Abstract
Background
The hallmark of thoracic aortic aneurysms and dissections (TAAD) is progressive medial degeneration, which can result from excessive tissue destruction and insufficient repair. Although multipotent stem cells (SCs) are important in tissue repair, their role in TAAD is unknown. We sought to determine whether SCs are more abundant in TAAD tissue than in controls, and whether SCs within the diseased aortic wall differentiate into functionally relevant cell types.
Methods
Using immunohistochemistry, we compared the abundance of STRO-1+ cells, c-kit+ cells, and CD34+ cells in aortic tissue from patients with descending thoracic aortic aneurysms (n=12), patients with chronic descending thoracic aortic dissections (n=18), and age-matched organ donors (n=5). Using double immunofluorescence staining, we evaluated SC differentiation into smooth muscle cells (SMCs), fibroblasts, and macrophages.
Results
STRO-1+ cells, c-kit+ cells, and CD34+ cells were significantly more abundant in the media and adventitia of TAAD tissues than in controls. We identified subsets of STRO-1+ cells, c-kit+ cells, and CD34+ cells that also expressed the SMC marker SM22-α or fibroblast specific protein-1, suggesting SC differentiation into SMCs or fibroblasts. Other STRO-1+ cells expressed the macrophage marker CD68, suggesting differentiation into inflammatory cells.
Conclusions
SCs are more abundant in TAAD tissue compared to normal aortic tissue. Differentiation of SCs into SMCs, fibroblasts, and inflammatory cells within the diseased aortic wall suggests that SCs might be involved in both reparative and destructive remodeling processes in TAAD. Understanding the regulation of SC-mediated aortic remodeling will be a critical step toward designing strategies to promote aortic repair and prevent adverse remodeling.
Keywords: Aneurysm, aorta, aortic dissection, stem cells
Introduction
Despite significant improvements in the diagnosis and surgical repair of thoracic aortic aneurysms and dissections (TAAD), the associated morbidity and mortality remains high [1]. Degenerative remodeling within the medial layer [2] of TAAD tissue is characterized by loss of smooth muscle cells (SMCs) [3] and destruction of the extracellular matrix (ECM) [2]. This medial degeneration leads to weakening and progressive dilatation of the vascular wall and, ultimately, results in aortic dissection or aneurysm rupture. Understanding the pathobiology of destructive tissue remodeling is necessary to develop better treatment options for patients with TAAD.
The degenerative remodeling seen in TAAD can result from a combination of excessive destruction and insufficient repair. For example, repetitive hemodynamic stress or biologic insult can lead to chronic inflammation and excessive medial destruction within the aortic wall. When tissue is injured, inflammatory cells infiltrate the injured area and remodel the ECM to clear damaged or dead cells and degraded proteins. Whether stem cells (SCs) also aid in repairing or replacing damaged thoracic aortic tissue is unknown.
Multipotent SCs play an important role in tissue repair and regeneration. Both c-kit+ [4, 5] and CD34+ SCs [6-8] have been shown to play a critical role in tissue repair in the cardiovascular system. These SCs have the capacity to differentiate into multiple cell lineages, including endothelial cells, SMCs, and cardiomyocytes [9]. In addition, SCs can recruit and stimulate the proliferation of resident SCs [10, 11], creating a favorable microenvironment for cardiac or vascular repair [12]. Although SCs play an important role in the repair and regeneration of various cardiovascular tissues [13, 14], whether SCs participate in a reparative process that attempts to maintain structural and functional integrity of the aortic wall in TAAD is unknown. To begin exploring the hypothesis that SCs facilitate aortic wall repair, we first sought to determine whether SCs are more abundant in TAAD tissue than in normal aortic tissue, and whether SCs within the diseased aortic wall differentiate into functionally relevant cell types.
Patients and Methods
Patient Enrollment and Tissue Collection
The study was approved by the institutional review board at Baylor College of Medicine. Informed written consent was obtained from all patients. We enrolled 30 patients who underwent elective surgical repair of either a descending thoracic aortic aneurysm without dissection (TAA; n=12) or a chronic descending thoracic aortic dissection (TAD; n=18). We excluded patients with acute symptoms (<14 days); bicuspid aortic valve; heritable connective tissue disease (eg, Marfan syndrome); TAAD related to trauma, aortitis, or infection; and first-degree relatives who had TAA or TAD. During aneurysm repair, samples of the posterior-lateral aortic wall were excised from the site of maximal aortic dilatation. In cases of aortic dissection, samples were obtained from the outer wall of the false lumen. The mean interval between the onset of dissection and operation was 5.1 ± 4.5 years. Control descending thoracic aortic tissue (n=5) was collected from age-matched organ donors without aortic aneurysm, dissection, coarctation, or previous aortic repair (International Institute for the Advancement of Medicine, Jessup, PA). Characteristics of enrolled patients are presented in Table 1. Compared to control subjects, TAAD patients were more likely to have a history of smoking but less likely to have a history of stroke.
