Abstract
The selective serotonin reuptake inhibitor fluvoxamine reduces responding for ethanol at lower doses than responding for food when each is available in separate components or separate groups of rats. However, when both are available concurrently and deliveries earned per session are equal, this apparent selectivity inverts and food-maintained behavior is more sensitive than ethanol-maintained behavior to rate-decreasing effects of fluvoxamine. Here, we investigate further the impact concurrent access to both food and ethanol has on the potency of fluvoxamine. Fluvoxamine (5.6-17.8 mg/kg) potency was assessed under conditions where food and ethanol were available concurrently and response rates were equal (average variable intervals (VI) 405-s and 14-s for food and ethanol, respectively), as well as when density of food delivery was increased (average VI 60-s food & VI 14-s ethanol). The potency of fluvoxamine was also determined when only ethanol was available (food extinction and average VI 14-s ethanol) and under a multiple VI (VI 30-s food and ethanol) where either food or ethanol was the only programmed reinforcer available during each component. Fluvoxamine was less potent at decreasing ethanol self-administration when food was available concurrently (ED50 [95% C.L.]: 8.2 [6.5-10.3] & 10.7 [7.9-14.4]) versus when ethanol was available in isolation (ED50: 4.0 [2.7-5.9] & 5.1 [4.3-6.0]). Effects on food were similar under each condition where food was available. The results demonstrate that the potency of fluvoxamine to reduce ethanol-maintained behavior depends on whether ethanol is available in isolation or in the context of concurrently scheduled food reinforcement.
Keywords: alcoholism, SSRI, concurrent, reinforcement, selective, pharmacotherapy, Fluvoxamine, ethanol, food, reinforcement schedule, rat
Introduction
Drugs that reduce behavior maintained by ethanol more than behavior maintained by an alternative are considered selective. Such selective drug effects are thought to be an important characteristic of putative pharmacotherapies for alcoholism and could also provide insight into the neurochemical mechanisms of alcohol reinforcement. Typically, these studies are performed in separate groups of animals responding for either ethanol or some other alternative, such as food. For example, the selective serotonin reuptake inhibitor fluvoxamine selectively reduces ethanol-maintained behavior compared with food-maintained behavior when food and ethanol are tested in separate groups of rats (Lamb and Järbe, 2001). In order to examine whether unmatched ethanol histories or baseline response rates were responsible for this selective effect, (Ginsburg et al., 2005) established a multiple schedule of food- and ethanol-maintained behavior. In the multiple schedule, food and ethanol were made available to each rat in distinct components during the same daily session. Again, fluvoxamine selectively reduced responding maintained by ethanol over responding maintained by food. The authors concluded the selective effect of fluvoxamine on ethanol-maintained behavior was therefore not due to different ethanol histories or different baseline response rates in the between-subjects procedure. Further, the selective effect of fluvoxamine was not due to an interaction between fluvoxamine and ethanol, as the procedure allowed for fluvoxamine effects on food-maintained behavior to be evaluated both before and after ethanol self-administration (Ginsburg et al., 2005). Taken together, these results suggest that fluvoxamine might be an effective pharmacotherapy for alcoholism. Indeed, early clinical reports were consistent with this possibility (Balldin et al., 1994; Angelone et al., 1998).
However, even as positive preclinical results were being published, new evidence was accumulating indicating that the clinical benefits of serotonin reuptake inhibitors in alcoholism were limited (Pettinati, 2001). Thus, selective drug effects in common animal models may not predict therapeutic benefit. This may be due to the exclusion of certain critical processes in the animal model that are important to human behavior. For example, in common animal models, multiple concurrently scheduled events are rarely provided; rather, drug (or an alternative) is only available in isolation. Yet some have suggested that alcoholism and other drug addictions are due to disordered choice behavior, where seeking and consumption of drugs occupy increasing amounts of time to the exclusion of other more adaptive behavior in an environment where multiple reinforcers are concurrently scheduled (Vuchinich and Tucker, 1988; Kalivas and Volkow, 2005). This can only occur in an environment in which multiple reinforcing events are concurrently scheduled. It is possible that by integrating concurrent reinforcement into the preclinical model, results might be more predictive of the outcome in humans. This concept has been integrated into studies related to treatment of cocaine and opioid addiction (e.g. Negus, 2003, 2006).
In an effort to extend this approach to the preclinical study of alcohol use and abuse, Ginsburg and Lamb (2006) developed a procedure in which subjects could earn ethanol or food while both were available concurrently under fixed-ratio schedules. Conditions were adjusted so that rats earned equal numbers of food and ethanol deliveries throughout the 30-min session by increasing the response requirement for food relative to ethanol. Under these conditions, fluvoxamine selectively decreased food-maintained behavior, compared to ethanol-maintained responding (Ginsburg and Lamb, 2006). The behavioral mechanisms responsible for the inversion of fluvoxamine selectivity under concurrent versus multiple or separate schedules remain unknown.
