Abstract
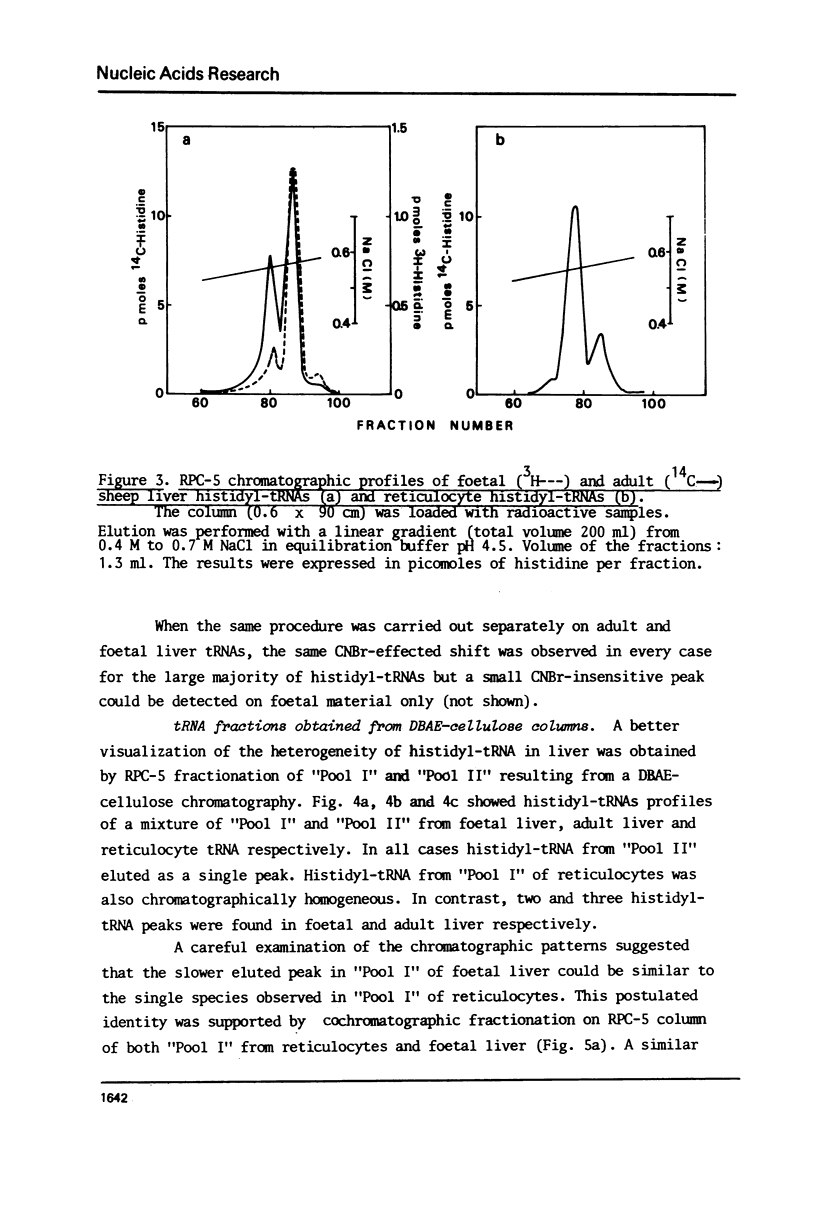
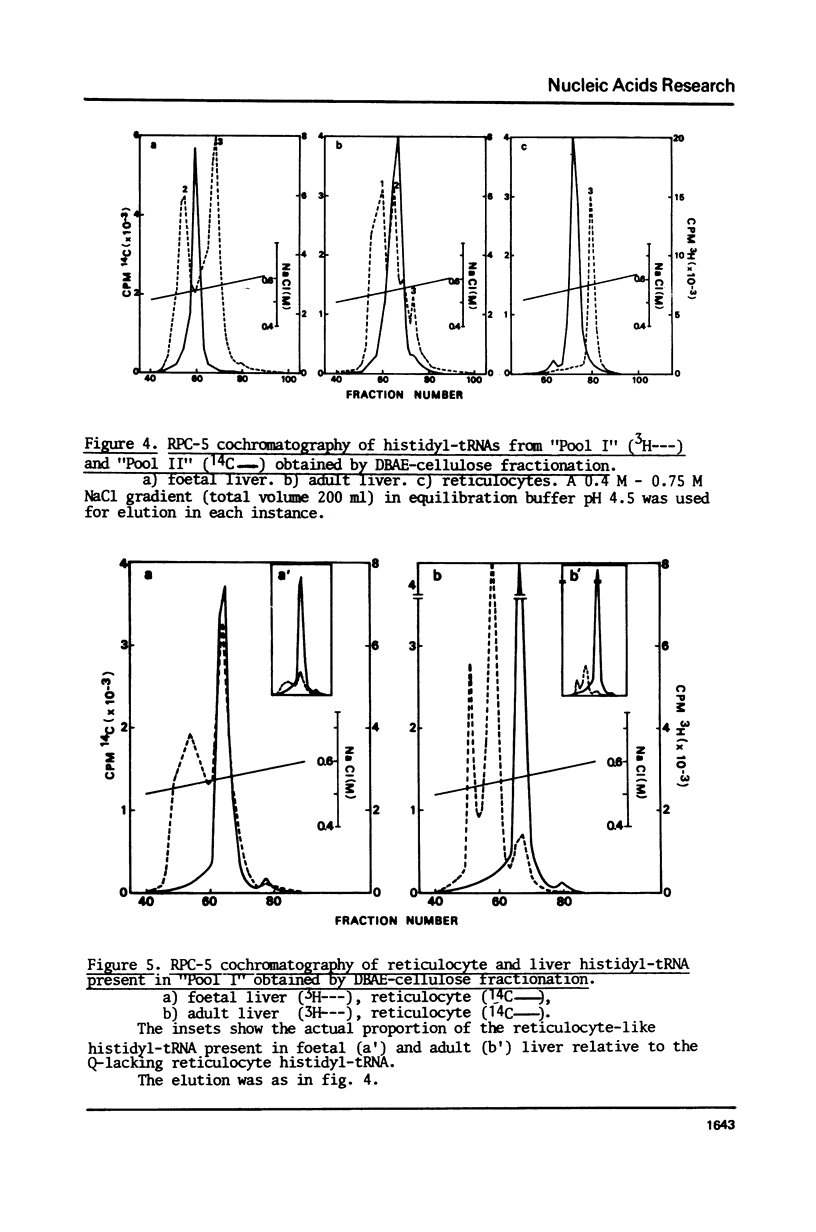
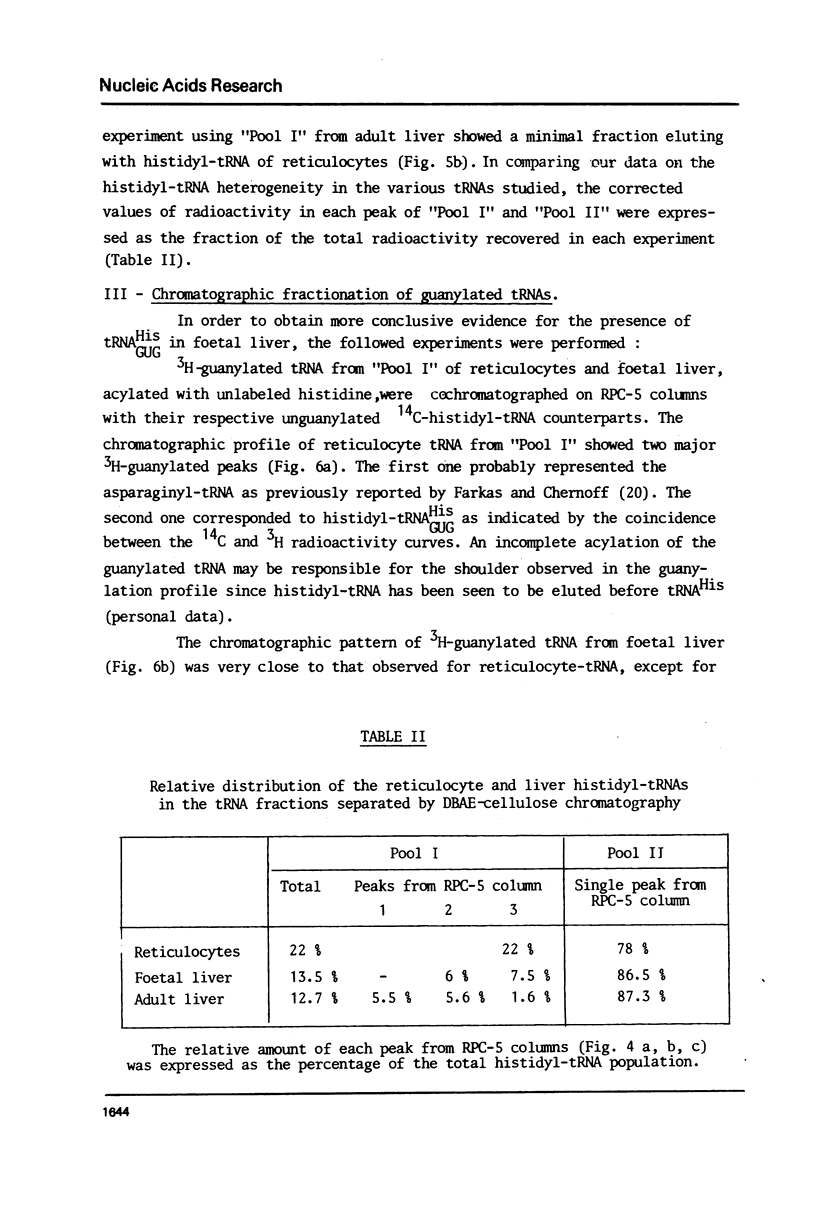
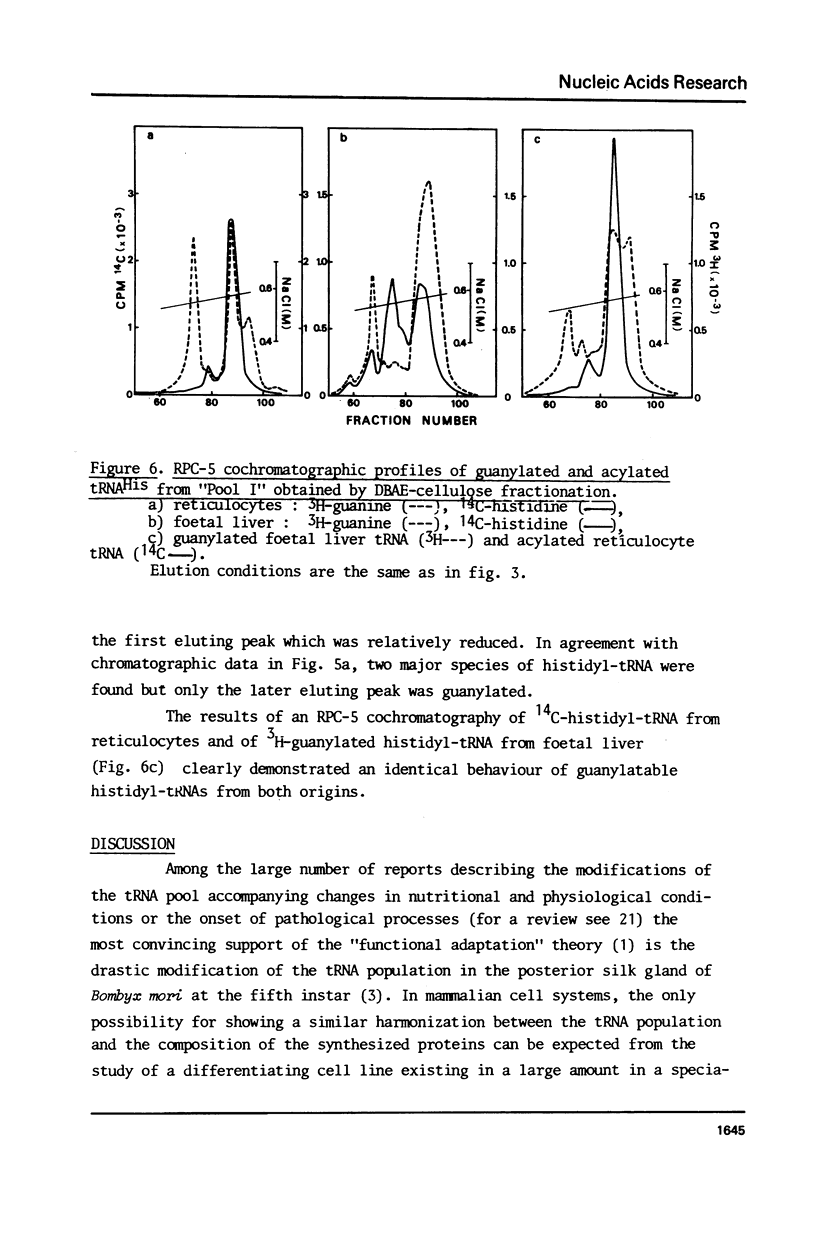
Histidyl-tRNAs from foetal and adult sheep liver were compared to their reticulocyte counterparts. The combination of various techniques revealed the existence of two histidyl-tRNA species in reticulocytes, one of which was not retained on acetylated DBAE-cellulose columns and was guanylatable. Three histidyl-tRNA isoacceptors were identified in foetal liver. Two of these species were not adsorbed on acetylated DBAE-cellulose but only one was found to be guanylatable. An identical chromatographic behaviour on RPC-5 columns was observed for guanylated histidyl-tRNAs from both origins. These results suggest the occurrence of a GUG anticodon in these guanine-accepting tRNAs. In foetal liver the amount of guanylatable histidyl-tRNA was estimated to be 7% of the total tRNA population. This observation is in agreement with the erythropoietic function of liver during the foetal life.
