Abstract
Autophagy is an important mechanism used by macrophages to kill intracellular pathogens. The results reported here demonstrate that autophagy is also involved in the macrophage killing of the extracellular enteropathogen Citrobacter rodentium following phagocytosis. The process was significantly impaired in macrophages isolated from mice chronically infected with the helminth parasite Heligmosomoides polygyrus. The H. polygyrus-mediated inhibition of autophagy was Th2-dependent since it was not observed in macrophages isolated from helminth infected STAT6-deficient mice. Moreover, autophagy of Citrobacter was inhibited by treating macrophages with IL-4 and IL-13. The effect of H. polygyrus on autophagy was associated with decreased expression and processing of LC3, a key component of the autophagic machinery. The helminth-induced inhibition of LC3 expression and processing was STAT6-dependent and could be recapitulated by treatment of macrophages with IL-4 and IL-13. Knock-down of LC3 significantly inhibited autophagic killing of Citrobacter, attesting to the functional importance of the H. polygyrus-mediated down-regulation of this process. These observations reveal a new aspect of the immunosuppressive effects of helminth infection and provide mechanistic insights into our earlier finding that H. polygyrus significantly worsens the in vivo course of Citrobacter infection.
Keywords: Parasitic helminth, alternatively activated macrophage, autophagy
Introduction
Helminths are estimated to infect 3 billion people worldwide (1, 2). The distribution of several helminth pathogens coincides geographically with many devastating microbial diseases including HIV, malaria and tuberculosis, and it is possible that the strong immunomodulatory effects of helminths on host responses may have a significant impact on such coincident infections(3, 4). Indeed, we have found that infection of mice with the intestinal helminth parasite Heligmosomoides polygyrus exacerbates the colitis caused by concurrent infection with Citrobacter rodentium, a Gram-negative organism used as a model of non-invasive, attaching-effacing bacterial enteropathogens such as enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC) (5). The severity of the colitis in the co-infected mice was correlated with high Citrobacter loads in the gut, translocation of the bacteria into mucosal and systemic immune compartments and uncontrolled bacterial growth (5, 6). Subsequent investigations from our laboratory showed that the increased bacterial translocation and replication were associated with a significantly impaired ability of macrophages from helminth-infected animals to kill phagocytosed C. rodentium, an abnormality that was dependent on intact Th2 responses (7). The mechanisms responsible for the helminth-induced impairment of bacterial killing by macrophages are yet to be elucidated.
Macrophages can contribute significantly to the effector phase of the host anti-microbial response, i.e. elimination of bacteria. Recent experiments indicate that one of the important aspects of this process is autophagy. Autophagy is a fundamental cellular homeostatic mechanism that has long been known to be involved in degrading and recycling proteins and organelles. Studies carried out over the last few years have shown that it is also involved in destroying several types of intracellular pathogens, including Mycobacterium tuberculosis, Shigella flexneri, Salmonella typhimurium and Toxoplasma gondii (8–11). During autophagy, cytosolic proteins and organelles are sequestered by a double membrane. The resulting vacuoles, i.e. autophagosomes, go through a series of maturation steps and eventually fuse with lysosomes for degradation. By a similar process, autophagy can capture and eliminate intracellular pathogens, acting as an important innate immune effector mechanism. Autophagy can be monitored by changes in the microtubule-associated light chain protein 3 (LC3/Atg8), which is converted from the 16 kDa LC3 I form to the lipidated 14 kDa LC3 II form and is recruited to the autophagosomal membrane (12, 13). In addition to its involvement in the removal and destruction of intracellular microbial pathogens, autophagy also plays a role in antigen presentation (the delivery of microbial peptides to endosomes or MHC II loading compartments) and, thus, in the activation of adaptive immunity (reviewed in (14)). Recent genome-wide association studies have provided evidence linking autophagy and Crohn’s disease (15–17). It has been speculated that mutations in the autophagy pathway may alter the normal gut response to intestinal commensals or pathogens (15–17).
In the present work, we show that macrophages normally eliminate phagocytosed Citrobacter by autophagy, and that this process is inhibited, in a Th2-dependent fashion, in animals infected with H. polygyrus. The underlying mechanism involves helminth-induced down-regulation of LC3 expression and processing. These results reveal a novel immunosuppressive consequence of helminth infection.
Materials and Methods
Mice
Six to 8 week old female BALB/c ByJ and STAT6 KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME). They were fed autoclaved food and water and were housed in a specific pathogen-free facility at Massachusetts General Hospital. All animal experiments were approved by the Institutional Subcommittee on Research Animal Care.
H. polygyrus infection
H. polygyrus was propagated as previously described and stored at 4°C until use (5). Mice were inoculated orally with 200 third-stage larvae (L3). Seven days following parasitic infection, a subset of the H. polygyrus-infected mice was fed C. rodentium.
C. rodentium infection
Mice were orally inoculated with C. rodentium (strain DBS100 from American Type Culture Collection). Bacteria were grown overnight in Luria broth (LB) and resuspended in PBS before infecting the mice (0.5 ml/mouse; 5 × 108 CFU of C. rodentium). GFP-expressing C. rodentium (GFP-C. rodentium) (provided by Dr. L. Bry at Brigham and Women’s Hospital, Boston, MA) were grown overnight in LB containing carbenicillin (100 µg/ml) and resuspended in PBS before in vitro infection assays.
Real-time quantitative RT-PCR
Total RNA was prepared from thioglycollate-elicited peritoneal macrophages using TRIzol reagent (Invitrogen Life Technologies) following the manufacturer’s recommendations. cDNA was synthesized using 2 µg of total RNA (Ready-to-Go kit; GE Healthcare). The cDNA samples were then tested for the expression of FIZZ1, YM1, and arginase 1 (Arg1) by real-time RT-PCR. Real-time RT-PCR was performed using SYBR Green PCR Master Mix for 38 cycles on an Opticon II DNA engine (MJ Research). GAPDH was used as an internal control. Negative controls included the amplification of samples without prior reverse transcription. LightCycler relative quantification software was used to normalize data to the same GAPDH mRNA level. Samples were run in triplicate. The sequences for the sense and antisense primers used to quantify mRNA were: GAPDH, 5'-TGGAATCCTGTGGCATCCATGAAAC-3' and 5'-TAAAACGCAGCTCAGTAACAGTCCG-3'; Arg1, 5'-CAGAAGAATGGAAGAGTCAG-3' and 5'-CAGATATGCAGGGAGTCACC-3'; Ym1, 5'-TCACAGGTCTGGCAATTCTTCTG-3' and 5'-TTTGTCCTTAGGAGGGCTTCCTC-3'; Fizz1, 5'-TCCCAGATACTGATGAGA-3' and 5'-CCACTCTGGATCTCCCAAGA-3'; and iNOS2, 5'-CTGGAGGAGCTCCTGCCTCATG-3' and 5'-GCAGCATC CCCTCTGATGGTG-3'; and mLC3, 5'-GACCGCTGTAA-3' and 5'-CTTGACCAACT-3'.
