Abstract
Background
Bacterial translocation is a frequent event in cirrhosis leading to an increased inflammatory response. Splanchnic adrenergic system hyperactivation has been related with increased bacterial translocation. We aim at evaluating the interacting mechanism between hepatic norepinephrine and inflammation during liver damage in the presence of bacterial-DNA.
Animals and Methods
Forty-six mice were included in a 16-week protocol of CCl4-induced cirrhosis. Laparotomies were performed at weeks 6, 10, 13 and 16. A second set of forty mice injected with a single intraperitoneal dose of CCl4 was treated with saline, 6-hydroxidopamine, Nebivolol or Butoxamine. After 5 days, mice received E. coli-DNA intraperitoneally. Laparotomies were performed 24 hours later. Liver bacterial-DNA, norepinephrine, TNF-alpha, IL-6 and beta-adrenergic receptor levels were measured.
Results
Bacterial-DNA translocation was more frequent in CCl4-treated animals compared with controls, and increased as fibrosis progressed. Liver norepinephrine and pro-inflammatory cytokines were significantly higher in mice with vs without bacterial-DNA (319.7±120.6 vs 120.7±68.6 pg/g for norepinephrine, 38.4±6.1 vs 29.7±4.2 pg/g for TNF-alpha, 41.8±7.4 vs 28.7±4.3 pg/g for IL-6). Only beta-adrenergic receptor-1 was significantly increased in treated vs control animals (34.6±7.3 vs 12.5±5.3, p = 0.01) and correlated with TNF-alpha, IL-6 and norepinephrine hepatic levels in animals with bacterial-DNA. In the second set of mice, cytokine levels were increased in 6-hydroxidopamine and Nebivolol (beta-adrenergic receptor-1 antagonist) treated mice compared with saline. Butoxamine (beta-adrenergic receptor-2 antagonist) didn’t inhibit liver norepinephrine modulation of pro-inflammatory cytokines.
Conclusions
Beta-adrenergic receptor-1 mediates liver norepinephrine modulation of the pro-inflammatory response in CCl4-treated mice with bacterial-DNA.
Introduction
Cirrhosis represents the end-stage of any chronic liver disease, characterized by the most advanced stage of fibrosis, distortion of the liver parenchyma associated with septae and nodule formation, altered blood flow and the potential development of liver failure at long term. Bacterial translocation (BT) is a common and recurrent event occurring in decompensated cirrhosis and constitutes the current pathogenic theory for the onset of bacterial infections in this setting [1]–[3]. Intestinal bacterial overgrowth, impairment in permeability of the intestinal mucosal barrier, and deficiencies in local host immune defences are the major mechanisms postulated to favour BT in cirrhosis [4], [5].
Experimental chronic liver damage is commonly associated with a several-fold increase of BT [6], [7]. Recently, our group showed that bacterial DNA (bactDNA) translocation incidence to mesenteric lymph nodes (MLNs) could be correlated with grade of fibrosis in mice treated with weight-controlled CCl4 increasing amounts during a 16-week study [8]. This was associated with changes in gut microbiota content favoring a pro-inflammatory TNF-α and IL-6 up-regulation. These cytokines had already been implicated in fibrosis progression in the past [9]–[11]. Promotion of hepatic fibrosis includes, though, many other players among which the sympathetic nervous system (SNS) is a prominent one [12]–[15].
Evidence of an enhanced SNS in patients with established decompensated cirrhosis has been reported in the past [16], [17]. SNS plays an immunosuppressive role, mainly through norepinephrine (NE), in chemotaxis and phagocytosis [18]–[20], which are relevant activities in host response against Gram-negative microorganims. In fact, it has recently been shown in rats with CCl4-induced cirrhosis that hyperactivity of the splanchnic SNS contributes to the translocation of E coli but not S aureus to MLNs and extraintestinal sites. Also, splanchnic sympathectomy reduced bacterial translocation to MLN in ascitic cirrhotic rats from 45% to 17% [21]. Related with all this, propanolol decreases the rates of bacterial overgrowth and translocation in cirrhotic rats with ascites [22], and the use of beta-blockers has been reported to prevent SBP in patients with cirrhosis and ascites, independent of haemodynamic response [23].
Since we have demonstrated the increasing incidence of BT, mainly by E. coli, during the induction of liver cirrhosis in the mouse experimental model of chemically-induced liver damage, we hypothesize that SNS must be hyperactivated, not only favouring fibrosis but also a progressively augmented BT rate in these animals. This study will provide insight on interactions between bactDNA translocation, NE and inflammation activity at different stages in the liver, the main organ of inflammation in cirrhosis, as progressive damage is induced by CCl4 administration in mice and may offer rationale for new therapeutic strategies aim at preventing BT in cirrhosis.
Methods
Ethics Statement
Animals received care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals. The study was approved by the Animal Research Committee of Universidad Miguel Hernandez (Alicante, Spain) with approval number HA-RFG-002-09.
Animals and Study Design
Female Balb/c 8-week old mice (Harlan, Barcelona, Spain) were included in a 16-week study protocol for induction of chronic liver damage (Protocol I) or in a 2-week protocol for chemical sympathectomy (Protocol II). Mice were caged at a constant room temperature of 21°C and exposed to a 12∶12 light/dark cycle.