Full text
PDF



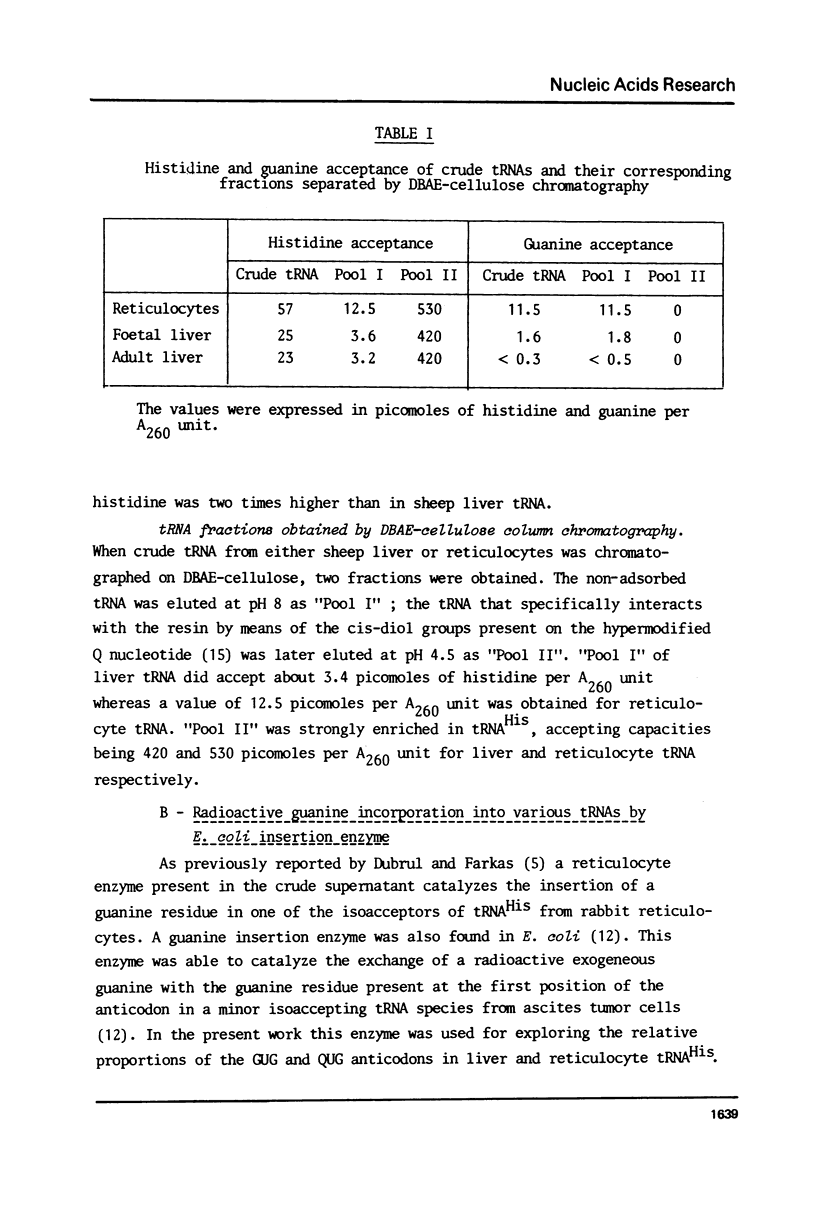

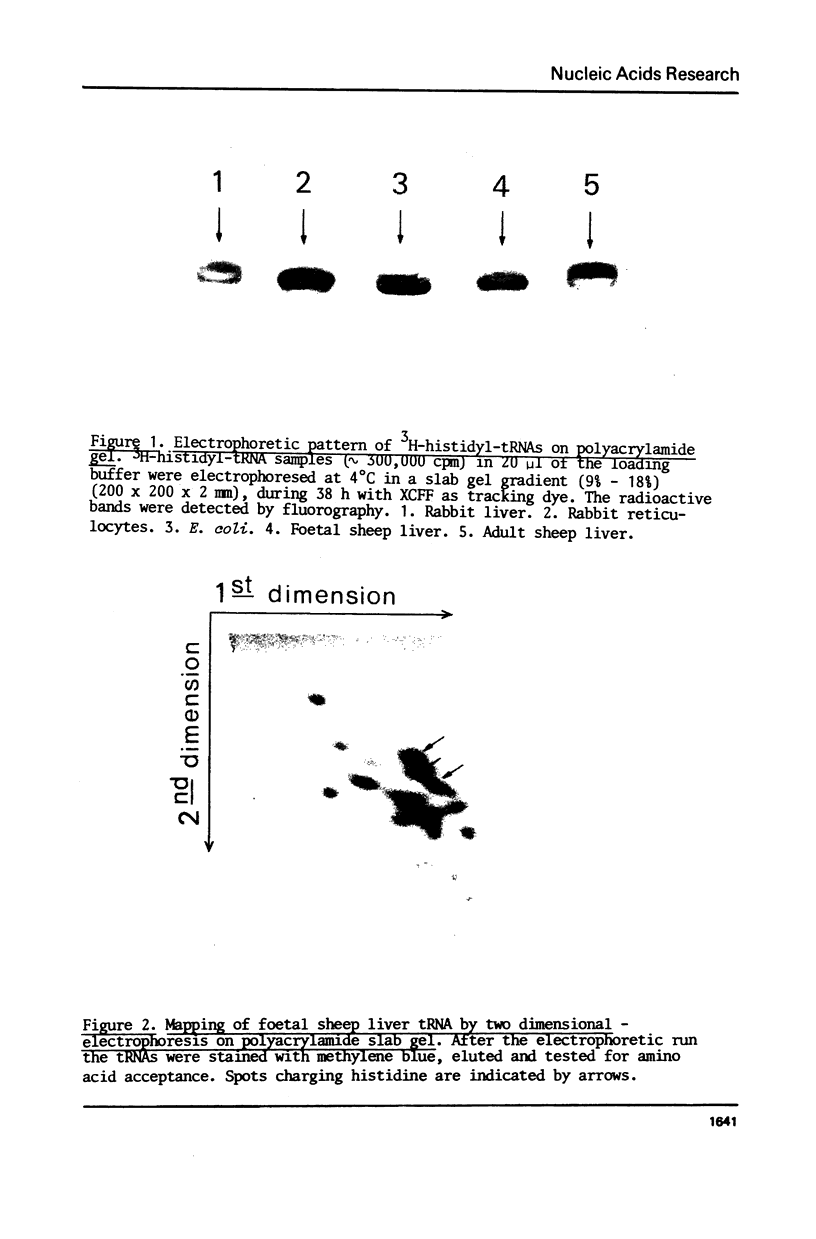



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Garel J. P. Functional adaptation of tRNA population. J Theor Biol. 1974 Jan;43(1):211–225. doi: 10.1016/s0022-5193(74)80054-8. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Heindell H. C., Liu A., Paddock G. V., Studnicka G. M., Salser W. A. The primary sequence of rabbit alpha-globin mRNA. Cell. 1978 Sep;15(1):43–54. doi: 10.1016/0092-8674(78)90081-8. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density in tissue culture to the isoaccepting spectra of the nucleoside Q containing tRNAs: tRNATyr, tRNAHis, tRNAAsn and tRNAAsp. Nucleic Acids Res. 1978 Jul;5(7):2513–2524. doi: 10.1093/nar/5.7.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Sato S., Itoh Y. H., Oda K., Nishimura S. Detection of unique tRNA species in tumor tissues by Escherichia coli guanine insertion enzyme. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4247–4251. doi: 10.1073/pnas.75.9.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrissant G., Boisnard M., Puissant C. Large scale preparation of rabbit liver tRNA. Isolation of tRNAs specific for methionine, phenylalanine, serine, tryptophan and valine. Biochimie. 1971;53(10):1105–1109. doi: 10.1016/s0300-9084(71)80200-6. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Meltzer V. N., McNamara A. L. A comparison of rabbit liver and reticulocyte transfer RNA: evidence of unique species in reticulocytes. Biochim Biophys Acta. 1974 May 31;349(3):366–375. doi: 10.1016/0005-2787(74)90123-3. [DOI] [PubMed] [Google Scholar]
- Sprague K. U., Hagenbüchle O., Zuniga M. C. The nucleotide sequence of two silk gland alanine tRNAs: implications for fibroin synthesis and for initiator tRNA structure. Cell. 1977 Jul;11(3):561–570. doi: 10.1016/0092-8674(77)90074-5. [DOI] [PubMed] [Google Scholar]
- Sprague K. U. The Bombyx mori silk proteins: characterization of large polypeptides. Biochemistry. 1975 Mar 11;14(5):925–931. doi: 10.1021/bi00676a008. [DOI] [PubMed] [Google Scholar]