One possible explanation for this inversion may relate to differences in the density of the reinforcement between the multiple and concurrent schedule conditions. The ability of some events, such as pre-feeding or extinction, to disrupt behavior has been related to the reinforcement density that maintains the behavior (Nevin and Grace, 2000). Greater reinforcement density results in greater resistance to disruption by pre-feeding or extinction, and these results have been extended to the use of drugs as disruptors with mixed results (Cohen, 1986; Harper, 1999). Indeed, work from our own laboratory has not demonstrated compelling evidence that the disruptive effects of fluvoxamine depend on reinforcement density (Lamb and Ginsburg, 2005, 2008; Ginsburg and Lamb, 2008). However, these results have only been compared in studies in which behavior was maintained by differing densities of food. Here, we extend these results to studies in which differing densities of food are concurrently scheduled with ethanol.
Thus, the present experiment was conceived to examine the interplay between ethanol- and food-maintained behavior as density of food reinforcement was varied (or eliminated) during concurrent access to both. In the previous study, rats earned equal numbers of food and ethanol deliveries under a concurrent fixed-ratio (FR) schedule. Under these conditions, the rate of responding for food was substantially higher than the rate of responding for ethanol, which could explain the inversion of fluvoxamine selectivity, as high rates of responding are often reduced more by drug administration (Kelleher and Morse, 1968). Using variable interval (VI) schedules allowed us to better equate rates between conditions. If the Matching Law holds under this condition in which two different commodities are concurrently scheduled under VI schedules (see Anderson et al., 2002), then the value or density of food and ethanol reinforcement should be equated under a schedule that maintains equal response rates (Herrnstein, 1970). Additionally, variable interval schedules are more often used in behavioral studies of resistance to change (e.g., Nevin and Grace, 2000), allowing us to better interpret our results within that conceptual model. Finally, because our original multiple-schedule work was done with fixed-ratio schedules, we systematically replicated that study using multiple schedules to provide a multiple-schedule comparison for our concurrent study.
Methods
Subjects
Concurrent VI experiments were conducted with eight male Lewis rats (Harlan, Inc, Indianapolis, IN). Each weighed 250g upon arrival and was housed singly with free access to food and water for two weeks or until their weight was over 320g, whichever was longer. Multiple VI experiments were conducted with a separate group of five male Lewis rats (Harlan, Inc, Indianapolis, IN). For both groups, food was restricted to 12-15g/day in order to maintain body weights of approximately 330g. Water was available continuously in the home cage. Lights in the colony room where the rats were housed operated on a 14:10 light:dark cycle; sessions were conducted during the light phase. Experimental procedures were approved by the Institutional Animal Use and Care Committee of the University of Texas Health Science Center at San Antonio.
Apparatus
Experiments were conducted using a commercially available apparatus (Standard Rat Chamber, Med Associates, St. Albans, VT, USA). On one wall of the chamber, two response levers were arranged horizontally, one on each side of the wall. Above each lever was a stimulus lamp covered by a translucent white cap. Equidistant between the levers was a receptacle that provided access to 45 mg pellets (Bio-Serve, Frenchtown, NJ) via a pellet dispenser and to solutions via a 0.1-ml dipper. A clear lamp was mounted on the wall opposite the levers near the ceiling and served as a houselight. White noise was present in the room where experiments were conducted to mask extraneous sounds.
Concurrent VI
Rats were trained to respond on a lever for a sucrose solution (8% w/v) when the stimulus light above it was illuminated during 30-min sessions. Over several days, the FR value was gradually increased from one to five. Then, ethanol was faded into the solution over the next several weeks until the solution consisted of 8% sucrose, 8% (w/v) ethanol. Finally, sucrose was slowly faded out of the solution so that the rats were responding for an 8% (w/v) solution of ethanol under a FR5 schedule. At this point, a second 30-min session was introduced immediately after the ethanol self-administration session in which the stimulus light above the second lever was illuminated and presses on that lever produced food. Rats were required to respond once for two food pellets, then the response requirement was increased over the next few days to FR5. Once rats responded under FR5 for ethanol and food in separate sessions, rats were exposed to a single 30-min session each day in which the lights above both levers were illuminated. By the end of training, responses on the food-associated lever were reinforced under a FR25 schedule, while responses for ethanol were reinforced under a FR5 schedule. Delivery of either food or ethanol was accompanied by turning off both lever lights and turning on a houselight above the chamber. Completion of a fixed ratio did not reset the ratio on the second lever, and no changeover delay was imposed.
Once responding stabilized under the concurrent FR schedule, contingencies were changed such that rats responded under a concurrent variable-interval (VI) schedule for food and ethanol. A 3-s changeover delay was imposed if subjects responded on the alternative lever after the VI had expired. Initially, all rats responded under a concurrent VI 30-s for ethanol - VI90-s for food. The VI for food was then adjusted by increasing the average interval for food and decreasing the average interval for ethanol for each subject until the number of responses per session for food and ethanol did not differ significantly (p>0.05) over five consecutive days. The final VI values are shown in Table 1 (see “Equal-rate” conditions).
Table 1.
