, 1973; Wit et al., 1979; De Biasi et al., 1984). Valvular interstitial cells (VICs) containing smooth-muscle alpha-actin and in close proximity to nerve terminals and blood vessels (Filip et al., 1986; Chester and Taylor, 2007) create a ubiquitous network throughout the leaflet. Activation of these contractile tissues, in response to cardiac depolarization, circulating agents and/or neural signaling, could alter leaflet stiffness.
Our recent studies (Krishnamurthy et al.; Swanson et al.; Itoh et al., 2009; Krishnamurthy et al., 2009b) have shown that MV leaflets can indeed change stiffness in the beating heart, with these studies demonstrating that: 1. The intact AML in the beating heart is two orders of magnitude stiffer than ex vivo leaflets; 2. AML stiffness changes substantially throughout systole and this change can be altered by pharmacologic agents; and 3. Electrical pacing of the region of aorto-mitral continuity, known to be a densely innervated region, greatly increases AML stiffness.
Thus, given that multiple contractile systems are present in the leaflet, and that leaflet stiffness can be rapidly altered by electrical and pharmacologic stimulation, we reasoned that the parasympathetic nerve fibers observed in the AML may serve, at least in part, to alter the inotropic state of one or more of the leaflet contractile elements. Because the vagus nerve is the source of parasympathetic cardiac innervation and post-ganglionic parasympathetic fibers are seen in close proximity to the MV contractile tissue (cardiac myocytes and VICs), we tested the hypothesis that remote central vagal stimulation would immediately change leaflet stiffness.
MATERIALS & METHODS
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and also in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (U.S. Department of Health and Human Services, NIH Publication 85-23, Revised 1985). This study was approved by the Stanford Medical Center Laboratory Research Animal Preview Committee, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, and conducted according to Stanford University policy.
The methodology used for this experiment has been previously described (Itoh et al., 2009; Krishnamurthy et al., 2009b), thus will only be briefly outlined here, with changes described in detail.
Surgical Preparation
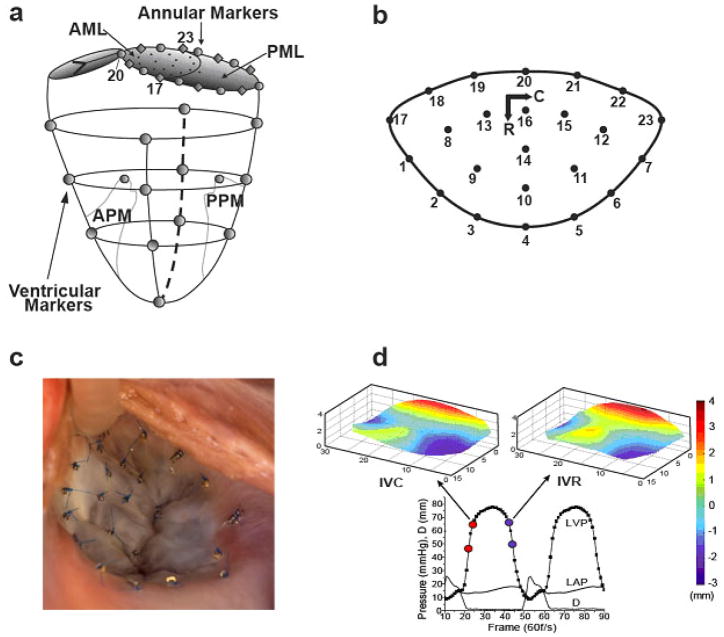
Eleven adult, Dorsett-hybrid, male sheep (49±5 kg) were pre-medicated, anesthetized (isoflurane), mechanically ventilated and instrumented for hemodynamic monitoring. Thirteen miniature radiopaque tantalum markers were surgically implanted into the subepicardium to silhouette the left ventricular chamber along four equally spaced longitudinal meridians (“Ventricular Markers”, Figure 1a). On cardiopulmonary bypass a total of 35 additional radiopaque tantalum markers were sewn to the following sites: one at the tip of each papillary muscle (APM, PPM, Figure 1a), 16 around the mitral annulus (Annular Markers, Fig 1a), 16 on the atrial aspect of the AML (Fig 1b), and 1 on the central edge of the middle scallop of the posterior mitral leaflet (PML, Figure 1a). A single tantalum loop was used for each leaflet marker (Figure 1c). The left vagus nerve was isolated and equipped with a bipolar stimulating electrode via a cervical incision.
Figure 1.