Gentamicin protection assay
The peritoneal macrophages were collected from mice infected with helminths (3 wk after H. polygyrus infection) or uninfected control mice. After incubation in complete DMEM for 2 h, non-adherent cells were removed by washing and the cells were cultured overnight. The adherent cells were incubated in complete DMEM at 37°C overnight and then infected for 1 h with 107 C. rodentium (multiplicity of infection of 10:1) in antibiotic-free medium. After completion of the infection period the cells were washed with cold PBS (×3) and incubated with gentamicin-containing medium (100 µg/ml) for 2 h, which kills the extracellular bacteria. Because gentamicin is not cell permeable, intracellular bacteria are not killed by this antibiotic. The cells were then washed (×3) with sterile PBS and then lysed immediately in 0.2 ml of sterile 1% Triton X-100 in water or after a further 4 h in medium containing 10 µg/ml gentamicin. The lysates were mixed with 0.8 ml of PBS and serial dilutions were made before plating 100 µl of the appropriate dilutions on LB agar. Colonies were counted after overnight incubation at 37°C and the number of bacteria present inside the cells at each time point was calculated.
Immunofluorescence microscopy and LC3 detection
Peritoneal macrophages were collected from normal and helminth-infected mice (day 7 to 14 post-infection), grown on coverslips, and infected with GFP-expressing C. rodentium for 1 h, followed by incubation with gentamicin-containing medium for 2 h (as described above). After fixation, the cells were stained with rabbit anti-mouse LC3B primary antibody (Cell Signaling), followed by incubation with anti-rabbit IgG-Cy3 (Cedarlane Laboratories). Sections were analyzed by immunofluorescence microscopy (Nikon ECLIPSE 80i).
siRNA-mediated silencing of LC3
LC3-specific and irrelevant siRNAs were purchased from Dharmacon Inc. siRNAs were transfected using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Analysis of LC3 expression and bacterial killing was performed 2 days after transfection.
Western blot analysis of LC3
CD11b+ peritoneal macrophages were isolated from normal and H. polygyrus infected BALB/c or STAT 6 KO mice and the cells were pretreated with Th1 (IFN-γ) or Th2 (IL-4, IL-13) cytokines overnight. The cells were then exposed to C. rodentium for 1 h. Cellular lysates were prepared and protein content was determined using BCA protein assay (Bio-Rad Laboratories). Proteins were separated by SDS-PAGE for western blot analysis. LC3 protein expression was determined by immunoblotting with polyclonal rabbit anti-mouse LC3B primary antibody (1:2000, Cell Signaling), followed by a goat anti-rabbit antibody conjugated to horseradish peroxidase (1: 5000 dilution, Biosource). Each blot was analyzed for GAPDH protein expression as an internal loading control using a specific rabbit anti-mouse GAPDH antibody (1:5000).
Statistical analysis
All results were expressed as the mean ± SD. N refers to the number of mice used. Statistical differences were determined using one-way analysis of variance test (Tukey’s multiple comparison test) with GraphPad Prism. A value for p< 0.05 was considered significant.
Results
Autophagy is involved in macrophage killing of Citrobacter and is enhanced by IFN-γ
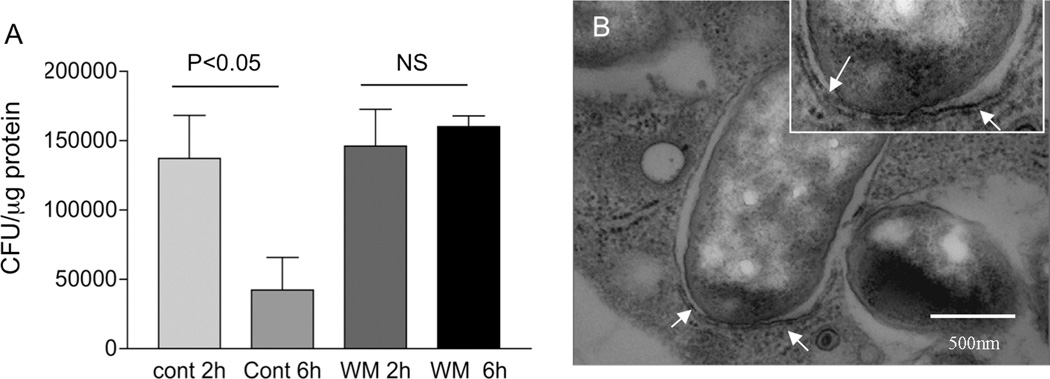
Although C. rodentium typically colonizes the intestinal epithelial cell surface, bacterial translocation occurs in hosts with a dysregulated or compromised immune system (5, 7). Therefore, tissue resident macrophages can be involved in the elimination of the translocated bacteria. We carried out experiments to determine whether autophagy plays a role in this process. We isolated peritoneal macrophages from normal BALB/c mice and incubated the cells in the absence or presence of the phosphatidylinositol (PI) 3-kinase inhibitor wortmannin, a pharmacologic strategy that is often used to evaluate the involvement of autophagy (18). After overnight incubation, cells were infected with C. rodentium (or GFP-expressing C. rodentium) for 1 h, followed by a gentamicin protection assay (7). Our results show that macrophages from normal mice are able to effectively control internalized C. rodentium as evidenced by a time-dependent reduction in intracellular bacterial numbers (Figure 1 A). This reduction was abolished in the wortmannin treated cells (Figure 1A). The results, therefore, suggest a potential role for autophagy in the control of C. rodentium by macrophages. Similar results were reported recently for another non-invasive bacterial enteropathogen, Helicobacter pylori (19). Autophagy involves the formation of a double membrane structure called the autophagosome (20). Accordingly, we used electron microscopy to analyze Citrobacter infected macrophages, and found characteristic autophagic double-membrane structures partially enclosing the internalized bacteria (Figure 1B). This observation further supports the idea that autophagy is involved in killing phagocytosed C. rodentium.
Figure 1.
Autophagy-mediated bacterial killing is impaired in macrophages from helminth-infected mice. A: Treatment of macrophages with wortmannin, an autophagy inhibitor, resulted in impaired bacterial killing. Peritoneal macrophages were isolated from normal BALB/c mice and exposed to C. rodentium (10 bacteria/cell) for 1 h. At different time points (2 and 6 h) after infection, the number of viable intracellular bacteria was determined by plating the cell lysates onto LB plates. The data shown represent the mean ± SD of triplicate cultures, and are from one of three experiments showing similar results. Additional cultures of macrophages without infection by C. rodentium showed no detection of viable bacteria (data not show). B: Autophagic double-membrane structures are present in macrophages with phagocytosed C. rodentium. Peritoneal macrophages were isolated from normal BALB/c mice, pre-treated with IFNγ (100 U/ml) overnight and exposed to C. rodentium for 1 h. The ultra-structure of the cells was examined by transmission electron microscopy (images shown are 60,000×). Arrows (and the inset) indicate the presence of double-membrane structures surrounding the internalized bacteria. Scale bar represents 500 nm.