Control response rates, number of reinforcer deliveries earned, and VIs in concurrent procedure
| Subject | Ethanol | Food | Condition | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Response rate (resp/min) | Deliveries earned | VI (sec) | Response rate (resp/min) | Deliveries earned | VI (sec) | ||
| 1094 | 4.3 | 54 | 7 | 4.0 | 3 | 600 | Equal rates |
| 2.9 | 42 | 7 | 1.3 | 0 | ~ | Extinguished food | |
| 2.5 | 41 | 7 | 5.1 | 20 | 60 | Enriched food | |
| 1095 | 6.5 | 50 | 15 | 5.7 | 6 | 420 | Equal rates |
| 4.0 | 39 | 15 | 0.1 | 0 | ~ | Extinguished food | |
| 4.9 | 43 | 15 | 9.5 | 23 | 60 | Enriched food | |
| 1096 | 3.7 | 39 | 15 | 3.8 | 6 | 300 | Equal rates |
| 3.3 | 33 | 15 | 0.1 | 0 | ~ | Extinguished food | |
| 3.0 | 34 | 15 | 3.8 | 20 | 60 | Enriched food | |
| 1097 | 5.7 | 64 | 7 | 5.6 | 5 | 540 | Equal rates |
| 4.0 | 42 | 7 | 0.3 | 0 | ~ | Extinguished food | |
| 5.0 | 55 | 7 | 8.1 | 22 | 60 | Enriched food | |
| 1098 | 3.7 | 41 | 15 | 4.1 | 8 | 180 | Equal rates |
| 4.9 | 33 | 15 | 0.4 | 0 | ~ | Extinguished food | |
| 3.4 | 36 | 15 | 9.0 | 34 | 30 | Enriched food | |
| 1099 | 5.5 | 34 | 30 | 5.2 | 8 | 240 | Equal rates |
| 2.9 | 23 | 30 | 0.2 | 0 | ~ | Extinguished food | |
| 4.4 | 31 | 30 | 7.7 | 22 | 60 | Enriched food | |
| 1102 | 3.0 | 43 | 7 | 4.5 | 15 | 540 | Equal rates |
| 1.7 | 25 | 7 | 0.6 | 0 | ~ | Extinguished food | |
| 3.7 | 44 | 7 | 6.5 | 24 | 60 | Enriched food | |
| 1104 | 4.5 | 44 | 15 | 4.4 | 5 | 420 | Equal rates |
| 4.9 | 36 | 15 | 0.7 | 0 | ~ | Extinguished food | |
| 3.7 | 41 | 15 | 8.0 | 20 | 60 | Enriched food | |
|
| |||||||
| Group: | Mean (SD) | ||||||
| Equal rates | 4.6 (1.2) | 46 | 14 | 4.7 (0.7) | 7 | 405 | |
| Extinguished food | 3.6 (1.1) | 34 | 14 | 0.5 (0.4) | 0 | ~ | |
| Enriched food | 3.8 (0.9) | 41 | 14 | 7.2 (2.0) | 23 | 56 | |
Fluvoxamine dose-effects were determined, then the VI programmed for food deliveries was adjusted. In half of the rats [1095, 1098, 1102, 1104], the VI was lowered to 30-s or 60-s (effectively increasing the density of reinforcement). In the other four rats [1094, 1096, 1097, 1099], responses on the food-associated lever had no programmed consequence, i.e. extinction. Under the extinction condition, both stimulus lights above the levers were illuminated, but only responses on the ethanol-associated lever were reinforced. Following determination of fluvoxamine dose-effect curves, food VIs were changed to the other (enriched or extinguished) condition. As shown in Table 1, the VI values for ethanol were not altered throughout the experiment.
Multiple VI
As this group of rats had prior experience with ethanol and food contingencies, no training was needed. Eat rat was placed on a three-component schedule. The first and last components arranged a VI 30-s schedule of food delivery (named Food 1 and Food 2, respectively); the middle component arranged an identical, but independent, VI 30-s schedule of ethanol delivery (named Ethanol). Each component was signaled by illumination of the house light and the light above the active lever and lasted for 5 min, excluding the duration of the post-reinforcement timeout of 10-s. Depending on the component, the first lever press to occur after the interval timer expired resulted in delivery of either two food pellets or 8% (w/v) ethanol. Rats were allowed 10 seconds to consume either food or ethanol, during which time the house light flashed at 0.5-s intervals, before the schedule resumed. Consumption time did not count toward the 5 min component duration. Any time remaining on the interval timer at the end of Food 1 was used at the start of the Food 2 component later in the session. When each component ended, there was a 30-s inter-component interval where all stimulus lights were extinguished and there were no programmed consequences for lever presses. Ethanol delivery was contingent on responses on the left lever for rats #2 and #5 and on the right lever for rats #3, #6, and #7. Food delivery was contingent on responses on the other lever.
Interval Values
Interval values were randomly selected from a list generated using the algorithm of Fleshler and Hoffman with 30 or 20 elements for the concurrent or multiple procedures, respectively (Fleshler and Hoffman, 1962)
Drug
Fluvoxamine maleate (Solvay Inc., Weesp, The Netherlands) was dissolved in physiological saline to an injection volume of 1 ml / kg body weight. Drug or vehicle injections (i.p.) occurred 30 min prior to daily sessions on Tuesdays and Fridays of each week. For multiple VI studies, two determinations of each dose and vehicle were made. Initial tests were conducted in a mixed order determined individually for each rat; the order was reversed for the second determination. Vehicle was administered each Thursday and results served as control values.