Radiopaque marker sites on (a) left ventricle, mitral annulus, and leaflets, and (b) mitral valve anterior leaflet, with circumferential (C) and radial (R) axes. (c) Intra-operative photograph of markers sewn to the mitral valve anterior leaflet and annulus. (d) Representative left ventricular pressure (LVP), left atrial pressure (LAP), and inter-leaflet distance (D) data, showing the LVP interval during which IVC stiffness is measured (red dots) and anterior leaflet shape at end IVC (upper left); and the pressure-matched LVP interval during which IVR stiffness is measured (blue dots) and anterior leaflet shape at the beginning of IVR (upper right). Leaflet vertical dimension color-coded from −3 to +4 mm to illustrate leaflet shape. Annular saddle-horn is in the middle of the red edge.
Data Acquisition
After weaning from cardiopulmonary bypass, the animals were transferred to the experimental catheterization laboratory for open-chest data acquisition. Videofluoroscopic images (60 frames/sec) of all radiopaque markers were acquired in multiple-beat runs using biplane videofluoroscopy with the heart in normal sinus rhythm and ventilation transiently arrested at end expiration. Images were obtained before (CTRL) and immediately after left vagus nerve stimulation (VNS, 600 min−1, current 4.5±0.8 mA (range 4.0–6.0 mA) to achieve a 15% reduction in heart rate). Marker coordinates from each view were then merged to yield the 3-D coordinates of the centroid of each marker in each frame. Left ventricular pressure (LVP), left atrial pressure (LAP), aortic pressure (AoP) and electrocardiographic voltage signals were digitally recorded simultaneously during marker data acquisition and synchronized with the images.
Leaflet Shape Analysis
Leaflet shape was characterized at end systole (ES) for each beat (B=1,2,3) in each run (R= CTRL, VNS) and each heart (H=1,2, …,11) by the distances DES(H, R, B, M) from each leaflet marker (Figure 1b; M=1, 2, …,16) to a best-fit plane through all the markers delineating the mitral annulus. This distance was averaged for each run and each marker in each heart as DESavg(H,R,M) = [DES(H,R,1,M) + DES(H,R,2,M) + DES(H,R,3,M)]/3. Leaflet shape change from CTRL to VNS was then computed as ΔDESavg(H,M) = DESavg(H,VAGAL,M) − DESavg(H,CTRL,M) which was considered significant if |ΔDESavg(H,M)| > 0.5mm and p<0.01.
Finite Element Model
Three consecutive beats in sinus rhythm were selected for analysis for the CTRL and VNS runs from each heart. Cardiac cycle timing was determined as previously described(Itoh et al., 2009). Finite element models of the AML were individually developed for both the IVC and IVR intervals for each beat analyzed. The initial geometry for each model was defined by the x, y, z coordinate values for each leaflet, annular and papillary tip marker. A leaflet surface was generated from these marker positions (Fig. 1d). Previously measured ovine leaflet thickness values were used in an orthotropic linear elastic material model (Krishnamurthy et al., 2009a).
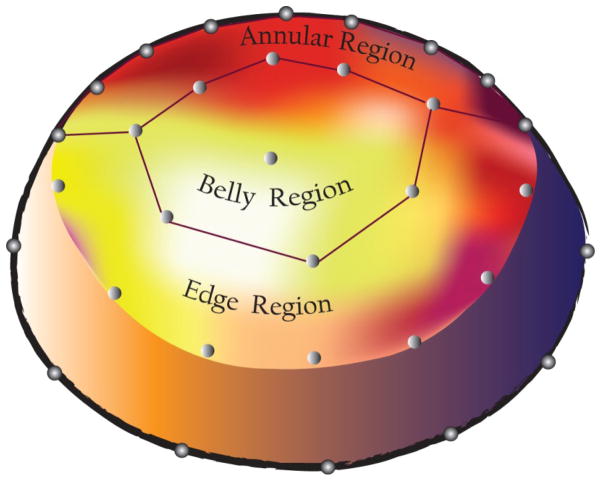
The model was then meshed with approximately 2200 elements and strut chordae were modeled as rope-like structures radiating from the papillary muscle tips to the leaflet belly. The model used the experimental displacements of the papillary muscle tips and leaflet edge and annular markers as displacement boundary conditions, along with experimentally measured LVP and LAP as hemodynamic boundary conditions, while allowing the leaflet belly to displace according to the circumferential (Ecirc) and radial (Erad) elastic modulus (stiffness) values entered into the model. Three leaflet regions (Annulus, Belly, Edge, Figure 2) were identified based on marker locations as previously described(Krishnamurthy et al., 2009b).
Figure 2.
Schematic of the mitral orifice illustrating the three regions for which the radial and circumferential stiffness were determined. (Posterior mitral leaflet not shown.)