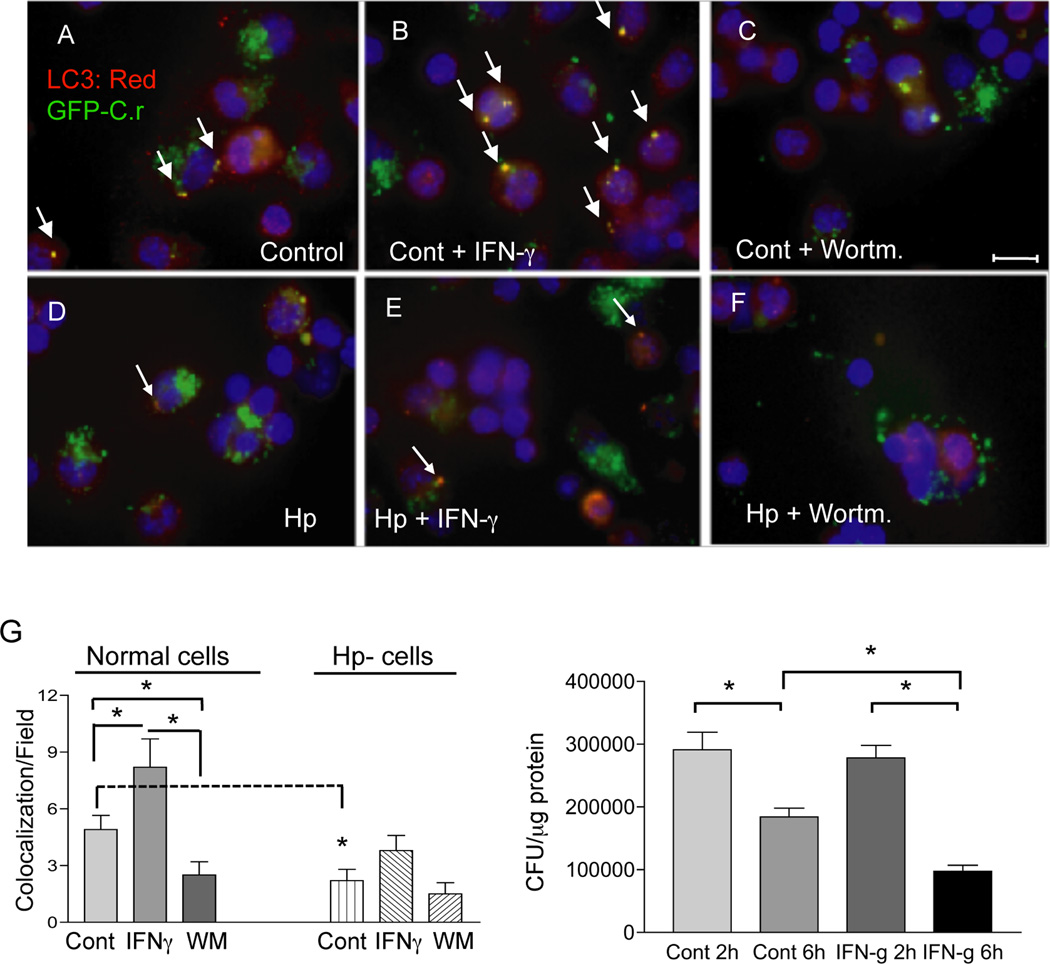
Autophagy is commonly characterized by the redistribution of the LC3 protein onto autophagosomes (12, 13, 21, 22). Immunostaining for LC3, therefore, is a relatively simple method to detect autophagy (23). To further assess the role of autophagy in the control of phagocytosed C. rodentium, we examined LC3 distribution and co-localization with GFP-expressing bacteria in macrophages. The results showed that co-localization of LC3 with the GFP-Citrobacter was readily detectable in macrophages isolated from normal mice (Figure 2 A & G). In support of the idea that this co-localization represented the formation of autophagosomes, we found that it was enhanced by IFN-γ, a known inducer of autophagy (23), whereas it was inhibited by wortmannin, an autophagy inhibitor (Figure 2 A, B, C and G). Moreover, the changes in co-localization correlated with intracellular bacterial numbers (Figure 2 H). These results provide further evidence that autophagy is involved in the control of phagocytosed C. rodentium by macrophages. The observations expand our current understanding of the mechanisms by which macrophages limit the replication of internalized bacterial enteropathogens.
Figure 2.
Helminth-infection resulted in reduced co-localization of LC3 and C. rodentium in macrophages. A–F: Immunofluorescence microscopy data show the detection of LC3, an autophagy marker, internalized GFP-expressing C. rodentium and colocalization of LC3 and GFP-C. rodentium in peritoneal macrophages (stained with anti-LC3 and Cy3: red) from Normal (A, B and C) and H. polygyrus-infected (D, E and F) mice at 6 h after C. rodentium-GFP infection. Wortmannin-treated cells displayed reduced LC3-GFP bacterial colocalization (C and F). IFNγ treatment resulted in an increase in LC3 expression and colocalization of LC3 with GFP-C. rodentium (B and E). Scale bar represents 10 µm. G: Frequency of LC3-GFP-C. rodentium colocalization within macrophages isolated from normal and helminth-infected BALB/c mice with and without wortmannin and IFNγ. The data shown are from one of three experiments showing similar results. Arrows indicate the presence of co-localization of LC3 and GFP-expressing C. rodentium. *p < 0.05 for a comparison of two groups. H: IFNγ treatment of the cells results in enhanced bacterial killing. Peritoneal macrophages were isolated from normal BALB/c mice, and then exposed to C. rodentium for 1 h with or without overnight IFNγ pretreatment. The number of viable intracellular bacteria recovered from the macrophages was determined by plating the cell lysates onto LB plates 6 h after gentamicin treatment. The data represent the mean ± SD of triplicate cultures. *p < 0.05 for a comparison of two groups.