Statistical analysis
Response rates were calculated by dividing the number of responses by the number of seconds during which ethanol and/or food was available during the session or component (excluding periods where reinforcement was unavailable following delivery of ethanol or food). Doses were converted to Log(dose) and response rates for each subject under each condition were normalized and expressed as a percent of Thursday control response rate. ED75 and ED50 values were then derived for fluvoxamine effects under each condition, using the method described by (Tallarida and Murray, 1981). Briefly, a linear regression was performed relating dose and group effect. The dose that resulted in 50% or 75% reduction in responding was calculated from the resulting regression equation. The sum of squares differences from the mean effect for each dose included in the calculation was determined. This sum was converted into a standard error by dividing by the total number of observations minus 2 then taking the square root. Additionally, the sum of squares differences from the mean dose included in the calculation was determined. A standard error estimate of dose was similarly calculated and the product of the error estimates for dose and effect represents the upper and lower 95% confidence limits of the derived ED50 or ED75. ED75 was calculated because no dose of fluvoxamine tested resulted in less than 50% reduction for ethanol responding in the concurrent VI group under the extinguished food condition. Likewise, ED50 was calculated because no dose of fluvoxamine resulted in a 75% or greater reduction of responding for ethanol in the concurrent group under the equal-rate condition. For these analyses, only doses that resulted in group effects that bracketed 25% or 50% (for ED75 and ED50, respectively) were included in the analysis. There were two exceptions to this procedure, both in the concurrent group. Under the equal-rate condition, 17.8 mg/kg fluvoxamine resulted in 37% control responding for the group, and the ED75 was extrapolated from the regression through all three doses (5.6, 10, and 17.8 mg/kg). Similarly, under the extinguished food condition, 5.6 mg/kg fluvoxamine resulted in 40% of control responding for the group, so the ED50 was extrapolated from all three doses.
For concurrent VI studies, comparisons among ED50 or ED75 values on responding for either reinforcer were made against values and confidence limits of the equal-rate condition. ED50 or ED75 values for other conditions that fell outside of the confidence limits for the equal-rate condition are considered significant at α=0.05. For Multiple VI studies, comparisons between effects on responding for ethanol versus food in each component were made using confidence limits derived for responding for ethanol. Comparison of fluvoxamine effects on responding for food between the two food components were made against the confidence limits derived for the first food component. Equal-rate condition response rates were compared using paired Student’s t-tests with p<0.05 considered significant.
Results
Control response rates
In the concurrent VI group, control rates of responding for each subject under each condition are shown in Table 1. Compared with response rate under the equal-rate condition, response rates for ethanol (4.6 resp/min) were significantly lower under the extinguished (3.5 resp/min), but not the enriched (3.8 resp/min) food condition, though the latter comparison fell just short of our significance criteria (paired Student’s t-test: t=2.9 & 2.2, p<0.02 & p<0.06, respectively). Thus, removing concurrent food availability led to a reduction in the response rate for ethanol. Response rates for food were significantly higher under the enriched-food condition (7.2 resp/min) and significantly lower under the extinguished-food (0.5 resp/min) condition, compared with the equal-rate condition (4.7 resp/min; t= -4.7 & 11.9, p<0.005, respectively). Response rates for ethanol and food were not significantly different under the equal-rate condition, (t=-0.26), but were significantly greater for food under the enriched-food condition (t= -6.6, p<0.005) and significantly greater for ethanol than for food under the extinguished-food condition (t=7.2, p<0.005). In the multiple VI group, response rates for each component are shown in Table 2. Rates for ethanol (8.5 resp/min) were significantly lower than for food (20.1 and 21.8 resp/min) in either component (p<0.01). Rates in the two food components did not differ.
Table 2.
Response rates (resp/min) for subjects in Multiple VI procedure
| Food 1 | Ethanol | Food 2 | |
|---|---|---|---|
| RE2 | 18.15 | 10.15 | 19.60 |
| RE3 | 24.47 | 7.20 | 23.80 |
| RE5 | 16.95 | 13.00 | 22.25 |
| RE6 | 17.25 | 4.90 | 20.90 |
| RE7 | 23.73 | 7.00 | 22.20 |
|
| |||
| Mean (SD) | 20.11 (3.69) | 8.45 (3.16) | 21.75 (1.58) |
Fluvoxamine effects
Effects of fluvoxamine on responding for ethanol or food under each condition are shown in Fig. 1. Fluvoxamine administration produced dose-dependent reductions in response rate for food and ethanol under all conditions.
Fig. 1.