Inverse Finite Element Analysis Algorithm
An inverse finite element analysis was performed to determine in vivo MV stiffness(Itoh et al., 2009; Krishnamurthy et al., 2009b). Briefly, stiffness values were entered into the finite element model and a simulation was run using the appropriate displacement and hemodynamic boundary conditions for that beat, time interval, and region. Leaflet displacements in the model were then compared to the actual experimental leaflet displacements and stiffness values iterated using an optimization algorithm and solver until minimum error was reached between the simulated and measured leaflet displacements. The stiffness values ((Ecirc) and (Erad) elastic moduli) for each region in the model were then interpreted as the in vivo anterior MV leaflet stiffness values for that region. This was done for both the IVC and IVR time intervals during each cardiac cycle, for three beats, for each heart, and both CTRL and VNS.
Statistical Analysis
All values are given as group mean ± 1standard deviation (SD). Three-beat averages were used to characterize the data for each animal. Data were compared using Student’s paired t-test (Microsoft Office Excel 2007, Microsoft Corp., Bellingham, WA), after proving data had a normal distribution. Statistical significance was set at p<0.05, unless otherwise noted.
RESULTS
Epicardial echocardiography confirmed that no animal in the study had substantial mitral regurgitation (0.32±0.25 using 0–4+ American Society of Echocardiography MR scale) prior to the CTRL run. Technical limitations prevented acquiring echocardiographic data during the VNS run. Postmortem examination confirmed that all markers were present and appropriately placed.
Hemodynamic Response
All hearts remained in sinus rhythm throughout the study. Remote stimulation of the left vagus nerve caused immediate hemodynamic changes, thus VNS data were acquired within seconds after stimulation. Table 1 shows the hemodynamic parameters for CTRL and VNS. Mean heart rate decreased from 94±9 to 82±10 min−1 (p<0.001). Left ventricular (LV) dP/dt-max and LV end-systolic pressure decreased slightly with VNS in all hearts. LV end-systolic volume decreased and LV stroke volume increased.
Table 1.
Hemodynamic Response to Vagal Stimulation
| HR (min−1) | dP/dtmax (mmHg s−1) | LVEDV (mL) | LVESV (mL) | LVSV (mL) | LVEDP (mmHg) | LVESP (mmHg) | |
|---|---|---|---|---|---|---|---|
| Control | 94±9 | 1330±332 | 144±15 | 111±15 | 34±10 | 11±5 | 89±4 |
| VNS | 82±11 | 1187±348 | 146±14 | 109±15 | 37±7 | 11±4 | 83±6 |
| p= | <0.001 | <0.001 | n.s. | 0.017 | 0.019 | n.s. | <0.001 |
VNS=vagal nerve stimulation HR=heart rate dP/dt max=change in left ventricular (LV) pressure per second maximum LVEDV= LV end diastolic volume LVESV=LV end systolic volume SV= LV stroke volume LVEDP=LV end diastolic pressure LVESP=LV end systolic pressure Paired t-test, p<0.05 significant.
Leaflet Shape Response
Group mean distance from all leaflet markers (Figure 1b; M=1, 2, …,16) to a best-fit annular plane at end systole changed by only −0.08±0.17mm (N.S.) from CTRL to VNS. Thus, leaflet shape did not change significantly from CTRL to VNS.
Anterior Mitral Leaflet Stiffness Response
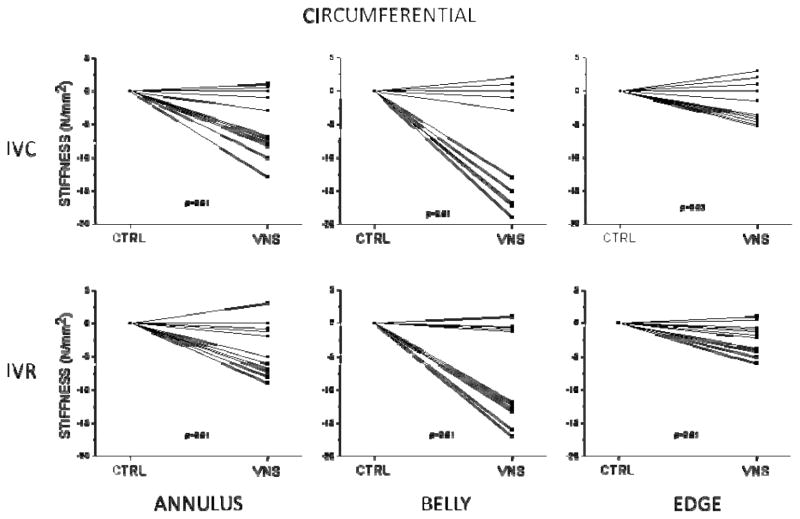
Mean circumferential (Ecirc) and radial (Erad) AML stiffness for all three regions are shown for isovolumic contraction (table 2) and isovolumic relaxation (table 3). In figures 3 and 4 the control stiffness is normalized to zero and the change in stiffness in response to VNS is shown for circumferential and radial stiffness for each heart.