Protocol I
Induction of liver damage and laparotomies
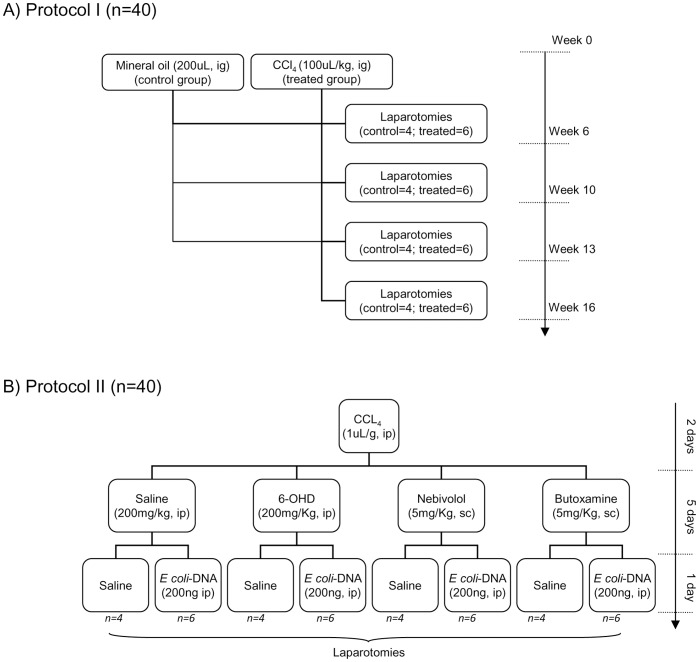
Mice weighting 18–20 g were fed standard rodent chow and were treated with 0.25 mmol/L phenobarbital in tap water along the study protocol. After a 4-week housing in those conditions, liver damage was induced by intragastrical CCl4 administration and laparotomies were performed at 6, 10, 13 and 16 weeks in a subgroup of treated mice (n = 6/week) and at 0, 6, 10, 13 and 16 weeks in a subgroup of control animals (n = 4/week) (Figure 1A), as previously described [8].
Figure 1. Design of study protocols.
(A) Study Protocol I. Balb/c mice were included in a 16-week protocol for induction of liver cirrhosis by oral administration of CCl4. A subgroup of animals (six CCl4-treated and four control mice) was subjected to laparotomy at different study weeks to follow progression of fibrosis, inflammation, bactDNA translocation and NE hepatic levels. CCl4: carbon tetrachloride; ig.: intragastrically. (B) Study Protocol II. CCl4-treated Balb/c mice were distributed to receive saline, 6-OHD for sympathetic denervation, Nebivolol for beta-adrenergic receptor (ADRB)-1 blockade, or Butoxamine for ADRB-2 blockade. Within each group, animals were further distributed to receive saline or E. coli-DNA. Laparotomies were performed in all subgroups. CCl4: carbon tetrachloride; ip: intraperitoneally; 6-OHD: 6-hydroxydopamine; sc: subcutaneously;
Sample collection
The liver was perfused in situ with 6 ml of Hanks balanced salt solution (HBSS) without Ca2+ and Mg2+ at 37°C at a rate of 1.5 ml/min. This was followed by perfusion with 12 ml of HBSS containing 0.02% collagenase and 100 mM CaCl2 solution at the same perfusion rate. The liver was then removed and rinsed with 3 mL of HBSS. A liver fraction was immediately preserved with EDTA 1 mM and sodium metabisulfite 4 mM and frozen to avoid catecholamine degradation. Histopathological, microbiological and molecular studies were performed in all collected liver samples. Spleen was also collected.
Histological analysis
Liver biopsy specimens between 10 to 15 mm in size were fixed in buffered formalin and embedded in paraffin. Histologic changes were first evaluated by routine hematoxilin and eosin (H&E) in four-micrometer thick sections. We estimated the severity of hepatic fibrosis and architectural distortion with the connective tissue stain Masson trichrome. The amount of fibrosis was blindly assessed semiquantitatively based on the Ishak score [24] by a single senior pathologist (GP), with a conventional light microscope (Olympus BX50, Barcelona, Spain).
Gene expression analysis of pro-fibrogenic markers
Total cellular RNA was isolated from 20–30 mg of frozen liver preserved in RNAlater RNA stabilization reagent (Qiagen, Hilden, Germany) disrupted by sonication (Hielscher UP100H Ultrasonic Processor, Teltow, Germany) and handled according to QuantiTect SYBRGreen RT-PCR kit (Qiagen) manufacturer’s instructions. Quantitative PCR was used to determine expression of profibrogenic Procollagen α-1(1) (ProCol-1), tumour growth factor beta (TGF-β)-1, tissue inhibitor of metalloproteinase (TIMP)-1 matrix metalloproteinase (MMP)-2 and adrenergic receptor beta (ADRB)-1, -2 and -3 genes as previously described [8]. Gene expression levels were normalized to β2-microglobulin. Primer-pair sequences used in the study can be followed in Table S1.
Identification of bacterial DNA
MLNs and livers were disrupted by sonication in a Tissue Lyser II (Qiagen). Total DNA was isolated by using the QIAamp DNA Tissue kit (Qiagen) according to the manufacturer’s instructions. Bacterial DNA presence was identified by polymerase chain reaction (PCR) followed by partial nucleotide sequencing of 16SrRNA gene according to the methodology described elsewhere [25].