Table 1.
Patient Characteristics
| Characteristics | Control (n=5) |
TAA (n=12) |
TAD (n=18) |
P values |
|---|---|---|---|---|
| Age (years) | 61.9±4.4 | 66.0±5.5 | 62.5±5.5 | 0.2 |
| Men | 3 (60%) | 5 (42%) | 13 (72%) | 0.3 |
| Hypertension | 4 (80%) | 10 (83%) | 18 (100%) | 0.2 |
| COPD | 1 (20%) | 4 (33%) | 6 (33%) | 0.8 |
| Diabetes | 2 (40%) | 2 (17%) | 1 (6%) | 0.1 |
| Stroke | 3 (60%) | 1 (8%) | 2 (11%) | 0.02 |
| Coronary artery disease |
0 | 2 (17%) | 3 (17%) | 0.6 |
| Peripheral artery disease |
0 | 2 (17%) | 2 (11%) | 0.6 |
| Smoking history | 1 (20%) | 11 (92%) | 11 (61%) | 0.02 |
| Lipid-lowering medication |
3 (60%) | 6 (50%) | 6 (33%) | 0.5 |
| Aneurysm diameter at sample site (cm) |
NA | 5.8±1.2 | 6.2±1.4 | 0.4 |
Note: Age was compared by using ANOVA. All other variables were compared using chi-square.
COPD, chronic obstructive pulmonary disease.
Immunohistochemistry
For immunohistochemical staining, formalin-fixed, paraffin-embedded aortic sections were deparaffinized and rehydrated before antigen retrieval in citrate buffer (92–98°C for 12 min). Endogenous peroxidase activity was quenched by incubating the slides with 3% hydrogen peroxide for 10 min, and nonspecific staining was reduced by blocking with 5% normal blocking horse serum. The sections were incubated with the primary antibodies at 4°C overnight and then incubated with peroxide-conjugated anti-mouse or anti-rabbit IgG secondary antibody. The sections were stained with 3, 3-diaminobenzidine by using the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA). Nuclei were counterstained with hematoxylin. Slides treated only with normal IgG were used as negative controls. The primary antibodies used were anti-STRO-1 (R&D Systems, Minneapolis, MN), anti-c-kit (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-CD34 (Santa Cruz Biotechnology). The density of positive cells from three randomly selected 400X microscopic fields per sample were counted and normalized with the aortic area.
Double Immunofluorescence Staining
For double immunofluorescence staining, formalin-fixed, paraffin-embedded tissues were cut into 5 um sections, deparaffinized, rehydrated, and then subjected to antigen retrieval. Tissue sections were incubated with the primary antibody overnight at 4°C, followed by incubation with secondary antibody for 1 hour at 37°C. The nuclei were counterstained for visualization with DAPI. The primary antibodies used were anti-STRO-1 (R&D Systems), anti-c-kit (Santa Cruz Biotechnology), anti-CD34 (Santa Cruz Biotechnology), anti-SM22-α (Abcam, Cambridge, MA), anti-fibroblast specific protein (FSP)-1 (Abcam), and anti-CD68 (Santa Cruz Biotechnology). The secondary antibodies used were Alexa Fluor 488-, Alexa Fluor 568-, and Alexa Fluor 647-conjugated anti-IgG (Invitrogen, Carlsbad, CA). Slides treated with normal IgG only were used as negative controls.
Statistical Analysis
All quantitative data are presented as the mean ± standard deviation (SD). Data were analyzed with SPSS software, version 11.0 (SPSS Inc., Chicago, IL). The differences between the 3 groups were evaluated by chi-square for categorical variables and by one-way analysis of variance (ANOVA) for continuous variables. Two-tailed P values are reported.