Table 2.
Mitral Leaflet Stiffness during Isovolumic Contraction
| Isovolumic Contraction
| ||||||
|---|---|---|---|---|---|---|
| Annular | Belly | Edge | ||||
|
| ||||||
| Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | |
| Control | 60±14 | 19±4 | 46±11 | 13±3 | 53±12 | 17±4 |
| VNS | 55±13 | 18±3 | 37±12 | 11±2 | 51±12 | 16±3 |
| p-value | 0.01 | 0.05* | 0.01 | 0.01 | 0.03 | n.s. |
VNS= vagus nerve stimulation; Annular, Belly and Edge refer to leaflet regions illustrated in figure two; Ecirc=mitral leaflet stiffness in the circumferential direction, N/mm2; Erad=mitral leaflet stiffness in the radial direction, N/mm2; p<0.05 significant.
Absolute value is less than <0.05.
Table 3.
Mitral Leaflet Stiffness during Isovolumic Relaxation
| Isovolumic Relaxation
| ||||||
|---|---|---|---|---|---|---|
| Annular | Belly | Edge | ||||
|
| ||||||
| Ecirc | Erad | Ecirc | Erad | Ecirc | Erad | |
| Control | 45±11 | 13±3 | 35±11 | 9±3 | 40±10 | 11±3 |
| VNS | 41±11 | 12±3 | 28±12 | 7±2 | 38±11 | 11±3 |
| p-value | 0.01 | 0.04 | 0.01 | 0.04 | 0.01 | n.s. |
VNS= vagus nerve stimulation; Annular, Belly and Edge refer to leaflet regions illustrated in figure two; Ecirc=mitral leaflet stiffness in the circumferential direction, N/mm2; Erad=mitral leaflet stiffness in the radial direction, N/mm2; p<0.05 significant.
Figure 3.
Annular, belly and edge regional circumferential anterior mitral leaflet stiffness with control (ctrl) values normalized to zero and the change for each heart in response to vagal nerve stimulation (VNS) shown. IVC = isovolumic contraction; IVR = isovolumic relaxation.
Figure 4.
Annular, belly and edge regional radial anterior mitral leaflet stiffness with control (ctrl) values normalized to zero and the change for each heart in response to vagal nerve stimulation (VNS) shown. IVC = isovolumic contraction; IVR = isovolumic relaxation.
The statistically significant decrease in leaflet circumferential stiffness due to VNS is seen in figure 3. All three regions decreased, however the belly region had the largest reduction in what appeared to be a subset of the hearts at both IVC and IVR. The effect was smaller in the annular and edge leaflet regions. The decrease in leaflet radial stiffness is seen in figure 4, where the annular and belly regions were statistically significantly reduced at both IVC and IVR by VNS (p<0.05). Edge region stiffness was unchanged with VNS (p=n.s.).
DISCUSSION
Left vagus nerve stimulation decreased MV leaflet stiffness. Leaflet stiffness was immediately reduced in both the circumferential and radial directions at both time points (IVC and IVR) during the cardiac cycle. The stiffness reduction in the belly region was greater than the reduction in the annular region, which was greater than the reduction at the edge. This stiffness change supports the hypothesis that remote vagal stimulation immediately decreases the stiffness of the AML and for the first time demonstrates a functional role for the parasympathetic nerve fibers in the AML.
The heterogeneous regional response to vagal stimulation, with maximal stiffness reduction in the belly region, may be due to regional heterogeneity of vagal innervation of the leaflet or contractile cell density and/or orientation. Though histologic studies have shown a great nerve plexus density in the annular region of the leaflet, this is structural and does not necessarily correlate with function (Ellison and Hibbs, 1973; Hibbs and Ellison, 1973; De Biasi et al., 1984; Williams et al., 1990b; Kawano et al., 1993; Marron et al., 1996).