Measurement of catecholamine and cytokine levels in control and treated mice livers
The 3-CAT Research ELISA kit (LDN, Nordhorn, Germany) and the Mouse Quantikine ELISA kits (R&D Systems, Minneapolis, MN) were used to determine norepinephrine (NE), and TNF-alpha and IL-6 levels respectively, in disrupted livers from control and treated mice, according to manufacturer’s instructions. Protein hepatic levels of ADRB-1, ADRB2 and ADRB3 were also evaluated by ELISA kits (USCNK, Wuhan, PR China) according to manufacturer’s instructions. Measurements were read by triplicate in a Tecan Sunrise automated microplate reader (Männedorf, Switzerlad).
Protocol II
A second set of female Balb/c 8-week old mice (n = 40) was included for additional experiments on NE-mediated effects over the inflammatory response. To resemble the chemically-induced liver damage of the first protocol, mice received an intraperitoneal single dose of CCl4 (1uL/g body weight) [26]. Forty-eight hours later, a blood sample was obtained by venopunction of the tail vein to confirm liver damage through alanine aminotransferase (ALT) and total billirrubin (TBIL) levels using Mammalian Liver Profile for VetScan VS2 (Abaxis, Union City, CA). Mice were then injected either saline, 6-hydroxidopamine (6-OHD) (Sigma, Spain) (200 mg/Kg) [27] intraperitoneally for chemical sympathectomy, Nebivolol or Butoxamine (Sigma) (5 mg/Kg every 12 h, each) subcutaneously for the selective blockade of ADRB1 and ADRB2, respectively. After 5 days, animals from all groups received E. coli bactDNA (200 ng) intraperitoneally. A subgroup of animals remained without E. coli injection as controls. Laparotomies were performed at 24 hours after E. coli-DNA injection (Figure 1B). Liver and MLNs were collected as described above.
BactDNA was evaluated in all specimens, and measurement of catecholamines and cytokines in the liver was performed as described above.
Statistical Analysis
Continuous variables are reported as mean±standard deviation and categorical variables as frequency or percentages. Statistical differences between groups were analyzed using the chi-square test for categorical data and the Mann-Whitney U test for quantitative data. Statistical differences between 3 or more groups were analyzed using the Kruskal–Wallis test. Bivariate correlations between continuous variables were calculated using the Spearman test. Multiple comparisons were analyzed using pairwise comparisons using Mann-Whitney U test and Bonferroni correction to determine if the post-hoc tests were significant. All reported P values are 2-sided, and P values lower than.05 are considered to indicate significance. All calculations were performed using the SPSS 19.0 software (SPSS, Inc, Chicago, IL).
Results
Characteristics of Animals and Progressive Liver Injury
A total of 62 animals were included in the protocol for induction of cirrhosis (Protocol I). Forty of them were weekly treated with CCl4 and 22 mice constituted the control group. Sixteen animals died during liver damage induction (treated group). Cause of death was CCl4 toxicity and liver insufficiency. None of the control animals died during the study protocol. According to previous results [8], animals were grouped in basal and two protocol stages based on fibrosis degree: one from the beginning of liver bactDNA evaluation (week 6) to week 10, defined by mild fibrosis (Stage-1), and a second one from week 13 to week 16 characterized by severe fibrosis (Stage-2). Characteristics of control and treated mice at all these stages can be followed in Table 1.
Table 1. Characteristics of CCl4-treated mice included in the study at different Stages.
| Basal (week 0) | Stage 1 (weeks 6–10) | Stage 2 (weeks 13–16) | |
| control (n = 6) | control (n = 8) | control (n = 8) | |
| Body weight (gr) | 19,80±0,30 | 22,17±0,74 | 23,28±0,55 |
| Liver weight (gr) | 0,92±0,12 | 0,89±0,06 | 1,04±0,05 |
| Spleen weight (gr) | 0,08±0,03 | 0,10±0,02 | 0,11±0,02 |
| TGFB-1 mRNA Rel Exp | 1,20±0,14 | 1,21±0,18 | 1,28±0,15 |
| mmp2 mRNA Rel Exp | 1,14±0,12 | 1,36±0,14 | 1,29±0,12 |
| Proc-1 mRNA Rel Exp | 1,12±0,14 | 1,15±0,16 | 1,28±0,19 |
| TIMP-1 mRNA Rel Exp | 1,11±0,13 | 1,24±0,25 | 1,27±0,23 |
| CCl4 (n = 12) | CCl4 (n = 12) | ||
| Body weight (gr) | 21,48±1,23 | 21,48±1,44 | |
| Liver weight (gr) | 1,28±0,15* | 1,35±0,15* $ | |
| Spleen weight (gr) | 0,13±0,03* | 0,15±0,01* $ | |
| Accum CCl4 dosage (uL) | 631,50±60,65 | 1348,26±103,50 | |
| TGFB-1 mRNA Rel Exp | 4,84±2,52* | 29,81±4,31* $ | |
| mmp2 mRNA Rel Exp | 6,85±3,51* | 41,50±4,24* $ | |
| Proc-1 mRNA Rel Exp | 10,20±5,20* | 42,40±6,15* $ | |
| TIMP-1 mRNA Rel Exp | 14,16±8,56* | 45,90±7,82* $ | |
| Fibrosis Grade (Ishak) | 0±0 | 2,50±0.92 (2–3) | 5,12±0,54 (4–5) |
p<0,05 compared with Basal and Stage control group
p<0,05 compared with Stage 1
Gene markers of fibrosis were significantly increased from Stage-1 when compared with levels in non-treated mice. Their highest relative expression and the most severe liver fibrosis, as evaluated by the Ishak Scale, corresponded with Stage-2. Accumulated dose of CCl4 correlated with relative expression of all markers of fibrosis (vs TGF-B, r = 0.815; vs MMP-2, r = 0.794; vs ProCol-1, r = 0.817; vs TIMP-1, r = 0.893; p<0.001 in all cases).