Macrophages from H. polygyrus infected mice have compromised autophagic killing of Citrobacter
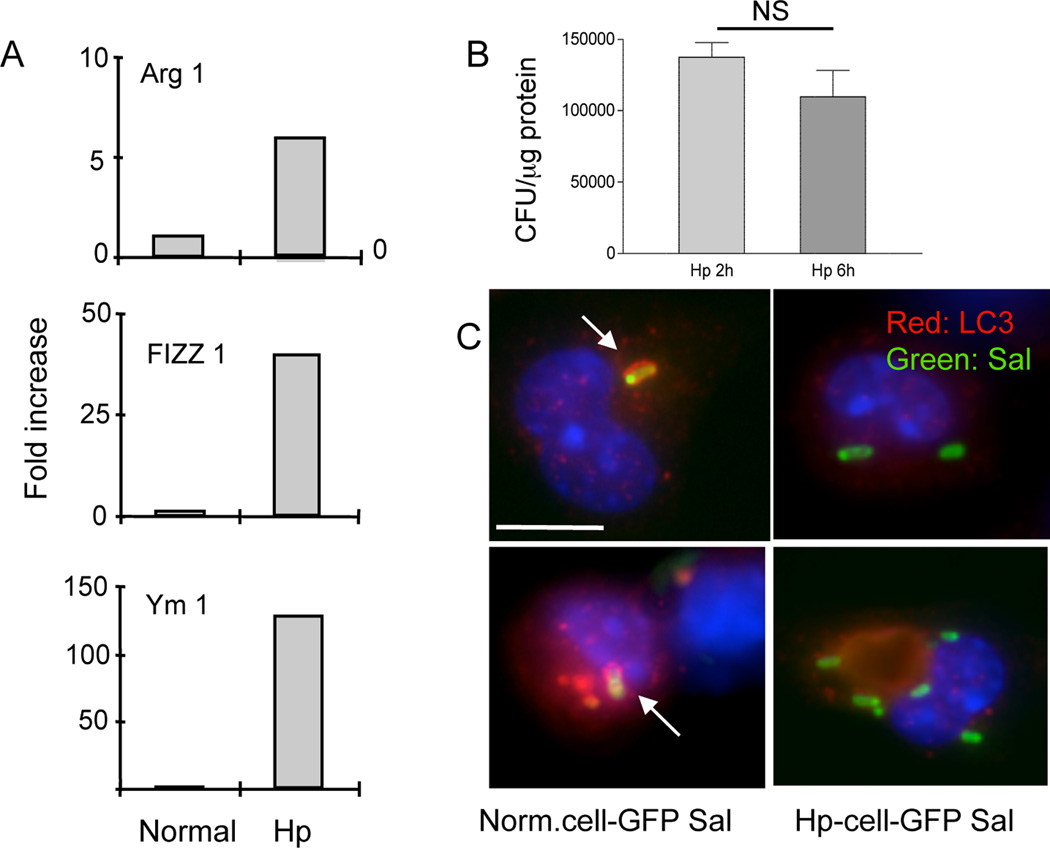
Intestinal helminth infections have been shown to have a significant impact on the occurrence and course of a number of other illnesses. This may be particularly important in the developing world where chronic helminth infections coexist commonly with other pathogens. Helminths are classical Th2 inducers and helminth infection has been shown to dampen Th1 responses to other infections (24–27). The impairment of the bacterial killing capacity of macrophages has been attributed to the alternative activation of these cells in the mucosal immune compartment by helminth infection (7). Based on this information and on the results described above, we carried out experiments to determine whether an on-going helminth infection might influence autophagic killing of bacteria by macrophages. First, we examined phenotypic alterations of peritoneal macrophages induced by helminth infection. Our real-time RT-PCR analysis showed that peritoneal macrophages isolated from helminth-infected mice displayed an alternatively activated phenotype as indicated by the up-regulation of Ym1, Arg1, and Fizz1, genes that are characteristic of this phenotype (28, 29) (Figure 3A). This finding is consistent with our earlier observation that macrophages isolated from the gut-associated lymphoid tissue of H. polygyrus infected mice were alternatively activated (7).
Figure 3.
A. H. polygyrus infection up-regulates Ym1, Arg1 and Fizz1 expression in peritoneal macrophages and impairs bacterial killing by macrophages. Peritoneal macrophages were isolated from normal and helminth-infected BALB/c mice at 7 days post infection. Total RNA was isolated from the cells. Ym1, Arg1 and Fizz1 expression was determined using real-time RT-PCR. Values are the fold increase compared with baseline obtained from uninfected control mice. B. H. polygyrus infection impairs bacterial killing by macrophages. Peritoneal macrophages were isolated from H. polygyrus infected mice and used for gentamicin protection assay. The results show similar numbers of viable internalized bacteria recovered in macrophages isolated from heminth-infected mice at 2 and 6 h post bacterial infection in vitro. P > 0.05. C. Immunofluorescence microscopy shows LC3-Samonella co-localization in macrophages from control and helminth-infected mice. Scale bar indicates 10 µm. The data shown are from one of three experiments with similar results.
We went on to evaluate the impact of helminth infection on the ability of peritoneal macrophages to kill phagocytosed Citrobacter. Macrophages from control and helminth-infected mice were exposed to C. rodentium (about 10 bacteria/cell) and a gentamicin protection assay was performed to enumerate intracellular bacteria surviving at different times after infection (7). In contrast to macrophages from normal mice, which showed an autophagy-mediated killing of bacteria (Figure 1A), macrophages that were isolated from helminth infected hosts had impaired killing of internalized C. rodentium, as indicated by unchanged levels of intracellular bacteria at 2 time points (2 h and 6 h) (Figure 3 B). Furthermore, the co-localization of LC3 with GFP-expressing Citrobacter was significantly reduced in macrophages from H. polygyrus-infected mice, even when the cells were treated with IFNγ (Figure 2 D, E, G). Co-localization was further reduced when the cells were treated with wortmannin (Figure 2 F, G). Transmission electron microscopic analysis failed to detect the characteristic, autophagic double membrane structure surrounding intracellular bacteria in cells isolated from helminth-infected mice (data not shown). Macrophage infection with Salmonella has been used as one of the models for studying the protective role of autophagy in immune defense against intracellular pathogens (10). To further evaluate the impact of helminth infection on autophagy, we examined co-localization of LC3 with GFP-expressing Salmonella. We observed clear co-localization of LC3 and GFP-Salmonella in macrophages from control mice, whereas co-localization in macrophages from helminth-infected mice was extremely infrequent (Figure 3 C). Taken together, our results indicate clearly that helminth infection impairs the ability of macrophages to kill phagocytosed bacteria by autophagy.
The effect of H. polygyrus on the autophagy of C. rodentium is mediated by a Th2 response
The enteric pathogens involved in this study induce distinct immune responses, with H. polygyrus and C. rodentium inducing strong Th2 and Th1 responses respectively (5). Th1/Th2 cytokine responses play a regulatory role in the autophagy process (30). The Th1 cytokine IFN-γ, through mechanisms that are not yet fully understood, induces or augments autophagy, and thus contributes to the elimination of intracellular bacteria (Figure 2B and 2G) (9). Impaired Th1 immunity observed in mice that were co-infected with Th2-inducing H. polygyrus has been suggested to contribute to their enhanced susceptibility to C. rodentium and intestinal injury (7). The Th2 cytokines IL-4 and IL-13 have been shown to abrogate autophagy and autophagy-mediated killing of intracellular bacteria in macrophages (31). Recently, we have shown that helminths impair host defense against enteric bacterial infection through a Th2-dependent dependent mechanism (7). Therefore, we carried out experiments to determine if the effects of H. polygyrus infection on autophagy are dependent on Th2 responses.
