Abstract
ALK1 (ACVRL1) is a member of the TGFβ receptor family and is expressed predominantly by arterial endothelial cells (EC). Mutations in ACVRL1 are responsible for Hereditary Hemorrhagic Telangiectasia Type 2 (HHT2), a disease manifesting as fragile vessels, capillary overgrowth, and numerous arterio-venous malformations (AVMs). Arterial EC also express EphrinB2, which has multiple roles in vascular development and angiogenesis and is known to be reduced in ACVRL1 knockout mice. Using an in vitro angiogenesis model we find that the Alk1 ligand BMP9 induces EphrinB2 in EC, and this is entirely dependent on expression of Alk1 and at least one of the co-receptors BMPRII or ActRII. BMP9 induces both ID1 and ID3, and both are necessary for full induction of EphrinB2. Loss of Alk1 or EphrinB2 results in increased arterial-venous anastomosis, while loss of Alk1 but not EphrinB2 results in increased VEGFR2 expression and enhanced capillary sprouting. Conversely, BMP9 blocks EC sprouting and this is dependent on Alk1, BMPRII/ActRII and ID1/ID3. Finally, notch signaling overcomes the loss of Alk1 – restoring EphrinB2 expression in EC, and curbing excess sprouting. Thus, in an in vitro model of HHT2, loss of Alk1 blocks BMP9 signaling, resulting in reduced EphrinB2 expression, enhanced VEGFR2 expression, and misregulated EC sprouting and anastomosis.
Introduction
Hereditary Hemorrhagic Telangiectasia (HHT) represents a spectrum of diseases characterized by fragile telangiectatic vessels in numerous mucosal tissues, and arteriovenous malformations (AVMs), predominantly in lungs, liver, gastrointestinal tract, and brain. These defects manifest as recurrent nosebleeds (epistaxis), and in severe cases, ischemic injuries or stroke. HHT occurs with a frequency of approximately 1:5,000 individuals [1], with some regional differences [2], and is inherited as an autosomal dominant trait [3]. HHT1 and HHT2 are caused by mutations in the endoglin (ENG) and Alk1 (ACVRL1) genes, respectively [4, 5], each of which are involved in TGF- -family signaling [6, 7].
Although the genetic underpinnings of HHT are known, the specific cellular and molecular mechanisms are not well understood, especially why only some vascular beds are affected, and what the relevant ligands are for these receptors. Recent work in a mouse model for HHT2 has provided compelling evidence that environmental factors such as wounds, in addition to mutation or loss of Alk1, are required for the formation of AVMs in adult mice [8], however there is still much to be learned about the molecular role of Alk1 in vascular homeostasis and/or angiogenesis. In addition there is mounting evidence for involvement of modifier genes, although these are much less well characterized [9, 10].
Alk1 is one of seven type I receptors for the TGF family of signaling molecules, and is expressed predominantly by endothelial cells (EC) where it is largely restricted to the arterial side of the vasculature, except during angiogenesis, where it is also expressed on activated EC [11]. Alk1, a serine/threonine kinase, has been shown to bind TGF 1 as well as activins and recent work has also identified it as a receptor for BMP9 and BMP10 [12–16]. Type I receptors bind ligand in conjunction with type II receptors, also serine/threonine kinases, which triggers phosphorylation of the smad signaling intermediates, which in the case of Alk1 are smad1, 5, and 8. Interestingly, other TGF receptors, such as Alk5, signal through smad2 and smad3 [15, 17]. Type II receptors expressed by EC include TGF RII, BMPRII and ActRII. EC also express a type III receptor, ENG, mutations in which are responsible for HHT1 [5, 15, 17]. ENG is expressed on both sides of the vascular tree and it is thought to potentiate binding of ligand to the type I and II receptors.
The effect of TGF signaling on EC in culture appears to depend on a number of factors, including, concentration, duration of signaling, the type of EC used and possibly their length of time in culture [15]. Generally, TGF is thought to block proliferation and migration and to induce matrix proteins such as fibronectin and collagens I, IV and V. Defining the downstream targets and effects of Alk1 has been even more perplexing, particularly because of the conflicting data on the expression and role of Alk5 in EC [18, 19]. Several groups have used constitutively active (CA) forms of these receptors, but have rarely reported congruent results [20–24]. For example, Ota et al [24] found that both Alk1 and Alk5 blocked proliferation of HUVEC, but only CA-Alk5 blocked tube formation in collagen gels. Goumans et al, on the other hand, found that CA-Alk1 increased migration of mouse embryonic endothelial cells, whereas CA-Alk5 decreased migration [20]. Others have reported that both CA-Alk1 and CA-Alk5 block vascular sprouting from embryoid bodies [23]. A model has been proposed to reconcile these disparate results based on the idea of Alk1 and Alk5 balancing responses to TGF family ligands in response to different ligand concentrations [25, 26]. This balance hypothesis is, however, challenged by studies in zebrafish – where the loss of Alk1 (violet beauregarde) results in increased numbers of EC, suggesting that Alk1 normally acts to block EC proliferation [27]; and in mice – where a lacZ knock-in indicated that, at least during early development, Alk5 is not expressed by EC but is restricted to the vessel media, especially smooth muscle cells [19]. Thus, on balance, it appears likely that Alk1 acts to suppress EC migration and possibly proliferation, thereby helping to maintain them in a quiescent state. Several recent in vitro studies also support this interpretation [14, 21, 23, 28].
The idea that TGF is the predominant ligand for Alk1 has been challenged in a number of studies. For example, whereas vascular-specific targeting of Alk1 recapitulates the phenotype of HHT2 [29], vascular-specific targeting of TGF RII, which is the major type II receptor for TGF on EC, has no vascular phenotype [18]. Similarly, vascular targeting of Alk5, also has no effect on blood vessel development [18]. Consistent with these findings, BMP9 and BMP10 have been identified as two high-affinity ligands for Alk1 [13, 14, 16] and the BMP receptor, BMPRII, is expressed by EC. BMP9 is present in human serum at around 2–12 ng/ml (40- 250pM), which is several-fold higher than the Kd for the receptor (reported to be around 1pM) suggesting that Alk1 may be tonically activated to maintain vascular quiescence [13]. BMP9 stimulation of EC has been shown to induce a number of genes, including ID1, and to inhibit EC proliferation of pulmonary EC [30].
Previous studies of Alk1 knockout mice noted loss of EphrinB2 expression by EC [29]. EphrinB2 and its ligand EphB4 define arteries and veins respectively, and are thought to have a role in preventing direct arterio-venous anastomoses [31, 32]. Interestingly, although notch1 and notch4 are also essential for maintaining vessel identity, Alk1 mutants show strong similarities to notch gain-of-function mutants [33]. Recently, several reports have suggested that EphrinB2 also has functions aside from maintaining vessel identity, including roles in EC morphology and motility [34, 35], possibly downstream of effects on VEGF receptor internalization and signaling [36, 37].
We have investigated the relationship between BMP9, Alk1 and EphrinB2 in an in vitro angiogenesis model of HHT2 and find that BMP9 induces EphrinB2 expression through an Alk1-ID1/3 pathway. Moreover, loss of Alk1 in EC (mimicking HHT2) results in a loss of BMP9-induced EphrinB2, up regulation of VEGFR2 expression, and misregulated vascular sprouting and anastomosis. Finally, the loss of EphrinB2 expression in Alk1 knock-out EC can be overcome by enhanced notch signaling.