Evidence of bactDNA Translocation in Liver of Treated Animals
MLN bactDNA translocation was more frequently observed in treated animals compared with control mice (14/24, 58.3% vs 3/22, 13.6%, p = 0.01). MLN bactDNA sequencing analysis identified Escherichia coli in 11 samples (9 treated, 2 control), Streptococcus pneumoniae in 3 samples (2 treated, 1 control), and Staphylococcus aureus, Shigella flexneri and Campylobacter jejuni in 1 sample of treated mice. Interestingly, bactDNA was also found in disrupted liver samples of treated animals (10/24, 41.6%) and Escherichia coli (8 samples), Streptococcus pneumoniae (1 sample) and Shigella flexneri (1 sample) were identified from treated mice. Staphylococcus aureus was identified in the single bactDNA-positive liver from control mice. All bacteria identifications in liver samples corresponded with those in MLNs, either from control or treated animals.
BactDNA translocation rate in control mice was not increased along the study weeks. Among treated animals, percentage of bactDNA translocation in the liver was significantly incremented at Stage-2 (66% liver bactDNA) compared with Stage-1 (25% liver bactDNA, p = 0.04), as can be followed in Table 2.
Table 2. Presence of bactDNA in MLNs and livers of control and CCl4-treated mice at different Stages.
| Control mice (n = 22) | Treated mice (n = 24) | ||||
| MLNs | Liver | MLNs | Liver | ||
| Basal (week 0) | 0/6 (0%) | 0/6 (0%) | – | – | |
| Stage 1 (weeks 6–10) | 1/8 (12,5%) | 1/8 (12,5%) | 4/12 (33%)* | 3/12 (25%)* | |
| Stage 2 (weeks 13–16) | 1/8 (12,5%) | 0/8 (0%) | 10/12 (83%)* | 8/12 (66%)* | |
p<0,05 compared with the stage control group; MLNs: mesenteric lymph nodes
Hepatic NE and Inflammatory Cytokine Levels during Induction of Cirrhosis
In Stage-1, liver NE, TNF-α and IL-6 levels were significantly increased in treated animals compared with the basal and the stage control mice (Table 3). Regarding Stage-2, a further statistically significant increment in hepatic NE levels was observed compared with treated animals in Stage-1, whereas TNF-α and IL-6 showed no further differences. A positive correlation between hepatic NE levels and TGF-beta gene expression levels was present in CCl4-treated animals (r = 0.772, p = 0.01). No correlations between fibrosis progression, as determined either by profibrogenic gene expression levels or Ishak scores, and proinflammatory cytokines were observed.
Table 3. Liver NE, cytokine and ADRB levels in control and CCl4-treated mice in basal and protocol Stages.
| Basal (week 0) | Stage 1 (weeks 6–10) | Stage 2 (weeks 13–16) | ||
| Control mice | n = 6 | n = 8 | n = 8 | |
| Norepinephrine (pg/g) | 33,86±19,70 | 30,12±22,40 | 38,72±12,47 | |
| TNF-alpha (pg/g) | 21,04±5,36 | 19,61±5,28 | 20,35±4,87 | |
| IL-6 (pg/g) | 17,69±3,56 | 16,43±3,38 | 16,92±3,89 | |
| ADRB1 (ng/g) | 13,93±5,69 | 14,79±4,97 | 12,53±5,27 | |
| ADRB2 (ng/g) | 21,30±7,60 | 22,50±10,46 | 19,63±4,69 | |
| ADRB3 (ng/g) | 18,15±5,90 | 20,06±10,11 | 19,12±8,42 | |
| CCL4 mice | n = 12 | n = 12 | ||
| Norepinephrine (pg/g) | 105,75±66.73* | 334,74±134,6* $ | ||
| TNF-alpha (pg/g) | 34,08±10.62* | 28,67±4.93* | ||
| IL-6 (pg/g) | 38,99±15.2* | 32,88±8.17* | ||
| ADRB1 (ng/g) | 13,84±7,48 | 34,64±7,31* $ | ||
| ADRB2 (ng/g) | 18,07±5,87 | 17,73±4,51 | ||
| ADRB3 (ng/g) | 19,11±4,60 | 18,04±4,95 |
p<0,05 compared with basal and the stage control group;
p<0,05 compared with CCl4-mice in Stage 1
Stage 1: liver bactDNA≤25%; Stage 2: liver bactDNA>65%; ADRB: beta-adrenergic receptor
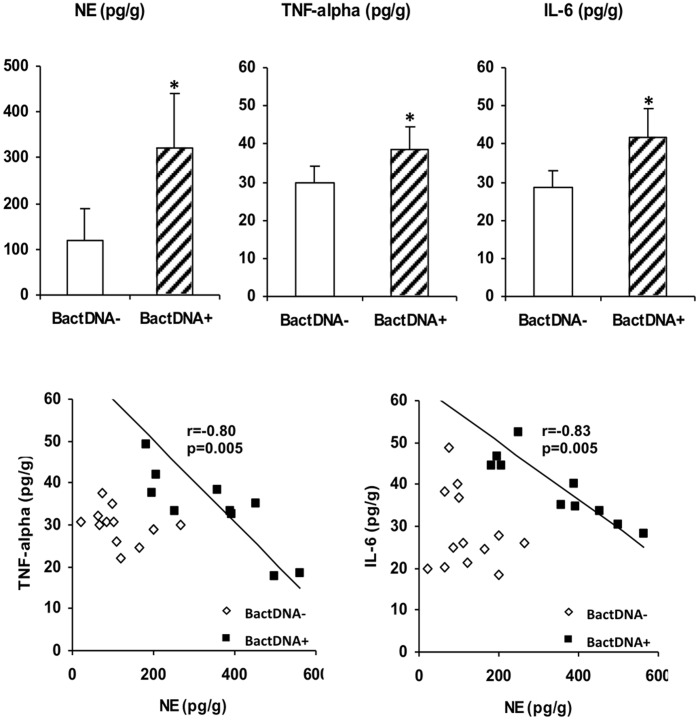
NE and inflammatory cytokine levels were significantly higher in the liver of treated mice with bactDNA translocation compared with treated animals without bactDNA (Figure 1). An inverse correlation was found between NE and all cytokines studied in the liver of treated animals with bactDNA, whereas no correlation was observed between the hepatic levels of NE and inflammatory cytokines in animals without bactDNA translocation into liver (Figure 2).