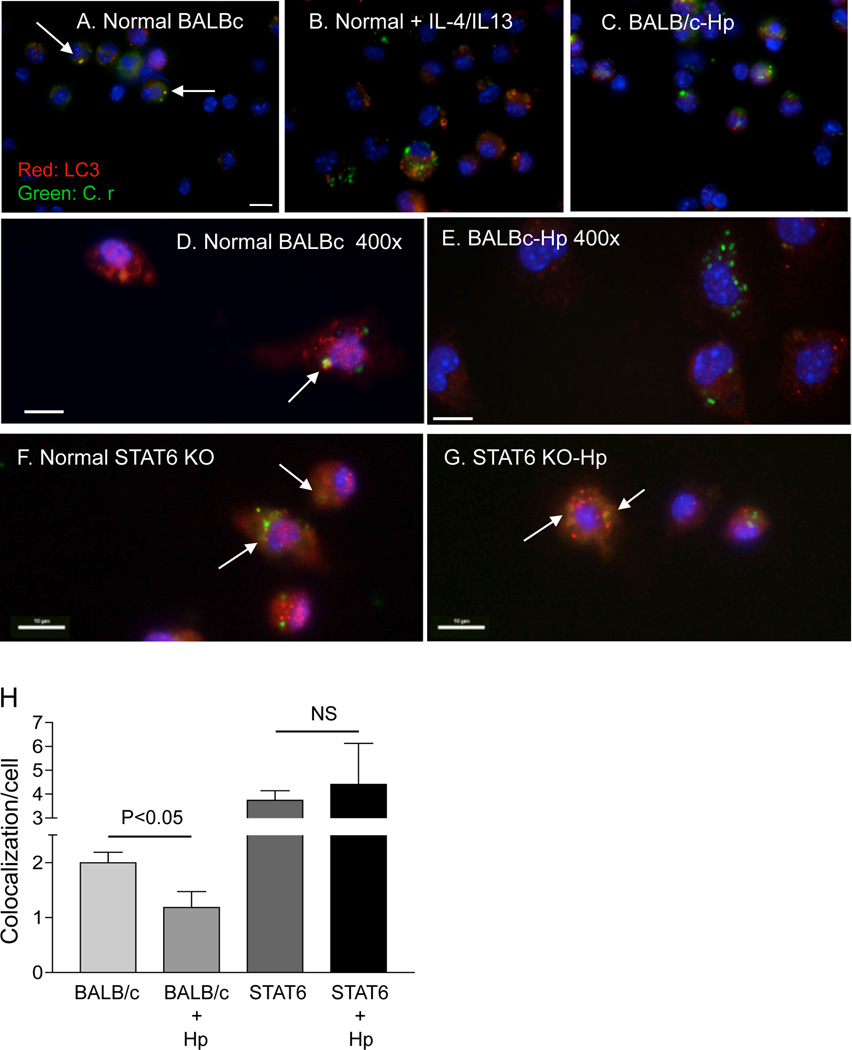
Accordingly, we treated peritoneal macrophages in vitro with Th2 cytokines (IL-4/IL-13), or left them untreated (as controls) and infected the cells with GFP-C. rodentium. Our immunofluorescence analysis showed, similar to the results presented in Figure 2, that co-localization of LC3 and GFP-bacteria was detected in cells isolated from normal mice (Figure 4 A & D). In contrast, co-localization of LC3 and GFP-bacteria was markedly reduced in cells that were pre-treated with Th2 cytokines (IL-4/IL-13), similar to what was seen in cells isolated from H. polygygrus infected mice (Figure 4 B, C & E). These observations raise the possibility that the helminth-induced impairment of autophagic bacterial killing may be mediated by the effects of the Th2 response in worm-infected animals. To test this hypothesis, we infected STAT6 KO mice with H. polygyrus, isolated macrophages from both helminth-infected and un-infected hosts and infected with GFP-Citrobacter (Figure 4 F & G). The immunofluorescence analysis revealed that co-localization of LC3 and GFP-Citrobacter is restored to normal in macrophages from helminth-infected STAT6 KO mice (Figure 4 H).
Figure 4.
The effect of H. polygyrus on autophagy of C. rodentium is mediated by a Th2 dependent mechanism. A–G: Immunofluorescence microscopy data show the detection of LC3, an autophagy marker, internalized GFP-expressing C. rodentium and colocalization of LC3 and GFP-C. rodentium in peritoneal macrophages isolated from normal BALB/c (A & D), H. polygyrus-infected BALB/c (C & E), normal STAT6 KO (F) or H. polygyrus-infected STAT6 KO mice (G). B: Peritoneal macrophages were isolated from normal BALB/c mice, pre-treated with IL-4/IL-13 (3 ng/ml) overnight and then exposed to GFP-C. rodentium for 1 h. Cells were collected 2 h after infection, fixed and stained for LC3. Scale bar represents 10 µm. For a detailed description, see Figure 1 C–H. H. Frequency of LC3-GFP-C. rodentium colocalization within macrophages isolated from normal and helminth-infected BALB/c and STAT6 KO mice. The data represent the mean (co-localization of LC3 and GFP-C. rodentium/cell) ± SD obtained from 30–50 cells per slide (1 slide from each of the two experiments was examined).
The effect of H. polygyrus on autophagy of C. rodentium is associated with a decrease in LC3 expression and processing
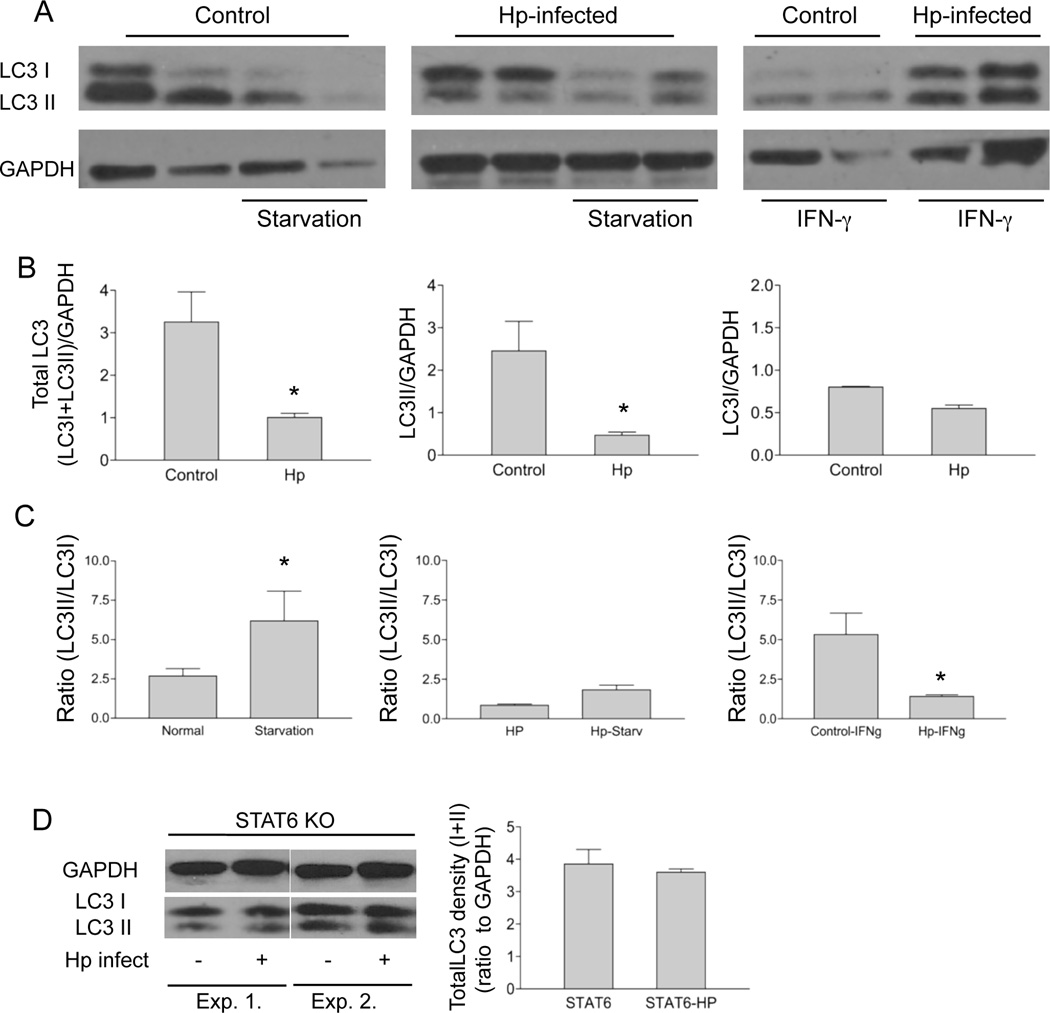
During autophagy, the cytosolic form of LC3 (LC3I) is conjugated to phosphatidylethanolamine to form LC3II, which is recruited to autophagosomal membranes. In the next set of experiments, we determined whether the ongoing helminth infection influences the expression of LC3I and LC3II in vivo. We isolated macrophages from helminth-infected and control mice and cultured the cells in vitro with and without IFNγ or starvation treatment, both of which can induce autophagy (16). Immunoblotting with an anti-LC3 antibody revealed two species of LC3, as can be seen in Figure 5A: a 16 kDa band corresponding to unmodified LC3I and a lower band corresponding to LC3II. Our results show that treatment of macrophages isolated from un-infected control mice with IFN-γ or starvation resulted in decreased detection of the unmodified LC3I (Figure 5 A). Strikingly, total LC3 levels as well as processing to LC3 II (LC3II/GAPDH) (in response to IFN-γ treatment or starvation) were significantly reduced in cells isolated from helminth-infected mice as compared to cells isolated from control animals (Figure 5B & C). These results suggest that the induction of autophagy in macrophages can be negatively regulated by an ongoing helminth infection.