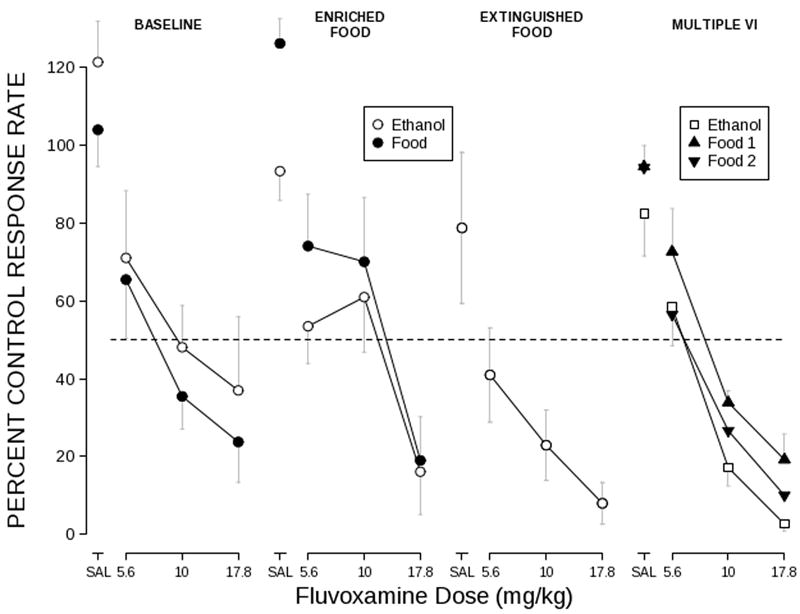
Effects of fluvoxamine on responding maintained by food and ethanol under concurrent VI conditions (equal rate, enriched food, and extinguished food) and multiple VI conditions. Response rate is expressed as a percentage of control rates determined on Thursdays. Saline and fluvoxamine were administered on Tuesdays and Fridays. Points represent the mean ± S.E.M. for n=8 (concurrent VI) or n=5 (multiple VI) rats. The dashed line represents 50% of control responding.
Selectivity
Fluvoxamine exerted a selective effect (i.e. fluvoxamine potency was greater) on responding for ethanol compared with food when ethanol was available alone, and a non-selective effect when ethanol was concurrently scheduled with food. This can be seen by comparing food and ethanol dose-effect curves across panels in Fig. 1. When both ethanol and food were available concurrently and response rates were equated (panel 1), the ED75 and ED50 values for food fell within the 95% confidence limits for effects on responding for ethanol, as shown in Table 3. The same held true for ED75 values when the density of food reinforcement was increased (Ethanol ED75 [95% confidence limits]: 17.7 [12.5 – 25.1]; Food ED50: 18.5), but not for ED50 values (Ethanol ED50 [95% confidence limits]: 8.2 [6.4-10.4]; Food ED50: 11.0), where fluvoxamine effects were more potent on responding for ethanol compared with responding for food (panel 2). In contrast, under extinguished-food conditions, the increase in fluvoxamine potency to disrupt responding for ethanol led to an apparent selective effect on responding for ethanol compared with fluvoxamine effects on responding for food under the equal-rate condition (Table 3: extinguished ethanol versus equal-rates food and panel 3). However, this comparison is somewhat limited as effects on responding for food alone were not assessed in these animals.
Table 3.
ED75 and ED50 for fluvoxamine effects on responding for ethanol and food
| ED50 | ||||||
|---|---|---|---|---|---|---|
| Ethanol | Food | |||||
|
|
||||||
| Concurent | Mean | 95% C.L. | Mean | 95% C.L. | ||
| Equal Rates | 10.7 | [7.9 – 14.4] | 7.9 | [6.5 – 9.6] | ||
| Enriched | 8.2 | 11.0 | ||||
| Extinguished | 4.0 | |||||
| Multiple | ||||||
| Ethanol component | 5.1 | [4.3 – 6.0] | Food 1 | 7.2 | [6.1 – 8.5] | |
| Food 2 | 6.0 | |||||
| ED75 | ||||||
| Ethanol | Food | |||||
|
|
||||||
| Concurent | Mean | 95% C.L. | Mean | 95% C.L. | ||
| Equal Rates | 25.0 | [13.1 – 47.7] | 15.8 | [12.4 – 20.2] | ||
| Enriched | 17.7 | 18.5 | ||||
| Extinguished | 9.6 | |||||
| Multiple | ||||||
| Ethanol component | 9.8 | [8.4 – 11.4] | Food 1 | 15.5 | [12.1 – 19.7] | |
| Food 2 | 11.6 | |||||
In the multiple VI schedule, fluvoxamine did exert a consistent, though small, selective effect on responding for ethanol, consistent with previous studies, and with the effects under the extinguished-food condition in the concurrent VI schedule. As shown in panel 4 of Fig. 1, in the multiple schedule, the fluvoxamine curve for ethanol responding falls to the left of the curves for the two food components (with Food 1 to the right of Food 2). This is borne out by ED75 comparisons between ethanol and each food component, where the ED75 for the ethanol component is significantly lower than the ED75 in either food component. Comparisons between ED50 values show the same pattern, though the ED50 for fluvoxamine during Food 2 is the upper confidence limit for effects on responding for ethanol. However, paired t-tests comparing effects of 10 or 17.8 mg/kg fluvoxamine on responding during the ethanol component versus either of the two food components were not significant. The apparent changes in selectivity depending on the concurrent availability of food led us to examine whether selectivity was altered due to changes in potency on ethanol-maintained responding, food-maintained responding, or both.