Figure 2. Inflammatory and sympathetic activities in the liver.
(A) Liver levels of NE, TNF-alpha and IL-6 in CCl4-treated mice according to bactDNA translocation into liver. *p<0.05 compared with bactDNA-negative CCl4-treated mice. (B) Correlations between liver NE, TNF-alpha and IL-6 in CCl4-treated mice according to bactDNA translocation into liver. r: Spearman’s rank correlation coefficient in bactDNA+ mice; NE: norepinephrine; bactDNA: bacterial DNA.
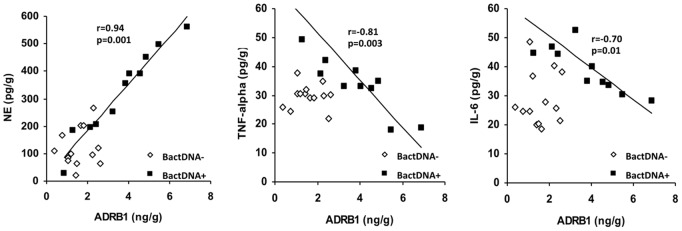
We then evaluated beta-adrenergic receptor levels in the liver of animals at different stages of cirrhosis induction (Table 3). ADRB1 was the only receptor showing a statistically significant increment between treated and control animals at Stage 2. Besides, ADRB1 protein levels correlated with the two cytokines studied and with NE hepatic levels in the subgroup of animals with liver bactDNA translocation, whereas this correlation was not found in animals without bactDNA (Figure 3). ADRB1 increment was also present at mRNA level, showing an inverse correlation with TNF-alpha (r = −0.75, p = 0.01), IL-6 (r = −0.72, p = 0.01) and NE (r = 0.88, p = 0.001) in animals with bactDNA. No correlations were observed for ADRB2 or ADRB3 in any case (Table S2).
Figure 3. Interaction between inflammatory and sympathetic activities in the liver.
Correlations between ADRB1 levels and NE, TNF-alpha and IL-6 levels in the liver of CCl4-treated mice according to bactDNA translocation. r: Spearman’s rank correlation coefficient in bactDNA+ mice; NE: norepinephrine; ADRB1: beta-1 adrenergic receptor; bactDNA: bacterial DNA.
NE Modulates the Liver Pro-inflammatory Response to bactDNA through ADRB1
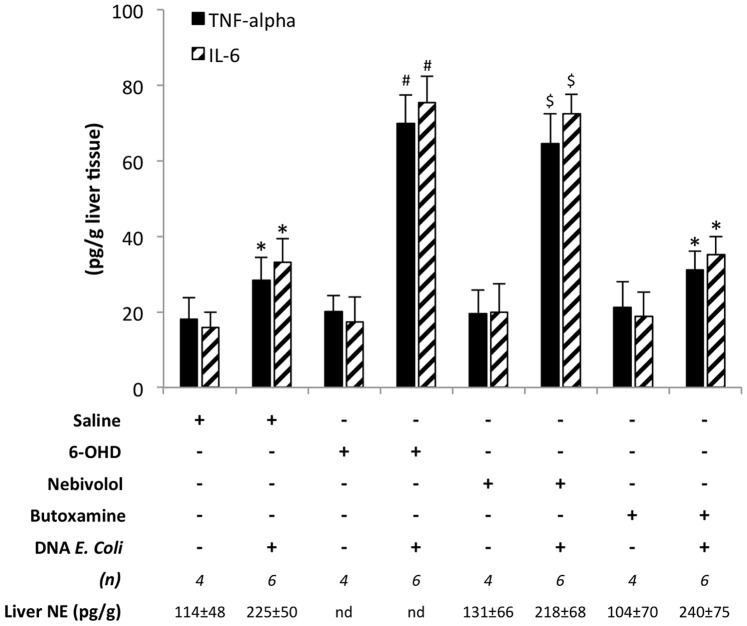
To confirm the interaction between NE and the inflammatory response in the presence of bactDNA in the liver, a second protocol involving chemical sympathectomy and the use of selective ADRB1 (Nebivolol) and ADRB2 (Butoxamine) antagonists was run in mice treated with CCl4 (Figure 1B). After 6-OHD-induced denervation, saline, Nebivolol or Butoxamine treatment in a CCl4 environment, animals received E. coli-DNA to evaluate the inflammatory response to this bacterial antigen. Forty mice were included in this protocol. Hepatic injure was confirmed in all cases (ALT>2000 U/L; TBIL>0.5 mg/dL). mRNA expression of profibrogenic genes, as markers of liver damage, didn’t show statistically significant differences between treatments with saline, 6-OHD and ADRB1 or ADRB2 antagonists (Table S3). NE levels in the liver were undetectable in all mice treated with 6-OHD. PCR and sequencing analysis confirmed bactDNA presence from E. coli in all mice.