Figure 5.
H. polygyrus infection reduces LC3 expression and LC3 II conversion in macrophages via a STAT6 dependent mechanism. A. Peritoneal macrophages were isolated from normal and H. polygyrus infected BALB/c mice (2 weeks after infection). Autophagy was induced in macrophages by incubating the cells overnight in the presence and absence of recombinant IFNγ (100u/ml) or by amino acid and serum starvation at 37°C for 3 h. Cell lysates were collected for detection of LC3 (LC3I and LC3II) by western-blotting. GAPDH was used as the loading control. Each lane represents the sample from an individual mouse. B and C. The bar graphs show the total LC3 density (LC3I+LC3II), LC3I, LC3II density and the LC3II/LC3I ratio (mean density + SD from 3 experiments) based on the western blotting analysis of LC3 expression (in A). D. Western blotting analysis of LC3 expression in peritoneal macrophages isolated from normal and H. polygyrus infected STAT 6 KO mice.
It has been shown previously that the effects of Th2 cytokines on starvation-induced autophagy is dependent on signaling via the Akt pathway, whereas the effect of Th2 cytokines on IFNγ-induced autophagy is Akt-independent and STAT 6 dependent (30). To directly demonstrate the influence of the helminth-induced Th2 cytokine response on LC3 during C. rodentium infection, in our next set of experiments, we utilized peritoneal macrophages isolated from helminth-infected or non-infected Th2-deficient, STAT6 KO mice (BALB/c mice used as control) and examined the influence of ongoing helminth-infection on LC3 expression. In contrast to what was observed in WT mice (Figure 5B), helminth-induced down-regulation of LC3 levels was not seen in cells isolated from STAT6 KO mice (Figure 5D). These results suggest that the helminth-induced Th2 response inhibits autophagic killing of phagocytosed bacteria by reducing LC3 expression. Our findings are consistent with a previous report showing that STAT6 is required for the suppression of IFNγ-induced autophagy (31).
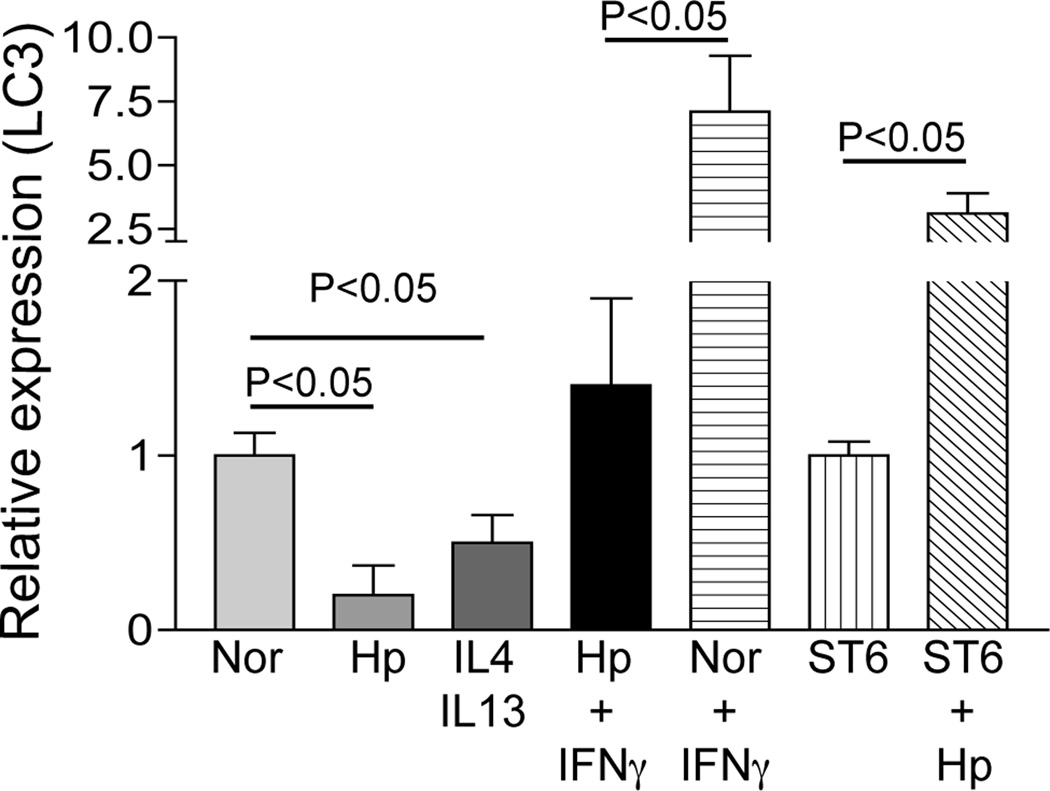
To further elucidate the mechanism responsible for the reduction in LC3 protein expression in peritoneal macrophages from helminth-infected mice, we next examined LC3 mRNA levels in macrophages that were isolated from STAT6 KO and WT mice with and without H. polygyrus infection. The real-time RT-PCR analysis showed a marked reduction of LC3 mRNA in macrophages from helminth-infected WT mice, an effect that was not seen in helminth-infected STAT 6 KO mice (Figure 6). IFNγ up-regulated LC3 mRNA expression in macrophages from uninfected mice, whereas this effect was blunted in macrophages from helminth-infected animals. In contrast to IFNγ, IL-4/IL-13 treatment of macrophages down-regulated LC3 mRNA. These results indicate a marked influence of Th1 and Th2 cytokines on autophagy and also support the notion that the effects of helminth infection on autophagy are mediated by the worm-induced Th2 response.
Figure 6.