Results
STRO-1+ Cells Were Abundant in TAA and TAD Tissue (Fig 1)
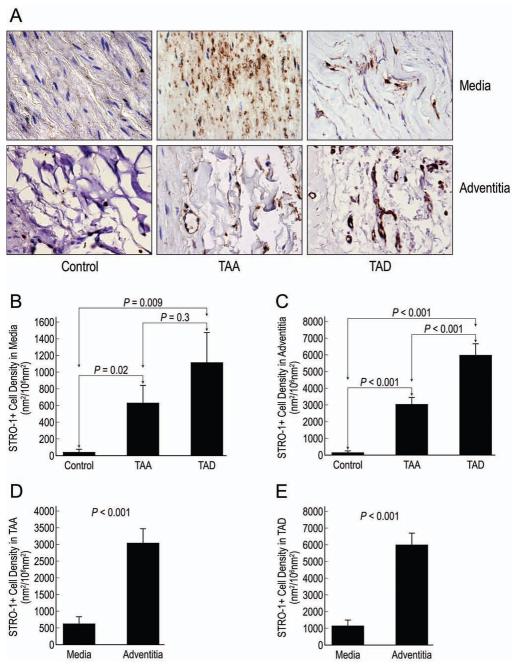
Figure 1.
Representative images of STRO-1+ cells detected by immunohistochemical staining in the medial and adventitial layers of control aortic, TAA, and TAD tissues (A; magnification, 400X). The mean densities of STRO-1+ cells in the media (B) and adventitia (C) were compared in the 3 groups. The relative distribution of STRO-1+ cells in the media and adventitia was compared in TAA (D) and TAD (E) tissues.
We found that TAA and TAD tissue had significantly more STRO-1+ cells in both the medial and adventitial layers compared with control tissues. The density of STRO-1+ cells in the medial layer was similar in TAD and TAA tissues; however, the adventitial layer contained significantly more STRO-1+ cells in TAD tissue than in TAA tissue. Examination of the distribution of STRO-1+ cells showed significantly more STRO-1+ cells in the adventitial layer than in the medial layer in both TAA and TAD tissues.
C-kit+ Cells Were Abundant in TAA and TAD Tissue (Fig 2)
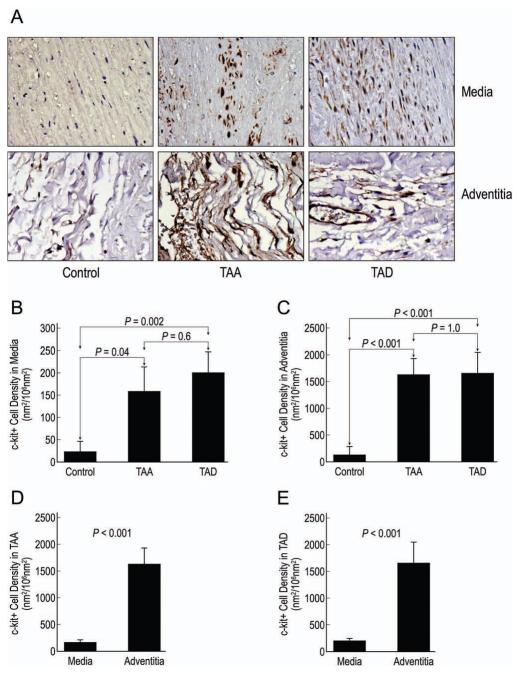
Figure 2.
Representative images of c-kit+ cells detected by immunohistochemical staining in the medial and adventitial layers of control aortic, TAA, and TAD tissues (A; magnification, 400X). The mean densities of c-kit+ cells in the media (B) and adventitia (C) were compared in the 3 groups. The relative distribution of c-kit+ cells in the media and adventitia was compared in TAA (D) and TAD (E) tissues.
We found that tissue from TAA and TAD patients had significantly more c-kit+ cells in both the medial and adventitial layers compared with control tissues. The density of c-kit+ cells in the TAA and TAD tissues was similar. Analysis of the distribution of c-kit+ cells showed that there were significantly more c-kit+ cells in the adventitial layers than in the medial layers in both TAA and TAD tissues.