Although group leaflet stiffness reduction achieved statistical significance, leaflet stiffness in some hearts was minimally reduced or did not change, as shown in Figures 3 and 4. These leaflets did not respond to VNS, despite hemodynamic changes confirming that the vagus nerve had been stimulated. This may demonstrate that leaflet stiffness is not directly coupled to hemodynamic changes, as both the responders and non-responders had similar hemodynamic responses. Interestingly, one heart (No. 5) had no change in heart rate, but had a substantial change in leaflet stiffness. Taken together, the responders and non-responders may reflect variability in cardiac innervation, e.g. variability in the distal post-ganglionic parasympathetic fibers in terms of their input from the left vs. right vagus nerves. The non-responders may also reflect limitations in the methodology we employed for nerve stimulation in this study. The vagus nerve carries parasympathetic efferent fibers from the brain stem to ganglia in the cardiac fat pads, then post-ganglionic fibers carry parasympathetic output to the heart. Vagus nerve afferent fibers, which originate peripherally and account for 80–90% of vagus fibers, can also modulate efferent sympathetic and parasympathetic function centrally and at the level of the baroreceptors(Olshansky et al., 2008). It is very likely, then, that whole vagus nerve stimulation, as performed in this study, stimulated both efferent and afferent fibers and caused complex changes in neurotransmission to both the sympathetic and parasympathetic systems. Collecting data quickly after the initiation of VNS was intended to minimize the effects of these feedback-loops and thus isolate (as much as possible) the efferent pathway. The data presented here, however, must be interpreted in this more complex context.
Histological studies have confirmed that there are parasympathetic nerve fibers in the AML of the MV in all species studied. The predominant neurotransmitter in the parasympathetic nervous system is acetylcholine (Ach). Sonnenblick and coworkers took freshly isolated, oxygenated strips of canine MV leaflets and mounted them in a myograph(Sonnenblick et al., 1967). They found that the leaflet strips contracted rhythmically at baseline and that when they were electrically stimulated the strength of contraction could be augmented with norepinephrine and lessened with Ach, which could then be reversed with atropine. This suggests that: 1.Leaflet inotropy can be manipulated; and, 2.The results we report herein are consistent with a cholinergic effect and likely do not reflect a non-cholinergic vagus nerve response. Interestingly, ex vivo neuronal stimulation of aortic valves cusps was shown to cause cusp relaxation by a nitric oxide dependent mechanism (Chester et al., 2008). Additionally, aortic valve cusp ex vivo material properties have been shown they can be manipulated by both contractile and relaxing stimuli arising from the endothelium (El-Hamamsy et al., 2009). The results reported here may be mediated by neural-endothelial interactions, however this has yet to be demonstrated in the mitral valve.
The intra-beat change in leaflet stiffness (IVC ~1.3x stiffer than IVR) suggests that the contractile cells in the leaflet actively stiffen with each beat, which is to say the MV possesses intrinsic function. Moreover, given that the leaflet myocardium is electrically coupled to the left atrium, cardiac myocyte contraction is likely responsible for IVC stiffness. If this is true, IVR stiffness must be the result of the smooth muscle and VIC contractile states, because the cardiac muscle must be in a refractory or relaxed state at this point in the cardiac cycle.
The anterior leaflet shape did not change from CTRL to VNS in spite of the leaflet stiffness decrease associated with vagal stimulation. There are at least two possible explanations for this finding. First, besides increasing leaflet compliance, vagal stimulation also decreased left ventricular pressure and these are offsetting variables in terms of leaflet displacement. Second, we have recently shown that the distinctive shape of the anterior leaflet produces both compressive and tensile stresses that are also offsetting variables in terms of LV pressure-induced leaflet displacements (Stevanella et al., 2011).
Mitral Valve Neural Control System Hypothesis
The present study has shown that VNS can decrease the stiffness of the MV while not disrupting the leaflet’s intra-beat stiffness variation. This demonstrates that remote structures can manipulate valve function and suggests that there may be central mechanisms, the normal physiologic vagal stimulus, which play a role in MV function. The vagus nerve may be part of a control system that, together with the sympathetic chain and local feedback loops, has the ability to modify valve function. Histological evidence supports the presence of afferent fibers with specialized sensory nerve endings arising in the valve leaflets, chords and papillary muscles. These may allow for the assessment of local conditions, which are then relayed centrally, whereupon the appropriate response can be generated and transmitted to the valve leaflets, chordae and papillary muscles through the efferent limbs. Given the complexity of the vagus system, there are likely several levels at which local feedback loops could dovetail with a central control system. For example, at the level of the parasympathetic peripheral ganglia baroreceptor input, which alters post-ganglionic neurotransmission.
This is not the first time the concept of a neural control system for heart valve function has been advanced (Sonnenblick et al., 1967; Curtis and Priola, 1992; Marron et al., 1996; Williams and Jew, 2004; Borin et al., 2006; Itoh et al., 2009). However, this is the first evidence that the previous elegant morphologic studies can be combined with physiologic data measured in a beating heart in support of this hypothesis. This now provokes a further question: Are there disease states that are primarily due to a disturbance in the neural control of the valve rather than the structure of the valve or altered geometry of the MV complex?