H. polygyrus infection down-regulates LC3 gene expression in wild-type mice but not in STAT6 KO mice. Peritoneal macrophages were isolated from normal or H. polygyrus-infected wild-type and STAT6 KO BALB/c mice. After overnight incubation in vitro in the presence or absence of Th1 or Th2 cytokines, total RNA was isolated. LC3 expression was determined using real-time RT-PCR. Values are the fold decrease or increase compared with baseline obtained from uninfected control mice. The data shown are from one of two experiments showing similar results.
siRNA-mediated suppression of LC3 in RAW264.7 cells and primary macrophages results in reduced autophagic killing of bacteria
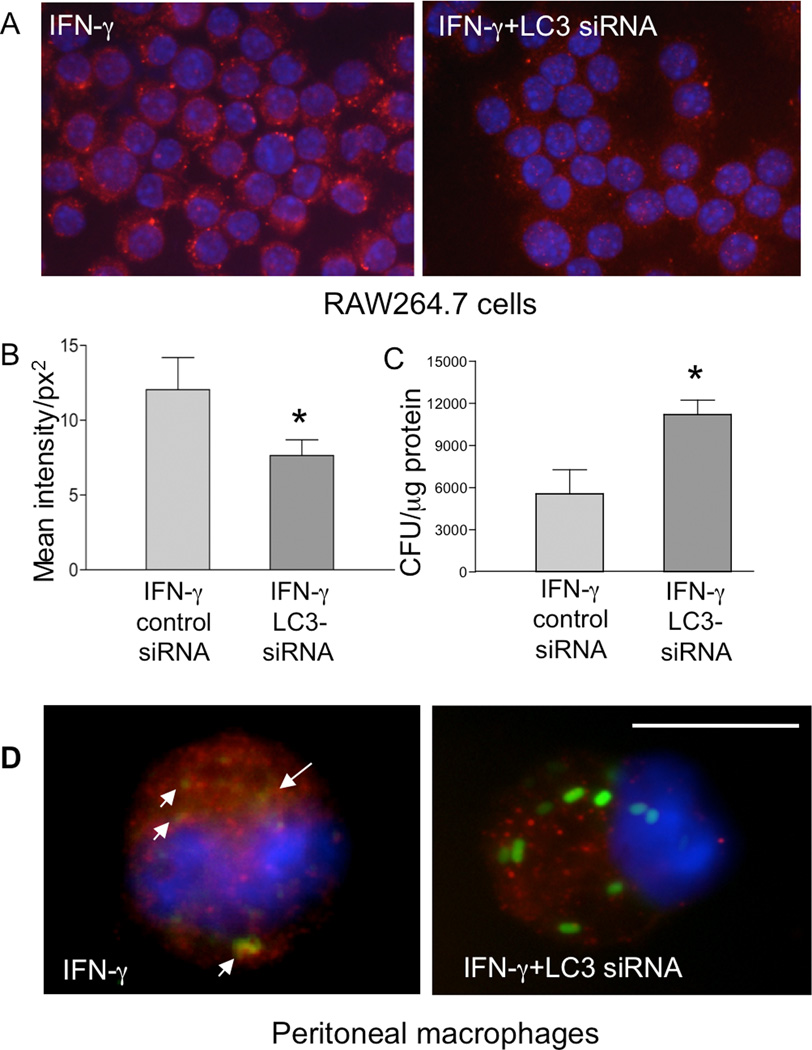
To determine whether the observed inhibition of LC3 expression by helminth-infection can influence autophagy, which in turn resulted in reduced bactericidal activity of macrophages, the effects of siRNA-mediated silencing of LC3 in the RAW264.7 macrophage cell line were analyzed. Immunofluorescence microscopy indicated that the LC3-specific siRNA used in our experiments effectively down-regulated LC3 expression (Figure 7A). Digital quantification of LC3 fluorescence intensity confirmed that LC3 expression was significantly reduced in the cells transfected with the LC3-specific siRNA (Figure 7B). The results from a gentamicin protection assay showed that siRNA-mediated knock-down of LC3 in macrophages was associated with impaired bacterial killing, as indicated by an increase in intracellular Citrobacter numbers at 6 hours post-infection in IFNγ-treated cells (Figure 7C). We also examined the effects of LC3 silencing in peritoneal macrophages. We collected macrophages from normal BALB/c mice, pre-treated the cells with IFNγ and transfected the cells with siRNA before GFP-Citrobacter exposure. Similar to the results in the RAW264.7 macrophage cell line, a marked reduction in LC3 intensity was observed in the siRNA-treated cells (Figure 7D), which correlated with an increased frequency of GFP-Citrobacter in the cells (Figure 7 C). These observations support the idea that reduced LC3 expression is responsible for the impairment of autophagy-mediated bacterial killing in macrophages from helminth-infected mice.
Figure 7.
siRNA-mediated knock-down of LC3 leads to impaired bacterial killing in RAW264.7 cells and peritoneal macrophages in vitro. A. RAW264.7 cells, with or without overnight IFNγ pretreatment, were transfected with or without an siRNA specific for LC3. The cells were then stained with anti-LC3 antibody as described in Figure 1. Results show that IFNγ-induced LC3 expression was effectively decreased by the siRNA (400 ×). B. Quantification of LC3 fluorescence intensity in RAW264.7 cells transfected with either a control or LC3-specific siRNA. The LC3 fluorescence intensity per cell was measured digitally using the Graph digitizing software (Nikon Imaging Software Elements). The data shown represent the mean values of fluorescence intensity obtained from 50–60 cells per slide (1 slide from each of the two experiments was examined). *p < 0.05 for a comparison of two groups. C. After knocking down LC3 expression with the siRNA, the RAW264.7 cells were used for gentamicin protection assay. The data shown represent the mean ± SD of triplicate cultures. *p < 0.05 for a comparison of two groups. D: Peritoneal macrophages were isolated from normal BALB/c mice and treated with IFNγ overnight in vitro. The cells were transfected with siRNA specific for LC3 and infected 48 h later with C. rodentium for 1 h. LC3, GFP-Citrobacter and colocalization of LC3 and bacteria were examined (1000 ×). Scale bar represents 10 µm.
Discussion
Helminth infection has been known to alter, and in some cases to compromise, the immune response to other pathogens (5, 24, 27, 32). Our own work in a mouse co-infection model has demonstrated that infection with the helminth H. polygyrus significantly impairs the ability of the host to eliminate the bacterial enteropathogen C. rodentium (5, 6). One aspect of this impairment is the helminth-induced, Th2-dependent alternative activation of macrophages, which inhibits the ability of these cells to kill Citrobacter (7). The results of the present study extend these observations to shed light on one of the macrophage anti-microbial mechanisms that is affected by helminth infection. Our observations indicate that autophagy plays an important role in macrophage killing of phagocytosed Citrobacter, an observation that is consistent with increasing evidence that this process mediates disposal of a wide variety of pathogens (8–11, 27, 33, 34). They are also in keeping with a recent study showing that in vivo treatment of mice with wortmannin impaired clearance of Citrobacter, although this effect was attributed to alterations in phosphatidylinositol-3-kinase- and Akt-mediated intestinal epithelial proliferation (35). Interestingly, we found that autophagy-mediated elimination of Citrobacter was significantly compromised in macrophages isolated from helminth-infected mice. The underlying mechanism appears to involve decreased expression and processing of LC3, a key component of the autophagy machinery (36). These effects were dependent on the helminth-induced Th2 response, as indicated by their absence in macrophages from STAT6-deficient mice and by their induction by the Th2 cytokines IL-4 and IL-13. The molecular details of how IL-4 and IL-13 down-regulate LC3 expression are yet to be elucidated and are the subject of on-going studies in our laboratory.