CD34+ Cells Were Abundant in TAA and TAD Tissue (Fig 3)
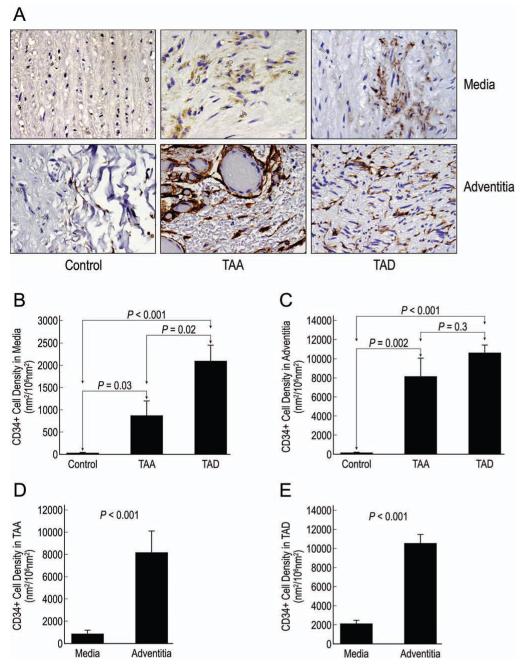
Figure 3.
Representative images of CD34+ cells detected by immunohistochemical staining in the medial and adventitial layers of control aortic, TAA, and TAD tissues (A; magnification, 400X). The mean densities of CD34+ cells in the media (B) and adventitia (C) were compared in the 3 groups. The relative distribution of CD34+ cells in the media and adventitia was compared in TAA (D) and TAD (E) tissues.
We found a significant increase in the density of CD34+ cells in the medial and adventitial layers of both TAA and TAD tissues compared with control tissues. Although similar levels of CD34+ cells were seen in the adventitia of TAA and TAD tissues, the medial layer of TAD tissues contained significantly more CD34+ cells than did the medial layer of TAA tissues. The distribution analysis showed that significantly more CD34+ cells were present in the adventitia than in the media in both TAA and TAD tissues.
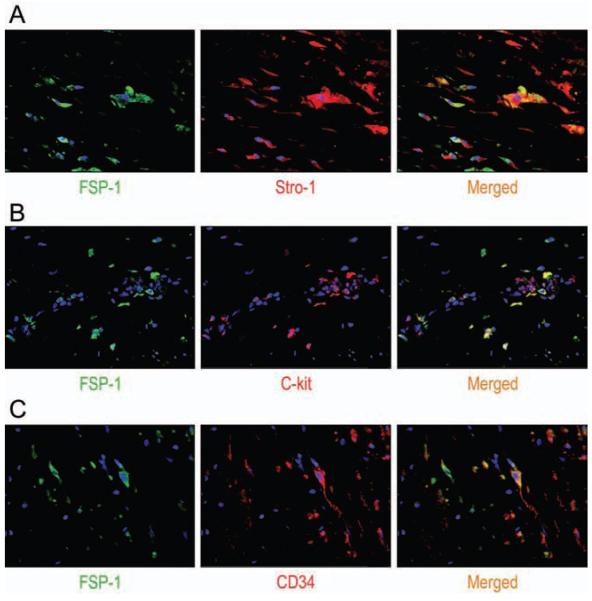
Differentiation of SCs Into SMCs (Fig 4)
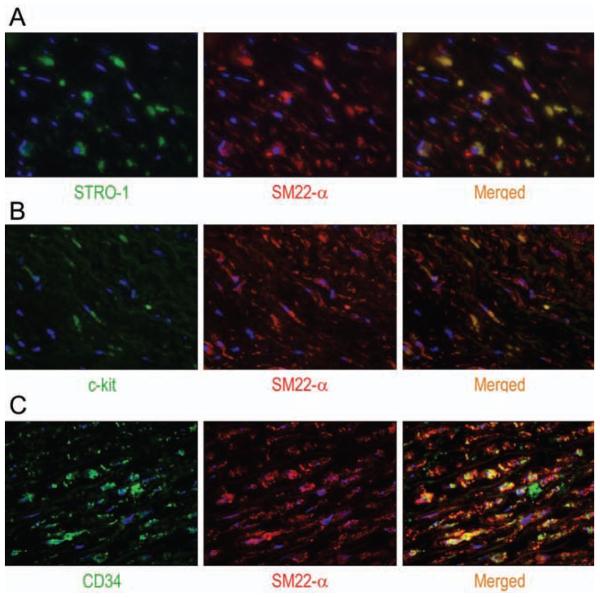
Figure 4.
Representative images (magnification, 400X) after double immunofluorescence staining showing the colocalization of the SMC marker SM22-α with STRO-1 (A), c-kit (B), or CD34 (C) in the medial layer of TAA tissue. Nuclei were counterstained with DAPI (blue).