The Mitral Valve and Autonomic Nervous System
The clinical implications of alterations in the balance of sympathetic vs. parasympathetic output have garnered broad interest. Mitral valve prolapse (MVP), a heterogeneous disease for which the etiology is poorly understood in some subgroups (“primary MVP”, “MVP syndrome” and “Idiopathic MVP”) has been found to be associated with increased parasympathetic activity(Frisinghelli et al., 1992). Oka and colleagues considered patients with anorexia nervosa, a population that is known to have elevated vagal tone based on heart rate variability testing(Oka et al., 1987). They assessed the presence of MVP and found that it was present in 19 of 23 patients (82.6%) and in 10 of the MVP patients double leaflet prolapse was present. Importantly, there were no differences in cardiac size or other echocardiographic parameters between the MVP+ and MVP− patients. The patients with double leaflet prolapse had lower heart rates then the single leaflet prolapse patients whose heart rates were lower than the MVP- patients. These results are particularly interesting in light of the findings reported here, whereby increased parasympathetic output decreases mitral leaflet stiffness.
Chronically, decreased MV stiffness could predispose to leaflet prolapse. When leaflet stiffness decreases, leaflet strain per mmHg pressure is increased, which could lead to leaflet remodeling and subsequent prolapse. This is only one potential mechanism; however, given that MVP (unrelated to chordal rupture) is not well understood in the “passive, connective tissue MV” paradigm, it may be useful to consider prolapse in the context of the newer “active, neurally controlled MV” paradigm.
Other conditions, such as heart failure, are also thought to have a neuro-humoral component(Olshansky et al., 2008). Heart failure patients have an increased sympathetic to parasympathetic ratio, which could cause altered mitral leaflet stiffness and thus strain patterns that could in turn be a causative factor behind the leaflet remodeling and regurgitation observed in some patients(Grande-Allen et al., 2005a; Grande-Allen et al., 2005b). Dal-Bianco and colleagues demonstrated that the MV actively adapts to tethering in an animal model and Chaput et al. showed in patients with dilated cardiomyopathy and MV tethering that leaflets may adapt constructively (“stretch” or “grow”) to accommodate annular dilatation and avoid the development of mitral regurgitation(Chaput et al., 2008; Dal-Bianco et al., 2009). A greater understanding of the intrinsic AML function and its perturbations may shed light on previously poorly understood clinical phenomena.
CONCLUSIONS
The AML contains contractile tissues and there is growing evidence that these tissues lend intrinsic function to the valve. An example of this intrinsic function is the dynamic change in leaflet stiffness during each cardiac cycle, wherein leaflet stiffness is increased by ~30% during isovolumic contraction and decreased during isovolumic relaxation. For the first time we demonstrate here one potential functional role for the parasympathetic nerves in the AML: to decrease leaflet stiffness. These findings support the concept of a central neural control system serving to alter valve stiffness to adapt to changing hemodynamic demands. Clinical disease states that involve altered autonomic neural function may also alter MV function that may be initially unrecognized, but may result in clinically significant valvulopathy.
Acknowledgments
We thank Koji Arata and Paul Chang for acting as perfusionists in this study. We also thank Paul Chang, Lauren R. Davis, Eleazar P. Briones, Sigurd Hartnett and Kathy Vo for technical assistance in the operating room and catheterization laboratory, Maggie Brophy and Sigurd Hartnett for careful marker digitization, and George T. Daughters for extraction of 4D data from marker coordinates. Without the initial conceptualization of how to determine MV stiffness in vivo and the tireless assistance of Dr Ingels throughout this study, this work would not have been possible.
FUNDING
This work was supported in part by National Heart, Lung, and Blood Institute Grants [RO1 HL-29589, RO1 HL-67025]; a fellowship from the Western States Affiliate of the American Heart Association [to J.C.S.]; a Stanford University Bio-X Graduate Student Fellowship [to G.K.]; a Deutsche Herzstiftung (Frankfurt, Germany) Research Grant [S/06/07 to W.B.]; and grants from U.S.-Norway Fulbright Foundation and the Swedish Heart-Lung Foundation [to JP.E.K.].