Materials and Methods
Cell culture and other reagents
Human Umbilical Vein Endothelial Cells (HUVEC) and Endothelial Colony-forming Cell derived EC (ECFC-EC) cell lines were isolated from umbilical cords obtained from local hospitals under University of California, Irvine Institutional Review Board approval. Human Umbilical Artery Endothelial Cells (HUAEC) were purchased from Lonza (Allendale, NJ). BMP9 and antibody for EphrinB2 and -actin were from R&D systems (Minneapolis, MN), Santa Cruz Biotechnology Inc (Santa Cruz, CA), and Abcam (Cambridge, MA). The siRNA for control, ALK1, ID1, ID3, ActRII, BMPRII and EphrinB2 were purchased from Ambion (Austin, TX). The ALK1 expression constructs were generous gifts from Dr. Paul Oh (U. Florida). Where used, BMP9 was added in serum-free medium.
Fibrin gel sprouting assay
The 3D in vitro fibrin gel sprouting assay model of angiogenesis was performed as described previously [38]. Briefly, primary HUVEC, HUAEC or ECFC-EC (200 cells/bead)-coated Cytodex3 beads (Sigma-Aldrich Co., St. Louis, MO) in fibrin gels were cultured in 24well plates (250 beads/well) in EGM2 medium with normal human lung fibroblasts (NHLF, 20,000cells/well) plated on top of the gel. After 6–8 days the beads were photographed using an Olympus IX70 inverted phase contrast/fluorescence microscope (Melville, NY), and the sprouts whose lengths were greater than the diameter of the bead were counted. Lumen formation was quantified by counting the number of sprouts that had formed lumens and results are expressed as a percentage. Counts were performed blinded to the treatment group. To investigate anastomosis using HUVEC and HUAEC, we generated retrovirus stocks using packaging plasmid pBMN-GFP and mCherry with Phoenix retrovirus packaging system from Orbigen, and transduced the HUVEC and HUAEC with GFP and mCherry, respectively. After transfection of siRNA for control, EphrinB2, EphB4 and ALK1, beads coated with HUVEC or HUAEC were mixed and cultured in fibrin gels with human lung fibroblasts on top of the gel. The sprouts that formed connections between HUVEC- and HUAEC-coated beads were counted.
Transfection of plasmids and siRNAs
HUVEC, HUAEC and ECFC-EC (1.5×106 cells/100-mm dish) cells were seeded and transfected with either pDelta4 (5µg), pcDNA 3.1 (5µg) or siRNA (13pmole/6well plate) using the Lipofectamine 2000 reagent (Life Technologies). For the luciferase reporter assay, luciferase constructs (0.5 g) were cotransfected into EC. The cells were transfected in serum-free medium for 3 hr and then were switched to a medium containing 10% FBS. Cells were then incorporated into the fibrin gel sprouting assay, luciferase reporter assays, western blot analysis or qRTPCR analysis. For western blot analyses, whole-cell lysates were prepared after transfection of siRNA for control and ALK1. For luciferase activity, cell extracts were assayed using luciferin (Sigma-Aldrich) as the substrate and normalized to protein concentration. Luciferase activities were expressed as the mean ± SD of measurements made from three identical wells.
Quantitative RT-PCR
Quantitative Real Time-PCR was performed as described previously [39]. Briefly, RNA isolation and cDNA synthesis were performed using the Trizol (Qiagen) and the iScript cDNA Synthesis kit (Bio-Rad, Madison, WI). The cDNA was synthesized from 1 µg of total RNA in a 25-µl volume. The reaction was incubated at 25 °C for 5 min and at 37 °C for 30 min and then inactivated at 85 °C for 5 min. Real time PCR analysis was performed on an IQ5 real time PCR (Bio-Rad) using the following primers: For EphrinB2 (GenBank accession number, NM_004093), 5’- TTCGA CAACA AGTCC CTTTG-3’ (sense) and 5’- GATGT TGTTC CCCGA ATGTC -3’(antisense) ; for ALK1(GenBank accession number, NM_000020), 5’- TTGGG CACCA CATCA TAGAA - 3’ (sense) and 5’- GACTG ACATC TGGGC CTTTG -3’(antisense) ; for ENG (GenBank accession number, NM_001114753.1), 5’- GGGTC TCAAG ACCAG GAAGTC -3’ (sense) and 5’- CAGAG TGCAG CAGTG AGCA -3’(antisense) ; and for GAPDH (GenBank accession number, NM_002046.3), 5’- AATCC CATCA CCATC TTCCA -3’ (sense) and 5’- TGGAC TCCAC GACGT ACTCA -3’(antisense). To avoid amplification of genomic DNA, primers for each gene were chosen from different exons. A 2-µl aliquot from the cDNA synthesis from 20 ng of RNA/µl of reaction was used in 20 µl of PCR buffer containing Brilliant SYBR Green (Stratagene) with a 0.1 nM concentration of each primer. Amplification was performed in triplicate using GAPDH, as a control. The change in fluorescence of SYBR Green in every cycle was monitored by the IQ5 system software.
Cloning of the EphrinB2 promoter region and luciferase reporter assay
To analyze the EphrinB2 promoter, the 5'-flanking region (−929 ~ +176) of the human EphrinB2 gene (GenBank accession number: AJ563723) was amplified from human genomic DNA (Sigma) and cloned into the SmaI site of the luciferase reporter vector pGL3-Basic (Promega). The DNA sequence of the EphrinB2 promoter was confirmed by DNA sequencing.
Microscopy
Visualization of fibrin gel sprouting assays was performed using brightfield images collected on an inverted microscope (Olympus IX70) with a SPOT Idea 3.0 megapixel color mosaic camera and SPOT software (SPOT Imaging Solutions, Sterling Heights MI). For confocal images, cells were stained with DAPI and Texas Red-X phalloidin (Invitrogen, St. Louis, MO). Confocal images were collected using an Olympus Fluorview FV1000 confocal microscope. Images were processed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA) to adjust contrast and color balance. All images in a given experiment were treated similarly.
Statistical analysis
Data are shown as the mean ± SEM. For statistical significance between groups, we used a paired Student t-test with P values less than 0.05 being considered significant.
Results
For these studies we used three types of endothelial cells (EC) to investigate the Alk1-EphrinB2 pathway – human umbilical vein EC (HUVEC), human umbilical artery EC (HUAEC) and cord blood endothelial colony-forming cell-derived EC (ECFC-EC). As expected, HUAEC expressed high levels of EphrinB2 and low levels of the vein markers EphB4 and COUPTFII (Fig. S1). HUVEC expressed EphB4, COUPTFII, and moderate levels of EphrinB2. ECFC-EC had what appeared to be a non-committed phenotype, expressing EphrinB2 at levels comparable to HUAEC, and EphB4 and COUPTFII at levels comparable to HUVEC (Fig. S1).