Using double immunofluorescence staining, we examined the potential differentiation of STRO-1+ cells, c-kit+ cells, and CD34+ cells into SMCs in the medial layer of diseased aortic tissue. We found a subset of STRO-1+ cells that also stained positive for SM22-α in both TAA and TAD samples. Similarly, we identified a small number of c-kit+ cells and CD34+ cells that stained positive for SM22-α in TAA and TAD tissues. These findings suggest potential differentiation of these SCs into SMCs and participation in aortic repair.
Differentiation of SCs Into Fibroblasts (Fig 5)
Figure 5.
Representative images (magnification, 400X) after double immunofluorescence staining showing the colocalizaton of the fibroblast marker FSP-1 with STRO-1 (A), c-kit (B), or CD34 (C) in the adventitial layer of TAA tissue. Nuclei were counterstained with DAPI (blue).
We also examined the potential differentiation of STRO-1+ cells, c-kit+ cells, and CD34+ cells into fibroblasts in the adventitial layer of diseased aortic tissue. We detected the expression of FSP-1 in STRO-1+ cells, c-kit+ cells and CD34+ cells in both TAA and TAD samples. These findings suggest that these SCs may potentially differentiate into fibroblasts and participate in aortic remodeling.
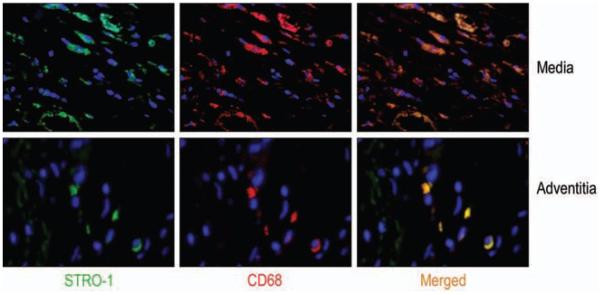
Differentiation of STRO-1+ Cells Into Macrophages (Fig 6)
Figure 6.
Representative images (magnification, 400X) of double immunofluorescence staining showing the colocalization of the macrophage marker CD68 with STRO-1 in the media and adventitia of TAA tissue. Nuclei were counterstained with DAPI (blue).
Using double immunofluorescence staining, we also examined the potential ability of stem cells to differentiate into macrophages within the diseased aortic wall. We found that large numbers of STRO-1+ cells, but not c-kit+ cells or CD34+ cells, also expressed the macrophage marker, CD68. The double-positive cell populations were seen in both the medial and adventitial layers in TAA and TAD tissues, and were especially abundant around areas of inflammatory cell infiltration.
Comment
Multipotent SCs are known to play an important role in arterial remodeling after injury. The presence of circulating endothelial progenitor cells has been previously reported in a murine model of abdominal aortic aneurysms [15], in patients with abdominal aortic aneurysms [16], and, recently, in patients with ascending aortic aneurysms [17]. In this study, we have shown that SCs are abundant in two other forms of aortic disease: descending TAA and chronic TAD. Specifically, we found that there were significantly more STRO-1+ cells, c -kit+ cells, and CD34+ cells and in the media and adventitia of aortic tissue from TAA and TAD patients than in control aortic tissue. Furthermore, subsets of STRO-1+ cells, c-kit+ cells, and CD34+ cells appeared to differentiate into SMCs and fibroblasts, and a large number of STRO-1+ cells exhibited differentiation into macrophages. The presence of multipotent SCs at sites of aneurysm and dissection formation that can further differentiate into SMCs suggests the existence of an active repair process involving SCs.
STRO-1+ cells are early mesenchymal stromal precursor cells. Although STRO-1+ cells have been suggested to have multilineage differentiation capacity [18] and paracrine activity [19], their role in tissue repair is less clear and remains controversial [20]. We found that STRO-1+ cells were abundant in TAA and TAD tissues. Interestingly, the STRO-1 marker colocalized with markers for SMCs, fibroblasts, and macrophages, indicating the potential of STRO-1+ cells to differentiate into these cell types. Our findings indicate that stem cells not only may promote vascular repair by differentiating into vascular SMCs and fibroblasts, or secreting growth factors, but also may facilitate tissue damage by differentiating into inflammatory cells. The greater amount of STRO-1+ cells in the adventitia of TAD tissue compared to TAA tissue suggests differing remodeling processes.