Administration of E. coli-DNA induced a significant upregulation of TNF-α and IL-6 in all studied conditions. However, both the ablation of SNS and the selective blockade of ADRB1 with Nebivolol prior to administration of E. coli bactDNA induced further significantly higher levels of TNF-α and IL-6 in the liver than mice treated with saline or with butoxamine prior to administration of E. coli-DNA (Figure 4). On the other hand, no differences were observed between chemically sympathectomized animals and those receiving the selective ADRB1 antagonist. However, statistically significant differences in the hepatic levels of TNF-α and IL-6 were present between 6-OHD and butoxamine treated mice. These data suggest that NE is implicated in tempering down the pro-inflammatory response to bactDNA and that ADRB1 is selectively implicated in mediating this process.
Figure 4. Liver inflammatory levels after different treatments.
Levels of TNF-alpha and IL-6 in the liver of mice undergoing different experimental conditions. All mice were pretreated with an intraperitoneal single dose of CCl4 (1uL/g body weight) to generate liver damage. After 2 days, animals were grouped for a 5-day treatment with saline, 6-OHD (200 mg/Kg, intraperitoneal), Nebivolol (5 mg/Kg every 12 h, subcutaneous) or Butoxamine (5 mg/Kg every 12 h, subcutaneous). Then, animals received E. coli DNA (200ng, intraperitoneal). A subgroup of animals remained without E. coli DNA injection as controls. *p<0,05 compared with all E. coli DNA-negative conditions; #p<0,05 compared with the rest of conditions, except Nevibolol+/E. coli DNA+; $p<0,05 compared with the rest of conditions, except 6-OHD+/E. coli DNA+; 6-OHD: 6-hydroxidopamine; NE: norepinephrine.
Discussion
The present study demonstrates that hepatic NE exerts an immunomodulatory effect over the pro-inflammatory activity observed in the presence of liver bactDNA translocation and that this effect requires ADRB1 in CCl4-induced liver damage.
Experimental BT has been correlated in the last years both with an increased inflammatory response [7] and with an SNS hyperactivation [21] in advanced decompensated cirrhosis with ascites. BactDNA translocation into MLN can be found in preascitic cirrhotic rats associated with an enhanced cytokine profile at a systemic level through the activation of immune cells at the hepatic draining lymph nodes [28]. Also, our group showed the increasing incidence of bactDNA translocation in mice during progression of CCl4-induced liver damage and its association with the decrease of anti-inflammatory gut microbiota content [8].
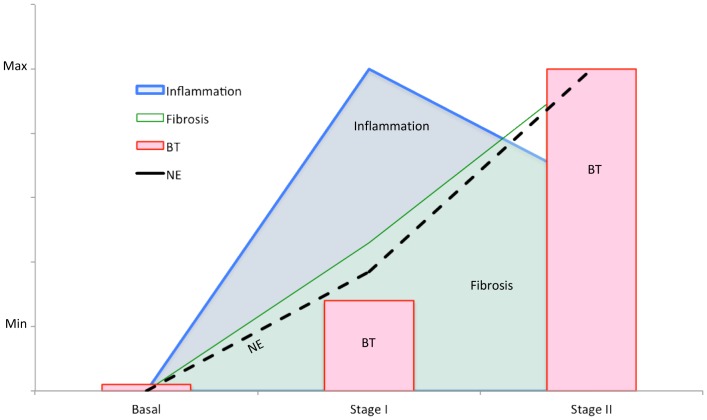
The staged analysis of liver damage induction has allowed us to evaluate the temporal evolution of inflammatory and sympathetic activities, fibrosis progression and bactDNA translocation. TNF-α and IL-6 had already been implicated in fibrosis progression in the past. In fact, if we consider only the beginning (basal) and the end (Stage 2, severe fibrosis) of the induction protocol, a correlation can be found between fibrosis and proinflammatory cytokines. However, intermediate stage results reveal an earlier activation of the inflammatory machinery that may be setting the favorable conditions for fibrosis progression. NE release would then be triggered to temper down the initial pro-inflammatory response and, consequently, BT would be facilitated (Figure 5).
Figure 5. Integration of different processes along study weeks.
Illustration of the temporal evolution on the interaction between fibrosis, bacterial translocation and pro-inflammatory and sympathetic activities in the experimental model of CCl4-induced liver damage. BT: bacterial translocation; NE: norepinephrine.
In the mice model of CCl4-induced liver damage, hepatic NE, TNF-α and IL-6 levels were significantly higher in bactDNA+ vs bactDNA– animals. Interestingly, hepatic levels of NE correlated directly with TGF-β gene expression and inversely with TNF-alpha and IL-6 concentrations in animals with bactDNA (Figure 1). Considering these results, NE would not only act as a profibrogenic agent, as described [12], [15] but also as a modulator of excessive pro-inflammatory response in the liver. This regulation of hepatic pro-inflammatory response might be facilitating bactDNA translocation into liver. In fact, NE has been reported to inhibit LPS-derived TNF-α and IL-6 levels in human whole blood [29].