BMP9 induces EphrinB2 through Alk1, BMPR-II and ActR-II
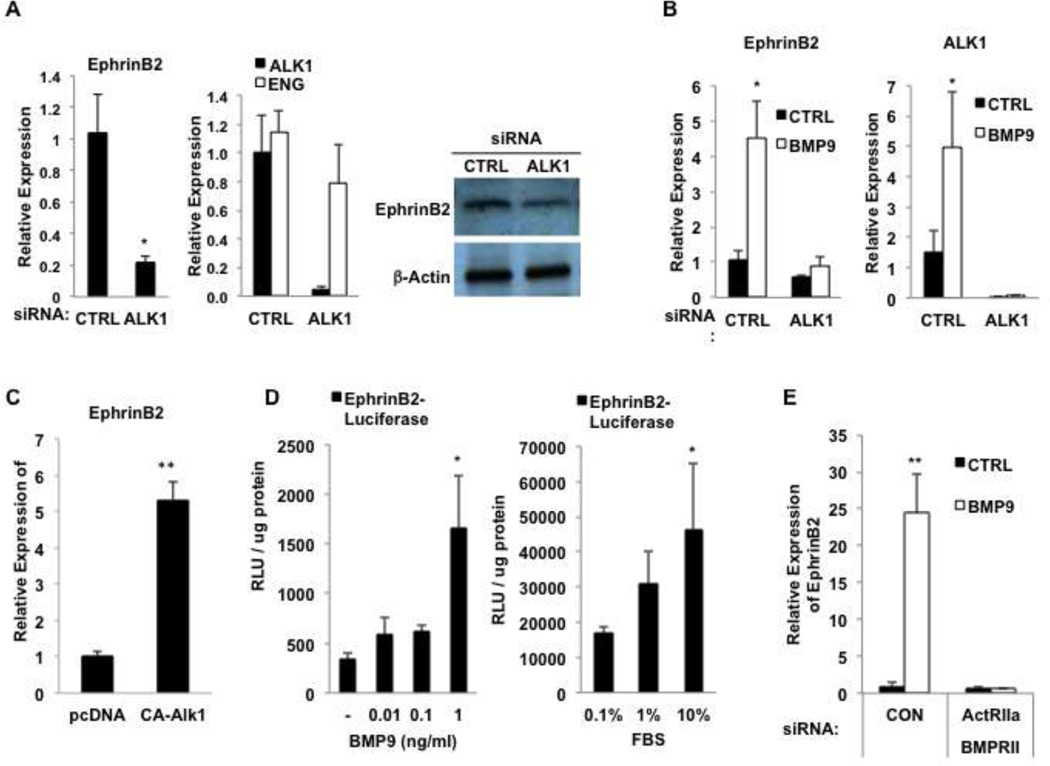
To confirm in human cells the previous finding in mice that cells lacking Alk1 have decreased expression of EphrinB2 we treated HUAEC with siRNA to Alk1. We confirmed successful and specific knockdown of the target mRNA, consistently finding greater than 80% reduction in the specific target, with no effect on the untargeted gene (Fig. 1A). Loss of Alk1 reduced EphrinB2 expression by close to 80% (Fig. 1A) consistent with previous work showing that a constitutively active Alk1 increased EphrinB2 expression in EC [24]. A second siRNA directed to Alk1 also reduced EphrinB2 expression, thereby excluding off-target effects as an explanation for this result (data not shown). EphrinB2 protein levels were also reduced by knockdown of Alk1 as demonstrated by Western blotting (Fig. 1A). BMP9 has been identified as a ligand for Alk1, and so we tested whether BMP9 can induce EphrinB2. As shown in Fig. 1B, this is indeed the case, and moreover, the activity of BMP9 is entirely dependent on the expression of Alk1 (Fig. 1B). A constitutively active (CA) form of Alk1, conversely, strongly induced EphrinB2 expression (Fig. 1C). As a further test of the ability of BMP9 to induce EphrinB2 expression we cloned 1.1 kb of the EphrinB2 proximal promoter upstream of luciferase and transfected this construct into EC. BMP9 strongly induced luciferase expression (Fig. 1D). BMP9 is present in serum at concentrations of 2–12ng/ml [13] and we confirmed that increasing doses of serum also induced the EphrinB2 promoter (Fig. 1D). In addition to signaling through the Type I receptor Alk1, BMP9 has also been reported to require the two Type II receptors, BMPR-II and ActR-II [30] and we confirmed that finding here – loss of both Type II receptors completely blocked BMP9 induction of EphrinB2 (Fig. 1E), whereas loss of either alone had no effect (data not shown). Thus, BMP9 induces EphrinB2 expression in EC through a combination of Alk1, and BMPR-II or ActR-II receptor signaling.
Fig. 1. Alk1 regulates EphrinB2 expression in EC.
A. HUAEC were transfected with control siRNA or siRNA to Alk1 and cultured for 48 hours. qRT-PCR was performed for Alk1, ENG and EphrinB2. Cell lysates were also examined by Western blot. B. HUAEC were transfected with control siRNA or siRNA to Alk1 and cultured for 48 hours in the presence or absence of BMP9 (5ng/ml). qRT-PCR was performed for Alk1 and EphrinB2. C. HUAEC were transfected with an expression plasmid encoding constitutively active (CA) Alk1 or a control (pcDNA3) and then harvested at 48 hours for qRT-PCR analysis of EphrinB2 expression. D.HUAEC were transfected with an EphrinB2 promoter-Luc construct and cultured for 48 hours in the presence of BMP9 or serum, as indicated. Luciferase activity was measured and is presented as RLU/ug protein. E. HUAEC were transfected with control siRNA, or siRNA to Alk1, or ActRII (ACVR2A) and BMPRII and cultured for 48 hours in the presence or absence of BMP9. qRT-PCR was performed for EphrinB2. All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01
BMP9 induction of EphrinB2 depends on ID1 and ID3
Previous studies have shown that BMP9 and Alk1 induce expression of the transcription factors ID1 and ID3 [20, 22, 24, 28]. Moreover, mice deficient in both ID1 and ID3 have severely compromised physiologic and pathologic angiogenesis [40]. We confirmed that both ID1 and ID3 are dose-dependently induced by BMP9 in HUVEC (data not shown) and in HUAEC (Fig. 2A). To determine whether ID1 and ID3 mediate BMP9/Alk1 induction of EphrinB2 we transfected HUAEC with control siRNA or siRNA to ID1 or ID3 and then stimulated them with BMP9. Knockdown of ID1 and ID3 was confirmed by qRT-PCR. Loss of either ID1 or ID3 singly reduced BMP9-induced EphrinB2 expression by about 50%, whereas knockdown of both ID1 and ID3 reduced induction by up to 90% (Fig. 2B). Conversely, overexpression of ID1 induced EphrinB2 (data not shown). Thus, EphrinB2 expression is transcriptionally upregulated in EC through a BMP9-Alk1-BMPR-II/ActR-II-ID1/3 pathway.
Fig. 2. BMP9 induces EphrinB2 through ID1/3.