C-kit+ cells have been shown to respond to tissue injury [21]. C-kit+ cells can induce the secretion of angiogenic cytokines such as vascular endothelial growth factor (VEGF) [4] and stimulate host stem cell proliferation and differentiation [12]. Moreover, they have been reported to differentiate into endothelial cells, cardiomyocytes, and SMCs [22, 23]. In a mouse study, bone marrow-derived c-kit+ cells promoted an angiogenic response in infarcted myocardium [4], and mutations in the c-kit receptor prevented angiogenesis and tissue repair, which resulted in cardiac failure and death [4]. In the present study, we observed an increase in c-kit+ cells in both TAA and TAD tissues and showed that c-kit+ cells also expressed a SMC marker or a fibroblast marker. Our results suggest that c-kit+ cells may have the capacity to differentiate into SMCs and contribute to aortic repair or may differentiate into fibroblasts and participate in aortic remodeling.
Like c-kit+ cells, CD34+ cells respond to tissue injury [24]. CD34+ cells have paracrine activity [24] and can secrete VEGF [25]. In addition, CD34+ cells have been reported to differentiate into endothelial cells [6-8, 26-28], SMCs, and cardiomyocytes [6, 7]. In experimental studies, CD34+ cells promoted neovascularization, improved wound healing in ischemic limbs [28, 29], and participated in cardiomyogenesis [8, 25, 30-32]. In the present study, CD34+ cells were abundant in TAA and TAD tissues and showed the potential to differentiate into SMCs and fibroblasts. In contrast to our findings with c-kit+ and STRO-1+ cells, we found a significant difference in medial CD34+ cell density between aneurysm and dissection tissue. The higher density of CD34+ cells in TAD tissue than in TAA tissue may be attributed to the paracrine effect of CD34+ cells, the larger area of damaged aortic wall (circumferential and longitudinal) in TAD, or the presence of chronic hematoma within the false lumen.
Our observation that SCs are highly abundant in TAA and TAD tissues raises many interesting questions. First, the origin of the SCs needs to be clarified. SCs involved in aortic repair may be recruited from the bone marrow or activated after residing in a quiescent state in the vascular wall. Second, the mechanisms of SC involvement in aortic repair and remodeling need to be investigated. For example, it is unclear whether these SCs participate in aortic repair and remodeling by differentiating into vascular cells, by producing growth factors that create a favorable environment, or through both mechanisms. Third, the specific role of stem cells in aortic wall remodeling remains unknown. We have shown that SCs can differentiate into SMCs, fibroblasts, or inflammatory cells; each of these cell types has a different function and could lead to effective repair, maladaptive remodeling, or further aortic damage. Finally, the factors and signaling pathways involved in regulating SC recruitment, survival, proliferation, growth factor production, and differentiation are unknown. Manipulation of these pathways by stimulating SC recruitment, activating paracrine activity, and directing differentiation may promote aortic repair. For instance, manipulation of SDF-1/CXCR axis[33,34] can potentially increase efficient homing of stem cells to damaged myocardium. Growth factors such as VEGF, granulocyte colony-stimulating factor , transforming growth factor-β and insulin-like growth factor-1 have been shown to be involved in homing of bone marrow-derived SCs to injured tissue, and can be potentially used for endogenous stem cell recruitment [35-38]. A potential treatment strategy also lies in manipulation of SC signaling pathway. For instance, Li and colleagues [36] recently demonstrated that altering the Notch1 signaling pathway in bone marrow-derived mesenchymal SCs improves their capacity to repair ischemic myocardium in a mouse model. Even though cell based therapy through manipulation of endogenous stem cells is an attractive option, the mechanism responsible for SC chemotaxis, homing, and activation remain poorly understood. Understanding the regulation of SC-mediated aortic remodeling will be a critical step toward designing strategies to promote aortic repair and prevent adverse remodeling.
This observational study has several important limitations. Because the samples we examined represent end-stage disease, we were not able to study the abundance or differentiation of SCs during the early stages of TAAD formation. Furthermore, the samples in this study ultimately represent failed tissue repair; the characteristics of aortic SCs in small stable aneurysms (representing successful tissue repair) are likely to be very different. Finally, there were important differences in the clinical characteristics of the TAAD patients and the control subjects; because the impact of various clinical factors—such as smoking and diabetes—on SC recruitment, activation and differentiation is unknown, these factors may have contributed to the differences we observed.