Footnotes
Conflict of Interest: none to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed A, Johansson O, Folan-Curran J. Distribution of PGP 9.5, TH, NPY, SP and CGRP immunoreactive nerves in the rat and guinea pig atrioventricular valves and chordae tendineae. J Anat. 1997;191:547–560. doi: 10.1046/j.1469-7580.1997.19140547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin C, Vanhercke D, Weyns A. Innervation of the atrioventricular and semi-lunar heart valves: a review. Acta Cardiol. 2006;61:463–469. doi: 10.2143/AC.61.4.2017309. [DOI] [PubMed] [Google Scholar]
- Boucek RJ, Bouckova B, Levy S. Anatomical arrangement of muscle tissue in the anterior mitral leaflet in man. Cardiovascular Research. 1978;12:675–680. doi: 10.1093/cvr/12.11.675. [DOI] [PubMed] [Google Scholar]
- Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation.[see comment] Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester AH, Kershaw JD, Sarathchandra P, Yacoub MH. Localisation and function of nerves in the aortic root. J Mol Cell Cardiol. 2008;44:1045–1052. doi: 10.1016/j.yjmcc.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Chester AH, Taylor PM. Molecular and functional characteristics of heart-valve interstitial cells. Philos Trans R Soc Lond B Biol Sci. 2007;362:1437–1443. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T, Napolitano LM, Fitzgerald MJ, Moore KE, Daggett WM, Willman VL, Sonnenblick EH, Hanlon CR. Structural basis of cardiac valvar function. Archives of Surgery. 1966;93:767–771. doi: 10.1001/archsurg.1966.01330050071010. [DOI] [PubMed] [Google Scholar]
- Curtis MB, Priola DV. Mechanical properties of the canine mitral valve: effects of autonomic stimulation. American Journal of Physiology. 1992;262:H56–62. doi: 10.1152/ajpheart.1992.262.1.H56. [DOI] [PubMed] [Google Scholar]
- Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, Levine RA, Carpentier A. Active Adaptation of the Tethered Mitral Valve. Insights Into a Compensatory Mechanism for Functional Mitral Regurgitation. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.846782. CIRCULATIONAHA.108.846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Vitellaro-Zuccarello L, Blum I. Histochemical and ultrastructural study on the innervation of human and porcine atrio-ventricular valves. Anat Embryol (Berl) 1984;169:159–165. doi: 10.1007/BF00303145. [DOI] [PubMed] [Google Scholar]
- Ehinger B, Falck B, Persson H, Sporrong B. Adrenergic and cholinesterase-containing neurons of the heart. Histochemie. 1968;16:197–205. doi: 10.1007/BF00307848. [DOI] [PubMed] [Google Scholar]
- El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, Yoganathan AP, Chester AH. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53:1448–1455. doi: 10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Ellison JP, Hibbs RG. The atrioventricular valves of the guinea-pig. I. A light microscopic study. American Journal of Anatomy. 1973;138:331–345. doi: 10.1002/aja.1001380304. [DOI] [PubMed] [Google Scholar]
- Fenoglio J, Jr, Tuan DP, Wit AL, Bassett AL, Wagner BM. Canine mitral complex. Ultrastructure and electromechanical properties. Circ Res. 1972;31:417–430. doi: 10.1161/01.res.31.3.417. [DOI] [PubMed] [Google Scholar]
- Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986;59:310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Frisinghelli A, Turiel M, Milletich A, Crema C, Malliani A. The role of mitral regurgitation in the neurovegetative regulation of mitral valve prolapse. Cardiologia. 1992;37:781–783. [PubMed] [Google Scholar]
- Grande-Allen KJ, Barber JE, Klatka KM, Houghtaling PL, Vesely I, Moravec CS, McCarthy PM. Mitral valve stiffening in end-stage heart failure: evidence of an organic contribution to functional mitral regurgitation. Journal of Thoracic & Cardiovascular Surgery. 2005a;130:783–790. doi: 10.1016/j.jtcvs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, Borowski AG, Troughton RW, Houghtaling PL, Dipaola NR, Moravec CS, Vesely I, Griffin BP. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005b;45:54–61. doi: 10.1016/j.jacc.2004.06.079. [DOI] [PubMed] [Google Scholar]
- Hibbs RG, Ellison JP. The atrioventricular valves of the guinea-pig. II. An ultrastructural study. Am J Anat. 1973;138:347–369. doi: 10.1002/aja.1001380305. [DOI] [PubMed] [Google Scholar]