A. HUAEC were cultured for 24 hours in BMP9 (0, 0.1, 1, 5ng/ml) and then harvested for qRT-PCR analysis of ID1 and ID3 expression. B. HUAEC were transfected with control siRNA or siRNA to ID1 or ID3, alone or together (at half dose each) and cultured for 48 hours in the presence or absence of BMP9 (5ng/ml). qRT-PCR was performed for EphrinB2. C. HUAEC were transfected with control siRNA or siRNA to Alk1, along with an expression plasmid for dll4 or a control plasmid, and cultured for 48 hours. qRT-PCR was performed for Alk1, ENG and EphrinB2. D. HUAEC were transfected with an expression plasmid for dll4 or a control plasmid, along with a HES1 promoter-Luc reporter. Luciferase activity was measured at 48 hours. All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01
Notch signaling rescues EphrinB2 expression in Alk1 knockdown cells
It has been noted that in mice lacking Alk1, or overexpressing activated notch, similar arteriovenous malformations (AVMs) are formed – in one case loss of Alk1 results in arteries taking on a venular phenotype (EphrinB2–), while in the other, notch activation drives veins toward an arterial phenotype (EphrinB2+) [33]. We wondered, therefore, whether activation of the notch pathway in EC could rescue EphrinB2 expression in cells lacking Alk1. To this end we transfected EC with control siRNA or siRNA to Alk1, and then mixed these with cells transfected with an expression plasmid for the notch ligand dll4. In cells receiving control siRNA dll4 stimulated a robust 2 to 3-fold increase in EphrinB2 expression (Fig. 2C). As before, siRNA to Alk1 reduced EphrinB2 expression. Remarkably, dll4-mediated notch signaling was able to completely overcome the effect of Alk1 knockdown and restore EphrinB2 expression (Fig. 2C). Efficient Alk1 knockdown was confirmed as was dll4 induction of the well-characterized notch target HES-1 (Fig. 2D). Thus, our data show that loss of Alk1 signaling to EphrinB2 can be overcome by enhanced dll4-mediated notch signaling.
Loss of Alk1 increases endothelial sprouting
To determine the role of Alk1 on endothelial sprouting we used an in vitro assay that recapitulates several of the necessary steps of angiogenesis, including sprouting, migration, alignment, proliferation, and tube formation [38]. HUAEC were transfected with either control siRNA or siRNA specific to Alk1, allowed to attach to beads and then plated into fibrin gels. Knockdown of Alk1 resulted in a significant 2 to 3-fold increase in both the number of sprouts and the number of tip cells – an indicator of branching (Fig. 3A, D). Sprouts that form in this assay invariably remain attached at their base to the bead and single migrating cells are rarely seen. In contrast to this we noted a significant increase in single cells migrating away from the bead when Alk1 expression was reduced, (Fig. 3B, D). HUVEC, unlike most veinderived EC also express Alk1 although to lesser levels than arterial cells (data not shown). HUVEC also showed enhanced sprouting when Alk1 expression was knocked down (Fig. 3E, H) and similar to HUAEC, loss of Alk1, led to a significant number of single EC migrating away from the beads (Fig. 3F, H). Finally, we also tested EC derived from cord blood endothelial cell colony-forming cells (ECFC-EC), which have a phenotype reflecting characteristics of both HUAEC and HUVEC (Fig. S1). These also showed enhanced sprouting in response to loss of Alk1 (Fig. 3I, L), as well as dramatically increased migration of single cells (Fig. 3J, L). In all cases we confirmed specific and efficient knockdown of the target gene (Fig. 3C, G, K). Thus in three different EC lineages loss of Alk1 results in enhanced sprouting and the appearance of single migrating cells.
Fig. 3. Alk1 regulates EC sprouting.
Either HUAEC, HUVEC or ECFC-EC were transfected with control siRNA or siRNA to Alk1 and then used in an in vitro angiogenesis assay (D, H, L). After 8 days the number of sprouts and the number of tip cells were counted (A, E, I). Individual cells that had left the bead and migrated into the gel were also enumerated (B, F, J). Cells were also harvested to confirm by qRT-PCR that target genes were knocked down (C, G, K). All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01
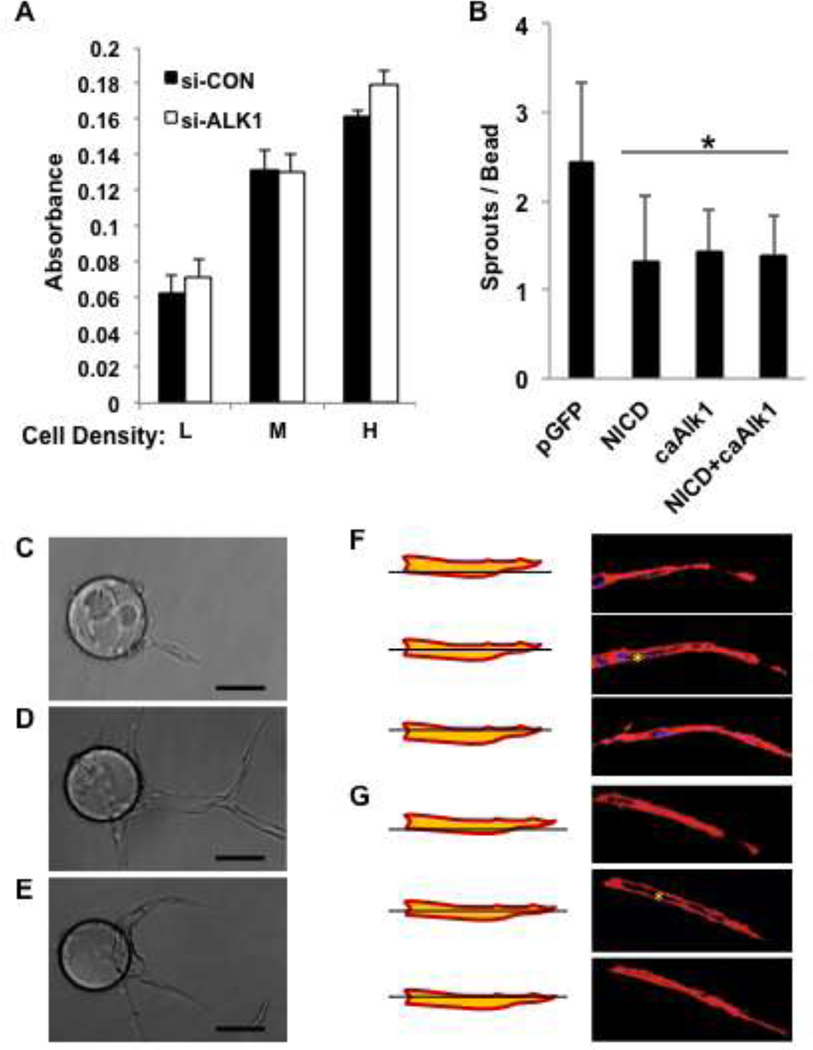
To determine whether the increased sprouting was due to an increase in cell proliferation we cultured siRNA-treated HUAEC at low, medium or high density and measured cell number by MTT assay after three days of culture. As shown in Fig. 4A, loss of Alk1 had no effect on EC proliferation, suggesting that the increased number of sprouts is not merely due to an increase in cell number.
Fig. 4. EphrinB2 and notch regulate EC sprouting.