Fluvoxamine effects on ethanol self-administration
Generally, when food was concurrently scheduled, responding for ethanol was less sensitive to fluvoxamine, especially the 10 mg/kg dose, than when ethanol was available in isolation. This can be seen in Fig. 1 by comparing fluvoxamine effects on responding for ethanol (open symbols) in the left two panels (where two of the three doses tested were at or above 50% of control rate) versus the right two panels (where all doses tested except for 5.6 mg/kg resulted in greater than 50% reduction in response rate). As shown in Table 3 (Ethanol column), both the ED75 and the ED50 were significantly lower under the extinguished food condition and during the ethanol component of the multiple VI compared to the equal rate or enriched food conditions under the concurrent VI. Increasing the density of food reinforcement in the concurrent VI schedule did not further reduce fluvoxamine potency; ED75 and ED50 values for ethanol self-administration did not differ between equal-rate and enriched-food conditions.
Fluvoxamine effects on food-maintained behavior
Generally, increasing the density of food reinforcement did not alter fluvoxamine potency with one exception. ED50 values for food-maintained behavior differed under the equal-rate and enriched conditions. However, the same relationship was not present for ED75 measures. This can be seen in Table 3 (Food column) by comparing fluvoxamine potency under the equal-rate condition (VI ~400-sec), enriched condition (VI ~56-sec), and the Food 1 component of the multiple VI (VI 30-sec with no concurrent reinforcement). While each of these conditions results in substantially different reinforcement densities, ED75 values do not differ, nor (with one exception) do ED50 values differ. Under the multiple schedule, the potency of fluvoxamine during the Food 2 component was lower than during the Food 1 component, though the density of reinforcement was the same during both components. Interpretation of this difference is further complicated by previous results where fluvoxamine effects were consistent across both food components in a similar procedure using fixed-ratio schedules (Ginsburg et al., 2005). Taken together these results show that food reinforcement density does not consistently alter fluvoxamine potency to disrupt responding for food.
Discussion
This study provides evidence that concurrent availability of food increases the resistance of ethanol-maintained responding to disruption by the selective serotonin reuptake inhibitor fluvoxamine. Extinguishing concurrent food-maintained behavior increased the potency of fluvoxamine to reduce responding for ethanol. Results from a multiple schedule study where food and ethanol were available successively (not concurrently) were consistent with this finding. However, the converse relationship does not appear to be true: concurrent ethanol availability does not alter the potency of fluvoxamine to decrease responding for food. These results replicate and extend previous studies where the apparent selectivity of fluvoxamine to reduce ethanol-maintained relative to food-maintained responding depends on whether each is available alone or concurrently (Lamb and Järbe, 2001; Ginsburg et al., 2005; Ginsburg and Lamb, 2006), and indicate that ethanol self-administration can be more susceptible to disruption in laboratory studies when ethanol is the only explicit event maintaining behavior.
More generally, other research shows the importance of reinforcement context in determining the potency of drugs to reduce ethanol-maintained behavior. Samson and Grant (1985) found that chlordiazepoxide was more potent in reducing responding for ethanol when ethanol was available concurrently with water than with sucrose. Interestingly, water maintained little behavior in that study, suggesting it may not have functioned as a reinforcer, while sucrose maintained a high rate of responding, suggesting it did function as a reinforcer. If our interpretation is correct, then ethanol was essentially available in isolation when offered with water, but in the context of other reinforcement when offered with sucrose; and, these results with chlordiazepoxide mirror those we found with fluvoxamine.
In attempting to identify what might explain the present effects, several possible explanations are considered. These include unlikely explanations related to differences in rates of responding or rates of reinforcement (i.e. behavioral momentum). More likely explanations include post-prandial polydipsia, schedule-induced polydipsia, and adventitious reinforcement.
Changes in the relative potency of fluvoxamine are unlikely to be due to differences in the control response rate across various studies. In an earlier series of studies, we found that fluvoxamine potency is influenced only modestly by control response rate (Lamb and McMillan, 1986; Ginsburg and Lamb, 2008; Lamb and Ginsburg, 2008). In the present study, response rates for food varied among the different test conditions (Tables 1 and 2). Even so, the potency of fluvoxamine to reduce responding for food was generally not different. In contrast, response rates for ethanol were similar across different concurrent VI conditions, yet the potency of fluvoxamine to disrupt responding for ethanol was significantly greater under the extinguished-food condition, suggesting that observed potency differences are unlikely to be due to rate differences.
Another possible explanation for the present results is increased resistance to change owing to overall increases in reinforcement density in situations where both food and ethanol are available. Such results appear in line with behavioral momentum theory, that disruption of ongoing behavior is negatively related to the density of reinforcement (Nevin and Grace, 2000). For example, responding for ethanol is more resistant to disruption by extinction when response-independent food was concurrently delivered than in a component where ethanol alone is available (Shahan and Burke, 2004). However, the extent to which disruption of responding by drug treatment conforms to behavioral momentum theory remains uncertain, as results have been mixed (Cohen, 1986; Harper, 1999; Jimenez-Gomez and Shahan, 2007; Pinkston et al., 2009). In particular, fluvoxamine effects on overall responding under fixed-interval or fixed-ratio schedules do not appear to depend on reinforcement density (Lamb and Ginsburg, 2005, 2008; Ginsburg and Lamb, 2008). Further, it should be noted that behavioral momentum has most commonly been studied using multiple schedules in which reinforcement density is varied across components during a single session (Cohen, 1998). Changes in reinforcement density across separate sessions over days or weeks do not generally yield similar results.