Despite these limitations, our study demonstrates that STRO-1+ cells, c-kit+ cells, and CD34+ cells are abundant in human TAA and TAD tissues, and that these SCs can differentiate into SMCs, fibroblasts, or macrophages within the diseased aortic wall. These findings suggest the potential for SCs to participate in both reparative and destructive aortic remodeling processes. Further studies are needed to examine the mechanisms involved in SC differentiation in the diseased aortic wall and to determine whether this process can be manipulated to favor aortic wall repair.
Acknowledgments
This study was supported by grant R01 HL085341 (to S.A.L.). The Thoracic Aortic Disease Tissue Bank at Baylor College of Medicine was supported in part through the Tissue Banking Core of the Specialized Center of Clinically Oriented Research in Thoracic Aortic Aneurysms and Dissections (NIH P50 HL083794). Darrell Wu was supported by a training grant (NIH T32 HL007676) through the Department of Molecular Physiology and Biophysics at Baylor College of Medicine. We thank Ludivine Russell, MS, and Laura C. Palmero, MPH, for enrolling patients and collecting tissue samples and corresponding clinical data, and thank Mary T. Nguyen and Pingping Ren, MD, for assisting with immunostaining. We gratefully acknowledge Scott A. Weldon, MA, CMI, of Baylor College of Medicine, for assistance with illustrations, and Rebecca A. Bartow, PhD, of the Texas Heart Institute at St. Luke’s Episcopal Hospital, for providing editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting: Presented at the 58th Annual Meeting of the Southern Thoracic Surgical Association, San Antonio, Texas, November 10, 2011
References
- 1.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. [PubMed] [Google Scholar]
- 2.Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39:21–6. doi: 10.1016/s0002-9149(77)80005-2. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 4.Fazel S, Cimini M, Chen L, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dentelli P, Rosso A, Balsamo A, et al. C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–71. doi: 10.1182/blood-2006-06-029603. [DOI] [PubMed] [Google Scholar]
- 6.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 7.Wojakowski W, Tendera M, Michalowska A, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zhang S, Rabinovich B, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–11. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji H, Miyoshi S, Ikegami Y, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106:1613–23. doi: 10.1161/CIRCRESAHA.109.205260. [DOI] [PubMed] [Google Scholar]
- 12.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 14.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:1916–21. doi: 10.1161/01.ATV.0000142805.20398.74. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J, Tooze J, Cockerill G, Choke E, Loftus I, Thompson MM. Endothelial progenitor cells and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:327–30. doi: 10.1196/annals.1383.011. [DOI] [PubMed] [Google Scholar]
- 17.Parietti E, Pallandre JR, Deschaseaux F, et al. Presence of circulating endothelial progenitor cells and levels of stromal-derived factor-1alpha are associated with ascending aorta aneurysm size. Eur J Cardiothorac Surg. 2011;40:e6–12. doi: 10.1016/j.ejcts.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009;18:487–96. doi: 10.1089/scd.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psaltis PJ, Paton S, See F, et al. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol. 2010;223:530–40. doi: 10.1002/jcp.22081. [DOI] [PubMed] [Google Scholar]
- 20.Bensidhoum M, Chapel A, Francois S, et al. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–9. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- 21.Limana F, Zacheo A, Mocini D, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–65. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 22.Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–13. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahoo S, Klychko E, Thorne T, et al. Exosomes From Human CD34+ Stem Cells Mediate Their Proangiogenic Paracrine Activity. Circ Res. 2011;109:724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshioka T, Ageyama N, Shibata H, et al. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23:355–64. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 27.Hildbrand P, Cirulli V, Prinsen RC, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–9. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 28.Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–64. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 29.Madeddu P, Emanueli C, Pelosi E, et al. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. Faseb J. 2004;18:1737–9. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- 30.Ott I, Keller U, Knoedler M, et al. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. Faseb J. 2005;19:992–4. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 31.Shintani S, Kusano K, Ii M, et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S123–8. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 33.Bernal A, San Martin N, Fernandez M, et al. L-selectin and SDF-1 enhance the migration of mouse and human cardiac mesoangioblasts. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theiss HD, Vallaster M, Rischpler C, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res. 2011;7:244–55. doi: 10.1016/j.scr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Tang JM, Wang JN, Zhang L, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–11. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krankel N, Spinetti G, Amadesi S, Madeddu P. Targeting stem cell niches and trafficking for cardiovascular therapy. Pharmacol Ther. 2010;129:62–81. doi: 10.1016/j.pharmthera.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–8. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]