A. HUAEC were transfected with control siRNA or siRNA to Alk1 and then plated at low, medium or high density and cultured for 4 days. Cell number was assessed by MTT assay. B. HUAEC were transfected with expression plasmids for GFP, notch-ICD, CA-Alk1 or notch-ICD and CA-Alk1 (at half dose each) and then used in an in vitro angiogenesis assay. After 4 days the number of sprouts was counted. All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01. C, D, E. Phase contrast images of sprouting EC treated with control, Alk1 or EphrinB2 siRNA. F, G. Confocal images of sprouting EC pre-treated with siRNA to Alk1 or EphrinB2, stained with phalloidin to show actin fibers. Asterisk indicates the presence of a lumen.
As further confirmation of the role of Alk1 in regulating sprouting we transfected EC with expression plasmids encoding a constitutively active form of Alk1 (CA-Alk1) or, as a positive control, constitutively active notch (NICD), and examined these cells in a sprouting assay. As predicted, both constructs significantly suppressed sprouting compared to a GFP control (Fig. 4B), consistent with the loss of Alk1, or the suppression of notch signaling inducing augmented sprouting (Fig. 3 and [41]). Given that both Alk1 signaling and notch signaling suppress sprouting we wondered whether inhibition of notch signaling would relieve the suppressive effects of Alk1. We therefore transfected EC with control or Alk1 expression plasmids and treated the cells with vehicle or the notch inhibitor DAPT. Loss of notch signaling did not relieve inhibition by Alk1 (Fig. S2A). In a reverse experiment, we looked at whether loss of Alk1 would reverse the suppressive effect of notch signaling on sprouting. Cells were transfected with control or Alk1 siRNA and with control or dll4 expression plasmids. Dll4-mediated notch signaling strongly suppressed sprouting even in the absence of Alk1 (Fig. S2B). Therefore, inhibition of sprouting by Alk1 signaling is not relieved by loss of notch, nor is inhibition by notch signaling relieved by loss of Alk1, suggesting that the two pathways act to suppress sprouting largely independently of each other, although they may have some redundant overlap (see below).
Under some culture conditions sprouting occurs normally and yet the neovessels fail to form lumens [42]. We examined sprouts formed by control , or Alk1 or EphrinB2 knockdown EC by phase contrast (Fig. 4C, D, E) and confocal (Fig. 4F, G) microscopy for evidence of lumen formation. Neither loss of Alk1 or EphrinB2 compromised the ability of vessels to undergo lumen formation. We conclude, therefore, that Alk1 and EphrinB2 are not involved lumenogenesis. Our previous work similarly ruled out a role for notch in this process [41].
In summary, Alk1 signaling normally acts to suppress EC sprouting. The loss of Alk1 results in enhanced sprouting, and this can be reversed by notch signaling.
BMP9 acts through Alk1 to block EC sprouting
It has been shown that BMP9 blocks EC migration [13, 43], and our data show that siRNA to Alk1 enhances EC sprouting, while CA-Alk1 blocks sprouting, suggesting that BMP9 should block sprouting. To test this directly we added BMP9 to EC embedded in fibrin gels and then counted the number of sprouts. Confirming our findings with siRNA to Alk1 and CA-Alk1, BMP9 dose-dependently blocked sprout formation in both HUAEC (Fig. 5A) and in HUVEC (Fig. 5B). Pretreatment of the EC with siRNA to Alk1 blocked the ability of BMP9 to suppress sprouting, again confirming the necessity of Alk1 for BMP9 effects on EC. Similarly, knockdown of ID1/ID3 also blocked the ability of BMP9 to inhibit sprouting (data not shown). Our data suggest therefore that BMP9 acts through Alk1 to suppress EC sprouting. As shown above using CA-Alk1 and NICD (Fig. S2), each pathway appears to act largely independently to suppress sprouting. We confirmed that finding using BMP9 in place of CA-Alk1. As shown in Fig. S3, inhibition of sprouting by BMP9 (as measured by the number of tip cells) could not be overcome by concomitant loss of notch signaling in the presence of DAPT.
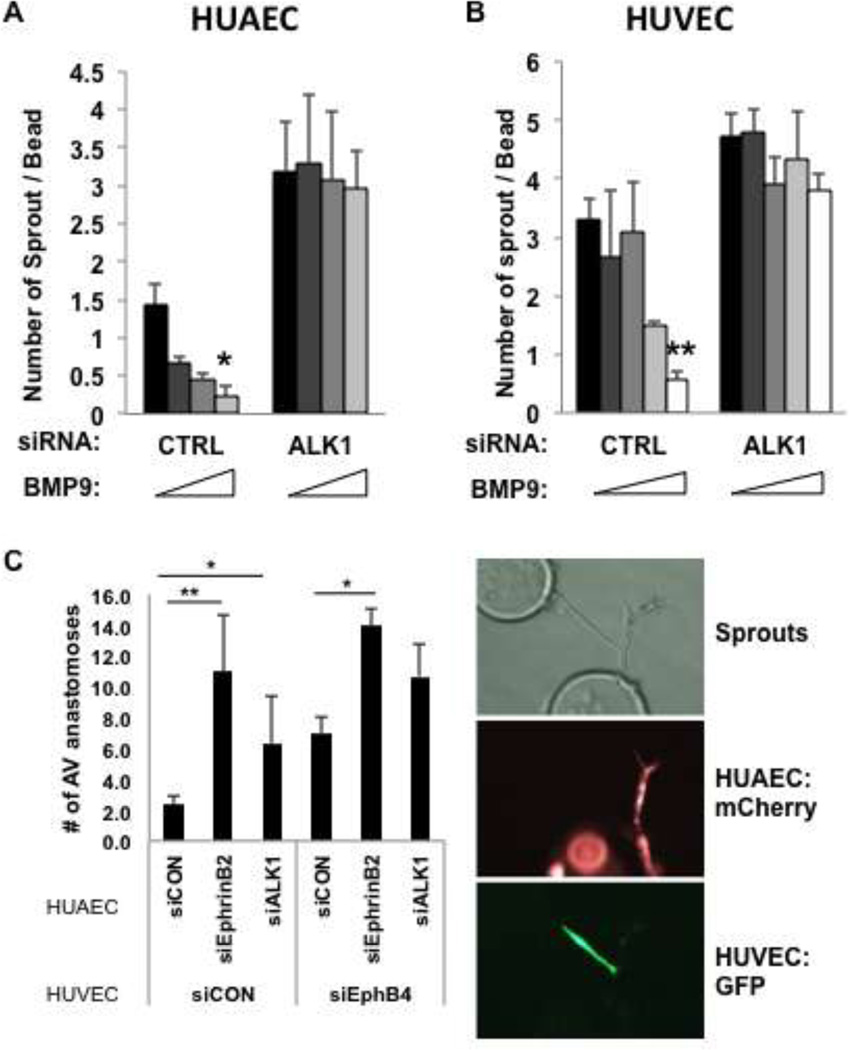
Fig. 5. BMP9 suppresses EC sprouting through Alk1.