It is possible that the concurrent presence of food decreased the potency of fluvoxamine to disrupt responding for ethanol due to post-prandial polydispsia. Simply stated, rats tend to drink after eating. Increased tendency to drink in the presence of concurrently scheduled food could have decreased the potency of fluvoxamine to disrupt responding for ethanol. If this occurred, the potency of fluvoxamine should increase when food was removed (as did happen). It also follows that the potency of fluvoxamine should further decrease when more food was available, but this did not happen. Clearly the rats were eating substantially more food under the “enriched” condition, and thus presumably engendered even more feeding-related thirst, but ethanol-maintained behavior was not more resistant to fluvoxamine under those conditions (potency did not change). This suggests that post-prandial polydipsia does not explain observed changes in fluvoxamine potency.
Another possible explanation for the apparent selectivity of fluvoxamine on ethanol-maintained behavior in the presence or absence of concurrently scheduled food is schedule-induced polydipsia. This is a well-known phenomenon where periodic presentations of small portions of food can induce overdrinking (for a review, see Falk and Tang, 1988). Schedule-induced polydipsia is seen when rats respond for food pellets under variable- or fixed-interval schedules in which the interpellet interval is 45-s or more (Falk, 1967). Schedule-induced polydipsia is not a result of restricted food access per se. Rats maintained at 80% of their free-feeding body weight with food available under a CRF schedule did not develop schedule-induced polydipsia, but when food was availability under a variable-interval schedule, schedule-induced polydipsia developed (Falk, 1966). In the present study, ethanol-maintained behavior was increased when food was concurrently scheduled under VI schedules. Certainly the present results cannot exclude a role for polydipsia; it is possible some of the behavior maintained by ethanol was due to schedule-induced drinking, and so it remains an important consideration in interpreting these findings.
Finally, it is possible that responses on the ethanol-associated lever were strengthened by delivery of food after a chain of responses that included responses on the ethanol-associated lever. In an earlier study, fluvoxamine effects on responding for ethanol relative to food were diminished under a concurrent fixed-ratio procedure when compared with a multiple fixed-ratio procedure (Ginsburg et al., 2005; Ginsburg and Lamb, 2006). No changeover delay was imposed under the concurrent fixed-ratio schedule, which likely increases the chances of adventitious strengthening of ethanol responding, perhaps leading to the observed change in relative fluvoxamine potency. In the present study, there was a changeover delay, which should decrease adventitious strengthening of ethanol responding (Shull and Pliskoff, 1967). Still, the changeover delay in this study was modest, and it remains likely that responding for ethanol was strengthened by proximal food delivery. Indeed, the separation between relative potencies of fluvoxamine disruption of responding for ethanol and food was greater in the previous study, which had no changeover delay, compared with the present study, which imposed a changeover delay. This is consistent with the notion that adventitious strengthening of responding for ethanol decreased the potency of fluvoxamine. The extent to which this influenced the potency of fluvoxamine in the present study remains unclear. However, simply increasing the magnitude of reinforcement (and presumably increasing response strength) does not have profound effects on fluvoxamine potency to disrupt overall responding (Lamb and Ginsburg, 2005, 2008; Ginsburg and Lamb, 2008).
An important implication of our results is that drugs that appear to selectively decrease responding for ethanol may lose that selectivity when ethanol is available concurrently with food. The list of drugs that appear to selectively reduce ethanol self-administration in separate groups is long, yet few if any of these candidates have shown substantial promise as pharmacotherapies for alcoholism. Alcoholism develops and persists in an environment where many different potential reinforcing events are available in addition to ethanol. Better homology between animal models and human conditions that promote or maintain alcoholism could help refine these models so that they better predict beneficial treatments. Thus, providing animals with a concurrently contingent reinforcer in addition to ethanol provides a more homologous condition to the human situation.
The most important result of the present studies is that presence or absence of another concurrently contingent reinforcer can influence the potency with which fluvoxamine reduces ethanol self-administration. Increasing the density of food delivery did not further reduce fluvoxamine potency; however, this could be due to a ceiling effect where fluvoxamine potency was already diminished as much as possible by the lowest density of food provided. In general, our findings emphasize the importance of examining potential therapeutics in multi-reinforcement settings and suggest such studies will be important in evaluating the validity of current and future pharmacotherapies. Concurrent access to ethanol and other sources of reinforcement is more homologous with the human situation and could improve the specificity of ethanol self-administration procedures at identifying potential pharmacotherapies.
Acknowledgments
The authors would like to thank Gerardo Martinez for technical assistance and PHS for funding through grants AA015993 (B.C.G) and AA012337 (R.J.L.)