ADRB are widely expressed on immune cells and play a role in modulating macrophagic function. In fact, ADRB1/ADRB2−/− mice exhibit increased TNF-alpha and decreased IL-10 serum levels in response to LPS challenge relative to wild type animals [30]. In our study, ADRB1 levels in the liver of CCl4-treated mice were significantly increased, they correlated directly with hepatic NE levels and inversely with TNF-α and IL-6 in animals with liver bactDNA translocation (Figure 2). These data suggest the involvement of ADRB1in NE modulation of the inflammatory response to bactDNA translocation. In fact, levels of TNF-α and IL-6 were significantly increased only after a selective blockade of ADRB1 with Nebivolol (Figure 3). These data confirm first, an NE-inflammation crosstalk in the presence of bactDNA, and second, the prominent role of ADRB1 as receptor in the NE modulation of hepatic TNF-α and IL-6 levels. NE signalling through ADRB1 is a well-known mechanism in which adenylate cyclase is activated resulting in a cyclic adenosine monophosphate (cAMP) intracellular increase. cAMP binds protein kinase A (PKA) and their interaction downregulates nuclear factor kappa B (NF-kB) signalling, therefore decreasing pro-inflammatory cytokine secretion [31].
From a clinical standpoint, it has been reported the beneficial effects of non-selective beta-blockers in patients with cirrhosis that have recently been associated with a reduction in community-acquired SBP, independently of the hemodynamic response [23], [32]. However, the use of beta blockers has been associated with poor survival in patients with cirrhosis and refractory ascites [33]. NE is a vasoconstrictor agent that can be used as an alternative to terlipressin in the treatment of hepatorenal syndrome [34]. The possible effect of NE or terlipressin administration on an increased BT rate and subsequent hemodynamic changes is yet to be elucidated in this setting. Although our data suggest that NE administration and the subsequent ADRB1 activation would facilitate increased rates of BT, specific studies would be necessary to ascertain whether administration of terlipressin could have the same effect, as terlipressin acts through different receptor mechanisms. Considering all this data, it would be interesting that new studies using beta-blockers or NE evaluate bactDNA translocation events in decompensated cirrhosis. Data would also support the startup of studies on novel therapeutic strategies based on ADRB antagonists aimed at preventing BT in this setting. In fact, a recent study has demonstrated a beneficial effect of a beta-1 blocker on survival over septic rats through preservation of gut barrier function [35].
In conclusion, the present investigation demonstrates a specific interaction between hepatic NE and the pro-inflammatory response in mice with CCl4-induced liver damage and bactDNA presence that is mediated through ADRB1.
Supporting Information
Primer-pair sequences used in the study.
(DOC)
Correlation scores between ADRB2 and ADRB3 with TNF-alpha, IL-6 and NE.
(DOC)
mRNA expression of profibrogenic genes in animals treated with saline, 6-OHD, ADRB1 and ADRB2 antagonists.
(DOC)
Acknowledgments
In memoriam: Authors are indebted to Dr. Miguel Pérez-Mateo, who recently passed away after a lifetime dedicated to the practice and research on gastroenterology, for his continuous support and advice.
Funding Statement
This work has been supported by grants CP05/0005, PI10/0340 and PI11/0962 from Instituto de Salud Carlos III, Madrid, Spain; AP-026/09 and AP-162/11 from Consellería de Sanitat, Generalitat Valenciana, Spain; C-6/11 from Fundación FCVI-HGUA, Alicante, Spain; and Diputación Provincial de Alicante, Alicante, Spain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Berg RD (1992) Bacterial translocation from the gastrointestinal tract. J Med 23: 217–244. [PubMed] [Google Scholar]
- 2. Garcia-TSAO G (2001) Bacterial translocation: cause or consequence of decompensation in cirrhosis? J Hepatol34: 150–155. [DOI] [PubMed] [Google Scholar]
- 3. Wiest R, Garcia-TSAO G (2005) Bacterial translocation (BT) in cirrhosis. Hepatology 41: 422–433. [DOI] [PubMed] [Google Scholar]
- 4. Guarner C, Runyon BA, Young S, Heck M, Sheikh MY (1997) Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 26: 1372–1378. [DOI] [PubMed] [Google Scholar]
- 5. Guarner C, Soriano G (2005) Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol 17: 27–31. [DOI] [PubMed] [Google Scholar]
- 6. Frances R, Chiva M, Sanchez E, Gonzalez-Navajas JM, Llovet T, et al. (2007) Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol 46: 797–803. [DOI] [PubMed] [Google Scholar]
- 7. Guarner C, Gonzalez-Navajas JM, Sanchez E, Soriano G, Frances R, et al. (2006) The detection of bacterial DNA in blood of rats with CCl(4)-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology 44: 633–639. [DOI] [PubMed] [Google Scholar]
- 8. Gomez-Hurtado I, Santacruz A, Peiro G, Zapater P, Gutierrez A, et al. (2011) Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS One6: e23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kershenobich SD, Weissbrod AB (2003) Liver fibrosis and inflammation. A review. Ann Hepatol 2: 159–163. [PubMed] [Google Scholar]
- 10. Marra F (2002) Chemokines in liver inflammation and fibrosis. Front Biosci 7: d1899–d1914. [DOI] [PubMed] [Google Scholar]