HUAEC (A) and HUVEC (B) were transfected with control siRNA or siRNA to Alk1 and then seeded in an in vitro angiogenesis assay and treated different concentrations of BMP9 (0, 0.1, 1, 5ng/ml for HUAEC; 0, 0.1, 0.5, 1, 5ng/ml for HUVEC). After 8 days the number of sprouts was counted. C. HUAEC or HUVEC were transduced with retroviruses expressing mCherry or GFP, respectively, and then transfected with the indicated siRNAs. EC were coated onto separate beads and the beads were then mixed in a sprouting assay. Anastomoses between HUAEC (red) and HUVEC (green) sprouts were counted after 8 days. All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01
As EphrinB2 is implicated in maintenance of vessel identity [44] and both EphrinB2 and Alk1 knockout mice display AVMs [29, 33] we tested whether loss of Alk1 or EphrinB2 would alter artery-vein anastomosis in vitro. Sprouting assays were established containing mCherry-expressing HUAEC and GFP-expressing HUVEC, treated with either control, Alk1, EphrinB2 or EphB4 siRNAs. Inter-bead anastomoses were then counted under fluorescent illumination. As shown in Fig. 5C, loss of Alk1 in HUAEC led to a significant increase in anastomosis with control HUVEC. Direct knockdown of EphrinB2 similarly increased anastomosis. In both cases, simultaneous loss of the EphrinB2 receptor EphB4 in the HUVEC potentiated the increase, although not to a statistically significant level. A-V connections were still relatively rare, however – approximately 10-fold less frequent than either A-A or V-V connections, the rate of which was not altered (data not shown). Thus, loss of Alk1 in arterial EC mirrors the loss of EphrinB2 in promoting anastomosis between arterial and venular EC.
Loss of Alk1 or EphrinB2 suppresses induction of a VEGF-target gene
Sprouting angiogenesis is strongly dependent on VEGF signaling through VEGFR2, the expression of which is enriched in tip cells [45]. We looked, therefore, at whether loss of Alk1 affected VEGFR2 expression. As shown in Fig. 6A, treatment of EC with siAlk1 strongly induced VEGFR2 expression, whereas loss of EphrinB2 had no effect. Our previous work has shown that notch signaling induces expression of the bHLH transcription factor HESR1 (HEY1) and that this down regulates VEGFR2 transcription [46–48]. Here we find that BMP9 also induces HESR1 expression (Fig. 6B), providing a molecular link between loss of Alk1 and up regulated VEGFR2. Finally, we tested whether increased VEGFR2 expression in response to loss of Alk1 correlated with a change in the level of sprouting at different concentrations of VEGF, and indeed it did. We saw a dramatic increase in responsiveness, especially at higher concentrations of VEGF (Fig. 6C).
Fig. 6. Alk1 regulates VEGFR2 expression.
A. HUAEC were transfected with control siRNA or siRNA to Alk1 or EphrinB2 and cultured for 2 days. Expression of the VEGF receptor VEGFR2 was then assessed by qRT-PCR. B. HUAEC were treated for 18 hours with 1 ng/ml BMP9 and HESR1 expression was assessed by qRT-PCR. C. HUAEC were transfected with control siRNA or siRNA to Alk1, established in an in vitro angiogenesis assay, and cultured with VEGF at the indicated concentrations. Sprouts were counted after 8 days. All data are shown as mean and SEM. p values are for Student t-tests. * p < 0.05; ** p < 0.01
Discussion
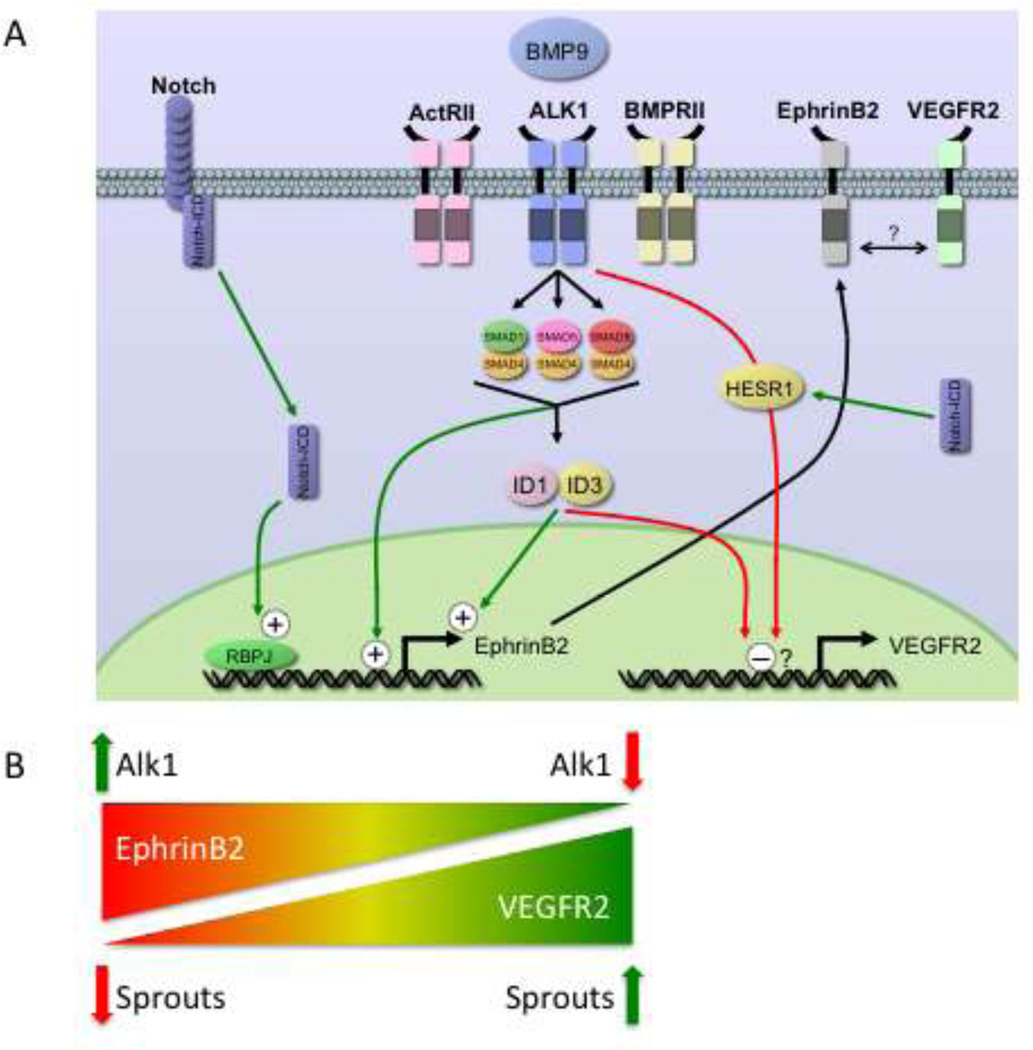
Our findings provide further evidence that Alk1 is a critical type I receptor for BMP9 on human EC, although a role for Alk5 (or other Alk receptors) has not been ruled out. We find that BMP9 signaling through Alk1, in combination with BMPRII or ActRII, up regulates ID1 and ID3, which in turn cooperate to induce EphrinB2 expression. Loss of Alk1, results in enhanced EC sprouting, and this correlates with enhanced responsiveness of EC to VEGF. We now provide a mechanistic link between Alk1 and EphrinB2, through ID1 and ID3, and also demonstrate that the loss of EphrinB2 expression downstream of defective Alk1 can be rescued by dll4- mediated notch signaling (Summarized in Fig. 7).