Footnotes
Disclosure: Drs. Ginsburg, Pinkston, and Lamb have no conflicts to report.
References
- Anderson KG, Velkey AJ, Woolverton WL. The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2002;163:319–326. doi: 10.1007/s00213-002-1012-7. [DOI] [PubMed] [Google Scholar]
- Angelone SM, Bellini L, Di Bella D, Catalano M. Effects of fluvoxamine and citalopram in maintaining abstinence in a sample of Italian detoxified alcoholics. Alcohol and Alcoholism. 1998;33:151–156. doi: 10.1093/oxfordjournals.alcalc.a008371. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berggren U, Engel J, Eriksson M, Hård E, Söderpalm B. Effect of citalopram on alcohol intake in heavy drinkers. Alcohol Clin Exp Res. 1994;18:1133–6. doi: 10.1111/j.1530-0277.1994.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Cohen SL. A pharmacological examination of the resistance-to-change hypothesis of response strength. Journal of the Experimental Analysis of Behavior. 1986;46:363–379. doi: 10.1901/jeab.1986.46-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL. Behavioral momentum: the effects of the temporal separation of rates of reinforcement. J Exp Anal Behav. 1998;69:29–47. doi: 10.1901/jeab.1998.69-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. The motivational properties of schedule-induced polydipsia. J Exp Anal Behav. 1966;9:19–25. doi: 10.1901/jeab.1966.9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL. Control of schedule-induced polydipsia: type, size, and spacing of meals. J Exp Anal Behav. 1967;10:199–206. doi: 10.1901/jeab.1967.10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk JL, Tang M. What Schedule-Induced Polydipsia Can Tell Us About Alcoholism. Alcoholism Clin Exp Res. 1988;12:577–585. doi: 10.1111/j.1530-0277.1988.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–30. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Fluvoxamine effects on concurrent ethanol- and food-maintained behaviors. Exp Clin Psychopharmacol. 2006;14:483–92. doi: 10.1037/1064-1297.14.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Reinforcement magnitude modulation of rate-dependent effects of fluvoxamine and desipramine in the rat. Behav Pharmacol. 2008;19:829–835. doi: 10.1097/FBP.0b013e32831d9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Koek W, Javors MA, Lamb RJ. Effects of fluvoxamine on a multiple schedule of ethanol- and food-maintained behavior in two rat strains. Psychopharmacology (Berl) 2005;180:249–57. doi: 10.1007/s00213-005-2156-z. [DOI] [PubMed] [Google Scholar]
- Harper DN. Behavioral resistance to haloperidol and clozapine. Behavioural Processes. 1999;46:1–13. doi: 10.1016/S0376-6357(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. J Exp Anal Behav. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez C, Shahan TA. Resistance to change of alcohol self-administration: effects of alcohol-delivery rate on disruption by extinction and naltrexone. Behav Pharmacol. 2007;18:161–169. doi: 10.1097/FBP.0b013e3280f2756f. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Fluvoxamine and desipramine on fixed-ratio responding: effects of reinforcement magnitude. Behav Pharmacol. 2005;16:573–578. doi: 10.1097/01.fbp.0000181594.01244.a2. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Ginsburg BC. Reinforcement magnitude modulates the rate-dependent effects of fluvoxamine and desipramine on fixed-interval responding in the pigeon. Behav Pharmacol. 2008;19:51–60. doi: 10.1097/FBP.0b013e3282f3d093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TU. Effects of fluvoxamine on ethanol-reinforced behavior in the rat. J Pharmacol Exp Ther. 2001;297:1001–9. [PubMed] [Google Scholar]
- Lamb RJ, McMillan DE. The effects of some putative antidepressant agents on the schedule-controlled behavior of the pigeon. Psychopharmacology (Berl) 1986;88:368–73. doi: 10.1007/BF00180840. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Grace RC. Behavioral momentum and the law of effect. Behav Brain Sci. 2000;23:73–90. doi: 10.1017/s0140525x00002405. discussion 90-130. [DOI] [PubMed] [Google Scholar]
- Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62(Suppl 20):26–31. [PubMed] [Google Scholar]
- Pinkston JW, Ginsburg BC, Lamb RJ. Examination of reinforcement magnitude on the pharmacological disruption of fixed-ratio performance. Exp Clin Psychopharmacol. 2009;17:237–246. doi: 10.1037/a0016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Grant KA. Chlordiazepoxide effects on ethanol self-administration: dependence on concurrent conditions. J Exp Anal Behav. 1985;43:353–364. doi: 10.1901/jeab.1985.43-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, Burke KA. Ethanol-maintained responding of rats is more resistant to change in a context with added non-drug reinforcement. Behav Pharmacol. 2004;15:279–285. doi: 10.1097/01.fbp.0000135706.93950.1a. [DOI] [PubMed] [Google Scholar]
- Shull RL, Pliskoff SS. Changeover delay and concurrent schedules: some effects on relative performance measures. J Exp Anal Behav. 1967;10:517–527. doi: 10.1901/jeab.1967.10-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations: With Computer Programs. Springer-Verlag; New York: 1981. [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol abuse. J Abnorm Psychol. 1988;97:181–95. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]