- 11. Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubuisson L, Desmouliere A, Decourt B, Evade L, Bedin C, et al. (2002) Inhibition of rat liver fibrogenesis through noradrenergic antagonism. Hepatology 35: 325–331. [DOI] [PubMed] [Google Scholar]
- 13. Hsu CT (1992) The role of the sympathetic nervous system in promoting liver cirrhosis induced by carbon tetrachloride, using the essential hypertensive animal (SHR). J Auton Nerv Syst 37: 163–173. [DOI] [PubMed] [Google Scholar]
- 14. Hsu CT (1995) The role of the autonomic nervous system in chemically-induced liver damage and repair–using the essential hypertensive animal model (SHR). J Auton Nerv Syst 51: 135–142. [DOI] [PubMed] [Google Scholar]
- 15. Oben JA, Roskams T, Yang S, Lin H, Sinelli N, et al. (2004) Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 53: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henriksen JH, Moller S, Ring-Larsen H, Christensen NJ (1998) The sympathetic nervous system in liver disease. J Hepatol 29: 328–341. [DOI] [PubMed] [Google Scholar]
- 17. Henriksen JH, Ring-Larsen H, Kanstrup IL, Christensen NJ (1984) Splanchnic and renal elimination and release of catecholamines in cirrhosis. Evidence of enhanced sympathetic nervous activity in patients with decompensated cirrhosis. Gut 25: 1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia JJ, del Carmen SM, De la FM, Ortega E (2003) Noradrenaline and its end metabolite 3-methoxy-4-hydroxyphenylglycol inhibit lymphocyte chemotaxis: role of alpha- and beta-adrenoreceptors. Mol Cell Biochem 254: 305–309. [DOI] [PubMed] [Google Scholar]
- 19. Garcia JJ, del Carmen SM, De la FM, Ortega E (2003) Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol Cell Biochem 254: 299–304. [DOI] [PubMed] [Google Scholar]
- 20. Shilov JI, Orlova EG (2003) Role of adrenergic mechanisms in regulation of phagocytic cell functions in acute stress response. Immunol Lett 86: 229–233. [DOI] [PubMed] [Google Scholar]
- 21. Worlicek M, Knebel K, Linde HJ, Moleda L, Scholmerich J, et al. (2010) Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut 59: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 22. Perez-Paramo M, Munoz J, Albillos A, Freile I, Portero F, et al. (2000) Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology 31: 43–48. [DOI] [PubMed] [Google Scholar]
- 23. Senzolo M, Cholongitas E, Burra P, Leandro G, Thalheimer U, et al. (2009) beta-Blockers protect against spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. Liver Int 29: 1189–1193. [DOI] [PubMed] [Google Scholar]
- 24. Ishak K, Baptista A, Bianchi L, Callea F, De GJ, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22: 696–699. [DOI] [PubMed] [Google Scholar]
- 25. Such J, Frances R, Munoz C, Zapater P, Casellas JA, et al. (2002) Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology 36: 135–141. [DOI] [PubMed] [Google Scholar]
- 26. Pritchard MT, Cohen JI, Roychowdhury S, Pratt BT, Nagy LE (2010) Early growth response-1 attenuates liver injury and promotes hepatoprotection after carbon tetrachloride exposure in mice. J Hepatol 53: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Straub RH, Pongratz G, Weidler C, Linde HJ, Kirschning CJ, et al. (2005) Ablation of the sympathetic nervous system decreases gram-negative and increases gram-positive bacterial dissemination: key roles for tumor necrosis factor/phagocytes and interleukin-4/lymphocytes. J Infect Dis 192: 560–572. [DOI] [PubMed] [Google Scholar]
- 28. Ubeda M, Munoz L, Borrero MJ, Diaz D, Frances R, et al. (2010) Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology 52: 2086–2095. [DOI] [PubMed] [Google Scholar]
- 29. van der PT, Jansen J, Endert E, Sauerwein HP, van Deventer SJ (1994) Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun 62: 2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker-Brown J, Roberts MR (2009) Differential contribution of beta-adrenergic receptors expressed on radiosensitive versus radioresistant cells to protection against inflammation and mortality in murine endotoxemia. Shock 32: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES (2000) The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638. [PubMed] [Google Scholar]
- 32. Gonzalez-Suarez B, Guarner C, Villanueva C, Minana J, Soriano G, et al. (2006) Pharmacologic treatment of portal hypertension in the prevention of community-acquired spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 18: 49–55. [DOI] [PubMed] [Google Scholar]
- 33. Serste T, Melot C, Francoz C, Durand F, Rautou PE, et al. (2010) Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 52: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 34. Wong F (2012) Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol 9: 382–91. [DOI] [PubMed] [Google Scholar]
- 35. Mori K, Morisaki H, Yajima S, Suzuki T, Ishikawa A, et al. (2011) Beta-1 blocker improves survival of septic rats through preservation of gut barrier function. Intensive Care Med 37: 1849–1856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer-pair sequences used in the study.
(DOC)
Correlation scores between ADRB2 and ADRB3 with TNF-alpha, IL-6 and NE.
(DOC)
mRNA expression of profibrogenic genes in animals treated with saline, 6-OHD, ADRB1 and ADRB2 antagonists.
(DOC)