- Itoh A, Krishnamurthy G, Swanson JC, Ennis DB, Bothe W, Kuhl E, Karlsson M, Davis LR, Miller DC, Ingels NB. Active stiffening of mitral valve leaflets in the beating heart. American Journal of Physiology. 2009;296:H1766–H1773. doi: 10.1152/ajpheart.00120.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Kawai S, Shirai T, Okada R. Morphological study on vagal innervation in human atrioventricular valves using histochemical method. Jpn Circ J. 1993;57:753–759. doi: 10.1253/jcj.57.753. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G, Itoh A, Bothe W, Swanson JC, Kuhl E, Karlsson M, Craig Miller D, Ingels NB., Jr Stress-strain behavior of mitral valve leaflets in the beating ovine heart. Journal of Biomechanics. 2009a;42:1909–1916. doi: 10.1016/j.jbiomech.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G, Itoh A, Swanson JC, Bothe W, Karlsson M, Kuhl E, Craig Miller D, Ingels NB., Jr Regional stiffening of the mitral valve anterior leaflet in the beating ovine heart. Journal of Biomechanics. 2009b;42:2697–2701. doi: 10.1016/j.jbiomech.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy G, Itoh A, Swanson JC, Miller DC, Ingels NB., Jr Transient stiffening of mitral valve leaflets in the beating heart. American Journal of Physiology - Heart & Circulatory Physiology. 298:H2221–2225. doi: 10.1152/ajpheart.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron K, Yacoub MH, Polak JM, Sheppard MN, Fagan D, Whitehead BF, de Leval MR, Anderson RH, Wharton J. Innervation of human atrioventricular and arterial valves. Circulation. 1996;94:368–375. doi: 10.1161/01.cir.94.3.368. [DOI] [PubMed] [Google Scholar]
- Montiel MM. Muscular apparatus of the mitral valve in man and its involvement in left-sided cardiac hypertrophy. Am J Cardiol. 1970;26:341–344. doi: 10.1016/0002-9149(70)90727-7. [DOI] [PubMed] [Google Scholar]
- Oka Y, Ito T, Matsumoto S, Suematsu H, Ogata E. Mitral valve prolapse in patients with anorexia nervosa. Two-dimensional echocardiographic study. Japanese Heart Journal. 1987;28:873–882. doi: 10.1536/ihj.28.873. [DOI] [PubMed] [Google Scholar]
- Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- Sonnenblick EH, Napolitano LM, Daggett WM, Cooper T. An intrinsic neuromuscular basis for mitral valve motion in the dog. Circulation Research. 1967;21:9–15. doi: 10.1161/01.res.21.1.9. [DOI] [PubMed] [Google Scholar]
- Stevanella M, Krishnamurthy G, Votta E, Swanson JC, Redaelli A, Ingels NB., Jr Mitral leaflet modeling: Importance of in vivo shape and material properties. J Biomech. 2011;44:2229–2235. doi: 10.1016/j.jbiomech.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Swanson JC, Krishnamurthy G, Itoh A, Kvitting J-PE, Bothe W, Craig Miller D, Ingels NB., Jr Multiple mitral leaflet contractile systems in the beating heart. Journal of Biomechanics. 44:1328–1333. doi: 10.1016/j.jbiomech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JC, Krishnamurthy G, Kvitting J-PE, Miller DC, Ingels NB., Jr Electromechanical coupling between the atria and mitral valve. American Journal of Physiology - Heart & Circulatory Physiology. 300:H1267–1273. doi: 10.1152/ajpheart.00971.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TH, Folan JC, Jew JY, Wang YF. Variations in atrioventricular valve innervation in four species of mammals. American Journal of Anatomy. 1990a;187:193–200. doi: 10.1002/aja.1001870208. [DOI] [PubMed] [Google Scholar]
- Williams TH, Folan JC, Jew JY, Wang YF. Variations in atrioventricular valve innervation in four species of mammals. Am J Anat. 1990b;187:193–200. doi: 10.1002/aja.1001870208. [DOI] [PubMed] [Google Scholar]
- Williams TH, Jew JY. Is the mitral valve passive flap theory overstated? An active valve is hypothesized. Med Hypotheses. 2004;62:605–611. doi: 10.1016/j.mehy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Wit AL, Fenoglio JJ, Jr, Hordof AJ, Reemtsma K. Ultrastructure and transmembrane potentials of cardiac muscle in the human anterior mitral valve leaflet. Circulation. 1979;59:1284–1292. doi: 10.1161/01.cir.59.6.1284. [DOI] [PubMed] [Google Scholar]
- Wit AL, Fenoglio JJ, Jr, Wagner BM, Bassett AL. Electrophysiological properties of cardiac muscle in the anterior mitral valve leaflet and the adjacent atrium in the dog. Possible implications for the genesis of atrial dysrhythmias. Circulation Research. 1973;32:731–745. doi: 10.1161/01.res.32.6.731. [DOI] [PubMed] [Google Scholar]