Fig. 7. Summary of EphrinB2 and VEGFR2 regulation.
A. Schematic of BMP9 signaling in EC. BMP9, acting through Alk1/ActRII/BMPRII, targets ID1 and ID3, which then up regulate the EphrinB2 promoter, presumably indirectly. Smad1/5/8 downstream of Alk1 may regulate the EphrinB2 promoter directly as it contains numerous BMP response elements (see Fig. S4, S5). Notch signaling also up-regulates EphrinB2, through a 5’ RBPj binding site. Alk1 signaling down-regulates VEGFR2 mRNA, and this may be through direct binding of ID1/ID3 to the E-boxes in the VEGFR2 promoter region or by its induction of HESR1. EphrinB2 and VEGFR2 may interact at the cell surface to further modulate VEGF signaling. B. Alk1 balances EphrinB2 and VEGFR2 expression. Loss of Alk1 in EC results in decreased EphrinB2 expression but increased VEGFR2 expression. Loss of Alk1, and increased VEGFR2 expression, correlates with enhanced sprouting.
A recent study [33] noted the surprising finding that although notch signaling induces EphrinB2 expression, the phenotype associated with loss of EphrinB2 was mirrored best not by a notch loss-of-function (LOF) mutant but by a notch gain-offunction (GOF) mouse. These data imply that the phenotypes in the EphrinB2 LOF mice and the notch GOF mice arise by different mechanisms – potentially, a conversion of artery to vein in the former and conversion of vein to artery in the latter. Our data showing that notch activation downstream of over-expressed dll4 rescues EphrinB2 expression in EC (Fig. 2) suggest, therefore, that spatiallyrestricted activation of notch – in the arterial side of the vasculature only – might limit the formation of AVMs in an Alk1 mutant background (HHT2). Unrestricted expression, however, that included the venous side of the circulation, might also trigger AVMs as has previously been shown by induced expression of notch ICD in adult mice [49]. These data raise the possibility that components of the notch pathway may act as modifier genes in HHT [15, 50–52]. Such modifier genes have been suggested as an explanation for strain differences in the penetrance of the HHT1 phenotype in Endoglin haploinsufficient mice [10, 53, 54].
Our results on the role of BMP9 in inducing ID protein expression are consistent with the recent findings of Upton et al [30], who used pulmonary arterial EC. In that study BMP9 was found to induce both ID1 and ID2, through a pathway dependent on Alk1 and BMPRII or ActRII. In human umbilical artery EC we find that BMP9 induces ID1 and ID3 (and ID2, data not shown), and that similarly to the findings of Upton et al [30] the two type II receptors act redundantly – only loss of both receptors blocked BMP9 induction of EphrinB2 (Fig. 1D). This is consistent with mouse studies showing that loss of TGF RII in EC does not result in a vascular defect [18].
The effect of ID1/ID3 on EphrinB2 promoter activity is likely to be indirect as ID proteins usually act as negative regulators of transcription through their binding to E-boxes. BMP9-regulated Smads, however, may have direct effects as we identified numerous BMP-response elements [55] in the EphrinB2 proximal promoter (Fig. S4), at least some of which are conserved across multiple species (Fig. S5). The VEGFR2 promoter does, however, have numerous E-boxes [47] and may therefore be a direct target of negative regulation by ID1/ID3 (Fig. 7A).
A recent study in zebrafish reported that loss of Alk1 resulted in an increase in endothelial cell numbers in arteries, and that the formation of AVMs was a result of aberrant retention of venous-derived conduits [27, 56]. A possible role for enhanced EC proliferation was not confirmed, however other studies have noted decreased EC proliferation in response to BMP9 or CA-Alk1 [13, 21, 28]. Although it appears that the initial increase in vessel caliber in Alk1 mutants is independent of flow, the later dilation of arteries, and subsequently of draining veins, is flow-dependent [56]. An elegant study in mice, using a dorsal skin-fold model, reported similar findings – early A-V anastomoses preceding dilatation of postcapillary venules and remodeling of arterial vessels [8]. We also see enhanced anastomosis between artery and vein EC when Alk1 expression is reduced and this is phenocopied by loss of either EphrinB2 or EphB4 (Fig. 5C). BMP9 has also been reported to induce EC proliferation in vitro [57], however we were not able to reproduce those findings here. Indeed, our findings almost completely mirror those of Suzuki in that we see BMP9-mediated-inhibition of EC sprouting, and in the absence of Alk1 we see an increase in VEGFR2, rather than a decrease. These discrepancies may relate to species differences as all of our studies were done with human cells whereas Suzuki et al used mouse. A resolution to this will likely require head-to-head comparative studies.
Our data suggest that loss of Alk1 signaling has multiple consequences: these include not only the loss of EphrinB2 expression discussed above, but also the loss of proper regulation of VEGFR2. The interactions between Alk1 and notch signaling are complex. Down regulation of EphrinB2 as a consequence of Alk1 loss can be overcome by activating notch (Fig. 2C), indicating that notch can regulate EphrinB2 transcription independently of Alk1. Similarly, activated notch can suppress sprouting regardless of whether Alk1 signaling is active (Fig. S2). Both notch and BMP9 can induce HESR1 expression, and both can down regulate VEGFR2. In HHT2, therefore, the loss of Alk1-mediated BMP9 signaling could drive angiogenesis and vascular overgrowth through up regulation of VEGFR2 transcription in the absence of HESR1-mediated suppression (Fig. 7A). A prediction from this model would be that increasing notch signaling on an Alk1 knockdown background should suppress the excess sprouting by restoring HESR1 expression, and indeed, this is the case – dll4-mediated notch signaling is dominant over loss of Alk1 in regulation of sprouting (Fig. S2B). Similarly, enhanced sprouting as a consequence of notch inhibition is overcome by the addition of BMP9 (Fig. S3).
Intriguingly, recent reports have shown efficacy of bevacizumab, an anti-VEGF antibody, in treating epistaxis in HHT patients [58]. It is likely that the antibody blocks the angiogenesis phase of the perpetual cycle that is the hallmark of HHT patients suffering continual nose bleeding: damage to fragile vessels – bleeding – tissue damage – angiogenesis – vessel damage, etc. Clearly, regulation of VEGF signaling in HHT is critical.
Taken together, our data along with previously-published studies, suggest an underlying mechanism for the formation of telangiectasias and AVMs that involves altered VEGF signaling downstream of mutant Alk1, as a consequence of up regulated VEGFR2 expression, combined with reduced EphrinB2 expression. The outcome is enhanced sprouting, but misregulated anastomosis (Fig. 7B). Finally, it is possible that local enhancement of notch signaling may have some beneficial effects in HHT.
Supplementary Material
Acknowledgements
This work was supported by a grant from the HHT Foundation International (CCWH) and an NIH R01 Award (HL60067 - CCWH), and an NIH RC1 Award (ES018361 -SCG). We thank Dr. Paul Oh for the kind gift of Alk1 plasmids. We thank Linda Him and Matt Peacock for outstanding help with tissue culture.