To our knowledge, the results reported here represent the first demonstration that helminth infection impairs autophagy. They reveal a novel aspect of the immunomodulatory effects of helminth infection, and provide an in vivo, clinically relevant context to earlier observations showing that in vitro exposure of macrophages to IL-4 and IL-13 inhibited autophagy-mediated killing of mycobacteria(31). They also provide a mechanistic explanation for our previously reported finding that H. polygyrus significantly worsens the course of Citrobacter infection (5). Since autophagy is involved in defense against many different types of pathogens, our results have implications for a number of infectious diseases, particularly those that occur in areas of the world where chronic helminth infections are endemic. They could help to explain, for example, the increased incidence of tuberculosis in individuals chronically infected with helminth parasites (37). Defects in autophagy have also been implicated, on the basis of experimental models as well as genome-wide association studies in humans, in the pathogenesis of inflammatory bowel disease (IBD) (15–17). Although deliberate infection with helminths has demonstrated some success as a treatment for IBD (38), the data presented here raise the possibility that this approach may actually worsen the disease by impairing autophagy. Indeed, we have evidence suggesting that H. polygyrus infection can enhance the severity of intestinal inflammation in mouse models of IBD (unpublished data).
In summary, the results reported in this manuscript have added autophagy to the list of immunological processes that are affected by helminth infection. Determining the contribution of altered autophagy to the diverse immunomodulatory effects of helminths will be an interesting area for future investigation.
Acknowledgements
We are grateful for Mary McKee of the Microscopy Core of the Center for Systems Biology/Program in Membrane Biology for performing the electron microscopy studies.
Footnotes
This work was supported by grants from the National Institutes of Health (DK074727 and DK082427 to HNS, AI089700 to BJC), and from the Clinical Nutrition Research Center at Harvard (P30 DK040561 to WAW). Electron microscopy was supported by an Inflammatory Bowel Disease Grant DK43351 and a Boston Area Diabetes and Endocrinology Research Center Award DK57521.
References
- 1.Nokes C, Cooper E, Robinson B, Bundy D. Geohelminth infection and academic assessment in Jamaican children. Trans R Soc Trop Med Hyg. 1991;2:272–273. doi: 10.1016/0035-9203(91)90052-z. [DOI] [PubMed] [Google Scholar]
- 2.Crompton D, Nesheim M. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 3.Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, TB N. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS One. 2009;3:e489. doi: 10.1371/journal.pntd.0000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmby H. Gastrointestinal nematode infection exacerbates malaria-induced liver pathology. J Immunol. 2009;182:5663–5671. doi: 10.4049/jimmunol.0803790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C-C, Louie Steve, McCormick Beth A., W WA, Shi HN. Concurrent infection of an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infection and Immunity. 2005;73:5468–5481. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C-C, Louie Steve, McCormick Beth A., W AW, Shi HN. Helminth-primed dendritic cells alter the host response to enteric bacterial infection. J. Immunol. 2006;176:472–483. doi: 10.4049/jimmunol.176.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng M, Huntley Deke, Huang I-Fei, Foye-Jackson Ondulla, Wang Lijian, Sarkissian Aliese, Zhou Qingping, Walker W. Allan, Cherayil Bobby J., Shi HN. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, F DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa M, Yoshimori T, Suzuki T, Sagara H, M N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 10.Birmingham CL, Smith AC, Bakowski MA, Y T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G. Methods for assessing autophagy and autophagic cell death. Methods Mol. Biol. 2008;445:29–76. doi: 10.1007/978-1-59745-157-4_3. [DOI] [PubMed] [Google Scholar]
- 14.Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr. Opin Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath R, Xavier R. Autophagy, immunity and human disease. Curr Opin Gastroenterol. 2009;25:512–520. doi: 10.1097/MOG.0b013e32833104f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cadwell K, Patel Khushbu K., Maloney Nicole S., Liu Ta-Chiang, Ng Aylwin CY, Storer Chad E., Head Richard D., Xavier Ramnik, Stappenbeck ST, et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Gorvel JP, Chu YT, Wu JJ, Lei H. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS One. 2010;27:e10844. doi: 10.1371/journal.pone.0010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima N, Ohsumi Y, T Y. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 21.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. The International Journal of Biochemistry & Cell Biology. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J. Patholo. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima N. Methods for monitoring autophagy. Int. J. Biochem. Cell. Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Actor JK, Shirai M, Kullberg MC, Buller ML, Sher A, Berzofsky JA. Helminth infection results in decreased virus specific CD8+ cytotoxic T cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce E. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect Immun. 2001;69:1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady M, O'Neill SM, Dalton JP, Mills K. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect Immun. 1999;67:5372–5378. doi: 10.1128/iai.67.10.5372-5378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansfield L, Gauthier DT, Abner SR, Jones KM, Wilder SR, Urban J. Enhancement of disease and pathology by synergy of Trichuris suis and Campylobacter jejuni in the colon of immunologically naive swine. Am J Trop Med Hyg. 2003;68:70–80. [PubMed] [Google Scholar]
- 28.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RM, Urban JF, Farhang A, Hamed HA, Rozo CT, Boucher J, Van Rooijen a N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris J, Master SS, De Haro SA, Delgado M, Roberts EA, Hope JC, Keane J, D V. Th1–Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet Immunol Immunopathol. 2009;15:37–43. doi: 10.1016/j.vetimm.2008.10.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris J, De Haro Sergio A, Master Sharon S, Keane Joseph, Roberts Esteban A, D M, Deretic V. T Helper 2 Cytokines Inhibit Autophagic Control of Intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce EJ. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect Immun. 2001;69:1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter Berón MGG, Rabinovitch Michel, Colombo Maria I. Coxiella burnetii Localizes in a Rab7-Labeled Compartment with Autophagic Characteristics. Infect Immun. 2002;70:5816–5821. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méabh Cullinane LG, Li Xuelei, Adler Natalie-Lazar, Tra Thien, Wolvetang Ernst, Prescott Mark, Boyce John. D, Devenish Rodney J, Adler Ben. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–753. doi: 10.4161/auto.6246. [DOI] [PubMed] [Google Scholar]
- 35.Brown SL, C P, Goretsky T, Managlia E, Grimm GR, Ryu H, Zadeh M, Dirisina R, Barrett TA. Epithelial phosphatidylinositol-3-kinase signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection. Infect Immun. 2011;79:1863–1872. doi: 10.1128/IAI.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanida I. Autophagy basics. Microbiol Immunol. 2011;55:1–11. doi: 10.1111/j.1348-0421.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 37.Elias DBS, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–584. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 38.Summers RW, E D, Qadir K, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]