References
- 1.HHT Foundation International website. 2011
- 2.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood reviews. 2010;24(6):203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17(7):860–871. doi: 10.1038/ejhg.2009.35. PMCID: 2986493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 1996;13(2):189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 5.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994;8(4):345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 6.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274(2):584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 7.Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. Journal of cellular physiology. 2005;204(2):574–584. doi: 10.1002/jcp.20311. [DOI] [PubMed] [Google Scholar]
- 8.Park SO, Wankhede M, Lee YJ, Choi EJ, Fliess N, Choe SW, et al. Realtime imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest. 2009;119(11):3487–3496. doi: 10.1172/JCI39482. PMCID: 2769195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonyadi M, Rusholme SA, Cousins FM, Su HC, Biron CA, Farrall M, et al. Mapping of a major genetic modifier of embryonic lethality in TGF beta 1 knockout mice. Nat. Genet. 1997;15(2):207–211. doi: 10.1038/ng0297-207. [DOI] [PubMed] [Google Scholar]
- 10.Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, Wanless IR, Letarte M. Potential role of modifier genes influencing transforming growth factor-beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. The American journal of pathology. 2001;158(6):2011–2020. doi: 10.1016/s0002-9440(10)64673-1. PMCID: 1891990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptorlike kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93(7):682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- 12.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20(3):203–212. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, et al. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102(8):914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109(5):1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 15.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102(6):637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 16.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, et al. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280(26):25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 17.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGFbeta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20(9):556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, et al. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111(2):633–642. doi: 10.1182/blood-2007-08-107359. PMCID: 2200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki T, Hong KH, Oh SP. Nonoverlapping expression patterns of ALK1 and ALK5 reveal distinct roles of each receptor in vascular development. Laboratory investigation; a journal of technical methods and pathology. 2006;86(2):116–129. doi: 10.1038/labinvest.3700376. [DOI] [PubMed] [Google Scholar]
- 20.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. Embo J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamouille S, Mallet C, Feige JJ, Bailly S. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100(13):4495–4501. doi: 10.1182/blood.V100.13.4495. [DOI] [PubMed] [Google Scholar]
- 22.Lux A, Salway F, Dressman HK, Kroner-Lux G, Hafner M, Day PJ, et al. ALK1 signalling analysis identifies angiogenesis related genes and reveals disparity between TGF-beta and constitutively active receptor induced gene expression. BMC Cardiovasc Disord. 2006;6:13. doi: 10.1186/1471-2261-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallet C, Vittet D, Feige JJ, Bailly S. TGFbeta1 induces vasculogenesis and inhibits angiogenic sprouting in an embryonic stem cell differentiation model: respective contribution of ALK1 and ALK5. Stem Cells. 2006;24(11):2420–2427. doi: 10.1634/stemcells.2005-0494. [DOI] [PubMed] [Google Scholar]
- 24.Ota T, Fujii M, Sugizaki T, Ishii M, Miyazawa K, Aburatani H, et al. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-beta in human umbilical vein endothelial cells. J Cell Physiol. 2002;193(3):299–318. doi: 10.1002/jcp.10170. [DOI] [PubMed] [Google Scholar]
- 25.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13(7):301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 26.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129(12):3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- 28.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120(Pt 6):964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 29.Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26(3):328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- 30.Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J Biol Chem. 2009;284(23):15794–15804. doi: 10.1074/jbc.M109.002881. PMCID: 2708876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230(2):151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 32.Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230(2):139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 33.Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48(3):146–150. doi: 10.1002/dvg.20599. PMCID: 2849749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Ephreceptor binding. J Cell Sci. 2010;123(Pt 8):1235–1246. doi: 10.1242/jcs.061903. PMCID: 2848112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heroult M, Schaffner F, Pfaff D, Prahst C, Kirmse R, Kutschera S, et al. EphB4 promotes site-specific metastatic tumor cell dissemination by interacting with endothelial cell-expressed ephrinb2. Molecular cancer research : MCR. 2010;8(10):1297–1309. doi: 10.1158/1541-7786.MCR-09-0453. [DOI] [PubMed] [Google Scholar]
- 36.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, et al. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465(7297):487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 38.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1 small star, filled. Microvasc Res. 2003;66(2):102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Zhao Y, Pan X, He X, Gilbert HF. The unfolded protein response is necessary but not sufficient to compensate for defects in disulfide isomerization. J Biol Chem. 2009;284(16):10400–10408. doi: 10.1074/jbc.M900377200. PMCID: 2667727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts [see comments] Nature. 1999;401(6754):670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 41.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, et al. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19(8):1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 42.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Molecular biology of the cell. 2011;22(20):3791–3800. doi: 10.1091/mbc.E11-05-0393. PMCID: 3192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David L, Mallet C, Vailhe B, Lamouille S, Feige JJ, Bailly S. Activin receptor-like kinase 1 inhibits human microvascular endothelial cell migration: potential roles for JNK and ERK. J Cell Physiol. 2007;213(2):484–489. doi: 10.1002/jcp.21126. [DOI] [PubMed] [Google Scholar]
- 44.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson AM, Wang SJ, Taylor AC, Aitkenhead M, Hughes CC. The basic helix-loop-helix transcription factor HESR1 regulates endothelial cell tube formation. J Biol Chem. 2001;276(9):6169–6176. doi: 10.1074/jbc.M008506200. [DOI] [PubMed] [Google Scholar]
- 47.Holderfield MT, Henderson Anderson AM, Kokubo H, Chin MT, Johnson RL, Hughes CC. HESR1/CHF2 suppresses VEGFR2 transcription 25 independent of binding to E-boxes. Biochem Biophys Res Commun. 2006;346(3):637–648. doi: 10.1016/j.bbrc.2006.05.177. [DOI] [PubMed] [Google Scholar]
- 48.Taylor KL, Henderson AM, Hughes CC. Notch Activation during Endothelial Cell Network Formation in Vitro Targets the Basic HLH Transcription Factor HESR-1 and Downregulates VEGFR-2/KDR Expression. Microvasc Res. 2002;64(3):372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 49.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, et al. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc. Natl. Acad. Sci. 2005;102(28):9884–9889. doi: 10.1073/pnas.0504391102. PMCID: 1175015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leblanc GG, Golanov E, Awad IA, Young WL. Biology of vascular malformations of the brain. Stroke. 2009;40(12):e694–e702. doi: 10.1161/STROKEAHA.109.563692. PMCID: 2810509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104(5):576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 52.ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132(Pt 12):3231–3241. doi: 10.1093/brain/awp246. PMCID: 2792368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell. 2009;16(2):209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Marchuk DA, Srinivasan S, Squire TL, Zawistowski JS. Vascular morphogenesis: tales of two syndromes. Hum. Mol. Genet. 2003;12(Spec No 1):R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 55.Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin CH, et al. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39(20):8712–8727. doi: 10.1093/nar/gkr572. PMCID: 3203580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, et al. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development. 2011;138(8):1573–1582. doi: 10.1242/dev.060467. PMCID: 3062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J. Cell Sci. 2010;123(Pt 10):1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 58.Davidson TM, Olitsky SE, Wei JL. Hereditary hemorrhagic telangiectasia/avastin. The Laryngoscope. 2010;120(2):432–435. doi: 10.1002/lary.20757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.